Chapter 37 Spheno-Orbital Meningioma
The original description of sphenoid ridge meningiomas by Cushing and Eisenhardt in 1938 defines a global type, which grows outward into the sylvian fissure from its attachment, and an en-plaque type, which has a carpet-like growth pattern and evokes hyperostotic changes in the adjacent bone.1 Since the original description, myriad synonyms have been used in the literature to further describe hyperostosing meningiomas found along the sphenoid ridge, such as pterional tumors en plaque,2 intraosseous meningiomas,3 osteomeningiomas, hyperostosing lesions of the ala magna, hyperostosing meningiomas of the sphenoid wing,4,5 invading meningiomas of the sphenoid wing,6 and spheno-orbital meningiomas (SOMs).7
SOMs may be either globoid or en plaque; may invade the orbit, superior orbital fissure, or cavernous sinus; and may extend into the paranasal sinuses. Hyperostosis can occur regardless of lateralization on the sphenoid wing or invasion into the cavernous sinus.8 Typically, SOMs exhibit hyperostosis of the squamosal temporal bone, greater sphenoid wing, lateral orbital wall and orbital roof, lesser sphenoid wing, anterior clinoid, and middle fossa floor. Structures involved may include the basal foramina, superior orbital fissure, optic canal, and ethmoid and sphenoid sinuses. The hyperostotic bone has been shown to be secondary to tumor invasion in the vast majority of cases.9,10 The intradural component can be either globoid or carpet-like; can be attached to the convexity dura, basal sphenoid, and floor of the middle fossa dura; and can invade the cavernous sinus and superior orbital fissure. An intraorbital tumor can be extra- or intraperiorbital, can be extra- or intraconal, and can invade the orbital apex. Extracranial invasion of the temporalis muscle and the infratemporal fossa can occur.
Early management of these complex, hyperostosing meningiomas was conservative. In Castellano’s early report in 1952, he discloses a mortality rate of 13.3% in 15 patients undergoing surgery for hyperostosing sphenoid wing meningiomas.2 Based on this high perioperative mortality, the discomforts of severe proptosis and even unilateral vision loss were insufficient reasons to warrant surgical intervention.2,11 It was not until the work of Derome12 and others6,13,14 in the 1970s that more complete resections were safely achieved with use of a coronal incision, pericranial grafting, and reconstruction of the anterior skull base with iliac bone or split calvarial bone grafts. Today, with greater understanding of surgical anatomy and the development of advanced skull base techniques,15–19 the perioperative mortality has dropped from 20%2,6,11,13 to 0% to 4%.3,7,8,20–24 Even so, with efforts to preserve neurologic function,25 residual tumors involving the cavernous sinus, orbital apex, and superior orbital fissure remain problematic surgical limitations. These areas are major sites for residual tumors, recurrence, and continued neurologic deterioration.4–6,8,22–24,26 Adjuvant radiotherapy for residual tumors or recurrence commonly forms part of modern management plans,27 but specific indications remain controversial.22,26,28
The most common presenting symptom of a SOM is by far unilateral eye bulging with the corresponding physical finding of proptosis.2 Hyperostosis of the lateral orbital wall, orbital roof, and intraorbital tumor invasion contribute to proptosis. Other common symptoms include decreased vision, double vision, headache, temporal swelling, facial numbness, and seizures. Correlative neurologic findings include optic atrophy, decreased acuity and deficits of visual field, cranial nerve (CN) paresis, and trigeminal hypesthesia. CN III, IV, and VI paresis is the result of cavernous sinus, superior orbital fissure, or both types of invasion. However, diplopia can also result from rectus muscle compression and restricted movement from orbital invasion by tumor. Facial hypesthesia in the V1 distribution typically indicates superior orbital fissure invasion. Tumor invasion in the cavernous sinus or hyperostotic stricture of the basilar foramina can cause facial hypesthesia in the V2 or V3 distributions. Presenting symptoms and neurologic findings are summarized in Tables 37-1 and 37-2.
Table 37-1 Symptoms in Patients with Hyperostosing SOMs
| Symptom | Percentage of Patients (mean) |
|---|---|
| Eye bulging | 36-100 (78)2,5,8,10,21,24 |
| Decreased vision | 16-60 (32)2,5,8,10,24 |
| Double vision | 5-21 (14)5,10,21,24 |
| Headache | 8-41 (25)2,5,8,10,24 |
| Mass or swelling | 7-61 (27)2,5,21,24 |
| Facial numbness or pain | 2-5 (3)5,8,24 |
| Seizures | 1-16 (8)2,5,8,21,24 |
Table 37-2 Findings in Patients with Hyperostosing SOMs
| Neurologic Finding | Percentage of Patients (mean) |
|---|---|
| Proptosis | 36-100 (79)2,5,8,21,24 |
| Optic atrophy | 16-29 (23)2,5 |
| Decreased acuity | 24-58 (43)2,5,24 |
| Visual field deficit | 10-32 (18)2,5,24 |
| CN paresis | 21-33 (26)2,5,24 |
| Temporal swelling | 7-61 (27)2,5,21 |
| Trigeminal hypesthesia | 3-8 (5)2,5,24 |
Data from the Central Brain Tumor Registry of the United States reveals that the most frequently reported primary brain tumors are meningiomas, at 33.4%. They are the most common type of tumor from age 35 on and occur more often in females than males, with a 2.2:1 (cbtrus.org). In Castellano’s 1952 report of 608 cases of verified meningiomas,2 18.4% (n = 111) were located along the sphenoid ridge and 2.5% (n = 15) were associated with hyperostosis. Other series report a range of 4% to 9% for hyperostosis associated with sphenoid wing meningiomas.5,29
Presurgical Management
Physical Examination
Patients with hyperostosing sphenoid wing meningioma need to undergo a thorough physical evaluation. Due to the variable degree of hyperostosis, soft tissue invasion, and extracranial extension, a detailed neurologic examination is requisite. Patients may have deficits in CN II, III, IV, and V (V1, V2, and V3) and in CN VI. If a portion of the tumor is globoid, contralateral hemiparesis may exist, as may deficits of speech and language. Careful attention should be given to the patient’s appearance, and proptosis and temporal bulging should be noted.
Neurologic Imaging
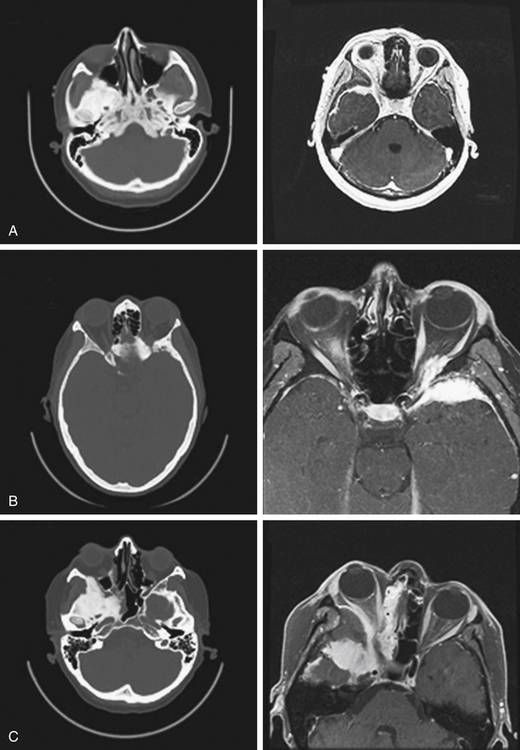
Neurologic imaging should begin with high-resolution thin section computed tomography (CT) scan of the head and skull base. Assessment of the bony windows identifies the affected hyperostotic bone. Structures commonly involved include the orbital roof and lateral wall, greater and lesser sphenoid wing, anterior clinoid, temporal squamosal bone, body of the sphenoid bone, and lateral wall of the sphenoid and ethmoid sinuses. Brain magnetic resonance imaging (MRI) with gadolinium enhancement gives greater detail of intradural and dural involvement. Fat-suppressed T1 sequences are essential when evaluating intraorbital invasion. Preoperative angiography is typically not necessary. Figure 37-1 demonstrates the variable radiologic presentations found with SOMs.
Operative Techniques
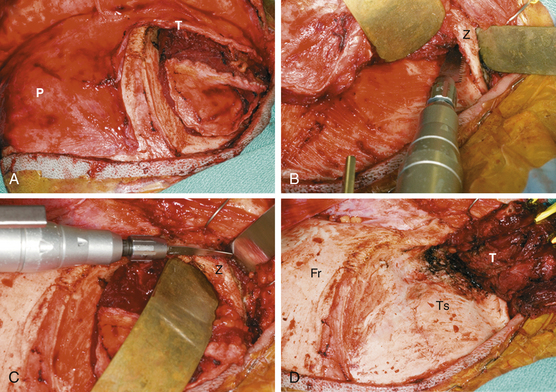
The surgical approach for SOMs is typically frontotemporal with possible modifications to include transzygomatic, orbitozygomatic, and orbital frontal approaches. The initial approach is extradural, which consistently enables access to the orbit and middle fossa for removal of hyperostotic bone and decompression of the optic canal. This also allows coagulation and control of extradural arterial blood supply and minimizes brain retraction during intradural tumor removal.8,19 Accessing of the superior orbital fissure, cavernous sinus, and intraorbital compartment is facilitated by this approach as well. A coronal incision allows harvest of a vascularized pericranial graft for reconstruction purposes. The patient is typically positioned in the supine position. The head is rotated 30 degrees to the contralateral side and fixed. The patient’s surgical position is then registered to the preoperative imaging data sets to allow intraoperative navigation. Preoperative high-resolution CT and MRI with gadolinium and fat suppression are coregistered. CT is utilized to assess intraoperative bone removal, and MRI with gadolinium and fat suppression is utilized to guide intradural and intraorbital tumor removal. An area of the abdomen is prepped for an autologous fat graft. The coronal incision is made, and the anterior branch of the superficial temporalis artery is preserved. Sharp dissection is utilized to elevate the scalp in the immediate subgaleal plane. The scalp is reflected anteriorly, leaving the periosteum and overlaying loose connective tissue layer attached to the calvarium. The posterior edge of the scalp is undermined 2 to 3 cm.
The temporalis fascia is incised 2 cm above the “keyhole” region and continued to the root of the zygoma (Fig. 37-2A). The periosteum is released posteriorly and then incised along the superior temporal line. Together, the periosteum and the superficial and deep layers of the temporalis fascia are elevated off the frontal bone, orbital rim, and zygoma while the frontalis branch of the facial nerve is protected. Subperiosteal dissection is utilized to elevate the temporalis muscle laterally and inferiorly, carefully preserving the blood supply and innervation. When infratemporal exposure is required, a zygomatic osteotomy is then performed, leaving the zygomatic arch attached to the masseter muscle (Fig. 37-2B and C). Tumor-infiltrated temporalis muscle is excised. Complete exposure of the frontal, temporal, and zygomatic bones is thus achieved (Fig. 37-2D). Intraoperative navigation is utilized to ensure sufficient exposure of involved bone, as well as underlying dural involvement.
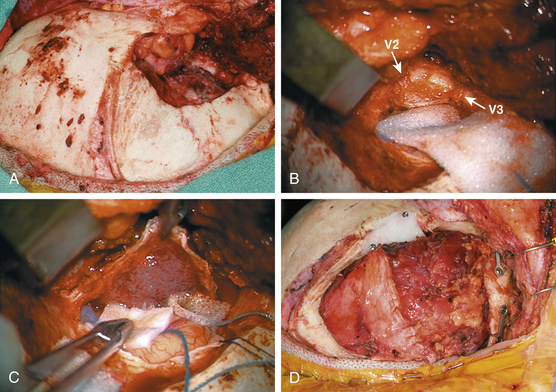
The hyperostotic greater sphenoid wing, lateral orbital wall and rim, and temporal squamosal bone are then removed with a high-speed drill and rongeurs (Fig. 37-3A). Bony removal is performed as indicated clinically and by the preoperative evaluation of the CT scan, as well as intraoperative use of CT-guided neuronavigation. The superior orbital fissure and basilar foramina (foramen rotundum, vidian canal, foramen ovale, and foramen spinosum) all can be completely skeletonized (Fig. 37-3B). When the lesser wing of the sphenoid bone, anterior clinoid, orbital roof, or optic canal is involved, an orbital frontal craniotomy is performed. Removal of the lesser wing and anterior clinoid and wide opening of the optic canal is performed with a high-speed diamond burr under constant irrigation and microscopic visualization. Involved dura is excised (Fig. 37-3C). Intradural or plaque-like tumor involvement is removed with sharp dissection and microsurgical techniques. When cavernous sinus invasion is noted, resection is carried forward to include the medial temporal dura of the lateral cavernous sinus wall. Meningiomas within the cavernous sinus can directly invade the CNs and the connective tissue planes between them.4,30 Thus, even with radical cavernous sinus resection, an oncologic cure is not possible and certainly not justified given the resultant morbidity.31 For these reasons, tumor within the cavernous sinus or superior orbital fissure is typically left in place to avoid neurologic complications. Once the intracranial portion has been excised, attention is turned to the intraorbital involvement. The dura is closed prior to the commencement of intraorbital tumor resection.
The dura is reconstructed with a portion of the pericranial graft, temporalis fascia, or allographic dural substitute. If the sphenoid or ethmoid sinuses are entered, these are obturated with autologous fat to prevent cerebrospinal fluid leak. If a periorbital defect is present, it is closed with locally harvested temporalis fascia. The pericranial graft is then rotated over the orbit. This helps compartmentalize the orbit from the intracranial space and avoid adhesion of the orbital tissues to the dura. Reconstruction of the orbital roof and lateral orbital wall is not necessary for a good cosmetic result32 if the periorbita is intact or repaired. If the superior or lateral orbital rim is removed, it can be reconstructed with split calvarial bone grafts or commercially available orbital prostheses. The orbital and zygomatic osteotomies are fixated with low-profile cranial plating. Remaining dead space from hyperostotic bone removal can be filled with autologous fat. If a temporal fossa defect exists, a cranioplasty is performed. Options for cranioplasty include polymethylmethacrylate, various commercially available bone cements, or commercially available prostheses. Alternatively, prior to bone removal, titanium mesh can be molded to the calvarium and then utilized to cover the bony defect at the end of the case.10 The temporalis muscle is reattached to a cuff of fascia, drill holes in the calvarium, or the reconstruction plates (Fig. 37-3D).
Outcomes
Mortality
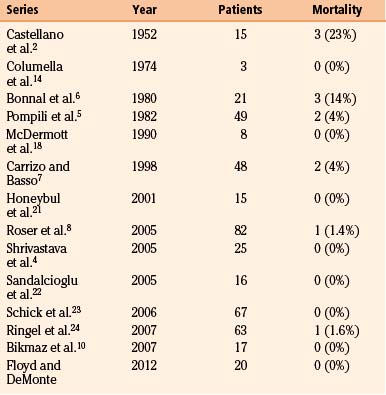
In modern case series, perioperative mortality rates vary from 0% to 4% (Table 37-3). Since 2001, only two perioperative deaths have been reported, one from pulmonary embolus8 and one from carotid laceration.24
Morbidity
Morbidity is inconsistently reported in the literature. Instances of subgaleal fluid collection, temporal hollowing, chemosis, meningitis, osteomyelitis, epidural hematoma, chronic subdural hematoma, retro-orbital hematoma, transient aphasia, brain edema, temporary hemiplegia, hemispheric stroke secondary to carotid injury, and blindness have all been reported.4,5,7,21,22,24
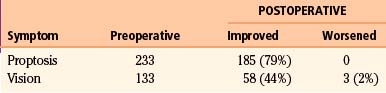
Specific outcomes related to the presenting clinical symptoms of proptosis, decreased vision, and cranial neuropathy have also been inconsistently detailed. Since 2001, 285 patients have been reported in case series.4,8,10,21–24 With the addition of 20 patients from our series, 305 patients are considered.
Proptosis
In the case series since 2001,4,8,10,21–24 there have been 233 patients with proptosis identified, either by clinical inspection or by formal measurements. Of this number, 79% (n = 185) have been reported to have had significant cosmetic improvement postoperatively (Table 37-4). Methods used to evaluate the degree of proptosis varied; patient questionnaires, patient perception, clinical examinations, and formal Hertel measurements were all used. Five patients developed enophthalmos (2%) that required additional corrective surgery, and the remaining patients had stable degrees of proptosis. Maroon et al.26 reported good cosmetic results without orbital reconstruction. However, no objective measurements or strict criteria were utilized in the evaluation of globe position. Subsequent reports that utilized objective measures emphasize that reconstruction of the orbit is necessary if more than one orbital wall is removed during surgery.20,21 DeMonte et al.,32 however, demonstrated that as long as the periorbita is intact, or has been primarily repaired, isolated bone defects of the medial or lateral orbital walls do not need to be reconstructed. Similarly, isolated defects of the orbital roof, or combined defects of the orbital roof and lateral wall, do not need reconstruction as long as the periorbita is intact or repaired. Large defects of the orbital floor always need reconstruction regardless of whether the periorbita is intact or repaired to prevent hypoglobus or enophthalmos.
Vision
In the literature reviewed,4,8,10,21–24 133 patients were reported to have decreased visual acuity at the time of diagnosis. Fifty-eight patients (44%) experienced an improvement in visual acuity postoperatively (see Table 37-4). Three patients (2%) were noted to have worsened visual acuity postoperatively.23,24 One new case of transient decreased acuity and a permanent temporal hemianopsia occurred when a skull base fracture extended through the optic canal when elevating the bone flap.21 The remaining patients retained stable vision after surgery. In Schick’s series,23 7 patients had no useful vision; of those, 1 patient improved postoperatively. Another 28 patients had decreased vision, and 11 of those patients improved. In our series of 20 patients, 2 patients had light perception vision only and did not improve. Another 4 patients had vision loss that did not exceed 20/400, and 3 of these 4 patients improved after surgery. Complete optic nerve decompression is imperative in improving and stabilizing vision. When hyperostosis involves the orbital roof, lesser sphenoid wing, and clinoid process, extra attention must be given to avoid bone fractures into the optic canal. This is generally avoided by utilizing a two-piece approach to the orbital osteotomy when an orbital frontal approach is indicated. However, even with excellent surgical technique, once vision has deteriorated to greater than 20/400 or to light perception only, it is unlikely that acuity will improve.
Cranial Nerve Function
Ocular motility is often disturbed in SOMs secondary to either extraocular muscle restriction or CN dysfunction. Either can cause diplopia as a presenting symptom. When diplopia is secondary to extraocular muscle restriction, symptoms typically resolve after orbital decompression and reconstruction. In Shrivastava et al.’s series,4 five patients presented with double vision, thought to be secondary to extraocular muscle restriction. All five patients eventually had significant improvement after surgery. In our series, nine patients presented with double vision. Five were secondary to extraocular muscle restriction and four due to CN dysfunction (CN III, IV, and IV palsies). Of the five patients with extraocular muscle restriction, four patients (80%) had improvement in double vision postoperatively. However, there was no improvement in patients who had preexisting CN palsies. Sandalcioglu et al.22 reported nine transient CN deficits postoperatively, including ptosis and CN III palsy in seven patients and facial hypesthesia in two patients. One patient had a preexisting CN III palsy, and one patient had a new permanent CN III palsy following surgery. Ringel et al.24 also reported eight transient CN III deficits, along with one CN VI and two CN IV deficits after surgery. In that series, there were eight additional permanent CN III palsies, one CN IV palsy, two CN VI palsies, and 10 cases of facial hypesthesia from CN V dysfunction. The number of CN deficits that may have been preexisting is unclear.
In summary, double vision when secondary to the restricted movement of the extraocular muscles improves with orbital decompression. When patients present with true CN III, IV, and VI deficits, these are unlikely to improve after decompression. True CN palsies are due to direct tumor invasion of the cavernous sinus, superior orbital fissure, or orbital apex and annulus of Zinn. Transient CN deficits occur most often with ptosis (CN III) and facial hypesthesia (CN V). New CN deficits are variably reported; however, it seems that CN III is most often reported.22,23
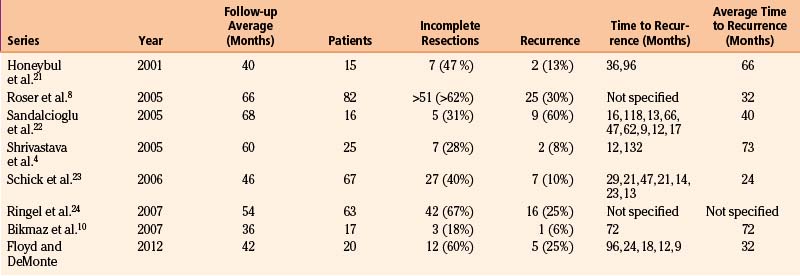
Long-Term Outcomes
The recurrence or progression rate for hyperostosing sphenoid wing meningiomas varies from 6% to 60%, with an average time to recurrence or progression of 24 to 73 months4,8,10,21–23 (Table 37-5). Bonnal et al.6 describe six early recurrences (within less than 2 years) in their series of 21 patients. Three areas attributed to the early recurrence, or more likely progression, are the cavernous sinus, sphenoid body, and annulus of Zinn. Shrivastava et al.4 also reported one early recurrence (within less than 1 year) from direct extension from the superior orbital fissure. Maroon et al. in 1994 indicated that recurrences are multifactorial, including failure of early diagnosis, subtotal resection when tumor invades the cavernous sinus and superior orbital fissure, and incomplete removal of hyperostotic bone.26 Today, it is clear that hyperostotic bone is tumor-invaded bone.9,10 With advanced imaging by means of high-resolution CT scans and gadolinium-enhanced, fat-suppressed MRIs, corrected diagnosis is made earlier and more extensive resections are achieved. However, tumor invasion in the cavernous sinus, superior orbital fissure, and orbital apex remains problematic and is the main reason for tumor progression.7,21,29 In our series of 20 patients followed for an average of 42 months, 5 patients developed tumor recurrence or progression at an average of 32 months. The areas of progression resulted from residual tumors in the cavernous sinus, superior orbital fissure, and intraorbital and intraconal locations.
Long-Term Management
Postoperative Follow-up
Patients with hyperostosing SOMs need indefinite close and careful follow-up in the postoperative period, given the possibility for recurrence many years after the primary resection. Patients with recurrent hyperostosing SOMs most often present with progressive proptosis, followed by progressive vision loss and CN dysfunction.23,26 For these reasons, serial ophthalmologic examinations should be part of the postsurgical evaluation of globe position, ocular motility, visual acuity, visual fields, and other signs of optic neuropathy. Early detection of a recurrent tumor can be made prior to clinical symptoms with high-resolution gadolinium-enhanced MRIs with fat-suppressed orbital sequences. This allows for the evaluation of intradural and intraorbital recurrences.
When faced with recurrence or progression of hyperostosing SOMs, the management goals are the same as with initial presentation: maximal tumor removal and reconstruction, preservation of ocular and CN function, and improvement in cosmesis with reduction of proptosis. The strategy varies according to the recurrence and symptomology. However, in most instances of recurrence, a second surgery for maximal tumor cytoreduction is indicated.8 Excellent cosmetic results and vision preservation can be achieved with a second surgery.26 Sandalcioglu et al. report that out of nine recurrences in their series, eight underwent reoperation; in three patients, a complete resection was achieved. In all eight patients, there were no additional neurologic deficits.22 For these reasons, and because these are slow-growing tumors, Honeybul et al. advocate a second surgery unless additional surgery poses greater risk to the patient.21 When this situation arises, either due to medical comorbidities or due to excessive neurologic risks, radiation treatment as primary therapy is a good option.
Radiation Treatment
Radiation treatment has been shown to decrease the recurrence rate and increase the time to progression for benign meningioma.28,33–35 Barbaro et al.36 demonstrated that in subtotally resected meningiomas, 60% of patients had tumor recurrence at an average of 66 months if no additional radiation was given. In patients with subtotally resected meningiomas who received postoperative radiation, the recurrence rate was 32% at an average of 125 months. Later, Goldsmith et al. demonstrated a 5-year 98% progression–free survival rate in patients who had undergone a subtotally resected benign meningioma.37 Soyuer et al.28 also demonstrated that the 5-year progression-free survival is significantly improved in patients who have had adjuvant radiotherapy for subtotally resected meningiomas versus those patients who have had subtotal resections without additional treatment. However, there was no overall survival advantage when patients were receiving immediate postoperative radiation. This significant finding indicates that delaying adjuvant radiation therapy does not compromise the overall survival and may have the added benefit of delaying treatment-related toxicities. At the time of recurrence, most often a second surgery is advocated. At that time, in most published clinical series, adjuvant radiation is given.4,8,22,23,26
Bikmaz K., Mrak R., Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(5):905-912.
Carrizo A., Basso A. Current surgical treatment for sphenoorbital meningiomas. Surg Neurol. 1998;50(6):574-578.
De Jesus O., Toledo M.M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001;55(5):265-269.
DeMonte F., et al. Ophthalmological outcome following orbital resection in anterior and anterolateral skull base surgery. Neurosurg Focus. 2001;10(5):E4.
Derome P. [Spheno-ethmoidal tumors. Possibilities for exeresis and surgical repair]. Neurochirurgie. 18(1), 1972. p. (suppl 1):1-164
Honeybul S., et al. Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir (Wien). 2001;143(8):749-757. discussion 758
Maroon J.C., et al. Recurrent spheno-orbital meningioma. J Neurosurg. 1994;80(2):202-208.
Pieper D.R. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44(4):742-746. discussion 746-747
Pompili A., et al. Hyperostosing meningiomas of the sphenoid ridge—clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol. 1982;17(6):411-416.
Ringel F., Cedzich C., Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60(4 suppl 2):214-221. discussion 221-222
Roser F., et al. Sphenoid wing meningiomas with osseous involvement. Surg Neurol. 2005;64(1):37-43. discussion 43
Sandalcioglu I.E., et al. Spheno-orbital meningiomas: interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005;33(4):260-266.
Schick U., et al. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104(2):208-214.
Shrivastava R.K., et al. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103(3):491-497.
Soyuer S., et al. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol. 2004;71(1):85-90.
1. Cushing H., Eisenhardt L. Meningiomas. Their Classification, Regional Behavior, Life History, and Surgical End Results. Springfield, IL: Charles C. Thomas; 1938.
2. Castellano F., Guidetti B., Olivecrona H. Pterional meningiomas en plaque. J Neurosurg. 1952;9(2):188-196.
3. Elder J.B., et al. Primary intraosseous meningioma. Neurosurg Focus. 23(4), 2007. p. E13
4. Shrivastava R.K., et al. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103(3):491-497.
5. Pompili A., et al. Hyperostosing meningiomas of the sphenoid ridge—clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol. 1982;17(6):411-416.
6. Bonnal J., et al. Invading meningiomas of the sphenoid ridge. J Neurosurg. 1980;53(5):587-599.
7. Carrizo A., Basso A. Current surgical treatment for sphenoorbital meningiomas. Surg Neurol. 1998;50(6):574-578.
8. Roser F., et al. Sphenoid wing meningiomas with osseous involvement. Surg Neurol. 2005;64(1):37-43. discussion 43
9. Pieper D.R. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44(4):742-746. discussion 746-747
10. Bikmaz K., Mrak R., Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(5):905-912.
11. Grant F.C. The surgery of meningiomas at the base of the brain. Clin Neurosurg. 1957;5:25-35. discussion 36-37
12. Derome P. [Spheno-ethmoidal tumors. Possibilities for exeresis and surgical repair.]. Neurochirurgie. 1972;18(1):1-164. p. suppl
13. Dolenc V. Microsurgical removal of large sphenoidal bone meningiomas. Acta Neurochir Suppl (Wien). 1979;28(2):391-396.
14. Columella F., Testa C., Andreoli A. Radical resection and reconstruction in spheno-ethmoidal-orbital tumors. Report of 3 cases. J Neurosurg Sci. 1974;18(3):198-205.
15. Jane J.A., et al. The supraorbital approach: technical note. Neurosurgery. 1982;11(4):537-542.
16. Al-Mefty O. Supraorbital–pterional approach to skull base lesions. Neurosurgery. 1987;21(4):474-477.
17. Alaywan M., Sindou M. Fronto-temporal approach with orbito-zygomatic removal. Surgical anatomy. Acta Neurochir (Wien). 1990;104(3-4):79-83.
18. McDermott M.W., et al. Combined frontotemporal–orbitozygomatic approach for tumors of the sphenoid wing and orbit. Neurosurgery. 1990;26(1):107-116.
19. Day J.D. Cranial base surgical techniques for large sphenocavernous meningiomas: technical note. Neurosurgery. 2000;46(3):754-759. discussion 759-760
20. Gaillard S., et al. [Long-term results of the surgical treatment of spheno-orbital osteomeningioma.]. Neurochirurgie. 1995;41(6):391-397.
21. Honeybul S., et al. Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir (Wien). 2001;143(8):749-757. discussion 758
22. Sandalcioglu I.E., et al. Spheno-orbital meningiomas: interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005;33(4):260-266.
23. Schick U., et al. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104(2):208-214.
24. Ringel F., Cedzich C., Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60(4 suppl 2):214-221. discussion 221-222
25. Ojemann R.G. Skull-base surgery: a perspective. J Neurosurg. 1992;76(4):569-570.
26. Maroon J.C., et al. Recurrent spheno-orbital meningioma. J Neurosurg. 1994;80(2):202-208.
27. Stieber V.W., Mehta M.P. Advances in radiation therapy for brain tumors. Neurol Clin. 25(4), 2007. p. 1005-1033, ix
28. Soyuer S., et al. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol. 2004;71(1):85-90.
29. De Jesus O., Toledo M.M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001;55(5):265-269.
30. Sen C., Hague K. Meningiomas involving the cavernous sinus: histological factors affecting the degree of resection. J Neurosurg. 1997;87(4):535-543.
31. DeMonte F. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81(2):245-251.
32. DeMonte F., et al. Ophthalmological outcome following orbital resection in anterior and anterolateral skull base surgery. Neurosurg Focus. 2001;10(5):E4.
33. Barbaro N.M., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20(4):525-528.
34. Goldsmith B.J., et al. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80(2):195-201.
35. Miralbell R., et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol, 1992;2(13):157-164