Chapter 128 Spinal Cord Stimulation for Chronic Pain
A theory of pain transmission published in 1965 inspired researchers to develop a reversible, nondestructive pain therapy that relied on equipment adapted from cardiac pacemaker technology to deliver electrical stimulation to the spinal cord. The initial results of this therapy, now known as spinal cord stimulation (SCS), were inconsistent, but some patients benefited dramatically. During the intervening decades, refinements in SCS techniques, equipment, and patient selection criteria (Table 128-1) have led to continually improving clinical results.
Table 128-1 Chronic Pain Patient Selection Criteria
| 1. An objective basis for the patient’s pain (with a specific diagnosis). The results of physical examinations and diagnostic imaging studies in patients with failed back surgery syndrome, for example, should be consistent with the reported distribution of radiating pain, and these findings should predominate over functional, nonphysiologic signs.205 |
| 2. Spinal cord stimulation (SCS) should be a late resort. Reasonable alternative xtreatments should be exhausted or comparatively unacceptable (e.g., repeated reconstructive spine surgery). |
| 3. A multidisciplinary evaluation, with specific attention to psychological issues, rules out any unresolved major psychiatric problem or personality disorder, significant issues of secondary gain, or major drug habituation problem. |
| 4. The technical feasibility of overlapping pain with paresthesia and the resulting relief of pain is demonstrated through a screening trial. |
| 5. No coagulopathy (that cannot be reversed during implantation), ulcers close to implantation sites, or chronic septicemia. |
| 6. The patient must be able to control the device. |
| 7. Patients with on-demand pacemaker, defibrillators, or the need for magnetic resonance imaging (MRI) require special attention. Most imaging centers refuse full-body MRI in patients with an SCS system. |
Background
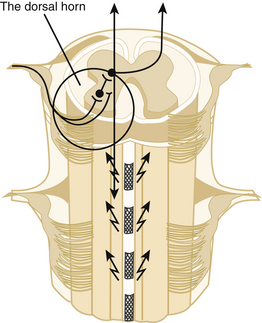
Melzack and Wall’s gate control theory provided a theoretical rationale for the use of electrical stimulation in the management of pain.1 The theory proposes that a neuronal “gate” controls the transmission of pain signals from the dorsal horn of the spinal cord to the brain. An excess of small-fiber afferent input opens the gate, and a dominance of large-fiber afferent activity closes it. (Actually, the gate concept is a bit similar to Head and Holmes’ 1911 proposal that parallel “epicritic and protopathic” input systems govern sensory influx.2 This hypothesis provided the physiologic basis for Gabriel Mazars’ therapeutic trials with sensory thalamic stimulation in Paris beginning in the 1960s, which were the first modern attempts to treat severe neuropathic pain with electric stimulation.3,4)
Because large fibers are more susceptible than small fibers to electrical depolarization, it seemed reasonable to attempt to close the gate and stop pain transmission with low-amplitude stimulation that would selectively recruit large-fiber activity in a mixed population of nerves. Electrical stimulation of mixed peripheral nerves can achieve this effect,5 but stimulation of peripheral nerves at an amplitude close to that required for a therapeutic sensory effect can cause unwanted motor effects. In addition, pain generally involves multiple peripheral nerves. Thus investigators decided to apply electrical stimulation to the spinal cord, where they could recruit primary large fiber afferents from multiple segments conveniently isolated in the posterior columns. As expected, antidromic activation of these primary afferents, whose collateral processes extend into the dorsal horn, yielded a wide area of paresthesia with, in successful cases, ensuing pain relief.
Mechanism of Action
The electrical stimulation techniques that grew out of the gate control theory have succeeded, but the theory remains controversial. One reason is that activation of peripheral large fibers might result in increased pain (hyperalgesia or allodynia) in some pathologic circumstances.6 Thus, peripheral nerve stimulation or SCS might relieve pain by blocking the conduction of primary afferents at the branch points of dorsal column fibers and their collaterals.6 The mechanism of action of SCS, however, cannot depend solely on blocking conduction (e.g., by impulse collision) because electrical stimulation does not inhibit all types of pain,8 and therapeutic SCS does not normally evoke the pain that would occur if SCS also activated small-diameter, high-threshold fibers in the spinothalamic tracts. Dorsal column activation is more successful than ventral stimulation, which is close to the spinothalamic tracts.9
Spinal Cord Stimulation Mechanisms in Neuropathic Pain
In neuropathic pain states, activation of peripheral nerve fibers increases the sensitivity and activity of wide dynamic range neurons in the superficial laminae of the corresponding dorsal horns, which in turn causes hyperalgesia (increased sensitivity to pain) and/or allodynia (normally nonpainful stimuli cause pain). In rat models of neuropathy that employ stimulation parameters similar to those used in humans, SCS effectively suppresses this heightened activity and relieves tactile hypersensitivity as reflected by responses to innocuous stimuli (which is similar to clinical allodynia).10
In a study that sought to determine if SCS suppresses long-term potentiation of wide dynamic range dorsal horn neurons, SCS gradually reduced the C-fiber response to the baseline level. A-fibers, on the other hand, were not potentiated by the conditioning stimulus or affected by SCS.11 This indication that SCS affects C-fiber responses is noteworthy because the findings of previous studies supported the view that SCS primarily influences A-fibers.
Investigators have used finite-element computer techniques to model the electrical fields SCS produces in the spinal cord.12–14 These models reveal distributions of current and voltage that agree with measurements from cadaver and primate spinal cords.15 The models and measurements predict that an electrode’s longitudinal position is the most important factor in achieving the desired segmental effect (fibers decrease in diameter as they ascend the fasciculus gracilis),16 that bipolar stimulation with contacts 6 to 8 mm apart provide the greatest selectivity for longitudinal midline fibers, and that the electrical field between two cathodes that bracket the midline does not sum constructively in the midline. Clinical experience confirms that the correct position and spacing of SCS electrodes is essential and that instead of expanding the area of paresthesia, positioning electrodes more cephalad than the target area commonly elicits unwanted local segmental effects.17
Psychophysical studies have found that stimulation induces a subtle loss of normal sensation in SCS patients but does not affect acute pain sensibility to an extent that could lead to undesirable side effects, such as Charcot joints.18,19 Side effects increase with increases in stimulation amplitude and in recruitment of nerve fibers; thus, psychophysical studies in individual patients should include quantitative measures of stimulation adjusted over the range of amplitudes from perception to motor threshold.20
To explain the sustained pain relief (often lasting from 1 to 3 hours) that patients experience following a 30-minute period of SCS, investigators have hypothesized that SCS affects the release of neurotransmitters in the dorsal horn and brain.21 This led to several lines of investigation, which revealed that SCS changes the concentration of neurotransmitters and their metabolites in cerebrospinal fluid9,22; administration of high doses of opioid antagonists, such as naloxone, does not affect the relief of pain achieved by SCS23,24; and both SCS and administration of γ-aminobutyric acid (GABA) agonists to neuropathic rats suppresses the allodynia that occurs from peripheral nerve lesions.25,26
SCS induces GABA release in the dorsal horn,27 and the pain-relieving effect of SCS depends on activation of the GABA-B receptor.25,26 In fact, for a period of time SCS inhibits the pathologic response properties of dorsal horn neurons often observed in allodynic rats after peripheral nerve injury (e.g., elevated firing frequency of wide dynamic range neurons, presence of after-discharge),10 conceivably because of an electrically induced increase in GABAergic activity.28
SCS likely prompts the release of a multitude of as-yet-unidentified transmitters and neuromodulators in the dorsal horn as well as supraspinally.8,27,29,30 In addition to GABA, animal and human studies indicate that SCS releases substance P, serotonin, glycine, adenosine, and noradrenaline in the dorsal horn.21,22,26 The resultant beneficial effect likely depends on a complicated interaction among several substances.31 Studies also indicate that the cholinergic system is involved in the SCS effect in painful neuropathy via activation of the muscarinic M4 receptors.32,33 Ongoing studies of descending inhibition from the brain stem, where several loci might be activated by the orthodromic SCS-induced impulses, show that SCS upregulates 5-HT activity in the dorsal horns in SCS-responsive rats34 and that the segmental serotonin-induced inhibition is likely mediated via GABA-B receptors on local dorsal horn cells.34
This emerging knowledge might be used to tailor adjunct pharmacologic therapy in patients whose response to SCS is less than optimum.35,36 In fact, the first clinical trial of adjunct pharmacologic therapy, conducted in 48 subjects who had neuropathic pain that had not responded well to SCS, found that intrathecal delivery of the GABA-B agonist baclofen in addition to SCS was beneficial in a subgroup (about 20%) of the subjects and that the effect was durable over a long time.37,38 Other drugs, like clonidine, which partly exerts its beneficial effect via the cholinergic system, have been effective in selected cases.39 The mechanisms discussed in this section are schematically outlined in Figure 128-1.
Spinal Cord Stimulation Mechanisms in Ischemic Pain
Peripheral Vascular Disease (Peripheral Arterial Occlusive Disease)
Ischemic pain is the only type of nociceptive pain known to respond to SCS, and the mechanisms involved in the stimulation-induced alleviation of ischemic pain differ fundamentally from those involved in the relief of neuropathic pain.9,28,40
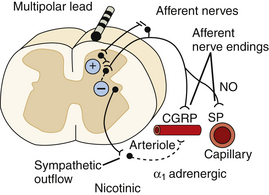
SCS appears to exert its beneficial effect in the treatment of ischemic extremity pain by reducing tissue ischemia through increased or redistributed perfusion to the ischemic area and/or by decreasing tissue oxygen demand. In peripheral arterial occlusive disease, the results of experimental studies favor the idea that SCS suppresses efferent sympathetic activity, particularly vasoconstriction maintained by nicotinic ganglionic receptors and mainly by α1-adrenoreceptors in the periphery.41,42 This reduced peripheral vasoconstriction results in reduced ischemia and secondary relief of pain.40 Antidromic mechanisms might also be activated by SCS at intensities far below the motor threshold,43–47 and this might result in peripheral calcitonin gene-related peptide (CGRP) and nitric oxide (NO) release, with subsequent peripheral vasodilatation. The balance between the two mechanisms seems to depend on the level of activity of the sympathetic system, SCS intensity, and individual patient factors (e.g., genetic differences, diet), but animal studies indicate that antidromic activation might be more important during an initial vasodilative period, whereas sympathetic inhibition appears to support persistence of increased peripheral blood flow.42,47
Investigation into the powerful therapeutic effect of SCS in vasospastic disorders (ischemic skin flaps induced in experimental animals48; patients with Raynaud’s syndrome8) shows that the mechanism of preemptive SCS might involve blocking or reducing vasospasm. This is consistent with theories that Raynaud’s syndrome is caused by a heightened sensitivity or increased density of α-adrenergic receptors49 in possible combination with dysfunction in the CGRP system.50 Consequently, a stimulation-induced “normalization” of function in each system could underlie the efficacy of SCS in treating this condition. Figure 128-2 illustrates the possible mechanisms of action of SCS in peripheral ischemia.
Complete lumbar sympathectomy in laboratory animals abolishes the beneficial vasodilative effects of SCS on skin and muscle tissue.51 In some of the animals, even an incomplete sympathetic denervation led to partial loss of SCS’s vasodilative benefit. This supports the notion that the beneficial effect of SCS depends on its action on the sympathetic system; however, because sympathetic blocks or even surgical sympathectomies are rarely complete, SCS can be tried in patients with previous sympathetic interventions.
Complex Regional Pain Syndrome
SCS therapy is often effective in complex regional pain syndrome (CRPS) accompanied by signs of dysautonomia. In principle, SCS could affect pain syndromes related to sympathetic hyperactivity by direct action on central hyperexcitability and/or by encouraging development (direct coupling) of de novo abnormal contacts between peripheral sympathetic and damaged somatosensory fibers.52 The indirect-coupling hypothesis, which proposed that damaged sensory neurons might become so hypersensitive to mild degrees of hypoxia that even moderate increases in sympathetic activity with peripheral vasoconstriction could excite the damaged afferents52,53 was not supported by the findings of a study in which SCS did not cause peripheral vasodilatation in subjects with CRPS type I.54
The mechanisms underlying the effects of SCS on pain due to ischemia in the extremities, whether from occlusive vascular disease or vasospasm, seem to rely on a rebalancing of oxygen supply and demand (i.e., relief of net ischemia). The mechanisms discussed in this section are schematically outlined in Figure 128-2. SCS-induced vasodilation in a situation with low sympathetic vasoconstrictor tone might also occur as a result of antidromic activation, whereas with a high level of sympathetic activity, SCS-induced sympathetic inhibition could also contribute to the effect.55–57
Angina Pectoris
The mode of action of SCS in otherwise refractory angina pectoris appears to be complex, and investigators have derived conflicting data from experimental and clinical studies. Although the first experimental studies revealed that SCS has a direct inhibitory effect on cardiac nociception,58 the clinical studies that followed clearly demonstrated that partial resolution of cardiac ischemia seems to be a pivotal factor in the antianginal effect of SCS. Some researchers believe that a stimulation-induced increase in blood flow or redistribution of the blood supply from well-perfused to ischemic regions in the heart is the cardinal underlying factor,59 whereas others credit a decrease in cardiomyocyte oxygen demand.60 In any case, this reduced ischemia is manifest as decreased ST changes on electrocardiography as well as reversal of lactate production to extraction. Experimental studies, however, have been unable to demonstrate a local blood flow increase in the myocardium61; instead, preemptive SCS seems to increase the myocardium’s resistance to critical ischemia.62
Another observation of possible importance is that local coronary ischemia excites the intrinsic cardiac nervous system, which consists of mixed somatosensory and autonomic ganglia located in fat pads on the exterior surface of the heart and mediates neural activity to and from the heart. This ischemia-induced excitation might lead to generalized ischemia by encouraging dysrhythmia. SCS seems to inhibit and stabilize the activity of these cardiac neurons, especially during an ischemic challenge.61,63,64 Indeed, SCS can counteract severe cardiac arrhythmia.65,66
The last word on the use of SCS in cardiac ischemia remains to be written, and the effects of stimulation might extend beyond the relief of pain to provide cardioprotection before the advent of additional chest pain.62,67
Spinal Cord Stimulation Devices
Electrode Placement and Design
The earliest applications of SCS involved high thoracic electrode placement in an attempt to treat pain in all caudal segments68; however, this strategy commonly caused excessive, uncomfortable radicular effects to occur before the desired segments could be recruited. When clinicians realized that stimulation paresthesia should overlap the distribution of pain, they adjusted the placement of electrodes to achieve this effect more selectively; for example, low thoracic electrode placement (T9 to T12) is most effective in the treatment of persistent low back and lower extremity pain following spine surgery (failed back surgery syndrome [FBSS]).69
In the late 1960s and early 1970s, SCS electrodes were two-dimensional and required a laminectomy or laminotomy for introduction into the epidural, endodural, or subarachnoid space.70–72 Use of these electrodes was problematic because clinicians had no way of determining the ideal spinal level for electrode placement in any given patient and because laminotomy under local anesthesia limits longitudinal access. Furthermore, even when electrodes are placed so that paresthesia overlaps the area of pain, not all patients report pain relief. For these reasons, test stimulation with a temporary electrode is desirable.
Accordingly, in the 1970s, investigators developed percutaneous techniques using a Tuohy needle to insert temporary catheter-type electrodes73–76 for a screening trial to establish the best level for electrode placement and to determine if SCS had the desired analgesic effect. Clinicians soon applied these percutaneous techniques to the implantation of electrodes for chronic use, thus avoiding the need for laminectomy.77,78 Use of a percutaneous technique to place multiple individual electrodes and achieve bipolar stimulation, however, increased the likelihood of electrode migration, compromising stimulation, thus reducing or eliminating pain relief, and requiring surgical revision.
In the early 1980s, in response to this problem, electrode manufacturers introduced percutaneous electrodes with arrays of contacts. If such an electrode migrates slightly, its implanted pulse generator can be reprogrammed with a different selection of stimulating anodes and cathodes to reestablish appropriate paresthesia. This noninvasive postoperative adjustment can be made with the patient in the upright or supine position (in which the device is ordinarily used, as opposed to the prone position in which it is usually implanted). Multicontact programmable systems rarely require surgical revision, and this development has led to significantly improved long-term clinical results.79–81
New electrode designs based on computer models of SCS13 are being tested clinically.82 These configurations should make it even easier to steer paresthesia to cover the painful area. Clinicians are also improving results by refining the method of anchoring percutaneous electrodes.83
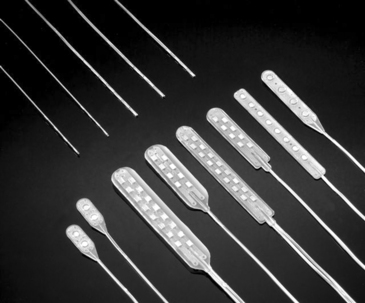
Despite these improvements in the use of percutaneous electrodes, properly placed laminectomy electrodes offer advantages. For example, a prospective randomized, controlled technical comparison involving 24 patients—half of whom received a four-contact percutaneous electrode and half a four-contact insulated laminectomy electrode84—yielded significantly superior results with the laminectomy electrode for paresthesia coverage of pain, at the same time reducing power requirements sufficiently to double battery life.
Figure 128-3 shows a sample of percutaneous and laminectomy electrodes as well as the oblique-lateral approach used to place a percutaneous electrode and a small laminotomy opening for a laminectomy electrode. The percutaneous electrode is inserted under local anesthesia, which does not interfere with the clinician’s ability to monitor paresthesia during test stimulation. The laminectomy electrode can be implanted with local anesthesia alone, using regional anesthesia (with paresthesia achieved at a slightly higher than normal stimulation threshold to guide electrode positioning) or even with spinal anesthesia to a degree allowing intraoperative paresthesia testing.85,86
Pulse Generators
The prototype SCS generator, used exclusively during the first decade of experience, was a passive implant powered to deliver stimulation pulses by an external radiofrequency transmitter. Although the implant contained no life-limiting components and thus avoided the expense and potential morbidity of eventual replacement, this system was cumbersome. An implanted pulse generator (IPG) powered by an internal battery was subsequently developed from pacemaker technology. Patients operate these systems and control the amplitude within preset limits with an external magnet or handheld remote control. The first IPGs were powered by nonrechargeable lithium cells that required replacement approximately every 4 years. To avoid such frequent surgical replacement of the battery, with attendant expense and risk, SCS device manufacturers developed IPGs with rechargeable batteries. This, of course, increases initial cost, and comparative cost effectiveness remains to be established by long-term study. The less expensive radiofrequency systems remain in use but are no longer manufactured.
Figure 128-4 shows representative pulse generators.
Computerized Methods
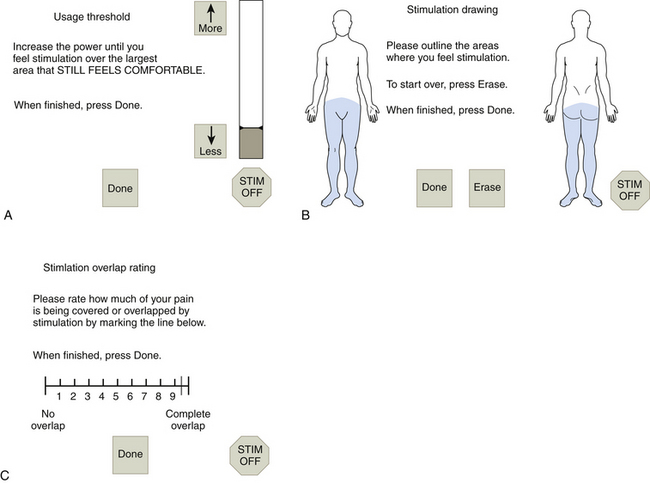
By scaling the amplitude from perception of pain and paresthesia overlap to stimulation of discomfort, we can compare the results of various electrode configurations and stimulation parameters at identical subjective stimulus intensities.87
Systematic quantitative assessment of these effects generates a large volume of data that would be prohibitively difficult to analyze without a computer.88,89 These data can be entered by a skilled operator working with the patient or, given a suitable means of control, by the patient alone. Figure 128-5 illustrates a computer system that presents the patient with a prespecified series of contact combination and pulse parameters. The patient adjusts the stimulation amplitude and draws the area of paresthesia for comparison with drawings of the painful area. Optimal settings are derived from analysis of these results. In a randomized, controlled trial involving 44 patients from two centers, the computerized system produced significantly better technical results at a significantly faster rate than did the manual adjustment method. This occurred regardless of practitioner experience, but results improved with patient experience.90 Use of the computerized system also allowed identification of new settings that improved expected battery life for 95% of the patients.91 With an assumed battery use of 24 hours per day, the average battery life predicted after manual settings was 25.4 ± 49.5 months versus 55.0 ± 71.7 months for the computerized settings. For 72% of the patients, the settings that extended battery life led to equivalent or improved technical results.
Screening Protocols
Percutaneous placement of a temporary epidural electrode for an SCS screening trial is a straightforward procedure that can take place in a fluoroscopy suite instead of an operating room and facilitates testing of electrode positions and contact combinations for optimal therapeutic effect. Indeed, most third-party payers in the United States and in some European countries require that patients complete a successful screening trial before undergoing implantation of an SCS system for chronic use. A brief period of intraoperative stimulation immediately before permanent implantation technically meets this requirement,92 but an extended trial allows the patient to assess stimulation effects while engaging in everyday activities.
The role of SCS trials was assessed critically in a retrospective comparison of 15-minute versus 5-day trials in 54 patients, in which the positive predictive value was equivalent for predicting SCS outcome.93 The trial success rate, however, was an extraordinarily high 47 of 52 at 5 days, and the number of patients who failed the prolonged trial (5) was significantly greater than the number who failed the on-table trial (1); thus, despite the equivalent predictive value of each trial, the prolonged trial identified more patients who would fail long-term therapy. Furthermore, if all clinicians obtained such a high trial success rate for patients with chronic low back pain and/or lower extremity pain, the trials would not be necessary. In fact, a lower trial success rate is likely to result in a higher long-term success rate, and some reports note that as few as 40% of patients undergoing temporary electrode placement proceed to permanent implantation.94
Whereas some clinicians go in the other direction and extend the SCS screening trial for as long as 2 months95 (indeed, some European health authorities require 30-day trials), the potential morbidity of infection and epidural scarring (which can compromise permanent device implantation) and the expense of such intensive follow-up must be balanced against the potential yield of a prolonged trial.
• Anchoring and removing an anchored electrode (if the trial fails) must take place in an operating room instead of a fluoroscopy suite. This increases the cost.
• Anchoring a temporary electrode implies a commitment on the part of the patient and the physician to proceed to internalization, which partially defeats the purpose of the trial.
• The physician cannot adjust the position of an anchored electrode at the bedside as the patient gains experience with the system. In contrast, if the physician places a temporary percutaneous array at the most cephalad position that shows promise during preliminary testing of the naive patient, the electrode can be incrementally withdrawn at the bedside (instead of in an operating room or fluoroscopy suite) for testing at more caudal positions. Plain radiographs can document successful repositioning.
• Pain from the anchoring incision might confound the results of the therapeutic trial.
• Placing a percutaneous extension and staging the implant increases the risk of infection.96,97
The criteria that have been used for proceeding from a trial to a system implanted for chronic use have varied from 30%98 to 75% reported pain relief.99–101 The first author (RBN) typically conducts 7- to 9-day trials, shortening or extending them as appropriate, with patients proceeding to implantation for chronic use after achieving at least 50% reported relief of pain with stable or improved levels of activity and analgesic use.
Indications for Spinal Cord Stimulation for Pain
Neuropathic Pain
Failed Back Surgery Syndrome
When FBSS causes a chief complaint of axial low back pain, achieving pain overlap by paresthesia is technically difficult and might require the use of complex electrode arrays (and/or the addition of subcutaneous electrodes).102,103 In addition, mechanical or nociceptive axial low back pain does not respond as well to SCS as does neuropathic pain.29,104
Initial case reports indicate that SCS is safe for FBSS patients with cardiac pacemakers or cardioverter defibrillators105 if the devices are managed appropriately.106
Peripheral Nerve Injury
SCS is also used to treat pain from peripheral nerve injury (e.g., postherpetic neuralgia and CRPS) with or without signs of disturbed sympathetic function. For CRPS, appropriate SCS electrode placement in the cervical or lumbar regions yields statistically equivalent results.107
SCS is used to treat both stump neuroma pain and phantom limb pain (the latter requiring that the phantom limb be covered with paresthesia). Pain from pressure applied directly onto a stump neuroma (e.g., from a prosthesis), however, does not respond adequately to SCS.108 This might be due to the technical difficulty of covering an entire phantom limb with paresthesia or to the degeneration of dorsal column fibers as a consequence of nerve damage.
Ischemic Pain
Peripheral Arterial Occlusive Disease
Clinicians have used SCS to treat pain arising from peripheral arterial occlusive disease (PAOD) since Cook and associates published their report in 1976.109 When several investigators presented encouraging results from confirmatory studies,110–115 the use of SCS for ischemic pain spread rapidly in Europe.
Thus, in many countries, the use of SCS for PAOD decreased considerably. During 1994, for example, Swedish neurosurgeons implanted only 13 SCS systems for PAOD.40 The fact that most PAOD patients have a satisfactory outcome and more than half achieve good pain control, however, has helped the application of SCS for PAOD to survive in a few centers, which follow strict patient selection criteria (Table 128-2).
Table 128-2 Additional Peripheral Vascular Disease Patient Selection Criteria
| 1. Severe pain at rest, with or without defined tissue loss (Fontaine grade III) |
| 2. Reconstructive vascular surgery is impossible or contraindicated. |
| 3. Life expectancy is more than 3 months. |
| 4. Any ischemic ulcer is less than 3 cm in diameter. |
| 5. If arrest of tissue loss, or use of SCS as an adjunct in ulcer healing, is the primary goal, it should be evaluated objectively. |
| 6. Any gangrene should be dry, and when patients have gangrene, SCS is regarded as a means of obtaining a more distal amputation site. |
| 7. Appropriate preoperative transcutaneous oxygen pressure (TcPo2) is used, measured apically on the diseased extremity,202 compared with the patient in supine versus seated position,203 or change is assessed while the patient breathes pure oxygen.204 |
| 8. During a screening trial, the patient should report a significant decrease in the ischemic component of the pain on a visual analog scale and/or demonstrate a clear increase in TcPo2 or in some other objective indicator of microflow concurrent with the therapy. |
| 9. The patient should be able to understand that SCS can alleviate ischemic pain but not nociceptive pain from ulcers and gangrene. |
SCS alleviates ischemic pain (and this effect is immediate only for vasospastic conditions and angina pectoris). Typical ischemia (e.g., of the foot) might induce ischemic, neuropathic, and nociceptive pain from ischemic ulcers and from the border of gangrenous zones.116,117
Clinicians who used SCS to treat ischemic pain conducted prospective randomized studies to determine the impact of SCS on tissue salvage118 and found statistically significant limb-saving effects of SCS only in subgroups of their subjects (see later section on Ischemic Pain under Clinical Results).
Despite a recommendation that clinicians should not offer SCS therapy to patients with major tissue loss,119 however, patients who reach stage IV on the Fontaine Classification System for Peripheral Artery Disease (tissue loss or ulceration) might benefit from SCS therapy, and patients with diabetes can do as well as those without this condition.120
Initial case reports indicate that a patient undergoing SCS for PAOD can later receive a cardiac pacemaker or a dual-chamber cardioconverter-defibrillator capable of delivering tiered therapies in both the atrium and ventricle with no adverse effect on either therapy,121 given proper programming of the devices (see earlier).
Angina Pectoris
Angina pectoris is often refractory to standard treatment (administration of appropriate pharmaceuticals and revascularization) and is a major reason for hospitalization. As more and more patients live longer with coronary artery disease, the number with refractory angina will increase. Many patients suffering from disabling angina (New York Heart Association [NYHA] Class III to IV) are elderly or have a comorbidity that makes them unsuitable candidates for invasive first-line treatment. Other patients have typical symptoms of angina but no signs of obstruction in cardiac circulation and are said to suffer from syndrome X, which has its physiologic basis in small vessel disease, vasospasm, or some other undetected anomaly.122
In the 1980s, transcutaneous electrical nerve stimulation became the first stimulation technique used to treat otherwise refractory angina pectoris,123,124 and the outcome was so promising that clinicians who were already using SCS to treat ischemic pain in the lower extremities began to position the electrode at the T1 to T2 level so they could induce paresthesia that would also overlap the pain of otherwise refractory angina.125,126
The initial use of SCS for angina caused concern that paresthesia would conceal the warning signs of a myocardial infarction, but paresthesia has neither this effect nor an adverse impact on arrhythmia.127,128
That thoracic SCS can be safely used in angina patients being treated concurrently with a pacemaker was demonstrated by researchers who conducted electrocardiographic monitoring in 18 subjects while increasing the pacemaker setting and SCS intensity to the maximum tolerated. The investigators also asked the subjects for information on any interference during long-term treatment. Nothing indicated an adverse reaction to this combination treatment, but the investigators recommended individual patient assessment and proposed a safety testing procedure.106
The first report of SCS to treat angina in a patient with a cardioverter-defibrillator appeared in 2007129 and was followed the next year by a report from the same investigators of a study demonstrating a time-dependent positive effect of SCS on the arrhythmic substrate in three such patients.130
Although thousands of SCS systems have been implanted for angina and the success rate is greater than 80%,131 the U.S. Food and Drug Administration has not approved the use of SCS specifically for this indication; however, long-standing approval for intractable pain of the trunk and limbs might apply.
Table 128-3 lists additional selection criteria for patients with angina who are being considered for SCS therapy.
| 1. Severe handicapping angina pectoris (New York Heart Association Class III or IV) |
| 2. Significant coronary artery disease or syndrome X refractory to conventional treatment. |
| 3. Further revascularization therapy not immediately applicable. |
| 4. Demonstrated reversible myocardial ischemia |
| 5. Pain alleviation with transcutaneous electrical nerve stimulation (not an absolute criterion) |
| 6. No recent (<6 mo) acute myocardial infarction |
| 7. No concurrent heart disease (e.g., pericarditis, myocarditis) |
Clinical Results
Neuropathic Pain
For an annotated bibliography of the studies that report the use of SCS to treat neuropathic pain, see www.neuromodfound.org.
Failed Back Surgery Syndrome
After retrospective studies indicated that SCS can produce better results with fewer risks than those associated with reoperation in FBSS patients,132,133 the first author’s (RBN’s) research group conducted the first prospective randomized, controlled trial in subjects whose FBSS caused unrelieved leg pain with or without low back pain. All subjects were eligible for a specific operation to relieve nerve compression and were randomized either to SCS or to the proposed reoperation. Subjects with unsatisfactory results could request crossover to the other treatment, and the frequency of this crossover was a primary outcome measure. Among 45 subjects (90%) available for a mean follow-up of 3 years, SCS was successful in 9 of 19, whereas reoperation was successful in only 3 of 26. Subjects randomized to SCS were also less likely to cross over (5 of 24) than were those randomized to reoperation (14 of 26).134 Each of these differences in outcome was statistically significant, favoring SCS. Six of the subjects who crossed to SCS from reoperation achieved success with SCS, bringing the success rate to 15 of 38 who received SCS as a final treatment. In contrast, no one who failed SCS achieved success with reoperation.
In an international multicenter randomized, controlled trial (the PROCESS study), 100 subjects with FBSS were randomized to conventional medical management (including medication, nerve blocks, physical therapy, massage, etc., n = 48) or SCS plus conventional medical management (n = 52).135 By 6 months, the subjects randomized to SCS achieved significantly greater pain relief and improved functional capacity and health-related quality of life than did those randomized to CMM. The investigators followed the 42 subjects randomized to SCS who actually received SCS for 24 months and found that leg pain relief, functional capacity, and quality of life had significantly improved in this group compared with their pretreatment status.136
Another international multicenter randomized, controlled trial (the EVIDENCE study) is now under way to test the reproducibility of North’s single-center randomized, controlled trial and to determine any effect of advances in surgical practice and in SCS on the comparative efficacy of these procedures.137
Peripheral Nerve Injury
An important randomized, controlled trial of the use of SCS to treat neuropathic pain did not offer crossover.138,139 Instead, the investigators randomized subjects with CRPS to SCS plus physical therapy (n = 36) or to physical therapy alone (n = 18). All of the study subjects had failed 6 months of physical therapy and other pain treatments, including transcutaneous electrical nerve stimulation and sympathetic block (which might reduce the therapeutic effect of SCS, see earlier). Only 24 (two thirds) of the subjects randomized to SCS passed the screening trial and received implants, but the intent-to-treat analysis (which included the SCS failures) showed significant improvements in pain intensity and global perceived effect in the SCS group versus the physical therapy group. Although this study was insufficiently powered for long-term follow-up, the investigators published a 5-year report.140 Among the problems with this report, the investigators present an “intent to treat” analysis that includes patients randomized to SCS whether or not they received a stimulator for chronic use and, oddly, excludes the four patients randomized to physical therapy who received SCS and the one patient randomized to SCS who received a “special” stimulator. We consider the results of this analysis to be of little value. More useful is the comparison of the remaining 20 patients randomized to SCS who actually received implanted SCS systems with the 13 remaining patients randomized to physical therapy. The patients treated with SCS had significantly better results in both outcome measures.140
In a nonrandomized study in patients with CRPS, favorable results were obtained both for persistent spontaneous pain and for the stimulus-evoked pain component (allodynia).141 It should be noted, however, that all the patients accepted for implant in this study had reacted positively to a sympathetic block.
Another neuropathic pain syndrome that often resists therapy is postherpetic neuralgia. One prospective study indicates that otherwise intractable pain from herpes zoster and postherpetic neuralgia responds so well to SCS that some patients were able to discontinue the therapy. In this study, the only patients who failed SCS treatment had serious comorbidities.142
As mentioned earlier, SCS can relieve phantom limb pain, and an analysis in 19 patients found that SCS relieved the pain in 6 (32%), sometimes producing a “dramatic effect,” with stimulation needed only infrequently. When SCS failed, brain stimulation was successful in 6 of 10 patients.143
SCS to treat pain after spinal cord injury is reportedly effective in patients with incomplete lesions or pain confined to the level of injury.81,144 In general, pain of central origin is difficult to treat with SCS.
Ischemic Pain
Peripheral Vascular Disease
Two studies compared results in PVD patients randomized to SCS or to conservative treatment. One included 51 patients and found a clear, though not significant, trend for a limb-saving effect of SCS.118 This study protocol, however, had technical problems: no percutaneous trial was performed, and 12% of the patients randomized to SCS did not receive implants for various reasons. The investigators also observed a tendency for a less-satisfactory result in patients with arterial hypertension (excluding the hypertensive patients from analysis significantly reduced the amputation rate among the remaining group). Other researchers consider arterial hypertension to have no impact on outcome of treatment with SCS.145
In the other prospective randomized, controlled trial—a Dutch multicenter study—120 patients with non-reconstructable PVD were randomized to SCS versus conservative treatment. The first data analysis considered only mortality and limb survival at 2-year follow-up and found no benefit to limb survival of SCS treatment.146 Additional analysis of data from baseline capillary microscopy, laser Doppler perfusion, and transcutaneous oxygen pressure (TcPo2) measurements, however, allowed investigators to classify the pretreatment microcirculatory status of their patients as poor, intermediate, or good.147 By 18 months’ follow-up, in each treatment group, those with poor baseline microcirculatory status had a high rate of amputation and those with good baseline microcirculatory status demonstrated a low rate of amputation. The only significant difference between the treatment groups was found in the patients with an intermediate microcirculatory baseline status (TcPo2 between 10 and 30 mm Hg). In this group, 24% of those who received SCS underwent amputation versus 48% of those who received standard treatment. By 24 months, however, the amputation rate had climbed to 48% for SCS and 54% for standard treatment.148 Each treatment reduced pain, but SCS patients required fewer analgesics. No other significant treatment differences were found in pain or quality of life.
A 2003 report from the European Peripheral Vascular Disease Outcome Study considered the effect of SCS treatment on limb survival in patients with critical leg ischemia and the effect of using TcPo2 as a screening tool for patient selection.149 Subjects with a baseline forefoot TcPo2 of less than 30 mm Hg were divided into three groups: one group (n = 41) obtained pain relief and more than 75% paresthesia coverage after an SCS screening trial lasting a minimum of 72 hours; the second group (n = 32) failed the trial; and the third group (n = 39) received no SCS treatment. The SCS treatment group obtained significantly improved pain relief and TcPo2 scores as well as significantly better limb survival at 12 months.
When the same authors reviewed TcPo2 data in cases of inoperable PVD,150 they reported that 1-year limb salvage was 83% in SCS patients whose baseline supine TcPo2 was greater than 10 mm Hg, baseline change in TcPo2 from supine to sitting was greater than 17 mm Hg, and treatment difference was greater than 4 mm Hg. The general SCS treatment salvage rate regardless of TcPo2 was 68%. The investigators proposed that these parameters would help guide patient selection when the goal of SCS is limb salvage.
A group of Italian investigators believes that a 50% improved TcPo2 score during a 2-week SCS screening trial predicts that SCS treatment leads to limb salvage, regardless of baseline score or disease stage.151–153 In the case of diabetes, however, the same investigators found that the stage of neuropathy was inversely related to SCS treatment success independent of the stage of the disease.154
These clinical analyses are helping investigators identify the optimal patient selection criteria for SCS treatment of PVD. Much remains to be determined, however. A meta-analysis, for example, that included six controlled studies of SCS versus conventional therapy for inoperable critical leg ischemia found positive results for the SCS patients in terms of limb salvage, pain relief, and Fontaine stage attained after treatment but no overall difference in ulcer healing.155 The only trial included in the meta-analysis that used presence of ischemic ulcers as an inclusion criterion occurred in 86 subjects who were undergoing a 21-day regimen of intravenous prostaglandin E1 therapy for nonhealing ulcers and were categorized as Fontaine stage IV with end-stage PVD. The investigators randomized these subjects to receive SCS (n = 45) or not (n = 41), and the SCS group showed significantly better total healing of foot ulcers (69% versus 17%; p < 0.0001) but no difference in amputation frequency at 1-year follow-up.156 In each group, healing of ulcers or amputation wounds was best among patients whose TcPo2 rose to an average of 26.0 ± 8.6 mm Hg.
The favorable impact of SCS on ulcer healing is echoed in a report of a patient with severe Raynaud’s syndrome of long duration in whom, by improving microcirculation, SCS treatment led to complete resolution of all fingertip ulcers as well as of pain.157
Every published study on the use of SCS to treat vasospastic conditions, such as Raynaud’s, demonstrates a positive effect of the therapy.158–162 This is not surprising because, compared with PAOD patients, patients with vasospastic conditions are relatively young, present with relatively few obliterative vessel wall processes, and have symptoms that are often temporarily relieved by destroying or blocking the corresponding sympathetic ganglia. The few published studies, however, involve only small numbers of patients.
Because the long-term results of SCS seem to be superior to those of many other therapies, clinicians should attempt to identify candidates whose ischemic pain has major vasospastic components. A comparison of the outcome of different interventions in a case of vasospasm, for example, revealed that sympathetic blockade and sympathectomy provided only temporary effects, whereas SCS produced a long-lasting increase in blood flow index.163 SCS has also been used successfully to treat pain in frostbite.164
Angina Pectoris
In general, 80% to 90% of angina patients treated with SCS report marked pain relief, with a diminished frequency of anginal attacks, a diminished need for short-acting nitrates (but other medication use is often unchanged), fewer visits to the emergency department, and enhanced quality of life.60,131,165
Many investigators report SCS-induced changes in various indices of coronary ischemia during workload, such as reduction in ST segment depression, reversal of cardiac lactate production to extraction, and an increase in working capacity.166–168 In these studies, the multipolar electrode was generally placed with its center near T1 to T2. Gonzalez-Darder and associates, however, reported positive effects of SCS with the electrode tip at C2 in 23 angina patients followed at least 3 months.169 In their patients, SCS decreased the number of angina attacks from an average of 124 per month to 8 per month and led to reclassification of most patients as NYHA class I from a pre-SCS class of III to IV.
A meta-analysis of treatment of angina with SCS from 114 centers with a total of 517 patients and a median follow-up of 23 months170 found that the cardiovascular mortality rate of approximately 5% per year was related to several factors, including sex, cardiovascular history, age older than 71 years, and the use of medications prescribed for severe disease. Nothing has indicated that SCS alters long-term mortality in this patient group.
The ESBY Study (electrical stimulation versus bypass surgery) randomized 104 patients with angina to SCS or to coronary artery bypass surgery and found a significant difference in mortality favoring SCS, with no significant difference in symptom relief in surviving patients.171 In the absence of SCS, only in the bypass group did exercise capacity increase significantly and various indices of ischemia change positively. (The investigators discontinued stimulation during the 24 hours before the exercise test because they believed the “anti-ischemic effect during ongoing stimulation” required no further documentation, and they wanted to determine if the anti-ischemic effect of SCS would continue despite discontinuation. They found that it did not.)
These results are supported by findings in a study of 15 angina patients who had been treated with SCS for a mean of 39 ± 27 months and were randomized to 48-hour ambulatory electrocardiograph monitoring with SCS stimulation on or off for 24 hours.172 During the off periods, the number and duration of ischemic events increased, as did the total ischemic burden (heart rate variability parameters were similar during both periods).
The impact of discontinuing stimulation in angina patients remains controversial, however, because the ESBY study also found that the number of anginal attacks decreased significantly in patients who underwent coronary artery bypass surgery and in those with temporarily disconnected SCS, which means that some benefits of SCS in angina extend beyond active stimulation.173
Another study indicated that temporary SCS dysfunction increases anginal symptoms. In this study, 32 patients had been treated with SCS for an average of 65 months before battery depletion or electrode failure (n = 7) led to complete cessation of stimulation. In all patients, the number of anginal attacks, consumption of nitrates, and physical dysfunction (assessed with the Seattle Angina Questionnaire) increased during the time without stimulation and decreased when stimulation was restored.174 Overall, we can conclude that at least some of the effects of SCS on angina rely on continuous stimulation, and patients do not become tolerant to, and thus do not cease to benefit from, this therapy.
A long-term (4.8 years) follow-up report on the 104 patients in the ESBY Study found no significant difference between the surviving patients treated with coronary artery bypass surgery and those treated with SCS in quality of life or survival.175 It is clear, then, that SCS is a reasonable therapeutic choice for patients who will not benefit from a major surgical procedure including those with syndrome X (see earlier section on Angina, under Indications).
In the case of syndrome X, SCS can improve the disabling anginal symptoms that have an adverse impact on quality of life. The first prospective study dealing solely with syndrome X patients treated by SCS followed seven patients for a mean of 11 months.176 All seven passed an SCS screening trial, and at last follow-up, SCS significantly reduced anginal symptoms and improved physical activity and quality of life. Only four of the patients, however, sustained this improvement throughout the follow-up period (for two this was 14 to 15 months). The three who lost benefit after several months of pain control reported that the distribution of their paresthesia suddenly changed, despite no radiographic evidence of a displaced electrode.
In general, very few complications are reported with SCS therapy for angina.177 For example, investigators conducting a retrospective multicenter review of 130 patients with a mean follow-up of 31.4 ± 25.9 months reported only a 6.8% incidence of minor complications and no major complications.178 In a subsequent prospective report, the most common complication was infection, which occurred in 6 of 104 patients followed for an average of 13.2 ± 8 months.179
Generally, it has been difficult to perform blinded placebo-controlled studies with SCS because the patients experience stimulation-induced paresthesia. Unlike SCS treatment of neuropathic pain, where perceptible pain and paresthesia overlap seems to be a requirement for a beneficial effect, SCS treatment of ischemic pain can lead to a peripheral blood flow increase without concomitant paresthesia.40
In a study on anginal patients responding favorably to SCS,180 stimulation below the perception threshold was compared with both the usual suprathreshold SCS and with placebo stimulation (0.1V). Both SCS settings were effective compared with placebo, which indicates that paresthesia might not be needed to achieve the beneficial effect in this indication.
Cost Effectiveness
The high cost of SCS equipment has prompted discussion about the cost effectiveness of SCS for various indications. Of course, the overall cost effectiveness of SCS treatment will increase if the application of SCS is directed to the right patients by the right clinicians using the right equipment and techniques. This will reduce the incidence of expensive clinical failures and complications.181
Kumar and colleagues published a retrospective study in 2006 that detailed the incidence and cost of complications from 160 patients treated during a 10-year period. Of the 51 adverse events that occurred in 42 patients, 39 were hardware-related and 12 were biological. The mean per-patient cost of treating the complications was $7092 in 2005 Canadian dollars. The authors suggested ways to reduce the complication rate and tested their own progress in this regard by comparing their experience in these 160 patients with that in all 424 SCS patients they had treated during a 23-year period. They found that the rate of every complication except infection had decreased in the 160 patients (infection increased from 3.5% to 4.4%).182
Another factor that will likely have a positive impact on the cost of SCS is the introduction of pulse generators with rechargeable batteries. Despite attempts to define the extent of this impact through modeling studies,183 however, the impact remains to be defined.
In 2008, the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom conducted a systematic review and technology assessment of the use of SCS as a treatment for neuropathic and for ischemic pain.184 This assessment included an economic evaluation using two different but similar models that assumed an implanted pulse generator longevity of 4 years and an SCS system price of ≤9000. In the case of SCS versus CMM and versus reoperation for FBSS and SCS versus CMM for CRPS, the model predicted that SCS would produce additional quality-adjusted life years at a cost the health service would be willing to pay. For ischemic pain, however, the investigators found insufficient evidence of comparative efficacy because the studies were underpowered. They did note, however, that SCS was economically dominant (cost less and provided more benefit) when compared with coronary artery bypass surgery.
Neuropathic Pain
Failed Back Surgery Syndrome
SCS is a valid alternative to reoperation in FBSS patients,134 and a modeling study that compared 5-year costs for 100 patients with FBSS with those for 100 patients receiving other therapies found that SCS was “cost-effective versus alternative therapies costing $20,000 per year or more, with 78% or less efficacy.”185 In addition, rehabilitation after SCS is much less expensive than rehabilitation after reoperation.
SCS is also less expensive than conventional medical pain therapy for FBSS. The first report supporting this finding was a 2-year analysis of a series of 14 FBSS patients in whom the decrease in the cost of medication and the ability to return to work more than compensated for the initial high cost of the SCS system.98 Another group of investigators conducted a 5-year trial in a consecutive series of 104 FBSS patients to compare the cost of SCS treatment (n = 60) with that of the best available conventional pain therapy in those who failed the SCS trial (n = 44 who did not obtain 50% pain relief with a temporary electrode).186 Each group had the same follow-up protocol in a controlled environment. The cumulative cost per patient in Canadian dollars of SCS treatment was $29,123 versus $38,029 for conventional pain therapy. SCS treatment was more expensive only during the initial half of the study period. SCS also improved productivity: 15% of the SCS patients and none of the conventionally treated patients returned to work.
To compare the cost of SCS treatment for FBSS with the mix of therapies commonly in use (chronic maintenance), including reoperation and medical management, a group of investigators187 applied the technique of cost-minimization analysis188 and found that SCS with an efficacy of 56% pays for itself within 2.3 years.
The choice of SCS equipment, the implantation technique, and clinician experience, obviously have a large impact on cost. Even when such issues lengthen cost recovery, however, the cost can still be recovered within 5 years. In a retrospective study of 20 patients who received SCS for FBSS conducted in the United Kingdom, for example, the mean cost of care in the year before SCS was ≤1954.18.189 Extrapolated over 5 years, this would be ≤9770.90 compared with ≤9782 for SCS. Despite an increase in capital outlay when 25% of generator batteries depleted prematurely, prompting a switch to externally powered generators, and despite other surgical adjustments to the SCS equipment in 20% of the patients, SCS became cost neutral after 5 years of treatment. The investigators estimated that without these changes they would have achieved cost neutrality in 3.4 years.
In 2004, French investigators reported the results of a multisite (nine hospitals) prospective cost-benefit analysis of SCS for FBSS that collected cost and pain data from 43 patients before SCS treatment and at 6-, 12-, and 24-month follow-up. At 24 months, SCS reduced the cost of pain treatment by a mean 64% per patient per year. Like many SCS cost studies, however, this does not include the cost of screening patients.190
The first author (RBN) published a cost study based on data from the first 40 of 42 (of 50) patients enrolled in the randomized, controlled trial of SCS versus reoperation. To determine cost effectiveness, the difference in the SCS and reoperation per-patient cost was divided by the difference in the proportion of patients achieving success, and utility scores were assigned to various health states to determine quality-adjusted life years.191 In every analysis (intention-to-treat, treated-as-intended with crossover counted as failure of randomized treatment, and final treatment), SCS achieved economic dominance by being more effective and less expensive.
Manca and colleagues used data from the randomized trial of SCS plus conventional medical management versus conventional medical management alone to conduct a cost -consequences study in which they compared cost, quality of life, and resource consumption from baseline to 6 months of treatment.192 As expected, at 6 months the cost of treating patients with SCS was significantly higher than treatment with CMM alone. This report offers some information on how variations in practice affect cost. For example, the mean duration of inpatient stay and the mean length of the SCS intervention were more than double the first author’s (RBN’s) experience. This obviously increased costs. The PROCESS clinicians reduced costs, however, by performing only a single imaging study (an x-ray in one patient) during the screening trial. The first author (RBN) requires an MRI of the thoracic spine before a patient undergoes a screening trial, which increases the SCS-related cost (while reducing the incidence of rare but costly neurologic complications).
Peripheral Nerve Injury
An evaluation of the cost of SCS in a randomized controlled trial (see earlier section on Clinical Results) in 54 patients with reflex sympathetic dystrophy (CRPS) compared costs before and after treatment.193 The investigators presented cost data in 1998 euros for three sets of patients. One group was the SCS plus physical therapy as-randomized group (n = 36); 24 underwent SCS and 12 failed SCS and underwent physical therapy. The others were the physical therapy group (n = 18) and the SCS plus physical therapy as-treated group (n = 24). The data on the as-randomized group (two thirds SCS, one third physical therapy) revealed a mean per-patient first-year cost of €9,805. The mean as-treated first-year cost per patient for SCS plus physical therapy, however, at €12,721, was more than twice the €5,741 cost of physical therapy alone. The initial year disadvantage for SCS was eliminated by analysis to expected time of death, which yielded a mean as-randomized cost per patient of €171,153 in the SCS plus physical therapy group versus €229,624 in the physical therapy alone group. These investigators concluded that in addition to being a more-effective therapy for CRPS, SCS becomes cost effective after 3 years.
Three years later, Harke and colleagues published a report on 29 patients severely disabled by CRPS who were enrolled in an SCS trial from 1995 to 2001.141 In this group, the average cost of the implantation procedure including hospitalization was €11,844, which is comparable with the €12,721 reported by Kemler and Furnee.193 What Harke and colleagues termed “aftercare charges” (follow-up, correction of electrode migration, device reimplantation, and hospitalization, which would be roughly comparable with Kemler and Furnee’s “complications” category) amounted to €1335 per patient per year, a little more than 10% of the implantation cost (versus the 30% to 50% assumed by Kemler and Furnee). Harke and colleagues reported significant reduction in pain as well as significant improvement in functional ability in these patients at a mean follow-up of nearly 3 years.
Ischemic Pain
Peripheral Vascular Disease
The first report on the cost of SCS in patients with critical limb ischemia evaluates data from the Dutch multicenter randomized trial (see earlier).146 The investigators collected health care resource use cost data and conducted a cost-minimization analysis, which assumes no difference in clinical outcome and is considered appropriate only when a study has been powered for equivalence (which did not occur in this case). The cost minimization analysis also ignored the slight between-group difference in quality-of-life scores, which favored SCS. In this study, the significantly higher mean per patient cost (adjusted for mortality) in the SCS group versus the CMM group (69,066 versus 52,407 Dutch guilders) was almost entirely accounted for by the cost of implanting the SCS system. The story could end with the conclusion that SCS is not cost effective in these patients except for three clinically important observations. First, at the time of this study, clinicians were only beginning to use microcirculatory skin blood flow to assist with appropriate patient selection. Second, 35% of SCS patients had undergone sympathectomy, which destroys neural substrate that is important for the success of SCS (see earlier). Third, during the clinical trial that generated the cost data, the investigators encountered expensive problems arising from now-outdated SCS techniques and equipment. Although the Dutch study does not, therefore, provide useful conclusions about the cost effectiveness of SCS for critical limb ischemia, it does serve as another effective example of the importance of looking at patient selection criteria and implantation techniques and equipment when determining the shelf-life of study results.
Unfortunately, even journal peer reviewers do not always critique the patient selection criteria used in a study that provides data for a cost analysis. Thus, in 2006, another version of Klomp’s group’s 1999 study appeared—this time claiming in the title that SCS is not cost effective for patients with critical limb ischemia.194 The only new data in this report consist of tables detailing costs that were summarized in the 1999 publication.
Angina
Several studies have demonstrated that SCS is a cost-effective treatment for angina. The first was a 1992 cost-utility analysis conducted by Rasmussen and colleagues that analyzed the cost of SCS and its effect on quality-adjusted life years in 16 consecutive patients with otherwise intractable angina who initiated SCS between August 1988 and December 1989.195 Despite the mean 9.4 days of postimplantation hospitalization (now routinely an overnight stay), SCS reduced the cost of health care (compared with costs from a year before SCS implantation) and improved the patients’ quality of life. It is noteworthy that SCS reduced angina-associated hospitalization costs by $5700 per year per patient and reduced home care costs by approximately $2300 per year per patient.
In 2004, Rasmussen and colleagues conducted their second cost-utility analysis, based this time on cost and quality of life data from 18 consecutive intractable angina patients who had undergone transcutaneous electric nerve stimulation for 2 to 11 months prior to SCS implantation. Data on the cost of medical treatment were gathered for the year before TENS treatment and the year after SCS implantation. By the year before TENS, the patients had exhausted all other expensive treatments, including coronary artery bypass grafting and angioplasty. The analysis assumes that the costs occurred during the year before TENS would be similar to those the patients would incur for the duration of their lives if they did not receive SCS treatment. As in the previous study, the investigators report that SCS reduced the number of hospitalizations and other medical expenses. Even including the cost of implantation, the cost of SCS treatment was recovered in the first year in these patients. In addition, the patients reported improvements in all dimensions of quality of life.196
Another study compared hospitalization rates from most recent revascularization until SCS implantation, and from implantation until the study date in 19 consecutive NYHA Class III and IV patients with three-vessel disease.197 Revascularization led to an annual admission rate of 0.97 per patient, whereas the rate following SCS implantation was 0.27. The annual duration of hospitalization was longer during the post-revascularization period (8.3 days) than in the SCS period (2.5 days). This result was underscored when the ESBY investigators (see earlier section on Clinical Results) conducted a 2-year follow-up of their 104 patients in which they considered hospital costs198 and found that the SCS patients spent significantly fewer days in the hospital, which made SCS significantly less expensive than bypass surgery. Among this group of patients, the initial cost of SCS was lower than the cost of the bypass surgery. Even though follow-up interventions cost more in the SCS group than in the bypass group, the overall cost of SCS treatment was significantly lower than that in the bypass group.
Two reports attempted to determine the cost recovery period of SCS for angina, which apparently is shorter than for FBSS. One relied on data from eight patients treated before April 1999 at a single hospital.199 The investigators compared costs associated with hospitalization duration and consumption of health care resources for the 12 months preceding and following attempted implantation in the six patients with successful implants with those in the two patients in whom implantation was “technically impossible.” The successful SCS patients had significantly fewer days of hospitalization and consumed significantly fewer resources in the period following implantation. The opposite was true for the two unsuccessful SCS patients. The investigators concluded that the cost of SCS implantation for angina is recovered in approximately 15 months.
The other study came to a nearly identical conclusion after conducting a retrospective comparison of hospitalization data before and after SCS implantation in 24 angina patients.200 The median annual per-patient duration of hospitalization increased during the 3 years prior to SCS implantation from 3 days to 10 and decreased significantly to a median of 0 days in the year after implantation. Thus, in this group, the cost of SCS was recovered within 16 months of implantation.
It is noteworthy that cost studies of SCS have been limited to health care costs. No study has taken into account the economic value or impact of the patient’s disability, as regards employment status, not to mention suffering and inability to engage in activities of daily living. The only study that followed patients long enough to show a survival benefit (because the alternative was a relatively morbid procedure) did not consider the economic benefit of survival.198 The true costs and benefits of SCS and alternative treatments remain to be assessed fully.
Conclusion
SCS is a relatively easily implemented, reversible technique with low morbidity for the management of chronic, intractable pain in selected patients, with efficacy proved during more than 40 years of clinical use. The number of annual implants in the United States alone is estimated to exceed 27,000.201 Percutaneous placement of electrode arrays, supported by programmable implanted electronics that allow noninvasive adjustment of anode and cathode positions, and the development of rechargeable implanted power sources have been important technical advances.
The initial cost of an SCS system has often been considered high, but several cost-effectiveness analyses have demonstrated that the total cost of health care is lower for SCS than alternative treatments. Although there are as yet no comparative data for rechargeable systems, experienced implanters believe that the cost effectiveness of SCS will continue to improve and will easily offset their increased initial cost.
Amann W., Berg P., Gersbach P., et al. European Peripheral Vascular Disease Outcome Study SCS-EPOS. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003;26(3):280-286.
Bell G.K., Kidd D., North R.B. Cost-effectiveness of spinal cord stimulation in treatment of failed-back surgery syndrome. J Pain Symptom Manage. 1997;13:286-295.
Cook A.W., Oygar A., Baggenstos P., et al. Vascular disease of extremities: electrical stimulation of spinal cord and posterior roots. NY State J Med. 1976;76:366-368.
Cui J.G., Linderoth B., Meyerson B.A. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms: an experimental study in the mononeuropathic rat. Pain. 1996;66:287-295.
deJongste M.J.L., Hautvast R.V.M., Hillege H.L., Lie K.I. Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: a prospective, randomized clinical study. J Am Coll Cardiol. 1994;23:1592-1597.
Ekre O., Eliasson T., Norrsell H., et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002;23(24):1938-1945.
Foreman R.D., Linderoth B., Ardell J.L., et al. Modulation of intrinsic cardiac neuronal activity by spinal cord stimulation: implications for its therapeutic in angina pectoris. Cardiovasc Res. 2000;47(2):367-375.
Gersbach P., Hasdemir M.G., Stevens R.D., et al. Discriminative microcirculatory screening of patients with refractory limb ischemia for dorsal column stimulation. Eur J Endovasc Surg. 1997;13:464-471.
Holsheimer J., Wesselink W.A. Effect of anode-cathode configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery. 1997;41:654-660.
Kemler M.A., de Vet H.C.W., Barendse G.A.M., et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;208:292-298.
Klomp H.M., Spincemaille G.H., Steyerberg E.W., et al. Spinal-cord stimulation in critical limb ischaemia: a randomised trial. ESES Study Group. Lancet. 1999;353:1040-1044.
Kumar K., Taylor R.S., Jacques L., et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762-768.
Lind G., Meyerson B.A., Winter J., Linderoth B. Intrathecal baclofen as an adjuvant therapy to enhance effect of spinal cord stimulation. Eur J Pain. 2004;8:377-383.
Linderoth B., Foreman R.D., Meyerson B.A. Mechanisms of action of spinal cord stimulation. In: Lozano A.M., Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. 2nd ed. Berlin: McGraw Hill/Springer-Verlag; 2009:2331-2347.
Lopshire J.C., Zhou X., Dusa C., et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286-294.
Mannheimer C., Eliasson T., Augustinsson L.E., et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: the ESBY Study. Circulation. 1998;97:1157-1163.
Melzack P., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150(3699):971-978.
North R.B., Ewend M.G., Lawton M.T., et al. Failed back surgery syndrome: five-year follow-up after spinal cord stimulation implantation. Neurosurgery. 1991;28:692-699.
North R.B., Kidd D., Shipley J., Taylor R. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized controlled trial. Neurosurgery. 2007;61(2):361-369.
Shealy C., Mortimer J., Reswick J. Electrical inhibition of pain by stimulation of the dorsal columns: a preliminary report. Anesth Analg. 1967;46:489-491.
Stiller C.O., Cui J.G., O’Connor W.T., et al. Release of GABA in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367-375.
Ubbink D.T., Gersbach P.A., Berg P., et al. The best TcPo2 parameters to predict the efficacy of spinal cord stimulation to improve limb salvage in patients with inoperable critical leg ischemia. Int Angiol. 2003;22(4):356-363.
Ubbink D.T., Vermeulen H.. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev, 2005;3:CD004001 2005
Wu M., Linderoth B., Foreman R.D. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9-23.
Yakhnitsa V., Linderoth B., Meyerson B.A. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79(2-3):223-233.
1. Melzack P., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150(3699):971-978.
2. Head H., Holmes G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102-254.
3. Mazars G., Mérienne L., Ciolocca C. Intermittent analgesic thalamic stimulation. Preliminary note. Rev Neurol (Paris). 1973;128:273-279.
4. Mazars G., Roge R., Mazars Y. Results of the stimulation of the spinothalamic fasciculus and their bearing on the physiopathology of pain. Rev Prat. 1960;103:136-138.
5. Shetter A.G., Racz G.C., Lewis R., Heavner J.E. Peripheral nerve stimulation. In: North R.B., Levy R.M. Neurosurgical Management of Pain. New York: Springer-Verlag; 1997:261-270.
6. Campbell J.N., Meyer R.A. Primary afferents and hyperalgesia. In: Yaksh T.L., editor. Spinal Afferent Processing. New York: Plenum; 1986:59-81.
7. Campbell J.N., Davis K.D., Meyer R.A., North R.B. The mechanism by which dorsal column stimulation affects pain: evidence for a new hypothesis. Pain. 1990;5(Suppl):228.
8. Linderoth B., Foreman R.D. Physiology of spinal cord stimulation. Review and update. Neuromodulation. 1999;2:150-164.
9. Linderoth B. Dorsal column stimulation and pain: experimental studies of putative neurochemical and neurophysiological mechanisms [Thesis]. Stockholm, Sweden: Karolinska Institute; 1992.
10. Yakhnitsa V., Linderoth B., Meyerson B.A. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79(2-3):223-233.
11. Wallin J., Fiska A., Tjolsen A., et al. Spinal cord stimulation inhibits long-term potentiation of spinal wide dynamic range neurons. Brain Res. 2003;973(1):39-43.
12. Coburn B., Sin W. A theoretical study of epidural electrical stimulation of the spinal cord. I: finite element analysis of stimulus fields. IIEEE Trans Biomed Eng. 1985;32:971-977.
13. Holsheimer J., Strujik J.J., Rijkhoff N.J.M. Contact combinations in epidural spinal cord stimulation: a comparison by computer modeling. Stereotact Funct Neurosurg. 1991;56:220-233.
14. Holsheimer J., Wesselink W.A. Effect of anode-cathode configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery. 1997;41:654-660.
15. Sances A., Swinotek T.J., Larson S.J., et al. Innovations in neurologic implant systems. Med Instrum. 1975;9:213-216.
16. Ohnishi A., O’Brien P.C., Okazaki H., Dyck P.J. Morphometry of myelinated fibers of fasciculus gracilis of man. J Neurol Sci. 1976;27:163-172.
17. Law J. Spinal stimulation: statistical superiority of monophasic stimulation of narrowly separated bipoles having rostral cathodes. Appl Neurophysiol. 1983;46:129-137.
18. Lindblom U., Meyerson B.A. Influence on touch, vibration and cutaneous pain of dorsal column stimulation in man. Pain. 1975;1:257-270.
19. Marchand S., Bushnell M.C., Molina-Negro P., et al. The effects of dorsal column stimulation on measures of clinical and experimental pain in man. Pain. 1991;45:249-257.
20. Law J.D., Kirkpatrick A.F. Pain management update: spinal cord stimulation. Am J Pain Manage. 1991;2:34-42.
21. Linderoth B., Gazelius B., Franck J., Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289-297.
22. Meyerson B.A., Brodin E., Linderoth B. Possible neurohumoral mechanisms in CNS stimulation for pain suppression. Appl Neurophysiol. 1985;48:175-180.
23. Freeman T.B., Campbell J.N., Long D.M. Naloxone does not affect pain relief induced by electrical stimulation in man. Pain. 1983;17:189-195.
24. Meyerson BA, Boethius J, Terenius L, Wahlström A. Endorphine mechanisms in pain relief with intracerebral and dorsal column stimulation. Presented at the 3rd Meeting of the European Society for Stereotactic and Functional Neurosurgery, Freiburg, Germany, 1977.
25. Cui J.G., Linderoth B., Meyerson B.A. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms: an experimental study in the mononeuropathic rat. Pain. 1996;66:287-295.
26. Cui J.G., O’Connor W.T., Ungerstedt U., et al. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87-95.
27. Stiller C.O., Cui J.G., O’Connor W.T., et al. Release of GABA in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367-375.
28. Meyerson B.A., Linderoth B. Spinal cord stimulation: mechanisms of action in neuropathic and ischemic pain. In: Simpson B.A., editor. Electrical Stimulation and the Relief of Pain. New York: Elsevier; 2003:161-182.
29. Linderoth B., Meyerson B.A. Spinal cord stimulation. I: mechanisms of action. In: Burchiel K., editor. Pain Surgery. New York: Thieme, 1999.
30. Linderoth B., Stiller C.O., Gunasekera L., et al. Release of neurotransmitters in the CNS by spinal cord stimulation: survey of the present state of knowledge and recent experimental studies. Sterotact Funct Neurosurg. 1993;61:157-170.
31. Cui J.G., Meyerson B.A., Sollevi A., Linderoth B. Effects of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by GABA B and adenosine receptor activation. Neurosci Lett. 1998;247:183-186.
32. Schechtmann G., Song Z., Ultenius C., et al. Cholinergic mechanisms in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139:136-145.
33. Song Z., Meyerson B.A., Linderoth B. Muscarinic receptor activation potentiates the effect of spinal cord stimulation on pain related behaviour in rats with mononeuropathy. Neurosci Lett. 2008;436:7-12.
34. Song Z., Ultenius C., Meyerson B.A., Linderoth B. Pain relief by spinal cord stimulation involves serotonergic mechanisms: an experimental study in a rat model of mononeuropathy. Pain. 2009;147:241-248.
35. Schechtmann G., Wallin J., Meyerson B.A., Linderoth B. Intrathecal clonidine potentiates suppression of tactile hypersensitivity by spinal cord stimulation in a model of neuropathy. Anesth Analg. 2004;99(1):135-139.
36. Wallin J., Cui J.-G., Yakhnitsa V., et al. Gabapentin and pregabalin suppress tactile allodynia and potentiate spinal cord stimulation in a model of neuropathy. Eur J Pain. 2002;6:261-272.
37. Lind G., Meyerson B.A., Winter J., Linderoth B. Intrathecal baclofen as an adjuvant therapy to enhance effect of spinal cord stimulation. Eur J Pain. 2004;8:377-383.
38. Lind G., Schechtmann G., Winter J., et al. Baclofen-enhanced spinal cord stimulation and intrathecal baclofen alone for neuropathic pain: long-term outcome of a pilot study. Eur J Pain. 2008;12:132-136.
39. Schechtmann G. Drug-enhanced spinal cord stimulation for neuropathic pain. Studies on neurochemical mechanisms and clinical applications. PhD thesis. Karolinska Institutet Stockholm. 2009:85.
40. Linderoth B. Spinal cord stimulation in ischemia and ischemic pain. In: Horsch S., Claeys L. Spinal Cord Stimulation III: An Innovative Method in the Treatment of PVD and Angina. Darmstadt, Germany: Steinkopff Verlag; 1995:19-35.
41. Linderoth B., Herregodts P., Meyerson B.A. Sympathetic mediation of peripheral vasodilatation induced by spinal cord stimulation: Animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35:711-719.
42. Tanaka S., Komon N., Barron K.W., et al. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Autonom Neursci. 2004;114:55-60.
43. Croom J.E., Foreman R.D., Chandler M.J., Barron K.W. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol. 1997;272:H950-H957.
44. Croom J.E., Foreman R.D., Chandler M.J., Barron K.W. Reevaluation of the role of the sympathetic nervous system in cutaneous vasodilatation during dorsal spinal cord stimulation: are multiple mechanisms active? Neuromodulation. 1998;1:91-101.
45. Tanaka S., Barron K.W., Chandler M.J., et al. Local cooling alters neural mechanisms producing changes in peripheral blood flow by spinal cord stimulation. Auton Neurosci. 2003;104(2):117-127.
46. Tanaka S., Barron K.W., Chandler M.J., et al. Low intensity spinal cord stimulation may induce cutaneous vasodilatation via CGRP release. Brain Res. 2001;896:183-187.
47. Tanaka S., Barron K.W., Chandler M.J., et al. Role of primary afferent in spinal cord stimulation-induced vasodilatation: characterization of fiber types. Brain Res. 2003;959(2):191-198.
48. Gherardini G., Lundeberg T., Cui J.G., et al. Spinal cord stimulation improves survival in ischemic skin flaps: an experimental study of the possible mediation via the calcitonin gene-related peptide. Plast Reconstr Surg. 1999;103(4):1221-1228.
49. Freedman R.R., Sabharwal S.C., Desai N., et al. Increased α-adrenergic responsiveness in idiopathic Raynaud’s disease. Arthritis Rheum. 1989;32:61-65.
50. Bunker C.B., Terenghi G., Springall D.R., et al. Deficiency of calcitonin gene-related peptide in Raynaud’s phenomenon. Lancet. 1990;336:1530-1533.
51. Linderoth B., Gunasekera L., Meyerson B.A. Effects of sympathectomy on skin and muscle microcirculation during dorsal column stimulation: animal studies. Neurosurgery. 1991;29:874-879.
52. Baron R., Binder A., Schattschneider J., Wasner G. Pathophysiology and treatment of complex regional pain syndromes. In: Dostrovsky J.O., Carr D.B., Koltzenburg M. Proceeding of the 10th World Congress on Pain. Seattle: IASP Press; 2003:683-704.
53. Häbler H.-J., Eschenfelder S., Brinker H., et al. Neurogenic vasoconstriction in the dorsal root ganglion may play a crucial role in sympathetic-afferent coupling after spinal nerve injury. In: Devor M., Rowbotham M.C., Wiesemfeld-Hallin Z. Progress in Pain Research and Management. Seattle: IASP Press; 2000:661-667.
54. Kemler M.A., Barendse G.A., van Kleef M., Egbrink M.G. Pain relief in complex regional pain syndrome due to spinal cord stimulation does not depend on vasodilation. Anesthesiology. 2000;92(6):1653-1660.
55. Linderoth B. Spinal cord stimulation: a brief update on mechanisms of action. Eur J Pain Suppl. 2009;3:89-93.
56. Linderoth B., Foreman R.D., Meyerson B.A. Mechanisms of action of spinal cord stimulation. In: Lozano A.M., Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. 2nd ed. Berlin-Heidelberg: McGraw Hill/Springer Verlag; 2009:2331-2347.
57. Wu M., Linderoth B., Foreman R.D. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9-23.
58. Chandler M.J., Brennan T.J., Garrison D.W., et al. A mechanism of cardiac pain suppression by spinal cord stimulation: implications for patients with angina pectoris. Eur Heart J. 1993;14:96-105.
59. Hautvast R. Spinal cord stimulation for chronic refractory angina pectoris [Thesis]. Groningen, the Netherlands: Rijksuniversiteit; 1997.
60. Eliasson T., Augustinsson L.E., Mannheimer C. Spinal cord stimulation in severe angina pectoris: presentation of current studies, indications and practical experience. Pain. 1996;65:169-179.
61. Kingma J.G.Jr., Linderoth B., Ardell J.L., et al. Neuromodulation therapy does not influence blood flow distribution or left-ventricular dynamics during acute myocardial ischemia. Auton Neurosci. 2001;91(1-2):47-54.
62. Cardinal R., Ardell J., Linderoth B., et al. Spinal cord activation differentially modulates ischemic electrical responses to different stressors in canine ventricles. Autonom Neurosci. 2004;111(1):34-47.
63. Foreman R.D., deJongste M.J.L., Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In: Armour J.A., Ardell J.L. Basic and Clinical Neurocardiology. London: Oxford University Press; 2004:153-186.
64. Foreman R.D., Linderoth B., Ardell J.L., et al. Modulation of intrinsic cardiac neuronal activity by spinal cord stimulation: implications for its therapeutic in angina pectoris. Cardiovasc Res. 2000;47(2):367-375.
65. deJongste MJL et al. Unpublished data.
66. Issa Z.F., Zhou X., Ujhelyi M.R., et al. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217-3220.
67. Lopshire J.C., Zhou X., Dusa C., et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286-294.
68. Shealy C., Mortimer J., Reswick J. Electrical inhibition of pain by stimulation of the dorsal columns: a preliminary report. Anesth Analg. 1967;46:489-491.
69. Barolat G., Massaro F., He J., et al. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J Neurosurg. 1993;78:233-239.
70. Burton C.V. Dorsal column stimulation: optimization of application. Surg Neurol. 1975;4:171-176.
71. Nashold B., Somjen G., Friedman H. Paresthesias and EEG potentials evoked by stimulation of the dorsal funiculi in man. Exp Neurol. 1972;36:273-287.
72. Sweet W., Wepsic J. Stimulation of the posterior columns of the spinal cord for pain control. Clin Neurosurg. 1974;21:278-310.
73. Burton C.V. Session on spinal cord stimulation: safety and clinical efficacy. Neurosurgery. 1977;1:164-165.
74. Erickson D.L. Percutaneous trial of stimulation for patient selection for implantable stimulating devices. J Neurosurg. 1975;43:440-444.
75. Hoppenstein R. Electrical stimulation of the ventral and dorsal columns of the spinal cord for relief of chronic intractable pain. Surg Neurol. 1975;4:187-194.
76. Hosobuchi Y., Adams J.E., Weinstein P.R. Preliminary percutaneous dorsal column stimulation prior to permanent implantation. J Neurosurg. 1972;17:242-245.
77. North R.B., Fischell T.A., Long D.M. Chronic stimulation via percutaneously inserted epidural electrodes. Neurosurgery. 1977;1:215-218.
78. Zumpano B.J., Saunders R.L. Percutaneous epidural dorsal column stimulation. J Neurosurg. 1976;45:459-460.
79. Leclercq T.A. Electrode migration in epidural stimulation: comparison between single electrode and four electrode programmable leads. Pain. 1984;20(Suppl 2):78.
80. North R.B., Ewend M.G., Lawton M.T., Piantadosi S. Spinal cord stimulation for chronic, intractable pain: superiority of “multichannel” devices. Pain. 1991;44:119-130.
81. North R.B., Kidd D.H., Zahurak M., et al. Spinal cord stimulation for chronic, intractable pain: two decades’ experience. Neurosurgery. 1993;32:384-395.
82. Holsheimer J., Nuttin B., King G.W., et al. Clinical evaluation of paresthesia steering with a new system for spinal cord stimulation. Neurosurgery. 1998;42:541-549.
83. Renard V.M., North R.B. Prevention of percutaneous electrode migration in spinal cord stimulation by a modification of the standard implantation technique. J Neurosurg Spine. 2006;4:300-303.
84. North R.B., Kidd D.H., Olin J.C., Sieracki J.M. Spinal cord stimulation electrode design: prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes. Part I: technical outcomes. Neurosurgery. 2002;51(2):381-389.
85. Kumar K., Lind G., Winter J., et al. Spinal cord stimulation: placement of surgical leads via laminotomy—techniques and benefits. In: Krames E., Peckham P.H., Rezai A. Neuromodulation. London: Academic Press/Elsevier; 2009:1005-1011.
86. Lind G., Meyerson B.A., Winter J., Linderoth B. Implantation of laminotomy electrodes for spinal cord stimulation in spinal anesthesia with intraoperative dorsal column activation. Neurosurgery. 2003;53:1150-1153.
87. Law J.D. A new method for targeting a spinal stimulator: quantitatively paired comparisons. Appl Neurophysiol. 1987;50:436.
88. North R.B., Fowler K.R., Nigrin D.A., et al. Automated “pain drawing” analysis by computer-controlled, patient-interactive neurological stimulation system. Pain. 1992;50:51-58.
89. North R.B., Fowler K.R., Nigrin D.A., Szymanski R.E. Patient-interactive, computer-controlled neurological stimulation system: clinical efficacy in spinal cord stimulation. J Neurosurg. 1992;76:689-695.
90. North R.B., Calkins S.K., Campbell D.S., et al. Automated, patient-interactive, spinal cord stimulator adjustment: a randomized controlled trial. Neurosurgery. 2003;52(3):572-580.
91. North R.B., Brigham D.D., Khalessi A., et al. Spinal cord stimulator adjustment to maximize implanted battery longevity: a randomized controlled trial using a computerized, patient-interactive programmer. Neuromodulation. 2004;7(1):13-25.
92. Feler C., Kaufman S. Spinal cord stimulation: one stage? Acta Neurochir. 1992;117:91.
93. Weinard M., Madhusudan H., Davis B., Melgar M. Acute vs. prolonged screening for spinal cord stimulation in chronic pain. Neuromodulation. 2003;6:15-19.
94. de la Porte C., Siegfried J. Lumbosacral spinal fibrosis (spinal arachnoiditis): its diagnosis and treatment by spinal cord stimulation. Spine. 1983;8:593-603.
95. Meglio M., Cioni B., Rossi G.F. Spinal cord stimulation in management of chronic pain: a 9-year experience. J Neurosurg. 1989;70:519-524.
96. Koeze T.H., Williams A.C., Reiman S. Spinal cord stimulation and the relief of chronic pain. J Neurol Neurosurg Psychiatry. 1987;50:1424-1429.
97. Law J: Results of treatment for pain by percutaneous multicontact stimulation of the spinal cord. Presented at the American Pain Society meeting, Chicago, November 11-13, 1983.
98. Bel S., Bauer B.L. Dorsal column stimulation: cost to benefit analysis. Acta Neurochir. 1991;52(Suppl):121-123.
99. de la Porte C., Van de Kelft E. Spinal cord stimulation in failed back surgery syndrome. Pain. 1993;52:55-61.
100. Leibrock L., Meilman P., Cuka D., Green C. Spinal cord stimulation in the treatment of chronic low back and lower extremity pain syndromes. Nebraska Med J. 1984;69:180-183.
101. Meilman P.W., Leibrock L.G., Leong F.T.L. Outcome of implanted spinal cord stimulation in the treatment of chronic pain: arachnoiditis versus single nerve root injury and mononeuropathy. Clin J Pain. 1989;5:189-193.
102. Law J.D. Targeting a spinal stimulator to treat the “failed back surgery syndrome.”. Appl Neurophysiol. 1987;50:437-438.
103. North R.B., Kidd D.H., Olin J., et al. Spinal cord stimulation for axial low back pain: a prospective, controlled trial comparing dual with single percutaneous electrodes. Spine. 2005;30:1412-1418.
104. Meyerson B.A. Electric stimulation of the spinal cord and brain. In: Bonica J.J., Loeser J.D., Chapman R.C., Fordyce W.E. The Management of Pain. 2nd ed. Philadelphia: Lea & Febiger; 1990:1862-1877.
105. Monahan K., Casavant D., Rasmussen C., Hallet N. Combined use of a true-bipolar sensing implantable cardioverter defibrillator in a patient having a prior implantable spinal cord stimulator for intractable pain. Pacing Clin Electrophysiol. 1998;21:2669-2672.
106. Ekre O., Börjesson M., Edvardsson N., et al. Feasibility of spinal cord stimulation in angina pectoris in patients with chronic pacemaker treatment for cardiac arrhythmias. Pacing Clin Electrophysiol. 2003;26:2134-2141.
107. Forouzanfar T., Kemler M.A., Weber W.E., et al. Spinal cord stimulation in complex regional pain syndrome: cervical and lumbar devices are comparably effective. Br J Anaesth. 2004;92(3):348-353.
108. Krainick J.U., Thoden U., Riechert T. Pain reduction in amputees by long-term spinal cord stimulation: long-term follow-up study over 5 years. J Neurosurg. 1980;52:346-350.
109. Cook A.W., Oygar A., Baggenstos P., et al. Vascular disease of extremities: electrical stimulation of spinal cord and posterior roots. NY State J Med. 1976;76:366-368.
110. Augustinsson L.E., Carlsson C.A., Holm J., Jivegard L. Epidural electrical stimulation in severe limb ischemia. Ann Surg. 1986;202:104-110.
111. Augustinsson L.E., Linderoth B., Mannheimer C., Eliasson T. Spinal cord stimulation in cardiovascular disease. Neurosurg Clin North Am. 1995;6:157-165.
112. Galley D., Elharrar C., Scheffer J., et al. Intérêt de la neurostimulation épidurale dans les artériopathies des members intétients. Arteres Veines. 1988;7:61-71.
113. Groth K.E.. Spinal cord stimulation for the treatment of peripheral vascular disease, Fields H.L., Dubner R., Cervera F., editors, Advances in Pain Research and Therapy, New York, Raven, 1985;Vol 9:861-870.
114. Jacobs M.J., Jorning P.J., Beckers R.C., et al. Foot salvage and improvement of microvascular blood flow as a result of epidural spinal cord electrical stimulation. J Vasc Surg. 1990;13:354-360.
115. Jivegard L., Augustinsson L.E., Carlsson C.A., Holm J. Long-term results of epidural spinal electrical stimulation (ESES) in patients with inoperable severe lower limb ischaemia. Eur J Vasc Surg. 1987;1:345-349.
116. Bonica J. Pain due to vascular disease. In: Bonica J.J., editor. The Management of Pain. 2nd ed. Philadelphia: Lea & Febiger; 1990:502-537.
117. Seijo F. Ischemic pain: nociceptive pain or deafferentation pain. In: Herreros J., et al. Spinal Cord Stimulation for Peripheral Vascular Disease: Advances and Controversies. Madrid: Editorial Libro del Año; 1994:25-29.
118. Jivegard L.E.H., Augustinsson L.E., Holm J., et al. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischemia: a prospective randomized study. Eur J Endovasc Surg. 1995;9:421-425.
119. TransAtlantic InterSociety Consensus Working Group. management of peripheral arterial disease (PAD): transAtlantic InterSociety Consensus. J Vasc Surg. 2000;31(1 Part 2; Suppl):39.
120. Horsch S., Schulte S., Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single-center study of 258 patients. Angiology. 2004;55(2):111-118.
121. Schimpf R., Wolpert C., Herwig S., et al. Potential device interaction of a dual chamber implantable cardioverter defibrillator in a patient with continuous spinal cord stimulation. Europace. 2003;5(4):397-402.
122. Kaski J.C., Russo G. Microvascular angina in patients with syndrome X. Z Kardiol. 2000;89(Suppl 9):121-125.
123. Mannheimer C., Carlsson C.A., Emanuelsson H., et al. The effects of transcutaneous electrical nerve stimulation in patients with severe angina pectoris. Circulation. 1985;71:308-316.
124. Mannheimer C., Carlsson C.A., Eriksson K., et al. Transcutaneous electrical nerve stimulation in severe angina pectoris. Eur Heart J. 1982;3:297-302.
125. Mannheimer C., Augustinsson L.E., Carlsson C.A., et al. Epidural spinal electrical stimulation in severe angina pectoris. Br Heart J. 1988;59:56-61.
126. Murphy D.F., Giles K.E. Dorsal column stimulation for pain relief from intractable angina pectoris. Pain. 1987;28:365-368.
127. Andersen C., Hole P., Oxhoj H. Does pain relief with spinal cord stimulation for angina conceal myocardial infarction? Br Heart J. 1994;71:419-421.
128. Eliasson T. Spinal cord stimulation in angina pectoris [Thesis]. Göteborg, Sweden: Stra Hospital; 1994.
129. Ferrero P., Grimaldi R., Massa R., et al. Spinal cord stimulation for refractory angina in a patient implanted with a cardioverter defibrillator. Pacing Clin Electrophysiol. 2007;30(1):143-146.
130. Ferrero P., Castagno D., Massa R., et al. Spinal cord stimulation affects T-wave alternans in patients with ischaemic cardiomyopathy: a pilot study. Europace. 2008;10(4):506-508.
131. Augustinsson L.E., Linderoth B., Eliasson T., Mannheimer C. Spinal cord stimulation in peripheral vascular disease and angina pectoris. In: Gildenberg P.H., Tasker R. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw-Hill; 1997:1973-1978.
132. North R.B., Ewend M.G., Lawton M.T., et al. Failed back surgery syndrome: five-year follow-up after spinal cord stimulation implantation. Neurosurgery. 1991;28:692-699.
133. North R.B., Kidd D.H., Campbell J.N., Long D.M. Dorsal root ganglionectomy for failed back surgery syndrome: a five-year follow-up study. J Neurosurg. 1991;74(2):236-242.
134. North R.B., Kidd D.H., Farrokhi F., Piantadosi S.A. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98-107.
135. Kumar K., Taylor R.S., Jacques L., Eldabe S., et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;32:179-188.
136. Kumar K., Taylor R.S., Jacques L., et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762-768.
137. North R.B., Kumar K., Wallace M., et al. Spinal cord stimulation versus reoperation in patients with failed back surgery syndrome: an international multicenter randomized controlled trial (EVIDENCE Study). Neuromodulation. 2011;14(4):330-335. discussion 335-6
138. Kemler M.A., Barendse G.A., van Kleef M., et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618-624.
139. Kemler M.A., de Vet H.C., Barendse G.A., et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55(1):13-18.
140. Kemler M.A., de Vet H.C.W., Barendse G.A.M., et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;208:292-298.
141. Harke H., Gretenkort P., Ladleif H.U., Rahman S. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical study. Eur J Pain. 2005;9:363-373.
142. Harke H., Gretenkort P., Ladleif H.U., et al. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002;94:694-700.
143. Katayama Y., Yamamoto T., Kobayashi K., et al. Motor cortex stimulation for phantom limb pain: comprehensive therapy with spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77(1–4):159-162.
144. Rogano L., Teixeira M.J., Lepski G. Chronic pain after spinal cord injury: clinical characteristics. Stereotact Funct Neurosurg. 2003;81(1-4):65-69.
145. Guarnera G., Mascellari L., Bianchini G., et al. The role of spinal cord electric stimulation in critical ischemia of the extremity. Minerva Cardioangiol. 1996;44(12):663-667.
146. Klomp H.M., Spincemaille G.H., Steyerberg E.W., et al. Spinal-cord stimulation in critical limb ischaemia: a randomised trial. ESES Study Group. Lancet. 1999;353:1040-1044.
147. Ubbink D.T., Spincemaille G.H., Prins M.H., et al. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the dutch multicenter randomized controlled trial. J Vasc Surg. 1999;30(2):236-244.
148. Spincemaille G.H., Klomp H.M., Steyerberg E.W., et al. Technical data and complications of spinal cord stimulation: data from a randomized trial on critical limb ischemia. Stereotact Funct Neurosurg. 2000;74:63-72.
149. Amann W., Berg P., Gersbach P., et al. European Peripheral Vascular Disease Outcome Study SCS-EPOS. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003;26(3):280-286.
150. Ubbink D.T., Gersbach P.A., Berg P., et al. The best TcPo2 parameters to predict the efficacy of spinal cord stimulation to improve limb salvage in patients with inoperable critical leg ischemia. Int Angiol. 2003;22(4):356-363.
151. Petrakis I.E., Sciacca V. Spinal cord stimulation in critical limb ischemia of the lower extremities: our experience. J Neurosurg Sci. 1999;43(4):285-293.
152. Petrakis I.E., Sciacca V. Spinal cord stimulation in diabetic lower limb critical ischaemia: transcutaneous oxygen measurement as predictor for treatment success. Eur J Vasc Endovasc Surg. 2000;19(6):587-592.
153. Petrakis I.E., Sciacca V. Transcutaneous oxygen tension (TcPo2) in the testing period of spinal cord stimulation (SCS) in critical limb ischemia of the lower extremities. Int Surg. 1999;84(2):122-128.
154. Petrakis I.E., Sciacca V. Does autonomic neuropathy influence spinal cord stimulation therapy success in diabetic patients with critical lower limb ischemia? Surg Neurol. 2000;53(2):182-188.
155. Ubbink D.T., Vermeulen H.. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev, 2005;3:CD004001 2005
156. Claeys L.G., Horsch S. Transcutaneous oxygen pressure as predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. Int Angiol. 1996;15(4):344-349.
157. Neuhauser B., Perkmann R., Klingler P.J., et al. Clinical and objective data on spinal cord stimulation for the treatment of severe Raynaud’s phenomenon. Am Surg. 2001;67(11):1096-1097.
158. Augustinsson L.E., Linderoth B., Mannheimer C. Spinal cord stimulation in various ischaemic conditions. In: Illis L., editor. Spinal Cord Dysfunction. III: Functional Stimulation. Oxford, UK: Oxford Medical Publications; 1992:272-295.
159. Barolat G., Myklebust J.R., Wenninger W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. App Neurophys. 1988;51:29-44.
160. Francaviglia N., Silvestro C., Maiello M., et al. Spinal cord stimulation for the treatment of progressive systemic sclerosis and Raynaud’s syndrome. Br J Neurosurg. 1994;8:567-571.
161. Ktenidis K., Claeys L., Bartels C., Horsch S. Spinal cord stimulation in the treatment of Buerger’s disease. In: Horsch S., Claeys L. Spinal Cord Stimulation: An Innovative Method in the Treatment of PVD and Angina. Darmstadt, Germany: Steinkopff Verlag; 1995:207-214.
162. Robaina F.J., Dominguez M., Diaz M., et al. Spinal cord stimulation for relief of chronic pain in vasospastic disorders of the upper limbs. Neurosurgery. 1989;24:63-67.
163. Lepantalo M., Rosenberg P., Pohjola J., et al. Epidural spinal cord stimulation in the treatment of limb threatening vasospasm—Report of a case with a five year follow-up. Eur J Endovasc Surg. 1996;11:368-370.
164. Arregui R., Morandeira J.R., Martinez G., et al. Epidural neurostimulation in the treatment of frostbite. PACE. 1989;12:713-717.
165. deJongste M.J.L., Hautvast R.V.M., Hillege H.L., Lie K.I. Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: a prospective, randomized clinical study. J Am Coll Cardiol. 1994;23:1592-1597.
166. Hautvast R., deJongste M.J.L., Blanksma P.K., et al. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 1996;77:462-467.
167. Mannheimer C., Eliasson T., Andersson B., et al. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. BMJ. 1993;307:477-480.
168. Sanderson J.E., Brooksby P., Waterhouse D., et al. Epidural spinal electrical stimulation for severe angina: a study of its effects on symptoms, exercise tolerance and degree of ischaemia. Eur Heart J. 1992;13:628-633.
169. Gonzalez-Darder J.M., Canela P., Gonzalez-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg. 1991;56:20-27.
170. Eliasson T., Andersen C., deJongste M.J.L., et al. Long-term mortality and morbidity with treatment of severe angina pectoris with spinal cord stimulation. Hygiea. 1997;106:364.
171. Mannheimer C., Eliasson T., Augustinsson L.E., et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: the ESBY Study. Circulation. 1998;97:1157-1163.
172. Di Pede F., Zuin G., Giada F., et al. A. Long-term effects of spinal cord stimulation on myocardial ischemia and heart rate variability: results of a 48-hour ambulatory electrocardiographic monitoring. Ital Heart J. 2001;2(9):690-695.
173. Norrsell H., Pilhall M., Eliasson T., Mannheimer C. Effects of spinal cord stimulation and coronary artery bypass grafting on myocardial ischemia and heart rate variability: further results from the ESBY study. Cardiology. 2000;94(1):12-18.
174. Ekre O., Norrsell H., Wahrborg P., et al. Temporary cessation of spinal cord stimulation in angina pectoris-effects on symptoms and evaluation of long-term effect determinants. Coron Artery Dis. 2003;14(4):323-327.
175. Ekre O., Eliasson T., Norrsell H., et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002;23(24):1938-1945.
176. Lanza G.A., Sestito A., Sandric S., et al. A. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J. 2001;2(1):25-30.
177. Andersen C. Complications in spinal cord stimulation for treatment of angina pectoris. Differences in unipolar and multipolar percutaneous inserted electrodes. Acta Cardiol. 1997;52(4):325-333.
178. Romano M., Auriti A., Cazzin R., et al. Epidural spinal stimulation in the treatment of refractory angina pectoris. Its clinical efficacy, complications and long-term mortality. An Italian multicenter retrospective study. Ital Heart J. 2000;1(Suppl 1):97-102.
179. Di Pede F., Lanza G.A., Zuin G., et al. Immediate and long-term clinical outcome after spinal cord stimulation for refractory stable angina pectoris. Am J Cardiol. 2003;91(8):951-955.
180. Eddicks S., Maier-Hauff K., Schenk M., et al. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart. 2007;93:585-590.
181. Kumar K., Buscher E., Linderoth B., et al. Spinal cord stimulation: avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007;10:24-33.
182. Kumar K., Wilson J.R., Taylor R.S., Gupta S. Complications of spinal cord stimulation suggestions to improve outcome and financial impact. J Neurosurg. Spine. 2006;5(3):191-203.
183. Hornberger J., Kumar K., Verhulst E., et al. Rechargeable spinal cord stimulation versus nonrechargeable system for patients with failed back surgery syndrome: a cost-consequences analysis. Clin J Pain. 2008;24(3):244-252.
184. Simpson E.L., Duenas A., Holmes M.W., et al. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 13(17), 2009.
185. Health Technology Assessment Information Service. Spinal cord (dorsal column) stimulation for chronic intractable pain. Plymouth Meeting, PA: ECRI; 1993.
186. Kumar K., Malik S., Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51(1):106-115.
187. Bell G.K., Kidd D., North R.B. Cost-effectiveness of spinal cord stimulation in treatment of failed-back surgery syndrome. J Pain Symptom Manage. 1997;13:286-295.
188. Eisenberg J.M. Clinical economics. JAMA. 1989;262:2879-2886.
189. Budd K. Spinal cord stimulation: cost-benefit study. Neuromodulation. 2002;5(2):75-78.
190. Blond S., Buisset N., Dam Hieu P., et al. Cost-benefit evaluation of spinal cord stimulation treatment for failed-back surgery syndrome patients. Neurochirugie. 2004;50(4):443-453.
191. North R.B., Kidd D., Shipley J., Taylor R. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized controlled trial. Neurosurgery. 2007;61(2):361-369.
192. Manca A., Kumar K., Taylor R.S., et al. Quality of life resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12(8):1047-1058.
193. Kemler M.A., Furnee C.A. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59(8):1203-1209.
194. Klomp H.M., Steyerberg E.W., van U.r.k., et al. Spinal cord stimulation is not cost-effective for non-surgical management of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2006;31:500-508.
195. Rasmussen M.B., Andersen C., Andersen P., Frandsen F. Cost-utility analysis of electric spinal cord stimulation for treatment of angina pectoris. Ugeskr Laeger. 1992;154:1180-1184.
196. Rasmussen M.B., Hole P., Andersen C. Electric spinal cord stimulation (SCS) in the treatment of angina pectoris: a cost-utility analysis. Neuromodulation. 2004;7(2):89-96.
197. Murray S., Carson K.G., Ewings P.D., et al. Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart. 1999;82(1):89-92.
198. Andréll P., Ekre O., Eliasson T., et al. Cost-effectiveness of spinal cord stimulation versus coronary artery bypass grafting in patients with severe angina pectoris: long-term results from the ESBY study. Cardiology. 2003;99(1):20-24.
199. Merry A.F., Smith W.M., Anderson D.J., et al. Cost-effectiveness of spinal cord stimulation in patients with intractable angina. N Z Med J. 2001;114(1130):179-181.
200. Yu W., Maru F., Edner M., et al. Spinal cord stimulation for refractory angina pectoris: a retrospective analysis of efficacy and cost-benefit. Coron Artery Dis. 2004;15(1):31-37.
201. Prager J. Estimates of annual spinal cord stimulator implant rises in the United States. Neuromodulation. 2010;13:68-69.
202. Favre J.P., Richard A., Gournier J.P., Barral X. Value of spinal cord stimulation for limb salvage in patients with graft failure. In: Horsch S., Claeys L. Spinal Cord Stimulation. II: An Innovative Method in the Treatment of PBD and Angina. Darmstadt, Germany: Steinkopff Verlag; 1995:137-145.
203. Gersbach P., Hasdemir M.G., Stevens R.D., et al. Discriminative microcirculatory screening of patients with refractory limb ischemia for dorsal column stimulation. Eur J Endovasc Surg. 1997;13:464-471.
204. Kumar K., Toth C., Nath R.K., et al. Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation. A prospective study. J Neurosurg. 1997;86:662-669.
205. Waddell G., McCulloch J.A., Jummel E.G., Venner R.M. Non-organic physical signs in low back pain. Spine. 1980;5:117-125.