Sleep Alterations and Management
Nurses who have an appreciation of the importance of sleep place a higher priority on protection of patients’ sleep.1 Health care providers interrupt patients’ sleep for assessment, treatments, or interventions, and environmental noise, pain, or anxiety can also disturb it.2 Although prioritizing care is essential, the consequence of sleep interruptions is not merely sleep-deprived patients; alterations in sleep patterns can result in chronic sleep problems, poor recovery, and decreased quality of life.1 To facilitate sleep and healing, critical care nurses need to understand the essentials of sleep and chronobiology, the effect of pharmacologic therapy on sleep, and the consequences of disrupted sleep. The purpose of this chapter is to acquaint nurses with the characteristics of normal human sleep and chronobiology, changes in sleep associated with aging and pharmacologic treatment, and abnormal sleep patterns that may affect critically ill patients. Evidence-based nursing care for critically ill patients with sleep disturbances is discussed.
Normal Human Sleep
Sleep Physiology
Humans spend about one third of their lives engaged in a process known as sleep. Although little is now known about the physiologic process or the depths to which it affects us, researchers are learning more about sleep every day. The behavioral definition of sleep is a reversible behavioral state of perceptual disengagement from and unresponsiveness to the environment.3 Sleep is a basic human need, just as food and water are. For patients to regain and maintain their optimal physical and emotional health, they must be able to get adequate amounts of quality sleep. To help patients obtain their optimal amount of sleep, a nurse must first understand what constitutes normal sleep and how the nursing plan of care can contribute to accomplishing this goal.
Polysomnography (PSG) is the collection of multiple channels of physiologic data to assess sleep and its disorders using various electrodes.4 Electroencephalographic (EEG) electrodes are attached to the patient’s scalp to measure brain waves. Changes in the EEG frequency (number of waveforms) and amplitude (height of waveform) over the course of the study allow the sleep to be scored into stages. Sleep stages are distinguished primarily by the EEG waveforms they produce. Sleep is scored by each 30-second epoch or segment of the tracing. The criteria for scoring sleep in infants differ from those used for adults.4
Electrooculography (EOG) measures eye movement activity. The study can help to determine when the patient is in rapid eye movement (REM) sleep; it also can establish when sleep onset occurs as reflected by slow, rolling eye movements.4 Electromyography (EMG) involves leads placed over various muscle groups. When placed over the chin, the leads can help detect muscle atonia associated with REM sleep. Intercostal leads detect respiratory effort, whereas leads over the anterior tibialis detect leg movements that may be causing the patient to arouse. The electrocardiogram (ECG) shows any cardiac abnormalities, oximetry monitors the oxygen saturation levels, and piezoelastic bands around the chest and abdomen detect respiratory disorders such as apnea. Thermocouples are used to monitor airflow through the nose and mouth.4
Sleep Stages
Humans experience three states of being. They are awake, in REM sleep, or in non–rapid eye movement (NREM) sleep. NREM sleep usually occupies 70% to 75% of the sleep cycle, with REM sleep comprising 20% to 25%.5 Some theorize that NREM sleep is a restorative period that relieves the stresses of waking activities, whereas REM sleep serves to refuel creative brain stores.
Non-Rapid Eye Movement Sleep
Stage N1 comprises 2% to 5% of a night’s sleep and is demonstrated by an EEG pattern of low-voltage, mixed-frequency waveforms with vertex sharp waves.5 The EOG during stage N1 may demonstrate slow, side-to-side eye movements. A patient with severely disrupted sleep may experience an increase in the amount of stage N1 sleep throughout the sleep cycle. As a patient makes the transition from awake to asleep, a brief memory impairment may occur.4 As a result, the patient may not remember educational or care instructions given by the nurse during the transition between sleep and wake states. Stage N2 sleep occupies about 45% to 55% of the night, with sleep deepening and a higher arousal threshold being required to awaken the patient. Changes seen in the EEG pattern include sleep spindles and K complexes.5 As stage N2 continues, high-voltage, slow-wave activity begins to appear.4 When these slow waves represent 20% of the EEG activity per page, the criteria are met for stage N3 sleep, which constitutes 15% to 20% of the cycle.5 In stage N3 sleep, slow waves continue to develop until 50% of the EEG waveforms are slow wave. This stage of sleep is often referred to as slow-wave sleep.5
NREM sleep is dominated by the parasympathetic nervous system. The body tries to maintain a homeostatic regulation, and this causes a decreased level of energy expenditure. Blood pressure, heart and respiratory rates, and the metabolic rate return to basal levels. EMG levels are lower in NREM as opposed to wake states but not as low as in REM sleep. Sweating or shivering that a patient may experience with temperature extremes occurs during NREM sleep but ceases during REM sleep.3 During slow-wave sleep, 80% of the total daily growth-stimulating hormone is released, which works to stimulate protein synthesis while sparing catabolic breakdown. The release of other hormones, such as prolactin and testosterone, suggests that anabolism is occurring during slow-wave sleep. Cortisol release peaks during early morning hours, whereas melatonin is released only during darkness, and thyroid-stimulating hormone is inhibited during sleep. The activities associated with stage N3 sleep include protein synthesis and tissue repair, such as the repair of epithelial cells and specialized cells of the brain, skin, bone marrow, and gastric mucosa.6
Rapid Eye Movement Sleep
REM sleep occupies about 20% to 25% of the night in healthy young adults and is sometimes known as the dream stage. However, dreaming is not the exclusive property of any one stage. REM can be viewed as a highly active brain in a paralyzed body and is frequently referred to as a paradoxic sleep. The paradox is that some areas of the brain remain very active, whereas others are suppressed. EEG waveforms are relatively slow voltage and sawtooth waves are present. Increased cortical activity occurs, with the EEG pattern resembling those of the wake state. Synchronized bursts of rapid, side-to-side eye movements with suppressed EMG activity (muscle atonia) are seen, indicating functional paralysis of the skeletal muscles.7
The sympathetic nervous system predominates during REM sleep. Oxygen consumption increases, and blood pressure, cardiac output, and respiratory and heart rates become variable. The body’s response to decreased oxygen levels and increased carbon dioxide levels is lowest during REM sleep. Cardiac efferent vagus nerve tone is generally suppressed during REM sleep, and irregular breathing patterns can lead to oxygen reduction, particularly in patients with pulmonary or cardiac disease. An increase in premature ventricular contractions and tachydysrhythmias may be associated with respiratory pauses during REM sleep. Arterial pressure surges and increases in heart rate, coronary arterial tone, and blood viscosity may cause the combination of plaque rupture and hypercoagulability in persons with cardiac disease.7
Sleep Cycles
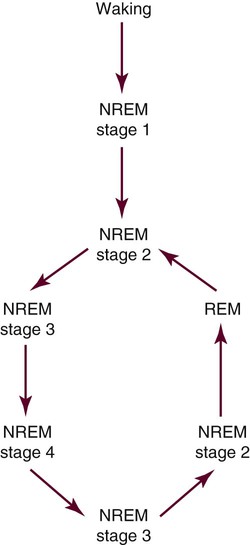
NREM and REM sleep cycles alternate (Fig. 7-1) throughout the night. Sleep onset usually occurs in stage 1 sleep, progressing through stages 2 and 3, then going back to stage 2, at which time the person usually enters REM. This first cycle typically takes about 70 to 100 minutes, with later cycles lasting 90 to 120 minutes. Four to five cycles are completed during normal adult sleep. NREM sleep predominates during the first third of the night, whereas REM is more prominent during the last third. Brief episodes of wakefulness (usually less than 5%) tend to intrude later into the night and are usually not remembered the next morning.5
Chronobiology
Circadian System
Many body systems cycle with approximately a 24-hour period, hence the name circadian rhythm (from circa [“about”] and dies [“day”]).8 Among these systems is the sleep-wake rhythm. A bundle of cells in the anterior hypothalamus, known as the suprachiasmatic nucleus, functions as the pacemaker for these rhythms. The circadian system facilitates cycling of the prescribed functions within a predictable period, but the functions are also influenced by other conditions, such as social activity, posture, and physical environment.8
Under normal conditions, a person’s rhythms interact and influence one another. For example, when body temperature is becoming lower, a person is more likely to sleep, and as the body temperature rises in early morning hours, people awaken. Another example is the melatonin cycle, which tends to run in synchrony with the sleep-wake cycle.8
External influences such as posture, exercise, and light also influence the sleep circadian rhythm. These external influences, known as zeitgebers, can shift the rhythm, causing it to peak at different times, or fragment it. Light is the most influential zeitgeber for sleep8; critical care nurses therefore need to limit the light in the environment during nocturnal hours to facilitate sleep and circadian continuity in their patients.9
Homeostatic Mechanism
The recent history of the sleep obtained by an individual also influences timing and depth of sleep. Known as the homeostatic process of sleep regulation, this determinant of sleep is linked to how much sleep the individual has had previously. Essentially, someone who is sleep-deprived will sleep more readily, regardless of circadian phase, whereas someone who is well rested will not fall asleep readily.10,11 The amount of slow-wave sleep (stage N3) reflects sleep intensity, and individuals recovering from sleep deprivation have increased amounts of slow-wave sleep.11
Circadian and Homeostatic Interaction
Circadian and homeostatic processes function together to ensure optimal sleep for an individual. In essence, these studies have shown that homeostatic processes primarily regulate slow-wave activity and that the ratio of REM to NREM sleep is primarily regulated by the interaction of circadian and homeostatic processes.11
Pharmacology
Hypnotic benzodiazepines remain the medications of choice to treat insomnia. Insomnia is a patient complaint of inability to initiate or maintain sleep, and prescriptions for treatment of insomnia cost more than $1 billion annually.12 Acute stress, such as admission to a critical care unit, may cause some patients to experience acute sleep-onset insomnia. Hypnotics tend to promote lighter sleep stages and have a higher lipophilicity, which can cause the elderly to experience an increased drug half-life.12 Care should be used in the administration and dosage of hypnotics in the elderly. This age group may experience night terrors, nightmares, and increased agitation. The metabolism of hypnotics in elderly patients can be inhibited by the use of steroids, or it can be accelerated in those who smoke. Hypnotics may also produce anterograde amnesia, which is a memory failure of information processed after the medication is consumed. Patients with normal ventilation should not be affected by the mild respiratory depression caused by hypnotics, although patients with chronic obstructive pulmonary disease (COPD) or sleep-disordered breathing may be affected.13
Wake-promoting medications produce increased arousal, behavioral activation, and alertness. They can be divided into three classes: direct-acting sympathomimetics (e.g. phenylephrine), indirect-acting sympathomimetics (e.g., methylphenidate, amphetamine, mazindol), and stimulants that are not sympathomimetics (e.g., caffeine). Common side effects can include irritability and sweating, with talkativeness, anorexia, gastrointestinal complaints, dyskinesias, insomnia, and palpitations occurring less frequently.14 Wake-promoting medications can be used for patients who experience disabling symptoms of sleepiness resulting from narcolepsy, idiopathic central nervous system hypersomnia, or sleep deprivation. Wake-promoting medications should be used only when sustained alertness is required for the individual or for reasons of public safety.
Wake-promoting medications possess a high abuse potential. A sequence of euphoria, dysphoria, paranoia, and psychosis can occur after a single exposure, and sustained use can lead to cognitive and behavioral disorders. Proper dosing and a structured management plan are recommended for wake-promoting medication use. This includes providing patient education with treatment goals, beginning with low doses, emphasizing good sleep hygiene such as naps, and adjusting the dosage according to clinical information. Wake-promoting medications should be included as part of a treatment plan only to decrease excessive somnolence. Sustained use of high-dose wake-promoting medications can lead to cognitive and behavioral disorders.15 Effective sleep hygiene and attention to other substances or medications that can affect sleep may be beneficial for patients who want to avoid the abuse potential.
Many people use alcohol to assist them in falling asleep. Alcohol is a central nervous system sedative and will cause suppression of REM sleep. More than two alcoholic drinks may cause an increase in NREM stages 1 and 2 and decrease the onset of slow-wave sleep. Alcohol also may cause shallow, fragmented sleep and may precipitate or aggravate an existing obstructive sleep apnea (OSA) condition. In the 2008 National Sleep Foundation survey, 8% reported self-medicating for insomnia with alcohol. Of those polled, 20% reported using alcohol, over-the-counter medications (7%), prescription sleep medications (3%), or alternative therapy or herbal supplements (2%).16
Patients with illness may respond differently to medications than do healthy patients. Patients may come to the critical care unit with impaired sleep or poor cognitive function. Beta-blockers, a commonly used class of medications in critical care, are known to produce nightmares and have disruptive effects on sleep quality in some individuals.17 The effect of various drug combinations are not well known.
The critical care nurse should assess the patient’s need for sedative and analgesic medications. The nurse has a responsibility to administer these medications in the most efficient manner to promote sleep and to monitor effectiveness. This can be achieved through assessment, including a medication history, diagnostic test results, and review of the patient’s medical history. Information from that assessment assists the nurse in formulating a nursing diagnosis with outcome criteria and interventions. Evaluation of the patient ensures attainment of the desired outcomes.18
Sleep Pattern Disturbance
Description
Sleep disturbance in critically ill patients is defined as insufficient duration or stages of sleep that results in discomfort and interferes with quality of life. When ill, most people need more sleep than usual, and sleep seems to promote recovery. Studies have demonstrated that the nocturnal sleep of patients in critical care units is severely disturbed, even though many receive medications to promote sleep.1,19,20
Etiology and Pathophysiology
Normal sleep is a period of decreased physiologic workload for the cardiovascular system. Insufficient sleep in acutely and critically ill patients has been associated with physiologic and psychologic exhaustion and may delay recovery from illness. These effects include mental status changes resulting in delirium.21 A general consensus among sleep experts and researchers is that sleep deprivation results in psychologic alterations such as changes in mood and performance, fatigue, increased irritability, and feelings of persecution.1,22
The intensification of pain related to sleep disturbance is a significant problem in acutely and critically ill patients. Sunshine and colleagues related a potential theory for pain alleviation from massage therapy that is linked to quiet or restorative sleep. During deep sleep, somatostatin is normally released. Without this substance, pain is experienced. Substance P is released when an individual is deprived of deep sleep, and substance P is noted for causing pain. When people are deprived of deep sleep, they may have less somatostatin and increased substance P, which results in greater pain and more sleep disruption.23
Sleep disturbance in critically ill patients may stem from psychologic stress associated with critical illness and the critical care environment, surgical stress, noise, interruptions for care, painful procedures or physiologic processes, excessive bright light, and muscular and joint discomfort resulting from bed rest.22 Of 84 patients’ recollections about sleep-disturbing factors in the critical care unit, the most frequently mentioned factors included an inability to get comfortable or lie comfortably (recalled by 70% of patients), inability to perform one’s usual routine before going to sleep (57%), anxiety (55%), and pain (54%).24 The stressful nature of the critical care environment and uncertainty and worry regarding the outcomes of a critical illness may explain why some patients have such difficulty sleeping while hospitalized. These concerns are not culturally isolated. A Swedish study identified pain, anxiety, and environmental noise among factors that interfered with sleep.25 However, perhaps because of staffing issues and general work flow, nurses tend to provide labor-intensive tasks during early morning hours.2 This trend is clearly counterproductive and should be discouraged. Another source of sleep disturbance in critically ill patients is surgical stress. A general inflammatory response caused by the heart-lung machine or the incisions, altered endocrine neurometabolism control, and the effects of medications such as benzodiazepines, barbiturates, scopolamine, and systemic opioids may disturb sleep.26
Bright nocturnal light, excessive noise, and frequent interruptions for care procedures also may disturb sleep in critically ill patients. In a study of light and sound levels and interruptions to sleep in medical and respiratory critical care units, light levels maintained a day-night rhythm, with peak levels dependent on window orientation. Peak sound levels were extremely high in all areas and exceeded recommendations of the Environmental Protection Agency as acceptable for a hospital. Patient interruptions for care procedures tended to be variable but ubiquitous, leaving little time for condensed sleep.1,2,22 However, one group determined that noise and nursing interventions explained fewer than 30% of sleep interruptions.27 Identifying additional factors that create these sleep disruptions remains an area of potential research.
Not surprisingly, mechanical ventilation and the required care associated with it contribute to sleep disturbances. Cooper and colleagues studied 20 subjects who were receiving mechanical ventilation and classified as critically ill; based on PSG-accepted criteria, none of these patients had normal sleep. Twelve did not experience sleep at all, and the remaining eight demonstrated PSG findings consistent with severely disrupted sleep. The magnitude of sleep disruption found in the latter group was similar to the excessive daytime sleepiness and cognitive impairment of patients with untreated OSA.28 Ventilatory modes may also influence sleep patterns. Bosma and associates compared the effects of pressure support and proportional assist ventilation (PAV) on sleep. PAV resulted in greater patient-ventilator synchrony and therefore improved sleep.29 A comparison of assist control ventilation (AC) and low levels of pressure support also found that use of AC improved sleep quality. The authors suggested that ventilating patients at night may improve weaning outcomes. However, additional evidence suggests that sleep disturbances associated with prolonged mechanical ventilation do not resolve with extubation and discharge.30 These findings emphasize the nursing responsibility to promote and protect the sleep of all patients in the critical care environment.
Nursing Management
If one of the primary causes of the sleep pattern disturbance for a patient hospitalized in the critical care unit is a state of heightened anxiety and discomfort,31 nursing interventions, such as massage, that promote relaxation and comfort may be effective. In a review of 22 articles investigating the effects of massage on relaxation, comfort, and sleep, massage consistently reduced anxiety and pain.33 Another research-based intervention is playing audio of ocean sounds or other relaxing auditory sounds. Williamson found that an audiotape of the ocean or the rain significantly increased sleep quality in patients in a progressive care unit.34 Providing a relaxed, caring environment that encourages confidence in care providers may also assist the patient to relax. Allowing close family members to sit quietly at the bedside while the patient rests may comfort the family and the patient and allow the patient to rest better. Stanchina and colleagues found that white noise machines in patient rooms decreased the variance in peak noises and increased arousal thresholds for patients.35
Nurses should limit interruptions for care procedures and should coordinate the care among other disciplines to allow patients time for consolidated nocturnal sleep and a daytime nap. Draperies or blinds should be opened during the day to allow patients to receive bright natural light and to help orient them to time of day, with lights dimmed at night. Noise from staff, squeaky carts, alarms, televisions, slamming doors, and ringing phones should be minimized. Offering the patient earplugs may help decrease noise and promote sleep. Outcomes of these nursing interventions can be assessed and documented on the 24-hour flow sheet. One group reported that an enforced afternoon quiet time resulted in benefits for patients as well as the multidisciplinary team.36
Sleep Apnea Syndrome
Description
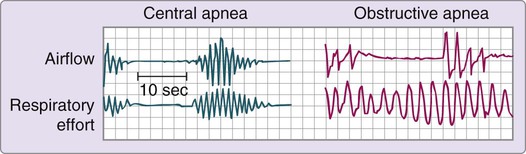
Sleep apnea syndrome, sometimes called sleep-disordered breathing, occurs when airflow is absent or reduced. Apnea during sleep can be divided into three types: 1) obstructive; 2) central; and 3) mixed. In obstructive apnea, the absence of airflow is caused by an obstruction in the upper airway. Complete obstruction lasting 10 seconds or longer is referred to as an obstructive apnea, whereas a partial obstruction is known as a hypopnea (Fig. 7-2). In central apnea, airflow is absent because of lack of ventilatory muscle effort. The third type of sleep apnea syndrome, mixed apnea, occurs when a combination of obstructive and central patterns occurs in a single apneic event. An apnea-hypopnea index (the number of apneas and hypopneas per hour divided by the hours of sleep) of 5 or greater is diagnostic of sleep apnea syndrome. With new understandings of alterations in ventilatory effort, researchers have developed the respiratory distress index (RDI). To calculate the index, the total number of apneas and hypopneas plus the number of respiratory effort–related arousals (RERAs) or other respiratory events is divided by the hours of sleep. An RDI greater than 5 in addition to reports of daytime sleepiness supports a diagnosis of sleep apnea.37
All types of sleep apnea syndrome are accompanied by arterial desaturation and potentially by hypoxemia, which may cause pulmonary vasoconstriction and an increased systemic vascular resistance. However, desaturation and hypoxemia are most severe in the obstructive type. Although the pathophysiology of OSA is unclear, hypotheses suggest that the various types of sleep apnea are all part of a disease continuum. Failure of the central respiratory rhythm control center to generate a stable rhythm is thought to be the basic defect responsible for sleep apnea syndrome. Cyclic oscillations occur with greater frequency at night and are further exacerbated by mouth breathing.38
Nursing management of the patient with sleep apnea incorporates a variety of diagnoses (Box 7-1). Nursing interventions include optimizing oxygenation and ventilation, providing comfort and emotional support, maintaining surveillance for complications, and educating the patient and family (Box 7-2).
Obstructive Sleep Apnea
Etiology and Pathophysiology
OSA syndrome occurs when at least five apnea or hypopnea events per hour of sleep occur as the result of an obstruction in the upper airway. In the general population, between 3% and 7% of people have severe OSA.39 The incidence of OSA is believed to increase with age. Consequences include chronic hypoventilation syndrome; arousals that fragment sleep; cardiovascular changes such as hypertension, stroke, ischemic heart disease, insulin resistance, ventricular hypertrophy, and nocturnal angina.40 Because of the cardiovascular complications and accidents caused by sleepiness, OSA is a significant condition that should be effectively evaluated.
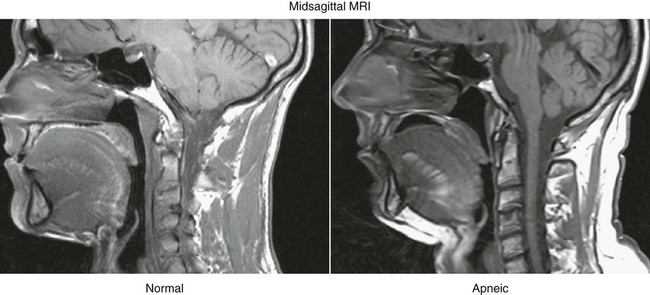
The cause of OSA is not entirely understood; however, upper airway structure, hormonal balance, and neural control are implicated. Factors that contribute to OSA are: 1) anatomic narrowing of the upper airway; 2) increased compliance of the upper airway tissue; 3) reflexes affecting upper airway caliber; and 4) pharyngeal inspiratory muscle function.41 Computed tomographic studies of awake subjects have shown that patients with OSA have narrower airways than normal subjects do. The narrower the airway, the more easily it may become obstructed (Fig. 7-3).
Upper airway patency is also affected by upper airway function, which is under the control of the respiratory motor neurons. During sleep, this control varies and causes decreased neural activity, thereby narrowing the airway. This effect is especially prevalent during REM sleep, when the motor neurons are hypotonic. Unstable control of the respiratory nerves of the diaphragmatic, intercostal, and upper airway muscles can cause sleep apneas.41 Hypothyroidism can alter respiratory controls and thereby contribute to OSA. Other contributing disorders are exogenous obesity, kyphoscoliosis, and autonomic dysfunction.42
With obstruction, inspiratory subatmospheric intrathoracic pressures are abnormally elevated. This leads to a tendency for airways to collapse, resulting in hemodynamic and electrocardiographic changes. The extremely elevated pressures that occur in individuals with OSA who have apneic episodes in REM and NREM stages cause systemic and pulmonary hypertension. Systemic pressures of 200/120 mm Hg (awake control: 130/80 mm Hg) and pulmonary artery pressures of 80/54 mm Hg (awake control: 30/20 mm Hg) have been reported.43 Cardiac dysrhythmias associated with obstructive apnea include bradycardias, sinus arrest, and occasionally, second-degree heart blocks. After resumption of airflow, tachycardias commonly occur. Bradycardia-tachycardia syndrome is associated with OSA.44
Assessment and Diagnosis
Careful monitoring of oxygen saturation and breathing patterns can help the critical care nurse identify patients with this syndrome and assist in its diagnosis and treatment. Patients at risk for OSA may have the following symptoms: snoring; obesity; short, thick neck circumference; cardiovascular disease; systemic hypertension; pulmonary hypertension; sleep fragmentation; gastroesophageal reflux; and an impaired quality of life. Apneas may occur in people whose throats are abnormally small or collapsible. Muscles that would normally hold the throat open relax while the patient is asleep. Snoring is caused when those soft tissues in the throat vibrate. Snoring often precedes the complaint of daytime sleepiness, and the intensity increases with weight gain and alcohol ingestion.42 Men with a collar size of 17 or greater and women with a size 16 or greater are thought to have an increased incidence of apnea. Friedman and others showed a clinical correlation between Modified Mallampati grade (MMP), tonsil size, body mass index, and the severity of apnea. These assessments are used by anesthesiologists to determine intubation difficulty.42 There is an increased incidence of sleep apnea among African Americans as well as Mexican Americans, and an increased incidence is observed among those with metabolic syndrome.
Medical Management
CPAP via nasal mask is the treatment of choice. CPAP machines are simply pressure generators with the effective pressure being determined during the titration part of the PSG. This holds the airway open and prevents collapse. The patient wears a small, triangle-shaped mask over the nose or uses nasal pillows if the mask is not tolerated. CPAP treats the obstruction and the snoring, choking, and gasping that accompany it, and it provides cardiovascular benefits. Although CPAP is the treatment of choice, it is only effective if the patient is compliant with therapy. If a patient cannot tolerate the continuous pressure of CPAP, bimodal positive airway pressure (BiPAP), which provides separate pressures for inspiration and expiration, may be tried.45
Various surgical interventions are available for the treatment of apnea and snoring. Patients with mild OSA or snoring alone may undergo an outpatient procedure called laser uvulopalatopharyngoplasty (LAUP), which uses lasers to remove excess tissue at the soft palate level. For patients who snore but do not have apnea, somnoplasty may provide relief. Somnoplasty involves inserting a small electrode into the soft palate and heating the tissue, causing the area to shrink and tighten.46
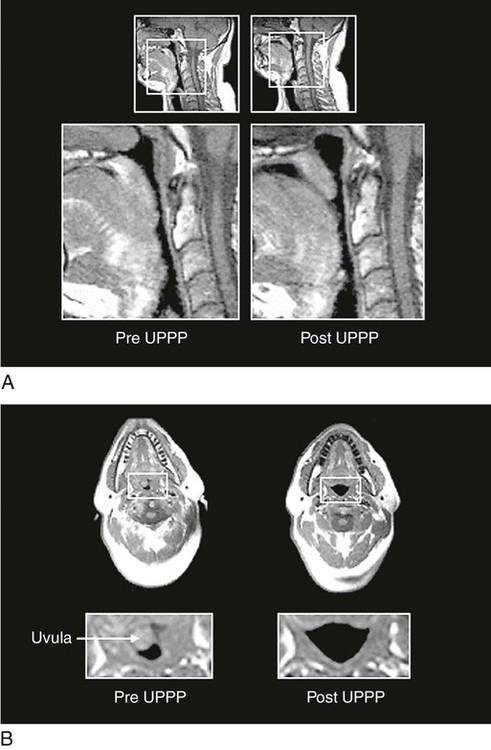
Uvulopalatopharyngoplasty (UPPP) was one of the earlier surgeries used to treat OSA. Essentially, a large tonsillectomy is performed and all redundant tissue is removed (Fig. 7-4). Reports of success from UPPP vary widely, with 40% to 80% of patients experiencing sleep apnea improvement.47 Complications include speech impairment, inability to eat, postoperative bleeding, and infection. Severe pain after UPPP is documented and may continue well into the postoperative period.47 UPPP patients are not usually admitted to critical care areas, because the postoperative recovery does not require critical care.
Although tracheostomy was the original surgery procedure used to treat OSA, it is now used only in the most severe cases of apnea that do not respond to other treatments. Bariatric surgery is an effective means to facilitate weight loss and subsequent improvement in OSA.48
Central Sleep Apnea
Etiology and Pathophysiology
Central sleep apnea (CSA) can be seen on PSG as an absence of airflow and respiratory effort for at least 10 seconds. A complete loss of electromyographic activity by the respiratory muscles would be expected, because CSA is defined as a pause in respiration without ventilatory effort.49
A chemoreceptor sensitive to the levels of CO2 resides within the brain. When CO2 levels become excessive, ventilatory efforts are increased to blow off the excess CO2. This negative-feedback loop exists to provide a homeostatic balance in the carbon dioxide and oxygen levels of the body. Whereas OSA results from an obstructed or collapsed airway, patients with CSA suffer from a lack of ventilatory effort. This can be observed in patients with cardiopulmonary disease (e.g., COPD) or heart failure, because their chemoreceptors have become adjusted to an increased CO2 level.50 However, the uniting factor is cessation of breathing momentarily during sleep due to the transient withdrawal of central nervous system drive to the muscles of respiration.51 It is not uncommon for a patient who experiences CSA to also have some obstructive events.
CSA may result from many physiologic or pathophysiologic events.51 Possible causes of nonhypercapnic CSA include periodic breathing at high altitude, renal or metabolic disturbances, and idiopathic central apnea seen at sea level. Hypercapnic CSA can occur in many neuromuscular conditions such as spinal cord or brain injury, encephalitis, brainstem neoplasm or infarcts, muscular dystrophy, myasthenia gravis, bulbar poliomyelitis, and postpolio syndrome.
Assessment and Diagnosis
Clinical characteristics of hypercapnic CSA include respiratory failure, cor pulmonale, peripheral edema, polycythemia, daytime sleepiness, and snoring. Patients with nonhypercapnic CSA have clinical features very similar to those of OSA. Nonhypercapnic CSA characteristics include daytime sleepiness, insomnia or poor sleep, mild or intermittent snoring, and awakenings accompanied by choking or feeling short of breath; frequently, the patients are of normal body weight. Diagnosis is made by overnight PSG or sleep study, which will determine the respiratory and sleep patterns of the patient.51
Medical Management
Because there are two types of CSA, there are two therapeutic approaches depending on the cause of the apnea. The hypercapnic patient who has worsening hypoventilation during sleep is best served by nocturnal ventilation. Most such patients experience some respiratory muscle failure. One treatment for the nonhypercapnic or heart failure patients is nasal CPAP, which also may provide a beneficial cardiovascular effect. Nocturnal oxygen supplementation may be effective as well. If CPAP is not tolerated, pharmacologic management may be tried. Medroxyprogesterone, a respiratory stimulant, may improve ventilation in selected patients. Acetazolamide, a carbonic anhydrase inhibitor that can result in metabolic acidosis, also may decrease the frequency of apneic episodes. Several studies have reported the development of obstructive apnea in patients successfully treated for central apnea.52
Nursing Management
For the nurse caring for a patient with CSA, patient and family education about the patient’s condition and treatment regimen can help to ensure patient compliance. The nurse needs to address any fear or anxiety about going to sleep. Nurses also need to caution patients to avoid alcohol and sedative medications. Weight loss is recommended if the patient is obese. The nurse needs to carefully monitor and assess the respiratory status of the patient. For more information, see the Case Study on Sleep Alterations (Box 7-3).
Summary
Normal Human Sleep
• The behavioral definition of sleep is a reversible behavioral state of perceptual disengagement from and unresponsiveness to the environment.
• Humans experience three states of being: awake, REM sleep, or NREM sleep.
• A sufficient amount of sleep has been achieved when one awakens without external stimuli and gets through the day without feeling sleepy.
• Circadian and homeostatic processes function together to ensure optimal sleep for an individual.
Pharmacology
• Hypnotic benzodiazepines remain the medications of choice to treat insomnia.
• Wake-promoting medications produce increased arousal, behavioral activation, and alertness.
• Patients with illness may respond differently to medications than do healthy patients.
• The nurse has a responsibility to administer these medications in the most efficient manner to promote sleep and to monitor effectiveness.
Sleep Pattern Disturbance
• Sleep disturbance in critically ill patients is defined as insufficient duration or stages of sleep that results in discomfort and interferes with quality of life.
• Insufficient sleep in acutely and critically ill patients has been associated with physiologic and psychologic exhaustion and may delay recovery from illness.
• Sleep disturbance in critically ill patients may stem from psychologic stress associated with critical illness and the critical care environment, surgical stress, noise, interruptions for care, painful procedures or physiologic processes, excessive bright light, and muscular and joint discomfort that result from bed rest.
• Medical management of sleep pattern disturbance in critically ill patients consists of the administration of sedative-hypnotics.
Sleep Apnea Syndrome
• Sleep apnea syndrome, sometimes called sleep-disordered breathing, occurs when airflow is absent or reduced and can be divided into three types: 1) obstructive; 2) central; and 3) mixed.
• All types of sleep apnea syndrome are accompanied by arterial desaturation and potentially by hypoxemia, which may cause pulmonary vasoconstriction and an increased systemic vascular resistance.
• Medical management focuses on correcting the underlying cause, supporting ventilation with CPAP, and evaluating for the patient for surgery.
• Nursing interventions include optimizing oxygenation and ventilation, providing comfort and emotional support, maintaining surveillance for complications, and educating the patient and family.