FIGURE 3.1 Schematic of epidermis. The basal cell layer is the deepest layer of the epidermis differentiating to spinous cells then to granular cells and eventually terminally differentiating to stratum corneum.
The SC is the outer layer of the epidermis and serves as the main functional barrier. A theoretical model is a brick-and-mortar-like structure where bricks represent terminally differentiated nonviable keratinocytes, also known as corneocytes, embedded in intercellular lipid membranes (3). As corneodesmosomes (protein bridges between corneocytes) degrade, lacunar spaces are created within the SC referred to as an aqueous pore pathway. These spaces can extend and form continuous networks, creating a pathway for penetration across the SC (4).
The major components of the SC lipid membranes are free fatty acids, ceramides, and esterols (5). Melanocytes are neural crest-derived, pigment-synthesizing dendritic cells that reside primarily in the basal layer. Merkel cells are mechanosensory receptors also present in the basal layer. Langerhans cells are dendritic antigen-processing and antigen-presenting cells in the epidermis (6). They form 2–8% of the total epidermal cell population, mostly found in a suprabasal position. The dermal–epidermal junction (DEJ) is a basement membrane zone that forms the interface between the epidermis and the dermis. The major functions of the DEJ are to attach the epidermis and dermis to each other and to provide resistance against external shearing forces.
Dermis
The dermis is an integrated system of fibrous cellular and acellular matrices that accommodates nervous and vascular structures as well as epidermally derived appendages. Many cell types reside in the dermis including fibroblasts, macrophages, mast cells, and circulating immune cells. The dermis is responsible for skin elasticity, pliability, and tensile strength. It provides protection against mechanical injury, retains water, and aids in thermal regulation. It also contains and supports receptors of sensory stimuli and is a key element in wound healing (7).
Hypodermis
The hypodermis is primarily composed of adipose tissue, which insulates the body and serves as a reserve energy supply. It cushions and protects the skin and supports nerves, vessels, and lymphatics located within the septa, supplying the overlying region.
Skin Appendages
Skin appendages include nails, hair, sebaceous glands, eccrine (sweat) glands, and apocrine glands. They have two distinct components: superficial and deeper components in the dermis, which are downgrowths of the epidermis. The dermal component regulates differentiation of the appendage. During embryonic development, dermal–epidermal interactions are critical for the induction and differentiation of these structures.
Skin as a Route of Entry
The SC is 3–20 μm in thickness, composed of 15–25 layers of corneocytes. It provides an effective barrier against transcutaneous water loss and entry of exogenous materials.
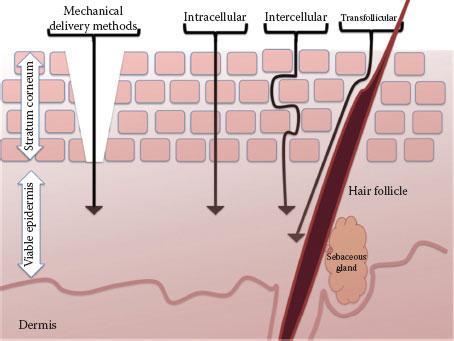
Extracellular lipids contribute to the barrier function and the route taken through the SC by all molecules. Arrangement of extracellular lipids, their hydrophobicity, their composition, and distribution of key components (ceramides, cholesterol, and free fatty acids) provide more barrier function (8). Skin absorption varies between different body parts and between individuals; these regional intra- and inter-individual variations are partly related to variations in lipid composition and SC thickness (8). A range of biological factors can influence the rate and extent of percutaneous penetration including the anatomical site, age, appendageal density, SC morphology, and composition. Routes through which chemicals can cross the SC (Figure 3.2) include the following (9):
• Intercellular (or extracellular), in which chemicals pass exclusively through the lipid matrix
• Intracellular or transcellular, in which chemicals pass both through the lipid matrix and through the corneocytes themselves
FIGURE 3.2 Schematic pathways of penetration into the skin with arrangement of corneocytes in a brick-and-mortar model.
• Through skin appendages
• Mechanical methods to remove SC such as stripping, ablation, and microneedles
In vivo measurement of skin absorption to quantify and rate the extent of absorption is of fundamental importance in the risk assessment of compounds that are active via the dermal route of entry, although the details are beyond the scope of this chapter. The skin as part of the somatosensory system is reviewed in the following chapter.
Measuring the Skin
Measuring the physical characteristics of the skin using biophysical instruments provides key information about various skin parameters. Many noninvasive techniques and equipment are available with increasing applications within dermatotoxicology, allowing the study of the skin in real time and providing objective, quantitative data, which can also be used in the evaluation of individuals affected by sensitive skin.
Parameters measured with these techniques provide information about a particular aspect of the skin. Using multiple parameters measured simultaneously, along with clinical assessment, provides more of a comprehensive analysis. Histological studies may be used to complement these analyses. Few parameters and techniques are briefly introduced in this chapter, and additional texts are available for comprehensive study (10,11). Raman spectroscopy is discussed in detail later in the book.
Skin Surface pH
Skin pH is normally acidic, ranging in pH values of 4–6, while the pH in the internal environment of the body is near neutral, ranging from 7 to 9 (12). The term acid mantle refers to the inherent acidic nature of the SC. Skin pH affects barrier function and SC cohesion. The elevation of pH in the normal skin creates a disturbed barrier (13). Measurement of skin surface pH is used to assess the acidity of the surface of the skin, which can vary according to the time of the day, the skin site, and between individuals. There are several instruments available for the measurement of skin pH; basically, any standard, portable pH meter with a planar electrode should suffice.
Sebum
Sebum is a light yellow viscous fluid, composed of triglycerides, free fatty acids, squalene, wax and sterol esters, and free sterols. Sebum is produced by sebaceous glands and contributes to moisture balance in the SC. Sebum production is mainly influenced by androgens and varies among individuals and races, but its average rate in adults is approximately 1 mg/10 cm2 every 3 hours (14). Sebum production less than 0.5 mg/10 cm2 every 3 hours is associated with dry skin, and values of 1.5–4.0 mg/10 cm2 every 3 hours are associated with seborrhea (15). Sebum can be measured using a gravimetric or a variety of photometric techniques.
Methods used in the past for the measurement of skin lipids included skin swabbing by absorbing pads followed by soaking them in solvents or by weighing the absorptive tapes (gravimetric analysis) (16). Also, photometric analyses such as grease-spot photometry and UV-visible spectrophotometry are used. Photometric techniques can provide additional information, such as droplet size and distribution (8). Multiple commercially available devices can provide quantitative measures of sebum secretion.
Desquamation
Loss of superficial SC is a natural process called desquamation, which can be affected in certain diseases. Desquamation is used to assess the structure and biological dynamics of SC and to investigate the effect of drugs and topical products on the skin. Techniques for desquamation measurement usually involve stripping the surface layers for analysis or visual assessment through skin imaging.
Squamometry involves stripping of corneocytes from the surface of the SC using adhesive disks followed by staining with toluidine blue-basic fuchsin ethanol-based solution and reading the colorimetric variable (17). Image analysis systems are also used to assess desquamation (18–20). These techniques are rapid, reliable, and sensitive.
Thickness
Epidermal and SC thickness can be measured in vivo using noninvasive methods such as ultrasound-based devices, confocal microscopy, and spectroscopic techniques (21–25). These measurements have investigative and clinical applications.
The Measurement of Transepidermal Water Loss
Transepidermal water loss (TEWL) is the amount of water that passively evaporates through the skin to the external environment due to water vapor pressure gradient on both sides of the skin barrier and is used to characterize skin barrier function. The average TEWL in humans is about 300–400 mL/day; however, it can be affected by environmental and intrinsic factors. In high humidity, the amount of water loss will decrease due to the drop in the water vapor pressure gradient. TEWL varies in different anatomic sites and is inversely related to the corneocyte size. Skin sites with smaller corneocytes have higher TEWL values (26–28). Multiple instruments are commercially available to measure TEWL, providing valuable data with applications in clinical settings, toxicology, and product development. TEWL is a sensitive indicator of skin irritation and is widely used in objective analysis of irritancy potential or protective properties of topical products. The accuracy of TEWL measurements can be influenced by environmental factors such as humidity, temperature, and ventilation and intrinsic factors. It is essential that these measurements be conducted under standard conditions (29).
Hydration Measurement
The water content of SC in normal conditions is estimated to be around 5–10% in the outer layer and about 30% near the viable epidermis (30). The water contents of the skin influence its physical properties, viscoelastic characteristics, and functional properties such as drug penetration and barrier function. In disease states such as atopic dermatitis, SC cannot properly stay hydrated. Hydration status of SC is considered a measure of toxicity. Hydration measurements are extensively used in the assessment of topical product safety and efficacy.
Various methods have been introduced to indirectly measure hydration. One method uses electrical properties of the skin such as capacitance, impedance, resistance, and conductance to calculate hydration levels (31). Another method measures transient thermal transfer by using a probe to transfer a constant generated thermal pulse to the epidermis, while it simultaneously obtains high-precision measurements of skin temperature. The water content of SC is calculated based on the changes detected in the temperature (32). The third technique, nuclear magnetic resonance (NMR) spectroscopy, directly measures the total water content of the epidermis and the outer dermis, using magnetic fields (33). NMR provides direct measurements and is considered a reference technique for skin hydration studies. However, all the available techniques can complement each other (34).
Measurement of Vascular Perfusion
Skin microcirculation and perfusion can be affected by a number of exogenous and endogenous factors. Changes in cutaneous vascular perfusion may reflect a physiologic response such as thermoregulation or a pathologic response such as inflammation caused by exposure to chemical irritants and concomitant release of inflammatory mediators. Also, exposure to topical vasoactive drugs can affect skin circulation. Laser Doppler instruments measure the Doppler shift induced by the laser light that is being scattered by moving red blood cells. A signal is quantified based on the average red blood cell concentration and velocity. Since this measure is not an exact measure, it is referred to as flux, which has a linear relationship with the actual flow (35–37). Two distinct laser Doppler tools are used: the first is the laser Doppler flowmetry (LDF), which has a small probe touching the skin, measuring blood flow over a small volume (1 mm3 or smaller), and the second method is laser Doppler imaging (LDI), in which the laser beam is emitted at a certain distance above the skin surface and reflected by a computer-driven mirror to scan an area of the skin. LDI provides two-dimensional images mapping the perfusion but is a slow method. Quantifying fast changes in blood flow is easier with LDF compared to LDI (35).
Laser speckle contrast imaging (LSCI) is a noncontact imaging technique that provides information on skin perfusion over large areas (up to 100 cm2) with a high frequency (up to 100 images per second). These images are obtained based on the generation of a high-contrast grainy pattern when a laser hits a matte surface. This pattern is called the speckled pattern, which fluctuates when objects move. The images created by LSCI reflect fast changes in skin blood flux over wide skin areas (35,38).
These techniques along with the other methods briefly mentioned in this chapter are among the many tools used to assess skin parameters and to provide objective measures for investigators and clinicians. Many bioengineering methods are subject to operational guidelines and have limitations. Data obtained from various methods can complement each other.
REFERENCES
1. Weinstein G, Van Scott E. Autoradiographic analysis of turnover times of normal and psoriatic epidermis. J Invest Dermatol. 1965;45(4):257–262.
2. Bergstresser P, Taylor J. Epidermal “turnover time”—A new examination. Br J Dermatol. 1977;96(5): 503–509.
3. Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: Theory and in vitro experimental measurements. AICHE J. 1975;21(5):985–996.
4. Menon GK, Elias PM. Morphologic basis for a pore-pathway in mammalian stratum corneum. Skin Pharmacol. 1997;10(5–6):235–246.
5. Lee D, Ashcraft JN, Verploegen E, Pashkovski E, Weitz DA. Permeability of model stratum corneum lipid membrane measured using quartz crystal microbalance. Langmuir. 2009;25(10):5762–5766.
6. Mutyambizi K, Berger CL, Edelson RL. The balance between immunity and tolerance: The role of Langerhans cells. Cell Mol Life Sci. 2009;66(5):831–840.
7. Chu DH. Chapter 7. Development and structure of skin. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K., eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York: McGraw-Hill; 2012.
8. Elias PM. The epidermal permeability barrier: From the early days at Harvard to emerging concepts. J Invest Dermatol. 2004;122(2):xxxvi–xxxix.
9. Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–124.
10. Fluhr WJ, Elsner P, Berardesca E, Maibach HI. Bioengineering of the Skin: Water and the Stratum Corneum, 2nd Edition (Dermatology: Clinical & Basic Science). 2nd ed. Boca Raton, FL: CRC Press; 2004.
11. Agache P, Humbert P. Measuring the Skin. Heidelberg: Springer; 2004.
12. Ali SM, Yosipovitch G. Skin pH: From basic science to basic skin care. Acta Derm Venereol. 2013; 93(3):261–267.
13. Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121(2):345–353.
14. Plewig G, Kligman A. Acne and Rosacea. Berlin: Springer; 2000.
15. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. China: Elsevier; 2012.
16. Pande SY, Misri R. Sebumeter. Indian J Dermatol Venereol Leprol. 2005;71(6):444–446.
17. Pierard-Franchimont C, Henry F, Pierard GE. The SACD method and the XLRS squamometry tests revisited. Int J Cosmet Sci. 2000;22(6):437–446.
18. Black D, Boyer J, Lagarde JM. Image analysis of skin scaling using D-Squame samplers: Comparison with clinical scoring and use for assessing moisturizer efficacy. Int J Cosmet Sci. 2006;28(1):35–44.
19. Wilhelm KP, Kaspar K, Schumann F, Articus K. Development and validation of a semiautomatic image analysis system for measuring skin desquamation with D-Squames. Skin Res Technol. 2002;8(2):98–105.
20. Xhauflaire-Uhoda E, Loussouarn G, Haubrechts C, Leger DS, Pierard GE. Skin capacitance imaging and corneosurfametry: A comparative assessment of the impact of surfactants on stratum corneum. Contact Dermatitis. 2006;54(5):249–253.
21. El Gammal S, El Gammal C, Kaspar K, Pieck C, Altmeyer P, Vogt M, Ermert H. Sonography of the skin at 100 MHz enables in vivo visualization of stratum corneum and viable epidermis in palmar skin and psoriatic plaques. J Invest Dermatol. 1999;113(5):821–829.
22. Kaspar K, Vogt M, Ermert H, Altmeyer P, El Gammal S. 100 MHz sonography in the visualization of the palmar stratum corneum after application of various creams and ointments. Ultraschall Med. 1999;20(3):110–114.
23. Corcuff P, Bertrand C, Leveque JL. Morphometry of human epidermis in vivo by real-time confocal microscopy. Arch Dermatol Res. 1993;285(8):475–481.
24. Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ. In vivo confocal Raman microspectroscopy of the skin: Noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001;116(3):434–442.
25. Egawa M, Hirao T, Takahashi M. In vivo estimation of stratum corneum thickness from water concentration profiles obtained with Raman spectroscopy. Acta Derm Venereol. 2007;87(1):4–8.
26. Rougier A, Lotte C, Corcuff P, Maibach HI. Relationship between skin permeability and corneocyte size according to anatomic site, age and sex in man. J Soc Cosmet Chem. 1988;39(1):15–26.
27. Machado M, Salgado TM, Hadgraft J, Lane ME. The relationship between transepidermal water loss and skin permeability. Int J Pharm. 2010;384(1–2):73–77.
28. Hadgraft J, Lane ME. Transepidermal water loss and skin site: A hypothesis. Int J Pharm. 2009; 373(1–2):1–3.
29. Sotoodian B, Maibach HI. Noninvasive test methods for epidermal barrier function. Clin Dermatol. 2012;30(3):301–310.
30. Blank IH, Moloney J 3rd, Emslie AG, Simon I, Apt C. The diffusion of water across the stratum corneum as a function of its water content. J Invest Dermatol. 1984;82(2):188–194.
31. Berardesca E, Borroni G. Instrumental evaluation of cutaneous hydration. Clin Dermatol. 1995;13(4): 323–327.
32. Berardesca E, Fideli D, Borroni G, Rabbiosi G, Maibach H. In vivo hydration and water-retention capacity of stratum corneum in clinically uninvolved skin in atopic and psoriatic patients. Acta Derm Venereol. 1990;70(5):400–404.
33. Foreman MI. A proton magnetic resonance study of water in human stratum corneum. Biochim Biophys Acta. 1976;437(2):599–603.
34. Girard P, Beraud A, Sirvent A. Study of three complementary techniques for measuring cutaneous hydration in vivo in human subjects: NMR spectroscopy, transient thermal transfer and corneometry—Application to xerotic skin and cosmetics. Skin Res Technol. 2000;6(4):205–213.
35. Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci. 2013;34(7):373–384.
36. Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975; 254(5495):56–58.
37. Ahn H, Johansson K, Lundgren O, Nilsson GE. In vivo evaluation of signal processors for laser Doppler tissue flowmeters. Med Biol Eng Comput. 1987;25(2):207–211.
38. Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22(4):R35–R66.