Chapter 14 Spinal and Epidural Anesthesia
1. What term is used to collectively describe spinal and epidural anesthesia?
2. Where is medicine deposited during performance of spinal anesthesia? With epidural anesthesia? With caudal anesthesia?
3. What are some advantages of spinal anesthesia when compared with epidural anesthesia?
Anatomy
5. What is the relative clinical significance of the curvatures of the spinal canal with respect to spinal and epidural anesthesia?
6. What is the number of each type of vertebrae composing the vertebral column?
7. Describe the anatomic parts of a vertebra by answering the following questions: What are the two parts that make up a vertebra? From what parts of the vertebra does the transverse process arise? From what parts of the vertebra does the spinous process arise?
8. How do the different characteristic features of the spinous processes and laminae of the thoracic and lumbar vertebrae impact clinical performance of neuraxial blocks?
10. What are some important surface landmarks used to identify specific spinal interspaces to guide placement of a spinal or epidural needle?
11. How are the laminae of adjacent vertebrae connected?
12. How are the tips of the spinous processes of adjacent vertebrae connected?
13. What passes through the intervertebral foramina?
14. What are the rostral and caudal limitations of the spinal canal? What accounts for the disparity between the vertebral level and spinal level?
15. What is the cauda equina, and what characteristic features are relevant to spinal anesthesia?
16. What are the three meningeal layers surrounding the spinal cord?
17. Where is cerebrospinal fluid relative to the meningeal layers? What are two interchangeable terms for this space?
18. What structures form the boundaries of the epidural space?
19. What structures are contained within the epidural space?
20. As the nerves pass through the intervertebral foramen they become encased by the dura, arachnoid, and pia, forming what three components of a peripheral nerve?
21. Where do the preganglionic nerves of the sympathetic nervous system originate, and what is their course of travel after leaving the spinal cord?
22. What is the plica mediana dorsalis? What might be its clinical significance?
23. Describe the blood supply of the spinal cord. Which area of the cord is most vulnerable to ischemic insult?
Preoperative preparation
25. What are the potential complications that should be discussed with the patient before proceeding with a spinal or epidural anesthetic?
26. What are the common indications for spinal anesthesia?
27. What are the common indications for epidural anesthesia?
28. What are the absolute contraindications to neuraxial anesthesia?
29. Does bacteremia preclude performance of a neuraxial technique?
30. Does chronic back pain preclude performance of a neuraxial technique?
31. What cardiac disorders warrant specific concern with respect to neuraxial anesthesia?
32. Do coagulation abnormalities preclude performance of a neuraxial anesthetic?
Spinal anesthesia
33. What are the common positions patients are placed in for administration of a spinal anesthetic?
34. What are some advantages and disadvantages of having a patient in a sitting position during performance of a spinal anesthetic?
35. Why might an anesthetist choose to administer a spinal with a patient in the lateral decubitus position?
36. What vertebral level is crossed by a line drawn across the patient’s back at the level of the top of the iliac crests? What interspace is located directly above this line? What interspace is located directly below this line?
37. What is the reason for placing a spinal anesthetic at a level below the L2 vertebra?
38. On what basis are spinal needles generally classified?
39. Which characteristics of a spinal needle will result in the lowest incidence of postdural puncture headache?
40. What are the two approaches used for spinal anesthesia, and what are their relative advantages and disadvantages?
41. When a midline approach is chosen, what are the tissue planes that will be traversed as the needle is advanced toward the subarachnoid space?
42. What accounts for the “pop” the anesthetist may feel when advancing a spinal needle into the subarachnoid space?
43. How is subarachnoid placement of the spinal needle confirmed?
44. How can the spinal needle be handled to stabilize the needle after proper placement in the subarachnoid space is confirmed?
45. After the syringe containing the local anesthetic solution for administration into the subarachnoid space is attached to the spinal needle, how can continued subarachnoid placement of the spinal needle be confirmed?
46. What should be done if blood-tinged cerebral spinal fluid (CSF) appears at the hub of the needle?
47. Describe the lumbosacral (Taylor) approach. When is this approach advantageous?
48. What are the three factors that most influence the distribution of the local anesthetic solution in cerebrospinal fluid after its administration into the subarachnoid space?
49. How is the baricity of a local anesthetic solution to be administered into the subarachnoid space defined? Why is this clinically important?
50. What are the two things that most influence the duration of a spinal anesthetic?
51. What is the baricity of the most commonly used spinal anesthetics? What is added to local anesthetics for spinal anesthesia to make the solution hyperbaric? What is the principal advantage of these solutions?
52. What role does the contour of the vertebral canal play in anesthetic distribution, and hence, level of spinal block?
54. What situations might warrant use of a hypobaric solution?
55. What are the relative advantages and disadvantages of isobaric solutions?
56. What is the purpose of adding a vasoconstrictor to the local anesthetic solution used for spinal anesthesia? What is their mechanism of action?
57. What are the two potentially useful effects derived from adding an opioid to the local anesthetic used for spinal anesthesia? What is their mechanism of action?
58. How do spinal anesthetics regress during the recovery from spinal anesthesia?
59. What recent events have led to concern regarding the use of lidocaine for spinal anesthesia? What are some modifications in practice that have been suggested should lidocaine be used for this purpose?
60. What is TNS, and what are the factors that increase the risk of its occurrence following spinal anesthesia with lidocaine?
61. List the rank order for the relative incidence of TNS with the following local anesthetics used for spinal anesthesia: bupivacaine; chloroprocaine; lidocaine; mepivacaine; prilocaine; and procaine.
62. What are some important restrictions with respect to the anesthetic solution if using chloroprocaine off-label for spinal administration?
63. What are some distinguishing characteristics between bupivacaine and tetracaine with respect to spinal anesthesia?
64. What is the temporal order of blockade of the motor, sensory, and sympathetic nerves after the administration of a spinal anesthetic?
65. What is a useful way to gain an early indication of the level of spinal anesthesia?
66. How do the ultimate dermatomal levels of motor, sensory, and sympathetic block compare during spinal anesthesia?
67. How is the extent of motor block produced by a spinal anesthetic generally assessed?
68. What surface landmarks are used to determine the approximate level of spinal anesthesia?
69. What are some advantages and disadvantages of a continuous spinal technique?
70. Why were microcatheters used for spinal anesthesia withdrawn from the U.S. market?
71. What is the likely mechanism of injury associated with microcatheters?
72. Has removal of microcatheters eliminated the risk of neurologic injury?
73. What elements of the continuous spinal technique are important to prevent neurologic injury?
74. What dose of anesthetic should be used when repeating a spinal because of a failed block?
75. What are the physiologic effects on the respiratory system of an appropriately instituted spinal anesthetic?
76. What are some physiologic effects on the gastrointestinal tract and the genitourinary system that result from a spinal anesthetic?
77. What is the effect of spinal anesthesia on blood pressure and what accounts for this effect?
78. How is spinal anesthetic-induced hypotension treated?
79. What is the effect of spinal anesthesia on heart rate, and what is believed to be the underlying mechanism?
80. How are spinal anesthetic-induced perturbations in heart rate treated?
81. What is the cause and typical onset of a postdural puncture headache?
82. What is the hallmark feature of a postdural puncture headache?
83. What are some of the other characteristic features of a postdural puncture headache?
84. What serious complications may result from a postdural puncture headache?
85. Which patients are most at risk for development of a postdural puncture headache?
86. What features of a spinal needle will impact the incidence of a postdural puncture headache?
87. What are some of the commonly used treatment options for a postdural puncture headache?
88. What are the likely predominant mechanism(s) by which an epidural blood patch may relieve a postdural puncture headache?
90. How should a total spinal be managed?
91. What are two possible causes of nausea that present soon after the administration of a spinal anesthetic?
92. What might contribute to backache occurring in a patient who has received a spinal for surgical anesthesia?
Epidural anesthesia
93. Is it acceptable to place a lumbar epidural after the induction of general anesthesia?
94. Is it acceptable to place a thoracic epidural after the induction of general anesthesia?
95. What are the advantages and disadvantages of epidural catheters that have an inner stainless steel core?
96. What are the comparative advantages and disadvantages of epidural catheters with a closed versus open tip?
97. Which approach (midline or paramedian) is most often used for placement of a thoracic epidural, and why is it chosen?
98. What is the “loss-of-resistance” technique?
99. What is the “hanging-drop” technique?
100. What are the potential advantages and disadvantages of the single-injection versus catheter technique for epidural anesthesia?
101. What is the technique used for placement of a catheter following identification of the epidural space?
102. What is the “test dose” for an epidural catheter? What is it testing for? How long must the anesthetist wait after administering the test dose to make this determination?
103. Why is the anesthetic administered in incremental doses?
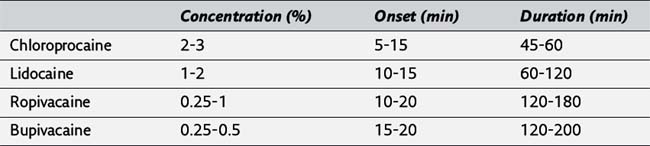
104. Complete the following table of local anesthetics used for epidural anesthesia:
105. Why are tetracaine and procaine rarely used for epidural anesthesia?
106. How is the caudal epidural space identified?
107. What anatomic variations may impact success and complications with caudal anesthesia?
108. What are the major factors affecting the spread of epidural anesthesia?
109. What patient-related factors might influence spread of epidural anesthesia?
110. How do patient position and anesthetic baricity influence the spread of epidural anesthesia?
111. What are the principal factors affecting the duration of epidural anesthesia?
112. What are some of the potential advantages of adding epinephrine to an anesthetic solution used for epidural anesthesia?
113. What is the effect of sodium bicarbonate when added to the local anesthetic solution used for epidural anesthesia?
114. For which local anesthetics is sodium bicarbonate commonly used as an additive?
115. How does lipophilicity affect the choice of opioids used for epidural administration?
116. What are some potential causes of failure of an epidural block?
117. What are the options for managing a failed epidural block?
118. What is the major site of action of local anesthetics administered epidurally?
119. How do the risks of epidural anesthesia compare with that of spinal anesthesia?
120. What are the commonly used management options following a “wet-tap”?
121. What are the risks associated with accidental subarachnoid injection of an epidural dose of anesthetic, and how should this complication be managed?
122. How do the hemodynamic effects of an epidural compare with those of spinal anesthesia?
123. Why does epidural anesthesia differ from spinal anesthesia with respect to systemic local anesthetic toxicity?
124. What are the clinical characteristics of an accidental subdural injection of anesthetic intended for the epidural space?
125. What is the significance of a paresthesia occuring during performance of an epidural anesthetic?
126. What is “combined spinal-epidural” anesthesia, and in what clinical setting is it commonly used?
127. What are the potential advantages of a combined epidural-general technique?
Answers*
1. Spinal and epidural anesthesia are collectively referred to as central neuraxial block. These procedures are subcategories or specific types of regional or conduction anesthesia. (253)
2. In spinal anesthesia, medicine is deposited into the cerebrospinal fluid within the subarachnoid space, with very rare exception at the lumbar level. In epidural anesthesia, the drug is deposited within the epidural space, and is commonly performed at both the lumbar and thoracic level. In caudal anesthesia, medicine is also deposited in the epidural space but the needle used to inject the medicine approaches the epidural space via the sacral hiatus. (253)
3. When compared with epidural anesthesia, spinal anesthesia takes less time to perform, causes less discomfort during placement, requires less local anesthetic, and produces more intense sensory and motor block. In addition, correct placement of the needle in the subarachnoid space is confirmed by a clearly defined endpoint (appearance of cerebral spinal fluid). (253)
4. Advantages of epidural anesthesia include a decreased risk of a postdural puncture headache (assuming a negligible incidence of inadvertent dural puncture), a lower incidence of systemic hypotension, the ability to produce a segmental sensory block, and greater control over the intensity of sensory anesthesia and motor block achieved by adjustment of the local anesthetic concentration. The routine placement of catheters for epidural anesthesia imparts additional benefit by allowing titration of the block for the duration of surgery. Additionally, maintenance of a catheter provides a means for long-term administration of local anesthetics or opioid-containing solutions (or both), which are highly effective for control of postoperative or obstetric pain. (253)
Anatomy
5. On a lateral view, the vertebral canal exhibits four curvatures, of which the thoracic convexity (kyphosis) and the lumbar concavity (lordosis) are of major importance to the distribution of local anesthetic solution in the subarachnoid space. In contrast, these curves have little effect on the spread of local anesthetic solutions in the epidural space. (253, Figure 17-1)
6. The vertebral column is composed of 7 cervical vertebrae, 12 thoracic vertebrae, and 5 lumbar vertebrae, as well as the 5 fused sacral and 4 fused coccygeal vertebrae. (253, Figure 17-1)
7. A vertebra is made up of the vertebral body and the bony arch. The transverse process arises from the junction of the pedicle and laminae. The spinous process arises posteriorly from the joining of the laminae. (253, Figure 17-2)
8. The nearly perpendicular orientation of the spinous process in the lumbar area and the downward angular orientation in the thoracic area define the angle required for placement and advancement of a needle intended to access the vertebral canal. The wide interlaminar space in the lumbar spine reflects the fact that the lamina occupies only about half the space between adjacent vertebrae. In contrast, the interlaminar space is just a few millimeters wide at the level of the thoracic vertebrae. (254, Figure 17-5)
9. The opening between the unfused lamina of the fourth and fifth sacral vertebrae is called the sacral hiatus. There is considerable anatomic variability in the features of the dorsal surface of the sacrum. Indeed, the sacral hiatus is absent in nearly 8% of adult subjects, thereby preventing entry through the sacrococcygeal ligament into the sacral canal and performance of caudal anesthesia. (255, Figure 17-4)
10. Surface landmarks are used to identify specific spinal interspaces. The most important surface landmarks include a line drawn between the iliac crests, which generally traverses the body of the L4 vertebra and is the principal landmark used to determine an appropriate level for insertion of a spinal needle; the C7 spinous process appreciated as a bony knob at the lower end of the neck; and a line drawn between the lower limits of the scapulae roughly correlating with the T7-8 interspace that is often used to guide needle placement for passage of a catheter into the thoracic epidural space. (255, Figure 17-6)
11. The laminae of adjacent vertebrae are connected by the ligamentum flavum. (256, Figure 17-3)
12. The tips of the spinous processes of adjacent vertebrae are connected by the supraspinous ligaments. (256, Figure 17-3)
13. The spinal nerves pass through the intervertebral foramina and supply a specific dermatome. (258, Figure 17-3)
14. The spinal cord begins at the rostral border of the medulla and, in the fetus, extends the entire length of the vertebral canal. However, because of disproportionate growth of neural tissue and the vertebral canal, the spinal cord generally terminates around the third lumbar vertebra at birth and at the lower border of the first lumbar vertebra in adults. As a further consequence of this differential growth, the spinal nerves become progressively longer and more closely aligned with the longitudinal axis of the vertebral canal. (256)
15. The cauda equina—so named because of its resemblance to a horse’s tail—is the collection of lumbar and sacral nerves that extend beyond the end of the spinal cord as a collection of nerves in the spinal canal before exiting via the intervertebral foramina at their respective vertebral column levels. The nerve roots of the cauda equina move relatively freely within the CSF, a fortunate arrangement that permits them to be displaced rather than pierced by an advancing needle. (256)
16. The outermost meningeal layer, the dura mater, is a tough fibroelastic membrane that provides structural support. It originates at the foramen magnum and continues caudally to terminate between S1 and S4. Closely adherent to the inner surface of the dura lies the arachnoid membrane. Though far more delicate than the dura, the arachnoid serves as the major pharmacologic barrier preventing movement of the drug from the epidural to the subarachnoid space. The innermost layer of the spinal meninges, the pia, is a highly vascular structure closely applied to the cord that forms the inner border of the subarachnoid space. (258, Figures 17-9 and 17-10)
17. Cerebrospinal fluid is contained between the pia and arachnoid, consequently referred to as the subarachnoid space. Another term for this anatomic compartment is the intrathecal space. (258, Figure 17-10)
18. The epidural space lies between the dura and the wall of the vertebral canal. It is bounded cranially by the foramen magnum, caudally by the sacrococcygeal ligament, anteriorly by the posterior longitudinal ligament, laterally by the vertebral pedicles, and posteriorly by both the ligamentum flavum and vertebral lamina. (259)
19. Although often referred to as a “potential space,” the epidural space is actually an irregular column of fat, lymphatics, and blood vessels. It is not a closed space but communicates with the paravertebral spaces by way of the intervertebral foramina. (259)
20. The dura, arachnoid, and pia encasement of the peripheral nerve are the origins of the epineurium, perineurium, and endoneurium, respectively. (258, Figure 17-10)
21. Preganglionic nerves of the sympathetic nervous system originate from the spinal cord at the T1 to L2 levels. From there they travel with the spinal nerves before separating to form the sympathetic chain, and more distant sites such as the celiac plexus. (258, Figure 17-11)
22. There is controversy regarding the existence and clinical significance of a connective tissue band (plica mediana dorsalis) extending from the dura mater to the ligamentum flavum and hence dividing the posterior epidural space into two compartments. Anatomic studies have suggested the presence of this structure and have led to speculation that this tissue band may occasionally be responsible for difficulty threading a catheter into the epidural space or the occurrence of a unilateral sensory block. However, some investigators have been unable to confirm the presence of this structure.
23. The blood supply of the spinal cord arises from a single anterior and two paired posterior spinal arteries (Figure 17-13). The posterior spinal arteries emerge from the cranial vault and supply the dorsal (sensory) portion of the spinal cord. Because they are paired and have rich collateral anastomotic links from the subclavian and intercostal arteries, this area of the spinal cord is relatively protected from ischemic damage. This is not the case with the single anterior spinal artery that originates from the vertebral artery and supplies the ventral (motor) portion of the spinal cord. (260, Figure 17-13)
24. The anterior spinal artery receives branches from the intercostal and iliac arteries, but these branches are variable in number and location. The largest anastomotic link, the radicularis magna (artery of Adamkiewicz), arises from the aorta in the lower thoracic or upper lumbar region. The vessel is highly variable but, most commonly, is on the left and enters the vertebral canal through the L1 intervertebral foramen. The artery of Adamkiewicz is critical to the blood supply of the lower two thirds of the spinal cord, and damage to it will produce characteristic bilateral lower extremity motor loss (anterior spinal artery syndrome). (260, Figure 17-13)
Preoperative preparation
25. Relevant complications that should be discussed with the patient include (1) those that are rare but serious, including nerve damage, bleeding, and infection, and (2) those that are common but of relatively minor consequence, such as a postdural puncture headache. There are no common serious complications (if there were, these techniques would not be used in clinical practice), and the infrequent minor problems are not of sufficient concern to warrant specific discussion. The possibility of a failed block should be discussed, and the patient should be reassured that in such circumstances, alternative anesthetic techniques will be provided to ensure their comfort. (262)
26. Spinal anesthesia is generally used for surgical procedures involving the lower abdominal area, perineum, and lower extremities. Although the technique can also be used for upper abdominal surgery, most consider it preferable to administer a general anesthetic to ensure patient comfort. In addition, the extensive block required for upper abdominal surgery and the nature of these procedures may have a negative impact on ventilation and oxygenation. (262)
27. Epidural anesthesia, like spinal anesthesia, is often used as the primary anesthetic for surgeries involving the lower abdomen or lower extremities. However, because of its segmental nature, anesthesia provided by lumbar epidural anesthesia may be suboptimal for procedures involving the lower sacral roots. Epidural anesthesia is also frequently used as a supplement to general anesthesia, particularly for thoracic and upper abdominal procedures. In such cases, significant benefit derives from the ability to provide continuous epidural anesthesia postoperatively to facilitate effective treatment of postoperative pain, as numerous studies confirm the superiority of epidural techniques compared to parenteral opioids. Similarly, continuous epidural anesthesia is very effective and widely used for the control of labor pain. (262)
28. Absolute contraindications to neuraxial anesthesia include patient refusal, infection at the site of planned needle puncture, elevated intracranial pressure, and bleeding diathesis. Patients should never be encouraged against their wishes to accept a regional anesthetic technique. (262)
29. Bacteremia does not necessarily mitigate against performance of a regional anesthetic technique. Although concern that an epidural abscess or meningitis might result from the introduction of infected blood during the procedure, clinical experience suggests that the risk is small and can be weighed against the potential benefit. In such cases, there is evidence to suggest that institution of appropriate antibiotic therapy before the block may decrease the risk for infection. (262)
30. Chronic back pain does not represent a contraindication to neuraxial anesthetic techniques, although they may be avoided because patients may perceive a relationship between postoperative exacerbation of pain and the block, even though they are not causally related. (262)
31. Patients with mitral stenosis, idiopathic hypertrophic subaortic stenosis, and aortic stenosis are intolerant of acute decreases in systemic vascular resistance. Thus, though not a contraindication, neuraxial block should be used cautiously in such cases. (262)
32. The decision to use a neuraxial block in patients with abnormal coagulation, either endogenous or produced by the administration of anticoagulants, must be based on a risk-benefit assessment and include discussion with the patient and the surgical team. Guidelines developed by the American Society of Regional Anesthesia (www.asra.com) are updated periodically based on evolving literature and changes in clincal practice, and can thus provide valuable guidance in the management of these patients. (262)
Spinal anesthesia
33. Spinal anesthesia can be performed with the patient in the lateral decubitus, sitting, or less commonly, the prone position. To the extent possible, the spine should be flexed by having the patient bend at the waist and bring the chin toward the chest, which will optimize the interspinous space and the interlaminar foramen. (263)
34. The sitting position encourages flexion and facilitates recognition of the midline, which may be of increased importance in an obese patient. Because lumbar cerebral spinal fluid (CSF) is elevated in this position, the dural sac is distended, thus providing a larger target for the spinal needle. This higher pressure also facilitates recognition of the needle tip within the subarachnoid space, as heralded by the free flow of CSF. When combined with a hyperbaric anesthetic, the sitting position favors a caudal distribution; the resultant anesthesia is commonly referred to as a “saddle block.” However, in addition to being poorly suited for a heavily sedated patient, vasovagal syncope can occur. (263)
35. The lateral decubitus position is more comfortable and more suitable for the ill or frail. It also enables the anesthetist to safely provide greater levels of sedation. (263)
36. A line drawn across the patient’s back at the level of the top of the iliac crests is generally considered to identify the L4 vertebral level. The interspace palpated directly above this line would be L3-4, and the interspace palpated directly below this line would be L4-5. However, this is not invariant, and not uncommonly, use of this conceptual line will result in estimates that are inaccurate by as much as two interspaces. (263)
37. The caudal limitation of the spinal cord in an adult usually lies between the L1 and L2 vertebrae. For this reason, spinal anesthesia is not ordinarily performed above the L2-3 interspace. Nevertheless, some risk remains because the spinal cord extends to the third lumbar vertebra in approximately 2% of adults. (263)
38. A variety of needles are available for spinal anesthesia and they are generally classified by their size (most commonly 22 to 25 gauge) and the shape of their tip. The two basic designs of spinal needles are (1) an open-ended (beveled or cutting) needle and (2) a closed tapered-tip pencil-point needle with a side port. (263, Figure 17-14)
39. The incidence of postdural puncture headache varies directly with the size of the needle, and it is also lower when a pencil-point (Whitacre or Sprotte) rather than a beveled-tip (Quincke) needle is used. Consequently, a 24- or 25-gauge pencil-point needle is usually selected when spinal anesthesia is performed on younger patients in whom postdural puncture headache is more likely to develop. (263, Figure 17-14)
40. Spinal anesthesia can be accomplished using a midline or a paramedian approach. The midline approach is technically easier, and the needle passes through less sensitive structures, thus requiring less local anesthetic infiltration to ensure patient comfort. However, the paramedian approach is better suited to challenging circumstances when there is narrowing of the interspace or difficulty in flexion of the spine. (264)
41. As the spinal needle progresses toward the subarachnoid space, it passes through the skin, subcutaneous tissue, supraspinous ligament, interspinous ligament, ligamentum flavum, and the epidural space to reach and pierce the dura/arachnoid. (264, Figure 17-3)
42. The anesthetist may feel a characteristic “pop” just before accessing the subarachnoid space as the spinal needle is being advanced. This “pop” is produced by the spinal needle passing through the dura mater. (265)
43. Subarachnoid placement of the spinal needle is confirmed by the appearance of cerebrospinal fluid in the hub of the spinal needle. (265)
44. After proper positioning in the subarachnoid space, the spinal needle may be stabilized by holding the hub of the spinal needle between the anesthesiologist’s thumb and forefinger and resting the dorsum of the same hand on the patient’s back. When the spinal needle is held in this manner, it should remain stabilized even with patient movement. (265)
45. After the syringe containing the local anesthetic solution for administration into the subarachnoid space is attached to the spinal needle, the anesthetist typically aspirates back on the syringe to confirm continued subarachnoid placement of the spinal needle tip. Confirmation is made by the characteristic swirl in the syringe as cerebrospinal fluid enters the syringe and mixes with the local anesthetic solution. The local anesthetic solution can then be deposited into the subarachnoid space over approximately 3 to 5 seconds. After completion of the deposition of the local anesthetic solution into the subarachnoid space, cerebrospinal fluid can again be aspirated to verify delivery of the anesthetic. The spinal needle and syringe should be removed together as a single unit, and the antiseptic wiped from the patient’s back. (265)
46. Occasionally, blood-tinged CSF initially appears at the hub of the needle. If clear CSF is subsequently seen, the spinal anesthetic can be completed. Conversely, if blood-tinged CSF continues to flow, the needle should be removed and reinserted at a different interspace. Should blood-tinged CSF still persist, the attempt to induce spinal anesthesia should be terminated. (265)
47. The Taylor approach (first described by Dr. John A. Taylor, a urologist) describes the paramedian technique to access the L5-S1 interspace. Though generally the widest interspace, it is often inaccessible from the midline because of the acute downward orientation of the L5 spinous process. The spinal needle is passed from a point 1 cm caudad and 1 cm medial to the posterior superior iliac spine and advanced cephalad at a 55-degree angle with a medial orientation based on the width of the sacrum. The Taylor approach is technically challenging but very useful because it is minimally dependent on patient flexion for successful passage of the needle into the subarachnoid space. (265, Figure 17-15)
48. The three factors that most influence the distribution of local anesthetic solution in the subarachnoid space are the baricity of the solution, the contour of the spinal canal, and the position of the patient during, and for the first few minutes after, its administration. (266)
49. Local anesthetic solutions are classified as hypobaric, isobaric, and hyperbaric based on their density relative to the density of CSF. Baricity is an important consideration because it predicts the direction that local anesthetic solution will move after injection into the CSF. (266)
50. The two things that most influence the duration of a spinal anesthetic are the particular drug selected and whether a vasoconstrictor, such as epinephrine or phenylephrine, is present in the local anesthetic solution. (266)
51. The most commonly selected local anesthetic solutions for spinal anesthesia are hyperbaric (achieved by the addition of glucose), and their principal advantage is the ability to achieve greater cephalad spread of anesthesia. Commercially available hyperbaric local anesthetic solutions include 0.75% bupivacaine with 8.25% glucose and 5% lidocaine with 7.5% glucose. Tetracaine is formulated as a 1% plain solution and is most often used as a 0.5% solution with 5% glucose, which is achieved by dilution of the anesthetic with an equal volume of 10% glucose. (266)
52. The contour of the vertebral canal is critical to the subarachnoid distribution of hyperbaric local anesthetic solutions. For example, in the supine horizontal position, the patient’s thoracic kyphosis will be dependent relative to the peak created by the lumbar lordosis. Anesthetic delivered cephalad to this peak will thus move toward the thoracic kyphosis, which is normally around T6-8. Placing the patient in a head-down (Trendelenburg) position will further accentuate this cephalad spread of local anesthetic solution. (266, Figure 17-1)
53. Hyperbaric local anesthetic solutions can be administered with the patient seated and this position maintained during the initial movement of anesthetic to deliberately encourage restricted sacral anesthesia (referred to as a “saddle block,” reflecting sensory anesthesia of the area that would be in contact with a saddle). (266)
54. Hypobaric local anesthetic solutions find limited use in clinical practice and are generally reserved for patients undergoing perineal procedures in the “prone jackknife” position or undergoing hip arthroplasty where anesthetic can “float up” to the nondependent operative site. (267)
55. Isobaric local anesthetic solutions undergo limited spread in the subarachnoid space, which may be considered an advantage or disadvantage depending on the clinical circumstances. Because the distribution of local anesthetic solutions is not affected by gravity, spinal anesthesia can be performed without concern that the resultant block might be influenced by patient position. Isobaric spinal anesthesia is particularly well suited for perineal or lower extremity procedures, as well as surgery involving the lower part of the trunk (hip arthroplasty, inguinal hernia repair). (267)
56. Vasoconstrictors are frequently added to spinal anesthetic solutions to increase the duration of spinal anesthesia. This is most commonly achieved by the addition of epinephrine (0.1 to 0.2 mg, which is 0.1 to 0.2 mL of a 1:1000 solution) or phenylephrine (2 to 5 mg, which is 0.2 to 0.5 mL of a 1% solution). Increased duration of spinal anesthesia is believed to result from a reduction in spinal cord blood flow, which decreases loss of local anesthetic from the perfused areas and thus increases the duration of exposure to local anesthetic. However, with epinephrine, there may be a small contribution as a result of its α2-adrenergic analgesic activity. (267)
57. Opioids may be added to local anesthetic solutions to enhance surgical anesthesia and provide postoperative analgesia. This effect is mediated at the dorsal horn of the spinal cord, where opioids mimic the effect of endogenous enkephalins. Commonly, fentanyl (25 μg) is used for short surgical procedures, and its administration does not preclude discharge home on the same day. The use of morphine (0.1 to 0.5 mg) can provide effective control of postoperative pain for roughly 24 hours, but it necessitates in-hospital monitoring for respiratory depression. (267)
58. During recovery from spinal anesthesia, regression of the anesthetic is from the highest dermatome in a caudad direction. (266)
59. Recent reports of major and minor complications associated with spinal lidocaine have tarnished its reputation and jeopardize its continued clinical use. Initial reports of permanent neurologic deficits were restricted to its use for continuous spinal anesthesia, where extremely high doses were administered. However, subsequent reports suggest that injury may occur even with the administration of a dose historically recommended for single-injection spinal anesthesia. These injuries have led to suggested modifications in practice that include a reduction in the lidocaine dose from 100 mg to 60 or 75 mg and dilution of the commercial formulation of 5% lidocaine with an equal volume of saline or CSF before subarachnoid injection. (267)
60. Lidocaine has been linked to the development of transient neurologic symptoms or “TNS” (pain and/or dysesthesia in the back, buttocks, and lower extremities) in up to a third of patients receiving this anesthetic for spinal anesthesia. Factors that increase the risk for TNS in association with lidocaine spinal anesthesia include patient positioning (lithotomy, knee arthroscopy) and outpatient status. (268)
61. With respect to TNS, the approximate rank order with respect to its incidence is lidocaine>procaine, mepivacaine>prilocaine, bupivacaine, chloroprocaine. (268)
62. When using chloroprocaine off-lable for spinal administration, the solution should be preservative-free, and epinephrine should not be used. (268)
63. The recommended doses (5 to 20 mg) and reported durations of action (90 to 120 minutes) of bupivacaine and tetracaine are similar. However, bupivacaine produces slightly more intense sensory anesthesia (as evidenced by a lower incidence of tourniquet pain), whereas motor block with tetracaine appears to be slightly more pronounced. The more important distinction between these local anesthetics is that the duration of tetracaine spinal anesthesia is more variable and more profoundly affected by the addition of a vasoconstrictor. Consequently, tetracaine remains the most useful spinal anesthetic in circumstances in which a prolonged block is sought. Unfortunately, the inclusion of a vasoconstrictor with tetracaine results in a significant incidence of transient neurologic symptoms, as opposed to the rarity of these symptoms when tetracaine is used alone. (268)
64. Sympathetic nerves are blocked before both motor nerves and sensory nerves after the administration of a spinal anesthetic. (268)
65. A useful way to gain an early indication of the level of spinal anesthesia is by testing the patient’s ability to discriminate temperature in the relevant dermatomes. For example, in an unblocked area, an alcohol sponge will produce a cold sensation, whereas in the blocked areas the same alcohol sponge will feel warm or neutral. (268)
66. The dermatomal order of blockade produced by a spinal anesthetic, from highest to lowest, is sympathetic, sensory, then motor. (268)
67. Skeletal muscle strength can be tested by asking the patient to dorsiflex the foot (S1-2), raise the knees (L2-3), or tense the abdominal rectus muscles (T6-12). (269)
68. The surface landmarks and their respective dermatomal level most often used clinically are: nipple, T4-5; tip of xiphoid, T7; umbilicus, T10; inguinal ligament, T12. (269)
69. Inserting a catheter into the subarachnoid space increases the utility of spinal anesthesia by permitting repeated drug administration as necessary to maintain the level and duration of sensory and motor block. Anesthesia can thus be provided for prolonged operations without delaying recovery. An added benefit is the possibility of using lower doses of anesthetic. (With a catheter in place, smaller doses can be titrated to the patient’s response. In contrast, with the single-injection technique, relatively high doses must be administered to all patients to ensure successful anesthesia in a large percentage of cases.) However, use of large-bore epidural needles and catheters for continuous spinal anesthesia poses significant risk of postdural puncture headache. (269, Figure 17-4)
70. Microcatheters (27 gauge and smaller) used for continuous spinal anesthesia were withdrawn from clinical practice in the United States after reports of cauda equina syndrome associated with their use. (269)
71. It is likely that the injury associated with the use of microcatheters resulted from the combination of maldistribution and repetitive injection of local anesthetic solution. It is speculated that pooling of local anesthetic solution in the dependent sacral sac produced a restricted block that was inadequate for surgery. In response to inadequate anesthesia, injections were repeated and ultimately achieved adequate sensory anesthesia, but not before neurotoxic concentrations were reached in the caudal region of the subarachnoid space. It is possible that the microcatheter contributed to this problem because the long narrow-bore tubing creates resistance to injection and thereby results in a low flow rate that can encourage a restricted distribution. (269)
72. Removal of microcatheters from clinical practice has not eliminated risk. The problem of maldistribution is not restricted to microcatheters or lidocaine, and the same injuries have occurred with larger “epidural” catheters used for continuous spinal anesthesia and other local anesthetics. (269)
73. Guidelines for continuous spinal anesthesia include the following elements:
 If maldistribution is suspected, use maneuvers to increase the spread of local anesthetic (change the patient’s position, alter the lumbosacral curvature, switch to a solution with a different baricity)
If maldistribution is suspected, use maneuvers to increase the spread of local anesthetic (change the patient’s position, alter the lumbosacral curvature, switch to a solution with a different baricity) If well-distributed sensory anesthesia is not achieved before the dose limit is reached, abandon the technique (269, Figure 17-4)
If well-distributed sensory anesthesia is not achieved before the dose limit is reached, abandon the technique (269, Figure 17-4)74. Similar to continuous spinal anesthesia, single-injection spinal anesthesia may fail due to local anesthetic maldistribution. This issue becomes important when considering whether to repeat a “failed” spinal and, if so, the dose of anesthetic that should be used for the second injection. In the past, it was considered acceptable to readminister a “full dose.” However, if failure derives from maldistribution of the local anesthetic solution, this strategy may introduce a risk of injury. Accordingly, if a spinal anesthetic is to be repeated, it should be assumed that the first injection was delivered in the subarachnoid space as intended, and the combination of the two doses should not exceed that considered reasonable as a single injection for spinal anesthesia. (270)
75. Spinal anesthesia has little, if any, effect on resting alveolar ventilation (arterial blood gases unchanged), but high levels of motor anesthesia that produce paralysis of abdominal and intercostal muscles can lead to a decreased ability to cough and expel secretions. Additionally, patients may complain of difficulty breathing (dyspnea)—despite adequate ventilation—because of inadequate sensation of breathing from the loss of proprioception from abdominal and thoracic muscles. (270)
76. Spinal anesthesia above T5 inhibits sympathetic nervous system innervation to the gastrointestinal tract, and the resulting unopposed parasympathetic nervous system activity results in contracted intestines and relaxed sphincters. Similarly, the ureters are contracted, and the ureterovesical orifice is relaxed.
77. Hypotension (systolic blood pressure <90 mm Hg) is estimated to occur in about a third of patients receiving spinal anesthesia. This hypotension results from a sympathetic nervous system block that (1) decreases venous return to the heart and decreases cardiac output and/or (2) decreases systemic vascular resistance. Modest decreases in systemic blood pressure are most likely due to decreases in systemic vascular resistance, whereas large decreases in systemic blood pressure are believed to be the result of decreases in venous return and cardiac output. (270)
78. Spinal anesthesia–induced hypotension is treated physiologically by restoration of venous return to increase cardiac output. In this regard, the internal autotransfusion produced by a modest head-down position (5 to 10 degrees) will facilitate venous return without greatly exaggerating cephalad spread of the spinal anesthetic. Adequate hydration before the institution of spinal anesthesia is important for minimizing the effects of venodilation from sympathetic nervous system block. Sympathomimetics with positive inotropic and venoconstrictor effects, such as ephedrine (5 to 10 mg IV), are often chosen as first-line drugs to maintain perfusion pressure during the first few minutes after the institution of spinal anesthesia. Phenylephrine (50 to 100 μg IV) and other sympathomimetics that increase systemic vascular resistance may decrease cardiac output and do not specifically correct the decreased venous return contributing to the spinal anesthesia–induced hypotension. Nevertheless, anesthesiologists have long used phenylephrine successfully to treat decreases in systemic blood pressure associated with spinal anesthesia. Furthermore, this drug is of particular value when administration of ephedrine is associated with significant increases in heart rate. (270)
79. The heart rate does not change significantly in most patients during spinal anesthesia. However, in an estimated 10% to 15% of patients, significant bradycardia occurs. As with hypotension, the risk for bradycardia increases with increasing sensory levels of anesthesia. Speculated mechanisms for this bradycardia include the block of cardioaccelerator fibers originating from T1 through T4 and decreased venous return (Bezold-Jarisch reflex). (271)
80. Although bradycardia is usually of moderate severity and promptly responsive to atropine or ephedrine, there are reports of precipitous bradycardia and asystole in the absence of any preceding event. This catastrophic event can probably be prevented through maintenance of preload and reversal of bradycardia by aggressive stepwise escalation of treatment (ephedrine, 5 to 50 mg IV; atropine, 0.4 to 1.0 mg IV; epinephrine, 0.05 to 0.25 mg IV), whereas the development of profound bradycardia or asystole mandates immediate treatment with full resuscitative doses of epinephrine. (271, Figure 17-16)
81. Postdural puncture headache is a direct consequence of the hole in the dura, which results in the loss of CSF at a rate exceeding its production. Loss of CSF causes downward displacement of the brain and a resultant stretch on sensitive supporting structures. Pain also results from distention of the blood vessels, which must compensate for the loss of CSF because of the fixed volume of the skull. The pain associated with postdural puncture headache generally begins 12 to 48 hours after transgression of the dura, but it can occur immediately and has been reported to occur up to several months after the event. (271, Figure 17-17)
82. The hallmark of a postdural puncture headache is its postural component: it appears or intensifies with sitting or standing and is partially or completely relieved by recumbency. This feature is so distinctive that it is difficult to consider the diagnosis in its absence. (271)
83. Postdural puncture headache is typically occipital or frontal (or both) and is usually described as dull or throbbing. Associated symptoms such as nausea, vomiting, anorexia, and malaise are common. Ocular disturbances, manifested as diplopia, blurred vision, photophobia, or “spots,” may occur and are believed to result from the stretch of the cranial nerves, most commonly cranial nerve VI, as the brain descends because of the loss of CSF. Although symptomatic hearing loss is unusual, formal auditory testing will routinely reveal abnormalities. (271)
84. Though generally a transient problem, loss of CSF may rarely result in significant morbidity because caudal displacement of the brain can result in tearing of bridging veins with the development of a subdural hematoma. (271)
85. Age is one of the most important factors affecting the incidence of postdural puncture headache. Children are at low risk, but after puberty, risk increases substantially and then slowly declines with advancing age. Females have long been suspected to be at increased risk, and a recent meta-analysis confirms this impression, even in the absence of pregnancy. A previous history of postdural puncture headache places one at increased risk for the development of this complication after a subsequent spinal anesthetic. (272)
86. The incidence of postdural puncture headache varies directly with the diameter of the needle that has pierced the dura. The shape of the hole created by the needle also has an impact on loss of CSF; this has led to the development of “pencil-point” needle tips, which appear to spread the dural and arachnoid fibers, and to produce less tear and a smaller hole for a given diameter needle. (272)
87. Initial treatment of postdural puncture headache is usually conservative and consists of bed rest, fluids, analgesics, and possibly caffeine. More definitively, a blood patch can be performed in which 15 to 20 mL of the patient’s blood, aseptically obtained, is injected into the epidural space. (272)
88. The immediate effect is related to the volume effect of the injected blood, whereas long-term relief is thought to occur from sealing or “patching” of the dural tear. (272)
89. Total spinal anesthesia is the term applied to excessive sensory and motor anesthesia associated with a loss of consciousness. Apnea and loss of consciousness are often attributed to ischemic paralysis of the medullary ventilatory centers because of profound hypotension and associated decreases in cerebral blood flow. However, loss of consciousness may also be the direct consequence of local anesthetic effect above the foramen magnum inasmuch as patients may lose or fail to regain consciousness despite restoration of systemic blood pressure. Total spinal anesthesia is typically manifested soon after injection of the local anesthetic solution into the subarachnoid space. (272)
90. Treatment of high or total spinal anesthesia consists of maintenance of the airway and ventilation, as well as support of the circulation with sympathomimetics and intravenous fluid administration. Patients are placed in a head-down position to facilitate venous return. An attempt to limit the cephalad spread of local anesthetic solution in CSF by placing patients in a head-up position is not recommended, because this position will encourage venous pooling and potentially jeopardize cerebral blood flow, which may contribute to medullary ischemia. Tracheal intubation is usually warranted and is mandated for patients at risk of aspiration (e.g., pregnant women). It may be appropriate to administer an intravenous induction drug before tracheal intubation if consciousness is retained and cardiovascular status is acceptable. (273)
91. Nausea occurring after the initiation of spinal anesthesia must alert the anesthetist to the possibility of systemic hypotension sufficient to produce cerebral ischemia. In such cases, treatment of hypotension with a sympathomimetic should eliminate the nausea. Alternatively, nausea may occur because of a predominance of parasympathetic activity as a result of selective block of sympathetic nervous system innervation to the gastrointestinal tract. Similar to bradycardia, the incidence of nausea and vomiting parallels the sensory level of spinal anesthesia. (273)
92. Backache presenting after spinal anesthesia is frequently due to the position that was maintained during surgery. Patients with decreased sensory perception induced by a spinal anesthetic may remain in positions for long periods of time that may otherwise have been too uncomfortable, thus resulting in ligament strain that might not have otherwise occurred. In support of this is the fact that roughly 25% of patients complain of backache after surgery regardless of the anesthetic technique. (273)
Epidural anesthesia
93. Controversy exists regarding the wisdom of placing lumbar epidural catheters after the induction of general anesthesia. Although there is concern that an inability to elicit a patient response might increase the risk for neural injury, a retrospective review challenges this assertion. Nonetheless, many anesthesiologists believe that lumbar epidural anesthesia and catheter placement are best performed in a communicative patient. (273)
94. Performance of thoracic epidural anesthesia in an anesthetized patient should be avoided. However, the same considerations do not apply to pediatric anesthesia, where a conscious patient would probably impart no benefit but instead add substantial risk. Consequently, it is standard practice to place caudal, lumbar, and even thoracic epidural catheters in children after induction of general anesthesia. (273)
95. Some catheters have an inner stainless steel wire coil that imparts flexibility and prevents kinking. This characteristic makes them less likely to (1) pierce an epidural vessel, (2) find false passage into a fascial plane, or (3) be advanced out of the epidural space through the intervertebral foramen. However, their flexibility also makes them more difficult to thread into the epidural space. (274, Figure 17-18)
96. The tip of an epidural catheter may be open or have a closed “bullet” tip with proximal ports. Bullet-tipped or multiorifice catheters tend to produce more uniform distribution of local anesthetic solution, but they have the disadvantage of requiring greater insertion depth to ensure complete delivery of local anesthetic solution into the epidural space. (274, Figure 17-18)
97. In contrast to procedures performed in the lumbar area, thoracic epidural anesthesia is generally accomplished through a paramedian approach. In this region the spinous processes are angulated and closely approximated, which makes it difficult to avoid bony obstruction when approaching from the midline. (275, Figure 17-5)
98. With the loss-of-resistance technique, a syringe containing saline, air, or both is attached to the needle, and the needle is slowly advanced while assessing resistance to injection. One method is to use a syringe containing 2 to 3 mL of saline with a small air bubble (0.1 to 0.3 mL). If the needle is properly seated in the ligamentum flavum, it will be difficult to inject the saline or the air bubble, and the plunger of the syringe will “spring back” to its original position. The needle is advanced while continuous pressure is exerted on the plunger of the syringe. An abrupt loss of resistance to injection signals passage through the ligamentum flavum and into the epidural space, at which point the contents of the syringe are delivered. Another technique uses repeated advances of the needle without continuous pressure, and an assessment of the resistance to injection is made after each advancement. (275, Figure 17-19)
99. The “hanging-drop” technique is an alternative method for identifying the epidural space. With this technique, a small drop of saline is placed at the hub of the epidural needle. As the needle passes through the ligamentum flavum into the epidural space, the saline drop is retracted into the needle by the negative pressure in the epidural space. Interestingly, the hanging-drop technique can be used in the lumbar region despite the lack of negative pressure in the lumbar epidural space. In this region, the needle pushing the dura away from the ligamentum flavum creates negative pressure. Accordingly, this technique is likely to be associated with a higher incidence of accidental dura penetration (“wet tap”). (275)
100. The advantage of the single-injection technique is its simplicity, and the distribution of local anesthetic solution tends to be more uniform than when administered through an indwelling catheter. The disadvantage is the lack of ability to reinject, and thus the inability to provide prolonged anesthesia titrated to the duration of surgery. Additionally, with the catheter technique, anesthetic administration can extend into the postoperative period to provide highly effective postoperative analgesia. (276)
101. Following identification of the epidural space, the catheter is advanced 3 to 5 cm beyond the tip of the needle positioned in the epidural space. Further advancement increases the risk that the catheter might enter an epidural vein, exit an intervertebral foramen, or wrap around a nerve root. The epidural needle is withdrawn over the catheter, with care taken to not move the catheter. No attempt should be made to withdraw a catheter back through the needle because shearing (transection) of the catheter might result, with retention of the transected tip of the catheter in the epidural space. An empty 3-mL syringe is then attached to the distal end of the catheter, and negative pressure is applied to the syringe. Failure to aspirate CSF or blood helps rule out accidental subarachnoid or intravascular placement. It is also important to reconfirm negative aspiration of CSF or blood from the catheter before any subsequent dose of local anesthetic is administered. (276)
102. The test dose commonly used for an epidural catheter is 3 mL of 1.5% lidocaine with 1:200,000 epinephrine. Failure of the test dose to produce sensory and motor anesthesia is assessed after 3 minutes to rule out accidental subarachnoid injection (spinal anesthesia has a more rapid onset of sensory and motor block). If epinephrine has been included in the test dose, the heart rate is monitored to detect an increase that may signal accidental intravascular injection. The local anesthetic solution is then injected in fractionated doses (e.g., multiple injections of 5-mL aliquots) over a 1- to 3-minute period at an appropriate volume and concentration (dose) for the planned surgical procedure. (276)
103. Intermittent dosing is critical because a negative test result does not conclusively rule out intravascular placement. This becomes even more critical with the single-injection epidural technique, where the needle’s position may change during or between injections. (276)
104. The following are local anesthetics commonly used for epidural anesthesia, their concentration, time of onset, and duration of action. (277, Figure 17-5)
105. Tetracaine and procaine are rarely used for epidural anesthesia because of their slow onset of action. (277)
106. After sterile preparation, the sacral cornu (typically 3 to 5 cm above the coccyx) is identified by the anesthetist’s palpating fingers. The depression between the cornu is the sacral hiatus, and a skin wheal is raised. The needle is introduced perpendicular to the skin through the sacrococcygeal ligament (generally felt as a rather distinct pop) and advanced until the sacrum is contacted. The needle is then slightly withdrawn, the angle is reduced, and the needle is advanced about 2 cm into the epidural caudal canal. Confirmation that the needle is properly positioned can be obtained by rapidly injecting 5 mL of air or saline through the needle while palpating the skin directly covering the caudal canal. Subcutaneous crepitus or midline swelling indicates that the needle is positioned posterior to the bony sacrum and requires replacement. (276, Figure 17-20)
107. Subarachnoid injection may occur if the needle is advanced too far cephalad in the sacral canal, or it may result from anatomic variation (the dural sac extends beyond S2 in approximately 10% of individuals). Anatomic variation may also hinder success inasmuch as the sacral hiatus is absent in nearly 10% of patients.
108. The principal factors affecting the spread of epidural anesthesia are dose (volume times concentration) and site of injection. However, administration of an equivalent dose (mass) at lower concentration may foster greater spread, particularly with lower concentrations of local anesthetic. Cephalad-to-caudad extension of epidural anesthesia depends on the site of administration of the local anesthetic solution into the epidural space. Lumbar epidural injections produce preferential cephalad spread because of negative intrathoracic pressure transmitted to the epidural space, whereas resistance to caudad spread of local anesthetic solution is created by narrowing of the space at the lumbosacral junction. In contrast, thoracic injections tend to produce symmetric anesthesia and result in greater dermatomal spread for a given dose of local anesthetic. This latter effect results, at least in part, from the smaller volume of the thoracic epidural space. The site of placement of the local anesthetic solution in the epidural space also defines the area of peak anesthetic effect, which decreases with increasing distance from the injection site.
109. The spread of epidural anesthesia varies directly with age and inversely with height, although these effects are small, and likely to be overshadowed by interpatient variability. (277)
110. In contrast to spinal anesthesia, the baricity of local anesthetic solutions does not influence the level of epidural anesthesia. Likewise, patient position during performance of an epidural block is less important than with a spinal block, but the dependent portion of the body may still manifest more intense anesthesia than the nondependent side. This effect is most noticeable in a pregnant woman who has remained in a specific lateral position for a prolonged period during labor. (277)
111. The duration of epidural anesthesia, as with spinal anesthesia, is principally affected by the choice of local anesthetic and whether a vasoconstrictor drug is added to the anesthetic solution. (277)
112. The addition of epinephrine (generally 1:200,000; 5 μg/mL) decreases vascular absorption of the local anesthetic from the epidural space, thus maintaining effective anesthetic concentrations at the nerve roots for more prolonged periods. Decreased vascular absorption also serves to limit systemic uptake and reduce the risk of systemic anesthetic toxicity. In addition, the inclusion of epinephrine serves as a marker of intravascular injection that may occur with cannulation of an epidural vein. (277)
113. Local anesthetic effect requires transfer across the nerve membrane. Because local anesthetics are weak bases, they exist largely in the ionic form in commercial preparations. Adding sodium bicarbonate to the solution favors the nonionized form of the local anesthetic and promotes more rapid onset of epidural anesthesia. (278)
114. Most commonly, 1 mL of 8.4% sodium bicarbonate is added to 10 mL of a solution containing lidocaine or chloroprocaine. Alkalinization of a bupivacaine solution is not recommended because this local anesthetic precipitates at alkaline pH. (278)
115. In contrast to spinal administration, lipid solubility of the opioid is a critical factor in determining the selection and appropriate use of epidural opioids. For example, morphine, which is relatively hydrophilic, spreads rostrally within the CSF and can produce effective analgesia for thoracic surgery, even when administered into the lumbar epidural space. In contrast, a lipophilic opioid such as fentanyl is rapidly absorbed into the systemic circulation and exhibits little rostral spread. (278)
116. Failed epidural anesthesia may occur when local anesthetic solution is not delivered into the epidural space or because spread of the local anesthetic solution is inadequate to cover the relevant dermatomes. A false loss of resistance can occur in the interspinous ligament before entry into the ligamentum flavum or as the needle passes through fascial planes. In some cases, failure results from advancement of the catheter through an intervertebral foramen, which generally gives rise to a limited unilateral block. Fortunately, these blocks can often be salvaged by retracting the catheter a few centimeters. Opinion varies on the presence of a midline barrier to diffusion of local anesthetics. (278)
117. If epidural anesthesia is nearly adequate and there are concerns that additional local anesthetic would create a risk for systemic toxicity, small doses of chloroprocaine, which are rapidly hydrolyzed in plasma, may provide adequate extension to permit surgery. At other times, failure of epidural anesthesia may be managed by replacement of the epidural catheter or abandonment of the technique in favor of a general or spinal anesthetic. However, subarachnoid injection after a failed epidural produces unpredictable and often excessive spinal anesthesia. This effect probably results from compression of the dural sac by the volume of anesthetic solution in the epidural space. (278)
118. The major site of action of local anesthetic solutions placed in the epidural space appears to be the spinal nerve roots, where the dura is relatively thin. To a lesser extent, anesthesia results from diffusion of local anesthetic solutions from the epidural space into the subarachnoid space. (278)
119. Side effects of epidural anesthesia resemble those described for spinal anesthesia, with the added risks of accidental subarachnoid injection, and anesthetic systemic toxicity, the latter attributable to the high doses of local anesthetic required for the epidural anesthetic. Additional potential complications include epidural hematoma and epidural abscess, particularly in patients with preexisting coagulopathy or infection. Although postdural puncture headache is not an issue if the dura is not pierced, when dural puncture does occur inadvertently, the risk of headache is far greater than with spinal anesthesia owing to the larger diameter needles used for the epidural technique. (279)
120. Accidental dural puncture may be managed by converting to single-injection or continuous spinal anesthesia, or epidural anesthesia can be attempted at a different lumbar interspace. Either placement of an epidural catheter at another interspace or passage of a subarachnoid catheter may decrease the risk for postdural puncture headache. (279)
121. Accidental subarachnoid injection of large volumes of local anesthetic solution used for epidural anesthesia may produce rapid progression to total spinal anesthesia. Immediate treatment is focused on supporting ventilation and restoring or maintaining hemodynamics. However, in contrast to an excessive block produced during spinal anesthesia, the large epidural doses of local anesthetics injected into the subarachnoid space can result in permanent neurologic deficits because of the neurotoxic effects of these agents. Consequently, consideration should be given to irrigation of the subarachnoid space by removal of small volumes of CSF and repetitive injections of saline. This maneuver may circumvent or minimize neurologic injury. (280)
122. Because the onset of sympathetic nervous system block is slower in epidural versus spinal anesthesia, excessive decreases in systemic blood pressure do not usually accompany epidural anesthesia administered to normovolemic patients. (280)
123. The high doses of local anesthetics required for epidural anesthesia along with the presence of numerous venous plexuses in the epidural space create a risk of substantial systemic absorption of local anesthetic. Additionally, accidental intravascular injection of local anesthetic will produce high blood levels and predictable toxicity ranging from mild central nervous system symptoms (restlessness, slurred speech, tinnitus) to loss of consciousness, seizures, and cardiovascular collapse. (280)
124. The subdural space is difficult to enter deliberately because the arachnoid is generally closely adherent to the overlying dura. The rare occurrence of subdural injection is difficult to detect because CSF cannot be aspirated through the catheter and the usual test dose is negative. Subdural injection of a local anesthetic solution can produce an unusual block characterized by patchy sensory anesthesia and often unilateral dominance. (280)
125. Neural injury after an epidural anesthetic is very rare but seems to be more likely if a paresthesia occurs during performance of this technique. The development of paresthesia as a result of the advancing epidural needle reflects stimulation of a nerve root and is a signal to the anesthetist that the needle is not in the midline and needs to be redirected. As with spinal anesthesia, injection of local anesthetic solution in the presence of a paresthesia is contraindicated because nerve damage may be induced or enhanced by the injection. (280)
126. Combined spinal-epidural anesthesia is a technique in which a spinal anesthetic and an epidural catheter are placed concurrently. This approach combines the rapid onset and intense sensory anesthesia of a spinal anesthetic with the ability to supplement and extend the duration of the block afforded by an epidural catheter. The technique is commonly used in obstetric anesthesia, as well as orthopedic procedures such as hip and knee replacement. (280, Figure 17-21)
127. Advantages of epidural block during general anesthesia include less need for opioids, pain-free emergence from anesthesia, and block of the stress response that is nearly complete for most surgical procedures performed below the umbilicus. However, use of this technique demands heightened attention to fluid management and blood pressure. (281)