Sleep Apnea
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with sleep apnea.
• Describe the causes of sleep apnea.
• Describe how a sleep study is performed.
• List the cardiopulmonary clinical manifestations associated with sleep apnea.
• Describe the meaning of the apnea-hypopnea index and oxygen desaturation index.
• Describe the general management of sleep apnea.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Normal Sleep Stages
The following terminology (nosology) is now recommended to define the stages of sleep:
REM Sleep
REM sleep periods last 5 to 40 minutes and recur approximately every 60 to 90 minutes. The REM sleep periods lengthen and become more frequent toward the end of a night’s sleep. REM sleep constitutes about 20% to 25% of the total sleep time. Most studies show that it is more difficult to awaken a subject during REM sleep. Table 30-1 provides an overview of the electroencephalographic findings in the various stages of sleep.
Table 30-1
| Stage | Electroencephalogram (EEG) | Characteristics |
| Eyes open-wake (Stage W) | 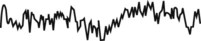 |
The EEG shows beta waves, and high-frequency, low-amplitude activity. The electrooculogram (EOG) looks very similar to REM sleep waves—low-amplitude, mixed-frequency, and saw-toothed waves. Electromyogram (EMG) activity is relatively high. |
| Eyes closed-wake (drowsy) | 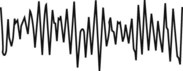 |
The EEG is characterized by prominent alpha waves (>50%). The EOG shows slow, rolling eye movements, and the EMG activity is relatively high. |
| Non-REM Sleep | ||
| Stage N1 (light sleep) | 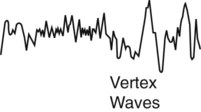 |
The EEG shows low amplitude alpha waves (8-13 Hz) that may be replaced by mixed frequency activity and theta waves (4-7 Hz). Vertex waves commonly appear. Vertex wave are sharp upward deflection EEG waves. The amplitude of many of the vertex sharp waves is greater than 20 µv. Vertex waves are usually seen at the end of stage N1. The EOG shows slow, rolling eye movements. The EMG reveals decreased activity and muscle relaxation. Respirations become regular, and the heart rate and blood pressure decrease slightly. Snoring may occur. If awakened, the person may state that he or she was not asleep. |
| Stage N2 (light sleep) | 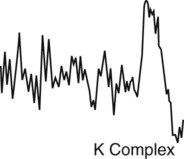 |
The EEG becomes more irregular and is composed predominantly of theta waves (4-7 Hz), intermixed with sudden bursts of sleep spindles (12-18 Hz), and one or more K complexes. Sleep spindles are a sudden burst of EEG activity in the 12-14 Hz frequency (6 or more distinct waves) with a duration of ≥ 0.5 to 1.5 seconds (not illustrated here). Vertex waves may also be seen during this stage. The EOG shows either slow eye movements or absence of slow eye movements. The EMG has low electrical activity. The heart rate, blood pressure, respiratory rate, and temperature decrease slightly. Snoring may occur. If awakened, the person may say he or she was thinking or daydreaming. |
| Stage N3 (slow wave sleep) | 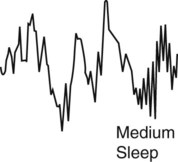 |
Slow wave activity 0.5 Hz-2.0 Hz and peak to peak amplitude > 75 µv. EOG shows little or no eye movement, and the EMG activity is low. Heart rate, blood pressure, respiratory rate, body temperature, and oxygen consumption continue to decrease. Snoring may occur, and there is no eye movement. Dreaming may occur, and the sleeper becomes more difficult to arouse. |
| (Deep sleep) | 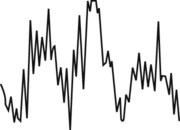 |
The EOG shows no eye movements, and the EMG has little or no electrical activity. The sleeper is very relaxed and seldom moves. The vital signs reach their lowest, normal level. Oxygen consumption is low. The patient is very difficult to awaken. Bed-wetting, night terrors, and sleepwalking may occur. |
| REM Sleep | ||
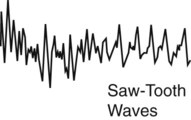 |
About 90 minutes into the sleep cycle, there is an abrupt EEG pattern change. The EEG pattern resembles the wakeful state with low-amplitude, mixed frequency EEG activity. Saw-toothed waves are frequently seen. Alpha waves may be seen. The respiratory rate increases, and respiration is irregular and shallow. The heart rate and blood pressure increase. Rapid eye movement occurs, and there is paralysis of most skeletal muscles. Most dreams occur during REM sleep. | |
Types of Sleep Apnea
Obstructive Sleep Apnea
It is estimated that more than 12 million people in the United States have OSA.
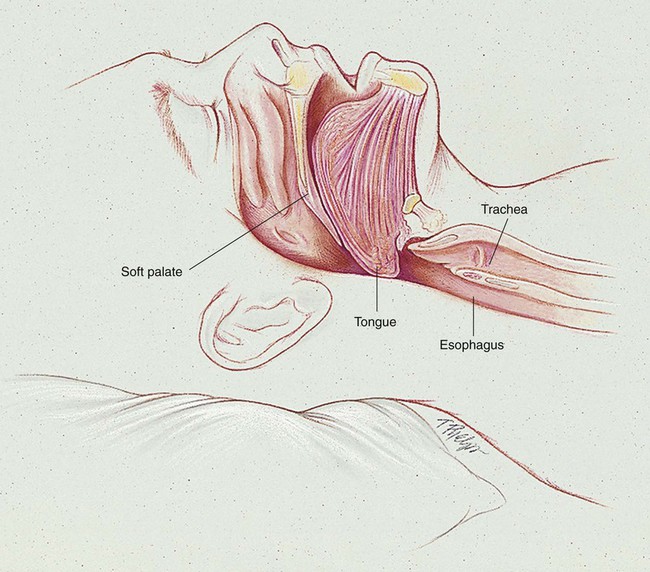
OSA is caused by an anatomic obstruction of the upper airway in the presence of continued ventilatory effort (see Figure 30-1). During periods of obstruction, patients commonly appear quiet and still, as though they are holding their breath, followed by increasingly desperate efforts to inhale. Often the apneic episode ends only after an intense struggle. A snorting sound called “fricative breathing” may be heard at the end of the apneic periods. In severe cases, the patient may suddenly awaken, sit upright in bed, and gasp for air. These events are called confusional arousals. Patients with OSA usually demonstrate perfectly normal and regular breathing patterns during the wakeful period.
In fact, a large number of patients with OSA demonstrate what is commonly called the Pickwickian syndrome (named after a character in Charles Dickens’s The Posthumous Papers of the Pickwick Club, published in 1837). Dickens’s description of Joe, “the fat boy” who snored and had excessive daytime sleepiness, included many of the classic features of what is now recognized as the sleep apnea syndrome. Box 30-1 provides common signs and symptoms associated with OSA. However, many patients with sleep apnea are not obese, and therefore clinical suspicion should not be limited to this group. Table 30-2 provides the more common risk factors associated with OSA.
Table 30-2
Risk Factors Associated with Obstructive Sleep Apnea
| Excess weight | More than 50% of the patients diagnosed with obstructive sleep apnea are overweight. It is suggested that fat deposits around the upper airway may obstruct breathing. |
| Neck size | Obstructive sleep apnea is often seen in the patients with large neck size. A neck circumference greater than 17 inches increases the risk for obstructive sleep apnea. |
| Hypertension | Obstructive sleep apnea is commonly seen in patients with high blood pressure. |
| Anatomic narrowing of upper airway | Common causes of anatomic narrowing of the upper airway include excessive pharyngeal tissue, enlarged tonsils or adenoids, deviated nasal septum, laryngeal stenosis, and vocal cord dysfunction. |
| Chronic nasal congestion | Obstructive sleep apnea occurs twice as often in patients with chronic nasal congestion from any cause. |
| Diabetes | Patients with diabetes are three times more likely to have obstructive sleep apnea than healthy individuals. |
| Male sex | Men are twice as likely to have obstructive sleep apnea as women. |
| Age older than 65 years | Obstructive sleep apnea is two to three times greater in people older than 65 years. |
| Age under 35 years, and black, Hispanic, or Pacific Islander heritage | Among individuals under the age of 35, the incidence of obstructive sleep apnea is greater in blacks, Hispanics, and Pacific Islanders. |
| Menopause | The risk of obstructive sleep apnea is greater after menopause. |
| Family history of sleep apnea | Individuals who have one or more family members who have obstructive sleep apnea are also at greater risk for developing obstructive sleep apnea. |
| Alcohol, sedatives, or tranquilizers | Depressive agents relax the muscles of the upper airway. |
| Smoking | Smokers are almost three times more likely to develop obstructive sleep apnea. |
Central Sleep Apnea
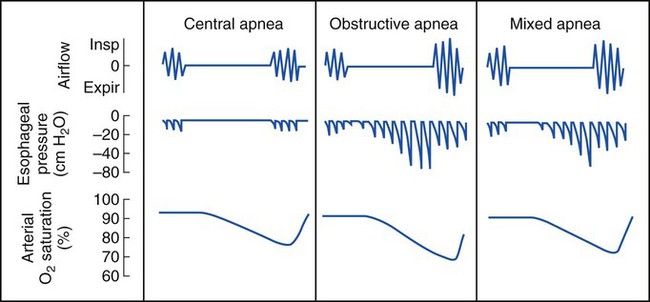
CSA is associated with cardiovascular, metabolic, and central nervous system disorders. A few brief central apneas normally occur with the onset of sleep or the onset of REM sleep. CSA, however, is diagnosed when the frequency of the apnea or hypopnea episodes is excessive (more than 30 in a 6-hour period). Box 30-2 provides a listing of clinical disorders associated with CSA.
Diagnosis
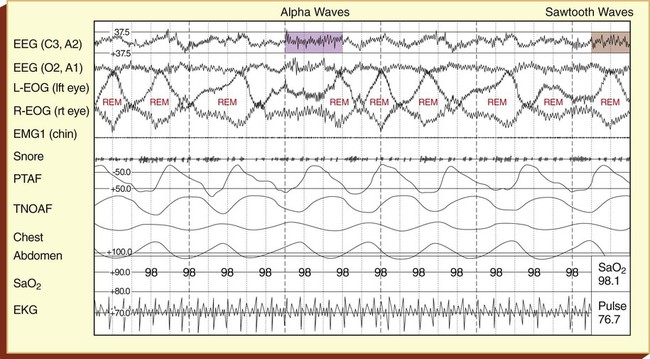
The diagnosis and type of sleep apnea are confirmed with multichannel polysomnographic sleep studies, which include (1) an EEG, which measures the electrophysiologic changes in the brain; (2) an electrooculogram (EOG), which monitors the movement of the eyes and identifies the sleep stages; (3) an electromyogram (EMG), which monitors muscle activity; (4) the absence or presence of snoring; (5) nasal and oral air flow; (6) chest and/or abdominal movement; (7) oxygen saturation; and (8) an ECG. Figure 30-3 provides a representative period of a sleep study polysomnogram (PSG) (called an epoch) of REM sleep.
Management of Sleep Apnea
Management of Obstructive Sleep Apnea
Continuous Positive Airway Pressure
The most common—and arguably the most effective—treatment for OSA is the use of a continuous positive airway pressure (CPAP) device. As discussed earlier, the cause of many OSAs is related to (1) an anatomic configuration of the pharynx and (2) the decreased muscle tone that normally develops in the pharynx during REM sleep. When the patient with OSA inhales, the pharyngeal muscles (and surrounding tissues) are sucked inward as a result of the negative airway pressure generated by the contracting diaphragm. Nocturnal CPAP therapy is useful in preventing the collapse of the hypotonic and obstructed airway and is the standard treatment for most cases of OSA (Figure 30-4). CPAP is not indicated in pure CSA.
Management of Central Sleep Apnea
VPAP Adapt SV and Adaptive Servo-Ventilation*
1. The patient’s recent average respiratory rate, including the inspiratory-expiratory ratio (I:E) and the time of any expiratory pause
2. The instantaneous direction of airflow, magnitude, and rate of change of airflow that are measured at specific points during each breath
The VPAP Adapt SV ensures that ventilatory support is synchronized with the patient’s ventilatory efforts by means of numbers 1 and 2 in this list. When the patient experiences an episode of central apnea or hypopnea, the pressure support initially works to reflect the patient’s recent ventilatory pattern. However, if the apnea or hypopnea persists, the VPAP Adapt SV increasingly uses the backup respiratory rate (number 3 in the list). Figure 30-5 illustrates this.
Therapeutic Strategies Used to Treat Sleep Apnea
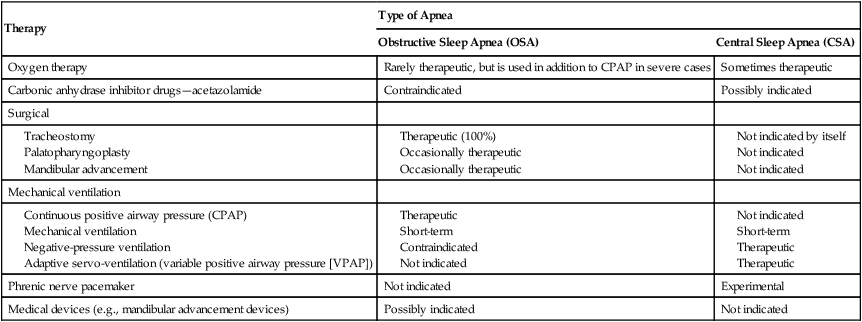
Over the past few decades, it has become apparent that many pathologic conditions are associated with sleep apnea, including hypoxemia, fragmented sleep, cardiac arrhythmias, and neurologic and psychiatric disorders. In general, the prognosis is more favorable for obstructive and mixed apneas than for CSA. Table 30-3 provides an overview of the various therapeutic strategies used for sleep apnea. Table 30-4 summarizes the major therapeutic modalities described in this chapter for obstructive and central apnea and their effectiveness.
Table 30-3
Therapeutic Strategies Used to Treat Sleep Apnea
| Weight reduction | Many patients with obstructive sleep apnea are overweight, and although the excess weight alone is not the cause of the apnea, weight reduction clearly leads to a reduction in apnea severity. The precise reason is not known. Weight reduction as a single form of therapy often fails. |
| Sleep posture | It is generally believed that most obstructive apnea is more severe in the supine position and, in fact, may be present only in this position (positional sleep apnea). Apnea and daytime hypersomnolence have significantly improved in some patients who have been instructed to sleep on their sides and avoid the supine posture. Others may benefit from sleeping in a head-up position (e.g., in a lounge chair). The effect of this change in sleeping habits can be documented by recording oximetry with the patient in the supine and lateral decubitus positions. |
| Oxygen therapy | Because of the hypoxemia-related cardiopulmonary complications of apnea (arrhythmias and pulmonary hypertension), nocturnal low-flow oxygen therapy is sometimes used to offset or minimize the oxygen desaturation, particularly in central sleep apnea (see Oxygen Therapy Protocol, Protocol 9-1). The reasoning behind the use of nasal oxygen therapy’s effectiveness is that the airway is continually “flooded” with oxygen, which will be inspired during the nonapneic episodes—in effect, “preoxygenating” the patient in anticipation of the apnea events. Usually, no improvement in sleep fragmentation or hypersomnolence occurs with the use of supplemental oxygen. |
| Drug therapy |
Drugs occasionally used to treat central sleep apnea include rapid eye movement (REM) inhibitors such as protriptyline (Vivactil). Acetazolamide (Diamox) is a carbonic anhydrase inhibitor that causes a bicarbonate diuresis and mild metabolic acidosis, which in turn stimulate respiration. It occasionally is also helpful in cases of central sleep apnea. Now that variable positive airway pressure (VPAP) therapy has become so successful in central sleep apnea, these drugs are rarely used. |
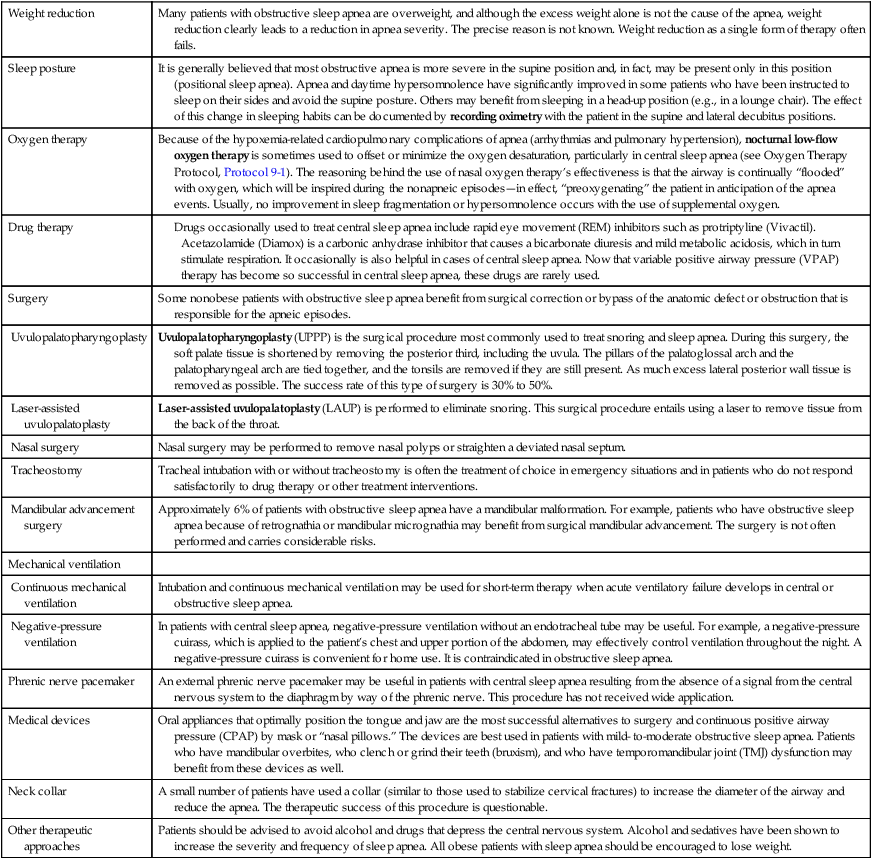
Table 30-4
Therapeutic Modalities for Obstructive Sleep Apnea and Central Sleep Apnea and their Effectiveness
| Therapy | Type of Apnea | |
| Obstructive Sleep Apnea (OSA) | Central Sleep Apnea (CSA) | |
| Oxygen therapy | Rarely therapeutic, but is used in addition to CPAP in severe cases | Sometimes therapeutic |
| Carbonic anhydrase inhibitor drugs—acetazolamide | Contraindicated | Possibly indicated |
| Surgical | ||
| Mechanical ventilation | ||
| Phrenic nerve pacemaker | Not indicated | Experimental |
| Medical devices (e.g., mandibular advancement devices) | Possibly indicated | Not indicated |
CASE STUDY
Obstructive Sleep Apnea
Admitting History
Respiratory Assessment and Plan
O Weight: 160 kg (355 lb); skin: flushed and cyanotic; distended neck veins and edema of feet and legs (4+) to midcalf; vital signs: BP 164/100, HR 78, RR 22, T normal; oropharyngeal exam typical for obstructive sleep apnea; diminished breath sounds, likely because of obesity; CXR: cor pulmonale; lungs appear normal; ABGs (on room air): pH 7.54, Paco2 58, 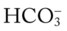 48, Pao2 52; Spo2 86%
48, Pao2 52; Spo2 86%
• Obstructive sleep apnea likely (history, cor pulmonale, ABGs, physical appearance)
• Acute alveolar hyperventilation superimposed on chronic ventilatory failure with moderate hypoxemia (ABGs and history)
P Place patient on alarming oximeter, set to alarm at 85%. Initiate Oxygen Therapy Protocol (venturi oxygen mask at Fio2 = 0.28). If obstructive sleep apnea is confirmed, start Continuous Positive Airway Pressure (via CPAP mask). Monitor and reevaluate (vital signs, ECG, ABGs and Spo2 q4h).
Respiratory Assessment and Plan
S “I’m breathing much better.”
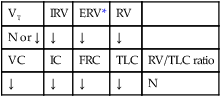
O Recent diagnosis: obstructive sleep apnea—more than 325 periods of obstructive apnea or hypopnea documented during sleep study; short muscular neck; narrow upper airway; obesity; Hct 51%; Hb 17 g/dL; PFTs: severe restrictive disorder; saw-tooth pattern seen on maximal inspiratory and expiratory flow-volume loops; no longer appearing short of breath; cyanotic appearance improved; clear but diminished breath sounds; ABGs (on room air): pH 7.38, Paco2 82, 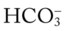 48, Pao2 66; Spo2 91%
48, Pao2 66; Spo2 91%
P Continue Oxygen Therapy Protocol. Start Continuous Positive Airway Pressure (12 cm H2O via mask). Ensure that patient sleeps in the head-up position and refrains from sleeping on his back. Monitor and reevaluate.
*It is not uncommon for patients to have 100 to 150 episodes of apnea and hypopnea per hour of sleep during a polysomnographic sleep study. Transient nocturnal Spo2 desaturations to levels <30% are occasionally seen in the polysomnograms of individuals with severe sleep apnea. Fortunately, these episodes are self-limited when the patient wakens at the end of the apnea.

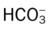

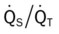
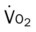
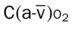
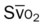

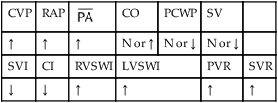
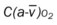 , Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 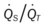 , pulmonary shunt fraction;
, pulmonary shunt fraction; 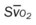 , mixed venous oxygen saturation;
, mixed venous oxygen saturation;  , oxygen consumption.
, oxygen consumption.
 × CaO2 × 10) falls, and results in electrocardiographic “brady-tachy syndrome” episodes and swings in blood pressure secondary to surges of adrenalin in an attempt to compensate for tissue hypoxia.
× CaO2 × 10) falls, and results in electrocardiographic “brady-tachy syndrome” episodes and swings in blood pressure secondary to surges of adrenalin in an attempt to compensate for tissue hypoxia.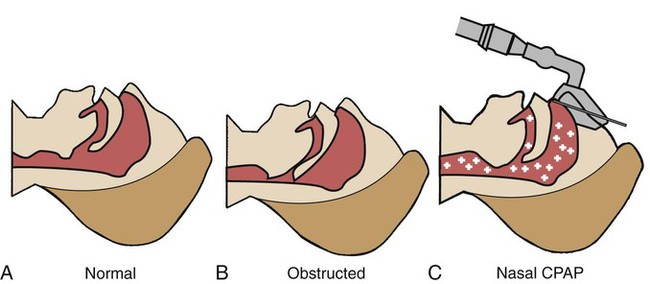

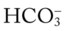 48, and Pa
48, and Pa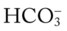 48, and Pa
48, and Pa