CHAPTER 36 Soft Tissue Coverage of the Elbow
INTRODUCTION
Difficult-to-manage soft tissue defects about the elbow occur as a result of trauma, infection, extravasation of chemotherapeutic agents, cutaneous ulceration or necrosis, and limb-sparing tumor surgery. Treatment options are many, and appropriate management requires careful consideration of all alternatives. Coverage choices may include primary closure, skin grafting, local cutaneous flaps, fasciocutaneous transposition flaps, island fascial or fasciocutaneous flaps, local or distant one-stage muscle or myocutaneous transposition, distant temporary pedicle flaps, and microvascular free tissue transfer. Historically, the reconstructive algorithm for providing elbow coverage has proceeded in a stepladder fashion, with the simplest procedures being used first to obtain coverage; however, with advancements in microsurgical techniques, the reconstructive ladder is often disregarded in preference for the surgical procedure, which provides the patient with the best form and function.
PATIENT ASSESSMENT
THE WOUND
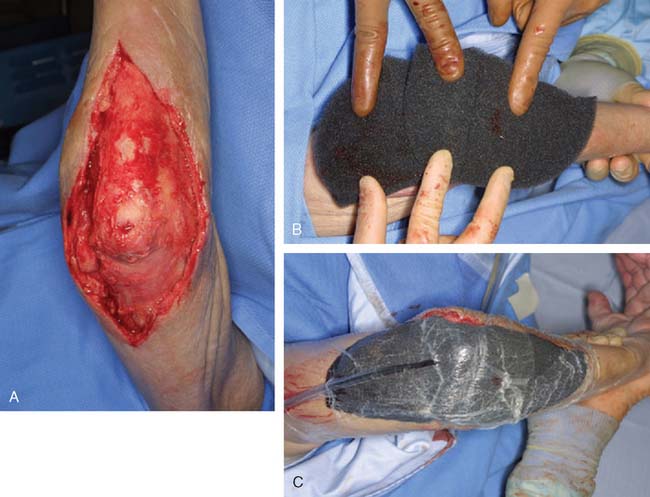
Most soft tissue defects benefit from early coverage. Advantages of expeditious closure include diminished edema, lower rates of wound infection, decreased scar formation and wound contraction, less pain, and enhanced upper limb function.65,69,106,110 Traumatic defects require thorough débridement and irrigation but often may be closed within 24 hours of injury, with salutary results.31,65 In contrast, complex wounds resulting from high-energy injury (electrical burns, crush, avulsion) and grossly contaminated wounds may require serial débridements and a delay to allow adequate assessment of tissue viability. Open packing, temporary coverage with biologic dressings or use of negative pressure therapy devices (vacuum-assisted closure [VAC]) may be required during this phase of management. VAC therapy has become an important adjunct in the management of major soft tissue injuries over the last 10 years. VAC therapy (V.A.C., KCI, San Antonio, TX) uses a polyurethane ether foam with a pore size of 400 to 600 mm, which is cut to size and applied to the wound with a transparent dressing to create an airtight seal. Controlled intermittent or continuous topical negative pressure is then delivered to the wound by incorporating a tube into the seal, and this is provided by a dedicated vacuum pump appliance. Exudate, edema, and bacterial load is reduced and the growth of granulation tissue is encouraged, and these properties make it a useful tool in any algorithm for soft tissue reconstruction because it has the potential to convert a difficult wound into one that may be closed or covered with a simpler technique. Its use is particularly important in two scenarios—when systemic comorbidity precludes a major lengthy reconstructive procedure; and when local wound factors, such as heavy contamination, necessitate multiple wound débridements before the definitive wound closure.3 In these situations, VAC therapy is not usually a substitute for surgery, but it offers a bridge until definitive surgery can proceed safely11 (Fig. 36-1).
RECONSTRUCTIVE METHODS
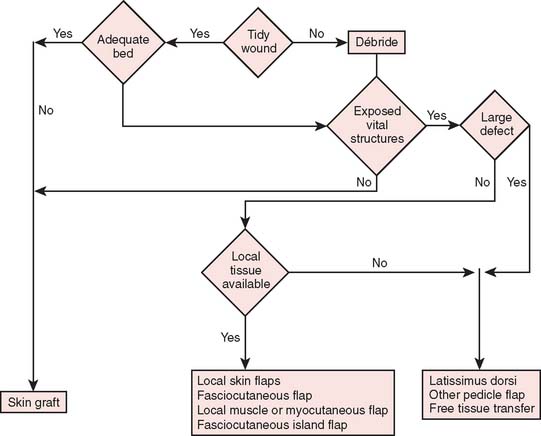
Reconstructive goals are to obtain optimal wound healing, restore function, and maximize aesthetic outcome, which should always be considered. Historically, the simplest method that provides adequate coverage and meets reconstructive needs is chosen. Thus, tension-free closure of an elbow defect by skin elevation and advancement is often performed. If tension-free closure is not possible, skin grafting can be considered for selected defects with well-vascularized tissue beds. Otherwise, the location, size, and tissue composition of a defect all in part dictate selection of an appropriate flap. Local flaps provide the best tissue match but are limited in size and can devascularize or weaken an already damaged limb. Larger defects and those that require composite tissues for primary reconstruction of tendon, muscle, bone, or sensibility demand consideration of other methods, including local island fascio-cutaneous flaps, local or distant pedicle muscle, myocutaneous transfer, and free tissue transfer. An algorithm for tissue coverage is useful in preoperative planning (Fig. 36-2).
SKIN GRAFTING
Indications
Skin grafting may be indicated for any defects with an acceptable bed. Exposed structures that will accept a graft include subcutaneous tissue, paratenon, and muscle. Other tissues, such as exposed bone, joint, tendon, and nerve, may be covered temporarily by graft used as a biologic dressing but will not support a graft for permanent coverage. Beds containing tissues of questionable viability, chronic granulation tissue, or frank infection must be appropriately débrided. Bacterial contamination, accumulation of serum, and a small amount of capillary bleeding are not contraindications for grafting. Tidy wounds that contain fewer than 105 bacteria per gram of tissue or that allow xenograft adherencewithin 24 hours allow successful skin grafting.27,52,61,93,107 Skin grafts are contraindicated in areas that are exposed to repetitive trauma or that lie over osseous prominences. In addition, skin grafting should be avoided in areas that may require secondary surgery for bone or nerve grafting. All split-thickness grafts will undergo some component of contracture over time; thus, if these grafts are placed over large areas of the antecubital fossa or olecranon, there is a risk of limitation in elbow motion. To improve durability, a collagen-glycoaminoglycan biodegradable matrix (Integra, Plainboro, NJ) can be grafted first to create a neo-dermis, then a split-thickness skin graft can be applied subsequently to improve durability of the graft.18
Technique
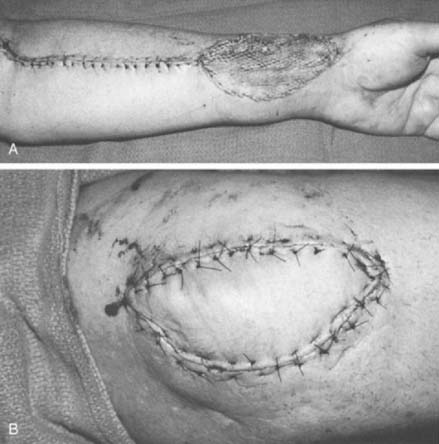
Small defects in areas where wound contracture is undesirable may be optimally covered with a full-thickness graft harvested from the inguinal crease. The thin, lax skin in this area provides excellent graft material and allows closure of the donor site with an inconspicuous linear scar. Split-thickness grafts that are harvested with a power-driven dermatome are used to cover larger areas. Any area may be used as a donor site; usually the proximal thigh is chosen because of its relatively flat contour and generally concealed location. The thickness of such grafts can range from 0.012 to 0.015 inch. Older adults and children with thin dermis should receive 0.012-inch thick graft to avoid full thick skin loss at donor site. A meshed graft allows expansion and is indicated for large or draining defects. The donor site is dressed with a plastic vapor-permeable dressing or fine mesh gauze, and exudate is removed from beneath the donor site with a syringe, as needed, or the gauze is dried with a heat lamp.45,96 The graft is secured with chromic sutures or stables and covered with Xeroform or some other form of nonadherent occlusive dressing. Immobilization of the graft during the early stages of healing is essential for complete graft survival and may be accomplished with either the use of a bolster or a VAC sponge. A cotton bolster may be created with gauze and saline-soaked cotton balls. Nylon sutures secured to the margin of the graft are tied over the cotton, firmly compressing the skin to its bed. The elbow is immobilized, and the graft is left undisturbed for 5 to 7 days. If VAC therapy is to be used to promote graft adherence the sponge is set to provide 75 mm Hg of constant suction for 5 days. This method has been shown to significantly improve skin grafting survival in a recent prospective randomized study.81
LOCAL FLAPS
Random Cutaneous Flaps
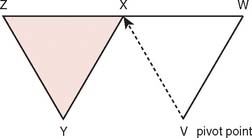
Often, small defects unsuitable for grafting may be covered by skin immediately adjacent to the primary defect. Such random cutaneous flaps carry their own blood supply through the dermal and subdermal plexuses and occasionally through a specific cutaneous artery oriented in the axis of the flap (axial flap). In general, random flap should be designed with a 1 : 1 length-width ratio. Such flaps are further categorized according to motion applied to the flap as it covers the defect; these flaps include transposition or translation flaps, advancement flaps, and rotation flaps.63 Transposition flaps are raised and moved laterally to an adjacent area, and the primary defect is closed by triangulating the defect and closing one angle of the triangle. The flap is then drawn as a parallelogram and raised (Fig. 36-3). Its pivot axis or critical line may be extended by lengthening or widening the flap or extending the cut parallel to the defect side (extension cut) or toward the defect (back cut).63 Closure of the secondary defect generallyrequires skin grafting. Other examples of transposition flaps include Z-plasties and rhomboid flaps (i.e., in the shape of an equilateral parallelogram). Rhomboid flaps are occasionally useful about the elbow when the defect may be excised or débrided to a rhomboid shape. A rhombus with 60- and 120-degree angles, the Limberg flap, is optimal, but other angles may be accommodated using Dufourmental’s method (Fig. 36-4).64 Such single rhomboid flaps have the disadvantages of creating tension at the flap tips and at the line of donor site closure, anatomic landmark displacement, and occasionally, cause difficulty in orientation for optimal scar appearance. A double-Z rhomboid may be a superior alternative for some defects (Fig. 36-5).21 Orientation of the long axis of the rhomboid to the relaxed skin tension line produces the least tension and “maximal cosmesis.”
Axial Fasciocutaneous Flaps
The above-mentioned random flaps are limited in their ability to cover large defects due to a poor random blood supply, limited mobility, and inadequate local skin elasticity. In contrast, the axial flap by inclusion of a known axial blood supply can allow safe extension of the length and narrowing of the base to improve coverage.79
The blood supply to the skin of the upper extremity was studied by Manchot71 more than a century ago and most recently by Le Huec and Liquois,59 by Salmon93a and Lamberty,56 and by others.94 A predictable pattern of cutaneous angiosomes exists that can be used in planning local flaps (Fig. 36-6). Blood from the named vessels supplying each of these regions may enter the skin from direct cutaneous vessels, musculocutaneous perforators, or fasciocutaneous vessels.19 These vessels form a plexus both subdermally and in deep fascia.108 Although forearm and elbow transposition flaps containing only subcutaneous tissue have been reported by Marty and colleagues, inclusion of deep fascia in transposition flaps improves viability.19,29,74,76 The bloodsupply to these areas of skin may come from multiple fasciocutaneous perforators, a single consistent and sizable pedicle, or multiple small branches from an underlying large vessel, the most dominant of which is the superior ulnar collateral artery.59
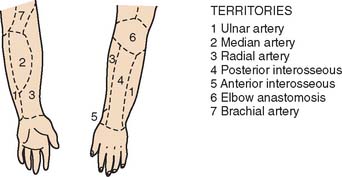
FIGURE 36-6 A reproduction of Manchot’s original diagrams of the forearm angiotomes.
(From Lamberty, B. G. H., and Cormack, G. C.: The forearm angiotomes. Br. J. Plast. Surg. 35:420, 1982.)
Fasciocutaneous transposition flaps may be raised from the medial arm based on the medial elbow anastomotic vessels (superior ulnar collateral artery and ulnar recurrent vessels), the lateral arm based on the terminal branches of the profunda brachii and their recurrent interosseous artery anastomoses, as well as forearm fasciocutaneous perforators from radial, ulnar, and anterior and posterior interosseous arteries.50,56,74,99 These perforators, arranged in four parallel rows, enter the forearm skin at regular intervals (Fig. 36-7).74 An example of such a flap is shown in Fig. 36-8, based on the posterior interosseous vessels and lateral elbow anastomotic blood vessels. Of all available forearm fasciocutaneous arteries, Lamberty and Cormack found that only the inferior cubital cutaneous artery, a branch of the radial recurrent artery, was sufficiently large and constant to reliably supply a large cutaneous area (most of the territory attributed to the median artery by Manchot) (see Fig. 36-6).56,57 Coverage of the olecranon is achieved reliably and easily with this tissue technique. In case of extensive trauma or reoperative surgery, it is advisable to use a hand-held Doppler device to confirmthe presence and extent of the vascular pedicle before raising the flap.
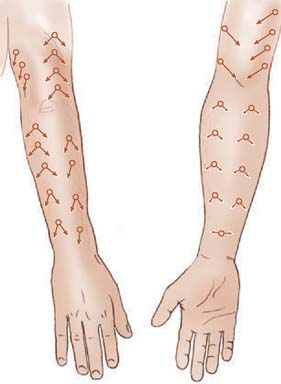
FIGURE 36-7 Location of cutaneous perforating vessels to the forearm.
(From Marty, F. M., Montandon, D., Gumener R., and Zbrodowski, A.: The use of subcutaneous tissue flaps in the repair of soft tissue defects of the forearm and hand: an experimental and clinical study of a new technique. Br. J. Plast. Surg. 37:95, 1984.)
Island flaps are the ultimate application of fasciocutaneous axial transposition flaps. Several upper extremity island flaps have been described in the past decade for use in orthograde or retrograde pedicle transposition or free tissue transfers. Island flaps are raised with a base consisting solely of a fully mobilized vascular pedicle, and they may include fascia, subcutaneous tissue, skin, and segments of vascularized muscle, bone, tendon, and cutaneous nerves, depending on the particular reconstructive needs. Some flaps used for elbow coverage are orthograde antecubital fasciocutaneous flap, radial forearm, ulnar forearm, and posterior interosseous flaps and retrograde medial and lateral arm flaps.* Their orthograde axial vascular pedicle allows the widest possible arc of rotation and present relatively little risk of circulatory embarrassment. Coverage of small and moderate defects on all surfaces of the elbow is possible without microvascular anastomoses and upper extremity mobilization.37
Antecubital Fasciocutaneous Flap
Described by Lamberty and Cormack,56,57 the antecubital fasciocutaneous flap is an axial-pattern, fasciocutaneous flap based on the inferior cubital artery. This vessel usually arises from the radial recurrent artery (17.5%) or from the radial artery proper (62.5%) and lies in the intermuscular septum between brachioradialis and flexor carpi radialis.70 It runs distally, paralleling the course of the cephalic vein in the superficial fascia. The vessels origin is 4 cm inferior to the midportion of the anterior interepicondylar line (Fig. 36-9). Venous drainage is provided principally by the cephalic vein.57 A 4:1 length-width-ratio flap may safely be raised.
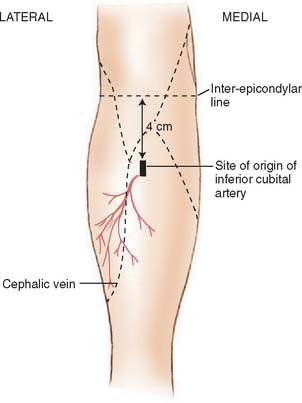
FIGURE 36-9 The distribution of the inferior cubital artery.
(From Lamberty, B. G. H., and Cormack, G. C.: The forearm angiotomes. Br. J. Plast. Surg. 35:420, 1982.)
Radial Forearm Island Flap
The cutaneous territory nourished by the radial artery includes the radial two thirds of the anterior forearm and its lateral aspect.56 A fasciocutaneous flap based on an orthograde (proximal) pedicle provides excellent elbow coverage of moderate-sized defects, including the posterior aspect of the region.2,24,28,35,63 Approximately 9 to 17 cutaneous perforators from the radial artery supply the skin, including musculocutaneous vessels proximally and fasciocutaneous vessels distally by means of a deep prefascial plexus and a subdermal plexus.105 Its chief advantages are its reliability, technical simplicity, and versatility, the results of its being a compound flap including fascia only, composite skin and fascia, vascularized brachioradialis, flexor carpi radialis, or palmaris longus tendon segment of the distal radius and its sensibility with lateral and medial antebrachial cutaneous nerves.26,37,82 Its arc of rotation allows coverage of all aspects of the elbow joint but is limited proximally by its point of rotation at the radial artery origin 10 cm below the elbow joint.105 The principal drawback of this method is the unsightly donor site, which requires split-thickness skin grafting unless a small area or fascia only is used.47 Although the size of the radial cutaneous territory may be as large as 15 by 25 cm, flaps generally no larger than 16 by 8 cm (usually smaller) are considered for elbow defects that measure 6 to 8 by 14 to 6 cm.24,26,56,104 A complete deep palmar arch is necessary for adequate perfusion of the hand by the ulnar and, occasionally, median arteries.
Other complications following radial forearm flap elevation can include decrease in radial nerve sensation, delayed healing at the skin graft site, decreased range of motion, and fractures of the radius following osteocutaneous flap elevation. Richardson found in a series of 100 patients that 13% experienced exposed tendons, 19% had delayed healing over the donor site and 32% had decrease sensation in the radial nerve distribution.91 Raising the flap with a portion of the radius has been associated with a high incidence of postoperative fractures.9,102,106
Attempts at limiting donor site morbidity have included modifications in flap elevation. The flap may be elevated as a pure facial flap, allowing for primary closure of the donor site.47,89 The flap may also be elevated suprafacially; this allows for fewer donor site complications with enhancement of skin graft take and elimination of tendon exposure.5,15,68 Further improvement to the donor site may be made by placing tissue expands before or following flap transfer to help with primary closure of the donor site.34,36,75 Also ulnar-based skin islands have been used to close the donor site.7,49 Despite donor site complications, the radial forearm flap has remained a workhorse flap for elbow coverage.
Technique
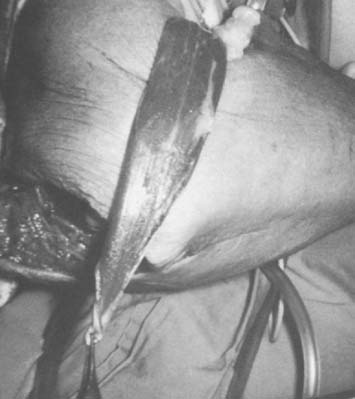
The course of the radial artery is marked with the help of a Doppler ultrasound probe, and a timed Allen’s test is performed to verify adequate distal circulation with the radial artery occluded. The donor skin area is outlined somewhat larger than the defect and as distal as possible to create a pedicle long enough to reach the elbow defect. It is centered over the radial artery and may include the cephalic vein. The flap is then raised from distal to proximal, protecting the superficial radial nerve distally but including the proximal cephalic vein and lateral antebrachial cutaneous nerve; this allows for the creation of a sensate flap. The margins are incised, including the antebrachial fascia. Distally, the radial artery is ligated and the flap, including the lateral intermuscular septum, radial vessels, and fasciocutaneous perforators, is raised as a proximally based island flap transposed to cover the elbow (see Fig. 36-9). Closure of the donor site requires split-thickness skin grafting (Fig. 36-10). Incomplete graft “take” is a common complication, but the risk may be minimized by preserving paratenon over distal tendons and by immobilizing the wrist and digits for several days after surgery.77,106 Further improvement in graft take may be accomplished by mobilization of the superficialis muscle belly over any component of exposed flexor carpi radialis tendon. We use a nonmeshed graft to improve donor site cosmesis.
Retrograde Lateral Arm Flap
The lateral arm flaps described by Katsaros and colleagues have been widely used for upper limb reconstruction as a free flap transfer based on its fasciocutaneous supply by means of a terminal branch of the profunda brachii artery, the posterior radial circumflex artery.51 This vessel contributes to the anastomotic blood supply of the elbow through the interosseous recurrent artery. Culbertson and Mutimer have described a retrograde island flap based on this distal circulation with reverse-flow venous drainage through venae comitantes in a fashion analogous to the reverse radial forearm flap (Fig. 36-11).20,62,82 It should be noted that any retrograde island flap may be prone to the development of venous congestion due to inadequate venous outflow. Venous congestion in such cases may require additional microvascular venous anastomosis to prevent partial flap failure.17
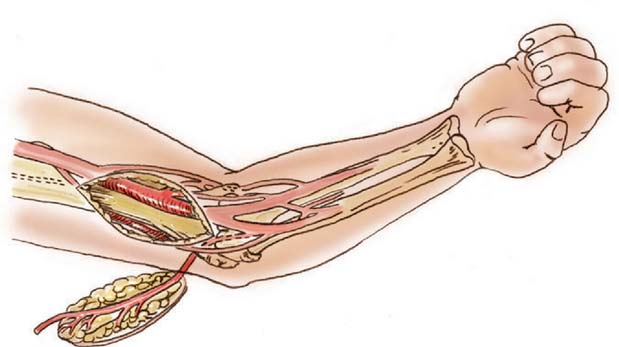
FIGURE 36-11 Design of a reverse lateral arm flap for elbow coverage.
(From Culbertson, J. H., and Mutimer, K.: The reverse lateral upper arm flap for elbow coverage. Ann. Plast. Surg. 18:62, 1987.)
The flap may include fascia only, skin up to 8 to 10 cm as well as sensation by neurotization of the posterior brachial cutaneous nerve, triceps tendon, or even a segment of vascularized humerus.51 Recently Kuek and Lanzetta and their respective colleagues revealed that local circulation supports greater distance to the forearm than had been previously described.53,58 This allows a broader indication than previously reported. As a retrograde flap, it covers all surfaces of the elbow and is indicated for burn cicatrix, olecranon area defects secondary to pressure sores, excision of rheumatoid nodules or bursitis, and local skin loss of other causes, provided that the anastomotic circulation is not disturbed. From measurements of the defects in flexion and extension a more accurate estimate of posterior coverage can be made.109 It has the significant advantages of a minimal donor site problem (if donor defect can be closed primarily) and no need to sacrifice a major vessel, which are two problems associated with the radial forearm flap. Yet, one recent study of almost 100 procedures reported concerns for appearance in 27% of patients and paresthesias in 59%, and the investigators recommended the procedures only for men.33
Other Fasciocutaneous Flaps
From least to most reliable option, the medial arm, posterior interosseous and ulnar forearm flaps also may be used for elbow coverage. The medial arm flap is not used often as a free flap because of its variable cutaneous blood supply through unnamed cutaneous arteries and biceps myocutaneous branches from the brachial artery. The anastomoses these vessels form with the superior ulnar collateral artery are various and not uniform.51,99 Its theoretical potential as a fasciocutaneous transposition flap for elbow coverage has been based on this anastomotic connection, but no clinical cases have been reported.50
The posterior interosseous flap described by Zancolli and Angrigiani in 1986, has been used primarily as a retrograde flap for hand coverage.12,87,112 It also may be used reliably as an orthograde fasciocutaneous flap based on some of the 7 to 14 fasciocutaneous perforators that arise from the posterior interosseous artery. These vessels run in the interval between the extensor carpi ulnaris and the extensor digiti minimi. The pivot of the flap is the point of emergence of the artery in the posterior forearm at the junction of the proximal and middle thirds of forearm, and the axis lies on a line drawn from the lateral humeral epicondyle to the ulnar head. A flap outlined in the distal third of the forearm will reach the antecubital fossa and olecranon (Fig. 36-12).87

FIGURE 36-12 An orthograde posterior interosseous flap for elbow coverage.
(From Penteado, C. V., Masquelet, A. C., and Chevrel, J. P.: The anatomic basis of the fasciocutaneous flap of the posterior interosseous artery. Surg. Radiol. Anat. 8:209, 1986.)
An orthograde ulnar artery forearm flap also may cover the elbow, and it offers the potential for sensibility and composite tissue inclusion.66 Its drawbacks are similar to those of the radial forearm flap, and it is seldom indicated.
PEDICLE MUSCLE FLAPS
For coverage about the elbow, a number of muscle flaps have been reported, including flexor digitorum superficialis, extensor carpi radialis longus, extensor carpi ulnaris, anconeus, brachioradialis, and latissimus dorsi.10,13,29,41,54,76,84 Indications for muscle rotation to cover a cutaneous defect include infection, dead space that must be filled, and loss of motor function (restored by functional muscle transfer). Muscle flaps have been shown to diminish the bacteria count and to survive better than skin flaps when placed over infected wounds.16 They are preferred in cases of infection. Their bulk is useful to fill defects. In addition, elbow flexion and extension and digit flexion have been restored by pedicled latissimus dorsi transfer.6,42,93,97,101,105,113 Because of significant functional loss with many of the aforementioned muscles or limited coverage secondary to small size and limited arc of rotation, only brachioradialis, anconeus, and latissimus dorsi transfers are used frequently about the elbow.10,41
Anconeus
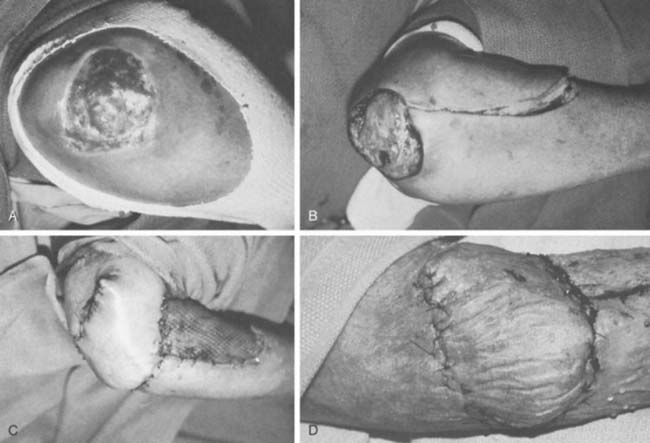
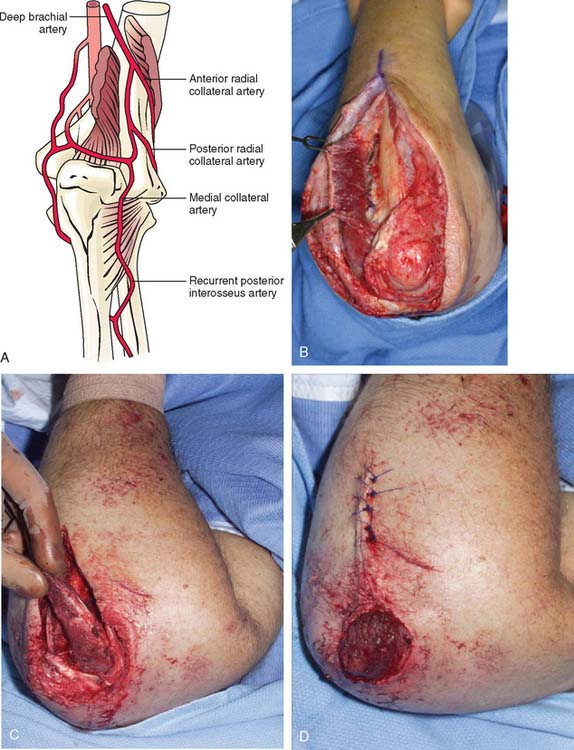
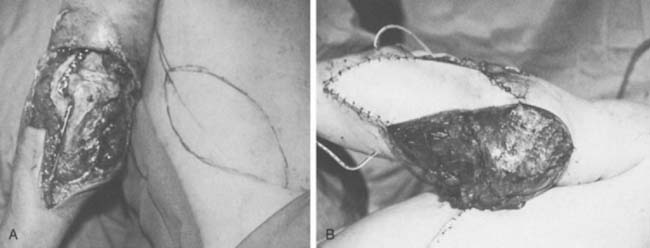
The anconeus muscle was first used for coverage in the elbow region by Cardany et al.14 in 1981. The technique of transfer and vascular anatomy were further delineated by Mathes and Nahai73 and Parry et al.86 in 1982 and 1988. The muscles size (4 × 8 cm) limits its use to small posterior defects over the radiocapitellar joint, the distal triceps tendon, and the olecranon.95 The anconeus only aids in the terminal 15 degrees of elbow extension and forearm supination and its sacrifice has not been shown to produce any decreased range of motion or strength.95 It receives its major blood supply via the recurrent posterior interosseus and medial collateral arteries43,86,95 (Fig. 36-13A). The medial collateral artery, a branch of the profunda brachii, enters pro-ximally, underneath the muscle and allows an easy and expeditious transposition or rotation of a proximally based flap over the defect (see Fig. 36-13B). Thus, it has the potential of being harvested under regional blockade in patients who cannot support a lengthy procedure under general anesthesia (Fig. 36-13C and D).
Extensor Carpi Radialis Longus
The ease of harvest and the extensive arc of rotation have made the extensor carpi radialis longus popular for limited coverage of the anterior aspect of the elbow.46 The minimal functional deficiency and ease of rotation are attractive for limited defects.
Brachioradialis
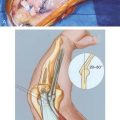
The brachioradialis is an expandable elbow flexor that arises from the upper two thirds of the lateral supracondylar ridge of the humerus and the anterior aspect of the lateral intermuscular septum. The muscle belly extends to the midforearm and ends in a broad, flat tendon that inserts at the radial styloid process. The blood supply to the muscle comes from several arterial pedicles.55 A consistent vascular anatomy includes a major pedicle found near the elbow joint.92 Its major pedicle arises from the radial recurrent artery located between brachioradialis and brachialis muscles, with additional smaller vessels from the radial artery (Fig. 36-14).54 The skin overlying the muscle belly in the distal arm and the proximal forearm may be included. This flap was first described by Lendrum60 and its relevant anatomy by Lai and colleagues.54 It is indicated for small defects in the antecubital and the lateral aspects of the elbow. It will not reach medially and will not reliably cover the olecranon or the posterior soft tissue.
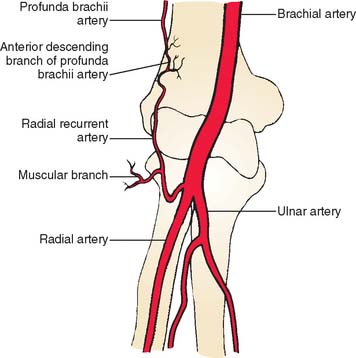
FIGURE 36-14 Location of radial recurrent artery and the muscular branch to brachioradialis.
(From Lai, M. F., Krishna, B. V., and Pelly, A. D.: The brachioradialis myocutaneous flap. Br. J. Plast. Surg. 34:431, 1981.)
Technique
The muscle is exposed through a longitudinal anterolateral forearm incision, including overlying skin if the surgeon desires. The tendon of insertion is divided, and the muscle is freed working from proximal to distal until the main pedicle from the radial recurrent vessel is reached. Several secondary pedicles are divided during the exposure. If needed, its origin may also be released to allow full rotation of the muscle about its major pedicle (Fig. 36-15).
Latissimus Dorsi Myocutaneous Pedicle Flap
The latissimus dorsi flap, along with the radial forearm flap, are the predominant workhorse flaps for coverage of large defects in the arm and elbow.15 The latissimus dorsi muscle is a broad, fan-shaped muscle with a single dominant thoracodorsal arterial pedicle in the axilla. The muscle originates from the lower six thoracic and lumbar vertebrae, the lower four ribs, and the posterior ilium, and ends in a broad tendon that inserts into the intertubercular groove of the humerus (Fig. 36-16).10 The entire muscle or its anterior half may be rotated on a posterior axillary axis point and tunneled into the arm. Fully mobilized, it extends well into the midforearm and covers all aspects of the elbow.69 A large skin paddle (up to 12 by 35 cm) may be included with primary closure of the recipient site (Fig. 36-17).76 Its use in an elbow flexorplasty or tricepsplasty is beyond the scope of this chapter, but simultaneous skin coverage and functioning muscle transfer occasionally is desirable.88 We have found this to be a major asset in certain instances. Its size, large skin paddle, enormous arc of rotation, simplicity, functional potential, and expendability as ashoulder extensor, adductor, and internal rotator are its principal virtues.76
FIGURE 36-16 The anatomy of the latissimus dorsi muscle and vascular pedicle. A, artery.
(From Bostwick, J., Nahai, F., Wallace, J. G., and Vasconez, L. D.: Sixty latissimus dorsi flaps. Plast. Reconstr. Surg. 63:31, 1979.)
Technique
The patient is positioned in the lateral decubitus position, and the involved extremity and ipsilateral hemithorax are prepared and draped from the iliac crest distally to the midline posteriorly and the nipple line anteriorly. The skin incision parallels the anterior border of the muscle several centimeters posterior to it, and a skin paddle is centered over the anterior muscle if needed. The incision is carried directly to muscle fascia and is directed anteriorly to identify the interval between latissimus and serratus anterior. The dissection then proceeds distally, deep to the latissimus from its free anterior edge at its midpoint. The latissimus is thus freed from its caudal and posterior insertions. It is then mobilized proximally at the scapula from the teres major by blunt dissection, and the neurovascular pedicles are identified. A constant branch of the thoracodorsal artery to the serratus anterior is divided, and proximal dissection is continued as required. The entire subscapular artery may be mobilized, if needed, by dividing the circumflex scapular artery in the axilla. The muscle is then rotated into the arm through a subcutaneous tunnel. If necessary, the humeral insertion may be divided for maximal mobility. Meticulous hemostasis is needed to minimize the risk of a seroma, which can be as high as 30%. The incidence of seroma formation at the donor site can be significantly reduced with the use of postoperative drains and internal quilt suturing, which coapt the deep dermis to the remaining underlying muscles.90
Distant Pedicled Flaps
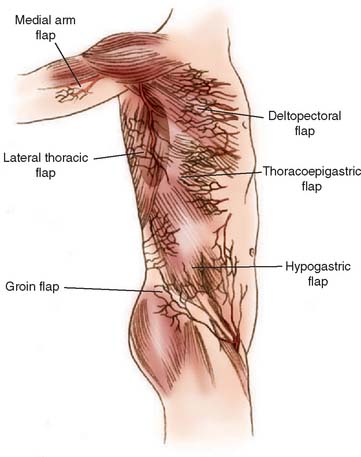
For many years, temporary pedicle flaps raised from the chest or abdominal wall were the mainstay for coverage of large elbow defects.18,102 Today, such distant flaps should be reserved for large defects of skin and subcutaneous tissue when a local flap is not available and free flap transfer is not appropriate because of general medical concerns. Their major drawback is the temporary pedicle itself, which demands a two-stage procedure, a prolonged hospital stay, and the need to splint the extremity to the thorax for at least 2 weeks. Joint stiffness and extremity edema may result.24 Flaps appropriate for emergency or salvage elbow coverage include thoracoepigastric flaps (based on internal mammary perforators), lateral thoracic flaps (thoracodorsal or lateral thoracic artery), external oblique fasciocutaneous flaps (myocutaneous perforators), pectoralis major flaps (muscle used as carrier for skin), and proximally based rectus abdominis pedicle flaps (Hartrampf’s flap).1,4,13,23,25,29,39,67,76,94,110 Several donor sites are illustrated in Fig. 36-18. The interested reader is referred to the original salvage reference or to the third edition of this book for details.
FREE TISSUE TRANSFER
Free flaps are assuming an ever-increasing role in reconstruction of upper extremity defects. The added operative time, need for microvascular anastomoses must be taken into consideration, but elective flap failure rates are now as low as 3% in most major centers.93 In certain circumstances, free tissue transfer is the procedure of choice, provided that the patient’s health, age, and injuries allow surgery and that a skilled microvascular surgical team is available. In many instances, simpler methods are either inappropriate or less desirable.22,40,111 For example, multiple tissue reconstruction may be possible in a single stage with a composite free flap. Distant pedicle flaps are contraindicated in a recipient site with marginal vascularity or when early limb mobilization is important. A local flap may be precluded by an external fixator, and free tissue transfer may be necessary (Fig. 36-19).
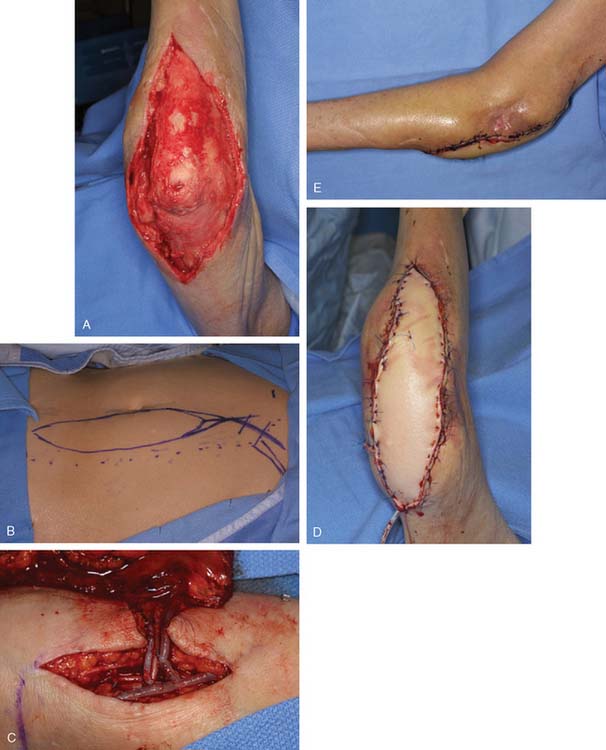
FIGURE 36-19 For large defects free tissue transfer often provides the best option for elbow coverage. A, Large soft tissue defect originally presented in Fig. 36-1 was covered with the use of a vertical rectus myocutaneous flap harvested from the patients abdomen (B). The use of a composite flap (including both skin and muscle) allowed for muscle coverage of devacularized and infected tissue, while the skin component of the flap will allow for motion across the elbow joint. Anastomosis is performed just distal to the level of the antecubital fossa (C). D and E, Appearance at final insetting.
The choice of a flap is dictated by the size of the defect, the type of tissue required, donor site morbidity, and the surgeon’s preference. Fasciocutaneous flap choices for the elbow include the anterolateral thigh, scapular or parascapular, and lateral arm flaps.8,28,38,40,44,51,80 Muscle is often preferable when dead space infection, or significant risk of infection is present, either with overlying skin or covered by a split-thickness graft. Manktelow and McKee pioneered free neurotized muscle transfers for functional reconstruction in the upper extremity.72 The large variety of potential donor muscles includes latissimus dorsi, serratus anterior, rectus abdominis, gracilis, and triceps.22,32,78,85,103 A composite lateral arm free flap with triceps tendon or triceps muscle has been possible without significant loss of extension strength.32
Vascularized bone is optimally provided with a composite groin flap based on the deep circumflex iliac vessels or a fibular flap including muscle, skin, or both, as needed. End-to-side arterial anastomosis to the brachial artery and end-to-end venorrhaphy to a vena comitante or cephalic vein is preferred for most elbow defects.30
1 Abu Dalu K., Muggia M., Schiller M. A bipedicled chest wall flap to cover an open elbow joint in a burned infant. Injury. 1982;13:292.
2 Akpuaka F.C. The radial recurrent fasciocutaneous flap for coverage of posterior elbow defects. Injury. 1991;22:332.
3 Argenta L.C., Morykwas M.J., Marks M.W., DeFranzo A.J., Molnar J.A., David L.R. Vacuum-assisted closure: State of clinic art. Plast. Reconstr. Surg. 2006;117:127S.
4 Ariyan S. The pectoralis major myocutaneous flap. Plast. Reconstr. Surg. 1979;63:73.
5 Avery C.M., Pereira J., Brown A.E. Suprafascial dissection of the radial forearm flap and donor site morbidity. Int. J. Oral. Maxillofac. Surg. 2001;30:37.
6 Axer A., Segal D., Elkon A. Partial transposition of the latissimus dorsi: a new operative technique to restore elbow and finger flexion. J. Bone Joint Surg. 1973;55A:1259.
7 Bardsley A.F., Soutar D.S., Elliot D., Batchelor A.G. Reducing morbidity in the radial forearm flap donor site. Plast. Reconstr. Surg. 1990;86:287.
8 Bartwick W.J., Goodkind D.J., Serafin D. The free scapular flap. Plast. Reconstr. Surg. 1982;69:779.
9 Boorman J.G., Brown J.A., Sykes P.J. Morbidity in the forearm flap donor arm. Br. J. Plast. Surg. 1987;40:207.
10 Bostwick J., Nahai F., Wallace J.G., Vasconez L.D. Sixty latissimus dorsi flaps. Plast. Reconstr. Surg. 1979;63:31.
11 Braakenburg A., Obdeijn M.C., Feitz R., van Rooij I.A., van Griethuysen A.J., Klinkenbijl J.H. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: A randomized controlled trial. Plast. Reconstr. Surg. 2006;118:390. discussion 398
12 Büchler U. Retrograde posterior interosseous flap. J. Hand Surg. 1991;16A:283.
13 Burstein F.D., Salomon J.C., Stahl R.S. Elbow joint salvage with the transverse rectus island flap: a new application. Plast. Reconstr. Surg. 1989;84:492.
14 Cardany C., Maxwell P., Gilbert A. The antecubital forearm flap. New York: Martin Dunitz, 1992.
15 Chang L.D., Goldberg N.H. Elbow defect coverage with a one-staged, tunneled latissimus dorsi transposition flap. Ann. Plast. Surg. 1994;32:496.
16 Chang N., Mathes S. Comparison of the effect of bacterial inoculation in musculocutaneous and random pattern flaps. Plast. Reconstr. Surg. 1982;70:1.
17 Chen H.C., Cheng M.H., Schneeberger A.G., Cheng T.J., Wei F.C., Tang Y.B. Posterior interosseous flap and its variations for coverage of hand wounds. J. Trauma. 1998;45:570.
18 Chou T.D., Chen S.L., Lee T.W., Chen S.G., Cheng T.Y., Lee C.H., Chen T.M., Wang H.J. Reconstruction of burn scar of the upper extremities with artificial skin [see comment]. Plast. Reconstr. Surg. 2001;108:378. discussion 385
19 Cormack G.C., Lamberty B.G.H. A classification of fasciocutaneous flaps according to their patterns of vascularization. Br. J. Plast. Surg. 1984;37:80.
20 Culbertson J.H., Mutimer K. The reverse lateral upper arm flap for elbow coverage. Ann. Plast. Surg. 1987;18:62.
21 Cuono C.B. Double Z-plasty repair of large and small rhombic defects: the double-Z rhomboid. Plast. Reconstr. Surg. 1983;71:658.
22 Daniel R.K., Weiland A.J. Free tissue transfers for upper extremity reconstruction. J. Hand Surg. 1982;7:66.
23 Davis W.M., McCraw J.B., Carraway J.H. Use of a direct, transverse thoracoabdominal flap to close difficult wounds of the thorax and upper extremity. Plast. Reconstr. Surg. 1977;60:526.
24 Fatah M.F., Davies D.M. The radial forearm island flap in upper limb reconstruction. J. Hand Surg. 1984;9B:234.
25 Fisher J. External oblique fasciocutaneous flap for elbow coverage. Plast. Reconstr. Surg. 1985;75:51.
26 Foucher G., Genechten E., Merle N., Michon J. A compound radial artery forearm flap in hand surgery: an original modification of the Chinese forearm flap. Br. J. Plast. Surg. 1984;37:139.
27 Freshwater M.F. Ten signs for successful skin grafting. Plast. Reconstr. Surg. 1983;72:491.
28 Gilbert A., Teot L. The free scapular flap. Plast. Reconstr. Surg. 1982;69:601.
29 Gilbert D.A. An overview of flaps for hand and forearm reconstruction. Clin. Plast. Surg. 1981;8:129.
30 Godina M. Preferential use of end to side arterial anastomoses in free flap transfers. Plast. Reconstr. Surg. 1979;64:673.
31 Godina M. Early microsurgical reconstruction of complex trauma to the extremities. Plast. Reconstr. Surg. 1986;78:285.
32 Gosain A.K., Matloub H.S. The composite lateral arm free flap: vascular relationship to triceps tendon and muscle. Ann. Plast. Surg. 1992;29:496.
33 Graham B., Adkins P. Complications and morbidity of the donor and recipient sites in. 123 lateral arm flaps. J. Hand Surg.. 1992;17B:189.
34 Hallock G.G. Free flap donor site refinement using tissue expansion. Ann. Plast. Surg. 1988;20:566.
35 Hallock G.G. Island forearm flap for coverage of the antecubital fossa. Br. J. Plast. Surg. 1986;39:533.
36 Hallock G.G. Refinement of the radial forearm flap donor site using skin expansion. Plast Reconstr Surg. 1988;81:21.
37 Hallock G.G. Soft tissue coverage of the upper extremity using the ipsilateral radial forearm flap. Contemp. Orthop. 1987;15:15.
38 Hamilton S.G., Morrison W.A. The scapular free flap. Br. J. Plast. Surg. 1982;35:2.
39 Hartrampf C.R., Scheflan M., Black P.W. Breast reconstruction with a transverse abdominal island flap. Plast. Reconstr. Surg. 1982;69:216.
40 Hing D.N., Buncke J.H., Alpert B.S., Gordon L. Free flap coverage of the hand. Hand Clin. 1985;1:741.
41 Hodgkinson D.J., Shepard G.H. Muscle musculocutaneous and fasciocutaneous flaps in forearm reconstruction. Ann. Plast. Surg. 1983;10:399.
42 Hovnanian A.P. Latissimus dorsi transplantation for loss of flexion or extension of the elbow. Ann. Surg. 1956;143:493.
43 Hwang K., Han J.Y., Chung I.H. Topographical anatomy of the anconeus muscle for use as a free flap. J. Reconstr. Microsurg. 2004;20:631.
44 Ikuta Y., Watari S., Kuwamura K. Free flap transfers by end-to-side arterial anastomosis. Br. J. Plast. Surg. 1975;28:1.
45 James J.H., Watson A.C.H. The use of Opsite, a vapor permeable dressing on skin graft donor sites. Br. J. Plast. Surg. 1975;28:107.
46 Janevicius R.V., Greager J.A. The extensor carpi radialis longus muscle flap for anterior elbow coverage. J. Hand Surg. 1992;17A:102.
47 Jin Y., Guam W., Shi T., Quian Y., Xu L., Change T. Reversed island forearm fascial flap in hand surgery. Ann. Plast. Surg. 1985;15:340.
48 Jones N.F., Hardesty R.A., Goldstein S.A., Ward W.T. Upper limb salvage using a free radial forearm flap. Plast. Reconstr. Surg. 1987;79:468.
49 Juretic M., Car M., Zambelli M. The radial forearm free flap: Our experience in solving donor site problems. J. Craniomaxillofac. Surg. 1992;20:184.
50 Kaplan E.N., Pearl R.M. An arterial medial arm flap: vascular anatomy and clinical applications. Ann. Plast. Surg. 1980;4:205.
51 Katsaros J., Schusterman M., Beppu M., Banis J.C., Ackland R.D. The lateral upper arm flap: anatomy and clinical applications. Ann. Plast. Surg. 1984;12:489.
52 Krizek T.J., Robson M.C., Kho E. Bacterial growth and skin graft survival. Plast. Surg. Forum. 1967;18:518.
53 Kuek L.B. The extended lateral arm flap: a detailed anatomical study. Ann. Acad. Med. Singapore. 1992;21:169.
54 Lai M.F., Krishna B.V., Pelly A.D. The brachioradialis myocutaneous flap. Br. J. Plast. Surg. 1981;34:431.
55 Lalikos J.F., Fudem G.M. Brachioradialis musculocutaneous flap closure of the elbow utilizing a distal skin island: a case report. Ann. Plast. Surg. 1997;39:201.
56 Lamberty B.G.H., Cormack G.C. The forearm angiotomes. Br. J. Plast. Surg. 1982;35:420.
57 Lamberty B.G.H., Cormack G.C. The antecubital fasciocutaneous flap. Br. J. Plast. Surg. 1983;36:428.
58 Lanzetta M., Bernier M. The lateral forearm flap: an anatomic study. Plast. Reconstr. Surg. 1997;99:460.
59 Le Huec J.C., Liquois F. A study of the fasciocutaneous vascularization of the arm. Surgical applications. Surg. Radiol. Anat. 1995;17:121.
60 Lendrum J. Alternatives to amputation. Ann. R. Coll. Surg. Engl. 1980;62:95.
61 Levine N.S., Lindberg R.B., Mason A.D., Pruitt B.A. The quantitative swab culture and smear: a quick simple method for determining the number of viable aerobic bacteria in open wounds. J. Trauma. 1976;16:89.
62 Lin S.D., Lai C.S., Chin C.C. Venous drainage in the reverse forearm flap. Br. J. Plast. Surg. 1981;34:431.
63 Lister G. The theory of the transposition flap and its practical application in the hand. Clin. Plast. Surg. 1981;8:115.
64 Lister G.D., Gibson T. Closure of rhomboid skin defects: the flaps of Limberg and Dufourmentel. Br. J. Plast. Surg. 1972;25:300.
65 Lister G., Schecker L. Emergency free flaps to the upper extremity. J. Hand Surg. 1988;13A:22.
66 Lovie M.J., Duncan G.M., Glasson D.W. The ulnar artery forearm free flap. Br. J. Plast. Surg. 1984;37:486.
67 Luce E.A., Gottlieb S.F. The pectoralis major island flap for coverage in the upper extremity. J. Hand. Surg. 1982;7:156.
68 Lutz B.S., Wei F.C., Chang S.C., Yang K.H., Chen I.H. Donor site morbidity after suprafascial elevation of the radial forearm flap: A prospective study in 95 consecutive cases. Plast. Reconstr. Surg. 1999;103:132.
69 Mackinnon S.E., Weiland A.J., Godina M. Immediate forearm reconstruction with a functional latissimus dorsi island pedicle myocutaneous flap. Plast. Reconstr. Surg. 1983;71:700.
70 Magden O., Icke C., Arman C., Atabey A. An anatomical study of the inferior cubital artery. Eur. J. Plast. Surg. 1997;20:24.
71 Manchot C. Die Hautarterien des Menschlichen Körpers. Leipzig: F. C. W. Vogel, 1889.
72 Manktelow R.T., McKee N.H. Free muscle transplantation to provide active finger flexion. J. Hand. Surg. 1978;3:416.
73 Mathes S., Nahai F. Clinical Applications for Muscle and Musculocutaneous Flaps. St Louis: CV Mosby, 1982.
74 Marty F.M., Montandon D., Gumener R., Zbrodowski A. The use of subcutaneous tissue flaps in the repair of soft tissue defects of the forearm and hand: an experimental and clinical study of a new technique. Br. J. Plast. Surg. 1984;37:95.
75 Masser M.R. The preexpanded radial free flap. Plast. Reconstr. Surg. 1990;86:295.
76 McCraw J.B., Dibbell D.G., Carraway J.H. Clinical definition of independent myocutaneous vascular territories. Plast. Reconstr. Surg. 1977;60:341.
77 McGregor A.D. The free radial forearm flap: the management of the secondary defect. Br. J. Plast. Surg. 1987;40:83.
78 Milloy F.J., Anson B.J., McAfee D.K. The rectus abdominis muscle and the epigastric arteries. Surg. Gynecol. Obstet. 1960;110:293.
79 Milton S.H. Pedicled skin flaps: the fallacy of the length-width ratio. Br. J. Surg. 1970;57:502.
80 Moffett T.R., Madison S.A. An extended approach for the vascular pedicle of the lateral arm free flap. Plast. Reconst. Surg. 1992;89:259.
81 Moisidis E., Heath T., Boorer C., Ho K., Deva A.K. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast. Reconstr. Surg. 2004;15:917.
82 Mühlbauer W., Herndl E., Stock W. The forearm flap. Plast. Reconstr. Surg. 1982;70:343.
83 Mühlbauer W., Herndl E., Stock W. The forearm flap. Plast. Reconstr. Surg. 1982;70:336.
84 Ohtsuka H., Imagawa S. Reconstruction of a posterior defect of the elbow joint using an extensor carpi radialis longus myocutaneous flap: case report. Br. J. Plast. Surg. 1985;38:238.
85 Onishi K., Yu M. Cutaneous and fascial vasculature around the rectus abdominis muscle: anatomic basis of abdominal fasciocutaneous flaps. J. Reconstr. Microsurg. 1986;2:247.
86 Parry S.W., Ward J.W., Mathes S.J. Vascular anatomy of the upper extremity muscles. Plast. Reconstr. Surg. 1988;81:358.
87 Penteado C.V., Masquelet A.C., Chevrel J.P. The anatomic basis of the fasciocutaneous flap of the posterior interosseous artery. Surg. Radiol. Anat. 1986;8:209.
88 Pruzansky M., Kelly M. Latissimus dorsi musculocutaneous flap for elbow extension. J. Surg. Oncol. 1991;47:62.
89 Reyes F.A., Burkhalter W.E. The fascial radial flap. J. Hand Surg. Am. 1988;13:432.
90 Rios J.L., Pollock T., Adams W.P.Jr. Progressive tension sutures to prevent seroma formation after latissimus dorsi harvest. Plast Reconstr. Surg. 2003;112:1779.
91 Richardson D., Fisher S.E., Vaughan E.D., Brown J.S. Radial forearm flap donor-site complications and morbidity: A prospective study. Plast. Reconstr. Surg. 1997;99:109.
92 Rohrich R.J., Ingram A.E.Jr. Brachioradialis muscle flap: clinical anatomy and use in soft tissue reconstruction of the elbow. Ann. Plast. Surg. 1995;35:70.
93 Romm S., Massac E. A guide to skin grafting. Contemp. Orthop. 1985;11:35.
93a Salmon M. Arteres de la Peau. Paris: Masson, 1936.
94 Sbitany U., Wray R.C.Jr. Use of the rectus abdominis muscle flap to reconstruct an elbow defect. Plast. Reconstr. Surg. 1985;77:988.
95 Schmidt C.C., Kohut G.N., Greenberg J.A., Kann S.E., Idler R.S., Kiefhaber T.R. The anconeus muscle flap: Its anatomy and clinical application. J. Hand Surg. Am. 1999;24:359.
96 Schoofs M., Bienfast B., Calteux N., Dachy C., Vandermaeren C., de Coninck A. Le lambeau aponéurotique de l’avantbras. Ann. Chir. Main. 1983;2:197.
97 Schottstaedt E.R., Larsen L.J., Bost F.G. Complete muscle transposition. J. Bone Joint Surg. 1955;37A:897.
98 Song R.S., Gao Y., Song Y., Yu Y., Song Y. The forearm flap. Clin. Plast. Surg. 1982;9:21.
99 Song R., Song Y., Yu Y., Song Y. The upper arm free flap. Clin. Plast. Surg. 1982;9:27.
100 Soutar D.S., Tanner S.B. The radial forearm flap in the management of soft tissue injuries of the hand. Br. J. Plast. Surg. 1984;37:18.
101 Stern P.J., Neale H.W., Gregory R.O., Kreilein J.G. Latissimus dorsi musculocutaneous flap for elbow flexion. J. Hand Surg. 1982;7:25.
102 Swanson E., Boyd J.B., Mulholland R.S. The radial forearm flap: A biomechanical study of the osteotomized radius. Plast. Reconstr. Surg. 1990;85:267.
103 Takayanagi S., Tsukie T. Free serratus anterior muscle and myocutaneous flaps. Ann. Plast. Surg. 1982;8:277.
104 Thornton J.W., Stevenson T.R., Vander Kolk C.A. Osteoradionecrosis of the olecranon: treatment by radial forearm flap. Plast. Reconstr. Surg. 1987;80:833.
105 Timmons M.J. The vascular basis of the radial forearm flap. Plast. Reconstr. Surg. 1986;77:80.
106 Timmons M.J., Missotten F.E.M., Pode M.D., Davies D.M. Complications of radial forearm flap donor sites. Br. J. Plast. Surg. 1986;39:176.
107 Tobin G.R. The compromised bed technique. Surg. Clin. North Am. 1984;64:653.
108 Tolhurst D.E., Haeseker B., Zeeman R.J. The development of the fasciocutaneous flap and its clinical applications. Plast. Reconstr. Surg. 1983;71:597.
109 Tung T.C., Wang K.C. Reverse pedicled lateral arm flap for reconstruction of posterior soft-tissue defects of the elbow. Ann. Plast. Surg. 1997;38:635.
110 Winspur I. Distant flaps. Hand Clin. 1985;1:729.
111 Wood M.B., Irons G.B. Upper extremity free skin flap transfer: results and utility as compared with conventional distant pedicle skin flaps. Ann. Plast. Surg. 1983;11:523.
112 Zancolli E.A., Angrigiani C. Colgajo dorsal de antebrazo (en “isla” con pediculo de vasos interosseos posteriores). Rev. Assoc. Arg. Orthop. Traumatol. 1986;51:161.
113 Zancolli E., Mitre H. Latissimus dorsi transfer to restore elbow flexion: an appraisal of eight cases. J. Bone Joint Surg. 1973;55A:1265.