Chapter 9 Skin Grafts
There are two basic types of skin grafts: full-thickness skin grafts (FTSG) and split-thickness skin grafts (STSG). FTSG are composed of epidermis and the full thickness of dermis, and STSG are composed of epidermis and partial-thickness dermis. This chapter will discuss the design of and considerations for these two types of skin grafts, including harvesting and placement techniques, modifications of FTSG such as composite grafts and free cartilage grafts, and advantages, disadvantages, and complications of FTSG and STSG.
FULL-THICKNESS SKIN GRAFTS
Design and Considerations
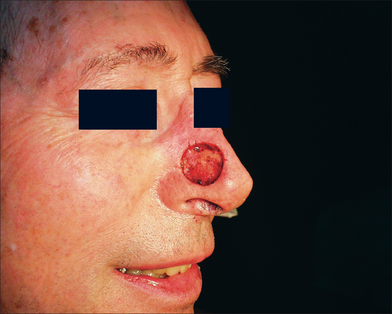
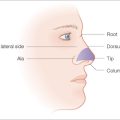
Full-thickness skin grafts are often used to reconstruct surgical defects resulting from skin cancer removal and can provide very good matching of color, thickness, and texture.1–6 Defects on the nasal tip, ala, lower eyelid, and ear, which may be difficult to close primarily or with a flap, may be excellent candidates for placement of a FTSG.3,7–10 FTSG can yield excellent results because they include full-thickness epidermis and dermis. They exhibit minimal wound contraction, and dermal adnexal structures and sensation usually remain intact.
FTSG have significant nutritional requirements and require a rich vascular supply both to promote capillary regrowth and to support the development of a collagenous matrix by fibroblasts, a requirement for graft adherence.11 Although FTSG can support small avascular areas through vascular bridging, the FTSG should not be used for large avascular areas such as exposed bone, cartilage, tendon, or nerve devoid of periosteum, perichondrium, peritenon, or perineurium.
Host factors can influence the success of the FTSG as well.1–3,12–14 The vascular and nutritional requirements for graft success may not be satisfied in a patient with diabetes mellitus or nutritional deficiencies such as protein deprivation or vitamin deficiencies, or in a cigarette smoker. Further, inherited or acquired bleeding disorders, uncontrolled hypertension, or use of anticoagulants such as nonsteroidal anti-inflammatory drugs or aspirin may lead to graft failure because of excessive bleeding and hematoma or clot formation beneath the graft. Immunosuppression may also interfere with graft survival.
Careful attention to matching the donor and recipient sites is essential for a good result. The surgeon must consider epidermal, dermal, and environmental factors. Epidermal and dermal thickness, dermal vascular pattern, sebaceous quality, and epidermal color and texture are also important.1,3,7,9,13,15 Environmental qualities such as the pattern of sun exposure and the ability to camouflage a donor scar site should also be considered.
Since donor site thickness and characteristics, irrespective of site, will vary from patient to patient, a variety of FTSG donor sites should be examined for each individual defect. At times, a regional approach may be used. Skin taken from a particular anatomic region may be used to repair defects in that same region.6 For example, grafts taken from redundant upper eyelid skin may be used to repair the lower eyelid, providing a good color and texture match as well as a well-camouflaged donor site scar. The nasolabial fold skin, in conjunction with a Burow’s triangle, can be used for nasal tip defects, and the donor site scar can be hidden in the remaining fold. Similarly, the postauricular sulcus is an excellent donor site for defects of the ear.
In cases where lack of available donor skin prohibits harvesting from the exact anatomic region, alternative FTSG donor sites are available. Color and texture matching are heavily influenced by sun exposure, and thus, donor sites for FTSG of the face are traditionally from sun-exposed areas above the shoulders. Thickness matching is also crucial to optimize the aesthetic result. The thinnest grafts are usually harvested from the upper eyelid or the postauricular sulcus. Medium thickness grafts are harvested from the preauricular and cervical regions. The supraclavicular region and nasolabial fold can be used for thicker grafts. The surgeon can utilize skin from the nasolabial fold, glabella, forehead, or conchal bowl to repair nasal defects requiring thick, sebaceous skin.6,15–17
Skin harvested from certain sun-exposed sites such as the postauricular sulcus and the preauricular region may be used as grafts for a variety of facial defects. The postauricular sulcus is a good source of donor tissue for the eyelid, medial canthus and ear. Given its sun-exposed location and thickness, the preauricular region often provides a good match for defects of the nasal dorsum, sidewalls, and tip. Donor site scars in these areas are well camouflaged and should not be very noticeable when healing is complete.18,19 Care must be taken to avoid the accidental inclusion of hair follicles within the graft. This often limits the size of the potential graft to be taken preauricularily to approximately 1.5 cm. If hair is accidentally or unavoidably included in the graft, it may be treated with electrolysis or laser hair removal when healing is complete.
Larger defects of the scalp and forehead may require large donor grafts of sun-damaged skin. In these cases, the supraclavicular region or lateral neck skin can be used.6 These donor sites are more difficult to camouflage and may not be covered by clothing, so the surgeon must orient the donor site scar carefully. Although color and texture match may not be optimal, areas below the neck with thin redundant skin, such as the upper inner arms, forearms, and inguinal areas, provide other donor site options.11 These donor areas are often used to graft defects on the dorsal hand and distal lower extremities.
Skin grafts go through several stages of wound healing in the early postoperative period. The first 24 hours after graft placement is the ischemic period, or the stage of plasmatic inhibition, during which fibrin glue attaches the graft to its recipient bed. Vessel anastomoses form during the next 48 to 72 hours, after which vascular proliferation occurs. Full circulation is not restored for 4–7 days.11 Limitation of activity is essential for graft survival, both to minimize shearing forces that would cause vessel disruption and to minimize the risk of overloading graft circulation in the early stages of healing. Vigorous exercise, heavy lifting, and bending should be eliminated for at least one to two weeks after skin grafting.1 Patients should therefore be counseled to avoid activities that elevate their blood pressure and pulse, including stair climbing, lifting heavy bags of groceries, or walking more than a few blocks at a moderate pace, for an additional one to two weeks after suture removal to maximize the likelihood of complete graft survival.
Technique
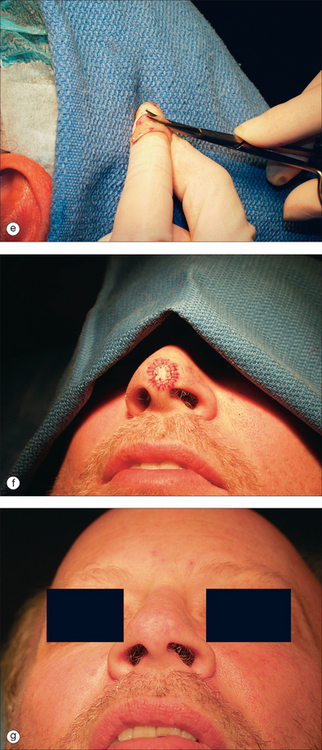
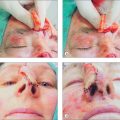
Although many techniques for harvesting and placing FTSG have been described, creating a template for the graft is usually the initial step.1,3–6 The authors’ technique is described below. The periphery of the donor site is inked with a waterproof pen to create an outline of the defect (Figure 9.1). A flexible material such as a telfa pad is pressed against the defect. The telfa may absorb some of the blood from the defect, as well as the ink surrounding it, providing a near exact image of the defect.20 To accommodate for graft shrinkage, a graft 3–5% larger than the template is often harvested. Grafts used for lower eyelid defects may be oversized by even more to allow for contraction and to avoid the possible side effect of ectropion.4 Increased tension on the graft because of inadequate sizing can lead to graft failure. However, oversizing may lead to pin-cushioning of the graft.
Injection of anesthesia should be delayed until the donor site is marked, so as to avoid incorrect marking due to tissue stretch from lidocaine injection (Figure 9.1b). Adrenaline (epinephrine) can be used and does not impair graft survival.21 Appropriate surgical prepping and draping should follow. The graft is then gently excised from the donor site to the level of the subcutaneous fat (Figure 9.1c), after which the donor defect is reapproximated with sutures (Figure 9.1d). Although it is probably best to use immediately, harvested grafts may be preserved for 1–2 hours by placing it in a dish with normal saline or on a saline-soaked gauze or, according to reports, up to 24 hours if refrigerated or kept on ice.6,11
Before transfer to the recipient site, the graft must be trimmed to remove the poorly vascularized adipose tissue. Excess adipose tissue will often not support vessel growth and can lead to graft failure. After defatting, the white glistening surface of the dermis remains (Figure 9.1e). The dermis may be further trimmed to match the thickness of the donor site, but excessive trimming may destroy the adnexal structures and lead to a poor cosmetic result. The graft is then placed in the recipient site, dermis down, and trimmed to size. Trimming and contouring the graft multiple times may be required to enhance the aesthetic result. Contouring may be challenging in locations such as nasal tip, ala, lateral sidewall, eyelid, and the helical rim of the ear, and grafts must be carefully sized in these areas.10,22 A graft that is too small may fail because of increased tension. Rough handling of the tissue, inadequate trimming of adipose tissue, excessive devitalized tissue in the recipient bed due to cautery, or alternatively, inadequate hemostasis can lead to graft failure.
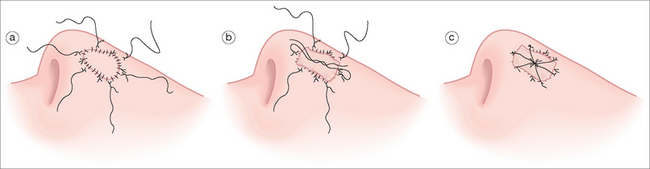
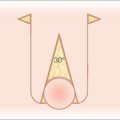
Proper anchoring of the graft is essential for graft survival. FTSG can be anchored with perimeter sutures, basting sutures, support dressings, or a combination of these modalities. Use of cyanoacrylate for skin graft fixation has also been described.23 Initially, four interrupted 5-0 or 6-0 sutures are placed in four quadrants, typically at 12, 3, 6, and 9 o’clock.11 The size of the suture and the location may vary, depending on the size of the graft. Simple interrupted or running sutures are placed at the remainder of the perimeter (Figure 9.1f). Perfect approximation of the graft and the skin of the recipient site should be attempted by introducing the needle first into the graft bed and then into the surrounding skin. Sutures must be tied securely, but not forcefully, to minimize graft movement, while avoiding damage to the graft.
Anchoring of the graft to the recipient site can be further aided by the use of bolsters, or tie-over dressings, which help to prevent hematoma or seroma formation (Figure 9.2).3,12,24,25 Xeroform gauze (Xeroform, Sherwood-Davis & Geck, St Louis, MO) or dental rolls provide simple and effective bolsters, and either sutures or adhesive wound closure tapes can be used to secure the bolster.6 The perimeter sutures placed at 12, 3, 6, and 9 o’clock can be tied to stabilize the bolster: the suture at 3 o’clock is left long and tied to its opposing suture at 9 o’clock, which is left uncut.
Over the bolster, a light dressing of telfa and hypafix (Hy-tape Corporation, Yonkers, NY), followed by a pressure dressing, is placed. The pressure dressing is not removed for 24 to 48 hours, and the bolster is left in place for one week.26 The patient must avoid excessive activity or direct trauma for two to three weeks after surgery to avoid graft failure because of vascular compromise. After 3–7 days, the bolster is removed. After suture removal, adhesive wound closure tapes may be applied to the donor site, and gentle cleansing with diluted hydrogen peroxide to remove all crusts. This is followed by a thin layer of petrolatum or antibiotic ointment.
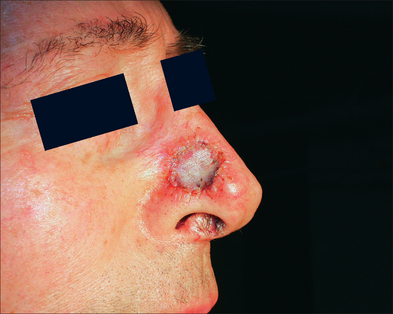
After the bolster is removed, the ideal graft is pink, but a variety of colors ranging from faint pink to blue may be acceptable.6 The color of the graft is dependent on the extent of revascularization and may be affected by ecchymosis or oozing following surgery, so a deep blue graft should not alarm the patient or physician. Black grafts, on the other hand, suggest necrosis. White grafts can imply necrosis as well; however, a white graft can also be caused by epidermal maceration. In some cases, there may be a good cosmetic result despite superficial necrosis of the epidermis since reepithelialization may occur from a healthy dermis and its adnexal structures. If a graft fails and becomes necrotic, the graft should not be debrided. The eschar provides a natural dressing and aids the healing process.
Modifications
Composite grafts, or grafts consisting of one or more types of contiguous tissues (cartilage, subcutaneous fat, overlying skin) are modified FTSG.27–34 Frequently used to repair small full-thickness defects of the nasal ala and the helical rim, composite grafts can also be used to fill partial-thickness defects that extend too deeply for a FTSG to heal without leaving a concave defect or contraction of the free margin. These grafts can be used as a single-stage repair for small defects, but may also be used to provide mucosal lining and structural support in combination with a nasolabial or forehead flap.
Since they are dependent on the bridging phenomenon for survival, composite grafts are generally small with graft diameters greater than 2 cm at higher risk for central necrosis.35 Composite grafts should never be more than approximately 1 cm from a vascular source, and, perhaps even more than FTSG, are threatened by any mechanism, such as shearing forces, that hinders revascularization.
Matching donor and recipient site color and texture allows for optimal cosmesis. Because of the morphological and structural flexibility of the ear, donor sites for composite grafts typically include the helical crus, helical rim, and conchal bowl.31,34,36–38 The helical rim or conchal bowl are typically used to repair more substantial defects, whereas small alar defects with cartilage loss utilize the crus. Most auricular donor sites can be allowed to granulate, although wedge excisions are often appropriate for helical donor sites.
Harvesting a composite graft may involve the inclusion of the cartilaginous portion of the graft beyond the margins of the overlying skin, to serve as stabilizing pegs once the graft is placed into the recipient site.36 Creating these pegs not only anchors the graft into the stable tissue surrounding the defect, but also directly apposes portions of the graft cartilage with tissue already fully vascularized. Some authors advocate a modified composite graft with dermal pedicles to improve graft survival.39 The graft is sutured with small tissue bites to minimize vessel strangulation and maximize the number of vessels available for reanastomosis. The cartilage does not need to be sutured, as it will heal on its own. Similar to FTSG, a pressure dressing or bolster is advisable, and if the graft is used to repair an alar defect, an intranasal antibiotic impregnated gauze should be used to stabilize the graft. Because of the high risk of failure with composite grafts and the high bacterial colonization around the nares, oral antibiotics are recommended.6 Some authors advocate the use of an ice pack over the graft for the first 24 hours, theorizing that cooling may slow autolysis and protect large grafts from necrosis while revascularization occurs.39
Yet another modification of the FTSG is the free cartilage graft, which consists of cartilage and its overlying perichondrium. Both functional and aesthetic concerns dictate the need for a free cartilage graft. These grafts are particularly useful for restoring the architecture of a site that has undergone significant cartilage loss and for maintaining the natural contour of an affected free margin during healing.32,40–44 They provide a rigid, but flexible framework, and can maintain airway patency in cases where collapse of the ala occurs after removal of tumor (Figure 9.3). They are also useful for defects that result in loss of cartilage at the distal nasal tip or ala, and for deep sidewall defects that involve loss of the lower lateral cartilage.43,45
Although hyaline cartilage, found in the nasal septum and costal joints, can be used for the free cartilage graft, elastic cartilage, found in parts of the ear such as the conchal bowl, is a superb choice for donor cartilage, as it has a high degree of memory and varied contours that can be matched to the desired nasal contours.32
The donor graft can be harvested from the conchal bowl using an anterior or a posterior approach. Use of the anterior surface provides for greater access during both the surgical procedure and postoperative wound care; however, the posterior approach allows for complete concealment of the donor site scar. After incision of the skin overlying the conchal bowl cartilage, a 3- to 6-mm strip or a larger disk or oblong-shaped piece of cartilage is harvested with its overlying perichondrium.6 The technique for securing the grafts varies based on the type of graft. For narrow graft strips, the soft tissue of the recipient bed is undermined medially and laterally and the ends of the graft inserted such that the graft interlocks with its recipient bed (Figures 9.4). Sutures are subsequently placed for additional security for this type of graft as well as for the disk or oblong-shaped grafts. A variation of the narrow strip graft, used to brace the side of the nose, involves the use of multiple cartilaginous strips aligned in parallel and secured with sutures. After the cartilage graft is placed, a flap or FTSG may be used to cover the defect.
In addition to composite grafts and free cartilage grafts, there are several other variations of the FTSG. The placement of a FTSG may be delayed to allow for granulation of the base of the recipient bed with minimal, if any, difference in the cosmetic or functional result.46 Alternatively, small dermal grafts may be used as tissue fillers prior to the placement of the FTSG, and may be especially useful for elevation of deep postsurgical defects of the nose and alar rim.47 These dermal grafts may be harvested from the dog-ears of FTSG donor sites. The epidermis should be removed prior to dermal graft placement to prevent the formation of epidermoid cysts at the graft site.
Burow’s grafts may be used to repair defects of the nasal sidewall and dorsum, and sometimes the forehead, lateral neck, or other areas.48 These grafts are obtained from the excised Burow’s triangle, and can provide outstanding results because they utilize skin immediately adjacent to the defect.49 Burow’s grafts eliminate the need for a separate donor site, but necessitate a larger scar line at the original defect site.
For large defects, or defects with exposed cartilage or bone at the edge of the wound, the use of the purse-string suture may decrease the chance of graft failure. The purse-string suture is a subcuticular stitch placed around the periphery of a circular or oval defect and allows partial closure of the defect by advancing skin from the entire periphery of the wound.50,51 The purse-string suture also decreases defect size, and thus enables the surgeon to use a smaller graft from preauricular, postauricular, or supraclavicular donor sites, which provide better matching of color and texture for defects of the face or scalp.
Advantages
The FTSG offers several advantages: it is functionally and cosmetically more appealing than the STSG.11 Texture, color, and thickness matching is significantly better and graft contraction is minimal. FTSG generally have good sensation and appendageal function, and excellent durability with low infection risk.
Disadvantages/Complications
The major disadvantage of FTSG is that they may not match the donor site in terms of skin tone, texture, or thickness as well as a local flap. If the donor site is a poor match, the graft is seen as a visible patch. In addition, grafts have high nutritional and vascular requirements and, as such, may not be appropriate for avascular tissue.11 Composite grafts have an even higher rate of graft failure because of the thicker piece of tissue that must be revascularized. This often limits the size of composite grafts.
In general, all grafts are at risk for both short- and long-term postoperative complications.3,12 Short-term problems include infection, hematoma, seroma, and mechanical shearing forces of the graft over the recipient bed. Although antibiotics are routinely given for composite grafts, traditional FTSG do not require antibiotics except in certain circumstances.6 Patients with diabetes mellitus or immunosuppression, or patients who were subject to a prolonged intraoperative period, may benefit from oral antibiotics covering staphylococcus and streptococcus.
Long-term complications of FTSG relate to cosmetic and functional problems. FTSG may take months to appear natural. In the first postoperative month, they may appear concave, although this depression usually corrects itself. Despite the surgeon’s best attempts at matching color, texture, and thickness, spot dermabrasion or laser resurfacing may be necessary six weeks to six months after the procedure to improve surface matching of the graft and the surrounding skin.52–54 Hyperpigmentation is another complication and may be treated with topical hydroquinone and/or tretinoin.55 Wound contraction is generally limited in FTSG and rarely leads to functional complications, but may occur in some cases, depending upon the thickness and elasticity of the donor site.2,56 When non-sun-exposed skin is used to repair a defect on the face, a color mismatch generally occurs. One way to minimize this is to use a variety of lasers to improve the quality of the surrounding donor skin. Vascular lasers may be used to remove sun-induced telangiectasia and pigmented lesion lasers may be used to remove solar lentigenes. This helps the grafts blend into the surrounding area and makes it much less noticeable.
SPLIT-THICKNESS SKIN GRAFTS
Design and Considerations
Split-thickness skin grafts consist of epidermis and a portion of the dermis. Thickness of the grafts varies, and they are classified as thin (0.013 to 0.033 cm), medium (0.033 to 0.046 cm), or thick (0.046 to 0.076 cm), depending on the amount of dermis included.6 Although less cosmetically appealing and less durable than the FTSG, STSG are more versatile. They are more likely to survive under conditions of vascular compromise and can be used to cover areas with limited vascular supply such as the periosteum, perichondrium, peritenon, and perineurium (Figure 9.5). They are easier to apply than FTSG and can be used for large defects, including those that cannot be covered by a flap or would heal too slowly by second intention.1,2,55,57 The STSG allows for early visualization of recurrent tumor and may be used to cover postsurgical defects at risk for tumor recurrence.
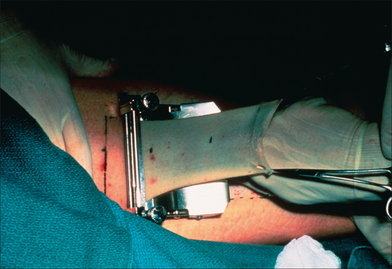
Figure 9.5 An electric dermatome is used to harvest a large STSG from the anterior thigh.
(Photo courtesy of Donald J Grande, MD.)
Optimal matching of color, texture, and thickness is not critical when selecting a donor site for a STSG. Rather, placement of the donor site scar should be carefully considered.58 STSG should be harvested from a broad area of skin that can be concealed beneath clothing. Wounds should not interfere with ambulation. Common donor sites include the anterior, medial, and lateral portions of the upper thigh, the inner and outer aspects of the upper arm, and the inner aspect of the forearm. The most common donor site is the anteromedial thigh. The buttock, although cosmetically ideal, may require assisted postoperative care. In addition to ease of postoperative care, the type of instrument used to harvest the graft may dictate the donor site. A Davol dermatome can be used in a variety of locations to harvest smaller grafts, whereas other power-driven dermatomes and large freehand knives are limited to donor sites such as the thighs, abdomen, and buttocks because they require larger flat donor surfaces (Figure 9.5).
Technique
There is not a consensus approach to harvesting and placing a STSG.1,2,5,6,59–61 Both electric dermatomes and freehand devices such as scalpel blades, double-edged razor blades, and knives, such as the Weck blade, can be used to harvest a graft; however, the freehand devices require more technical skill. Standard #15 or #15c blades may be used to harvest small medium-thickness STSG.6 After marking and anesthetizing the donor site, the donor site is scored lightly with the blade. The assistant applies tension to the donor site, and the blade is oriented parallel to the graft site, just beneath the surface, to harvest a medium-thickness STSG. Blade sharpness diminishes quickly, and several blades may be required to obtain the graft.
The electrically powered Zimmer dermatome has become the instrument of choice for STSG harvesting, as it is less operator-dependent than other electric dermatomes and tends to harvest uniform, consistent grafts of predetermined width and thickness.6 After anesthetizing, prepping, removal of excess surgical scrub with saline, and draping of the donor and recipient sites, the donor site is lubricated to ensure a smooth and steady pass of the dermatome over the skin. While the assistant applies traction around the donor area to create a flat, even surface, the surgeon guides the unit forward at a 30° to 45° angle to the donor site. The graft will emerge and is gently lifted away from the machine with forceps or hemostats. After a sufficiently sized graft is obtained, the dermatome is lifted from the skin, and the graft is placed in sterile saline.
Meshing a graft with scalpel slits allows for drainage of accumulated blood or serosanguineous material that could impair vascularization of the graft and allows the graft to stretch to cover a larger area. Meshing is generally performed after removal of the graft, before placing the graft on the donor site.62 After graft placement, the perimeter of the graft is secured with sutures or staples, and basting sutures are recommended to ensure central adherence of the graft to the recipient bed. A bolster, and often a pressure dressing, for additional security, are placed. Sutures or staples are removed after 7 to 10 days.
Depending on the thickness of the graft, healing of the STSG donor site occurs by secondary intention over 7 to 21 days. The postoperative care of the donor defect has been the subject of many articles, as these sites tend to cause more difficulties in the immediate postoperative period than the graft site. Application of ice, particularly in the first two days, may reduce postoperative donor site pain.63 Numerous wound care approaches have been promoted; however, the use of transparent, vapor-permeable dressings such as Opsite (Allerderm Laboratories, Inc, Petaluma, CA) has become more widespread over time. These dressings allow drainage at the donor site to collect and keep the wound moist, thereby shortening healing times.64,65 This type of dressing has other advantages, including the fact that it is inexpensive, transparent (permitting wound healing to be observed), and associated with a decreased incidence of infection and lower levels of pain.
Modifications
A modification of the STSG is the laminated graft. Using this technique, the graft is harvested in two stages.66 First, a thin STSG is cut with the dermatome set at 0.12 in (0.31 cm). Then the dermatome is immediately reset to 0.15 (0.38 cm) to 0.20 in (0.51 cm), and a deeper graft is taken. The deeper graft is applied to the donor site, and the thin superficial graft is reapplied to the recipient site. Advocates of this technique argue that it results in a better cosmetic result and less contraction at the donor site as contraction is related to the total percentage of dermis grafted rather than the total skin thickness. Additionally, the donor site heals faster. This modification may be ideal for patients with poor wound healing.
Advantages
The STSG is often the only choice for grafting very large defects or recipient beds with limited vascularity such as defects overlying exposed cartilage, bone, or tendon.11 It is a better choice for patients with nutritional deficiencies who may be unable to support a FTSG. The STSG is advantageous for those patients with cancers that are at a high risk of recurrence because recurrent cancer may be visualized under the split-thickness skin graft.
Disadvantages/Complications
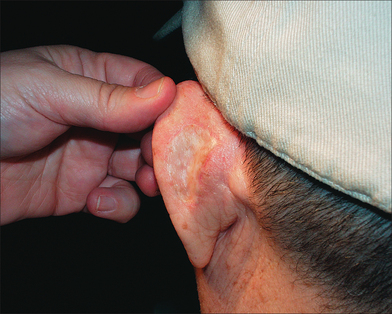
Early complications of STSG are similar to those of FTSG. Graft failure may occur because of hematoma, seroma, infection, or shearing forces. The late complications of the STSG are even more significant.1,2,3,5 Although the thinness of the graft provides the STSG with its versatility, this feature also lessens its cosmetic appearance and durability and contributes to graft contraction. The STSG tends to be pale or white, hairless, and smooth with impaired sweating since adnexal structures are not harvested in their entirety and do not survive (Figure 9.6). As a result, the STSG may contrast with surrounding skin and can produce a tire patch appearance.
In contrast to the FTSG, the STSG is at a higher risk of contracting, which may result in both cosmetic and functional complications if the STSG is placed near a free margin such as the nasal ala, eyelid, helical rim, or vermilion border.30,31 Hypertrophic scarring is also more likely with the STSG, and furthermore, since the STSG is thinner and less durable than the FTSG, it may require regrafting or healing by secondary intention.6 Another disadvantage of the STSG is that obtaining donor site tissue for large grafts requires special equipment and postoperative care of the granulating donor site wound.
CONCLUSION
This chapter emphasizes the versatility and range of skin grafts. FTSG may be a good cosmetic and functional repair choice for many well-vascularized defects, whereas STSG can survive on recipient beds with limited vascularity. The composite graft is a good choice for full-thickness nasal defects with significant alar or cartilage loss, whereas the free cartilage graft provides structural support for partial-thickness alar rim defects and allows the surgeon the flexibility to choose between flap and a graft. Grafting may be an option in a multitude of situations, and knowledge of the applications, advantages, and disadvantages of grafting is invaluable for the dermatologic surgeon.
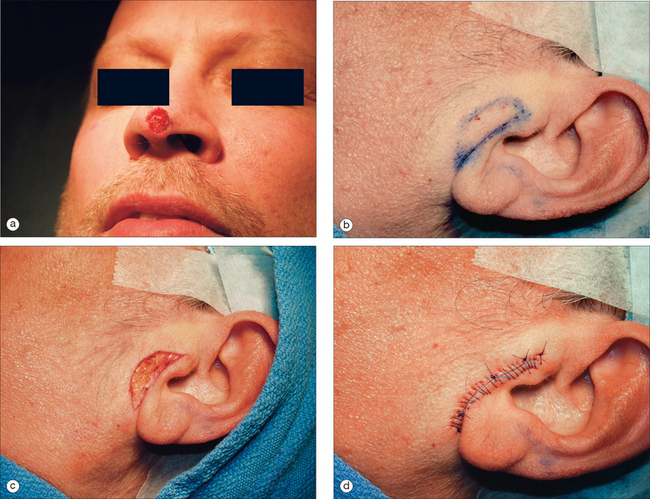
GRAFT COMPLICATION
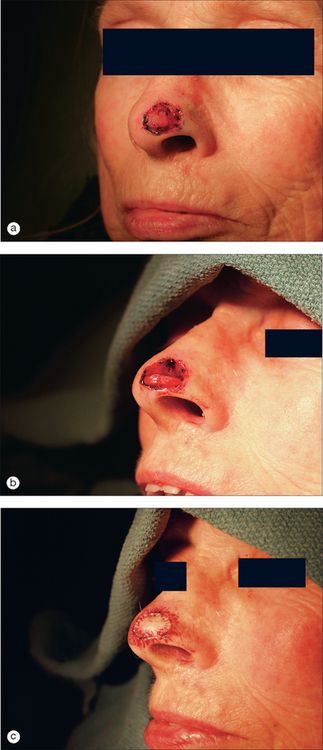
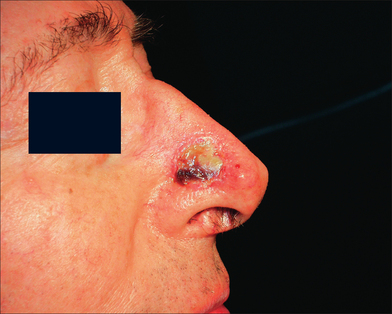
This 74-year-old man underwent Mohs surgery for a recurrent squamous cell carcinoma of his right lower nasal sidewall. After the tumor was cleared, the defect measured 2.0 × 1.9 cm2, and extended to the perichondrium of the lower lateral cartilage (Figure 9.7). The defect was repaired with a full-thickness skin graft taken from the supraclavicular region (Figure 9.8). The graft was pink at the time of suture removal one week after the surgery (Figure 9.9). The patient was advised that his graft had survived, but that he should avoid exercising for two additional weeks.
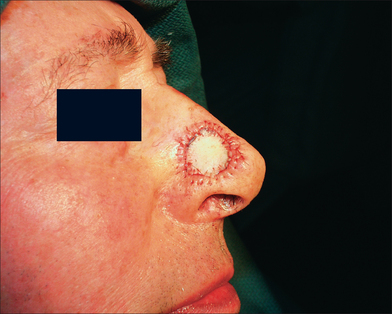
Figure 9.8 The defect was repaired with a full-thickness skin graft taken from the supraclavicular region.
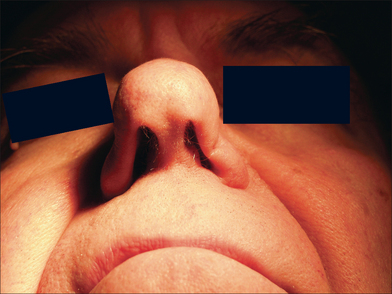
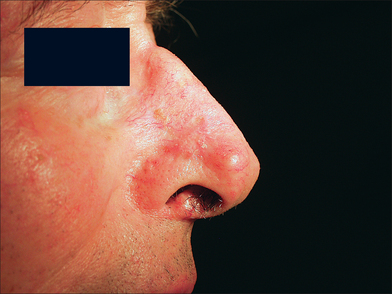
At the time of the patient’s three-week follow-up visit, a yellowish eschar was present over the superior half of the graft, and a brown eschar was present over the inferior half of the graft (Figure 9.10). The patient stated that he had been walking several miles per day since the time of his suture removal, although he had not been playing tennis. The eschar was not debrided, but was instead left in place to serve as a natural bandage, beneath which granulation could occur. The patient was advised to resume wound care twice daily with hydrogen peroxide and petrolatum ointment. He returned one month later for follow-up, at which time his graft site had healed completely (Figure 9.11).
1 Johnson TM, Ratner D, Nelson B. Soft tissue reconstruction with skin grafting. J Am Acad Dermatol. 1992;27:151-165.
2 Johnson T, Ratner D. Skin grafts. In: Ratz JL, editor. Textbook of dermatologic surgery. Philadelphia: Lippincott-Raven; 1998:201-221.
3 Ratner D. Skin grafting. From here to there. Dermatol Clin. 1998;16:75-90.
4 Tromovitch TA, Stegman SJ, Glogau RG. Flaps and grafts in dermatologic surgery. Chicago: Yearbook Medical, 1989;49-54. 65–67.
5 Wheeland RG. Skin grafts. In: Roenigk RK, Roenigk HH, editors. Dermatologic surgery: principles and practice. New York: Marcel Dekker; 1996:879-896.
6 Ratner D. Skin grafting. Semin Cutan Med Surg. 2003;22:295-305.
7 Walden JL, Garcia H, Hawkins H, Crouchet JR, Traber L, Gore DC. Both dermal matrix and epidermis contribute to an inhibition of wound contraction. Ann Plast Surg. 2000;45:162-166.
8 Gloster HMJr. The use of full-thickness skin grafts to repair nonperforating nasal defects. J Am Acad Dermatol. 2000;42:1041-1050.
9 Mellette JRJr., Swinehart JM. Cartilage removal prior to skin grafting in the triangular fossa, antihelix, and concha of the ear. J Dermatol Surg Oncol. 1990;16:1102-1105.
10 Hill TG. Reconstruction of nasal defects using full-thickness skin grafts: a personal reappraisal. J Dermatol Surg Oncol. 1983;9:995-1001.
11 Ratner D. Grafts. In: Bolognia J, Jorizzo J, Rapini R, editors. Dermatology. New York: Mosby; 2003:2305-2320.
12 Skouge J. Skin grafting. New York: Churchill Livingstone, 1991;81.
13 Goldminz D, Bennett RG. Cigarette smoking and flap and full-thickness graft necrosis. Arch Dermatol. 1991;127:1012-1015.
14 Harris DR. Healing of the surgical wound. II. Factors influencing repair and regeneration. J Am Acad Dermatol. 1979;1:208-215.
15 Beare RL, Bennett JP. The naso-labial full thickness graft. Br J Plast Surg. 1972;25:315-317.
16 Rohrer TE, Dzubow LM. Conchal bowl skin grafting in nasal tip reconstruction: clinical and histologic evaluation. J Am Acad Dermatol. 1995;33:476-481.
17 Lutz ME. Effective use of the glabella skin as a donor site for nasal tip grafts. Dermatol Surg. 2002;28:101-102.
18 Breach NM. Pre-auricular full-thickness skin grafts. Br J Plast Surg. 1978;31:124-126.
19 Corwin TR, Klein AW, Habal MB. The aesthetics of the preauricular graft in facial reconstruction. Ann Plast Surg. 1982;9:312-315.
20 Putterman AM. Blotter technique to determine the size of skin grafts. Plast Reconstr Surg. 2003;112:335-336.
21 Fazio MJ, Zitelli JA. Full-thickness skin grafts. Clinical observations on the impact of using epinephrine in local anesthesia of the donor site. Arch Dermatol. 1995;131:691-694.
22 Hill TG. Contouring of donor skin in full-thickness skin grafting. J Dermatol Surg Oncol. 1987;13:883-888.
23 Craven NM, Telfer NR. An open study of tissue adhesive in full-thickness skin grafting. J Am Acad Dermatol. 1999;40:607-611.
24 Hill TG. Enhancing the survival of full-thickness grafts. J Dermatol Surg Oncol. 1984;10:639-642.
25 Hill TG. Surgical gems. A simplified method for closure of full-thickness skin grafts. J Dermatol Surg Oncol. 1980;6:892-893.
26 Salasche SJ, Winton GB. Clinical evaluation of a nonadhering wound dressing. J Dermatol Surg Oncol. 1986;12:1220-1222.
27 Rudolph R. The effect of skin graft preparation on wound contraction. Surg Gynecol Obstet. 1976;142:49-56.
28 Rudolph R, Suzuki M, Guber S, Woodward M. Control of contractile fibroblasts by skin grafts. Surg Forum. 1977;28:524-525.
29 Gloster HMJr., Brodland DG. The use of perichondrial cutaneous grafts to repair defects of the lower third of the nose. Br J Dermatol. 1997;136:43-46.
30 Love CW, Collison DW, Carithers JS, Ceilley RI. Perichondrial cutaneous grafts for reconstruction of skin cancer excision defects. Dermatol Surg. 1995;21:219-222.
31 Symonds FC, Crikelair GF. Auricular composite grafts in nasal reconstruction: a report of 36 cases. Plast Reconstr Surg. 1966;37:433-437.
32 Otley CC, Sherris DA. Spectrum of cartilage grafting in cutaneous reconstructive surgery. J Am Acad Dermatol. 1998;39:982-992.
33 Haas AF, Glogau RG. A variation of composite grafting for reconstruction of full-thickness nasal alar defects. Arch Dermatol. 1994;130:978-980.
34 Field LM. Nasal alar rim reconstruction utilizing the crus of the helix, with several alternatives for donor site closure. J Dermatol Surg Oncol. 1986;12:253-258.
35 Ruch MK. Utilization of composite free grafts. J Int Coll Surg. 1958;30:274-275.
36 Ratner D, Katz A, Grande DJ. An interlocking auricular composite graft. Dermatol Surg. 1995;21:789-792.
37 Weisberg NK, Becker DS. Repair of nasal ala defects with conchal bowl composite grafts. Dermatol Surg. 2000;26:1047-1051.
38 Vecchione TR. Reconstruction of the ala and nostril sill using proximate composite grafts. Ann Plast Surg. 1980;5:148-150.
39 Chandawarkar RY, Cervino AL, Wells MD. Reconstruction of nasal defects using modified composite grafts. Br J Plast Surg. 2003;56:26-32.
40 Burget GC, Menick FJ. Nasal reconstruction: seeking a fourth dimension. Plast Reconstr Surg. 1986;78:145-157.
41 Burget GC, Menick FJ. Nasal support and lining: the marriage of beauty and blood supply. Plast Reconstr Surg. 1989;84:189-202.
42 Burget GC. Aesthetic reconstruction of the tip of the nose. Dermatol Surg. 1995;21:419-429.
43 Ratner D, Skouge JW. Surgical pearl: the use of free cartilage grafts in nasal alar reconstruction. J Am Acad Dermatol. 1997;36:622-624.
44 Byrd DR, Otley CC, Nguyen TH. Alar batten cartilage grafting in nasal reconstruction: functional and cosmetic results. J Am Acad Dermatol. 2000;43:833-836.
45 Park SS, White GJ, Cook TA, Wang TA, Kessler S, Cohen JI. Cartilage viability with interpolated skin flaps: an experimental study. Otolaryngol Head Neck Surg. 1997;116:483-488.
46 Lewis R, Lang PGJr. Delayed full-thickness skin grafts revisited. Dermatol Surg. 2003;29:1113-1117.
47 Meyers S, Rohrer T, Grande D. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
48 Zitelli JA. Burow’s grafts. J Am Acad Dermatol. 1987;17:271-279.
49 Krishnan R, Hwang L, Orengo I. Dog-ear graft technique. Dermatol Surg. 2001;27:312-314.
50 Harrington AC, Montemarano A, Welch M, Farley M. Variations of the pursestring suture in skin cancer reconstruction. Dermatol Surg. 1999;25:277-281.
51 Brady JG, Grande DJ, Katz AE. The purse-string suture in facial reconstruction. J Dermatol Surg Oncol. 1992;18:812-816.
52 Robinson JK. Improvement of the appearance of full-thickness skin grafts with dermabrasion. Arch Dermatol. 1987;123:1340-1345.
53 Nehal KS, Levine VJ, Ross B, Ashinoff R. Comparison of high-energy pulsed carbon dioxide laser resurfacing and dermabrasion in the revision of surgical scars. Dermatol Surg. 1998;24:647-650.
54 Bernstein LJ, Kauvar AN, Grossman MC, Geronemus RG. The short- and long-term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
55 Otley CC, Gayner SM, Ahmed I, Moore EJ, Roenigk RK, Sherris DA. Preoperative and postoperative topical tretinoin on high-tension excisional wounds and full-thickness skin grafts in a porcine model: A pilot study. Dermatol Surg. 1999;25:716-721.
56 Stephenson AJ, Griffiths RW, La Hausse-Brown TP. Patterns of contraction in human full thickness skin grafts. Br J Plast Surg. 2000;53:397-402.
57 Kirsner RS, Eaglstein WH, Kerdel FA. Split-thickness skin grafting for lower extremity ulcerations. Dermatol Surg. 1997;23:85-91. quiz 92–93.
58 Rigg BM. Importance of donor site selection in skin grafting. Can Med Assoc J. 1977;117:1028-1029.
59 Skouge JW. Techniques for split-thickness skin grafting. J Dermatol Surg Oncol. 1987;13:841-849.
60 Whitaker DC, Grande DJ, Koranda FC, Knabel MR. Rapid application of split-thickness skin grafts. J Dermatol Surg Oncol. 1982;8:499-504.
61 Glogau RG, Stegman SJ, Tromovitch TA. Refinements in split-thickness skin grafting technique. J Dermatol Surg Oncol. 1987;13:853-858.
62 Staiano JJ, Dhital SK. “Meshing” a small skin graft. Plast Reconstr Surg. 2004;113:465-466.
63 Akan M. Ice application to minimize pain in the split-thickness skin graft donor site. Aesthetic Plast Surg. 2003. November 12 (Online First).
64 James JH, Watson AC. The use of Opsite, a vapour permeable dressing, on skin graft donor sites. Br J Plast Surg. 1975;28:107-110.
65 Rakel BA, Bermel MA, Abbott LI, et al. Split-thickness skin graft donor site care: a quantitative synthesis of the research. Appl Nurs Res. 1998;11:174-182.
66 Chuenkongkaew T. Modification of split-thickness skin graft: cosmetic donor site and better recipient site. Ann Plast Surg. 2003;50:212-214.