Chapter 50 Spasticity
Classification, Diagnosis, and Management
• Spasticity is velocity-dependent resistance to passive muscle stretch that usually originates from pathological issues with the brain or spinal cord. Symptoms of spasticity include muscle tightness, cramping/pain, and fatigue. Spasticity is primarily due to an imbalance between activation and inhibition of muscle groups.
• Cerebral palsy is the most common cause of spasticity, especially in children. Stroke, multiple sclerosis, and brain or spinal trauma are common causes of spasticity in adults.
• Spasticity results from decreased inhibition of muscle groups and facilitation of hypertonia. The decreased inhibitory input into the motor unit results in coactivation of both agonistic and antagonistic muscle groups during volitional movement, and creates an imbalance of excitatory neurotransmitters in the spinal cord, specifically a lack of the inhibitory substance γ-aminobutyric acid (GABA).
• The Ashworth or Modified Ashworth scales are commonly used to classify the severity of spasticity. The Modified Ashworth scale1 ranges from 0 (no increase in muscle tone) to 4, in which affected part(s) are rigid in flexion or extension. Management of spasticity is often achieved with a combination of medications and surgical procedures.
• Intrathecal baclofen (ITB) pumps are most effective in pediatric patients older than 3 years when a response to a baclofen “test dose” is observed. ITB is effective in patients with spasticity in all limbs and in dystonia. Baclofen activates GABAB receptors, and benzodiazepines activate GABAA receptors; both provide compensatory inhibition in the brain and spinal cord.2 Spastic patients with intrathecal or oral balcofen withdrawal present with increased tone, pruritus, and anxiety. Severe withdrawal may include fever, seizures, hallucinations, and rarely death. Treatment of withdrawal includes administration of oral baclofen or intravenous benzodiazepines.
• Botulinum toxin injection is useful in treating spasticity of individual muscle groups or extremities.
• Selective dorsal rhizotomy is most effective in the treatment of spastic diplegia, and is more effective than physical therapy alone in selected patients. Cost-effectiveness studies have shown an advantage of selective dorsal rhizotomy (SDR) over ITB in patients with spastic diplegia due to reduced hospital readmissions for pump replacements and complications.
Spasticity is defined as a “velocity-dependent, increased resistance to passive muscle stretch.”3 It is distinguished from other common hypertonic movement disorders (such as dystonia) by quantifying the amount of abnormal movement: Spasticity is isokinetic (abnormal but not increased movements), while dystonia and other movement disorders are hyperkinetic (abnormal and increased movements).4 It is often associated with muscle tightness, cramping/pain, and fatigue, and its severity may range from mild to disabling. Even though spasticity is considered a diagnosis unto itself, it is always related to an underlying injury to the brain or spinal cord. The treatment of spasticity involves a combination of medical and surgical management.
Causes
Spasticity is typically defined by the causative diagnosis. The most common causes are cerebral palsy (CP),5 traumatic injury (including injuries to either the brain6,7 or spine8), stroke,9 and multiple sclerosis (MS);10 other inherited disorders (e.g., Wilson’s disease, Hallervorden-Spatz disease) are rare causes.
Cerebral Palsy
Cerebral palsy is the most commonly encountered spastic disorder, occurring in 2.4 per 1000 children and accounting for up to 75% of affected patients.11,12 Cerebral palsy is usually described as “a range of nonprogressive syndromes of posture and motor impairment that results from an insult to the developing central nervous system.”13 One or both of the following signs are required for diagnosis, according to the task force for pediatric hypertonia: (1) an increase in resistance to externally applied movement in the same direction as joint movement; and (2) a rapid rise in resistance to externally applied movement above a threshold speed or joint angle.5
Brain or Spinal Trauma
Injuries to either the brain or spinal cord can result in spasticity.6,7,14,15 These injuries include involvement of any of the following: basal ganglia, cerebellum, motor cortices, and spinal cord. It is estimated that up to 25% of patients with moderate or severe trauma develop spasticity symptoms.7
Stroke
Similar to traumatic spasticity, stroke-related spasticity is commonly encountered after lesions to either upper motor neurons or descending inhibitory systems (see later discussion). Approximately 15% to 40% of patients develop clinical spasticity disorders after major stroke.9,16,17
Multiple Sclerosis
Multiple sclerosis is a demyelinating disease often affecting upper motor neuron areas of the brain and spinal cord. A substantial proportion of MS patients develop spastic symptoms; one major study found that 84% of a large database of MS patients suffered from at least minimal spasticity during the disease course, with 34% reporting moderate to severe or disabling symptoms.10
Classification
Many practitioners further classify spasticity within the following anatomical categories, first described for CP:18
Pathophysiology
Understanding the neurophysiology of spasticity requires detailed knowledge of the control and regulation of movement and posture in the central nervous system. In normal conditions, a balance exists between alpha motor neurons in the spinal cord (activated by descending corticospinal tracts) and the descending inhibition created by the basal ganglia, cerebellum, and reticular activating system. These inhibitory systems exert their control on the spinal cord via descending input from the reticulospinal and other tracts, which synapse on the motor neurons or interneurons of the spinal cord. This system maintains baseline muscle tone and posture, as well as facilitates the complementary deactivation of antagonistic muscle groups via Ia and Ib afferents in the dorsal root of the motor unit. For example, flexion of the thigh is facilitated by positive input from the corticospinal tracts in conjunction with deactivation of extensors via descending inhibitory tracts.19,20
Spasticity results from decreased inhibition or facilitation of hypertonia.19,20 The decreased inhibitory input into the motor unit results in coactivation of both agonistic and antagonistic muscle groups during volitional movement, and creates an imbalance of excitatory neurotransmitters in the spinal cord (in particular, a lack of the inhibitory substance γ-aminobutyric acid [GABA]). Chronic spasticity may lead to denervation supersensitivity, and ultimately permanent soft tissue contractures requiring surgical release, especially in the ankle joint and Achilles tendon.21
Diagnosis
A careful history and physical examination is imperative for the diagnosis of both the cause and classification of spasticity. In pediatric patients with CP-related spasticity, a failure to meet motor system and other developmental milestones is often apparent by age 1 to 2 years, and is most severe by age 3.13 Often, symptoms of CP will decline over childhood, with one major study describing 66% of patients with spastic diplegia and 50% with CP of any kind “outgrowing” their symptoms by age 7.22 Older patients with alternative causes of spastic disorder will complain of characteristic muscle tightness, cramping, pain in the affected extremities, and generalized as well as focal fatigue.
Physical examination findings are often linked with the location of injury. Patients with CP often manifest with di- or tetraplegia of varying severity, often with disproportionate involvement of flexors, adductors, and internal rotators.4 In patients with cervical spinal cord injury, both flexor muscles in the arms and extensors in the leg (so-called antigravity muscles) are often affected, but lesions in the thoracic or lumbar spine typically affect only leg extensors. Cortical spasticity from stroke or trauma is often related to the laterality of injury, with contralateral extremities or a single extremity affected.23 Multiple sclerosis patients are heterogeneous in presentation depending on the areas of demyelination, but often present with leg adductor and extensor imbalance.
Resistance to passive muscle stretch correlating with the speed of the stretch is the hallmark of the physical examination in affected muscle groups in patients with spasticity. Several clinical scales have been developed to determine the severity of symptoms. The Ashworth scale grades spasticity as follows: 0 = normal muscle tone; 1 = slight increase in muscle tone, “catch” when limb moved; 2 = more marked increase in muscle tone, but limb easily flexed; 3 = considerable increase in muscle tone; and 4 = limb rigid in flexion or extension.24 The Modified Ashworth scale1 provides further precision, especially in patients with hemiplegia: 0 = no increase in muscle tone; 1 = slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion (ROM); 1+ = slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM; 2 = more marked increase in muscle tone through most of the ROM, but affected part(s) easily moved; 3 = considerable increase in muscle tone, passive movement difficult; 4 = affected part(s) rigid in flexion or extension. Most studies of the diagnosis and treatment of spasticity employ these scales, as well as other indices of motor function and dexterity, to grade the physical findings of individual patients.
Treatment
The treatment of spasticity almost always involves multidimensional or multimodality therapy that may include physiotherapy, medical therapy (oral medications, percutaneous injections), neurosurgical therapy (surgical implantation of intrathecal infusion pumps, permanent selective denervation procedures such as selective dorsal rhizotomy), and orthopedic procedures.25
Medical Therapy
The most common oral medications used in the treatment of spasticity include baclofen, benzodiazepines, tizanidine, dantrolene, gabapentin, and pregabalin. A summary of these medications is found in Table 50.1. They rely on two common mechanisms to reduce spastic symptoms throughout the body, often in combination: (1) compensating for reduced GABA or other inhibitory neurotransmitters in the brain or spinal cord, or (2) reducing the amount of excitatory neurotransmitters through direct inhibition or activation of inhibitory interneurons. The common mechanism of action of these medications also results in a similar side effect profile, including most commonly somnolence, ataxia, and muscle weakness.15,26
Baclofen, the most common oral medication in the treatment of spasticity, binds to GABAB receptors in Rexed laminae I to IV of the spinal cord.27 Activation of these metabotropic receptors leads to increased cell permeability of K+, causing cation efflux and resultant hyperpolarization of the cell membrane.12 This hyperpolarization leads to a reduction in the release of excitatory neurotransmitters (such as glutamate and aspartate), as well as substance P. Important side effects of baclofen include orthostatic hypotension and withdrawal symptoms such as seizures, fever, hallucinations, and rebound spasticity. One drawback of oral baclofen is poor penetration of the blood-brain barrier with cerebrospinal fluid (CSF) drug levels 10-fold lower than serum concentrations. It is common that oral dose escalation leads to side effects before full therapeutic efficacy is reached. Intrathecal delivery of baclofen is highly effective and is the favored modality of drug delivery when patients are unable to tolerate increased doses of oral baclofen (see later discussion).
Benzodiazepines facilitate the binding of existing GABA to the GABAA receptor,28 found in high concentrations in the reticular formation and polysynaptic spinal tracts. In contrast to the GABAB receptor, the GABAA receptor is ionotropic. Its activation results in the increase in cell permeability of Cl−, causing anion influx and hyperpolarization. This hyperpolarization increases presynaptic inhibition and reduces monosynaptic and polysynaptic reflexes throughout the spinal cord. A variety of different benzodiazepine compounds are available including oral, intravenous, and rectal routes of administration, with different durations due to the rate of metabolism. The most important (and often dose-limiting) side effect of this medication class is sedation. Rapid reversal of benzodiazepine toxicity can be achieved with the administration of flumazenil. Additionally, habituation and tolerance may develop, and prolonged use is associated with addiction.
Tizanidine and related medications (such as clonidine) provide an alternative mechanism to the preceding medications via the reduction of excitatory neurotransmitters. Tizanidine is an α2-adrenergic agonist that acts throughout the central nervous system (CNS) to reduce excitatory amino acid release from spinal interneurons.29,30 Tizanidine is particularly effective in adult spasticity from spinal disease or multiple sclerosis.31 Important adverse effects include nausea and vomiting, hypotension (clonidine), and sedation (tizanidine).
Dantrolene is unique among oral medications in the treatment of spasticity due to its effects on skeletal muscle. Dantrolene binds to skeletal muscle ryanodine receptors, preventing the release of calcium into the cytosol from its sequestration in the sarcoplasmic reticulum during motor unit activation. Thus, its action is defined as an “excitation-contraction decoupler” in skeletal muscle.32–34 It is also used in the emergency treatment of malignant hyperthermia and neuroleptic malignant syndrome. Although it is less sedative than other antispasticity agents, its effect on skeletal muscle may result in significant weakness of both volitional and (in severe cases) respiratory muscles.35 Another important side effect is the approximately 1.8% risk of hepatotoxicity, which is increased with coadministration of other agents (i.e., valproate, tizanidine).36 Some clinicians recommend routine surveillance of liver function tests in patients taking dantrolene.
Two newer medications, gabapentin and pregabalin, were originally developed as anticonvulsants but have found utility in the management of spasm and other disorders such as neuropathic pain. Both medications work in a similar fashion as GABA analogs that bind to the α2δ-subunit of voltage-gated calcium channels, inhibiting calcium influx. This mechanism reduces the concentration of excitatory neurotransmitters such as glutamate and aspartate in the CNS.37,38 Gabapentin is a well-established therapy for the treatment of spasticity of spinal origin39,40 and in the management of MS.41 Pregabalin is emerging as an alternative agent with similar properties.42 Both medications are well tolerated with minimal side effects, such as sedation, even after significant titration. Gabapentin has been associated with myopathy, especially in patients with renal failure, as it is renally excreted,43 and pregabalin is somewhat more sedating and may cause dose-limiting ataxia.44
Recent advances have been made in the treatment of spasticity of focal hypertonia using percutaneous injection of botulinum neurotoxin (BoNT), often in conjunction with the general effects of oral medications or surgical treatments,45 or as monotherapy for isolated limb spasticity.46–48 BoNT is derived from Clostridium botulinum exotoxin, and acts by cleaving polypeptides required for exocytosis, such as synaptosomal-associated protein (SNAP)-25, vesicle-associated membrane protein (VAMP), and syntaxin, within the cytosol of motor neurons.49,50 Cleavage of these peptides prevents the release of acetylcholine into the neuromuscular junction, relieving focal muscle hypertonia. Additional indirect beneficial effects on muscle tone, such as the suppression of other excitatory neurotransmitters and reduced autonomic activity, may occur. A single treatment remains effective for 2 to 4 months at a time,26 providing a useful adjunct treatment for persistent symptoms involving a single muscle group, such as the calf, upper extremity, leg adductor, or cervical musculature.
There are three main adverse effects of BoNT injection.45 First, inhibition of acetylcholine release may spread to neighboring nerve endings, including respiratory muscles. This has led to a black-box warning for all BoNT formulations regarding the risk of potentially life-threatening respiratory failure. Second, sustained or repeated administration to a single anatomical area may produce effects similar to denervation, such as muscle atrophy. Third, the patient’s host response may generate antibodies to the BoNT protein, leading to immunoresistance that prevents its association with neuronal membranes.51 These antibodies may lead to a habituation to BoNT injections over time.
Surgical Therapy
Intrathecal delivery of baclofen (ITB) has been shown to be safe, efficacious, and cost effective in the long-term treatment of both spasticity and dystonia.52–55 Patients report subjective improvement in spasticity symptoms in 60% to 85% of cases, and improvement in motor function in 20% to 33%; Ashworth scores are typically reduced by 1 to 1.8 points.56–60 Compared to oral baclofen administration, ITB delivers approximately four times the concentration of medication to the spinal cord with minimal systemic side effects, as plasma levels of intrathecal baclofen are undetectable.61,62 Most neurosurgeons advocate a screening protocol prior to pump implantation to predict clinical benefit.63 Baclofen is administered via lumbar puncture at a dose of 25 µg for children less than 40 lb and 50 µg for children or adults more than 40 lb. Postinfusion evaluations of spasticity are performed every 2 hours for 6 to 8 hours after injection, and patients with an improvement of one point or greater in the Ashworth score are considered candidates for pump implantation. A temporary intrathecal catheter connected to an external pump may be used for more prolonged evaluation, especially in adults or patients with dystonia in which a higher test dose is required.56
Pump implantation techniques have been thoroughly documented in the neurosurgery literature.64,65 The patient is placed in the lateral decubitus position to give clear access to the lumbar spine and abdomen. Meticulous sterile prep and draping are performed, and preoperative antibiotics administered. A 2-cm midline incision is made at the L4-L5 interspace, through the lumbosacral fascia. The thecal sac is accessed with a large-bore Tuohy needle, and the catheter is placed through the needle and advanced cephalad under fluoroscopy. Final catheter placement is based on the patient’s underlying disorder: for patients with spastic paraparesis, T10-T11; spastic quadraparesis, C6-T2; generalized secondary dystonia, C1-C4.64 The needle is removed, and the catheter is secured to the lumbosacral fascia with care to avoid kinking.
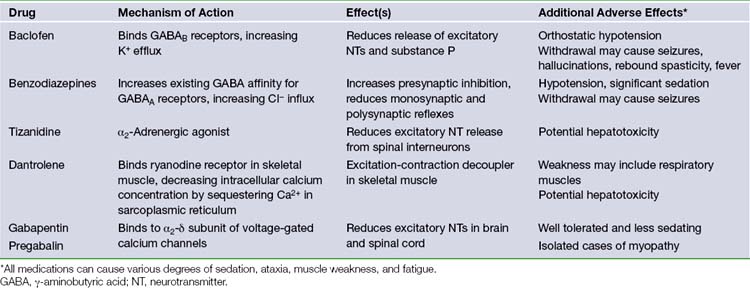
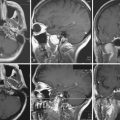
Following intrathecal placement, a subfascial pocket is made in the abdominal wall via an oblique subcostal incision. A pump is chosen from the available sizes based on patient weight and drug reservoir volume; proper selection is essential to prevent pressure on the wound. The pump reservoir is filled with baclofen, and sometimes placed in a Gore-Tex pouch before insertion into the subfascial pocket, to prevent pump rotation or malposition. A tunneling device is used to pass the catheter from the lumbar spine to the abdominal pocket, where it is connected to the pump. Careful irrigation and multilayered wound closure are performed at both surgical sites. Postoperative radiographs assess the position of the pump in the abdomen and the catheter in the spinal canal (Fig. 50.1A and B). The typical initial ITB dose is 100 µg per day, and may be titrated in the postoperative period to reach maximum clinical benefit. The pump reservoir is refilled by percutaneous injection, at an interval determined by the patient’s individual concentration and dosage needs. Pump battery life is usually 5 to 7 years.
Side Effects and Complications of Intrathecal Baclofen Therapy
The most common presenting sign of pump malfunction is baclofen withdrawal.2 Patients present with increased tone, pruritus, and anxiety. Severe withdrawal effects are similar to those with oral baclofen and may include fever, seizures, hallucinations, and rarely death.66,67 Treatment of withdrawal includes oral baclofen, intravenous benzodiazepines, or immediate pump revision. In contrast, ITB overdose results in hypotonia, sedation, cardiorespiratory depression, and coma; the most common cause of ITB overdose is improper pump programming. Treatment for overdose includes supportive care and resetting or deactivating the pump.
The technique for pump implantation is optimized to reduce the most common complications such as infection (3-15% overall; approximately 1% per year), catheter obstruction or disconnection (2-16%), and CSF leak (5-9%).68–71 Patients must be of adequate weight to permit subcutaneous abdominal pump implantation, usually age older than 3 years. Immediate, transient postoperative urinary retention is common for up to 72 hours after pump implantation, and resolves without intervention.56
The most common organism found in infected pump systems is Staphylococcus aureus; most infections occur within 6 to 8 weeks of implantation.68–71 Treatment involves removal of the pump and catheter, followed by long-term antibiotics and oral baclofen supplementation. Reimplantation may be considered at 8 to 12 weeks, and may occur in the same pump location.
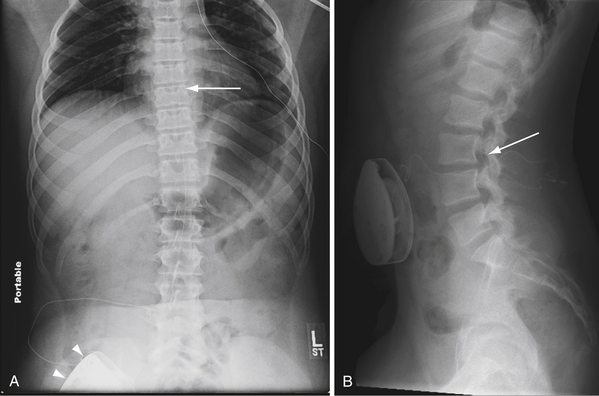
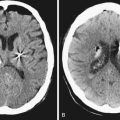
Catheter failure is usually the result of disconnection or retraction.56,68–70 Disconnection is the most common catheter-related complication, typically at the point of connection to the pump. Catheter retraction is caused by movement of the pump within its pocket, or by normal patient growth. The intrathecal end of the catheter may back out of the thecal sac and coil in the subcutaneous space (Fig. 50.2), or retract completely into the pump pocket. These failures occur early (within 3 months of implantation) and require surgical revision. For cases in which catheter malfunction is unclear, percutaneous injection of radiopaque contrast agent into the pump’s side port, followed by radiography or computed tomography (CT), can aid in the diagnosis.
CSF leaks occur around the intrathecal catheter entry site, and are more common after revision surgery or when multiple attempts are made to access the thecal sac during implantation. Treatment options for CSF leak include bed rest, pressure dressings, lumbar drain placement below the ITB catheter, epidural blood patch, or open revision. It is important to assess the patient for untreated hydrocephalus in the setting of persistent CSF leak.56
Selective Denervation Procedures
Foerster first reported transection of the posterior lumbar and sacral nerve roots as a treatment for spasticity in 1913;72 the procedure has since evolved to selective dorsal rhizotomy (SDR), the most common neurosurgical denervation procedure for spasticity. The lack of descending inhibition in spastic patients causes increased activation of antagonistic muscle groups via Ia and Ib reflexive afferents. By sectioning a portion of each dorsal root, SDR decreases the output of these Ia and Ib reflexive afferents, reducing spasticity.
Careful patient selection is necessary to determine which patients will derive maximum benefit from SDR. The optimal candidates are children older than 2 years of age with spastic diplegia, though some benefit is also seen in patients with spastic quadriplegia. Several randomized trials have demonstrated an advantage of SDR with physical therapy over physical therapy alone in appropriate patients. Ashworth scores may improve by 0.5 to 1.5 with SDR.73–75 Contraindications to SDR include severe muscle weakness; orthopedic deformity; and increased muscle tone secondary to hydrocephalus, intracranial infection, or traumatic brain injury.76
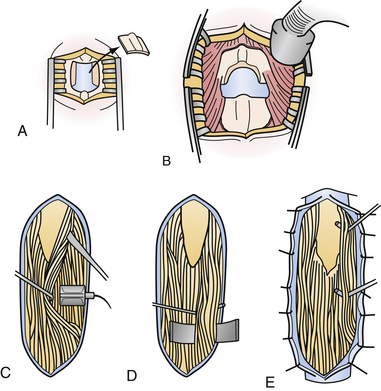
The SDR procedure has been refined over several decades, and requires coordination between neuroanesthesia, neurophysiology, and neurosurgery personnel.4,76 Once intubated, the patient is placed prone with the bed in a slight Trendelenburg position to reduce CSF loss. Neurophysiological monitoring is established and baseline measurements are taken. The level of the conus medullaris, having been identified on preoperative magnetic resonance imaging (MRI), is identified with interoperative fluoroscopy, typically between T12 and L3. A midline incision is made and a single-level laminectomy is performed (Fig. 50.3). The conus medullaris and cauda equina are usually identified with interoperative ultrasound prior to opening the dura.
Using the operative microscope, the dura is opened and the dorsal and ventral nerve roots are separated by following them back to their origins on the conus medullaris. A silastic sheet is inserted between the dorsal and ventral roots to prevent comingling of the roots during the procedure. First, each dorsal root is stimulated using a curved electromyographic (EMG) stimulation probe at increasing intensity until a reflex response is seen. Each dorsal root is then bluntly subdivided into three to five smaller rootlet fascicles, and the individual EMG reflex threshold of each rootlet is recorded. Once the reflex threshold is known, a tetanic stimulus is applied and the EMG response is graded on a scale from 0 to 4 (Table 50.2).76 A score of 0 indicates an unsustained or single discharge, and scores of 3 and 4 indicate sustained discharge with segmental spread and discharge from contralateral muscle, respectively.
TABLE 50.2 Grading Scale for EMG Response in Selective Dorsal Rhizotomy
| Grade | EMG Response |
|---|---|
| 0 | Unsustained or single discharge to a train of stimuli |
| 1 | Sustained discharges from muscles innervated through the segment simulated in the ipsilateral lower extremity |
| 2 | Sustained discharges from muscles innervated through the stimulated and immediately adjacent segments |
| 3 | Sustained discharges from segmentally innervated muscles and muscles innervated through segments distant from the one stimulated |
| 4 | Sustained discharges from contralateral muscles with or without sustained discharges from the ipsilateral muscles |
EMG, electromyography.
Adapted from Park TS, Johnston JM. Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note. Neurosurg Focus 2006;21:e7.
The rootlets at each spinal level are sectioned according to the following criteria: All rootlets with a 0 response are left intact, all with a 4 response are cut, and a number of rootlets with intermediate responses (grades 2 or 3) are also cut; the total number of rootlets sectioned is approximately 60%. To prevent complete sensory loss, at least one rootlet at each level is left unsectioned regardless of EMG response. The procedure is performed bilaterally on each dorsal root from L2-S2. Some authors section 50% of the L1 dorsal root, regardless of EMG response, to obtain reduced spasticity in the hip flexors.
Complications of Selective Dorsal Rhizotomy
Complications of SDR include paraplegia, sensory loss, hypesthesia, bowel or bladder incontinence, CSF leak, and infection.73–75,77,78 The most common complications include self-limiting urinary incontinence (4.4%) and dysesthesia/hypesthesia (8.9%) in the perioperative period. Permanent sensory changes and neurogenic bowel/bladder complications are found in 4% and 5.1% of patients, respectively.77 Cost-effectiveness studies have shown an advantage of SDR over ITB in patients with spastic diplegia owing to reduced hospital readmissions for pump replacements and complications.79
Albright A.L., Barron W.B., Fasick M.P., et al. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA. 1993;270:2475-2477.
Albright A.L., Turner M., Pattisapu J.V. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104:233-239.
Gooch J.L., Oberg W.A., Grams B., et al. Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg. 2003;39:1-6.
Park T.S., Johnston J.M. Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note. Neurosurg Focus. 2006;21:e7.
Steinbok P., Daneshvar H., Evans D., Kestle J.R. Cost analysis of continuous intrathecal baclofen versus selective functional posterior rhizotomy in the treatment of spastic quadriplegia associated with cerebral palsy. Pediatr Neurosurg. 1995;22:255-264. discussion 265
Please go to expertconsult.com to view the complete list of references.
1. Bohannon R.W., Smith M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206-207.
2. Zuckerbraun N.S., Ferson S.S., Albright A.L., Vogeley E. Intrathecal baclofen withdrawal: emergent recognition and management. Pediatr Emerg Care. 2004;20:759-764.
3. Sanger T.D., Delgado M.R., Gaebler-Spira D., et al. Disorders TFoCM: classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89-e97.
4. Albright A. Spasticity and movement disorders. In: Albright A., Pollack I., Adelson P., editors. Principles and Practice of Pediatric Neurosurgery. 2nd ed. New York: Thieme; 2007:1121-1140.
5. Bax M., Goldstein M., Rosenbaum P., et al. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571-576.
6. Elovic E.P., Simone L.K., Zafonte R. Outcome assessment for spasticity management in the patient with traumatic brain injury: the state of the art. J Head Trauma Rehab. 2004;19:155-177.
7. Zafonte R., Elovic E.P., Lombard L. Acute care management of post-TBI spasticity. J Head Trauma Rehab. 2004;19:89-100.
8. Taricco M., Adone R., Pagliacci C., Telaro E. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000:CD001131.
9. Sommerfeld D.K., Eek E.U., Svensson A.K., et al. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134-139.
10. Rizzo M.A., Hadjimichael O.C., Preiningerova J., Vollmer T.L. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004;10:589-595.
11. Boyle C.A., Yeargin-Allsopp M., Doernberg N.S., et al. Prevalence of selected developmental disabilities in children 3-10 years of age: the Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1991. MMWR CDC Surveill Summ. 1996;45:1-14.
12. Krach L.E. Pharmacotherapy of spasticity: oral medications and intrathecal baclofen. J Child Neurol. 2001;16:31-36.
13. Koman L.A., Smith B.P., Shilt J.S. Cerebral palsy. Lancet. 2004;363:1619-1631.
14. Adams M.M., Hicks A.L. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577-586.
15. Gracies J.M., Nance P., Elovic E., et al. Traditional pharmacological treatments for spasticity. Part II: general and regional treatments. Muscle Nerve Suppl. 1997;6:S92-S120.
16. Lundström E., Terént A., Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008;15:533-539.
17. Watkins C.L., Leathley M.J., Gregson J.M., et al. Prevalence of spasticity post stroke. Clin Rehab. 2002;16:515-522.
18. Horstmann H., Bleck E. Orthopaedic Management in Cerebral Palsy, 2nd ed, London: Mac Keith Press, 2008.
19. Burke D., Knowles L., Andrews C., Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain. 1972;95:31-48.
20. Dietz V., Quintern J., Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431-449.
21. Fergusson D., Hutton B., Drodge A. The epidemiology of major joint contractures: a systematic review of the literature. Clin Orthop Relat Res. 2007;456:22-29.
22. Nelson K.B., Ellenberg J.H. Children who “outgrew” cerebral palsy. Pediatrics. 1982;69:529-536.
23. Katz R.T. Management of spasticity. Am J Phys Med Rehab. 1988;67:108-116.
24. Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540-542.
25. Steinbok P. Selection of treatment modalities in children with spastic cerebral palsy. Neurosurg Focus. 2006;21:e4.
26. Verrotti A., Greco R., Spalice A., et al. Pharmacotherapy of spasticity in children with cerebral palsy. Pediatr Neurol. 2006;34:1-6.
27. Yang K., Wang D., Li Y.Q. Distribution and depression of the GABA(B) receptor in the spinal dorsal horn of adult rat. Brain Res Bull. 2001;55:479-485.
28. Davidoff R.A. Antispasticity drugs: mechanisms of action. Ann Neurol. 1985;17:107-116.
29. Coward D.M. Tizanidine: neuropharmacology and mechanism of action. Neurology. 1994;44:S6-S10. discussion S10-S11
30. Kamen L., Henney H.R., Runyan J.D. A practical overview of tizanidine use for spasticity secondary to multiple sclerosis, stroke, and spinal cord injury. Curr Med Res Opin. 2008;24:425-439.
31. Nance P.W. A comparison of clonidine, cyproheptadine and baclofen in spastic spinal cord injured patients. J Am Paraplegia Soc. 1994;17:150-156.
32. Ketel W.B., Kolb M.E. Long-term treatment with dantrolene sodium of stroke patients with spasticity limiting the return of function. Curr Med Res Opin. 1984;9:161-169.
33. Krause T., Gerbershagen M.U., Fiege M., et al. Dantrolene—a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364-373.
34. Pinder R.M., Brogden R.N., Speight T.M., Avery G.S. Dantrolene sodium: a review of its pharmacological properties and therapeutic efficacy in spasticity. Drugs. 1977;13:3-23.
35. Nance P.W., Young R.R. Antispasticity medications. Phys Med Rehabil Clin North Am. 1999;10:337-355.
36. Chan C.H. Dantrolene sodium and hepatic injury. Neurology. 1990;40:1427-1432.
37. Bryans J.S., Wustrow D.J. 3-Substituted GABA analogs with central nervous system activity: a review. Med Res Rev. 1999;19:149-177.
38. Fink K., Dooley D.J., Meder W.P., et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229-236.
39. Gruenthal M., Mueller M., Olson W.L., et al. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord. 1997;35:686-689.
40. Priebe M.M., Sherwood A.M., Graves D.E., et al. Effectiveness of gabapentin in controlling spasticity: a quantitative study. Spinal Cord. 1997;35:171-175.
41. Cutter N.C., Scott D.D., Johnson J.C., Whiteneck G. Gabapentin effect on spasticity in multiple sclerosis: a placebo-controlled, randomized trial. Arch Phys Med Rehab. 2000;81:164-169.
42. Bradley L.J., Kirker S.G. Pregabalin in the treatment of spasticity: a retrospective case series. Disabil Rehab. 2008;30:1230-1232.
43. Bookwalter T., Gitlin M. Gabapentin-induced neurologic toxicities. Pharmacotherapy. 2005;25:1817-1819.
44. Zaccara G., Gangemi P., Perucca P., Specchio L. The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52(4):826-836.
45. Simpson D.M., Gracies J.M., Graham H.K., et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1691-1698.
46. Lukban M.B., Rosales R.L., Dressler D. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: a summary of evidence. J Neural Transm. 2009;116:319-331.
47. Richardson D., Sheean G., Werring D., et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychiatry. 2000;69:499-506.
48. Simpson D.M., Gracies J.M., Yablon S.A., et al. Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. J Neurol Neurosurg Psychiatry. 2009;80:380-385.
49. Humeau Y., Doussau F., Grant N.J., Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427-446.
50. Simpson L.L. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167-193.
51. Dressler D., Hallett M. Immunological aspects of botox, dysport and myobloc/neurobloc. Eur J Neurol. 2006;13(Suppl 1):11-15.
52. de Lissovoy G., Matza L.S., Green H., et al. Cost-effectiveness of intrathecal baclofen therapy for the treatment of severe spasticity associated with cerebral palsy. J Child Neurol. 2007;22:49-59.
53. Hoving M.A., Evers S.M., Ament A.J., et al. Spasticity DSGoC: intrathecal baclofen therapy in children with intractable spastic cerebral palsy: a cost-effectiveness analysis. Dev Med Child Neurol. 2008;50:450-455.
54. Nance P., Schryvers O., Schmidt B., et al. Intrathecal baclofen therapy for adults with spinal spasticity: therapeutic efficacy and effect on hospital admissions. Can J Neurol Sci. 1995;22:22-29.
55. Russman B.S. Continuous intrathecal baclofen infusion for intractable spastic cerebral palsy—is it worth it? Nat Clin Pract Neurol. 2008;4:476-477.
56. Albright A.L., Ferson S.S. Intrathecal baclofen therapy in children. Neurosurg Focus. 2006;21:e3.
57. Campbell S.K., Almeida G.L., Penn R.D., Corcos D.M. The effects of intrathecally administered baclofen on function in patients with spasticity. Phys Ther. 1995;75:352-362.
58. Campbell W.M., Ferrel A., McLaughlin J.F., et al. Long-term safety and efficacy of continuous intrathecal baclofen. Dev Med Child Neurol. 2002;44:660-665.
59. Gilmartin R., Bruce D., Storrs B.B., et al. Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15:71-77.
60. Hoving M.A., van Raak E.P., Spincemaille G.H., et al. Efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy: a randomised controlled trial. Eur J Paediatr Neurol. 2009;13:240-246.
61. Albright A.L., Shultz B.L. Plasma baclofen levels in children receiving continuous intrathecal baclofen infusion. J Child Neurol. 1999;14:408-409.
62. Knutsson E., Lindblom U., Mårtensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci. 1974;23:473-484.
63. Albright A.L., Barron W.B., Fasick M.P., et al. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA. 1993;270:2475-2477.
64. Albright A.L., Turner M., Pattisapu J.V. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104:233-239.
65. Hsieh J.C., Penn R.D. Intrathecal baclofen in the treatment of adult spasticity. Neurosurg Focus. 2006;21:e5.
66. Alden T.D., Lytle R.A., Park T.S., et al. Intrathecal baclofen withdrawal: a case report and review of the literature. Childs Nerv Syst. 2002;18:522-525.
67. Green L.B., Nelson V.S. Death after acute withdrawal of intrathecal baclofen: case report and literature review. Arch Phys Med Rehabil. 1999;80:1600-1604.
68. Borowski A., Littleton A.G., Borkhuu B., et al. Complications of intrathecal baclofen pump therapy in pediatric patients. J Pediatr Orthop. 2010;30:76-81.
69. Fjelstad A.B., Hommelstad J., Sorteberg A. Infections related to intrathecal baclofen therapy in children and adults: frequency and risk factors. J Neurosurg Pediatr. 2009;4:487-493.
70. Gooch J.L., Oberg W.A., Grams B., et al. Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg. 2003;39:1-6.
71. Levin A.B., Sperling K.B. Complications associated with infusion pumps implanted for spasticity. Stereotact Funct Neurosurg. 1995;65:147-151.
72. Foerster O. On the indications and results of the excision of posterior spinal nerve roots in men. Surg Gynecol Obstet. 1913;5:463-474.
73. McLaughlin J.F., Bjornson K.F., Astley S.J., et al. Selective dorsal rhizotomy: efficacy and safety in an investigator-masked randomized clinical trial. Dev Med Child Neurol. 1998;40:220-232.
74. Nordmark E., Josenby A.L., Lagergren J., et al. Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr. 2008;8:54.
75. Steinbok P. Selective dorsal rhizotomy for spastic cerebral palsy: a review. Childs Nerv Syst. 2007;23:981-990.
76. Park T.S., Johnston J.M. Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note. Neurosurg Focus. 2006;21:e7.
77. Steinbok P., Schrag C. Complications after selective posterior rhizotomy for spasticity in children with cerebral palsy. Pediatr Neurosurg. 1998;28:300-313.
78. Trost J.P., Schwartz M.H., Krach L.E., et al. Comprehensive short-term outcome assessment of selective dorsal rhizotomy. Dev Med Child Neurol. 2008;50:765-771.
79. Steinbok P., Daneshvar H., Evans D., Kestle J.R. Cost analysis of continuous intrathecal baclofen versus selective functional posterior rhizotomy in the treatment of spastic quadriplegia associated with cerebral palsy. Pediatr Neurosurg. 1995;22:255-264. discussion 265