Soft tissue, musculoskeletal system, and miscellaneous targets
ASHOT E. SARGSYAN, GLYKERIA PETROCHEILOU, SERAFIM NANAS, ARIEL L. SHILOH, HEIDI LEE FRANKEL and DIMITRIOS KARAKITSOS
Overview
Besides encompassing specific focused techniques, the holistic approach (HOLA) ultrasound concept, introduced in Chapter 1, promotes generic scanning of any body part (head-to-toe ultrasound imaging) as modulated by current clinical indications. Any ultrasound view obtained through the skin contains some information about soft tissues. While serving as an imaging window and as anatomic reference structures in focused techniques (e.g., the chest wall in lung scanning), soft tissues per se are often a primary target (e.g., in extremity crush injury). Therefore mastery of fine anatomy is essential for the HOLA-level ultrasound operator, both in terms of tissue type (e.g., fascia, tendon, peritoneum), and in terms of named structures (e.g., basilic vein, gracilis muscle, median nerve). This chapter reviews nonspecific (generic) soft tissue and musculoskeletal (MSK) imaging and miscellaneous intensive care unit (ICU)-relevant HOLA targets.
• Visible bruising, swelling, deformity, redness, pulsation, or asymmetry
• Palpable mass, warmth, pulsation, crepitus, or other focal abnormality
• Known or suspected foreign body, including indwelling access devices
• Known or suspected MSK injury or history of a relevant event, such as a fall
• Traumatic or surgical wounds
• Spontaneous, traumatic, surgically placed, or postsurgical fistulas
• Ruling out pathologic lymph nodes
• Planning interventional procedures (e.g., vascular access)
• Known or suspected vascular pathology or access devices, including status post difficult vascular access
Equipment and technique
Any modern multipurpose system with a high-frequency (7-15 MHz) transducer is appropriate for most superficial targets. Lower frequencies (2-5 MHz) are used for deeper tissues in large subjects or large body parts (e.g., the thigh). More than one transducer can be used on the same region of interest (ROI) to exploit advantages of each. Some experts recommend broadband microconvex transducers (usually 5-8 MHz) for most soft tissue targets because they offer a good balance of resolution and penetration in a wide-angle view through a small footprint. With any transducer, both B-mode and color Doppler mode are often used. Although elastography, an emerging method to map elasticity of tissues, currently has limited use in the ICU, every superficial tissue scanning procedure includes elements of “visual elastography” (see Pathology section later).
Normal patterns
The uppermost layer of the image is a thin hyperechoic line that corresponds to the skin. Subcutaneous adipose tissue underneath is relatively hypoechoic, with linear echoes (septa). Fascia is a brightly echogenic line (perceived as a contiguous layer during scanning). Muscles appear as hypoechoic structures with organized echogenic fibroadipose septa between fasciculi; the septa merge as the transducer is moved toward a tendon (Figure 51 E-1). Tendons appear hyperechoic and fibrillar when scanning along their course and granular in cross sections; however, even a small deviation from the 90-degree orientation of the ultrasound beams relative to the fibers results in the loss of the echogenic pattern, mimicking a tear or defect. This feature is known as anisotropy (Figure 51-1)—dependence of the imaging pattern on the beam orientation. In tendons, the smooth type-1 collagen fiber bundles are good reflectors (direction dependent) but poor scatterers (direction independent). Peripheral nerves also have a fascicular appearance, but with less anisotropy, and are surrounded by loose connective tissue (see Figure 51-1). Vessels appear as anechoic (black) stripes across the image when the scanning plane aligns with their course. In transverse orientation, arteries are circular and may visibly pulsate. Veins have a near-circular appearance with less prominent walls, easily compress with transducer pressure, but fail to collapse entirely in case of thrombosis (see Figure 51-1). The bone surface is normally depicted as a brightly echogenic line with acoustic shadowing. The joint space is identified by a V-shaped discontinuity in the cortical line from adjacent bones, usually representing fibrocartilaginous tissue (e.g., a meniscus or a labrum). Random collagen fiber orientation in these structures determines their high, nonanisotropic echogenicity.1–3 Hyaline cartilage, on the other hand, looks like a thin hypoechoic rim over the bone (see Figure 51-1).
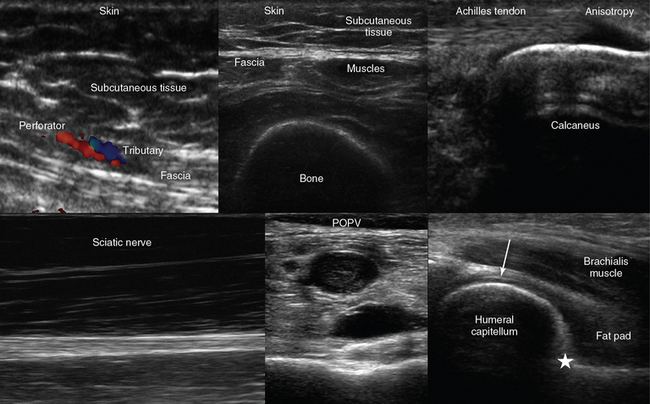
Figure 51-1 Top, left-to-right, Visualization of the skin, subcutaneous tissue, perforator (artery) and tributary (vein) exiting from the hyperechoic abdominal fascia (longitudinal plane); transverse view of the anterior thigh demonstrating the skin, subcutaneous tissue, fascia, muscles, and bone (femur); longitudinal view of the calcanear insertion of the Achilles tendon demonstrating anisotropy. Bottom, left to right, Longitudinal view of the sciatic nerve coursing the posterior thigh; incompressible popliteal vein (POPV) caused by thrombosis; medial sagittal plane of the anterior elbow joint showing the coronoid fossa (star = anterior coronoid recess) and the articular cartilage of the distal humeral epiphysis (arrow).
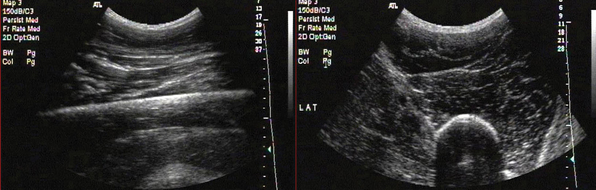
Figure 51 E-1 Longitudinal and transverse anteroposterior images of the thigh. Note the typical structure of a large skeletal muscle in the transverse view. Fascial borders of individual muscles and the compartment can be seen. Note the bright cortical reflection from femur. The hard bony cortex strongly reflects an ultrasonic wave, producing a bright, readily detectable curve or line on the image. This reflection is due to the large acoustic impedance gradient between bone and the overlying soft tissue. Although this makes the internal structure of bone difficult to visualize, it provides a very clear picture of the surface itself and therefore is a sensitive means of fracture detection. Because of the large size of the region of interest (ROI), a low-frequency convex transducer is used.
Pathology: High-level considerations
• Change in the relationships, shape, echostructure, or echogenicity of an anticipated (recognized) structure
• Focal change within an anticipated structure or its discontinuity
• Unexpected response of the structure(s) to transducer compression
• Appearance of an extraneous (unanticipated) structure within or between normal structures
• Consistent difference between the suspected pathologic area and the presumably normal contralateral site
• A trend in any of the above criteria relative to prior examinations
Pathology: Specific types
Hematomas are collections of extravascular blood caused by common and iatrogenic trauma, coagulation disorders, necrosis, and other mechanisms. They usually appear as poorly demarcated but structurally differing areas within or between anatomic structures, often distorting them (mass effect). A hematoma shows serial changes over time (Figure 51-2). Fresh blood initially looks anechoic and then becomes finely echogenic when clotted. After clot liquefaction, hematomas regain anechoic or hypoechoic appearance with diffuse internal echoes that may form a layer by sedimentation. Most hematomas resolve, but some organize into a chronic hyperechoic mass with echogenic inclusions or concentric layers from repeated bleeds.2–4 Color Doppler ultrasound helps demonstrate deviations of adjacent vessels; it is also helpful in distinguishing between a hematoma and a pathologic tissue.
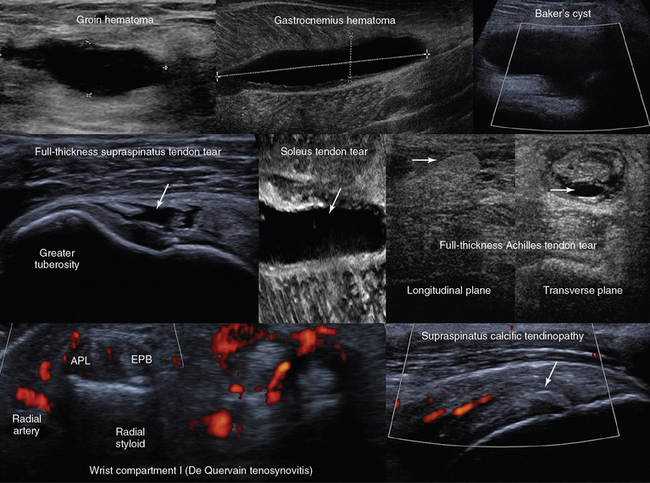
Figure 51-2 Top, left-to-right, Anechoic groin hematoma; gastrocnemius muscle hematoma after clot liquefaction; visualization of a cystic structure (no blood flow is evident) between the medial gastrocnemius and semimembranosus tendon in the popliteal fossa (Baker cyst). Middle, left-to-right, Visualization of a hypoechoic area in the supraspinatus tendon corresponding to a full-thickness tear (arrow); zoom image of a soleus muscle tear (arrow); longitudinal and transverse planes of a full-thickness Achilles tendon tear demonstrating Kager fat pad herniation (arrow) and a hypoechoic hematoma enhancing the conspicuity of the tear (arrow), respectively. Bottom, left-to-right, Focal thickening and increased vascularity surrounding the de Quervain tendons at the level of the radial styloid process (de Quervain tenosynovitis); anterior view of the shoulder depicting a thickened and heterogeneous supraspinatus tendon with a focus of calcification (arrow) and increased vascularity (calcific tendinopathy). APL, Abductor pollicis longus; EPB, extensor pollicis brevis. (Images of soleus muscle tear (middle) and de Quervain tendons (bottom) courtesy Dr. K. Stefanidis.)
Ultrasound imaging is valuable in musculotendinous injuries as part of the physical examination in trauma. Acute muscle contusion and hemorrhage appear hyperechoic, whereas later stages of injury exhibit mixed patterns. Partial or complete muscle tears with or without retractions are usually obvious (see Figure 51-2). Intramuscular hematomas may later evolve into seromas or intramuscular cysts (anechoic fluid collections) that may require aspiration or surgical drainage. Heterogeneous, grainy muscular patterns with a hypoechoic appearance of septa and fasciae are described in malignant hyperthermia. Hypoechoic muscular swelling with architectural disorganization may be observed in traumatic rhabdomyolysis.
Partial and complete tendon tears can be identified by dynamic ultrasound evaluation assessing the tendon’s integrity through the respective range of motion. Hypoechoic-to-anechoic intratendinal regions indicate partial tearing. Full-thickness tears may appear as tendon discontinuity with or without retraction and acoustic shadowing, whereas defects may be filled by hematomas or fat pad herniation (see Figure 51-2). An anechoic fluid cuff around a thickened, hyperechoic tendon with increased vascularity suggests tenosynovitis. Tendinopathy may appear as an intratendinous, hypoechoic patchy or hyperechoic calcific area with increased vascularity and loss of anisotropy and fibrillar pattern (see Figure 51-2). Ganglion cysts arise from the joint capsule or tendon sheaths and appear anechoic. The Baker cyst, first described by Dr. William Baker in 1877, is the most common mass in the popliteal fossa, which represents a fluid-distended gastrocnemio–semimembranosus bursa (see Figure 51-2).3
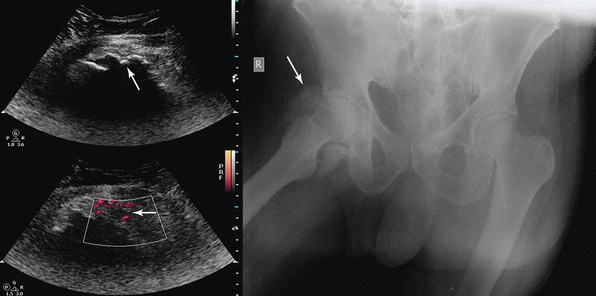
A cortical discontinuity with step-off deformity is the diagnostic hallmark of a bone fracture that is best seen in a plane perpendicular to the fracture line (Figure 51-3). Intensivists rarely deal with fracture diagnosis because patients arrive with a full set of radiographic imagery or after orthopedic surgery. Ultrasound imaging is relevant for monitoring adjacent soft tissue damage, such as hematomas or joint effusions (hemarthrosis). Monitoring can detect fracture nonunion and other complications (e.g., osteomyelitis) because ultrasound imaging depicts callus formation earlier than radiography, particularly in open injuries.2,3 Heterotopic ossification (HO) is usually observed in periarticular muscles of the limbs, mainly in neurologic states (spinal cord injury, hemiplegia after stroke, or traumatic brain injury) or after burns, trauma, and joint arthroplasty, and it can severely affect the joint’s range of motion. Early clinical features (pain, swelling, erythema, warmth) may resemble skin infection, deep vein thrombosis (DVT), or acute arthritis. Mineralization is detected by ultrasound earlier than in radiography as an inner hypoechoic core surrounded by a peripheral hyperechoic band of mineralized islands (“zone phenomenon”) with increased vascularity. In cases when calcifications have not yet occurred, HO may be confused with sarcomas (see Figure 51-3).
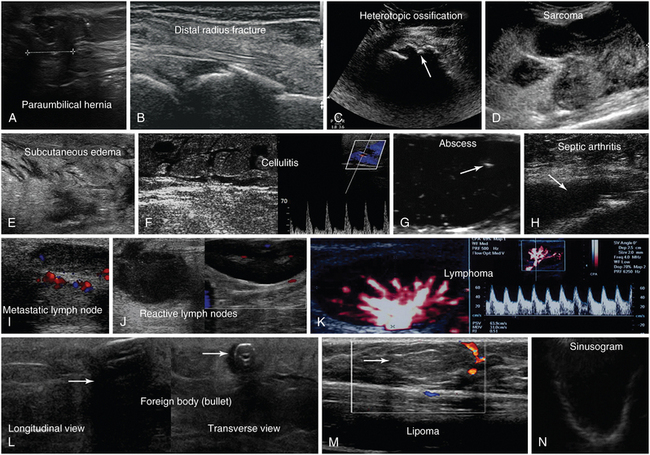
Figure 51-3 A, Longitudinal view of a paraumbilical hernia containing omentum (12-mm defect). B, Lateral/coronal long-axis scan of the distal forearm in a trauma patient. Note a comminuted distal radius fracture: four distinct segments of bone with mutual misalignment, with a hypoechoic area of a likely hematoma (note the extensor pollicis brevis tendon across the screen, parallel to the skin, and the general axis of the fractured bone). C, Visualization of the “zone phenomenon” in heterotopic ossification: an inner hypoechoic core is surrounded by hyperechoic mineralized islands (arrow) within the iliopsoas muscle adjacent to the hip joint. D, Heterogeneous groin mass with ill-defined borders (sarcoma). E, Cobblestone appearance of the subcutaneous tissue resulting from generalized interstitial edema in a heart failure patient. F, Cobblestone echotexture of the subcutaneous tissue in a region of the gastrocnemius muscle and depiction of unusually high flow (increased peak systolic Doppler velocity) in a local perforator (cellulitis). G, Zoom image of a subcutaneous hypoechoic abscess with hyperechoic punctiform material. H, hypoechoic knee effusion with echogenic material (arrow, septic arthritis). I, Hypoechoic metastatic arm lymph node in a patient with thyroid cancer. J, Reactive round- and oval-shaped groin lymph nodes. K, Oval-shaped groin lymph node with a Doppler-derived resistive index (RI) 5 0.51 (power Doppler mode), which was initially characterized as reactive, but eventually proved (biopsy) to be malignant (lymphoma) with cystic necrosis. L, Longitudinal and transverse planes of the thigh depicting a bullet casting an acoustic shadow (arrow) and the formation of “halo” (arrow), respectively. M, Longitudinal view of superficial lipoma (arrow) over the xiphoid process. N, Visualization of a totally fluid-filled sinus that appears hypoechoic, with its posterior and lateral walls delineated (sinusogram). (Images in A, courtesy Dr. K. Stefanidis; D, L, and M courtesy Dr. K. Shanbhogue; G, courtesy Dr. J. Poularas.)
Recently, ultrasound has been used to determine the type and extent of traumatic nerve injuries. In cases of nerve entrapment, associated changes in nerve contour and echotexture and causative extrinsic abnormalities may be depicted.5 The role of ultrasound in guiding regional anesthesia techniques is well established (see Chapter 53).
Ultrasonographic evaluation of infections in the ICU is another important application. Local spread of infection (e.g., involvement of muscles, joints, etc.), thrombophlebitis, causative factors (e.g., foreign bodies), or occult abscesses can be detected. Cellulitis is a diffuse infection of skin and subcutaneous tissue that may progress to abscess formation if left untreated. Initially, swelling with increased echogenicity of the involved area is depicted. Progressively, fluid-filled regions of hypoechoic edema create a cobblestone pattern. Detecting hypervascularity may be helpful to differentiate cellulitis from other causes of interstitial edema (e.g., congestive heart failure), which may also appear as anechoic, fluid-filled zones dissociating the connective septa (see Figure 51-3). Subcutaneous fat necrosis resulting from panniculitis or trauma resembles an ill-defined hyperechoic mass containing hypoechoic areas related to infarcted fat. Necrotizing fasciitis is a rapidly progressing and life-threatening infection involving deep fascia and muscle (usually located in the limbs, abdominal wall, or perineum). Thickened fascia with cloudy fluid collection (>4 mm in depth) and muscle swelling indicate the diagnosis, whereas subcutaneous gas can be identified as “dirty””acoustic shadowing with a reverberation artifact.2,6
Abscesses may exhibit a wide range of ultrasonographic patterns but are usually surrounded by an irregular rim that represents edematous soft tissue or cellulitis. An abscess cavity typically appears hypoechoic, although debris, septa, or bacterial gas can sometimes result in an isoechoic or hyperechoic appearance, making recognition difficult (see Figure 51-3). The increased through transmission effect and the movement of purulent material (induced with gentle transducer pressure) confirm the fluid nature. Color Doppler may depict hypervascularity within the abscess wall and in surrounding tissues while showing no Doppler signal within the abscess cavity. Abscesses and associated edema in skeletal muscle layers indicate pyomyositis (usually found in immunocompromised patients). Hypoechoic fluid collections adjacent to bone cortex (subperiosteal location in children), along with cortical irregularity, raise suspicion of osteomyelitis. Septic tenosynovitis may be encountered after penetrating injury and often shows fluid around the tendon and flow on color Doppler. Septic arthritis may be suggested by the presence of irregular internal echoes in a joint effusion (see Figure 51-3).2,6
Normal and reactive lymph nodes are usually oval shaped (short-to-long–axis ratio < 0.5); hypoechoic at the periphery compared with adjacent muscles, with echogenic medulla and hilum; and usually show low-resistive Doppler waveforms from the feeding artery (see Figure 51-3). Features favoring malignancy include larger size, rounded shape, eccentric versus concentric cortex thickening, heterogeneous echotexture with loss of identifiable medulla/hilum, presence of microcalcifications or cystic necrosis, and ill-defined borders. Malignant lymph nodes usually have high-resistance Doppler waveforms (resistive index [RI] > 0.8, pulsatility index [PI] > 1.5), but low-resistance flow patterns may be encountered in case of necrotic changes or advanced neovascularization (see Figure 51-3). The differential diagnosis of lymph node pathology (e.g., lymphoma, metastases) therefore requires an ultrasound-guided fine-needle aspiration (FNA) or excisional biopsy.2,4
Retained foreign bodies in soft tissues may lead to severe infections and disability. Ultrasound imaging detects even radiolucent foreign bodies (e.g., wood, plastic) and guides their removal. Most materials appear hyperechoic. Posterior acoustic shadowing or reverberation and comet-tail artifacts may be observed, depending on the size, shape, and structure; over time, edema, pus, or granulation tissue create a hypoechoic “halo” around the foreign body, enhancing its identification (see Figure 51-3).7 When searching for superficial foreign bodies in soft tissues of hand or foot, it is practical to scan the area immersed in saline bath to improve acoustical transmission and avoid pressure on the sensitive area.
Ultrasound can identify soft tissue tumors and assist in guided biopsy. Differentiating benign from malignant lesions may be facilitated by assessing size, border echogenicity, structure, adjacent tissues, vascularity, and Doppler-derived RI. Superficial lipomas usually do not require additional imaging or biopsy when demonstrating characteristic features (spindle shaped in cross sections; ovoid in the plane parallel to the skin; well-defined, elastic-compressible, with linear striations). Invasive tumors are typically hypoechoic heterogeneous masses with ill-defined borders; adjacent malignant lymph nodes may also be present (see Figure 51-3).4
Miscellaneous scanning targets
The HOLA ultrasound examination approach has a general head-to-toe sequence (see Chapter 1) and includes various tissue types and specific targets as modulated by the clinical circumstances. The examination may begin with facial structures, to assess soft tissues and facial bones as indicated. Eyelid, eye, and orbit scanning is discussed in Chapter 6. Ultrasound imaging is a valuable screening tool in intubated patients with maxillary bacterial sinusitis, which could be an occult cause of fever, nosocomial pneumonia, and meningitis.8,9 Radiography is unreliable in recumbent patients, whereas the costs and effort associated with computed tomography (CT) or magnetic resonance imaging (MRI) are not necessarily justified. The maxillary sinuses are scanned in sagittal and transverse planes just below the orbital rim (see Chapter 1). Normal air-filled sinuses elicit a reverberation artifact with parallel, evenly spaced echo lines. Totally fluid-filled sinuses allow a brightly echogenic line of the posterior wall and occasionally the two lateral walls to be seen, producing a pattern known as the “sinusogram” (see Figure 51-3). In partially fluid-filled sinuses, scanning with the patient in a more upright position results in periodic resonance artifact (as in a normal sinus) in the upper half (above the air-fluid level) and a posterior wall line in the lower, fluid-filled part of the image.8,9 Differentiation between tissue hypertrophy (significant mucosal thickening, nasal polyps) and fluid-filled sinus is achievable, but mucoid cysts or blood resulting from facial trauma (e.g., sinus wall fractures) are not easily differentiated. Other facial targets of interest are the parotid and submandibular glands. Parotitis is a rare complication of mechanical ventilation; the gland is easily identified in the preauricular area as hypoechoic and heterogeneous tissue with increased vascularity. Infection affecting the submandibular gland can also be ultrasonographically investigated. The mutual proximity of these two glands allows their side-by-side comparison; the pathologic gland is almost always relatively hypoechoic (darker). This side-by-side comparison of echogenicity is also highly useful in other anatomic sites such as testicular imaging.
Neck scanning zones are imaged in midsagittal and lateral planes, suprasternal views, as well as supraclavicular and infraclavicular approaches (Figure 51 E-2). Neurovascular structures, thyroid gland, extrathoracic trachea, lymph nodes, and soft tissues can be assessed. Ultrasound is useful in tracheal imaging and airway management. The longitudinal midline view shows tracheal rings as small well-aligned structures with a “string of beads” appearance (Figure 51 E-3). Beneath the cartilages, visualization of a brightly echogenic line and periodic resonance artifact correspond to the tracheal lumen. Additional oblique views may illustrate the entire tracheal anatomic configuration (see Figure 51 E-3). Preintubation scanning measures tracheal diameters (applied mainly in children), and aids in evaluating tracheal compression (e.g., diving goiter, lymph node) or stenosis (e.g., pharyngeal or laryngeal pathology). Landmarks (e.g., cricoid cartilage) that may be difficult to palpate (e.g., in obese patients, etc.) are depicted along with adjacent structures, such as the thyroid gland (e.g., thyroid isthmus, goiter, etc.) or vascular structures (e.g., aberrant brachiocephalic artery, innominate vein). This helps choose the tracheostomy and/or cricothyroidotomy site (see Chapter 52). Midline transverse planes can be used to depict the anterior jugular veins and tributaries of the venous jugular arch that remain major sources of hemorrhage when performing percutaneous tracheostomy in the ICU (see Figure 51 E-3). Postintubation scanning depicts the endotracheal tube and/or its cuff’s position in the midtrachea (suprasternal view). The tube is depicted as parallel echogenic lines with acoustic shadowing (see Figure 51 E-3), whereas the air-filled cuff is recognized by its curved contour and associated comet-tail artifacts (filling the cuff with saline improves visualization). Correct tube placement is also confirmed by its absence in the esophagus, presence of bilateral diaphragmatic motion (B-mode and M-mode) and bilateral lung sliding (pleural line movement) at the lung–chest wall boundary (see Chapters 1 and 19). Right mainstem bronchus intubation can be determined by the lack of motion of the left hemidiaphragm and a nonsliding left lung. Bilateral absence of lung sliding and paradoxic diaphragm motion during inspiration would indicate “esophageal intubation.”10 The tube will then be clearly seen in the esophagus and best approached in a transverse plane from the left side.
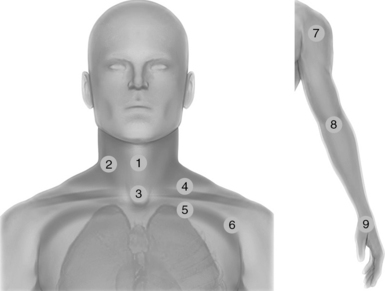
Figure 51 E-2 Neck scanning zones (left): Median line (1) and lateral (2) scanning zones; suprasternal view (3); supraclavicular (4) and infraclavicular (5) scanning approaches extending laterally (6); exploration of the upper limb (right) using the shoulder (7), elbow (8), and wrist joints (9) as landmarks.
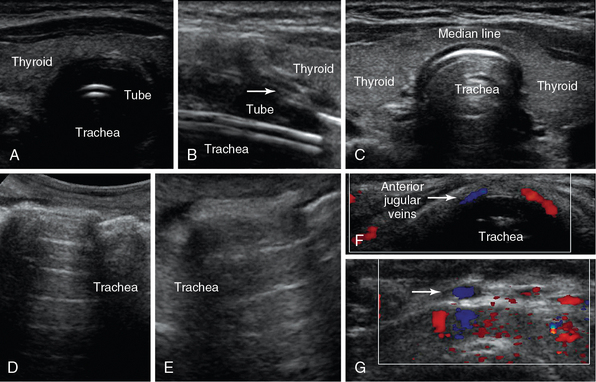
Figure 51 E-3 Median line plane: Transverse (A) and longitudinal (B) views of the intubated trachea depicting its rings as small hypoechoic structures with a “string of beads” appearance (arrow) and an endotracheal tube (series of echogenic parallel lines); transverse view at the level of the thyroid gland (median line) (C); oblique views of a normal trachea (D = median line, E = lateral); median-line transverse views depicting the anterior jugular veins (F) and tributaries (arrow) of the venous jugular arch, which may be helpful to identify when selecting optimal sites for performing percutaneous tracheostomy (G).
Thyroid gland ultrasound is a routine, highly revealing procedure and, in the ICU setting, is invaluable for screening for autoimmune thyroiditis, which is highly prevalent and underdiagnosed. The typical pattern is irregular, small, and hypoechoic and requires a certain level of experience to recognize. A normal pattern allows ruling out the condition, whereas positive or indeterminate results warrant laboratory assessment. The occasional detection of parathyroid tumors (homogeneously hypoechoic to the overlying thyroid gland) in acute hypercalcemia aids in prompt surgical intervention. CT is the preferred imaging modality for examining the retropharyngeal space; however, branchial cleft cysts, abscesses or hematomas can be evaluated by ultrasound. Supraclavicular and infraclavicular views (see Figure 51 E-2), which are extended laterally toward the axilla, may be used to evaluate tissues and structures, or plan and perform guided procedures (e.g., cannulation of central veins).
Scanning is extended to the upper limbs by using generic scanning approaches (applying longitudinal and transverse views bilaterally). Upper limb exploration uses MSK landmarks, such as shoulder, elbow, and wrist joints (Figures 51 E-4 and 51 E-5).1 Anterior and lateral dynamic shoulder views (Figure 51 E-6) are applied to detect the biceps tendon, tendons of the rotator cuff, and adjacent muscles. Scanning can then proceed to the arm to examine the brachial artery and vein (see Figures 51 E-4 and 51 E-7). The brachial artery can be followed distally to the anterior elbow, where it lies next to the median nerve. Anterior, lateral, and posterior views (see Figure 51 E-7) may assess the elbow and adjacent structures (e.g., effusion, tenosynovitis, muscle and tendon tears, etc.). Next, forearm exploration effectively reaches the wrist and the hand. By sweeping the transducer medially to examine the Guyon tunnel, the pisiform is detected adjacent to the ulnar artery, whereas lateral wrist exploration is facilitated by identifying the radial styloid adjacent to the radial artery (Figure 51 E-8). The latter view may evaluate the first wrist compartment (e.g., de Quervain disease). Moving the transducer distally from the level of the Lister tubercle, the scaphoid, the scapholunate ligament, and the fourth compartment of extensor tendons can be evaluated, whereas the median nerve is identified within the carpal tunnel, where it can be entrapped (see Figure 51 E-8). In the ICU, hand scanning has limited applications; however, it may be performed to evaluate traumatic injuries or other abnormalities (Figure 51 E-9 and see Figure 51 E-5). The veins of the upper extremity are often used for vascular access and may be evaluated for signs of phlebitis and/or thrombosis as clinically indicated.
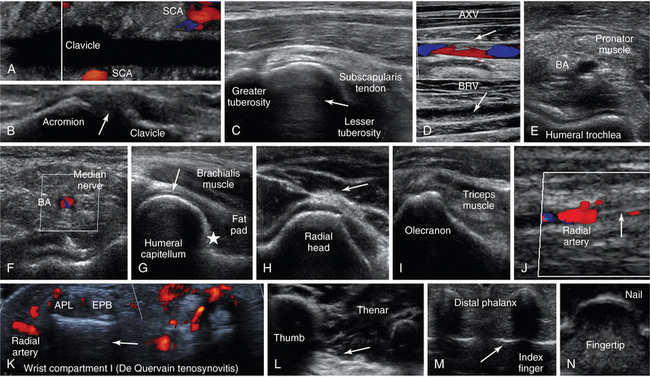
Figure 51 E-4 A, Oblique lower neck view depicting the subclavian artery (SCA) at the borders of the clavicular acoustic shadow. B, Coronal plane view over the acromioclavicular joint (arrow = joint space). C, Transverse view of the anterior shoulder depicting the biceps tendon’s long head (arrow) between the lesser and greater tuberosities. D, Partial flow in the axillary vein (AXV) resulting from thrombosis (arrows), which extends to the brachial vein (BRV). Transverse views of the anterior elbow depicting the humeral trochlea (E) and the brachial artery accompanied by the median nerve (F). G, Medial sagittal plane of the coronoid fossa (arrow = articular cartilage of distal humeral epiphysis, star = anterior coronoid recess). H, Lateral elbow view of radial head and posterior interosseous nerve (arrow). I, Posterior elbow (in flexion) view depicting the olecranon fossa. J, Longitudinal view of the lateral wrist depicting an arterial line (arrow) in the radial artery. K, Focal thickening and increased vascularity surrounding the de Quervain tendons abductor pollicis longus (APL) and extensor pollicis brevis (EPB) at the level of radial styloid process (de Quervain tenosynovitis). L, Oblique ventral view of the proximal palm showing the thenar eminence (arrow = fascia), M, Transverse view of the interphalangeal joints (arrow) of the index and middle fingers. N, Transverse inverted view of the index finger’s tip (“sonographic fingerprint”).
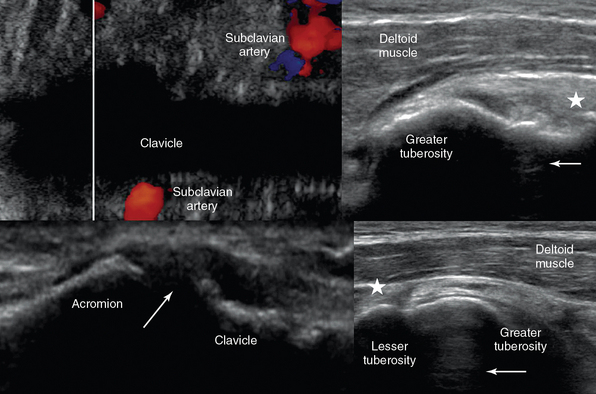
Figure 51 E-5 Longitudinal dorsal views of the second digit, which demonstrate minor trauma of the distal phalanx (top) and a normal phalanx (bottom), respectively. (Courtesy Dr. Hasmik Berberyan.)
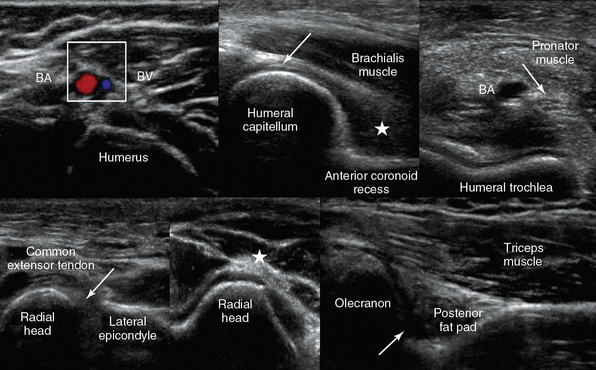
Figure 51 E-6 Top, Oblique lower neck views demonstrating that a vessel (subclavian artery) can still be visualized at the borders of a bone’s (clavicle) acoustic shadow (left); anterior shoulder view depicting the biceps (arrow) and supraspinatus (star) tendons adjacent to the greater tuberosity (right). Bottom, Coronal plane view over the acromioclavicular joint (arrow = joint space). By sweeping the transducer from this position anteriorly and posteriorly over the joint, the presence of an os acromiale may be demonstrated (left); anterior shoulder view with the arm in slight internal rotation (directed toward the contralateral knee) and the elbow flexed 90 degrees (palm up) depicting the biceps tendon between the lesser and greater tuberosities (star = subscapularis tendon; right).
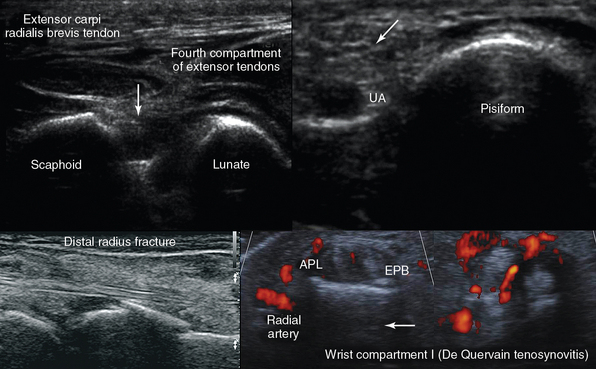
Figure 51 E-7 Top, left-to-right, Medial transverse view of the arm depicting the brachial artery and vein; anterior elbow views: medial sagittal plane of the coronoid fossa where a small amount of fluid may be normally seen between the fat pad (star) and the humerus (arrow = articular cartilage of distal humeral epiphysis); and transverse view depicting the pronator muscle, the brachial artery, and the median nerve (arrow) over the trochlea. Bottom, left-to-right, Lateral elbow planes depicting the lateral synovial fringe (arrow), between the radial head and the lateral epicondyle, and the posterior interosseous nerve (star); posterior view of the elbow (partial flexion) depicting the olecranon fossa, the triceps muscle, and the posterior olecranon recess (arrow).
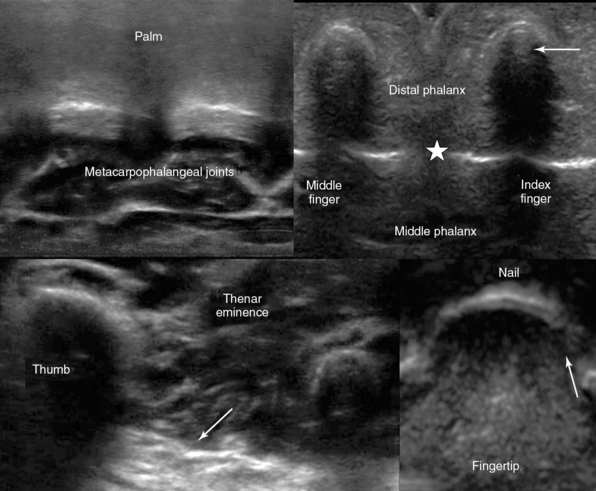
Figure 51 E-8 Top, left-to-right, Transverse dorsal view of the scapholunate ligament (arrow); medial wrist view of the ulnar artery (UA) and nerve (arrow) adjacent to the pisiform. Bottom, left-to-right, Distal radius fracture and de Quervain tenosynovitis.
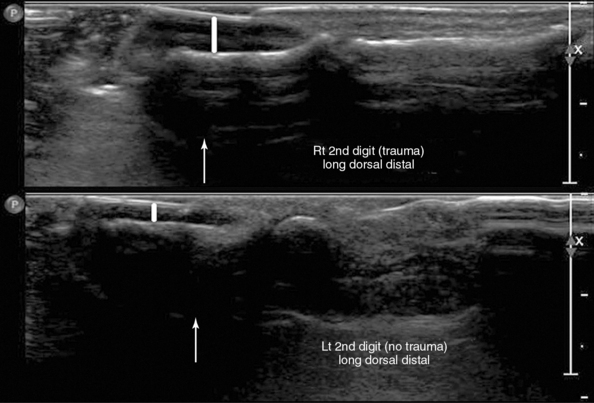
Figure 51 E-9 Suggested “fast” hand exploration by D. Karakitsos (the “h-scan”): Top, Transverse palmar view of metacarpophalangeal joints (left); transverse view of index and middle fingers (arrow = vincula tendinum and star 5 interphalangeal joints, right). Bottom, Oblique proximal palmar view of the thenar eminence (arrow = fascia, left); transverse inverted view of index finger’s (arrow = eponychium) distal phalanx (fingertip) and nail (“sonographic fingerprint”, right). Using these transverse planes as reference views, the operator can extend scanning accordingly to include longitudinal planes and detect thus even minor injuries (see Figure 53 E-5).
Surface ultrasound imaging of the thoracic and abdominal wall may reveal comet-tail vertical lines that start at a level external to the ribs and hide underlying structures. This finding signals parietal subcutaneous emphysema, a possible sign of pneumomediastinum or esophageal rupture. A focal region of abdominal tenderness and swelling may be due to a rectus sheath hematoma (e.g., bleeding disorders, postprocedural). Ultrasound delineates the entity within the abdominal wall and may guide its percutaneous drainage. Other abdominal wall abnormalities include hernias, lipomas, tumors, pseudoaneurysms, or dilatated superficial veins (Figure 51 E-10).2,4 A recanalized left umbilical vein is possible to follow to the teres ligament from the umbilical area in many patients with portal hypertension.
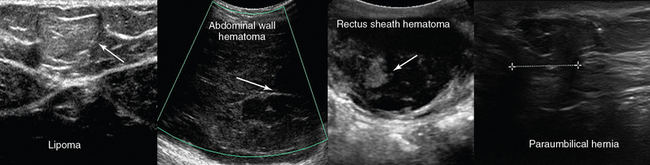
Figure 51 E-10 Left to right, Superficial lipoma (arrow); absence of flow in a superficial abdominal wall hematoma; depiction of a rectus sheath hematoma with mixed echogenicity pattern because of fibrin organization (arrow); longitudinal view of paraumbilical hernia containing omentum (12-mm defect).
Scanning is extended to the lower limbs via the groin (Figure 51 E-11). Several abnormalities may be encountered in the inguinal area, such as DVT, pseudoaneurysms, vascular bypass grafts, inguinal and femoral hernias, reactive and metastatic lymph nodes, lymphomas, abscesses, hematomas, tumors, and so forth (Figure 51 E-12). The exploration of lower limbs is facilitated by using MSK landmarks, such as hip, knee, and ankle joints. A low-frequency transducer (convex) is used when scanning the hip joint. By rotating the transducer between 45 to 60 degrees, and translating slightly forward, a longitudinal scan of the femoral neck can be obtained, and the femoral head can be identified Figure 51 E-13). Examination of deep veins to exclude DVT is a required examination in many intensive care units (see Chapter 9). Assessing other abnormalities that can mimic DVT (Baker cyst, hematomas, cellulitis, lymph nodes, heterotopic ossification, etc.) is equally important (see Figure 51 E-10). Dynamic MSK scanning in the lower limbs can be facilitated by a two-person technique as previously described. Scanning starts from the hip joint and continues caudally. The anterior and posterior aspects of the thigh are investigated (e.g., muscles, bones, neurovascular structures). The sciatic nerve can be depicted as it courses the posterior thigh (Figure 51 E-14). A microconvex, or even a convex probe, may be chosen for this area because of depth penetration challenges and the large structures that a linear probe may fail to adequately show. By sweeping the transducer toward the knee (anterior plane), the quadriceps tendon “anchoring” at the patella is depicted (see Figure 51 E-14). Anterior and lateral views (see Figures 51 E-14 and 51 E-15) may be used to evaluate the knee (e.g., effusions); posterior views access the popliteal fossa to evaluate major neurovascular structures (see Figure 51 E-15) or depict other abnormalities (e.g., popliteal cysts, etc.). As scanning advances distally toward the ankle, muscles (e.g., gastrocnemius muscle) and vessels (anterior tibial and peroneal veins) are accordingly explored. In supine ICU patients, midline longitudinal planes over the ankle dorsum identify the anterior recess of the tibiotalar joint. By sweeping the transducer medially and laterally, the talar dome is seen in 60% to 70% of cases (Figure 51 E-16).1 Lateral ankle planes evaluate the anterior talofibular ligament; medial ankle scanning is performed by rotating the foot slightly laterally, thus allowing visualization of medial malleolus and posterior tibial vessels (see Figure 51 E-16). Dorsal foot scanning offers a view of the small and tortuous dorsalis pedis artery, which may be used for placing an arterial line. Finally, the Achilles tendon, the most commonly injured ankle tendon, should be examined along its full course by using both longitudinal and transverse planes; using two or three operators and lateral positions may permit dynamic scanning (passive dorsal and plantar flexion to distinguish partial from complete Achilles tendon tears (see Figure 51-2).

Figure 51 E-11 Lower limb exploration: (1) Inguinal area and hip joint investigation that is extended distally using the knee (2) and ankle (3) joints as landmarks.
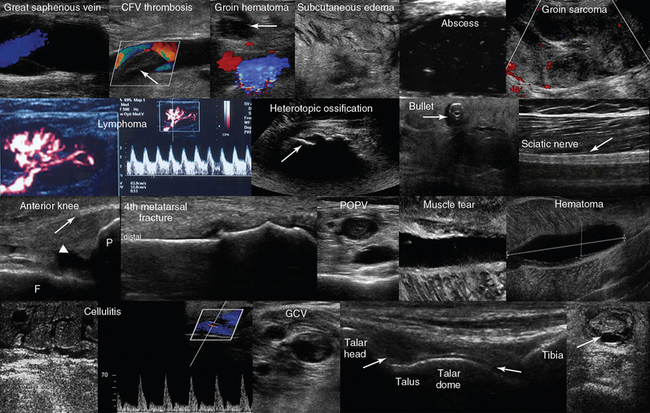
Figure 51 E-12 Top, left-to-right, Normal venous flow in the great saphenous vein; fresh thrombus in the common femoral vein; groin hematoma (arrow) over the common femoral artery and vein; cobblestone appearance of soft tissues (groin) resulting from generalized edema; hypoechoic cyst filled in with hyperechoic punctiform material (abscess); groin tumor of mixed echogenicity (sarcoma). Second line, left-to-right, Reactive groin lymph node; visualization of the “zone phenomenon” of heterotopic ossification: the inner hypoechoic core is surrounded by several hyperechoic mineralized islands (arrow) within the iliopsoas muscle; depiction of a bullet in the anterior thigh casting an acoustic shadow; posterior aspect of the thigh illustrating the sciatic nerve (arrow). Third line, left-to-right, Longitudinal view of the anterior knee depicting the femur (F), the patella (P), the suprapatellar synovial recess (arrowhead) and the distal third of the quadriceps tendon (arrow); longitudinal image (dorsal approach) of the fourth metatarsal bone depicting a cortical discontinuity with step deformity and adjacent tissue damage and edema (fracture). The thickness of overlying soft tissue can be compared with the contralateral side to appreciate the degree of edema (note the domelike curvature of the dorsal fascia and the curved tendon above the fracture); visualization of a popliteal vein thrombosis; zoom image of a soleus muscle tear; liquefying hematoma in the gastrocnemius muscle. Bottom line, left-to-right, Cobblestone appearance of the subcutaneous tissue and unusually high flow in a local perforator (cellulitis); visualization of a gastrocnemial vein thrombosis; midlongitudinal plane over the dorsum of the ankle depicting the anterior recess of the tibiotalar joint (arrows); transverse view of a ruptured Achilles tendon. (Top line image of groin hematoma courtesy Dr. K. Stefanidis; image of hyperechoic cyst courtesy Dr. J. Poularas; image of groin tumor courtesy Dr. K. Shanbhogue; second line image of bullet in anterior thigh courtesy of Dr. K. Shanbhogue.)
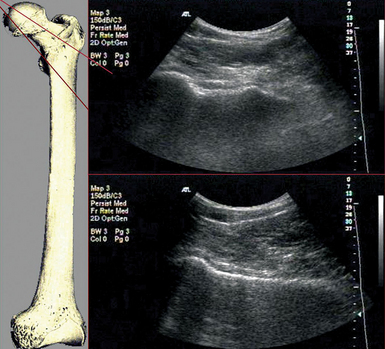
Figure 51 E-13 A low-frequency transducer (convex) is used to achieve a wide field of view and excellent penetration when scanning the hip joint. By rotating the transducer between 45 to 60 degrees and translate slightly forward, a longitudinal scan of the femoral neck can be obtained, and the femoral head can be identified. There is a range of possible transducer positions that covers the entire anterior aspect of the neck and should be explored if a fracture is suspected but not obvious. There are some irregularities of the normal femoral neck cortex that correspond with the tendinous insertions of the hip capsule. These may be confused with the cortical discontinuity of a hip neck fracture.
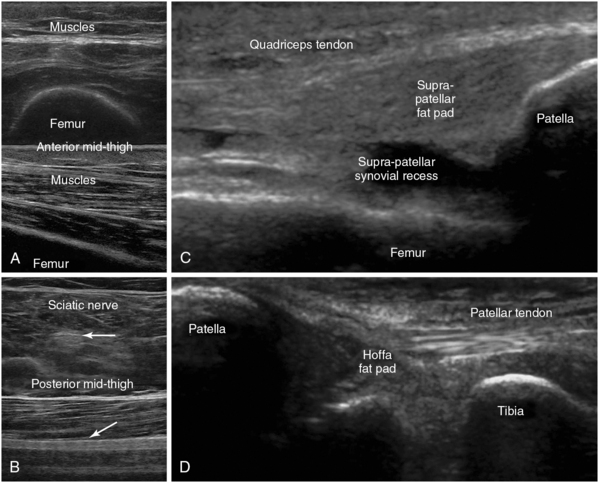
Figure 51 E-14 A, Transverse and longitudinal anteroposterior views of the thigh demonstrating the skin, subcutaneous tissue, muscles, and the underlying acoustic shadow of the femur. B, Transverse and longitudinal views of the sciatic nerve (arrows) coursing the posterior thigh; anterior knee views (longitudinal plane) depicting the femur, patella, suprapatellar synovial recess, and the distal third of the quadriceps tendon (proximal to the patella) (C), and as scanning is extended distally, permitting visualization of the patella, tibia, Hoffa fat pad, and the patellar tendon (D).
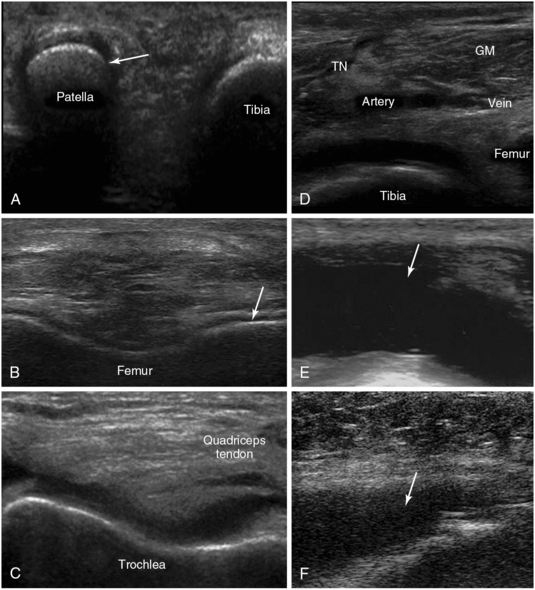
Figure 51 E-15 A, Medial knee (partially flexed) view demonstrating the medial facet of the patella (arrow). B, Depiction of the hyaline cartilage at the right lateral condyle. C, Transverse view of the V-shaped trochlea (knee partially flexed). D, Transverse view of the popliteal fossa demonstrating the gastrocnemius muscle and the popliteal vessels adjacent to the tibial nerve (TN), which appears rather blurred because of a recent guided nerve block. E, Posttraumatic knee joint effusion (arrow). F, Septic knee arthritis (arrow).
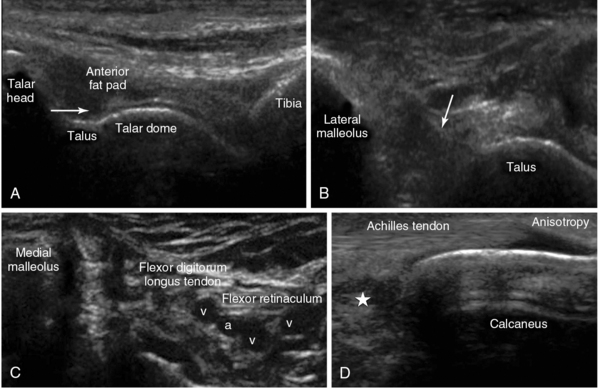
Figure 51 E-16 A, Midlongitudinal view of the anterior recess (arrow) of the ankle joint. By sweeping the transducer medially, the talar dome can be visualized (in 60%-70% of cases). B, Lateral ankle view of the anterior talofibular ligament (arrow). C, Medial ankle plane view (foot rotated laterally) depicting the medial malleolus and the posterior tibial vessels (a = artery, v = vein). D, Longitudinal view of the calcanear insertion of the Achilles tendon overlying the Kager fat pad (star).
Pearls and highlights
• Any ultrasound view obtained by placing the transducer on the skin includes potentially useful anatomic information.
• According to the HOLA concept, soft tissues may be a primary scanning target (e.g., in extremity crush injury) or may serve as an anatomic reference during imaging of deeper structures (e.g., skin, fat and fascia are identified over the vessel to be cannulated).
• Correct identification of anatomy is essential in both generic sense (types of tissues and layers) and site-specific sense (named vessels, muscles, nerves, bundles, compartments, joints).
• Ultrasound can greatly augment the physical examination of a conscious as well as sedated ICU patient and reliably identify soft tissue and MSK abnormalities.
• Hematomas usually appear as collections of extravascular fluid, showing serial changes in echogenicity over time (anechoic in acute phase and/or after clot liquefaction).
• An advanced HOLA protocol in crush injuries, accompanied by compartment syndrome can include assessment of the shape of the fascial compartment for outward convexity of the normally flat fascial borders, color and pulsed wave Doppler monitoring of the vessels feeding the compartment, renal imaging, and search for free abdominal fluid if the thigh and pelvis areas are involved.
• In the ICU, ultrasound is rarely used for the diagnosis of bone fractures, although ultrasonographic monitoring of fractures and their traumatic sequelae (e.g., hematomas, hemarthrosis, osteomyelitis) is useful.
• Heterotopic ossification can be detected by ultrasound imaging in its earliest stages.
• Ultrasound imaging detects local spread of infection (e.g., involvement of skin, muscles, joints), thrombophlebitis, potential causative factors (e.g., foreign bodies), or occult abscesses.
• Several ultrasound features may aid in differentiating malignant from benign lymph nodes and/or tumors of soft tissues, although biopsy is still required for definitive diagnosis.
• Ultrasound imaging detects maxillary sinusitis and aids in airway management.
• Ultrasound scanning of the upper and lower limb can be used to guide interventions, such as vascular access, placement of nerve blocks, or drainage of fluid collections. However, by applying the HOLA scanning concept, operators learn to appreciate all the structures in a ROI and thus avoid missing abnormalities during site-specific scanning.
References
1. European Society of Skeletal Radiology. Musculoskeletal ultrasound-technical guidelines. http://www.essr.org/cms/website.php?id=/en/essr_home.htm, March 25, 2013. [Accessed on].
2. Dewitz, A, Frazee, BW. Soft tissue. In: Ma OJ, Mateer JR, Blaivas M, eds. Emergency ultrasound. 2nd ed. New York: McGraw Hill; 2008:414–444.
3. Jacobson, J. Basic pathology concepts. In: Jacobson J, ed. Fundamentals of musculoskeletal ultrasound. Philadelphia: Saunders Elsevier; 2007:15–38.
4. Smith, SE, Salanitri, J, Lisle, D. Ultrasound evaluation of soft tissue masses and fluid collections. Semin Musculoskelet Radiol. 2007; 11:174–191.
5. Zhu, J, Liu, F, Li, D, et al. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011; 21:1097–1101.
6. Chau, CL, Griffith, JF, Musculoskeletal infections: ultrasound appearances. Clin Radio. 2005; 60:149–159.
7. Boyse, TD, Fessell, DP, Jacobson, JA, et al. Ultrasound of soft-tissue foreign bodies and associated complications with surgical correlation. Radiographics. 2001; 21:1251–1256.
8. Hilberg, G, Vargas, F, Valentino, R, et al. Comparison of B-mode ultrasound and computed tomography in the diagnosis of maxillary sinusitis in mechanically ventilated patients. Crit Care Med. 2001; 29:1337–1342.
9. Vargas, F, Boyer, A, Bui, HN, et al. A postural change improves the prediction of a radiological maxillary sinusitis by ultrasonography in mechanically ventilated patients. Intensive Care Med. 2007; 33:1474–1478.
10. Susti´c, A. Role of ultrasound in the airway management of critically ill patients. Crit Care Med. 2007; 35:S173–S177.