Chapter 177 Somatosensory-Evoked Potential for Spine Surgery
Because degenerative spinal conditions and deformities are relatively prevalent, surgical procedures for the spine are common. Furthermore, the evolution and understanding of spinal mechanics and physiology have allowed the introduction of many newer spinal surgical techniques. Nevertheless, a small proportion, less than 0.5%, of patients may develop a persistent neurologic deficit immediately after surgery. Careful surgical techniques, including stabilization of the spine during surgery, have helped reduce this complication somewhat. However, it is apparent that a neurologic injury related to such an intervention can be disabling. For this reason, the monitoring of somatosensory-evoked potentials (SSEPs) from peripheral nerve stimulation (posterior tiblial, peroneal, or median nerves) during spinal column or spinal cord surgery is common.1–31
The spinal cord and nerve roots are at risk during a variety of surgical procedures performed on the spinal cord and surrounding structures. The risk varies with the underlying disease, as well as the type and location of surgery.32–35 Patients with intramedullary tumors, syringomyelia, spinal arteriovenous malformation, thoracoabdominal aneurysms, and any other disorder associated with a baseline neurologic deficit are at greatest risk. The frequency of neurologic injury following scoliosis surgery, correction of congenital spinal deformities, and decompression (with and without spinal fusion) is low, but when damage to the spinal cord occurs, the resulting deficits are often severe, permanent, and devastating.35–37 The detection of significant changes in the monitored-evoked potentials (MEPs) can indicate damage to the motor pathway and may permit appropriate intervention to prevent spinal cord damage.
In the 1970s, SSEP monitoring was developed as an alternative to the wake-up test. SSEP recordings provided the means of monitoring spinal cord function continuously without interfering with surgery or producing additional risk. A large body of data, including clinical experience in thousands of patients, has provided significant information regarding the utility and limitation of SSEP monitoring during spinal surgery, but no prospective controlled trial of SSEP monitoring has ever been published.1,2,5,7,11,14,15,36,38
More recently, several studies from different institutes around the world have proven that a single method of potential recording usually carries a high incidence of misdiagnosing an injury. Based on this, the growing tendency has been to establish a multimodal intraoperative monitoring (MIOM) system that usually combines SSEPs with MEPs and sometimes other varieties. The use of MIOM has documented benefits on specificity and sensitivity as well as for clinical experience and outcome measurements during different spinal surgical procedures.39
Neuroanatomic and Functional Basis
SSEP monitoring evaluates the integrity of the dorsal column. Consequently, if the dorsal columns are preserved, injury to other important pathways could occur without a change in the SSEP.16,40,41
In addition, SSEPs recorded from upper-extremity stimulation do not reflect lower-extremity abnormalities. Posterior tiblial SSEP monitoring must also be recorded if there is concern for damage during surgery to the spinal cord below the midcervical level. Stimulation at the level of the medial malleolus generates afferent volleys that are transmitted by sensory fibers to the dorsal horn at the conus medullaris and then carried by the dorsal tract (fasciculus gracilis) to the dorsal medullary nucleus (nucleus gracilis). Cortical conduction is then achieved via the medial lemniscus and thalamus.
Methods of Monitoring
Noninvasive Techniques
The noninvasive techniques involve the monitoring of potentials generated by spinal, subcortical (brainstem), or cortical pathways from the skin surface or from subdermal needle electrodes. In all the noninvasive studies, peripheral nerves in the upper extremity (median or ulnar nerve) or lower extremity (posterior tiblial or peroneal nerve) are stimulated. Recordings outside the operating field (noninvasive technique) are by far the simplest and can be performed without disturbing the surgeon’s attention from the surgical field. Recordings are most commonly made from standard scalp derivations, usually Cz-Fz (International 10-20 System)42 with leg stimulation, and C3 (C4)-Fz with arm stimulation. Other reference electrodes, such as the ears, are also used. Most of the early studies of surgical monitoring used peripheral stimulation with scalp recording, which generally gives a well-defined, although unstable, response.
Invasive Techniques
A number of methods of recording in the operating field have been developed to facilitate recording closer to the neural tissue.4,12,17,25,43–45 These methods include subarachnoid, epidural, spinous process, and intraspinous ligament recordings. The spinal cord recording (not cortical potentials) facilitates direct evaluation of segmental changes that occur above and below the operative site. Dinner et al.4 assessed 70 of 100 scoliotic patients who were monitored with interspinous electrodes and confirmed that the spinal-evoked potentials were both reliable and reproducible, whereas the wires posed little risk to neurologic function. Lüders et al.17 successfully used spinal-evoked responses during 40 spinal procedures, 32 for scoliosis and Harrington rod placement and 8 for syrinx drainage and resection of tumors and arteriovenous malformations.
Monitoring Techniques
SSEPs are recorded from the cortex with only two of the many electrodes composing the cortical array used by the International 10-20 System.42 One electrode is placed in the midsagittal plane (Cz1), and the second is applied more ventrally in the midline. A third ground is always added (Fz). Placing an additional cervical needle electrode (at C2) helps confirm whether cortical changes reflect true spinal cord changes, as opposed to local cortical variations that may occur in response to alterations in anesthetic administration. Such needle electrodes may also be placed over a lumbar spinous process (L5) to differentiate between similar alterations. SSEP skin and surface electrodes are noninvasive and are applied far away from the operative field, and monitoring may begin before induction and continue through closing.
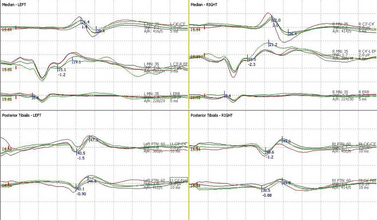
The large mixed peripheral nerves (median, ulnar, peroneal, or posterior tiblial nerves) receive short 200-msec pulses at rates of 3 to 5 per second. The larger-diameter peripheral sensory A alpha and A beta fast-conducting fibers are stimulated with intensities set at two or three times the motor threshold, sufficient to produce a motor twitch.46 Two hundred recordings are then averaged and passed through band-pass filters of 30 Hz to 3 kHz to improve signal-to-noise ratio. Alternate stimulation of the right and left sides allows both waveforms to be simultaneously monitored with a split-screen array. This requires 50 seconds (means of 200 recordings) for two extremities and 100 seconds for all four extremities. Findings may be reproduced by repeating stimulation of one or both sides, enabling the surgeon to be alerted to significant changes in any of the four extremities within minutes (Fig. 177-1).
MEPs can be obtained reliably and are useful for monitoring the motor pathway function. They are elicited by either electrical or magnetic stimulation of the cortex or the spinal cord itself. Recordings are obtained as neurogenic potentials in the distal spinal cord or peripheral nerves. They also can be recorded as myogenic potentials from the innervated muscle. Contraindications for MEPs include history of seizures, past surgical skull defects, and/or metal implants in the head.47 Electrical stimulation also can be applied directly over the cord with distal neurogenic potentials recordings if a surgical decompression has been performed.
Anesthesia
Anesthesia reduces the amplitude and increases the latency of cortical SSEP recordings. This is especially true in the presence of disease and in children and adolescents. Because the reduction of amplitude is directly related to depth of anesthesia, the level of anesthetic agents should be kept as light as possible. Anesthetic effect varies with the agent used, with halogenated anesthetics producing the greatest effects, followed by moderate changes with IV barbiturates and nitrous oxide, and the least with narcotics and benzodiazepines. Etomidate and ketamine have been shown to enhance the amplitude of the cortical SSEP potentials. All volatile anesthetics, as well as nitrous oxide, produce a dose-dependent reduction in MEP signal amplitude. Because the signal amplitudes of MEP recordings are already quite small, the effect of these inhalational agents can limit the practitioner’s ability to detect significant changes intraoperatively. These drugs can be used occasionally to record cortical potentials during surgery when responses are absent with standard anesthetics. Alterations in blood pressure can also reduce the amplitude of the evoked response, especially with mean blood pressures lower than 70 mm Hg. Similar effects can be found with hypothermia, which increases latency and decreases conduction velocity.
Interpretation
Animal and human studies have shown that SSEP changes can occur when there is injury to adjacent motor pathways at the spinal and brainstem levels.3,35,37,40 Assuming that appropriate stimulation and recording can be achieved, a major issue to be resolved is what constitutes a significant change and how reliably this can be detected. To further complicate matters, the primary disease often produces an SSEP abnormality that can be recognized in baseline recordings.4,12,17,25,43–45 Recording methods may have to be modified. Despite averaging, multiple sources of artifacts may result in unstable potentials that are different for each patient. It is essential to determine the limits of SSEP amplitude and latency variation with repeated samples during the early part of the surgery. Significance criteria can be determined that are beyond the baseline limits of variability. In patients with high-amplitude, well-defined potentials at peripheral, spinal, and cortical levels, a reproducible drop in amplitude of 50% or greater or an increase in latency of 2 msec or greater (or >5–10% prolongation of latency), or both, is considered significant. Equally, a complete loss of waveform not explainable by technical malfunction is considered significant.
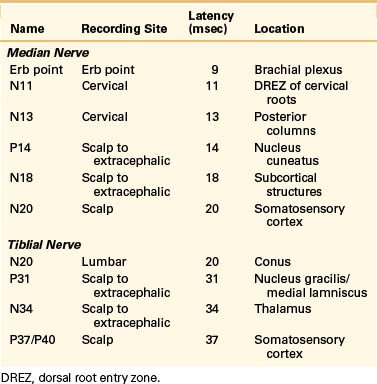
In the upper extremities, although the entire waveform from N10 (brachial plexus), N12a/N12b (segmental ascending dorsal column), N13a/N13b (dorsal horn/cuneate nucleus), and P14 (medial lemniscus) is recorded, the final cortical median N20 proves to be the most clinically relevant. Similarly, N22/P22 (dorsal horn T10 to L1), N29 (cervical gracile nucleus), P31 (medial lemniscus), and N34 (thalamus/brainstem) are noted, but the P38/N38 and P40 constitute the most used cortical potentials.
Cortical responses noted after tiblial nerve stimulation follow a similar but more prolonged pattern. The mean latency for the posterior tiblial response P40 is typically 38.8 msec. Multiple initial negative peaks may also be visualized with these responses. These varied responses reflect the different anatomic locations along the somatosensory pathway of the posterior tiblial nerve to the cauda equina, lumbosacral spinal cord, gracile nucleus, thalamus, and cortex (Table 177-1).
Resuscitative Measures
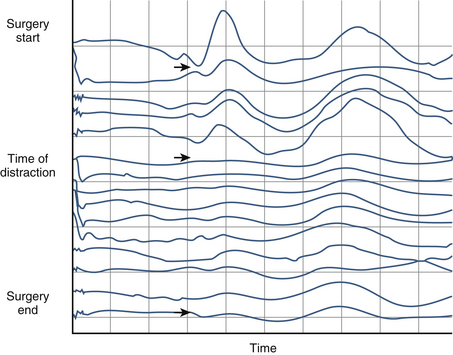
Surgical maneuvers may require cessation of surgical manipulation, release or elimination of distraction, removal of excessively large grafts resulting in overdistraction, and removal of instrumentation. An example of cortical SSEP changes during spine surgery for scoliosis in shown in Figure 177-2. For patients with ossification of the posterior longitudinal ligament, changes in SSEP responses are common. For these individuals, distraction is therefore avoided until the pathologic abnormality has been fully excised.
Summary
SSEP monitoring is a relatively new form of monitoring compared with other techniques such as ECG or even blood pressure monitoring. Despite its relatively recent introduction, SSEP monitoring has already contributed much to the ability of the spine surgeon to deal with difficult problems that only a few years ago were considered too risky to even consider. SSEP plays a valuable role during spine surgery by decreasing patient risk and improving outcome. It is essential that spine surgeons continue to improve intraoperative monitoring techniques to provide optimal care for their patients. Further information regarding this topic and future updates can be obtained from the web page of the recently created International Society of Intraoperative Neurophysiology (www.neurophysiology.org).
Allison T. Recovery functions of somatosensory evoked responses in man. Electroencephalogr Clin Neurophysiol. 1962;14:331.
Dinner D.S., Lüders H., Lesser R.P., et al. Intraoperative spinal somatosensory evoked potentials monitoring. J Neurosurg. 1986;65:807.
Giblin D.R. Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system. Ann NY Acad Sci. 1964;112:93.
Grundy B.L. Monitoring of sensory evoked potentials during neurosurgical operations: methods and applications. Neurosurgery. 1982;11:556.
Macon J.B., Poletti C.E., Sweet W.H., et al. Conducted somatosensory evoked potentials during spinal surgery. Part 2: Clinical applications. J Neurosurg. 1982;57:354.
Sutter M., Deletis V., Dvorak J., et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007;16(Suppl 2):S232.
1. Allen A., Starr A., Nudleman K. Assessment of sensory function in the operating room utilizing cerebral evoked potentials: a study of fifty-six surgically anesthetized patients. Clin Neurosurg. 1981;28:457.
2. Brown J.C., Axelgaard J., Rowe D.E. Monitoring of the human spinal cord. Orthop Trans. 1979;3:123.
3. D’Angelo C.M., VanGilder J.C., Taur A. Evoked cortical potentials in experimental spinal cord trauma. J Neurosurg. 1973;38:332.
4. Dinner D.S., Lüders H., Lesser R.P., et al. Intraoperative spinal somatosensory evoked potentials monitoring. J Neurosurg. 1986;65:807.
5. Engler G.L., Spielholz N.I., Bernhard W.N., et al. Somatosensory evoked potentials during Harrington instrumentation for scoliosis. J Bone Joint Surg [Am]. 1978;60:528.
6. Giblin D.R. Somatosensory evoked potentials in healthy subjects and in patients with lesions of the nervous system. Ann NY Acad Sci. 1964;112:93.
7. Grundy B.L. Monitoring of sensory evoked potentials during neurosurgical operations: methods and applications. Neurosurgery. 1982;11:556.
8. Grundy B.L., Nelson P.B., Doyle E., Procopio P.T. Intraoperative loss of somatosensory-evoked potentials predicts loss of spinal cord function. Anesthesiology. 1982;57:321.
9. Hahn J.F., Lesser R., Klem G., et al. Simple technique for monitoring intraoperative spinal cord function. Neurosurgery. 1981;9:692.
10. Halliday A.M., Wakefield G.S. Cerebral evoked responses in patients with dissociated sensory loss. Electroencephalogr Clin Neurophysiol. 1962;14:786.
11. Jones S.J., Edgar M.A., Ransford A.O., et al. A system for electrophysiological monitoring of the spinal cord during operations for scoliosis. J Bone Joint Surg [Br]. 1983;65:134.
12. Klem G., Andrish J., Gurd A., et al. Spinal cord evoked potentials recorded from ligamentum interspinalis. Electroencephalogr Clin Neurophysiol. 1980;50:221.
13. Kobrine A.I., Evans D.E., Rizzoli H.V. Correlation of spinal cord blood flow, sensory evoked response, and spinal cord function in subacute experimental spinal cord compression. Cervos-Navarro J., Betz E., Ebhardt G., et al, editors. Pathology of cerebrospinal microcirculation: advances in neurology. New York: Raven Press. 1978;vol 20:389.
14. LaMont R.L., Wasson S.L., Green M.A. Spinal cord monitoring during spinal surgery using somatosensory spinal evoked potentials. J Pediatr Orthop. 1983;3:31.
15. Larson S.J., Sances A.Jr., Christenson P.C. Evoked somatosensory potentials in man. Arch Neurol. 1966;15:88.
16. Lesser R.P., Raudzens P., Lüders H., et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22.
17. Lüders H., Gurd A., Hahn J., et al. A new technique for intraoperative monitoring of spinal cord function: multichannel recording of spinal cord and subcortical evoked potentials. Spine (Phila Pa 1976). 1982;7:110.
18. Maccabee P., Levine D.B., Kahanovitz N., et al. Monitoring of spinal and subcortical somatosensory evoked potentials during Harrington rod instrumentation. Orthop Trans. 1982;6:19.
19. MacEwen G.D., Bunnell W.P., Sriram K. Acute neurological complications in the treatment of scoliosis. A report of the Scoliosis Research Society. J Bone Joint Surg [Am]. 1975;57:404.
20. Macon J.B., Poletti C.E., Sweet W.H., et al. Conducted somatosensory evoked potentials during spinal surgery. Part 2: Clinical applications. J Neurosurg. 1982;57:354.
21. McCallum J.E., Bennett M.H. Electrophysiologic monitoring of spinal cord function during intraspinal surgery. Surg Forum. 1975;26:469.
22. Nash C.L.Jr., Lorig R.A., Schatzinger L.A., et al. Spinal cord monitoring during operative treatment of spine. Clin Orthop Relat Res. 1977;126:100.
23. Nuwer M.R., Dawson E.C. Intraoperative evoked potential monitoring of the spinal cord. A restricted filter, scalp method during Harrington instrumentation for scoliosis. Clin Orthop Relat Res. 1984;183:42.
24. Raudzens P.A. Intraoperative monitoring of evoked potentials. Ann NY Acad Sci. 1982;388:308.
25. Schramm J., Hashizume K., Fukushima T., et al. Experimental spinal cord injury produced by slow, graded compression. Alterations of cortical and spinal evoked potentials. J Neurosurg. 1979;50:48.
26. Spielholz N.I., Benjamin M.V., Engler G.L., et al. Somatosensory evoked potentials during decompression and stabilization of the spine. Methods and findings. Spine (Phila Pa 1976). 1979;4:500.
27. Tamaki T., Tsuji H., Inoue S., et al. The prevention of iatrogenic spinal cord injury utilizing the evoked spinal cord potential. Int Orthop. 1981;4:313.
28. Tsuji S., Lüders H., Lesser R.P., et al. Subcortical and cortical somatosensory potentials evoked by posterior tibial nerve stimulation: normative values. Electroencephalogr Clin Neurophysiol. 1984;59:214.
29. Tsuyama N., Tsuzuki N., Kurokawa T., et al. Clinical application of spinal cord action potential movement. Int Orthop. 1978;2:39.
30. Wilber R.G., Thompson G.H., Shaffer J.W., et al. Postoperative neurological deficits in segmental spinal instrumentation. A study using spinal cord monitoring. J Bone Joint Surg [Am]. 1984;66:1178.
31. Worth R.M., Markand O.N., DeRosa G.P., et al. Intraoperative somatosensory evoked response monitoring during spinal cord surgery. Courjon J., Mauguiere F., Revol M., editors. Clinical applications of evoked potentials in neurology: advances in neurology. New York: Raven Press. 1982;vol 22:p 367.
32. Bennett M.H. Effects of compression and ischemia on spinal cord evoked potentials. Exp Neurol. 1983;80:508.
33. Coles J.G., Wilson G.J., Sima A.F., et al. Intraoperative detection of spinal chord ischemia using somatosensory cortical evoked potentials during thoracic aortic occlusion. Ann Thorac Surg. 1982;34:299.
34. Croft T.J., Brodkey J.S., Nulsen F.E. Reversible spinal cord trauma: a model for electrical monitoring of spinal cord function. J Neurosurg. 1972;36:402.
35. Dolan E.J., Transfeldt E.E., Tator C.H., et al. The effects of spinal distraction on regional spinal cord in cats. J Neurosurg. 1980;53:756.
36. Allison T. Recovery functions of somatosensory evoked responses in man. Electroencephalogr Clin Neurophysiol. 1962;14:331.
37. Brodkey J.S., Richards D.E., Blasingame J.P., et al. Reversible spinal cord trauma in cats. Additive effects of direct pressure and ischemia. J Neurosurg. 1972;37:591.
38. McNeal D., Passoff T., Swank S., et al. Spinal cord monitoring using epidural electrodes for stimulation and recording. Orthop Trans. 1982;6:19.
39. Sutter M., Deletis V., Dvorak J., et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007;16(Suppl 2):S232.
40. Deecke L., Tator C.H. Neurophysiological assessment of afferent and efferent conduction in the injured spinal cord of monkeys. J Neurosurg. 1973;39:65.
41. Griffiths I.R., Trench J.G., Crawford R.A. Spinal cord blood flow and conduction during experimental cord compression in normotensive and hypotensive dogs. J Neurosurg. 1979;50:353.
42. Jasper H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371.
43. Cusick J.F., Myklebust J., Larson S.J., Sances A.Jr. Spinal evoked potentials in the primate: neural substrate. J Neurosurg. 1978;49:551.
44. Lüders H., Andrish J., Gurd A., et al. Origin of far-field subcortical potentials evoked by stimulation of the posterior tibial nerve. Electroencephalogr Clin Neurophysiol. 1981;52:336.
45. Nordwall A., Axelgaard J., Harada Y., et al. Spinal cord monitoring using evoked potentials recorded from feline vertebral bone. Spine (Phila Pa 1976). 1979;4:486.
46. Lesser R.P., Koehle R., Lueders H. Effect of stimulus intensity on short latency somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1979;47:377.
47. Greenberg M. Electrodiagnostics. Greenbery M., et al, editors, ed 7. Handbook of Neurosurgery. New York: Thieme, 2010. p 266