Objectives
• Explain the physiological effects that occur during rapid eye movement (REM) sleep.
• Describe changes in sleep resulting from the aging process.
• Name three commonly prescribed critical care medications that decrease REM sleep.
• Describe evidence-based practice methods for promoting sleep in critical care.
• Compare and contrast obstructive sleep apnea with central sleep apnea.
![]()
Be sure to check out the bonus material, including free self-assessment exercises, on the Evolve web site at http://evolve.elsevier.com/Urden/priorities/.
Nurses who have an appreciation of the importance of sleep place a priority on protection of patients’ sleep.1 Health care providers may interrupt a patient’s sleep for assessment or treatments; in addition, sleep can be disturbed by pain, anxiety, or environmental noise.2 A recent study indicated that critical care nurses “group” nursing care activities together and attempt to limit interruptions, especially during the night. The use of light among nurses during the nighttime in surgical intensive care units appears to be individualized. Sleep interruptions occur most frequently at the beginning and end of the nursing shifts.3 Although prioritizing care is essential, the consequence of sleep interruptions is not merely sleep-deprived patients; alterations in sleep patterns can delay physical and mental healing.1 To facilitate sleep and healing, critical care nurses need to understand the essentials of sleep and chronobiology, the effect of pharmacological therapy on sleep, and the consequences of disrupted sleep. The purpose of this chapter is to acquaint nurses with the characteristics of normal human sleep, changes in sleep associated with aging and pharmacological treatment, and abnormal sleep patterns that may affect critically ill patients. Evidence-based nursing care for critically ill patients with sleep disturbances is discussed.
Normal Human Sleep
Sleep Physiology
Humans spend about one third of their lives engaged in a process known as sleep. Although little is now known about the physiological process or the depths to which it affects us, researchers are learning more about sleep every day. The behavioral definition of sleep is a reversible behavioral state of perceptual disengagement from and unresponsiveness to the environment.4 Sleep is a basic human need, just as food and water are. For patients to regain and maintain their optimal physical and emotional health, they must be able to get adequate amounts of quality sleep. To help patients obtain their optimal amount of sleep, a nurse must first understand what constitutes normal sleep and how the nursing plan of care can contribute to accomplishing this goal.
Polysomnography (PSG) is the collection of multiple channels of physiological data to assess sleep and its disorders using various electrodes.5 Electroencephalographic (EEG) electrodes are attached to the patient’s scalp to measure brain waves. Changes in the EEG frequency (number of waveforms) and amplitude (height of waveform) over the course of the study allow the sleep to be scored into stages. Sleep stages are distinguished primarily by the EEG waveforms they produce. Sleep is scored by each 30-second epoch or segment of the tracing. The criteria for scoring sleep in infants differ from those used for adults.
Electrooculography (EOG) measures eye movement activity. The study can help to determine when the patient is in rapid eye movement (REM) sleep; it also can establish when sleep onset occurs as reflected by slow, rolling eye movements. Electromyography (EMG) involves leads placed over various muscle groups. When placed over the chin, the leads can help detect muscle atonia associated with REM sleep. Intercostal leads detect respiratory effort, whereas leads over the anterior tibialis detect leg movements that may be causing the patient to arouse. The electrocardiogram (ECG) shows any cardiac abnormalities, oximetry monitors the oxygen saturation levels, and piezoelastic bands around the chest and abdomen detect respiratory disorders such as apnea. Thermocouples are used to monitor airflow through the nose and mouth.
Sleep Stages
Non-Rapid Eye Movement Sleep
Humans experience three states of sleep. They are awake, in REM sleep, or in non-rapid eye movement (NREM) sleep. NREM sleep can be further divided into stages 1 through 4, with each stage being a progressively deeper sleep state. Adults usually enter sleep through NREM stage 1 sleep, which is a transitional, lighter sleep state from which the patient can be easily aroused by light touch or by someone softly calling his or her name. Stage 1 comprises 2% to 5% of a night’s sleep and is demonstrated by an EEG pattern of low-voltage, mixed-frequency waveforms with vertex sharp waves. The EOG during stage 1 may demonstrate slow, side-to-side eye movements. A patient with severely disrupted sleep may experience an increase in the length of stage 1 sleep throughout the sleep cycle. As a patient makes the transition from awake to asleep, a brief memory impairment may occur.6 As a result, the patient may not remember educational or care instructions given by the nurse during the transition between sleep and wake states. Patients sometimes may experience muscle jerks and recall vivid images on awakening. This is called hypnic myoclonia; although not pathological, it can cause the patient to awaken feeling frightened.
Stage 2 NREM sleep occupies about 45% to 55% of the night, with sleep deepening and a higher arousal threshold being required to awaken the patient. Changes seen in the EEG pattern include sleep spindles and K complexes. As stage 2 continues, high-voltage, slow-wave activity begins to appear. When these slow waves represent 20% of the EEG activity per page, the criteria are met for stage 3 sleep, which constitutes 3% to 8% of the cycle. In stage 3 NREM sleep, slow waves continue to develop until 50% of the EEG waveforms are slow wave; this is called stage 4 sleep. Stage 4 comprises 10% to 15% of the cycle. Stages 3 and 4 often are combined and referred to as slow-wave sleep, or delta sleep. Delta sleep has the highest arousal threshold. NREM sleep usually occupies 70% to 75% of the sleep cycle, with REM sleep comprising 20% to 25%.
NREM sleep is dominated by the parasympathetic nervous system. The body tries to maintain a homeostatic regulation, and this causes a decreased level of energy expenditure. Blood pressure, heart and respiratory rates, and the metabolic rate return to basal levels. EMG levels are lower in NREM as opposed to wake states, but not as low as in REM sleep. Sweating or shivering that a patient may experience with temperature extremes occurs during NREM sleep, but this ceases during REM sleep.4
During slow-wave sleep, 80% of the total daily growth-stimulating hormone is released, which works to stimulate protein synthesis while sparing catabolic breakdown. The release of other hormones, such as prolactin and testosterone, suggests that anabolism is occurring during slow-wave sleep. Cortisol release peaks during early morning hours, whereas melatonin is released only during darkness, and thyroid-stimulating hormone is inhibited during sleep. The activities associated with stage 4 NREM sleep include protein synthesis and tissue repair, such as the repair of epithelial cells and specialized cells of the brain, skin, bone marrow, and gastric mucosa.7 Some theorize that NREM sleep is a restorative period that relieves the stresses of waking activities, whereas REM sleep serves to refuel creative brain stores.
Rapid Eye Movement Sleep
REM sleep occupies about 20% to 25% of the night in healthy young adults and is sometimes known as the dream stage. However, dreaming is not the exclusive property of any one stage. REM can be viewed as a highly active brain in a paralyzed body and is frequently referred to as a paradoxical sleep. The paradox is that some areas of the brain remain very active, whereas others are suppressed. EEG waveforms are relatively slow voltage, and sawtooth waves are present. Increased cortical activity occurs, with the EEG pattern resembling those of the wake state. Synchronized bursts of rapid, side-to-side eye movements with suppressed EMG activity (muscle atonia) are seen, indicating functional paralysis of the skeletal muscles. Infants enter sleep onset through REM and spend about 50% of their night in REM sleep.
The sympathetic nervous system predominates during REM sleep.7 Oxygen consumption increases and blood pressure, cardiac output, and respiratory and heart rates become variable. The body’s response to decreased oxygen levels and increased carbon dioxide levels is lowest during REM sleep. Cardiac efferent vagus nerve tone is generally suppressed during REM sleep, and irregular breathing patterns can lead to oxygen reduction, particularly in patients with pulmonary or cardiac disease. An increase in premature ventricular contractions and tachydysrhythmias may be associated with respiratory pauses during REM sleep.7 Arterial pressure surges and increases in heart rate, coronary arterial tone, and blood viscosity may cause the combination of plaque rupture and hypercoagulability in persons with cardiac disease.8
Sleep Cycles
NREM and REM sleep cycles alternate throughout the night. Sleep onset usually occurs in stage 1 sleep, progressing through stages 2 to 4, and then going back to stage 2, at which time the person usually enters REM. This first cycle typically takes about 70 to 100 minutes, with later cycles lasting 90 to 120 minutes. Four to five cycles are completed during normal adult sleep. NREM sleep predominates during the first third of the night, whereas REM is more prominent during the last third. Brief episodes of wakefulness (usually less than 5%) tend to intrude later into the night and are usually not remembered the next morning.
The amount of sleep required is uncertain. No set number of hours has been established, and sleep length may be determined by many factors, including genetic predisposition. A sufficient amount of sleep has been achieved when one awakens without external stimuli and gets through the day without feeling sleepy.
Sleep Changes in Aging
Important changes in sleep occur with aging, and critical care nurses must consider these changes when planning care for older patients. Assessment for sleep disturbances in the older adult is essential, because lack of sleep can compromise daytime function, thereby lowering quality of life.9
Older adults most commonly complain about excessive sleepiness or insomnia, and current research offers justification for both of these complaints.10 Sleep pattern changes in older adults include fewer episodes of stages 3 and 4 NREM and REM sleep.11 Older adults also report that they do not sleep as soundly or feel as rested after awakening.12 One reason for this may be that older adults do not consolidate their sleep into one session. They may go to bed, awake 4 hours later, stay awake for an extended period, and then go back to sleep, resulting in fragmented sleep patterns.9 Many of the diseases associated with aging may contribute to these nocturnal arousals, including diabetes, nocturia, cardiovascular symptoms, chronic pain, and depression.9,13,14 Sleep-related respiratory disorders and increased incidence of periodic leg movements in older adults may further disrupt their sleep.15 Increasing age brings many physical and social changes with which older adults must cope. Assessment of older patients must always include sleep history as an indicator of mental health, because depression is a common struggle for older adults.16
In critical care areas, nurses need to identify these altered sleep patterns as well as other impediments to adequate rest and methods to minimize acute disruption of sleep while accommodating age-related changes in sleep patterns. Authorities on sleep17 recommend the use of nonpharmacological means to promote sleep, such as control of environmental noise and light, use of white noise, music, massage, and allowing specified blocks of time for sleep. It is also important for nurses to educate patients about the changes in sleep that result from aging and to teach sleep hygiene practices such as adhering to regular bedtimes and rising times and avoiding napping.
Pharmacology and Sleep
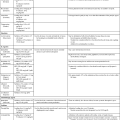
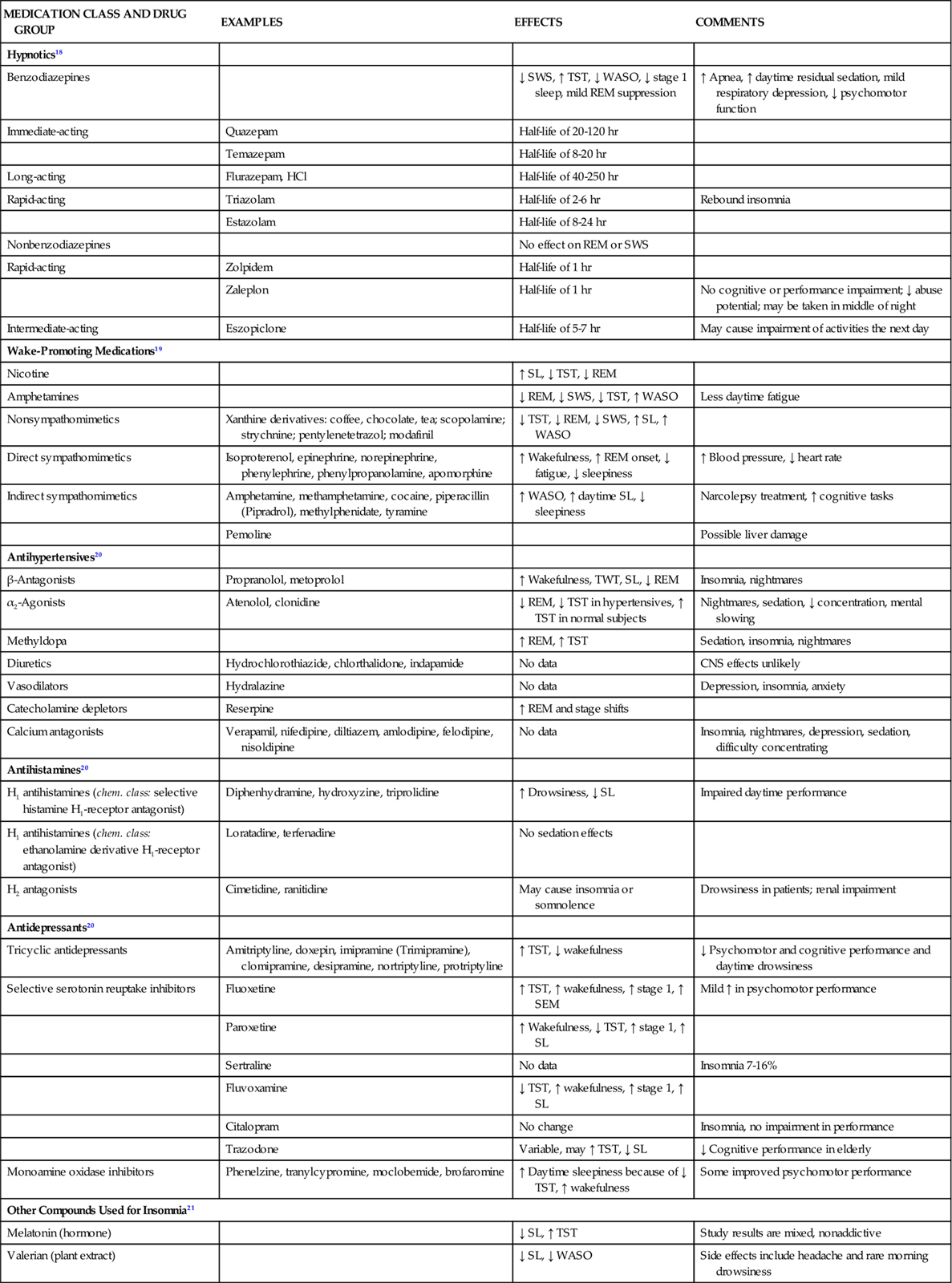
Many drugs essential to treatment of critically ill patients impact sleep quality (Table 5-1).18–21 It is important to understand the relationship between various medications and the sleep of patients in the critical care unit. Pathophysiology and age may profoundly affect not only medication absorption and elimination but also how patients cope with their illness and their ability to maintain health.
TABLE 5-1
PHARMACOLOGICAL MANAGEMENT: DRUGS THAT AFFECT SLEEP AND WAKEFULNESS
| MEDICATION CLASS AND DRUG GROUP | EXAMPLES | EFFECTS | COMMENTS |
| Hypnotics18 | |||
| Benzodiazepines | ↓ SWS, ↑ TST, ↓ WASO, ↓ stage 1 sleep, mild REM suppression | ↑ Apnea, ↑ daytime residual sedation, mild respiratory depression, ↓ psychomotor function | |
| Immediate-acting | Quazepam | Half-life of 20-120 hr | |
| Temazepam | Half-life of 8-20 hr | ||
| Long-acting | Flurazepam, HCl | Half-life of 40-250 hr | |
| Rapid-acting | Triazolam | Half-life of 2-6 hr | Rebound insomnia |
| Estazolam | Half-life of 8-24 hr | ||
| Nonbenzodiazepines | No effect on REM or SWS | ||
| Rapid-acting | Zolpidem | Half-life of 1 hr | |
| Zaleplon | Half-life of 1 hr | No cognitive or performance impairment; ↓ abuse potential; may be taken in middle of night | |
| Intermediate-acting | Eszopiclone | Half-life of 5-7 hr | May cause impairment of activities the next day |
| Wake-Promoting Medications19 | |||
| Nicotine | ↑ SL, ↓ TST, ↓ REM | ||
| Amphetamines | ↓ REM, ↓ SWS, ↓ TST, ↑ WASO | Less daytime fatigue | |
| Nonsympathomimetics | Xanthine derivatives: coffee, chocolate, tea; scopolamine; strychnine; pentylenetetrazol; modafinil | ↓ TST, ↓ REM, ↓ SWS, ↑ SL, ↑ WASO | |
| Direct sympathomimetics | Isoproterenol, epinephrine, norepinephrine, phenylephrine, phenylpropanolamine, apomorphine | ↑ Wakefulness, ↑ REM onset, ↓ fatigue, ↓ sleepiness | ↑ Blood pressure, ↓ heart rate |
| Indirect sympathomimetics | Amphetamine, methamphetamine, cocaine, piperacillin (Pipradrol), methylphenidate, tyramine | ↑ WASO, ↑ daytime SL, ↓ sleepiness | Narcolepsy treatment, ↑ cognitive tasks |
| Pemoline | Possible liver damage | ||
| Antihypertensives20 | |||
| β-Antagonists | Propranolol, metoprolol | ↑ Wakefulness, TWT, SL, ↓ REM | Insomnia, nightmares |
| α2-Agonists | Atenolol, clonidine | ↓ REM, ↓ TST in hypertensives, ↑ TST in normal subjects | Nightmares, sedation, ↓ concentration, mental slowing |
| Methyldopa | ↑ REM, ↑ TST | Sedation, insomnia, nightmares | |
| Diuretics | Hydrochlorothiazide, chlorthalidone, indapamide | No data | CNS effects unlikely |
| Vasodilators | Hydralazine | No data | Depression, insomnia, anxiety |
| Catecholamine depletors | Reserpine | ↑ REM and stage shifts | |
| Calcium antagonists | Verapamil, nifedipine, diltiazem, amlodipine, felodipine, nisoldipine | No data | Insomnia, nightmares, depression, sedation, difficulty concentrating |
| Antihistamines20 | |||
| H1 antihistamines (chem. class: selective histamine H1-receptor antagonist) | Diphenhydramine, hydroxyzine, triprolidine | ↑ Drowsiness, ↓ SL | Impaired daytime performance |
| H1 antihistamines (chem. class: ethanolamine derivative H1-receptor antagonist) | Loratadine, terfenadine | No sedation effects | |
| H2 antagonists | Cimetidine, ranitidine | May cause insomnia or somnolence | Drowsiness in patients; renal impairment |
| Antidepressants20 | |||
| Tricyclic antidepressants | Amitriptyline, doxepin, imipramine (Trimipramine), clomipramine, desipramine, nortriptyline, protriptyline | ↑ TST, ↓ wakefulness | ↓ Psychomotor and cognitive performance and daytime drowsiness |
| Selective serotonin reuptake inhibitors | Fluoxetine | ↑ TST, ↑ wakefulness, ↑ stage 1, ↑ SEM | Mild ↑ in psychomotor performance |
| Paroxetine | ↑ Wakefulness, ↓ TST, ↑ stage 1, ↑ SL | ||
| Sertraline | No data | Insomnia 7-16% | |
| Fluvoxamine | ↓ TST, ↑ wakefulness, ↑ stage 1, ↑ SL | ||
| Citalopram | No change | Insomnia, no impairment in performance | |
| Trazodone | Variable, may ↑ TST, ↓ SL | ↓ Cognitive performance in elderly | |
| Monoamine oxidase inhibitors | Phenelzine, tranylcypromine, moclobemide, brofaromine | ↑ Daytime sleepiness because of ↓ TST, ↑ wakefulness | Some improved psychomotor performance |
| Other Compounds Used for Insomnia21 | |||
| Melatonin (hormone) | ↓ SL, ↑ TST | Study results are mixed, nonaddictive | |
| Valerian (plant extract) | ↓ SL, ↓ WASO | Side effects include headache and rare morning drowsiness | |
Abnormal Sleep
Sleep Apnea Syndrome
Sleep apnea syndrome, sometimes called sleep-disordered breathing, occurs when airflow is absent or reduced. Apnea during sleep can be divided into three types: (1) obstructive, (2) central, and (3) mixed. In obstructive apnea, the absence of airflow is caused by an obstruction in the upper airway. Complete obstruction lasting 10 seconds or longer is referred to as an obstructive apnea, whereas a partial obstruction is known as a hypopnea. In central apnea, airflow is absent because of lack of ventilatory muscle effort. The third type of sleep apnea syndrome, mixed apnea, occurs when a combination of obstructive and central patterns occurs in a single apneic event. An apnea-hypopnea index (the number of apneas and hypopneas per hour divided by the hours of sleep) of 5 or greater is diagnostic of sleep apnea syndrome. With new understandings of alterations in ventilatory effort, researchers have developed the respiratory distress index (RDI). To calculate the index, the total number of apneas and hypopneas plus the number of respiratory effort-related arousals (RERAs) or other respiratory events is divided by the hours of sleep. An RDI greater than 5 in addition to reports of daytime sleepiness supports a diagnosis of sleep apnea.22
All types of sleep apnea syndrome are accompanied by arterial desaturation and potentially by hypoxemia, which may cause pulmonary vasoconstriction and an increased systemic vascular resistance. However, desaturation and hypoxemia are most severe in the obstructive type. Although the pathophysiology of OSA is unclear, research findings indicate that the various types of sleep apnea are all part of a disease continuum. Failure of the central respiratory rhythm control center to generate a stable rhythm is thought to be the basic defect responsible for sleep apnea syndrome. Cyclic oscillations occur with greater frequency at night and are further exacerbated by mouth breathing.23
Obstructive Sleep Apnea
Definition, Etiology, and Pathophysiology
OSA syndrome occurs when at least five apnea or hypopnea events per hour of sleep occur as the result of an obstruction in the upper airway. In the general population, between 3% and 7% of people have severe OSA.24 The incidence of OSA is believed to increase with age. Consequences include chronic hypoventilation syndrome; arousals that fragment sleep; cardiovascular changes such as hypertension, stroke, ischemic heart disease, insulin resistance, ventricular hypertrophy; and nocturnal angina.25 Because of the cardiovascular complications and accidents caused by sleepiness, such as motor vehicle accidents, job related injuries, falls, and other non-intentional accidents related to lack of concentration, OSA is a significant condition that should be effectively evaluated.
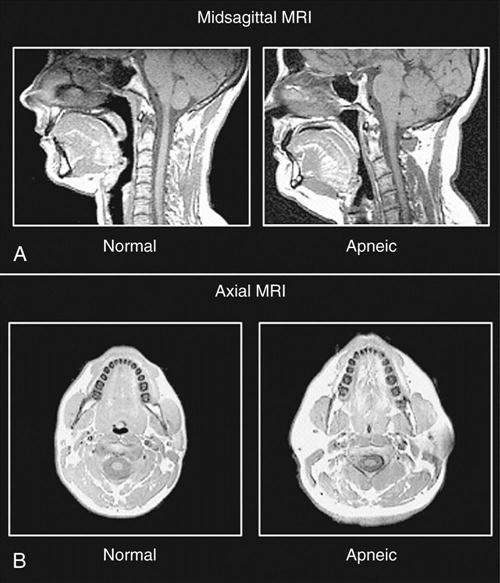
The cause of OSA is not entirely understood; however, upper airway structure, hormonal balance, and neural control are implicated. Factors that contribute to OSA are (1) anatomic narrowing of the upper airway, (2) increased compliance of the upper airway tissue, (3) reflexes affecting upper airway caliber, and (4) pharyngeal inspiratory muscle function.26 Computed tomographic studies of awake subjects have shown that patients with OSA have narrower airways than normal subjects do. The narrower the airway, the more easily it may become obstructed (Figure 5-1).
Upper airway patency is also affected by upper airway function, which is under the control of the respiratory motor neurons. During sleep, this control varies and causes decreased neural activity, thereby narrowing the airway. This effect is especially prevalent during REM sleep, when the motor neurons are hypotonic. Unstable control of the respiratory nerves of the diaphragmatic, intercostal, and upper airway muscles can cause sleep apneas.23 Hypothyroidism can alter respiratory controls and thereby contribute to OSA. Other contributing disorders are exogenous obesity, kyphoscoliosis, and autonomic dysfunction.
The patient with OSA develops cycles of hypoxemia, hypercapnia, and acidosis with each episode of apnea until he or she is aroused and airflow resumes. Alveolar hypoventilation accompanies each episode of apnea and results in hypercapnia. Between episodes, alveolar ventilation improves so that overall there is no retention of carbon dioxide (CO2).
With obstruction, inspiratory subatmospheric intrathoracic pressures are abnormally elevated. This leads to a tendency for airways to collapse, resulting in hemodynamic and electrocardiographic changes. The extremely elevated pressures that occur in individuals with OSA who have apneic episodes in REM and NREM stages cause systemic and pulmonary hypertension. Systemic pressures of 200/120 mm Hg (awake control: 130/80 mm Hg) and pulmonary artery pressures of 80/54 mm Hg (awake control: 30/20 mm Hg) have been reported.27 Cardiac dysrhythmias associated with obstructive apnea include bradycardias, sinus arrest, and occasionally, second-degree heart blocks. After resumption of airflow, tachycardias commonly occur. Bradycardia-tachycardia syndrome is associated with OSA.28
Assessment and Diagnosis
Careful monitoring of oxygen saturation and breathing patterns can help the critical care nurse identify patients with this syndrome and assist in its diagnosis and treatment. Patients at risk for OSA may have the following symptoms: snoring; obesity; short, thick neck circumference; cardiovascular disease; systemic hypertension; pulmonary hypertension; sleep fragmentation; gastroesophageal reflux; and an impaired quality of life. Apneas may occur in people whose throats are abnormally small or collapsible. Muscles that would normally hold the throat open relax while the patient is asleep. Snoring is caused when those soft tissues in the throat vibrate. Snoring often precedes the complaint of daytime sleepiness, and the intensity increases with weight gain and alcohol ingestion.26 Men with a collar size of 17 or greater and women with a size 16 or greater are thought to have an increased incidence of apnea. Friedman and others showed a clinical correlation between modified Mallampati grade (MMP), tonsil size, body mass index, and the severity of apnea. These assessments are used by anesthesiologists to determine intubation difficulty.26 There is an increased incidence of sleep apnea among African Americans as well as Mexican Americans, and an increased incidence is observed among those with metabolic syndrome.
OSA episodes frequently end in brief EEG arousals. Patients may experience hundreds of arousals and not even realize they awaken hundreds of times during the night. These arousals cause sleep fragmentation and daytime sleepiness, which can lead to irritability, poor job performance, troubled relationships, depression, and impaired quality of life.
The definitive diagnosis of OSA syndrome is made with PSG during an overnight sleep study. PSG is used to determine the number and length of apnea episodes and sleep stages, number of arousals, airflow, respiratory effort, and oxygen desaturation.
Medical Management
For patients with mild OSA (apnea-hypopnea index of 5 to 10), weight loss, sleeping on the side if apneas are associated with sleeping on the back, avoidance of sedative medications and alcohol before bedtime, and avoidance of sleep deprivation may be all that is necessary. Oral appliances may be prescribed to stabilize the jaw or retain the tongue. These devices must be fitted by a dentist and are not always as effective as continuous positive airway pressure (CPAP). Moderate to severe levels of apnea may be treated with mechanical, surgical, or pharmacological therapy. Treatment can vary depending on the type and severity of illness.
CPAP via nasal mask is the treatment of choice. CPAP machines are simply pressure generators with the effective pressure being determined during the titration part of the PSG. This holds the airway open and prevents collapse. The patient wears a small, triangle-shaped mask over the nose or uses nasal pillows if the mask is not tolerated. CPAP treats the obstruction and the snoring, choking, and gasping that accompany it, and it provides cardiovascular benefits. Although CPAP is the treatment of choice, it is effective only if the patient is compliant with therapy. Regular attendance at CPAP clinics can improve patient compliance.29 If a patient cannot tolerate the continuous pressure of CPAP, bimodal positive airway pressure (BiPAP), which provides separate pressures for inspiration and expiration, may be tried. Refer to Box 5-1 for nursing care of the patient using CPAP.
Various surgical interventions are available for the treatment of apnea and snoring. Patients with mild OSA or snoring alone may undergo an outpatient procedure called laser uvulopalatopharyngoplasty (LAUP), which uses lasers to remove excess tissue at the soft palate level. For patients who snore but do not have apnea, somnoplasty may provide relief. Somnoplasty involves inserting a small electrode into the soft palate and heating the tissue, causing the area to shrink and tighten.30
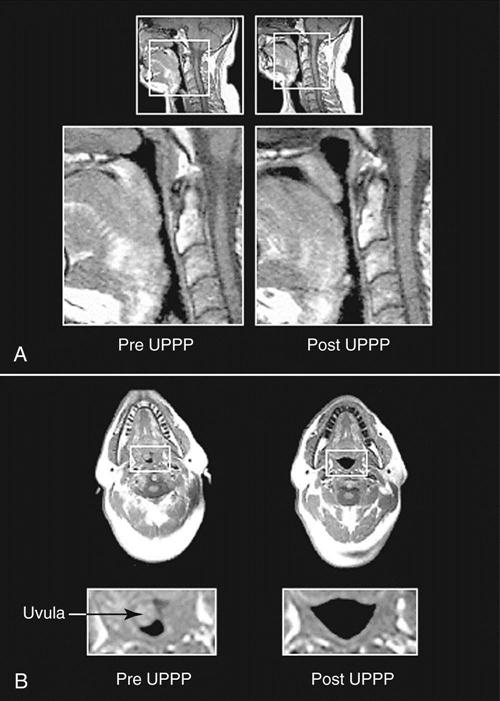
Uvulopalatopharyngoplasty (UPPP) was one of the earlier surgeries used to treat OSA. Essentially, a large tonsillectomy is performed and all redundant tissue is removed (Figure 5-2). Reports of success from UPPP vary widely, with 40% to 80% of patients experiencing sleep apnea improvement.30,31 Complications include speech impairment, inability to eat, postoperative bleeding, and infection. Severe pain after UPPP is documented and may continue well into the postoperative period.31 Although tracheostomy was the original surgical procedure used to treat OSA, it is now used only in the most severe cases of apnea that do not respond to other treatments. Bariatric surgery is an effective means to facilitate weight loss and subsequent improvement in OSA.32
Treatment of OSA with medication is usually a last resort and has proved to be very disappointing. Protriptyline has been shown to decrease apnea and reduce excessive daytime sleepiness by decreasing sleep apnea frequency that increases during REM sleep. Oxygen may be used to lower hypoxemia and nocturnal desaturations.
Nursing Management
The nurse’s role in the management of sleep apnea includes educating the patient and family about the syndrome and the consequences of nonadherence with treatment regimens. This education may also include preoperative teaching for any surgical procedures such as UPPP. Monitoring of patients with OSA while in critical care should include assessment of the breathing patterns, hours of sleep, and pulse oximetry. Cautious administration of narcotics to patients with sleep apnea is suggested because of the potential for respiratory depression, although the concern regarding adequate pain relief is not well studied.
Nasal CPAP is most effective when patients are properly fitted with the nasal mask and have clear instructions regarding its use. Several types and sizes of masks are available, including one type called a nasal pillow, which does not cover the nose but instead fits into the nostrils. If patients are admitted to the critical care unit with a history of OSA, they need to use their home CPAP mask and equipment as part of their regular sleep routine. The nurse can enhance compliance with the CPAP system. Nursing care includes ensuring proper fit of the CPAP mask, with no air blowing into the patient’s eyes, correct airway pressure, no pressure sores from the mask, and no gastric insufflation.
UPPP patients are not usually admitted to critical care areas because the postoperative recovery does not require intensive care.33 Postoperative monitoring after UPPP includes risk of aspiration, pain management, anxiety relief, patient education, and monitoring for respiratory complications, hemorrhage, infection, impaired speech, nutritional concerns, and sleep disturbance.
Central Sleep Apnea
Definition, Etiology, and Pathophysiology
Central sleep apnea (CSA) can be seen on PSG as an absence of airflow and respiratory effort for at least 10 seconds. A complete loss of electromyographic activity by the respiratory muscles would be expected since CSA is defined as a pause in respiration without ventilatory effort.34
A chemoreceptor sensitive to the levels of CO2 resides within the brain. When CO2 levels become excessive, ventilatory efforts are increased to blow off the excess CO2. This negative-feedback loop exists to provide a homeostatic balance in the carbon dioxide and oxygen levels of the body. Whereas OSA results from an obstructed or collapsed airway, patients with CSA suffer from a lack of ventilatory effort. This can be observed in patients with cardiopulmonary disease (e.g., COPD) or heart failure, because their chemoreceptors have become adjusted to an increased CO2 level. It is not uncommon for a patient who experiences CSA to also have some obstructive events.
CSA may result from many physiological or pathophysiological events.35 Possible causes of nonhypercapnic CSA include periodic breathing at high altitude, renal or metabolic disturbances, and idiopathic central apnea seen at sea level. Hypercapnic CSA can occur in many neuromuscular conditions such as spinal cord or brain injury, encephalitis, brainstem neoplasm or infarcts, muscular dystrophy, myasthenia gravis, bulbar poliomyelitis, and postpolio syndrome.
Assessment and Diagnosis
Clinical characteristics of hypercapnic CSA include respiratory failure, cor pulmonale, peripheral edema, polycythemia, daytime sleepiness, and snoring. Patients with nonhypercapnic CSA have clinical features very similar to those of OSA. Nonhypercapnic CSA characteristics include daytime sleepiness, insomnia or poor sleep, mild or intermittent snoring, and awakenings accompanied by choking or feeling short of breath; frequently, the patients are of normal body weight. Diagnosis is made by overnight PSG or sleep study, which will determine the respiratory and sleep patterns of the patient.
Medical Management
Because there are two types of CSA, there are two therapeutic approaches depending on the cause of the apnea. The hypercapnic patient who has worsening hypoventilation during sleep is best served by nocturnal ventilation. Most such patients experience some respiratory muscle failure. One treatment for nonhypercapnic or heart failure patients is nasal CPAP, which also may provide a beneficial cardiovascular effect. Nocturnal oxygen supplementation may be effective as well. If CPAP is not tolerated, pharmacologic management may be tried. Medroxyprogesterone, a respiratory stimulant, may improve ventilation in selected patients.34 Acetazolamide, a carbonic anhydrase inhibitor that can result in metabolic acidosis, also may decrease the frequency of apneic episodes.
Nursing Management
For the nurse caring for a patient with CSA, patient and family education about the patient’s condition and treatment regimen can help to ensure patient compliance. The nurse needs to address any fear or anxiety about going to sleep. Nurses also need to caution patients to avoid alcohol and sedative medications. Weight loss is recommended if the patient is obese. The nurse needs to carefully monitor and assess the respiratory status of the patient.