Chapter 30 Obstetrics
Physiologic changes in pregnant women
1. How do the maternal intravascular fluid, plasma, and erythrocyte volumes change during pregnancy?
2. How does the coagulation status change during pregnancy?
3. What is the average maternal blood loss during the vaginal delivery of a newborn? What is the average maternal blood loss during cesarean delivery?
4. How does the maternal cardiac output change from nonpregnant levels?
5. In an uncomplicated pregnancy, what changes occur in blood pressure, systemic vascular resistance, and central venous pressure?
6. What is the supine hypotension syndrome? What symptoms accompany the syndrome?
7. What compensatory mechanisms do most women have that prevents them from experiencing supine hypotension syndrome and how can maternal hypotension be minimized?
8. What are some aspects of the upper airway that undergo physiologic change in pregnancy? What are the clinical implications of these changes?
9. How is minute ventilation changed during pregnancy from nonpregnant levels? How does the resting maternal PaCO2 change as a result of the change in minute ventilation?
10. How do the binding characteristics of hemoglobin change during pregnancy?
11. What are the changes in maternal lung volumes that occur with pregnancy? What are the anesthetic implications of these changes?
12. How does maternal PaO2 change during pregnancy?
13. What are the gastrointestinal changes in pregnancy that render the woman vulnerable to regurgitation of gastric contents? What clinical implication does this have?
14. How do the epidural and subarachnoid spaces change in pregnancy? How is the sensitivity to local anesthetics different in the pregnant versus nonpregnant patient? How are the dosing requirements for neuraxial anesthesia affected by these changes?
15. How do renal blood flow and glomerular filtration rate change in pregnancy? At what gestational month of pregnancy is this change at a maximum? How does this affect the normal upper limits of creatinine and blood urea nitrogen in pregnant patients?
16. Does hepatic blood flow change during pregnancy? How are plasma protein concentrations and plasma cholinesterase activity altered by pregnancy?
Physiology of uteroplacental circulation
17. How are maternal and fetal blood delivered to the placenta?
18. What is uterine blood flow (UBF) at term?
19. What are the determinants of UBF?
20. What factors affect the transfer of oxygen between the mother and fetus?
21. What factors affect placental exchange of drugs and other substances? What is the most reliable way to minimize fetal transfer of a drug?
22. What common drugs used in anesthesia have limited ability to cross the placenta? Which readily cross the placenta?
Anatomy of labor pain
27. In the first stage of labor, describe the associated sensory levels and where the end organ afferent nerve impulses are initiated.
28. In the second stage of labor, describe the associated sensory levels and where the end organ afferent nerve impulses are initiated.
29. For each stage of labor, describe which analgesic techniques benefit the pregnant woman and why.
Methods of labor analgesia
Nonpharmacologic Techniques and Systemic Medications
30. Describe the different nonpharmacologic techniques used for labor and the efficacy of each.
31. List the different systemic medications used for labor analgesia and their active metabolites, if any.
33. How is remifentanil used as a labor analgesic and what are the indications for its use?
34. Are benzodiazepines used in pregnancy and if so, when?
35. When is ketamine used in labor and delivery and what additional benefits does it provide for pain control?
Neuraxial Analgesia and Neuraxial Techniques
36. List the different types of neuraxial analgesia?
37. When would you use each type of neuraxial analgesia for labor pain?
38. Should laboring women remain “nothing per oral (NPO)” after placement of an epidural or combined spinal and epidural (CSE)?
39. What is a walking epidural and what are the associated risks?
40. What drugs are used or being evaluated as adjuvant neuraxial drugs for labor analgesia?
41. Name the tissue layers and ligaments encountered when placing an epidural and in what order the anesthesiologist encounters each.
42. The American Society of Anesthesiologists (ASA) recommendations regarding aseptic technique for placement of neuraxial block include what specific precautions?
43. What are the interspaces where the neuraxial block for labor analgesia is placed and what are the risks of placing the neuraxial block higher or lower than this range of interspaces?
44. What is a “test dose” and what does it assess?
45. Can a test dose be used with a CSE?
46. What type of needle is used in placement of spinal analgesia and why?
47. What is a “saddle block” and when is it used during labor and delivery?
Contraindications and complications of neuraxial anesthesia
48. What are the contraindications to neuraxial procedures?
49. Is known infection with human immunodeficiency virus (HIV) a contraindication to epidural placement?
50. List the potential complications of a neuraxial block.
51. What is the occurrence rate of postdural puncture headache (PDPH)? What are the treatment options for PDPH?
52. What is the treatment for systemic local anesthetic toxicity of bupivacaine?
53. What physiologic effects do you expect to see with a high spinal or high epidural?
54. What are the important differences in performing advanced cardiac life support (ACLS) for a pregnant woman compared to a nonpregnant patient?
55. What is the rate of hypotension after neuraxial blockade?
56. What is the first-line pharmacologic treatment of hypotension after a neuraxial block?
57. How does epidural analgesia affect maternal body temperature?
Anesthesia for cesarean delivery
61. What are some benefits of regional anesthesia over general anesthesia for cesarean delivery?
62. What are the benefits of general anesthesia over regional anesthesia for cesarean delivery?
63. What are some advantages and disadvantages of spinal anesthesia for cesarean delivery compared to an epidural block?
64. What dermatome level of spinal anesthesia ensures patient comfort adequate for cesarean delivery? How can this be achieved?
65. What are some advantages and disadvantages of epidural anesthesia for cesarean delivery compared to spinal anesthesia?
66. Which local anesthetics, and corresponding doses, are typically administered to achieve an adequate density and dermatomal level of epidural anesthesia for cesarean delivery?
67. What is the advantage of the administration of morphine into the epidural space for cesarean delivery? What are some of the negative side effects that may accompany this route of morphine administration?
68. What are some indications for general anesthesia for cesarean delivery? What are some benefits of general anesthesia for cesarean delivery?
69. What are the main causes of increased morbidity and mortality associated with general anesthesia during pregnancy?
70. How should difficulty with endotracheal intubation be managed by the anesthesiologist?
71. What is the level of exposure of the fetus to thiopental after the administration of induction doses for general anesthesia? Is there an advantage to using propofol for the induction of general anesthesia?
72. What are some of the advantages and disadvantages of inducing general anesthesia for cesarean delivery with etomidate?
73. What are the effects of using volatile anesthetics for cesarean delivery on the fetus?
74. What neuromuscular agents are typically used for cesarean delivery with general anesthesia? Do they result in neuromuscular blockade of the fetus or relaxation of the uterus?
Abnormal presentations and multiple births
75. What percent of live births are twins and why is the number increasing? What are the complications that develop with multiple gestations?
76. What are the modes of delivery for twin pregnancies? What anesthetic techniques can be used to optimize delivery?
77. Describe external cephalic version and the associated risks.
78. What is a shoulder dystocia? What are the risk factors associated with the development of a shoulder dystocia? What are the risks to the fetus during a shoulder dystocia?
Hypertensive disorders of pregnancy
79. At what gestational age does gestational hypertension present?
80. What is the percent of preeclampsia in the general population? What are the risk factors for developing preeclampsia?
81. What are the criteria for the diagnosis of preeclampsia?
82. What are the criteria for severe preeclampsia?
84. What is the mechanism of preeclampsia?
85. How should patients with preeclampsia be managed for labor? What is the definitive treatment of preeclampsia?
86. How is magnesium sulfate infusion used and why? What are the signs of and treatment of magnesium sulfate toxicity?
87. What are the typical antihypertensive drugs used in preeclampsia?
Hemorrhage in pregnant women
88. What are some causes of hemorrhage in the pregnant patient? When do these typically manifest?
89. What is placenta previa? What are the associated risk factors?
90. If a massive postpartum hemorrhage is not controlled with standard measures (i.e., uterine massage, uterotonics), what invasive options can be considered by the obstetrician?
91. What is abruptio placentae? What are some risk factors for abruptio placentae?
92. What are some risk factors for uterine rupture? What is the incidence of uterine rupture associated with vaginal birth after a previous cesarean delivery?
93. What approximate percent of vaginal deliveries are associated with some amount of retained placenta? What are some options for the anesthetic management of patients with retained placenta?
94. What are some risk factors for uterine atony?
95. What medications are used to manage uterine atony? What are their side effects?
96. Define placenta accreta, increta, and percreta.
97. In a patient with known placenta previa, how does the risk of placenta accreta change with the number of prior cesarean deliveries?
Anesthesia for nonobstetric surgery during pregnancy
100. What are common nonobstetric surgeries that occur during pregnancy?
101. When should nonobstetric surgeries be performed during pregnancy?
102. What anesthetics are teratogenic?
103. How can intrauterine fetal hypoxia and acidosis be prevented?
104. When should fetal heart rate monitoring be done during nonobstetric surgery?
105. What is the usual etiology of premature labor that presents in the pregnant patient after having undergone nonobstetric surgery? How can premature labor be treated?
106. Can laparoscopic surgery be performed safely during the third trimester?
Diagnosis and management of fetal distress
107. Describe the frequency of normal uterine contractions and tachysystole. What is the treatment for tachysystole?
108. What is the normal baseline fetal heart rate (FHR)?
109. Define FHR variability in terms of absent, minimal, moderate, and marked.
110. What is the definition of a FHR acceleration?
111. What are late decelerations indicative of in the fetus?
112. What is a variable deceleration indicative of in the fetus?
113. What was fetal heart rate monitoring designed to assess?
Evaluation of the neonate and neonatal resuscitation
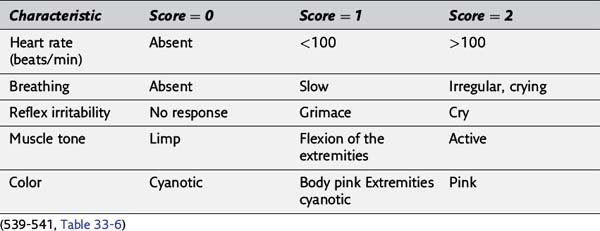
114. Define the values of each Apgar score category.
115. What are normal blood gas values for the umbilical artery and vein?
116. During neonatal evaluation and resuscitation, when is it appropriate to give positive pressure ventilation?
117. What is the dose of epinephrine given for neonatal resuscitation?
118. Should naloxone be given in the delivery room for neonatal resuscitation?
119. In neonates delivered with meconium-stained amniotic fluid, when should suctioning below the cords be instituted?
120. What have been significant advances in the prevention of meconium aspiration syndrome?
Answers*
Physiologic changes in pregnant women
1. During pregnancy the maternal intravascular fluid volume increases from its prepregnancy volume. The increase in intravascular volume begins in the first trimester of pregnancy. By term, the intravascular fluid volume has increased by about 35% above the prepregnancy state. The plasma volume increases by approximately 45% at term. The erythrocyte volume in the pregnant patient increases by approximately 20%. Because the plasma volume increases by over twice as much as the erythrocyte volume, the woman has a relative physiologic anemia. That is, the hematocrit of the pregnant patient is relatively less than her prepregnancy state. This is termed the physiologic anemia of pregnancy. (515, Table 33-1)
2. The pregnant woman at term is in a hypercoagulable state secondary to increases in factors I, VII, VIII, IX, X, and XII, and decreases in factors XI, XIII, and Antithrombin III. This results in an approximately 20% decrease in prothrombin time (PT) and partial thromboplastin time (PTT). Platelet count may remain normal or decrease 10% by term. (515)
3. The average maternal blood loss during vaginal delivery of a newborn is 300 to 500 mL. The average maternal blood loss during the delivery of a newborn by cesarean delivery is 800 to 1000 mL, but blood loss during a cesarean delivery is greatly variable. The increase in intravascular fluid volume and the hypercoagulable state of the mother help to counter the blood losses incurred during this time. The contracted uterus after either type of delivery creates an autotransfusion of approximately 500 mL of blood, which decreases the overall effect of the blood loss on the mother. (515)
4. Maternal cardiac output increases 10% by the tenth week of gestation, and at term pregnancy increases by approximately 40% to 50% of its prepregnancy value. Cardiac output is equal to the product of stroke volume and heart rate. The increase in cardiac output is primarily due to an increase in stroke volume. The increase in heart rate during pregnancy is less and is therefore only a minimal contributor to the increase in cardiac output. Labor is associated with further increases in cardiac output with output above prelabor values by 10% to 25% during the first stage and 40% in the second stage. The greatest increase in cardiac output occurs just after delivery, when it increases by as much as 80% above prelabor values. This is the maximal change in cardiac output in the woman. Cardiac output decreases substantially toward prepregnant values by 2 weeks postpartum. (515, Table 33-1)
5. The systolic blood pressure of the woman having an uncomplicated pregnancy does not exceed her prepregnancy blood pressure and typically decreases secondary to a 20% reduction in systemic vascular resistance at term. Systolic, mean, and diastolic blood pressure may all decrease 5% to 15% by 20 weeks gestational age and gradually increase toward prepregnant values as the pregnancy progresses towards term. Central venous pressure does not change during pregnancy despite the increased plasma volume because venous capacitance increases. (515-516, Table 33-1)
6. Supine hypotension syndrome, as the name implies, is the decrease in blood pressure seen when the pregnant patient lies in the supine position after midgestation. The supine hypotension syndrome occurs because of a decrease in cardiac output by approximately 10% to 20%. When the pregnant woman is in the supine position, the gravid uterus compresses the inferior vena cava, resulting in decreased venous return and decreased preload for the heart. Symptoms that accompany the hypotension include diaphoresis, nausea, vomiting, and possible changes in cerebration. Symptoms must be present for the patient to be considered susceptible to supine hypotension syndrome. (516, Figure 33-1)
7. Most pregnant women, when lying in the supine position, are able to compensate for the possible decrease in blood pressure that results from the compression of the inferior vena cava by the gravid uterus. One compensatory mechanism includes maintaining venous return by diverting blood flow from the inferior vena cava to the paravertebral venous plexus. The blood then goes to the azygos vein and returns to the heart via the superior vena cava. Dilation of the epidural veins may make unintentional intravascular placement of an epidural catheter more likely. A “test dose” is given before dosing an epidural catheter to decrease the likelihood of an unrecognized intravascular placement before initiating neuraxial blockade.
Another compensatory mechanism is an increase in peripheral sympathetic nervous system activity. This increases peripheral vascular tone and helps to maintain venous return to the heart. Regional anesthesia, however, can interfere with these compensatory mechanisms by causing sympathetic nervous system blockade, rendering the pregnant woman at term more susceptible to decreases in blood pressure. The gravid uterus can also compress the lower abdominal aorta and lead to arterial hypotension in the lower extremities, but maternal symptoms or decreases in systemic blood pressure as measured in the arms are often not reflective of this decrease. The major clinical significance of the aortocaval compression is the decrease in placental and uterine blood flow that results. The decrease in blood flow through the uteroplacental unit leads to a decrease in blood flow to the fetus. The aortocaval compression can be minimized by having the woman lie in the lateral position. Uterine displacement can also be used, typically with displacement being to the left because the inferior vena cava sits just to the right of and anterior to the spine. Left uterine displacement is easily accomplished by table tilt or the placement of a wedge or folded blanket under the right hip, elevating the hip by 10 to 15 cm. (516-517, Figures 33-1 and 33-2)
8. There is significant capillary engorgement of the mucosal layer of the upper airways and increased tissue friability during pregnancy. There is increased risk of obstruction from tissue edema and bleeding with instrumentation of the upper airway. Additional care is needed during suctioning, placement of airways (avoid nasal instrumentation if possible), direct laryngoscopy, and intubation. In addition, because the vocal cords and arytenoids are often edematous, smaller-sized cuffed endotracheal tubes (6.0 to 6.5 mm internal diameter) may be a better selection for intubation of the trachea for these patients. The presence of preeclampsia, upper respiratory tract infections, and active pushing with associated increased venous pressure further exacerbate airway tissue edema, making both intubation and ventilation more challenging. (517)
9. During pregnancy, the minute ventilation increases to about 50% above prepregnancy levels. This change occurs in the first trimester of pregnancy and remains elevated for the duration of the pregnancy. An increase in tidal volume is the main contributor to the increase in minute ventilation seen, with only small increases in respiratory rate from prepregnancy. During the first trimester, as a result of the increase in minute ventilation, the resting maternal PaCO2 decreases from 40 mm Hg to about 30 or 32 mm Hg. Arterial pH, however, remains only slightly alkalotic (7.42 to 7.44) secondary to increased renal excretion of bicarbonate ions. (517, Table 33-1)
10. Maternal hemoglobin has less of an affinity for binding oxygen during pregnancy, which facilitates downloading oxygen to the tissues and the fetus. The hemoglobin dissociation curve is thus shifted to the right with the P-50 increasing from 27 to approximately 30 mm Hg. (517)
11. Maternal lung volumes start to change in the second trimester. This is a result of mechanical compression by the gravid uterus as it enlarges and forces the diaphragm cephalad. This leads to a decrease in the woman’s functional residual capacity by approximately 20% at term. This decrease is a result of approximately equal decreases in both the expiratory reserve volume and residual lung volume. This can result in a functional residual capacity less than closing capacity and increased atelectasis in the supine position. There is no significant change in vital capacity seen during pregnancy. The rates of change in the alveolar concentration of inhaled anesthetics during induction and emergence from anesthesia are both increased secondary to the increase in minute ventilation and decrease in functional residual capacity. Clinically this, along with the decrease in MAC that accompanies pregnancy, leads to a more rapid achievement of an anesthetized state than when the patient is not pregnant. Apnea in the woman rapidly leads to arterial hypoxemia. There are at least two explanations for this. First, a decreased functional residual capacity and subsequent decreased oxygen reserve are contributors. Second, aortocaval compression and decreased venous return leading to decreases in cardiac output may also contribute. The decrease in cardiac output would lead to an increase in overall oxygen extraction and therefore decrease the level of oxygenation of blood returning to the heart. Third, maternal oxygen consumption is increased by 20% at term, with further increases noted during labor. Because of the rapid decrease in maternal PaO2 with apnea or hypoventilation, preoxygenation with 100% O2 for 3 minutes or four maximal breaths over the 30 seconds just prior to the induction of emergent general anesthesia is recommended. (517, Table 33-1)
12. Maternal PaO2 changes during the progression from early gestation to term. Early in gestation, the PaO2 in the mother is slightly increased over prepregnancy values to over 100 mm Hg breathing room air. This is secondary to maternal hyperventilation and subsequent decreased PaCO2 during this time. As the pregnancy progresses, the PaO2 is normal or even slightly decreased. The decrease in PaO2 during the course of pregnancy likely results from airway closure and associated intrapulmonary shunt. (517)
13. There are at least four gastrointestinal changes in pregnancy that render the woman significantly vulnerable to the regurgitation of gastric contents beyond midgestation. The enlarged uterus acts to displace the stomach and pylorus cephalad from its usual position. This repositions the intraabdominal portion of the esophagus into the thorax and leads to relative incompetence of the physiologic gastroesophageal sphincter. The tone of the gastroesophageal sphincter is further reduced by the higher progesterone and estrogen levels of pregnancy. Gastric pressure is increased by the gravid uterus. Gastrin secreted by the placenta stimulates gastric hydrogen ion secretion. The pH of the woman’s gastric fluid is predictably low as a result. Reflux and subsequent esophagitis are common during pregnancy. During labor, gastric emptying is delayed and intragastric fluid volume tends to be increased as a result. (Epidural analgesia alone does not alter gastric emptying.) Anxiety, pain, and the administration of opioids can further decrease gastric emptying. Clinically, this means that the pregnant patient must always be treated as if she has a full stomach. Regardless of what amount of time has elapsed since her last ingestion of solids, she is at increased risk of regurgitation and aspiration of gastric contents. This includes the routine use of nonparticulate antacids, rapid sequence induction, cricoid pressure, and cuffed endotracheal intubation as part of general anesthesia induction sequence in a pregnant woman after approximately 20 weeks gestational age.
14. During pregnancy, both the epidural and intrathecal spaces are decreased in volume from their prepregnancy state. This occurs because of the engorgement of epidural veins and the increased intraabdominal pressure resulting from the progressive enlargement of the uterus. However, CSF pressure does not increase with pregnancy. The decrease in the epidural space decreases the required volume of local anesthetic necessary to achieve a particular level of anesthesia by facilitating its spread in the epidural space. The decreased intrathecal space also facilitates the spread of spinal anesthetic and decreases the dose required from prepregnancy values.
15. Renal blood flow and glomerular filtration rate in the woman are both increased. By the third month of pregnancy the increase is about 50% to 60%. This results in a decrease in what is considered the normal upper limit of both the blood urea nitrogen and serum creatinine concentrations during pregnancy to about 50% of what it was in the prepregnancy state. (518)
16. Liver blood flow does not change significantly with pregnancy. Plasma protein concentrations are reduced in pregnancy secondary to dilution. The decreased albumin levels can create increased blood levels of highly protein bound drugs. Plasma cholinesterase, or pseudocholinesterase, decreases in activity by about 25% during pregnancy. This decrease in activity is first noted by about the tenth week of gestation and persists for as long as 6 weeks postpartum. There is no clinical manifestation of this change in plasma cholinesterase activity, and no significant change in the duration of action of succinylcholine. (518)
Physiology of uteroplacental circulation
17. The function of the placenta is to unite maternal and fetal circulations. The union allows for the physiologic exchange of nutrients and waste. Maternal blood is delivered to the placenta by the uterine arteries. Fetal blood is delivered to the placenta by the two umbilical arteries. Nutrient rich blood is returned from the placenta to the fetus via a single umbilical vein. The two most important determinants of placental function are uterine blood flow and the characteristics of the substances to be exchanged across the placenta. (519)
18. Uterine blood flow increases during gestation from approximately 100 mL/min before pregnancy to 700 mL/min at term. Adequate uterine blood flow must be maintained to ensure placental circulation is adequate and therefore guarantee fetal well-being. About 80% of the uterine blood flow perfuses the placenta and 20% supports the myometrium. (519)
19. During pregnancy uterine blood flow has limited autoregulation, and the uterine vasculature is essentially maximally dilated under normal pregnancy conditions. Uterine blood flow is proportional to the mean blood perfusion pressure to the uterus and inversely proportional to the resistance of the uterine vasculature. Decreased perfusion pressure can result from systemic hypotension secondary to hypovolemia, aortocaval compression, or decreased systemic resistance from either general or neuraxial anesthesia. Uterine blood flow also decreases with increased uterine venous pressure. This can result from vena caval compression (supine position), uterine contractions (particularly uterine tachysystole as may occur with oxytocin administration), or significant abdominal musculature contraction (Valsalva during pushing). Additionally, extreme hypocapnia (PaCO2 <20 mm Hg) associated with hyperventilation secondary to labor pain can reduce UBF to the point of fetal hypoxemia and acidosis. Epidural or spinal anesthesia does not alter UBF as long as maternal hypotension is avoided. Endogenous catecholamines induced by stress or pain and exogenous vasopressors have the capability of increasing uterine arterial resistance and decreasing UBF, although both ephedrine or phenylephrine are used clinically in moderate amounts to maintain uterine perfusion pressure when the pregnant patient is hypotensive. (519)
20. Transfer of oxygen to the fetus is dependent on a variety of factors including the ratio of maternal to fetal umbilical blood flow, the oxygen partial pressure gradient, the respective hemoglobin concentrations and affinities, the placental diffusing capacity, and the acid-base status of the fetal and maternal blood (Bohr effect).
21. Transfer of drugs and other substances less than 1000 Da from the maternal circulation to the fetal circulation and vice versa is primarily by diffusion. Some factors that affect the exchange of substances from the maternal circulation to the fetus include the concentration gradient of the substance across the placenta, maternal protein binding, molecular weight, lipid solubility, and degree of ionization of the substance. The most reliable way to minimize the amount of drug that reaches the fetus is by minimizing the concentration of the drug in the maternal blood. (519)
22. Nondepolarizing neuromuscular blocking drugs have a high molecular weight and low lipid solubility. These two characteristics together limit the ability of nondepolarizing neuromuscular blocking drugs to cross the placenta. Succinylcholine is highly ionized, preventing it from diffusing across the placenta despite its low molecular weight. Additionally, both heparin and glycopyrolate have significantly limited placental transfer. Placental transfer of barbiturates, local anesthetics, and opioids is facilitated by the relatively low molecular weights of these substances. (519)
Fetal uptake and distribution of drugs
23. Fetal blood is slightly more acidic than maternal blood, with a pH about 0.1 unit less than maternal blood pH. The lower pH of fetal blood facilitates the fetal uptake of drugs that are basic. Weakly basic drugs, such as local anesthetics and opioids that cross the placenta in the nonionized state, become ionized in the fetal circulation. This results in an accumulated concentration of drug in the fetus for two reasons. First, once the drug becomes ionized it cannot readily diffuse back across the placenta. This is known as ion trapping. Second, a concentration gradient of nonionized drug is maintained between the mother and the fetus. In the case of lidocaine administration, this may mean that if the fetus was distressed and acidotic and lidocaine was given in sufficient doses to the woman, lidocaine may accumulate in the fetus. (519)
24. First, about 75% of the blood that is coming to the fetus via the umbilical vein passes through the liver. This allows for a significant amount of metabolism of the drug to take place before going to the fetal arterial circulation and delivery to the heart and brain. Second, drug contained in the umbilical vein blood enters the inferior vena cava via the ductus venosus. This blood is diluted by drug-free blood returning from the lower extremities and pelvic viscera of the fetus, resulting in a decrease in the concentration of the drug that is in the inferior vena cava. In addition, despite decreased liver enzyme activity in comparison to adults, fetal/neonatal enzyme systems are adequately developed to metabolize most drugs. (520)
Stages of labor
25. Labor is a continuous process divided into three stages. The first stage is the onset of labor until the cervix is fully dilated. This first stage is further divided into the latent and active stage. The active phase begins at the point when the rate of cervical dilation increases (often between 3 and 5 cm.). The second stage of labor begins when the cervix is fully dilated and ends when the neonate is born. This stage is referred to as the “pushing and expulsion” stage. The third and final stage begins once the neonate is delivered and is completed when the placenta is delivered. (520)
26. If a woman fails to dilate adequately in the active phase despite pharmacologic interventions, it is considered active phase arrest and will result in a cesarean delivery. During the second stage of labor, the patient may not be able to “push” the neonate out of the pelvis. This is termed arrest of descent. If the neonate is low enough in the pelvis, the obstetrician can perform an instrumented vaginal delivery via vacuum or forceps, otherwise a cesarean delivery is required. (520)
Anatomy of labor pain
27. During the first stage of labor (cervical dilation), the majority of painful stimuli result in afferent nerve impulses from the lower uterine segment and cervix. This pain is typically visceral in nature (dull, aching, poorly localized). The nerve cell bodies are located at the dorsal root ganglia of T10 to L1 level. (520, Figure 33-3)
28. In the second stage of labor, afferents innervating the vagina, perineum, and pelvic floor travel primarily via the pudendal nerve to the dorsal root ganglia of the S2 to S4 levels. This pain is typically somatic in nature (sharp and well localized). (520, Figure 33-3)
29. The first and second stages of labor can employ neuraxial techniques such as an epidural or combined spinal epidural (CSE). Although used less frequently, a paracervical block can also be used during the first stage of labor. A single shot spinal or pudendal block can be used for the second stage of labor. Typically, the obstetrician performs both the paracervical and pudendal block. (520)
Methods of labor analgesia
Nonpharmacologic Techniques and Systemic Medications
30. A variety of nonpharmacologic techniques for labor analgesia exist. These include hypnosis, the breathing techniques described by Lamaze, acupuncture, acupressure, the LeBoyer technique, transcutaneous nerve stimulation, massage, hydrotherapy, vertical positioning, presence of a support person, intradermal water injections, biofeedback, and many others. A meta-analysis reviewing the effectiveness of a support individual (e.g., doula, family member) noted that women with a support individual used less pharmacologic analgesia methods, had a decreased length of labor, and a lower incidence of cesarean delivery. In a 2006 retrospective survey, nonpharmacologic methods of tub immersion and massage were rated more or equally effective in relieving pain compared to use of opioids in labor. (521)
31. Fentanyl is commonly used for labor analgesia because it is short acting with no active metabolite. Morphine was used more frequently in the past, but currently is rarely used. Its active metabolite (morphine-6-glucuronide) has a prolonged duration of analgesia, the half-life is longer in neonates compared to adults, and it produces significant maternal sedation. Meperidine is still one of the most frequently used opioids worldwide. Maternal half-life of meperidine is 2 to 3 hours with half-life in the fetus and newborn significantly greater (13 to 23 hours) and more variable. In addition, meperidine is metabolized to an active metabolite (normeperidine) that can significantly accumulate after repeated doses. With increased dosing and shortened time between doses, there are increased neonatal risks. (521-522)
32. In latent labor, obstetrical providers may use intramuscular morphine combined with phenergan for analgesia, sedation, and rest termed “morphine sleep.” This produces analgesia for approximately 2.5 to 4 hours with an onset of 10 to 20 minutes. (521)
33. Remifentanil patient controlled analgesia (PCA) has been considered for women who have contraindications to neuraxial blockade. Although pain was improved with remifentanil compared to those without pharmacologic intervention, a randomized control trial comparing epidural analgesia to remifentanil PCA had overall pain scores that were lower in the epidural group. More sedation and hemoglobin desaturation were noted during remifentanil analgesia, but there was no difference between groups in fetal and neonatal outcome measures. (522)
34. Diazepam is used in obstetrics; however, it will readily cross the placenta and yield roughly equal maternal and fetal blood levels. Since neonates have a limited ability to excrete the active metabolites, use of diazepam has been associated with neonatal respiratory depression. Midazolam is a shorter acting anxiolytic, but also rapidly crosses the placenta, and large induction doses have been associated with profound neonatal hypotonia. Their use has been controversial given their amnestic properties. In specific obstetrical situations, use of midazolam in small doses may be beneficial. (522)
35. During labor ketamine can be administered for urgent situations in divided IV doses (10 to 20 mg) totaling less than 1 mg/kg. Ketamine in these doses will provide rapid analgesia, and is useful for vaginal delivery and episiotomy. It has a rapid onset (30 seconds) and minimal duration of action (<5 minutes). When given with opioids, ketamine can act synergistically to reduce the amount of opioid necessary to produce adequate analgesia. (522)
Neuraxial Analgesia and Neuraxial Techniques
36. Neuraxial analgesia typically involves the administration of local anesthetics, often with the coadministration of opioid analgesics or other adjunctive analgesics. Bupivacaine and ropivacaine are the most commonly used local anesthetics. Epidural, spinal, and combined spinal and epidural (CSE) are the techniques typically used for labor analgesia. (522)
37. Anytime a woman in labor without contraindications requests neuraxial analgesia, regardless of the stage of her labor, a neuraxial blockade may be placed. The timing of placement does not depend on an arbitrary cervical dilation. A single shot spinal analgesic has a finite analgesic duration depending on the local anesthetic and this should be taken into account when using this technique. For example, a single shot spinal analgesic is ideal if an obstetrician is performing an instrumented vaginal delivery in a woman without previous neuraxial block. The other neuraxial techniques employ catheter delivery techniques and can be extended throughout the length of the labor. (523)
38. Otherwise healthy women in labor may have modest amounts of clear liquids. However, in a complicated labor (e.g., by morbid obesity, difficult airway, concerning fetal status), the decision to restrict oral intake should be individualized. (523)
39. Placing opioids alone in the epidural or intrathecal space (if placing a CSE) is considered a walking epidural. The local anesthetic solution, while providing analgesia, has minimal effects on sympathetic or motor nerves. This allows the woman to ambulate after tests for motor blockade indicate that she is not at risk of falling. Even so, the woman should be closely monitored and ideally should only ambulate when accompanied because proprioception and balance may be impaired. Use of neuraxial opioids is associated with dose-related maternal side effects including pruritus, sedation, and nausea. In addition, the administration of intrathecal opioids can result in fetal bradycardia independent of hypotension. The mechanism for fetal bradycardia is unclear, but may result from uterine hyperactivity following the rapid onset of analgesia. (522)
40. Clonidine and neostigmine are epidural adjuncts to local anesthetics and have been evaluated for use in neuraxial blockade. They prolong the blockade and limit the dose of local anesthetic infusion. Neostigmine is still undergoing evaluation for its use in obstetric anesthesia and currently is not recommended for standard practice. It increases acetylcholine binding within the spinal cord stimulating nitric oxide to produce analgesia. Neostigmine also produces refractory nausea and vomiting if given intrathecally, but minimal nausea and vomiting if given epidurally. Clonidine inhibits the release of substance P in the dorsal horn and produces analgesia. It also increases the level of acetylcholine in the cerebral spinal fluid. Intrathecal clonidine can produce excellent analgesia, but sedation and hypotension are common. (522-523)
41. When placing an epidural, the needle traverses the skin and subcutaneous tissues, supraspinous ligament, interspinous ligament, the ligamentum flavum, and is advanced into the epidural space. (523, Figures 33-4 and 33-5)
42. Aseptic technique should be used during neuraxial procedures, including (1) jewelry removal (e.g., rings and watches), hand washing, and use of caps, masks, and sterile gloves; (2) use of individually packaged chlorhexidine (preferred) or povidone-iodine (preferably with alcohol) for skin preparation, allowing for adequate drying time; (3) sterile draping; and (4) use of sterile occlusive dressings at the catheter insertion site. (523)
43. For neuraxial analgesia the needle is normally inserted between L1 and L4. If the needle is placed too high, there is a risk of puncturing the conus medullaris if the needle inadvertently punctures the dura. In addition, coverage of sacral roots needed during the second stage may be inadequate. If the catheter is placed lower than L4, the neuraxial block may not adequately cover the nerves that innervate the uterus and may not provide the necessary labor analgesia for uterine contractions and cervical dilation. (523)
44. Prior to the infusion of local anesthetic through an epidural catheter a test dose typically composed of 3 cc of 1.5% lidocaine with 1:200,000 epinephrine is administered. The anesthesia provider waits 3 minutes to confirm that the needle is not intravascular with no increase in heart rate or blood pressure, and no systemic symptoms of lidocaine infusion have resulted such as tinnitus or perioral tingling. Additionally, the patient is asked to move her lower extremities to confirm that the bolus was not placed intrathecally, which would result in motor blockade. (524-526)
45. A test dose can and should be used with the CSE. The test dose will both confirm whether the catheter is intravascular by changes in heart rate and blood pressure, and unintended intrathecal catheter placement can still be assessed as the patient should still be able to move her lower extremities after typical spinal local anesthetic doses in the spinal portion of the CSE. (524-526)
46. A 24 to 26-gauge “pencil point” spinal needle is commonly selected for CSE to reduce the risk of a postdural puncture headache. (526)
47. If primarily perineal anesthesia is needed (i.e., forceps delivery, perineal laceration repair), the patient may be left in the sitting position for 2 to 3 minutes after intrathecal injection with hyperbaric local anesthetic to concentrate the sensory block in the perineal region (“saddle block”). A true saddle block anesthetic (requiring more time in the sitting position) does not produce complete uterine pain relief because the afferent fibers (extending to T10) from the uterus are not blocked. (526)
Contraindications and complications of neuraxial anesthesia
48. Certain conditions contraindicate neuraxial procedures. These include: (1) patient refusal, (2) infection at the needle insertion site, (3) significant coagulopathy, (4) hypovolemic shock, (5) increased intracranial pressure from mass lesion, and (6) inadequate provider expertise. Other conditions such as systemic infection, neurologic disease, and mild coagulopathies are relative contraindications and should be evaluated on a case-by-case basis. (526)
49. Neither HIV nor hepatitis virus infection are contraindications to placement of neuraxial anesthesia. (526)
50. Possible complications of a neuraxial block include inadequate block, hypotension, intravascular catheter placement, systemic toxicity of local anesthetic, unintentional intrathecal catheter placement, excessive blockade, postdural puncture headache, epidural hematoma, epidural abscesses, meningitis, and nerve or spinal cord injury. Other side effects include pruritus, nausea, shivering, urinary retention, motor weakness, low back soreness, and a prolonged block. (526)
51. The rate of accidental dural puncture during epidural catheter placement is approximately 1% to 2%, and approximately half of these will result in a severe headache. PDPH are typically managed with analgesics, hydration, rest, caffeine, and an epidural blood patch, if necessary. (526)
52. If a local anesthetic overdose occurs, in addition to following standard ACLS algorithms for pregnancy, consider use of a 20% IV lipid emulsion to decrease toxicity. (527)
53. A high spinal (total spinal) can result from an unrecognized epidural catheter placed subdural, migration of the catheter during its use, or an overdose of local anesthetic in the epidural space (i.e., high epidural). Both high spinals and high epidurals can result in severe maternal hypotension, bradycardia, loss of consciousness, and blockade of the motor nerves to the respiratory muscles. (526-527)
54. ACLS guidelines for pregnancy include use of left uterine displacement, avoidance of lower extremity vessels for drug delivery, chest compressions positioned slightly higher on the sternum, and no modifications to the defibrillation protocol except removal of fetal and uterine monitors prior to shock. In any situation of maternal cardiac arrest with unsuccessful resuscitation, the fetus should be emergently delivered if the mother is not resuscitated within 4 minutes of the arrest. This guideline for emergent cesarean delivery increases the chances of survival for both the mother and neonate. Typically, at 20 weeks or greater gestational age, an emergent cesarean delivery will be performed to help save the life of the mother. Less than 24 to 25 weeks gestational age, the fetus is not viable, but in the setting of ACLS the delivery of the fetus regardless of viability improves the chances of survival for the woman. (527)
55. Hypotension (decrease in systolic BP >20%) secondary to sympathetic blockade is the most common complication of neuraxial blockade for labor analgesia with rates between 10% and 24%. Left uterine displacement and hydration are used to decrease hypotension associated with the initiation of neuraxial blockade. (527)
56. Small vasopressor boluses of either phenylephrine or ephedrine can be used to treat hypotension after a neuraxial block. Although ephedrine was historically used primarily, phenylephrine is now preferred as first-line treatment because it is associated with less fetal acidosis. If treated promptly, maternal hypotension does not lead to fetal depression or neonatal morbidity. (527)
57. During the first 5 hours of epidural analgesia, there is no significant rise in body temperature. Temperature increases at about 0.10° C/hr, and may reach 38° C in as many as 15% of women with a labor epidural compared with 1% without an epidural. Although the etiology of the maternal temperature rise remains uncertain, it does not increase the rate of neonatal sepsis and need not affect neonatal septic workup. (527)
Other techniques for labor analgesia
58. For achievement of a paracervical block, local anesthetic must be injected submucosal, lateral and posterior to the uterocervical junction bilaterally. Sensory fibers that come from the uterus, cervix, and upper vagina travel through this area. Therefore, this block is most effective to help provide analgesia during the first stage of labor and is usually not effective for the second stage of labor. The major disadvantage of a paracervical block is potential fetal bradycardia. In approximately 15% of laboring women who receive this block, fetal bradycardia develops 2 to 10 minutes after the local anesthetic solution is injected. Although the definitive cause of the bradycardia is not known, it is often associated with fetal acidosis. The bradycardia is normally limited to less than 15 minutes with supportive treatment as needed. (527)
59. A pudendal nerve block is useful for anesthesia of the lower vagina and perineum as needed for delivery with an episiotomy or low forceps delivery. The obstetrician typically performs this block, which is done transvaginally with the woman in the lithotomy position. The failure rate is high and complications include local anesthetic toxicity, ischiorectal or vaginal hematoma, and rarely, fetal injection of local anesthetic. (527)
60. The inhaled anesthetic that is most often used for analgesia in the pregnant woman is 50% nitrous oxide administered in a blend with oxygen. The other inhaled anesthetics are rarely used for this purpose. The inhalation of nitrous oxide for analgesia during labor and delivery should be self-administered after appropriate patient instruction. Optimal results from the administration of nitrous oxide during labor are obtained by having the woman inhale the nitrous oxide between contractions, so that an effective concentration of nitrous oxide is achieved for the uterine contraction. About 45 seconds of continuous breathing of the nitrous oxide are necessary for optimal labor analgesia with contractions. Maternal cardiovascular and respiratory depression is minimal, uterine contractility is not affected, and neonatal depression does not occur regardless of the duration of nitrous oxide administration. (527-528)
Anesthesia for cesarean delivery
61. Benefits of regional anesthesia over general anesthesia for cesarean delivery include the avoidance of the risks of general anesthesia, such as a decreased risk of pulmonary aspiration, difficult airway, decreased fetal depression from and exposure to anesthetic agents, the placement of neuraxial opioids for postoperative pain, and the maintenance of maternal awareness. No differences in neonatal outcome after cesarean delivery with regional anesthesia or general anesthesia have been shown. (528)
62. Benefits of general anesthesia over regional anesthesia for cesarean delivery include rapid and dependable onset, secure airway, controlled ventilation, and the potential for less hemodynamic instability. (528)
63. Advantages of spinal anesthesia for cesarean delivery include its technical ease of administration, low levels of systemic medication that decrease the risk of systemic toxicity and fetal drug levels, its low failure rate, and its rapid onset time. Disadvantages of spinal anesthesia for cesarean delivery include the finite duration of anesthesia provided, and its higher incidence of hypotension. It can be safely used in preeclamptic patients. (528)
64. Spinal anesthesia with a sensory level of T4 is usually sufficient for patient comfort during cesarean delivery. Exteriorization of the uterus or traction of the abdominal viscera may still lead to discomfort in the woman. A T4 sensory level can be achieved with the administration of hyperbaric bupivacaine 10 to 15 mg. The medication will flow with the spinal curvature to a position near T4. (528)
65. Advantages of epidural anesthesia for cesarean delivery include the ability to extend the duration of anesthesia if necessary, to control block height, and to slowly titrate the dose to avoid precipitous maternal hypotension. Disadvantages of epidural anesthesia for cesarean delivery include the potential for intravascular injection of toxic levels of local anesthetic and its technical difficulty, longer onset time, less reliability, and increased perception of visceral pain with peritoneal manipulation. (528)
66. An approximate volume of 15 to 20 mL of local anesthetic solution must be delivered in the epidural space to achieve the T4 sensory level of anesthesia necessary for cesarean delivery. Before the administration of such high volumes of local anesthetics a test dose for epidural catheter placement should be administered. Local anesthetics that can be administered for cesarean delivery by epidural catheter placement include 2% lidocaine, 0.5% bupivacaine, 0.5% ropivacaine, and 3% 2-chloroprocaine. Each of these should be administered in increments to further minimize the risk of accidental intravenous administration of toxic levels of local anesthetic. Addition of epinephrine (1:200,000) or fentanyl (50 to 100 μg) can enhance the intensity and duration of the block. For the rapid onset of analgesia with a lumbar epidural catheter, as in an urgent cesarean delivery, 2-chloroprocaine 3% has the most rapid onset. (528)
67. The administration of preservative-free morphine into the epidural space during cesarean delivery extends the duration of analgesia by up to 24 hours into the postoperative period. The dose of morphine commonly administered is 3 to 5 mg. Some negative effects of morphine that may accompany this route of administration include pruritus, nausea and vomiting, and, on rare occasions, delayed respiratory depression. (522, 528)
68. Indications for general anesthesia for cesarean delivery include fetal distress and required emergent delivery to prevent poor fetal outcome, maternal hemorrhage, and contraindications to regional anesthesia such as maternal coagulopathy or maternal refusal. Benefits of general anesthesia for cesarean delivery include a more reliable and rapid onset of anesthesia, less hypotension and hemodynamic instability than regional anesthesia, and control of the airway and ventilation. (529)
69. The main cause of maternal morbidity and mortality with general anesthesia are an eightfold increased risk of failed endotracheal intubation (approximately 1 in 500) and a threefold increased risk of pulmonary aspiration (1 in 650) compared with nonpregnant patients undergoing general anesthesia. Appropriate airway examination, preparation, and familiarity with techniques and an algorithm for the difficult airway are critical for providing a safe anesthetic. (528, Figure 33-6)
70. A major cause of morbidity and mortality for the woman with regard to general anesthesia is failed or difficult intubation of the trachea. Contributing factors include inadequate time for preoperative evaluation of the airway, unpredicted airway edema, and emergency situations. If a difficult airway is suspected preoperatively, an awake fiberoptic intubation of the trachea should be considered. The anesthesiologist must have a difficult airway algorithm to follow should she or he be confronted with a difficult or failed intubation. There should be equipment immediately available for the difficult airway, such as a variety of functioning laryngoscope blades, several sizes of endotracheal tubes, laryngeal mask airways, a fiberoptic bronchoscope, and the means to perform a cricothyrotomy. Extra help should be solicited. Numerous attempts at laryngoscopy should be avoided to prevent increasing airway edema and bleeding. If hypoxia should occur during attempted laryngoscopy, the patient should be hand ventilated with bag and mask while cricoid pressure is maintained. If intubation attempts fail, the cesarean delivery may proceed if the anesthesiologist communicates that she or he can reliably ventilate the mother with either facemask or laryngeal mask airway (LMA). Other options may include allowing the patient to resume spontaneous ventilation, waking the patient and doing an awake fiberoptic intubation of the trachea, or, in dire circumstances of inadequate ventilation and hypoxemia, proceeding to a surgical airway. (529, Figure 33-6)
71. The level of exposure of the fetus to thiopental after the administration of thiopental for the induction of general anesthesia is generally low as long as the dose administered to the woman is less than 6 mg/kg. It peaks in the umbilical vein blood in 1 minute and in the umbilical artery blood in 2 to 3 minutes, and has no significant clinical effect on neonatal well-being. The lack of neonatal effects in standard doses is unclear, but may be due to first pass metabolism as (1) the drug is partially cleared as it passes through the liver of the fetus before reaching the fetal heart, (2) there is rapid redistribution into maternal vascular rich tissue beds, (3) there is higher fetal brain water content, and (4) there is additional dilution by the fetal circulation as the blood reaching the fetal heart from the placenta is diluted by the blood from the fetal viscera and lower extremities. However, doses of 8 mg/kg can result in neonatal depression. Propofol is not superior to thiopental in maternal or neonatal outcome. Induction doses of 2.5 mg/kg have no significant effect on neonatal behavior scores, while larger doses (9 mg/kg) are associated with neonatal depression. (529)
72. Like thiopental, etomidate has a rapid onset because of its high lipid solubility, and redistribution results in a relatively short duration of action. At typical induction doses (0.3 mg/kg), unlike thiopental and propofol, etomidate has minimal cardiovascular effects, but is painful on injection, can cause muscle tremors, has higher rates of nausea and vomiting, and has increased risk of seizures in patients with decreased thresholds. (529)
73. Maintenance of anesthesia for cesarean delivery often includes the inhalation of a low concentration (<0.75 MAC) of halogenated anesthetic in combination with either nitrous oxide or propofol. The volatile anesthetic is an important component to decrease the incidence of maternal recall. Uterine tone after delivery is maintained when the concentration of volatile anesthetic used is low. Maternal blood loss is minimized, the uterine response to oxytocin is not altered, and little neonatal depression is seen. Placental transfer of volatile anesthetics is rapid because they are nonionized, highly lipid-soluble substances of low molecular weight. Fetal concentrations depend on the concentration and duration of anesthetic administered to the mother. There is no evidence to show that neuraxial anesthesia is superior to general anesthesia for neonatal outcome. However, emergent delivery of a depressed fetus often results in a depressed neonate. A long time from induction to delivery may result in a lightly anesthetized, but not an asphyxiated neonate. If excessive concentrations of volatile anesthetics are administered for prolonged periods, neonatal effects of these drugs, as evidenced by flaccidity, cardiorespiratory depression, and decreased tone, may be anticipated. It is important to recognize that if neonatal depression is due to transfer of anesthetic drugs, the infant is merely anesthetized and should respond easily to simple treatment measures such as assisted ventilation of the lungs to facilitate excretion of the volatile anesthetic. Rapid improvement of the infant should be expected, and if it does not occur, it is important to search for other causes of depression. (530-531)
74. Succinylcholine remains the neuromuscular blocking drug of choice for obstetric anesthesia because of its rapid onset and short duration of action. Because it is highly ionized and poorly lipid soluble, only small amounts cross the placenta. It is normally hydrolyzed in maternal blood by the enzyme pseudocholinesterase and does not generally interfere with fetal neuromuscular activity. If large doses are given (2 to 3 mg/kg), it results in detectable levels in umbilical cord blood, and extreme doses (10 mg/kg) are needed for the transfer to result in neonatal neuromuscular blockade. Rocuronium is an acceptable alternative. It provides adequate intubating conditions in approximately 60 seconds at doses of 1.2 mg/kg. Unlike succinylcholine it has a much longer duration of action, potentially decreasing maternal safety in the event the anesthesiologist is unable to intubate or ventilate the patient. Uterine smooth muscle is not affected by neuromuscular blockade. Under normal circumstances, the poorly lipid-soluble, highly ionized, nondepolarizing neuromuscular blockers do not cross the placenta in amounts significant enough to cause neonatal muscle weakness. This placental impermeability is only relative and when large doses are given over long periods, neonatal neuromuscular blockade can occur. (531)
Abnormal presentations and multiple births
75. Currently twin pregnancy accounts for 3.4 % of the live births in 2006. The vast majority of multiple gestations are twin (97% to 98%). Higher order multiples account for only 0.1% to 0.03% of the births. Multiple pregnancies account for a significant risk to both the mother and the fetuses, with a higher rate of preterm labor, preeclampsia, gestational diabetes, preterm premature rupture of membranes, intrauterine growth restriction, and intrauterine fetal demise. The United States is seeing an increase in multiple gestations with the expanded use of artificial reproductive technologies. (531-532)
76. The majority of twin pregnancies have vertex-vertex positioning of the fetuses, and can be delivered vaginally. If the second twin is breech, it is important to discuss the mode of delivery with the obstetricians and perinatologists as an emergent cesarean delivery might be required if the second twin changes position after delivery of the first or develops fetal bradycardia. Placement of an epidural can facilitate delivery and extraction of the second twin if it becomes necessary for the obstetrician to perform an instrumented delivery of the second twin. At the late second stage of delivery, a more concentrated local anesthetic will optimize the perineal anesthesia and relaxation during this critical portion of the delivery. At this time, the potential for head entrapment or fetal bradycardia is highest and a denser block allows for possible transition to cesarean delivery. (532)
77. Singleton breech presentation occurs in about 3% to 4 % of all pregnancies. External cephalic version (ECV) has a mean success rate of approximately 60%. The procedure involves rotating the fetus via external palpation and pressure of the fetal parts. Neuraxial analgesia may improve success of the ECV. The risks of ECV include placental abruption, fetal bradycardia, and rupture of membranes. The anesthesia provider should be immediately available if an ECV is performed in case an urgent or emergent cesarean delivery is needed. (532)
78. A shoulder dystocia is an obstetric emergency analogous to a difficult airway in anesthesia. After delivery of the fetal head, further expulsion of the infant is prevented by impaction of the fetal shoulders with the maternal pelvis. It occurs in approximately 1% to 1.5% of all deliveries. Risk factors include: macrosomia, diabetes, obesity, history of dystocia, labor induction, and instrumented delivery. Fetal pH declines 0.04 units/min between delivery of the head and trunk. Cases of shoulder dystocia 7 minutes or longer are associated with a significant increase in risk of neonatal brain injury. The final maneuver of the obstetrician is to push the fetus back into the uterus and proceed to emergent cesarean delivery. Among the fetal injuries and sequelae of shoulder dystocia are brachial plexus injury, neurologic injury from asphyxia, and broken clavicle. Often these neurologic injuries improve over time with roughly less than 10% having a permanent Erb’s palsy. (532)
Hypertensive disorders of pregnancy
79. Gestational hypertension is diagnosed in previously normotensive women who develop elevated blood pressure (SBP >140 or DBP >90) after 20 weeks gestational age without evidence of proteinuria. (532)
80. Preeclampsia affects 5% to 7% of pregnant women. Risk factors include primigravida, chronic hypertension, gestational/preexisting diabetes, obesity, preeclamptic family history, multiple gestation, and use of assisted reproductive technology. (532-533)
81. The diagnosis of preeclampsia requires both of the following: a blood pressure of 140 mm Hg systolic or higher, or 90 mm Hg diastolic or higher that occurs after 20 weeks of gestation in a woman with previously normal blood pressure, and proteinuria defined as urinary excretion of 0.3 g protein or higher in a 24-hour urine specimen (≥ 1 + urine dip). (533, Table 33-3)
82. Preeclampsia is considered severe if one or more of the following criteria are present:
 Blood pressure of 160 mm Hg systolic or higher or 110 mm Hg diastolic or higher on two occasions at least 6 hours apart while the patient is on bed rest.
Blood pressure of 160 mm Hg systolic or higher or 110 mm Hg diastolic or higher on two occasions at least 6 hours apart while the patient is on bed rest. Proteinuria of 5 g or higher in a 24-hour urine specimen or 3+ or greater on two random urine samples collected at least 4 hours apart.
Proteinuria of 5 g or higher in a 24-hour urine specimen or 3+ or greater on two random urine samples collected at least 4 hours apart.83. A subcategory of severe preeclampsia is HELLP syndrome, which is a constellation of Hemolysis, Elevated Liver enzymes, and Low Platelet count. (533)
84. Although the exact cause remains unknown, preeclampsia begins with the pathogenic maternal/fetal interface. During placental formation there is failure of complete trophoblast cell invasion of the uterine spiral arteries. The failure of spiral artery remodeling creates decreased placental perfusion, which may ultimately lead to early placental hypoxia. Ultimately there is upregulation of cytokines and inflammatory factors as seen in sepsis. (533)
85. The American College of Obstetricians and Gynecologists prefer neuraxial analgesia for labor in preeclamptics. Special concerns for neuraxial analgesia in this patient population include maintaining the uterine perfusion pressure through the avoidance of hypotension, and evaluation of the patient’s coagulations status and platelet levels both with the placement of the epidural and prior to pulling out the epidural catheter. Currently, the definitive treatment of preeclampsia is delivery. If the pregnancy is remote from term in the presence of severe preeclampsia, a determination must be made whether to deliver or expectantly manage. This requires repeated evaluation of the mother and fetus. It is critical for the anesthesiologist on labor and delivery to be aware of these patients and their clinical course, as they can rapidly deteriorate and require urgent or emergent delivery. (533)
86. Magnesium sulfate is used for seizure prophylaxis in preeclamptic women. The infusion usually is performed by loading 4 to 6 g over 20 to 30 minutes then continued magnesium sulfate infusion of 1 to 2 g/hr until 12 to 24 hours after delivery. The therapeutic blood level range for seizure prophylaxis is between 6 to 8 mg/dL. Monitoring for magnesium sulfate toxicity is important in all patients, but is especially important in patients with impaired renal function, since magnesium sulfate is renally excreted. Loss of deep tendon reflexes occurs at 10 mg/dL with prolonged PQ intervals and widening QRS on ECG. Respiratory arrest occurs at 15 to 20 mg/dL, and asystole occurs when the level exceeds 20 to 25 mg/dL. If toxicity occurs, IV calcium chloride (500 mg) or calcium gluconate (1 g) should be administered. (533)
87. Initial antihypertensive therapy for preeclampsia normally includes hydralazine and labetalol. In refractory severe hypertension, nitroglycerin and sodium nitroprusside may be used in the acute situation to prevent intracerebral hemorrhage. Current guidelines recommend treating SBP >160. (533- 534)
Hemorrhage in pregnant women
88. Placenta previa, abruptio placentae, and uterine rupture are major causes of bleeding in the third trimester and during labor. Postpartum hemorrhage occurs in 3% to 5% of all vaginal deliveries and is typically due to uterine atony, retained placenta, placenta accreta, or lacerations involving the cervix or vagina. (534)
89. Placenta previa is an abnormal uterine implantation of the placenta in front of the presenting fetus. The incidence is approximately 1 in 200 pregnancies. Risk factors include advanced age, multiparity, assisted reproductive techniques, prior hysterotomy, and prior placenta previa. Placenta previa classically presents as painless vaginal bleeding. This usually occurs around the thirty-second week of gestation when the lower uterine segment is beginning to form. The diagnosis of placenta previa can be confirmed by ultrasound examination of the placenta. (534)
90. If hemorrhage is not controlled with standard measures, the obstetrical team can consider (1) uterine artery ligation, (2) B-Lynch sutures, (3) an intrauterine balloon, (4) use of arterial embolization by interventional radiology if the patient is stable for transport, or (5) hysterectomy. (534)
91. Abruptio placentae is separation of the placenta after 20 weeks of gestation, but before delivery. The incidence is approximately 1 in 100 pregnancies. Risk factors include advanced age, hypertension, trauma, smoking, cocaine use, chorioamnionitis, premature rupture of membranes, and history of prior abruption. The woman often has painful, frequent uterine contractions. The separation can involve only the placental margin, presenting as vaginal bleeding. Abruptio placentae can also occur without vaginal bleeding. In these cases, blood can accumulate in large volumes and be entirely sequestered within the uterus. Therefore, the degree of vaginal bleeding may not reflect the total amount of blood loss from the placenta. (535)
92. Risk factors for uterine ruptures include prior uterine scar, rapid spontaneous delivery, motor vehicle trauma, trauma from instrumented vaginal delivery, large or malpositioned fetus, and excessive oxytocin stimulation. After previous cesarean delivery, vaginal birth is associated with a 0.4% to 1% incidence of uterine rupture. The presentation is variable, but may include vaginal bleeding, cessation of contractions, FHR deceleration, and abdominal pain normally not masked by neuraxial analgesia. Unfortunately, pain is not always a diagnostic finding. (535)
93. Retained placenta occurs when some portion of the placenta has not been spontaneously delivered within 1 hour of delivery of the fetus. Uterine bleeding continues due to the inability of the uterus to contract around adherent placenta. Approximately 1% of all vaginal deliveries are associated with some retained placenta. The treatment involves manual exploration of the uterus for the removal of retained placental parts. The anesthetic management of patients with retained placenta has as its goal uterine relaxation, as well as decreasing the pain and anxiety of the patient. Anesthetic methods that may be used to initially accomplish this typically include intravenous sedation (keeping airway reflexes intact) or dosing of a preexisting epidural catheter. If uterine relaxation is necessary, nitroglycerin (50 to 150 μg IV) is normally effective. Additionally, relocation to the operating room and placement of neuraxial analgesia may be beneficial for thorough evaluation. Rarely, induction of general anesthesia with endotracheal intubation and administration of a volatile anesthetic to provide uterine relaxation will be necessary. (535)
94. Risk factors for postpartum uterine atony include retained products, long labor, high parity, macrosomia, polyhydramnios, excessive oxytocin augmentation, and chorioamnionitis. (535)
95. The treatment of uterine atony is by the administration of agents that increase uterine tone. Oxytocin (20 to 40 U/L) is normally the initial treatment. This dilute solution of oxytocin exerts minimal cardiovascular effects, but rapid intravenous injection is associated with tachycardia, vasodilation, and hypotension. Methylergonovine (0.2 mg IM) is an ergot derivative. Due to the significant vasoconstriction, it is relatively contraindicated in preeclamptics and patients with cardiac disease. The prostaglandin F2α (0.25 mg IM) is associated with nausea, tachycardia, pulmonary hypertension, desaturation, and bronchospasm. It should be avoided in asthmatics. Prostaglandin E1 (600 μg oral/sublingual/rectal) has no significant cardiac effects, but may cause hyperthermia. (535)
96. Placental implantation beyond the endometrium gives rise to (1) placenta accreta vera, which is implantation and adherence onto the myometrium; (2) placenta increta, which is implantation into the myometrium; and (3) placenta percreta, which is penetration through the full thickness of the myometrium. With placenta percreta, implantations may occur onto bowel, bladder, ovaries, or other pelvic organs and vessels. (535-536, Figure 33-7)
97. In patients with placenta previa and no previous cesarean delivery, the incidence of accreta is approximately 3%. However, the risk of placenta accreta associated with placenta previa increases with the number of previous cesarean deliveries. With one previous uterine incision, the incidence of placenta accreta has been reported to be 11%, with two previous uterine incisions the rate is 40%, and with three or more prior uterine incisions, the incidence rises to more than 60%. (536)
Amniotic fluid embolism
98. The incidence of amniotic fluid embolism (AFE) is estimated between 1:20,000 and 1:80,000. Clinical features of AFE include the sudden onset of hypotension, respiratory distress, hypoxia, disseminated intravascular coagulopathy, altered mental status, and eventual maternal collapse. These signs must be differentiated from other more common morbidities of pregnancy and delivery, such as inhalation of gastric contents, air embolism, pulmonary thromboembolism, high spinal, anaphylaxis, and local anesthetic toxicity. (536)
99. The exact cause and pathogenesis of AFE remains uncertain, but it is thought to be a type of anaphylactoid reaction. The diagnosis of AFE is a clinical diagnosis of exclusion. Although in the past it had been believed that aspirating amniotic fluid debris such as fetal squamous cells from the maternal pulmonary circulation was diagnostic, the presence of fetal squames has been demonstrated in asymptomatic pregnant women, and no diagnostic laboratory test for AFE currently exists. Definitive diagnosis is extremely difficult or impossible, even with postmortem examination. There is no treatment of AFE other than supportive. (536)
Anesthesia for nonobstetric surgery during pregnancy
100. The overall incidence of nonobstetric surgery during pregnancy is 1 in 50 to 1 in 100, with trauma, appendicitis, and cholecystitis being the most frequent causes. (536)
101. Elective procedures should be delayed until 6 weeks postpartum. When possible, nonelective operations should be delayed until after the first trimester to minimize teratogenic effects on the fetus or spontaneous abortion. The second trimester is considered the optimal time for surgical intervention, because the risk of preterm labor is lowest. In the case of acutely urgent surgical procedures, their timing should mimic that of nonpregnant patients. (536-537)
102. The critical gestational period for organogenesis occurs between 15 and 56 days of gestation. This is important because drugs that are teratogenic will exert their most disastrous effects when they are administered to the pregnant woman during this period. Most data regarding the administration of anesthetics to pregnant women in the first trimester are retrospective. There is no evidence that any of the currently used anesthetics, administered during pregnancy, are teratogenic with the exception of cocaine. Neurodegeneration and widespread apoptosis following exposure to anesthetics has been clearly established in developing animals, and a few studies demonstrate cognitive impairment in adult animals after neonatal anesthetic exposure. Currently there are no data to extrapolate these animal findings to humans, and this phenomenon is difficult to study in humans as clinical evidence is still scarce and amounts to an associative and not causal relationship. (537)
103. Intrauterine fetal hypoxia and acidosis has been associated with maternal hypotension, arterial hypoxemia, and excessive changes in the PaCO2. Both hypercapnia and hypocapnia result in reduced uterine blood flow and fetal acidosis. During surgery, normocarbia should be maintained (30 mm Hg end-tidal CO2), adequate uterine perfusion pressure maintained using fluids and vasopressors, and uterine displacement maintained if after 20 weeks gestational age to optimize uterine blood flow and fetal well-being. It is recommended that the maternal inhaled concentration of oxygen should be at least 50%. High oxygen consumption of the placenta plus the uneven distribution of maternal and fetal blood flow in the placenta prevent fetal PaO2 from exceeding about 60 mm Hg even with high maternal arterial oxygen levels. (537)
104. FHR monitoring via Doppler is possible at 16 to 18 weeks gestational age, but variability as a marker of well-being is not established until 25 to 27 weeks. Fetal monitoring can display fetal compromise and allows further optimization of the maternal and fetal condition with in utero resuscitation maneuvers. Currently there is no evidence for the efficacy of FHR monitoring. In addition, interpretation is difficult since most anesthetics reduce FHR variability, placement and signal acquisition may be challenging, and a trained person is needed for interpretation. The decision of whether or not to monitor the fetal heart rate during nonobstetric surgery should be individualized case by case in discussion with an obstetrician and other perioperative team members. (537)
105. The usual cause of premature labor that presents in the pregnant woman after having nonobstetric surgery is the underlying pathologic process that led to the need for surgery and not the anesthetic technique. Postoperative monitoring that should be done in these circumstances, in addition to the routine monitoring, includes continuous fetal heart rate monitoring and monitoring of maternal uterine activity. Premature labor can be treated through the administration of tocolytics in consultation with an obstetrician. Common tocolytics include terbutaline, magnesium, indomethacin, and nifedipine. (537)
106. Laparoscopic surgery is as safe as an open approach during any trimester, and the indications for its use are the same as nonpregnant patients. A recent review which compared laparascopic surgery to open surgery found that the trimester did not influence the complication rate, the conversion to open was low (1%), there was a slightly higher fetal loss rate, but there was a lower preterm delivery rate. Most studies comparing laparoscopic to open techniques note no difference in fetal or maternal outcomes. (538)
Diagnosis and management of fetal distress
107. Normal uterine activity is five contractions or less in 10 minutes, averaged over a 30-minute window. Tachysystole while tachysystole is defined as more than five contractions in 10 minutes, averaged over a 30-minute window. If a tonic contraction or period of tachysystole occurs during labor, treatment with either sublingual or IV nitroglycerin can briefly relax the uterus and restore fetal perfusion. In addition, the obstetrician can give subcutaneous terbutaline. (538, Table 33-4)
108. The normal baseline FHR is between 110 and 160 beats/min.
109. Baseline variability is determined by examining fluctuations that are irregular in amplitude and frequency during a 10-minute window excluding accelerations and decelerations. Variability is classified as follows:
110. A FHR acceleration is an abrupt increase in FHR defined as an increase from the acceleration onset to the peak in greater than 30 seconds. In addition, the peak must be 15 beats/min or greater, and last 15 seconds or longer from the onset to return. Before 32 weeks of gestation, accelerations are defined as having a peak of 10 beats/min or more and a duration of 10 seconds or longer. (538)
111. Late decelerations are a result of uteroplacental insufficiency, causing relative fetal brain hypoxia during a contraction. The change results in sympathetic response and increased peripheral vascular resistance in the fetus, elevating the fetal blood pressure, which is detected by the fetal baroreceptors and results in slowing in the FHR. (539; Table 33-5)
112. Variable decelerations are generally synonymous with umbilical cord compression. (539; Table 33-5)
113. Intrapartum fetal monitoring was designed to detect hypoxia in labor and allow the clinicians to intervene prior to acidosis and long-term fetal CNS damage. The fetal brain responds to peripheral and central stimuli: (1) chemoreceptors, (2) baroreceptors, and (3) direct effects of metabolic changes within the CNS. FHR monitoring was developed as a crude, nonspecific method of tracking fetal oxygenation and distress. (538)
Evaluation of the neonate and neonatal resuscitation
115. Umbilical cord blood gas values:
| Mean Artery | Mean Vein | |
|---|---|---|
| pH | 7.27 | 7.34 |
| PCO2 | 50 | 40 |
| PO2 | 20 | 30 |
| Bicarbonate | 23 | 21 |
| Base excess | −3.6 | −2.6 |
(539, Table 33-6)
116. When evaluating the neonate, if breathing and crying does not occur, then clearing of the airway (mouth then nose) and repeated stimulation should be performed. Following this, the 1-minute Apgar score is determined with evaluation of the respirations, heart rate, and color. In the event of apnea or heart rate less than 100, positive pressure hand ventilation should be provided with 21% or up to 100% oxygen using a properly fitted facemask (avoiding excessive inspiratory pressure >30 cm H2O). Based on the current 2005 neonatal resuscitation guidelines, if the clinician begins with room air, it is recommended that supplemental oxygen be given if no improvement is seen within 90 seconds after birth. (540-541, Figure 33-9)
117. The dose of epinephrine for neonatal resuscitation is 0.1 to 0.3 mL/kg of a 1:10,000 solution given rapidly intravenously through an umbilical artery catheter inserted just below the abdominal skin (preferred) or via the trachea. The dose may be repeated every 3 to 5 minutes, if necessary. (541)
118. Naloxone is no longer recommended for use in newborns in the delivery room. Should the newborn manifest respiratory depression in the delivery room, appropriate ventilation should be maintained until the neonate is transported to the intensive care nursery, where naloxone can be given if determined to be necessary. (542)
119. Currently, neonates at delivery with meconium-stained amniotic fluid (MSAF) who are at term and vigorous should not be suctioned at the perineum, and once delivered do not require intubation. Intubation and suctioning should be performed in MSAF neonates if they are not vigorous (heart rate <100, decreased muscle tone, and ineffectual respirations). (542)
120. The most significant advances in the prevention of meconium aspiration syndrome are improved estimation of gestational age and use of an earlier gestational age for postdates induction. (542)