CHAPTER 16 SLEEP APNEA
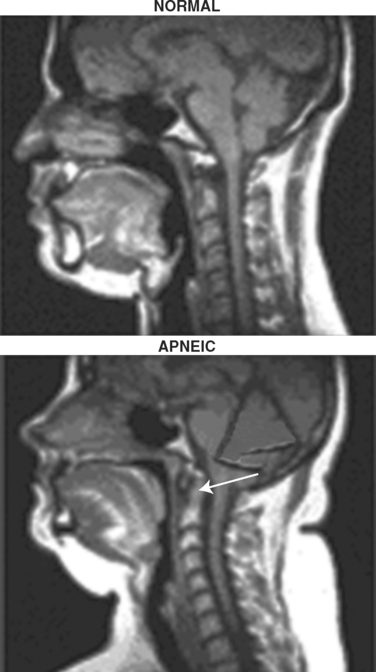
Sleep-disordered breathing is the broad term used to describe the endpoint of a number of conditions of diverse etiology that can disrupt breathing during sleep. Apnea is defined as the cessation of breathing for more than 10 seconds. Hypopnea refers to a reduction in tidal volume without total cessation of respiration. Degrees of hypopnea are recognized: either substantial (>50% reduction in airflow) or moderate (<50% reduction in airflow with desaturations of >3%, or electroencephalographic evidence of arousal). Episodes of apnea and hypopnea often, if not always, coexist; apnea represents the more severe end of the spectrum of reduction in tidal volume (Fig. 16-1).
OBSTRUCTIVE SLEEP APNEA-HYPOPNEA
Epidemiology
Obstructive sleep apnea-hypopnea (OSAH) is an increasingly important disease with numerous clinically relevant consequences, including neurocognitive and cardiovascular sequelae.1–3 The prevalence of this disease varies, depending on the definitions (of hypopnea) used. Young and colleagues4,5 showed that 4% of men and 2% of women in a middle-aged North American population had symptoms of OSAH and an apnea-hypopnea index exceeding 5. However, 24% of men aged 30 to 60 and 9% of women had an abnormal apnea-hypopnea index but without excessive sleepiness, which had been used to define the former statistics. Cardiovascular risk assessments, however, have shown a dose-response relationship between the apnea-hypopnea index and various sequelae; thus, the definition and epidemiology are still evolving (Young, Peppard, Gottlieb 2002).
Pathophysiology
Considerable progress has been made in understanding the genesis of obstructive events. The upper airway is anatomically small, and augmented pharyngeal dilator muscle activation maintains airway patency while the patient is awake but not while asleep, when an increase in upper airway resistance is found. Snoring, an important marker of increased upper airway resistance, is in part genetically determined,6 which perhaps reflects anatomical contributions such as a degree of retrognathia or overbite. Racial differences may be explained by this (apnea is more frequent among African Americans).7 Airway muscle tone insufficient for the airway size may allow intraluminal negative pressure to collapse the pharyngeal “tube.” Additional anatomical factors include enlarged tonsils or adenoids, vascular perfusion, the posture of the individual (supine versus lateral) and, of importance, fat accumulated in the pads in the lateral pharyngeal wall (Fig. 16-1).8
During wakefulness, augmented pharyngeal dilator muscle activity maintains airway potency. At sleep onset and/or during rapid-eye-movement sleep (with active inhibition of muscles), this reflex activity is diminished, and if airway anatomy is abnormal, the airway is compromised, which leads to hypopneas and/or apneas. As a result, hypoxia and hypercapnia occur; ventilation is stimulated, often with arousal from sleep; and airway patency is reestablished. With the return to sleep, the cycle is repeated. It is possible, then, to conceive of a continuum of disordered breathing from snoring alone to an inability to breathe and sleep at the same time. Additional risk factors for OSAH are obesity, male gender, and increasing age. Of patients with OSAH, 70% are obese (pharyngeal size is diminished); sleep laboratories report a fivefold or sixfold increased risk of OSAH in men in comparison with women; and the prevalence increases with age.9 An evolving literature10 also suggests an important concept of snoring-induced traumas causing sensory and/or motor neuronal damage, as well as actual damage to the muscle (Boyd, Petrof, and Hamid, 2004).
Clinical Manifestations/Sequelae
Poor sleep quality and daytime sleepiness are largely the results of sleep fragmentation by repetitive arousals. The neurocognitive sequelae of recurrent arousals also include reduced performance in neuropsychological tests, lengthened reaction times, altered quality of life, and an increased risk of vehicular accidents and work-related accidents.1,8 A causal relationship to all is supported by the response to treatment with continuous positive airway pressure (CPAP), which improves these sequelae.11–13 Because of its practical importance, more should be said about sleep apnea and driving.
 The fact that for 7 seconds, the driver could have clearly seen at the point of runoff or the object hit (which implies prolonged inattention rather than momentary distraction).
The fact that for 7 seconds, the driver could have clearly seen at the point of runoff or the object hit (which implies prolonged inattention rather than momentary distraction).Driver performance can be measured by simulators of varying degrees of sophistication, and some patients with OSAH perform as poorly as subjects intoxicated with alcohol. Beneficial effects of treatment, including CPAP and surgery, have also been shown with these simulators. The U.K. Driver and Vehicle Licensing Agency (DVLA) has a guide for medical practitioners in which it is pointed out that it is the duty of the license holder to notify the DVLA of any medical condition that may affect safe driving. There are some circumstances in which the license holder cannot, or will not, do this. Under these circumstances, the General Medical Council has issued clear guidelines:
At the same time, the associated hypoxemia promotes sympathetic activation and circulatory vasoconstriction. With the return of airflow, the augmented preload leads to increases in stroke volume and in systemic blood pressure. This occurs repeatedly, and the normal nocturnal fall in blood pressure may be lost. A delayed effect on diurnal blood pressure may then follow. Indeed, it is appreciated that as much as one third of “essential” hypertension is associated with OSAH.14,15 A causal relationship is, again, supported by the response to treatment (with CPAP)16 (Pepperell, 2002). The combination of immediate and delayed hemodynamic effects in OSAH have been associated with increased risk of myocardial infarction and congestive heart failure, and there is evidence of a link between these and stroke.17 Additional links have been demonstrated with insulin resistance.18 The combination of obesity, insulin resistance (with or without diabetes), hypertension, and cardiovascular disease is typical of the metabolic syndrome, or syndrome X. Because these may all be associated with OSAH also, OSAH should obviously be considered as well; some authorities refer to it as syndrome Z.19
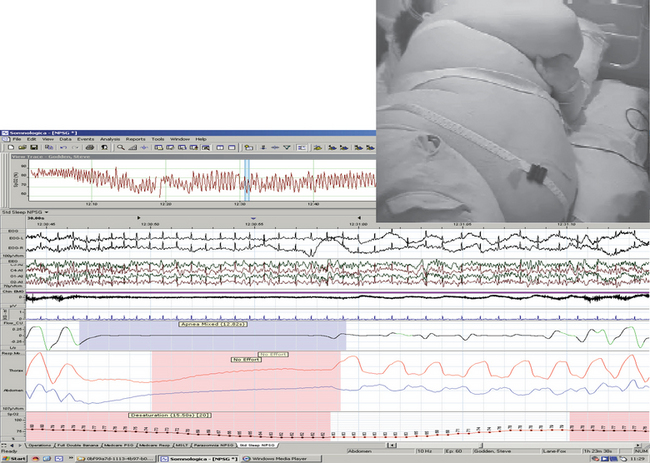
In the example shown in Figure 16-2, a sleep-onset central apnea is followed by a hypopnea associated with efforts to breathe, registered by abdominal and thoracic impedance plethysmography. The hypopnea in this instance, however, resulted from ineffectual diaphragm contraction, evidenced by the paradoxical inward movement of the abdomen, presumably caused by the excessive abdominal load.
Diagnosis
Investigations
Overnight oximetry is a useful screening test for patients suspected of having OSAH. It is highly specific and relatively inexpensive. It is simple to perform and noninvasive, and it can be performed reliably in the community. Its sensitivity has been reported to be as high as 70%, but its specificity is closer to 90%.20
Treatment
When the primary problem is an abnormally small upper airway, treatment is aimed at rectifying this. Cures may be potentially effected with surgical removal of obstructing tonsils and adenoids, substantial weight loss, and, in rare cases, prevention of supine sleep. There currently exist no drugs that increase further upper airway dilator activity.
Nasal Continuous Positive Airway Pressure
This remains the treatment of choice with substantial grade A evidence summarized by a Cochrane Review. Important immediate benefits are seen in sleepiness, cognition, quality of life, accidents, and blood pressure reduction, and reduced cardiovascular events are anticipated. However, compliance with CPAP is imperfect; approximately 20% of patients do not adhere to the CPAP regimen. Remedial, if imperfect, strategies include heated humidification, use of nasal decongestants, and intensive follow-up (cognitive behavioral therapy). Bilevel positive airway pressure is no better tolerated (Fig. 16-3).
Pragmatic Approach
For completeness, surgical treatment must be mentioned. The most common surgical procedure is uvulopalatopharyngoplasty, in which the uvula and redundant soft tissue of the soft palate are resected. The reduction in apnea-hypopnea index is, however, small; only 41% of patients who undergo this procedure have an apnea-hypopnea index of less than 20.21 Results of newer techniques such as radiofrequency ablation have also been disappointing. However, for the patient with the primary complaint of snoring with little or no apnea, these procedures may be considered.
CENTRAL SLEEP APNEA
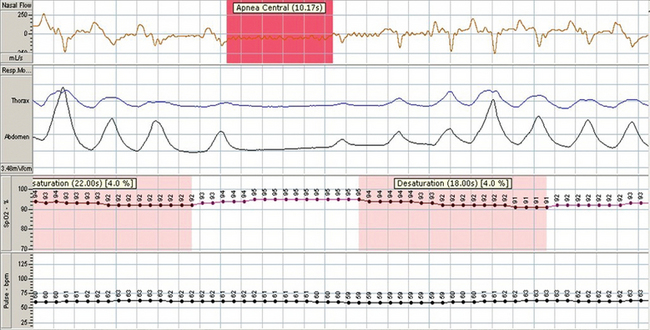
Unlike obstructive apnea, in which there is marked respiratory effort against a closed upper airway, central sleep apnea (CSA) involves repetitive cessation of airflow in the absence of respiratory effort (Fig. 16-4).
Etiology and Pathogenesis
CSR is characterized by alternating periods of hyperventilation and hypoventilation or apnea. It was first described by Hippocrates, but the classic descriptions were made by John Cheyne and William Stokes in the 19th century. The etiology and pathogenesis of CSR have been argued since the description by John Cheyne of a patient with both enlarged cerebral ventricles and heart disease who had periodic breathing. Autopsy studies have shown that most subjects with CSR have structural abnormalities of the brain. The disease is, however, seen frequently in patients with cardiovascular disease, particularly heart failure. Therefore, theories suggesting both neurological and cardiovascular mechanisms in the pathogenesis of the disease have been postulated. Proposed neurological abnormalities include cyclical medullary depression or medullary hyperexcitability alternating with periods of depression. Described cardiovascular abnormalities involve a circulatory delay related to heart failure.
Arousal and apnea termination are associated with the hyperventilation stage of CSR. Termination of apnea in patients with periodic breathing appears to be related largely to chemoreceptor input, which is in contrast to the proposed mechanism for apnea termination in OSAH, in which mechanoreceptor input from the lungs is believed to be of primary importance. Arousals disrupt sleep and are associated with lack of slow-wave sleep but surprisingly little daytime hypersomnolence (Cormican, Williams 2005).
Prevalence
Several studies have shown that significant left ventricular impairment is associated with sleep-disordered breathing, CSA, and CSR. Sleep-disordered breathing has been reported to occur in up to 50% of patients with stable congestive heart failure, and left ventricular systolic dysfunction may be an independent risk factor for sleep apnea in these patients.22 One small study showed that approximately 40% of patients on a heart transplantation waiting list had periodic breathing and CSA. In another study, patients with left ventricular impairment caused by ischemic heart disease were found to have cyclical oxygen desaturations with a frequency 10 times higher than those observed in healthy controls.23 Similar results suggesting a very high prevalence of CSR and central apnea have been reported in patients with dilated cardiomyopathy.
Morbidity and Mortality
In one relatively small study designed to determine the effect of CSA with or without CSR on morbidity and mortality, CSR was found in 60% of patients with CSA. Patients with severe CSR had more central apneas, more but shorter desaturations, and more awakenings and spent more time awake during the night. Heart failure was associated with CSR. Even though patients with severe CSR were at almost twice the risk of dying than were those with no apnea, CSR was not found to be an independent risk factor for increased mortality risk. On the basis of the limited information available, it appears that even though CSA is associated with significant morbidity, the prognosis in patients with CSA is largely dependent on the underlying disease and not on the presence of sleep apnea per se.
Treatment
1. Drugs
Respiratory stimulants such as methylxanthines appear to be a logical treatment for CSA, but large controlled studies have not been performed. In two small studies that included patients with heart failure, administration of theophylline resulted in a reduction in CSR and an improvement in oxygen desaturation events and sleep disruption.24,25 Aminophylline has also been reported to ameliorate CSA caused by structural brainstem disease. The tricyclic antidepressant imipramine may also reduce the number of apneic episodes and improve both nocturnal and diurnal symptoms in patients with CSA.
2. Noninvasive Continuous Positive Airway Pressure
The acute hemodynamic effects of nasal CPAP administration in patients with heart failure remain highly controversial; authors of a number of small studies have reported conflicting results. Some investigators have reported adverse hemodynamic effects, including a fall in cardiac index and a rise in systemic vascular resistance, and acute left ventricular failure occurring shortly after initiation of treatment has been described.26 Conversely, other studies have reported that CPAP either does not change or may improve the cardiac index in patients with left ventricular dysfunction and elevated pulmonary arterial wedge pressure.27
CONCLUSIONS
Sleep apnea is a common disturbance with many effects on sleep and daytime functioning. Obstructive sleep apnea is linked to many important adverse daytime consequences such as poor performance, accidents, hypertension, heart disease, stroke, and insulin resistance. The close association with obesity and the current epidemic of obesity mean that in the future, these disorders will become more prevalent, and thus clinicians need to remain alert to them and to be proactive in their evaluation of sleep. To this end, sleep-related questions should be routine and include those about snoring, daytime sleepiness (with the Epworth Sleepiness Scale [Table 16-1]), witnessed apneic events, nocturia, sleep duration, and sleep quality. Other parts of this book point out additional questions of importance, such as those aimed at identifying cataplexy and restless legs syndrome and injury in sleep. Simple approaches to diagnosis through nocturnal oximetry are encouraged, and referral for more complex studies should be contemplated if results are equivocal.
| How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how they would have affected you. Use the following scale to choose the most appropriate number for each situation: | |
| 0 = no chance of dozing | |
| 1 = slight chance of dozing | |
| 2 = moderate chance of dozing | |
| 3 = high chance of dozing | |
| Situation | Chance of Dozing |
| Sitting and reading | ___________ |
| Watching TV | ___________ |
| Sitting inactive in a public place (e.g., a theater or a meeting) | ___________ |
| As a passenger in a car for an hour without a break | ___________ |
| Lying down to rest in the afternoon when circumstances permit | ___________ |
| Sitting and talking to someone | ___________ |
| Sitting quietly after a lunch without alcohol | ___________ |
| In a car, while stopped for a few minutes in traffic | ___________ |
| Scoring | |
| Total the points from all situations. If your score is 1-6, you are getting enough sleep. A score of 7-8 is average. If your score is 9 and up, seek the advice of a sleep specialist in your area without delay. | |
Boyd J, Petrof B, Hamid Q, et al. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541-546.
Cormican L, Williams AJ. Sleep disordered breathing in congestive heart failure. Br J Cardiol. 2005;12:171-172.
Pepperell J, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomised parallel trial. Lancet. 2002;359:204-210.
Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
1 Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847-851.
2 Shahar E, Whitney CW, Redline S, et al. Sleep disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19-25.
3 Peppard P, Young T, Palta M, et al. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
4 Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;32:1230-1235.
5 Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
6 Desai A, Cherkas L, Spector T, et al. Genetic influences in self reported symptoms of OSA—a Twin Study. Twin Res. 2004;7:589-595.
7 Redline S, Tishler PV, Hans MG, et al. Racial differences in sleep-disordered breathing in African-Americans. Am J Respir Crit Care Med. 1997;155:186-192.
8 Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673-1689.
9 Bixler EO, Vgontzas AN, Ten Have T, et al. Effects of age on sleep apnea in men: prevalence and severity. Am J Respir Crit Care Med. 1998;157:144-148.
10 Boyd J, Petrof B, Hamid Q, et al. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541-546.
11 Jenkinson C, Davies RJ, Mullins R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100-2105.
12 Engleman HM, Martin SE, Kingshott RN, et al. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341-345.
13 Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnea. J Sleep Res. 1997;6:199-204.
14 Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654-675.
15 Williams AJ, Houston D, Finberg S. Sleep apnea and essential hypertension. Am J Cardiol. 1985;55:1019-1022.
16 Pepperell J, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomised parallel trial. Lancet. 2002;359:204-210.
17 Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
18 Ip M, Lam B, Ng M, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Res Crit Care. 2002;165:670-676.
19 Wilcox I, McNamara SG, Collins F, et al. Syndrome Z: the interaction of sleep apnea, vascular risk factors and heart disease. Thorax. 1998;53:S5-S28.
20 Williams AJ, Yu G, Santiago S, et al. Screening for sleep apnea using pulse oximetry. Chest. 1991;100:631-635.
21 Hudgel DW. Availability of a meta-analysis of the surgical treatment of obstructive sleep apnea. Chest. 1997;111:265-266.
22 Markides V, Williams AJ. Detection of sleep apnea in the cardiac care unit. In: Mohsenifar Z, Shah PK, editors. Practical Critical Care Cardiology. New York: Marcel Dekker; 1998:90-124.
23 Rasche K, Hoffarth HP, Marek W, et al. Nocturnal oxygen saturation in patients with coronary heart disease dependent on degree of left ventricular functional impairment. Pneumonologie. 1991;45:261-264.
24 Dowdell WT, Javaheri S, McGinnis W. Cheyne Stokes respiration presenting as sleep apnea syndrome. Am Rev Respir Dis. 1990;141:871-879.
25 Tomcsanyi J, Karlocai K. Effect of theophylline on periodic breathing in congestive heart failure measured by transcutaneous oxygen monitoring. Eur J Clin Pharmacol. 1994;46:173-174.
26 Liston R, Deegan PC, McCreery C, et al. Haemodynamic effects of nasal continuous positive pressure in severe congestive heart failure. Eur Respir J. 1995;8:430-435.
27 Naughton MT, Rahman MA, Hara K, et al. Effect of continuous airway pressure on left ventricular transmural pressure in patients with congestive cardiac failure. Circulation. 1995;91:1725-1731.