Solutions
Sudaxshina Murdan
Chapter contents
Advantages of pharmaceutical solutions
Enhancement of drug solubility
Key points
• All the components in a solution are dispersed as molecules or ions.
• As the drug is already dissolved, it is immediately available for action or absorption.
• Solutions have many advantages, for example, oral solutions are easy to swallow.
Introduction
This chapter concentrates on solution dosage forms and it is recommended that it be read in conjunction with Chapters 2 and 3 where the science of formation of solutions and their properties are discussed.
A solution, as defined in Chapter 2, is a homogeneous, molecular, mixture of two or more components. The simplest solution consists of two components, a solute dissolved in a solvent. The solute and the solvent could be in the solid, liquid or gaseous states of matter. Most commonly, pharmaceutical solutions are preparations in which the solid solutes, i.e. drug and excipients, are dissolved in a liquid solvent system. Water is the most common solvent, although organic solvents are used in combination with water or on their own. All the components of a solution are dispersed as molecules or ions, and the solution is optically clear. Solutions can be prepared by simple mixing of the solutes with the solvent system. In industry, solutions are prepared in large mixing vessels which are thermostatically controlled should a specific temperature be desired.
The solvent system
Aqueous solvents
The majority of pharmaceutical solutions are water-based. Water is the most commonly used solvent due to its many advantages, such as its lack of toxicity and low cost. Different types of ‘water’ have been defined in the pharmacopoeias, related to its purity. Those defined in the British Pharmacopoeia are given as representative examples in Table 24.1. Other pharmacopoeias, such as the United States Pharmacopoeia, have additional types, such as ‘Bacteriostactic Water for Injection’.
Table 24.1
Different types of water, as defined by the British Pharmacopoeia
| Type of water | Use |
| Purified Water | Used for the preparation of medicines that do not have to be sterile and apyrogenic. |
| Highly Purified Water | Used for the preparation of medicines where water of high biological quality is needed, except where Water for Injections is required. |
| Water for Injections | Used for medicines for parenteral administration. Must be pyrogen-free. |
| Sterilized Water for Injections | Used for medicines for parenteral administration. Water has been sterilized by heat and is suitably packaged. |
Tap (drinking) water is not normally used for the manufacture of pharmaceutical solutions or for extemporaneous compounding, as it contains dissolved substances which could interfere with the formulation, for example, reduce drug solubility and stability. Tap water is therefore purified, for example, by distillation, ion exchange or reverse osmosis to produce Purified Water. The latter is used for the preparation of non-parenteral solutions. For parenteral solutions, tap water is further purified in order to remove pyrogens (water-soluble, fever-producing compounds) thereby producing Water for Injections. In certain instances, for example, in extemporaneous dispensing, drinking tap (potable) water, freshly drawn from a mains supply, boiled and cooled, can be used to prepare oral or external solutions that are not intended to be sterile.
On its own, water does not dissolve many drug compounds to a sufficient degree to enable the preparation of a pharmaceutical solution. Other water-miscible liquids with greater drug solubility may therefore be added to water to enhance drug solubility. These liquids are called co-solvents. Commonly used examples include glycerol, propylene glycol, ethanol and poly(ethylene glycol). Co-solvents are generally less innocuous than water and the concentration used in an aqueous solution is limited primarily by their toxicity, by drug solubility in the formulation and finally cost. The mechanism of action of co-solvents is discussed in greater detail below.
Non-aqueous solvents
Non-aqueous solvent systems are used when the drug is insufficiently soluble or stable in aqueous systems, or when a solution is intended for specific properties, such as sustained drug absorption. Non-aqueous solutions are however limited to certain delivery routes, such as intramuscular and topical, due to their unpalatibility, toxicity, irritancy or immiscibility with physiological fluids. Although there is a huge number of organic liquids in which drugs can dissolve, the majority are toxic, and only a few are used in pharmaceutical solutions. Examples of commonly used organic liquids are shown in Table 24.2. These liquids are used as co-solvents with water, as co-solvents with other organic liquids, or on their own.
Table 24.2
Examples of non-aqueous solvents used in pharmaceutical solutions
| Solvent | Use |
| Alcohols, including polyhydric ones (i.e. those containing more than one hydroxyl group per molecule) | Ethanol is the most common organic solvent used in pharmaceutical solutions. It is often used as a co-solvent in oral, topical and parenteral solutions. Propylene glycol (CH3CH(OH)CH2OH) contains 2 hydroxyl groups per molecule. It is often used as a co-solvent in oral, topical, parenteral and otic solutions. Glycerol contains 3 hydroxyl groups per molecule. It is widely used as a solvent or co-solvent with water, in oral and parenteral solutions. Low molecular weight polyethylene glycols (PEGs) with the general formula HOCH2(CH2CH2O)nCH2OH. These are used as solvents or co-solvents with water or ethanol. Used in parenteral solutions. |
| Fixed vegetable oils | Fixed oils are expressed from the seeds, fruit or pit/stone/kernel of various plants. They are non-volatile oils and are mainly triglycerides of fatty acids. Examples include olive oil, corn oil, sesame oil, arachis oil, almond oil, poppyseed oil, soya oil, cottonseed oil, castor oil. Historically, they have been used for intramuscular administration. They are used to a lesser extent now due to their irritancy and the possibility of allergic reactions to certain oils. They are being replaced by synthetic alternatives such as ethyl oleate. |
| Esters, such as ethyl oleate, benzyl benzoate, ethyl ethanoate | These are used as a vehicle in certain intramuscular injections. |
| Dimethyl sulfoxide | Used as a carrier for idoxuridine for topical application to the skin. |
| Glycofurol | Used as a co-solvent in parenteral solutions for intramuscular or intravenous injection. |
| Ethyl ether | Used as a co-solvent with ethanol in collodions. |
The drug
The drug could be a small molecule like aspirin, or a large biotherapeutic molecule, such as insulin or an antibody. As defined in Chapter 2, the drug is present as molecules or ions throughout the solvent. It is usual to ensure that the drug concentration in a pharmaceutical solution is well below its saturation solubility in order to avoid the possibility of drug precipitating out of the solvent as a result of subsequent temperature changes during storage and use.
The excipients
Excipients – substances other than the drug or prodrug which are included in pharmaceutical solutions – are used for a number of reasons, such as to enhance product stability, bioavailability or patient acceptability, aid product manufacture and/or identification. Each excipient has a clear role in the product, thus, the nature of an excipient used depends on the requirements of the pharmaceutical product. The excipient must be non-toxic, nonsensitizing, nonirritating, as well as compatible with all the other components of the formulation. The route of administration is important; many excipients are acceptable by certain, but not all, routes. For example, the preservative benzalkonium chloride is used in oral, but not nebulizer, solutions, as it causes bronchoconstriction. Like the drug, excipients could be small (e.g. sucrose) or large (e.g. hydroxypropyl methylcellulose) molecules.
Pharmaceutical solutions
Solutions are one of the oldest pharmaceutical formulations. They are administered by many different routes; they are often therefore classified by the intended route (e.g. oral, otic (ear), parenteral). Solutions are also classified by the nature of the formulation, or by the traditional name which relates to the solvent system used, such as syrups, elixirs, spirits and tinctures. The latter terms are described in Table 24.3. While all pharmaceutical solutions must be stable, and acceptable to patients, other requirements of solutions administered by the different routes vary. For example, parenteral and ocular solutions must be sterile, oral solutions must be palatable, solutions which come into contact with body fluids must be isotonic and at physiological pH, especially if large volumes are used. Multidose products often contain preservatives to ensure that the growth of any microorganisms that are accidentally introduced during product use is inhibited. The requirements of the different types of pharmaceutical solutions are detailed in Table 24.4. These requirements are achieved by the inclusion of a number of excipients, as detailed in Table 24.5.
Table 24.3
| Traditional term | Description |
| Aromatic Waters | Saturated aqueous solutions of volatile oils or other aromatic or volatile substances. |
| Elixirs | Many oral solutions that contain alcohol as a cosolvent have traditionally been designated as elixirs. However, many other oral solutions containing significant amounts of alcohol are not designated as elixirs. |
| Spirits | Alcoholic or hydro-alcoholic solutions of volatile substances. Some spirits are used as flavouring agents, others are medicinal. |
| Syrups | Oral aqueous solutions containing high concentrations of sucrose or other sugars. ‘Syrup BP’ is a solution of sucrose (66.7%) in purified water; it promotes dental decay and is unsuitable for diabetic patients. ‘Sugar-free’ syrups are obtained by replacing sucrose with hydrogenated glucose, mannitol, sorbitol, xylitol, etc. |
| Tinctures | Alcoholic or hydro-alcoholic solutions prepared from vegetable materials or chemical substances. Due to the variability in the vegetable materials, drug concentration can vary. |
Table 24.4
Requirements of pharmaceutical solutions, with respect to their route of administration
| Route of administration | Requirements of the solution |
| Oral | |
| Oral solutions are swallowed, in which case, the drug may exert a local effect on the gastrointestinal tract or be absorbed into the blood and exert a systemic action. | Liquid oral solutions are aqueous formulations. To be acceptable to patients, these must be palatable. Flavouring, colouring and sweetening agents are therefore added to enhance their appearance and taste. Solution pH is usually 7.0, although a range of pH 2–9 can be tolerated. For convenience, the dose is usually in multiples of 5 mL, and the patient is given a 5 mL spoon with the solution. When smaller volumes are required, oral syringes are used. Viscosity should be appropriate for palatability and pourability. Solutions have a higher viscosity than water. |
| Oral Cavity | |
| Mouthwashes and gargles are used to treat local infection and inflammation in the oral cavity. Gingival solutions are applied to the gingivae. These are not intended to be swallowed and the drug exerts a local effect in the mouth. | Solutions are aqueous formulations. They must be palatable and acceptable to patients. Flavouring, colouring and sweetening agents are often added. As far as possible, the pH should be around neutral. |
| Topical Skin/Nail/Hair | |
| Solutions are applied to the skin for local and/or systemic effect. | The vehicle may be aqueous or non-aqueous, and different types of formulations are available. |
| An application frequently contains parasiticides. | |
| Solutions are also applied to the nail or hair for local effect. | A lotion is aqueous-based, and is intended for application without friction. A liniment is an alcoholic or oily solution (or emulsion) designed to be rubbed into the skin. Paints and tinctures are concentrated aqueous or alcoholic antimicrobial solutions. |
| Topical Skin/Nail/Hair (continued) | |
| Solutions are also applied to the nail or hair for local effect (continued). | A collodion is a solution of a polymer, usually Pyroxylin, in a volatile organic solvent system (a mixture of ethanol and ether). Following application to the skin, the solvents evaporate, leaving a polymeric film on the skin. Nail solutions are applied to the nail to treat nail diseases. All preparations must be acceptable to the patient. Formulations which are easy to transfer from the container and will spread easily and smoothly are preferred. Formulation must adhere to site of application, without being tacky or difficult to remove. |
| Otic (ear, aural) | |
| Solutions are instilled in the outer ear to exert a local effect. They are used to remove ear wax or to deliver anti-infective, anti-inflammatory and analgesic drugs. | May be aqueous or non-aqueous solutions. Water, glycerol, propylene glycol and oils may be used as solvents. Non-aqueous vehicles are predominantly used when ear wax removal is desired as ear wax can solubilize in them. As the residence time in the ear is higher for viscous solutions, the viscosity of aqueous solutions is increased by the use of polymers. Propylene glycol and glycerol solutions are naturally viscous and these enhance residence time. Solutions do not need to be isotonic as they are external preparations. |
| Ocular | |
| Eye drops are used to treat local disorders of the eye, e.g. infection. Ocular solutions may also be used to treat intraocular disorders, such as glaucoma. Eye lotions are solutions for rinsing or bathing the eye, or for impregnating eye dressings. |
Most ocular solutions are aqueous. They must be manufactured sterile as the product is to come in contact with tissues that are very sensitive to contamination. Once opened, a multidose ocular product should remain free from viable microorganisms during its period of use and must contain antimicrobial preservatives. Ideally the solution pH should be close to physiological pH of tears (pH 7.4) or slightly more alkaline to reduce pH-induced lacrimation, irritation and discomfort. Physiological pH may not however be the optimum pH for drug solubility, absorption and stability, or for the function of other components of the solution; thus ocular solutions do not always have a pH of about 7.4. Luckily, the eye can tolerate solutions with pH as low as 3.5 and as high as 9. Ideally, ocular solutions must be isotonic with the tears to minimize irritation and discomfort. Some deviation from isotonicity can be tolerated without marked discomfort when small volumes of solutions are administered, as the latter are rapidly diluted with tears. When large volumes are used, for example, to wash the eyes, the solution must be approximately isotonic. Most products have a viscosity of 15–25 mPa s (for comparison, the viscosity of water is given as 1 mPa s). An increase in viscosity prolongs the solution’s residence in the eye. |
| Nasal | |
| Nose drops, nasal sprays, used for local, e.g. decongestant effect, or for systemic drug delivery. | Nasal solutions are aqueous formulations. Solution pH is in the normal pH range of nasal fluids (pH 5.5 to 6.5). Solutions are usually isotonic to nasal fluids. Solution viscosity is similar to that of nasal mucus (which is higher than that of water). Flavouring or sweetening agents are sometimes used to mask taste, as a small proportion of nasal solution may be swallowed following nasal administration. Multidose solutions require preservatives. |
| Pulmonary | |
| Inhaled solutions are administered by pressurized metered-dose inhalers (pMDIs) or by nebulizers for local or systemic effect. | Solutions of drug and excipients dissolved in liquefied propellants, such as trifluoromonofluoroethane, are used in pMDIs. Solutions used in nebulizers are aqueous formulations. As relatively large volumes may be administered by nebulizers, the solutions must be isotonic and have a pH not lower than 3 and not higher than 8.5. Multidose preparations containing preservatives are available, although generally, sterile, single unit doses without a preservative are used. |
| Rectal | |
| Solution enemas are usually administered for local or systemic drug action. | Enemas can be aqueous or oily solutions. Micro-enemas have a volume of 1 to 20 mL, while macro-enemas have volumes of 50 mL or more. Macro-enemas should be warmed to body temperature before administration. |
| Vaginal | |
| Vaginal solutions are administered for local effect, for irrigation or for diagnostic purposes. | Vaginal solutions are aqueous. Excipients to adjust pH may be included. |
| Parenteral | |
| ‘Parenteral’ refers to the injectable routes of administration. Drugs are most commonly injected into the veins (intravenous), muscles (intramuscular) and into (intradermal) and under (subcutaneous) the skin, although they can also be injected into arteries, joints, joint fluid areas, spinal column, spinal fluid and the heart. | Parenteral solutions must be sterile and pyrogen-free. Preservatives, such as benzyl alcohol, are included under certain conditions such as in multidose products. Intravenous – the solution must be aqueous, as oil droplets can occlude the pulmonary microcirculation. Intramuscular and subcutaneous – The solution can be aqueous or non-aqueous. Ideally, a parenteral aqueous solution should have a pH close to physiological pH (which is 7.4), to avoid pain, phlebitis and tissue necrosis. A pH of 7.4 may not however be the optimum pH for drug solubility and product stability, and since a reasonably wide pH range can be tolerated, the pH of most licensed parenteral solutions is between 3 and 9. A wide pH range is tolerated as the administered solution is diluted upon administration, most notably with the intravenous route. Parenteral solutions must be isotonic when large volumes are administered by intravenous infusion. When smaller volumes are used, a wider range of tonicity can be tolerated as dilution with body fluids occurs. |
Table 24.5
| Excipients | Examples of excipients |
| Co-solvents | Ethanol, glycerol, propylene glycol. The concentration of ethanol should be limited as it exerts a pharmacological action following oral administration. |
| Flavouring Agents | Used to mask the taste of drugs, many of which have a very unpleasant taste. Synthetic or naturally occurring flavourings such as vanilla, raspberry, orange oil, lemon oil are used for oral solutions. Menthol is used in both oral and nasal solutions. Certain flavours appeal to certain patient populations and certain parts of the world; this must be borne in mind by the formulator. For example, fruit and bubble gum flavours are acceptable to children, whilst mint flavour is not. |
| Colouring Agents | A colouring agent should correlate with the flavouring agent, e.g. green with mint, red with cherry flavour. Like flavours, colour preference varies between cultures. |
| Sweeteners | Sucrose, sorbitol, mannitol, saccharin sodium, xylitol, high fructose corn syrup are used to improve the palatability of oral solutions. Sweetened, but sugar-free, preparations containing aspartame are suitable for diabetic patients and are not cariogenic. |
| Antimicrobial Preservatives | Used to preserve multidose preparations. Examples include benzalkonium chloride, benzyl alcohol, chlorobutanol, thimerosal, combinations of parabens (methyl, propyl, butyl). |
| Antioxidants | Sodium metabisulphite, sodium sulphite, sodium bisulfate ascorbic acid, used to stabilize solutions. |
| Chelating Agents | Disodium edentate, used to increase solution stability. |
| pH Adjusters | Acids, e.g. citric acid, buffers Alkali, e.g. sodium hydroxide, buffers. |
| Isotonicity Adjusters | Sodium chloride, potassium chloride, mannitol, dextrose, glycerol. |
| Viscosity Enhancers | Hypromellose, hydroxyethylcellulose, polyvinyl alcohol, povidone, dextran, carbomer 940. |
Advantages of pharmaceutical solutions
Solutions have several advantages, and for many drugs, a solution is the only available dosage form. Advantages of solutions include:
Disadvantages of solutions
The disadvantages of solutions compared with other dosage forms include:
• many drugs are poorly soluble in water. Their formulation as a solution is challenging (see below)
• liquids are bulky and less easy for the patient to carry, for example, the daily dose, compared to solid dosage forms. Liquids are also more expensive to transport, which increases the medicine’s cost. The packaging of pharmaceutical solutions requires materials of higher quality (see Chapter 47).
Solution stability
A pharmaceutical solution must be stable for the duration of its shelf-life (period of storage and use). That is, it must retain the same physical, chemical, microbiological, therapeutic and toxicological properties that it possessed at the time of its manufacture. The product’s physical properties (e.g. colour, clarity, viscosity, odour, taste) and efficacy must not change, and there should be no significant increase in toxicity. The product should remain sterile or resistant to microbial growth, and the drug’s chemical nature and potency must not change.
However, many drug molecules undergo chemical reactions, such as, hydrolysis, oxidation, decarboxylation, epimerization, dehydration, with hydrolysis, oxidation and reduction being the most common. Chemical reactions occur more readily at high temperature, at certain pHs, in the presence of UV light and of substances which can act as a catalyst, and in solutions, where the drug is present as molecules. The resulting loss of drug molecules can reduce the efficacy of the formulation and increase the latter’s toxicity if the products of the chemical changes are toxic. Pharmaceutical solutions are therefore formulated at the pH favouring drug stability, and often include excipients to enhance product stability. To reduce photo-oxidation, solutions are packaged in containers that do not allow light transmission. To reduce oxidation, antioxidants and/or metal chelators (as heavy metal ions catalyse oxidation) are used. Alternatively, oxygen can be excluded, by purging the solution with nitrogen and creating a nitrogen headspace within the container. To inhibit microbial growth during use, preservatives are used in multidose products. All the excipients used within a solution must be of suitable quality, non-toxic, compatible with the drug and with one another, and active at the solution pH. In addition, the excipient must remain in the solution throughout the shelf-life of the product. That is, the concentration of excipient must not decrease, which could happen, for example, if the excipient degraded or adsorbed onto the container walls.
Further detailed information about product stability can be found in Chapters 48, 49 and 50.
Enhancement of drug solubility
As mentioned above, water is the most commonly used vehicle in pharmaceutical solutions. Many drugs are water soluble, solubility being defined as the concentration of the drug in a solution when equilibrium exists between dissolved and undissolved drug. As described in Chapter 2, drug solubility in water depends on a number of factors such as the drug’s molecular structure, crystal structure, particle size, pKa and the pH of the medium (if the drug is a weak acid/base or a salt).
Unfortunately, many drugs are not sufficiently soluble in water and aqueous drug solubility must be increased by the inclusion of other solvents/chemicals. The nature of the solubility enhancer depends on the drug molecule and the route of administration, as well as the intended patient population. Certain enhancers may be safely administered via the oral route, but not parenterally due to their greater toxicity when administered parenterally. Others, e.g. ethanol, while widely used in medicines, should be avoided where possible in paediatric formulations. Different approaches to enhancing drug solubility in solutions are described below.
pH adjustment
Most existing drugs are either weak acids or weak bases. In solution, an equilibrium exists between the undissociated drug molecules and their ions. The equilibrium may be represented as:
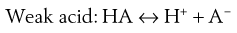 (24.1)
(24.1)
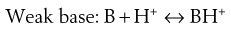 (24.2)
(24.2)
Depending on circumstances (discussed in Chapter 3) these equilibria will shift towards either the undissociated or dissociated forms.
Since ions are more soluble in water than neutral molecules, changing the pH of the medium to increase ionization of the drug is a common technique for increasing drug solubility in an aqueous medium. Weakly acidic drugs are ionized when the pH of the solvent is increased. Conversely, lowering pH favours ionization of weakly basic drugs. The pH required to achieve drug ionization can be calculated using the Henderson-Hasselbalch equations (see Chapter 3), and the pH can be adjusted using acids or alkali, or using buffers such as citrate, acetate, phosphate and carbonate buffers. Extremes of pH should be avoided however, so that the solution is physiologically acceptable; the pH ranges tolerated via the different routes are shown in Table 24.4.
In addition, the chosen pH should not adversely affect the stability of the drug and excipients. As mentioned above, the rate of chemical reactions which lead to degradation can be pH-dependent. The pH for optimal drug solubility may not be the same as that for optimal stability. pH can also be important for the optimal functioning of excipients. For example, the ionization, and subsequently the activity of a preservative may be influenced by the pH of the medium. Bioavailability of the drug should also not be compromised by a change in pH, unionized drug molecules being absorbed to a greater extent through biological membranes than their ionized counterparts. The pH of a pharmaceutical solution is thus a compromise between drug solubility, stability and bioavailability, the function of excipients, and physiological acceptability of the product.
Co-solvents
Co-solvents are often used to increase the water solubility of drugs which do not contain ionizable group(s) and whose solubility can thus not be increased by pH adjustment. The principle ‘like dissolves like’ was mentioned in Chapter 2. That is, polar drugs generally dissolve in polar solvents and non-polar drugs generally dissolve in non-polar solvents. Thus, non-polar drugs are poorly soluble in water – a polar solvent. To increase the solubility of such drugs in water, the latter’s polarity should be lowered. This can be achieved by adding a third component such as a water-miscible organic liquid with a low polarity. Such a liquid, when used in this context, is called a co-solvent.
Most water-miscible organic liquids are however toxic, and only a few are used as cosolvents in pharmaceutical solutions. Examples include glycerol, propylene glycol, ethanol and the low molecular weight poly(ethylene glycol)s. The solubility of non-polar drugs in water can be increased by several orders of magnitude using co-solvents. Typically, a linear increase in co-solvent fraction results in logarithmic increases in drug solubility. The concentration of the co-solvent is however limited by its physiological acceptability. The co-solvent must be non-toxic at the concentrations used, and by the route of administration.
Complexation with cyclodextrins
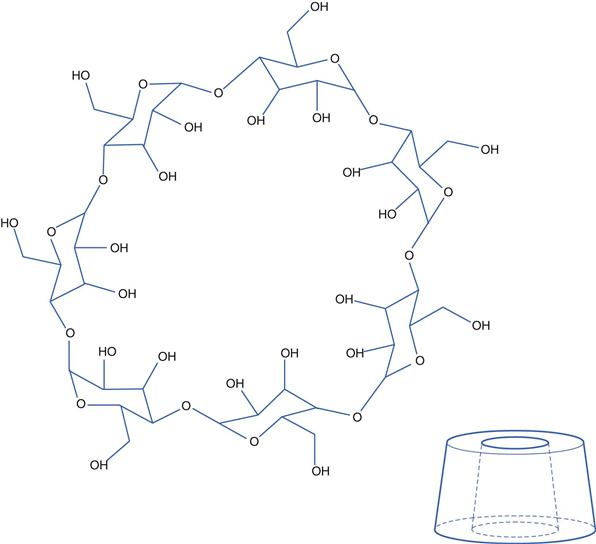
Cyclodextrins (CDs) are non-reducing cyclic glucose-based oligosaccharides, comprising a variable number of D-glucose residues linked by α-(1,4) glycosidic linkages. The three most important CDs are alpha, beta and gamma cyclodextrins which consist of 6, 7 and 8 D-glucopyranosyl units, respectively, arranged in a ring. Three-dimensionally, CDs can be visualized as a hollow truncated cone (Fig. 24.1). The cavity in the cone has different diameters dependent on the number of glucose units in the ring; α-CD has a cavity diameter of about 0.55 nm, β-CD about 0.70 nm and γ-CD about 0.90 nm, with cavity volumes of 0.10, 0.14 and 0.20 mL/g, respectively. The side rim depth is the same for all three (about 0.8 nm).
The interior cavity of cyclodextrins is apolar, while their exterior is hydrophilic. The representation in Figure 24.1 needs careful interpretation as the —OH groups shown are actually attached to the top and bottom rims of the structure and not to either the inside or outside walls. The hydrophobic nature of the inside surface arises from the location of the —O— and C—H bonds of the glucose molecules being orientated there.
The hydrophilic exterior results in CDs being soluble in water. Concurrently, the less polar interior can accommodate non-polar drug molecules via non-covalent interactions, thereby allowing the non-polar drug to be ‘hidden’, enabling it to be molecularly dispersed in water. Thus, drug inclusion within CDs effectively increases their aqueous solubility. Each cyclodextrin molecule can form complexes with one or more drug molecules. Drug-CD complexes can also self-associate, and the water-soluble structures formed can further solubilize the drug through non-inclusion complexation.
Upon administration, for example orally, of a solution containing a drug-CD complex, the drug can be released from the CD molecule and the free drug can then be absorbed through the gastrointestinal tract.
Surfactants and micelles
As described in Chapter 5, surfactants (surface-active agents) and amphiphiles are molecules which have two distinct regions in their chemical structure. One region is hydrophilic and the other hydrophobic. Because of this, such molecules tend to accumulate at the boundary between two phases, such as water-air or water-oil interfaces. They reduce the surface tension of liquids, and self-assemble to form micelles once the critical micellar concentration (CMC) is reached. Poorly water-soluble drugs can be solubilized in micelles to enhance their aqueous solubility. The location of the solubilisate (the drug which is solubilized within the micelles) depends on its nature: non-polar solubilisates being located within the micelles’ hydrophobic interior cores, solubilisates containing polar groups are oriented with the polar group at the micellar surface, while slightly polar solubilisate partition between the micelle surface and the core. Solubilisates may also be found in the palisade layer of non-ionic surfactant micelles. The maximum amount of solubilisate which can be incorporated into a given system at a fixed concentration is known as the maximum additive concentration (MAC).
The aqueous solubility of a wide range of drugs has been increased by surfactants, especially for oral and parenteral administrations. For example, solubilization of steroids with polysorbates has allowed their formulation in aqueous ophthalmic preparations, while solubilization of the water-insoluble vitamins A, D, E and K has enabled the preparation of aqueous injections. The surfactant chosen for a particular drug must solubilize the drug and be compatible with it, and all the other components of the solution. For example, the surfactant should not adversely influence the drug’s stability. The surfactant must also be non-toxic at the concentration used for the particular route of administration.
The different means of enhancing drug solubility are often used in combination, as one approach is often insufficient to achieve the target drug concentration in a pharmaceutical solution. For example, pH adjustment and co-solvents are often used in combination.
Bibliography
1. Council of Europe. European Pharmacopoeia. 7th edn Strasbourg: Directorate for the Quality of Medicines & Health Care of the Council of Europe; 2011.
2. Department of Health, HM Government. British Pharmacopoeia. London Stationery Office 2011.
3. Florence AT, Attwood D. Physicochemical Principles of Pharmacy. 5th edn London: Pharmaceutical Press; 2011.
4. Jones D. FASTtrack Pharmaceutics – Dosage Form and Design. London: Pharmaceutical Press; 2008.
5. Liu R. Water-Insoluble Drug Formulation. 2nd edn Boca Raton: CRC Press; 2008.
6. Rowe RC, Sheskey PJ, Cook WG, Fenton ME. Handbook of Pharmaceutical Excipients. 7th edn London: The Pharmaceutical Press; 2012.
7. Sweetman SC. Martindale, The Complete Drug Reference. 27th edn London: Pharmaceutical Press; 2011.
8. United States Pharmacopeial Convention. USP-NF. Rockville 2006.