CHAPTER 167 Spinal Cord Stimulation
Since its development 40 years ago, spinal cord stimulation (SCS) has become an increasingly used treatment for chronic pain. In one of the early examples of “bench-to-bedside” research translation, the gate theory of pain transmission was first put to use by Shealy’s development of SCS in 1967,1,2 a mere 2 years after it was proposed. However, pain relief through the use of electrical stimulation had not been an entirely novel idea. For centuries, eels, as biologic sources of electrical current, have been used as analgesic aids. This effect was largely written off as magical until the work of Melzack and Wall offered a reasonable explanation. The ensuing clinical experience and accumulated body of knowledge comprises one of the most successful examples of useful, translational research in the history of neurosurgery. This chapter outlines the proposed mechanisms by which SCS works and discusses contemporary issues and clinical uses of this technology.
Mechanism of Action
Gate Theory
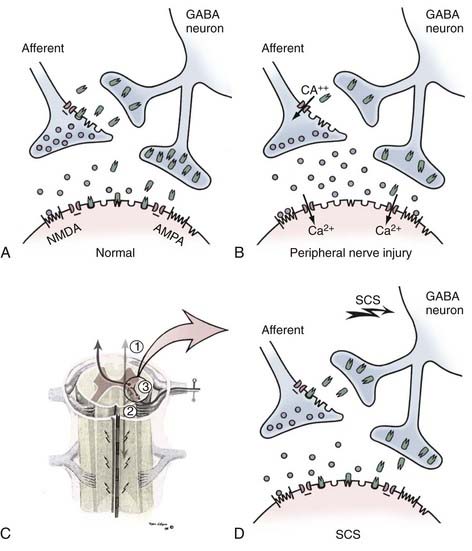
The gate-control theory of pain transmission proposed by Melzack and Wall3 in 1965 describes a balance between small and large sensory fibers, with positive and negative feedback loops controlling the activity of the dorsal horn cells of the spinal cord. The gate theory postulates that a predominance of small-diameter sensory fiber activity opens “gates” within the dorsal horn of the spinal cord, whereas large-fiber dominance closes them. Shealy reasoned that because large fibers have a lower threshold for depolarization by an electrical field applied to a peripheral nerve, they may be recruited selectively by an externally applied field. Moreover, because large-diameter sensory fibers within peripheral nerves are segregated into the dorsal columns, they may be more selectively activated by electrical stimulation of the dorsal aspect of the spinal cord. It is for this reason that the primary electrical effect of SCS has been assumed to be mediated by the dorsal columns.
As elegant as it is, the gate theory alone does not fully explain the clinical effect seen with SCS. For example, there are many pain conditions that are not effectively treated by SCS, including the pain of acute injury.4 In contrast, SCS is effective at treating hyperalgesia, which is signaled by large fibers. This may indicate that relief of pain by electrical stimulation is due to frequency-related conduction block, acting at primary afferent branch points where dorsal column fibers and dorsal horn collaterals diverge.5 This suggests that other mechanisms involving interneurons in the dorsal horn or involving descending fibers or sympathetic mechanisms may exist.6
One of the major limitations in our understanding of the mechanisms underlying SCS has been the paucity of animal models that reproduce the human chronic pain condition. Initial animal models using acute tissue injury have given way to those employing peripheral nerve injury, in an effort to create a reliable chronic pain model. However, it has been difficult to develop a rat model of chronic pain because rats generally recover from painful insults without long-term pain.5 Even in humans, peripheral nerve injury does not reliably result in neuropathic pain, and objective assessment of pain in the rat relies on behavioral changes. We therefore rely largely on the data accumulated over the past three decades of human use to further elucidate a possible mechanism.
Neurotransmitters
To better clarify the mechanism of action of SCS, investigators have also examined the role of local neurotransmitters within the spinal cord on pain conduction. Early studies explored opioid receptors as the conventional model of pharmacologic analgesia. It was found that administration of the narcotic antagonist naloxone had no effect on the relief of pain by SCS.7 Other studies revealed that substance P is increased in the cerebrospinal fluid (CSF) following SCS. It has also been noted that SCS causes a decrease in the release of excitatory amino acids, such as glutamate and aspartate, while at the same time increasing the release of γ-aminobutyric acid (GABA).6 This evidence suggests that neuropathic pain may be a state of imbalance between excitatory and inhibitory neurotransmitters and that SCS may restore that balance (Fig. 167-1).5 This is further corroborated by studies that show that the GABA agonist baclofen improves the effect of SCS, whereas GABA antagonists inhibit its effect.8 These lines of research leave open the door for the possibility of adjunctive pharmacotherapy.
Summary of Mechanisms
The mechanisms underlying SCS-induced analgesia are still open to question. However, it is clearly related to modulation of signals mediated by dorsally located fibers within the spinal cord. Neurohumoral changes in the spinal cord may be secondary to such activity modulation and may explain poststimulation analgesia, which is quite prominent in some patients.9
Indications and Outcomes
Early uses of SCS spanned the gamut of chronic pain etiologies, and outcomes were decidedly mixed. Clinicians soon learned that electrical stimulation was well suited for the treatment of certain diagnoses, whereas its use in others yielded disappointing results. With this in mind, before implantation of a spinal cord stimulator, the cause of the patient’s pain should be established. There should be appropriate, objective evidence of a pain disorder for which SCS has been shown to have efficacy. In general terms, pain conditions marked by nerve injury (so-called neuropathic pain) have had the most favorable track record following SCS. For an exhaustive description of indications and outcomes in SCS, the reader is encouraged to refer to Krames’ 1999 paper.10 In the sections that follow, we highlight a few of the most common diagnoses treated with SCS.
Failed Back Surgery Syndrome
Chronic neuropathic pain is most commonly located in the back and legs. Of patients undergoing lumbosacral spine surgery for treatment of this pain, 10% to 40% eventually develop persistent or recurrent pain.11 This postsurgery pain is often referred to as failed back surgery syndrome (FBSS). Patients commonly have complaints of axial low back pain and lower extremity pain. In general, SCS is indicated in patients with FBSS whose radicular leg symptoms are more severe than the component of axial back pain. Objective evidence for a source of their neuropathic pain should be sought, including radiographic evidence of an anatomically successful surgery for herniated disk with an appropriate radiculopathy, matching the pain complaint.
FBSS is the most common indication for SCS and has the most evidence supporting its use. The Prospective, Randomized, Controlled, Multicenter Study of Patients with Failed Back Surgery Syndrome (PROCESS) trial, a large, multicenter, randomized controlled trial of SCS, has provided some of the best evidence for the use of SCS in failed back surgery syndrome.12–14 The data emerging from this ongoing trial, first presented in 2005, followed pain relief and cost-effectiveness when compared with conventional medical management. Pain relief was significantly better in patients treated with spinal cord stimulators than in those managed conventionally.13 Patients also reported significant improvement in health care–related quality of life. However, the most recent data from this study show that, at 6 months, health care costs were significantly higher in the SCS group, likely related to the costs of the device and implantation.14 Longer follow-up of these patients is required to assess the true cost of this therapy.
The role of SCS in the management of recurrent back and leg pain following spine surgery has been examined in a randomized controlled trial comparing this therapy with repeat lumbar spine surgery.15 In this series of 42 patients with operable spinal pathology, SCS proved more effective than redo spine surgery. Moreover, a significant proportion of patients who had been randomized to spine surgery and failed to benefit from it subsequently achieved good results with SCS. Finally, the cost of therapy with SCS proved lower than repeat lumbar spine surgery, despite the significant cost of the hardware used.16
Complex Regional Pain Syndrome
SCS has been in use for the treatment of complex regional pain syndrome (CRPS). Also known as reflex sympathetic dystrophy, this is a condition for which there are few effective treatment options. The pathophysiology is unclear, with pain, dysfunction, and trophic changes occurring in an affected limb following trauma or surgery to that limb.17 Kemler and colleagues18 have recently released the 5-year follow-up results of a randomized, controlled trial comparing SCS with physical therapy to physical therapy alone. They found significantly better relief of symptoms in SCS patients than in those conservatively managed during the first 3 years after implantation. However, a spontaneous improvement in the control group appears to gradually diminish the effect of SCS over longer periods, with no statistical difference between the groups at 5 years. Despite these results, 95% of patients with SCS reported that they would undergo implantation again.
Ischemic Pain
It has been noted that SCS may alter vascular tone and improve tissue perfusion. It is thought that, through a combination of sympathetic outflow modulation and antidromic activation of sensory fibers, SCS acts to dilate peripheral vasculature.19 The use of SCS in the treatment of peripheral vascular disease was first proposed in the 1970s.20 Subsequently, stimulator placement in the low thoracic or lumbar region of the spine has been shown to be effective in the treatment of pain caused by lower extremity peripheral artery occlusive disease.21–23 Placement of the stimulator electrode at higher thoracic levels has been used in the treatment of intractable angina, with studies showing improved exercise tolerance and less ST-segment changes with SCS treatment.19 Recently, the effect of cervical SCS on the cerebral vasculature has been explored, with focus on cerebral vasospasm following subarachnoid hemorrhage.24
Psychological Screening
One of the practical issues in SCS is the screening of patients. Clearly, not every pain syndrome responds equally well to SCS. In the same vein, not every patient with chronic pain responds well to this therapy. In an effort to sort out who responds well to SCS, many practitioners have turned to psychological screening. Aside from its ability to identify patients with major psychiatric morbidity, psychological screening has been reported to have some predictive value in selecting patients who would benefit from the therapy.25 Some studies have found that it is important to assess the psychological profile of the patient to determine the suitability of SCS for treatment of the patient’s pain. In particular, preimplantation screening protocols have examined overall stability of the patient and the patient’s defensiveness, self-confidence, and self-efficacy. Other aspects examined are the patient’s response to and concern about the illness and the patient’s ability to cope with setbacks. Family support and a willingness to be an active participant in the patient’s care are also important.26 However, the utility of psychological screening is not uniformly accepted; there are also data that suggest that the predictive value of psychological testing is low.27 In summary, data regarding the usefulness of psychological screening of patients before SCS are mixed. However, this type of screening continues to be widely practiced.
Modern-Day Lead Types
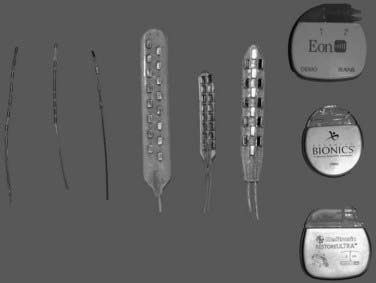
Contemporary stimulator lead types consist of either insulated wires containing anywhere from one to eight contacts at their tips in a linear array or paddle-shaped electrodes with two or three columns of disk-shaped electrodes (Fig. 167-2). The contacts are composed of a nonferromagnetic alloy such as platinum-iridium. In general, the insulating material is silastic based to maintain flexibility and durability. The percutaneous, wire-shaped electrodes can be placed through a needle under fluoroscopic guidance, allowing the surgeon broad access to multiple levels of the spinal cord in a minimally invasive fashion. However, by virtue of their cylindrical shape, these contacts radiate current in all directions, requiring more energy and potentially stimulating dorsally located nerve fibers in addition to the ventrally located spinal cord. The paddle-shaped leads allow for more focused dispersion of current, but require a more invasive implantation procedure.28
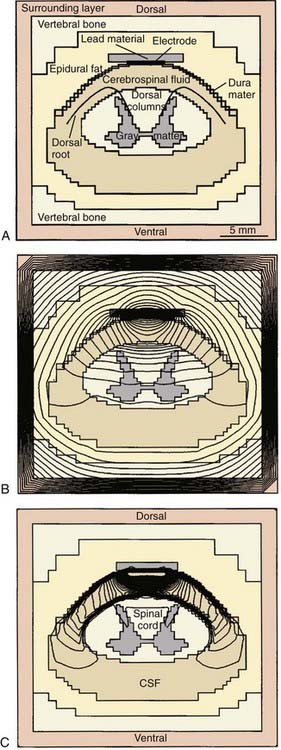
The percutaneous technique was initially used for temporary trials of stimulation before laminectomy and placement of a permanent lead. However, the percutaneous leads have since become popular for long-term use, owing to ease of placement. The earliest percutaneous electrodes had a single contact and required the placement of multiple leads to achieve bipolar stimulation. In the 1980s, leads were developed with increasing numbers of contacts, providing more maneuverability in stimulation. Earlier lead designs incorporated large contacts with long intercontact spacing because these were thought to broaden the effect of stimulation. However, more contemporaneous lead designs use smaller contacts with narrow intercontact spacing. Work with finite element modeling of the spinal canal has demonstrated that this narrow intercontact spacing provides superior targeting of the dorsal columns of the spinal cord (Fig. 167-3).29 In addition, it appears that a transverse orientation of anode and cathode minimizes uncomfortable radicular stimulation and maximizes stimulation of the dorsal columns. The “transverse tripole,” which involves a negative terminal flanked transversely by two positive terminals, is thought to provide for optimal stimulation characteristics.30 Accomplishing a transverse orientation requires that multiple percutaneous leads be placed parallel to each other or, alternatively, requires the placement of a paddle electrode with multiple linear electrode arrays.
Laminectomy Compared with Percutaneous Placement
The ability to implant leads for SCS by a minimally invasive, percutaneous technique has resulted in a profound increase in the popularity of this therapy during the past 20 years. During the past 5 years, however, a number of reports have surfaced suggesting that the leads implanted through laminectomy may deliver better results than those placed percutaneously.28,31 In the early 2000s, a randomized prospective controlled trial was conducted to compare both the technical and clinical results associated with the percutaneous and laminectomy electrodes. Initial technical data, published in 2002, touted the increased insulation of the paddle-style electrodes for longer battery life and the ability to use lower stimulation amplitudes.28 All patients participating in the trial initially underwent temporary percutaneous implantation, followed by randomization to either permanent implantation of percutaneous or laminectomy-placed leads. Patients who were able to compare the two types of leads reported better ratings of paresthesia coverage of pain, and patients reported improved low back coverage. Clinical data, published in 2005, revealed that this advantage was durable to a follow-up point of 2 years, but the statistical significance disappeared at a mean of 2.9 years of follow-up. The evidence therefore does not clearly support the theoretical and anecdotal reports of laminotomy lead superiority.31 Still, laminotomy leads are clearly indicated in patients in whom percutaneous implantation cannot be accomplished because of epidural adhesions and in patients who have experienced repeated percutaneous lead migration.
Generators
Implantable pulse generators became available in the 1980s with the advent of lithium ion battery technology (see Fig. 167-2). These have the obvious advantage of providing more convenience and a better cosmetic result. Battery life in older models is limited, however, and requires balancing stimulation parameters with battery life to achieve the best result. Patients must undergo surgical replacement of their implanted generators when the battery has been exhausted.
Although fully implanted pulse generators now make up the lion’s share of systems in clinical use, a new generation of rechargeable generators has entered the marketplace. These devices allow patients to enjoy the convenience of an internalized power source, without the need for frequent surgical procedures to replace the generator. To recharge these generators, the patient wears a radiofrequency-coupled external device periodically for several hours. Unfortunately, compliance with recharging these devices is very important; completely depleted, the rechargeable generator may be irreversibly damaged and require surgical replacement. The price of rechargeability therefore appears to be more significant patient compliance in a regular maintenance schedule, which interferes with the convenience of this device.32 Therefore, the new, rechargeable generators do not necessarily replace the primary cell, implantable generators that have dominated this clinical arena.
Surgical Technique
Electrode Placement
Determination of the spinal level for electrode insertion and placement is key to successful stimulation. The location of pain treated and the type of electrode being placed guide the level of insertion. Needle-type electrodes have more flexibility in that they may be advanced a number of levels cranially through the epidural space to ensure the best stimulation coverage. Conversely, paddle-type electrodes may generally be placed only one to two levels cranial to the location of the laminectomy. For upper extremity symptoms, cervical placement is required. One should be mindful of the cervical cord enlargement, and electrode insertion should ideally be performed below the T1-2 level. A practical rule of thumb is to insert the electrode at T2-3 or T3-4 for upper extremity pathology and at L1-2 or L2-3 for lower extremity targets. The target level for the electrical contacts varies by patient and pain region being treated. Common target levels for lower extremity coverage range from T8-T12, spanning the lumbar enlargement of the cord. Similarly, common levels for upper extremity symptoms range from C3 to C6, covering the cervical enlargement. Placement where the spinal cord is of small caliber may result in unpleasant local segmental effects. Barolat33,34 has published an exhaustive review of stimulation levels and their physiologic correlates.
Generator Placement
Internal pulse generators are implanted in a subcutaneous pocket in a position where they will not interfere with bony prominences. Two common locations are the lower quadrant of the abdomen or the buttock. It is important to consider the ease of the patient to access the generator for routine programming. Also, care should be taken to avoid placing the generator where undue pressure will be placed on it, causing skin breakdown from waistbands or sitting. Some practitioners advocate placing a generator in the region of the buttock because it is more convenient surgically and is cosmetically acceptable to most patients. However, there are data indicating that such generator placement increases the strain on the leads, increasing the propensity for lead migration or fracture.35
Complications
SCS has proved to be a safe technique for the management of chronic neuropathic pain. The incidence of life-threatening infection is low; the most severe complications require reoperation, whereas others may simply affect pain relief.35,36 Complications may be divided into two categories: technical and biologic. Technical complications relate to both the implantation technique and long-term durability of the hardware. A recent review of the literature found that one of the most frequent complications is electrode migration. Percutaneously placed electrodes are more susceptible to this issue than are paddle electrodes. Paralysis has been reported rarely, usually related to development of epidural abscess.37
Cautions and Contraindications
The presence of an implanted neurostimulator device warrants special consideration in medical and day-to-day situations. The manufacturers of these devices and the U.S. Food and Drug Administration (FDA) recommend caution with any sources of electrical or magnetic energy because they may cause uncontrolled heating of the electrode, leading to tissue damage. Obvious sources include surgical electrocautery and magnetic resonance imaging (MRI). Although most practitioners advocate avoiding monopolar cautery in the presence of spinal cord stimulators, it appears that this type of cautery in the operating room may be used safely but requires placing the grounding pad to direct the current field away from the electronic device and keeping the amplitude of electrocautery current at the lowest usable settings. Concerns about device failure and uncontrolled electrode heating have also prompted practitioners to avoid MRI. Although MRI is not currently recommended for patients with a neurostimulator device, a recent study cited no adverse events in a series of 31 patients who underwent 1.5-Tesla MRI.38
Other sources of radiofrequency energy outside the operating room or imaging suite may occasionally be encountered. For example, diathermy is a commonly used method of generating tissue heat, usually in coordination with physical therapy. This modality involves depositing significant energy to soft tissue, which may be transmitted to electrical devices, causing uncontrolled heating at the electrical contacts. There have been no reports of injury in patients undergoing diathermy in the presence of SCS. However, there have been a number of reports of patients with deep brain stimulators injured by diathermy.39,40 Nonmedical use of radiofrequency energy may also affect spinal neurostimulators; patients have been reported to experience alteration in stimulation while passing through antitheft devices, and a single death has been reported under this circumstance.41 It is therefore recommended that patients avoid proximity to these antitheft devices.
Barolat G. Experience with 509 plate electrodes implanted epidurally from C1 to L1. Stereotact Funct Neurosurg. 1993;61:60.
Cui JG, O’Connor WT, Ungerstedt U, et al. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87.
De Andres J, Valía JC, Cerda-Olmedo G, et al. Magnetic resonance imaging in patients with spinal neurostimulation systems. Anesthesiology. 2007;106:779.
De Vries J, De Jongste MJ, Spincemaille G, et al. Spinal cord stimulation for ischemic heart disease and peripheral vascular disease. Adv Tech Stand Neurosurg. 2007;32:63.
Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36:531.
Holsheimer J, Wesselink WA. Optimum electrode geometry for spinal cord stimulation: the narrow bipole and tripole. Med Biol Eng Comput. 1997;35:493.
Kemler M, De Vet H, Barendse G, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292.
Kumar K, Buchser E, Linderoth B, et al. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007;10:24-33.
Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51:106.
Kumar K, North R, Taylor R, et al. Neuromociulation. 2005;8:213.
Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179.
Kumar K, Wilson JR, Taylor RS, et al. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5:191.
Manca A, Kumar K, Taylor R, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12:1047-1058.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971.
North R. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106.
North RB, Kidd DH, Olin JC, et al. Spinal cord stimulation electrode design: prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes. Part I: Technical outcomes. Neurosurgery. 2002;51:381.
North R, Kidd D, Petrucci L, et al. Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes. Part II: Clinical outcomes. Neurosurgery. 2005;57:990.
North RB, Kidd D, Shipley J, et al. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurg Online. 2007;61:361.
North RB, Kidd DH, Wimberly RL, et al. Prognostic value of psychological testing in patients undergoing spinal cord stimulation: a prospective study. Neurosurgery Online. 1996;39:301.
Oosterga M, ten Vaarwerk IA, DeJongste MJ, et al. Spinal cord stimulation in refractory angina pectoris—clinical results and mechanisms. Z Kardiol. 1997;86(suppl 1):107.
Schechtmann G, Song Z, Ultenius C, et al. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;132:10-11.
Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489.
Takanashi Y, Shinonaga M. Spinal cord stimulation for cerebral vasospasm as prophylaxis. Neurol Med Chir. 2000;40:352.
Tulgar M, He J, Barolat G, et al. Analysis of parameters for epidural spinal cord stimulation. 3. Topographical distribution of paresthesiae—a preliminary analysis of 266 combinations with contacts implanted in the midcervical and midthoracic vertebral levels. Stereotact Funct Neurosurg. 1993;61:146.
Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108.
1 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489.
2 Shealy CN, Taslitz N, Mortimer JT, et al. Electrical inhibition of pain: experimental evaluation. Anesth Analg. 1967;46:299.
3 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971.
4 Kavar B, Rosenfeld JV, Hutchinson A. The efficacy of spinal cord stimulation for chronic pain. J Clin Neurosci. 2000;7:409.
5 Schechtmann G, Song Z, Ultenius C, et al. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;132:10-11.
6 Cui JG, O’Connor WT, Ungerstedt U, et al. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87.
7 Freeman TB, Campbell JN, Long DM. Naloxone does not affect pain relief induced by electrical stimulation in man. Pain. 1983;17:189.
8 Lind G, Meyerson B, Winter J, et al. Intrathecal baclofen as adjuvant therapy to enhance the effect of spinal cord stimulation in neuropathic pain: a pilot study. Eur J Pain. 2004;8:377.
9 Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108.
10 Krames E. Spinal cord stimulation: indications, mechanism of action, and efficacy. Curr Rev Pain. 1999;3:419.
11 Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51:106.
12 Kumar K, North R, Taylor R, et al. Spinal cord stimulation vs. conventional medical management: a prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS Study). Neuromodulation. 2005;8:213.
13 Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179.
14 Manca A, Kumar K, Taylor R, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12:1047-1058.
15 North R. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Acta Neurochir Suppl. 1995;64:106.
16 North RB, Kidd D, Shipley J, et al. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery Online. 2007;61:361.
17 Turner JA, Loeser JD, Deyo RA, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137.
18 Kemler M, De Vet H, Barendse G, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292.
19 Wu M, Linderoth B, Foreman R. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9.
20 Falowski S, Celii A, Sharan A. Spinal cord stimulation: an update. Neurotherapeutics. 2008;5:86.
21 De Vries J, De Jongste MJ, Spincemaille G, et al. Spinal cord stimulation for ischemic heart disease and peripheral vascular disease. Adv Tech Stand Neurosurg. 2007;32:63.
22 Oosterga M, ten Vaarwerk IA, DeJongste MJ, et al. Spinal cord stimulation in refractory angina pectoris—clinical results and mechanisms. Z Kardiol. 1997;86(suppl 1):107.
23 Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9.
24 Takanashi Y, Shinonaga M. Spinal cord stimulation for cerebral vasospasm as prophylaxis. Neurol Med Chir. 2000;40:352.
25 Doleys DM. Psychological factors in spinal cord stimulation therapy: brief review and discussion. Neurosurg Focus. 2006;21:E1.
26 Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481.
27 North RB, Kidd DH, Wimberly RL, et al. Prognostic value of psychological testing in patients undergoing spinal cord stimulation: a prospective study. Neurosurg Online. 1996;39:301.
28 North RB, Kidd DH, Olin JC, et al. Spinal cord stimulation electrode design: prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes. Part I: Technical outcomes. Neurosurgery. 2002;51:381.
29 Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36:531.
30 Holsheimer J, Wesselink WA. Optimum electrode geometry for spinal cord stimulation: the narrow bipole and tripole. Med Biol Eng Comput. 1997;35:493.
31 North R, Kidd D, Petrucci L, et al. Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes. Part II: Clinical outcomes. Neurosurgery. 2005;57:990.
32 Hornberger J, Kumar K, Verhulst E, et al. Rechargeable spinal cord stimulation versus non-rechargeable system for patients with failed back surgery syndrome: a cost-consequences analysis. Clin J Pain. 2008;24:244.
33 Tulgar M, He J, Barolat G, et al. Analysis of parameters for epidural spinal cord stimulation. 3. Topographical distribution of paresthesiae—a preliminary analysis of 266 combinations with contacts implanted in the midcervical and midthoracic vertebral levels. Stereotact Funct Neurosurg. 1993;61:146.
34 Barolat G. Experience with 509 plate electrodes implanted epidurally from C1 to L1. Stereotact Funct Neurosurg. 1993;61:60.
35 Kumar K, Buchser E, Linderoth B, et al. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007;10:24-33.
36 Kumar K, Wilson JR, Taylor RS, et al. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5:191.
37 Arxer A, Busquets C, Vilaplana J, et al. Subacute epidural abscess after spinal cord stimulator implantation. Eur J Anaesthesiol. 2003;20:755.
38 De Andres J, Valía JC, Cerda-Olmedo G, et al. Magnetic resonance imaging in patients with spinal neurostimulation systems. Anesthesiology. 2007;106:779.
39 Nutt JG, Anderson VC, Peacock JH, et al. DBS and diathermy interaction induces severe CNS damage. Neurology. 2001;56:1384.
40 U.S. Food and Drug Administration. FDA Public Health Notification: Diathermy Interactions with Implanted Leads and Implanted Systems with Leads. Available at http://www.fda.gov/cdrh/safety/121902.html, 2002.
41 Medtronic. Important Safety Information and Risks. Available at http://www.medtronic.com/neuro/paintherapies/pain_treatment_ladder/neurostimulation/risks/neuro_risk.html, 2004.