Skeletal Trauma
General Overview
The dictum “Children are not small adults” holds more weight than in any other situation with regard to skeletal trauma. Fracture patterns and fracture healing are different processes in children than in adults.1,2 Unfortunately, a fracture may interfere with subsequent normal growth of a bone.1,2 Fortunately, such complications are relatively uncommon. In healthy children, the process of fracture healing and remodeling is rapid, particularly in the vascularized metaphysis.3 Most posttraumatic deformities readily correct with healing and remodeling.1,2 The composition of a child’s bones and the presence of the growth process both predispose children to types and complications of fractures that are different from those seen in adults.1,2,4
General Etiologies, Pathophysiology, and Clinical Presentation (Mechanism, Healing, Complications)
Mechanism: Many mechanisms may play a role in pediatric trauma. Falls, injuries at play, and motor vehicle accidents account for a majority of childhood fractures.5 Unfortunately, younger children may suffer fractures as a consequence of nonaccidental trauma (child abuse). These injuries are often characteristic and are covered in Chapter 144. Children of all ages are increasingly involved in and dedicated to athletics and competitive sports. Certain types of fractures, including stress fractures, are often associated with sporting injury. These fractures are addressed Chapter 145. Pathologic fractures may be seen with bone tumors, and insufficiency fractures may be seen with metabolic bone disease (see Chapter 140).
Fracture Description and Nomenclature: Understanding basic fracture nomenclature is important for effective communication with clinicians and other radiologists. Fractures are subdivided into two basic categories: (1) incomplete (plastic) and (2) complete. Incomplete fractures include greenstick and buckle fractures. Complete fractures should be described based on orientation: transverse, oblique, longitudinal, and spiral. Spiral fractures are defined by an approximately 180-degree or greater twist in the fracture plane. For any fracture, angulation, displacement, diastasis, comminution, and impaction must be described. On follow-up radiographs, any change, in addition to the presence or absence of fracture healing, should be described. The term dislocation should be reserved for joints only, not fracture sites. In addition, whenever describing fractures, involvement of an open physis and articular surface should be described. For physeal fractures, it is acceptable to describe the fracture using the Salter-Harris classification.
Fracture Healing: Fracture healing in children has been described in three phases: (1) inflammatory, (2) reparative, and (3) remodeling (e-Fig. 143-1).6 At the time of fracture, bone and periosteum are disrupted. A hematoma is formed at the site of fracture enveloping the ends of the fractured bone. The hematoma may also contain necrotic fragments of bone, bone marrow, and adjacent tissues. An inflammatory response is initiated (inflammatory phase), and the organization of a hematoma then begins. Osteoclasts and osteoblasts arise from precursor cells of the involved tissues. Bone resorption occurs at areas of necrosis.7 This peaks 2 to 3 weeks after injury and appears as a poorly defined fracture line.8 Initial callus (immature woven bone) is formed during the reparative phase.6 Osteoid and chondroid material (callus) forming within the hematoma envelops the fracture fragments, joining and stabilizing them. Endosteal callus also forms within the fracture fragments and is seen as increased density on radiographs. Devitalized portions of bone at the margin of the fracture fragments may undergo resorption and appear demineralized on radiographs. An injury that is less than 5 to 7 days old will not show any of these described radiographic features.7 With time, the woven bone of callus is replaced by organized lamellar bone during the remodeling phase. With remodeling, excess thickness from the callus is resorbed and the medullary canal is reestablished. Remodeling lasts months, and occasionally years.6,7 The healing process is more rapid and complete in children than in adults. Most childhood fractures heal completely and without residual deformity.
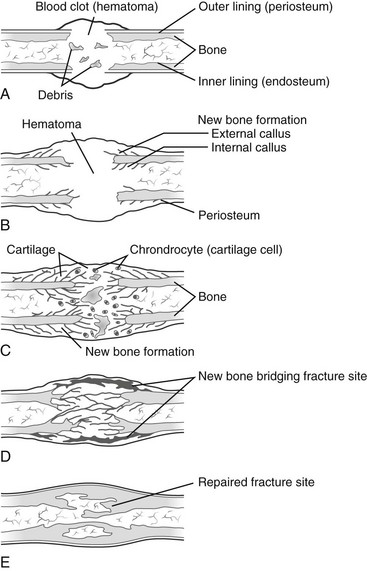
e-Figure 143-1 Stages in healing of a fracture.
A, Hematoma under the periosteum surrounding the fracture, communicating through the fracture with blood clot within the medullary cavity. B, Subperiosteal and endosteal cellular proliferation encroaching on the hematoma. Islands of cartilage may be present. Bone at the margins of the fracture has been devitalized. C, Callus formation from osteoblastic cells in the proliferating cellular masses. The intracellular substance becomes calcified to form woven bone. The dead bone at the margins of the fracture undergoes resorption. D, Replacement of woven bone by lamellar bone with bone continuity across the fractured area. E, Remodeling. Cortical bone is organized to conform to the cortex. Resorption of excess bone results in recanalization of the medullary cavity. (From Bleck EE, Nagel DA. Physically handicapped children. New York: Grune & Stratton; 1982.)
Complications: Complications may occur at the time of fracture, during treatment, or as a failure of normal, complete fracture healing. Box 143-1 lists potential complications of pediatric fractures. The incidence of a particular complication varies, depending on many factors, including the site and severity of the fracture, complicating factors (i.e., open fracture), the age and overall health of the patient, associated injuries, and the adequacy of therapy.9
In nonunion, healing stops before osseous continuity of the fracture fragments occurs. The fracture fragments may form a pseudoarthrosis. Pseudoarthrosis is most common in the clavicle, humerus, and tibia. Nonunion and pseudoarthroses are uncommon in normal children but may be seen with underlying abnormalities such as neurofibromatosis or congenital insensitivity to pain.10,11 The incidence of nonunion is increased with greater injury, comminution, and distraction and with open fractures complicated by substantial soft tissue injury, infection, or both.11 Delayed union is defined as failure of bone union to occur in the expected time. Malunion indicates fusion in a nonanatomic orientation. Mild degrees of malalignment are well tolerated and usually result in no permanent deformity and resolve overtime because of remodeling. Surgical interruption of the healing process to correct residual malalignment is occasionally necessary. Posttraumatic synostoses are most common in the paired bones of the forearm and leg. Infection may occur due to an open fracture or as a complication of surgery or percutaneous pinning.11
Fracture at certain sites may result in neurovascular injury; however, such injuries are rare and usually only seen with substantial displacement and deformity, as may occur with supracondylar fractures of the distal humerus.9 Compartment syndrome is an uncommon complication of extremity fractures, most commonly in the leg or with supracondylar elbow fractures, and may lead to Volkmann contracture caused by ischemia.9
Reflex sympathetic dystrophy syndrome (RSDS; also called “Sudeck atrophy”) is a poorly understood dysfunction of the autonomic nervous system after injury. RSDS most commonly affects the lower extremities in children. Patients present with pain, swelling, joint stiffness, and exquisite sensitivity to touch.12 Onset of symptoms is within 1 week to several months after injury.9 Radiographs show osteopenia that is difficult to distinguish from disuse osteoporosis. Magnetic resonance imaging (MRI) can show patchy bone marrow edema; however, at times it may be normal.13 Bone scintigraphy is usually abnormal. Early, increased activity is seen on perfusion, blood pool, and delayed phases. Later, decreased activity may be seen on both perfusion and blood pool phases, with increased activity remaining during the delayed phase for months.14 The characteristic imaging findings seen in adult patients may not be seen in children.15 RSDS has been more recently categorized under the more general term bone marrow edema syndrome, which includes other transient clinical conditions with an unknown underlying mechanism, such as transient osteoporosis of the hip.14
Premature fusion occurs in approximately 15% of physeal fractures.16 A bony “bridge” or “bar” forms across the physis.17 Prognosis depends on the involved bone, the extent and location of the physeal bar (central versus eccentric), and the amount of remaining growth.11,16 Morbidity is greater when the remaining growth potential is higher. The phalanges and distal radius are the most common sites of physeal fracture; however, growth arrest is rare.16 The distal femur and proximal tibia have high incidence of posttraumatic physeal fusion but are less common sites for physeal fracture.16 Premature fusion in the distal femur and tibia is also of greater clinical importance because of possible resultant leg length discrepancy or angular deformity.16 Indirect physeal insult such as burns, frostbite, and electrical injury, which are discussed later in this chapter, and other processes such as infection and secondary ischemic insult related to meningococcemia can also cause premature physeal fusion (Box 143-2).
Central fusions cause loss of growth potential. Central fusions result in a cupped appearance of the physis, with the epiphysis and physis invaginating into the center of the metaphysis.11,16 Peripheral fusions result in angular deformity.11,16 Premature physeal fusion usually occurs approximately 3 months after injury.16 Radiographs may show obliteration of the physeal clear space. Comparison views are often helpful, particularly when evaluating an older child in whom the time of normal physeal closure is near. A secondary sign of premature physeal fusion is tethering of growth lines.16 Growth lines normally form during the healing process. Normal growth lines parallel the physis, whereas with premature fusion, growth lines are angled toward a bony bar.16
Both computed tomography (CT) and MRI have been used to diagnose and map areas of premature fusion. With CT, a limited scan with narrow collimation is obtained through the physis. A standard protocol obtains 1.25-mm images overlapping at 0.625-mm intervals. Sagittal and coronal reformats will show the area of fusion. Small bars may be seen as a sclerotic band across the physis, whereas with larger areas of fusion, continuity of the marrow space across the fusion is seen.11,16,18 Cartilage-sensitive sequences (proton density with fat saturation, three-dimensional spoiled gradient recalled echo with fat saturation) can be employed on MRI to delineate the physis (Fig. 143-2). Areas of fusion will be seen as defects within the bright signal of the cartilaginous physis.16,19 These sequences can also be helpful to detect the potential fracture complication of trapped periosteum within the physis.20 With either CT or MRI, maps can be created showing the degree and site of fusion.
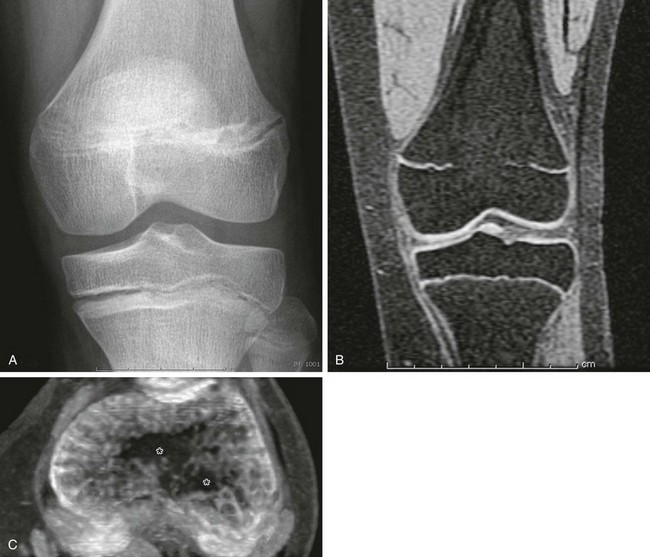
Figure 143-2 Premature growth plate fusion.
A, On radiography, the central portion of the distal femoral growth plate is poorly defined; however, the extent of fusion is poorly delineated. B, Coronal spoiled gradient recalled echo magnetic resonance image with fat saturation. The central growth plate is fused. C, Axial maximum intensity projection image constructed from the stack of coronal spoiled gradient recalled echo with fat saturation images. The area of growth plate fusion is mapped (asterisks). (Courtesy of Dr. K. Ecklund, Boston, MA.)
When a child is less than 2 years old or has 2 cm of growth remaining or when the bar involves less than 50% of the physis, resection of the bar can be considered.11,21 A plug of fatty tissue can be placed in the void.21 Premature fusion may recur. If greater than 50% of the physis is fused, resection of the bar may be impractical. Depending on the deformity and growth potential of the patient, other orthopedic techniques may be utilized to minimize morbidity from the premature fusion, including osteotomy and contralateral epiphysiodesis (surgical physeal fusion).11
Imaging: Radiographs are the mainstay of imaging of traumatic injuries to the pediatric skeleton. As a general rule, two orthogonal views are obtained to assess for fracture at nonosteoarticular locations and an additional oblique view at osteoarticular locations. At some locations, normal anatomy limits the value of orthogonal projections (i.e., the pelvis). At other locations, unique projections are helpful for delineation of anatomy (i.e., an axillary view of the shoulder, and a sunrise view of the knee). When imaging a long bone, both the proximal and distal joints must be included.
Contralateral comparison views are not routinely obtained, but frequently aid in differentiating normal developmental variation from pathology.22 Comparison views are most helpful in areas of complex anatomy such as the elbow. Normal variants are common and may mimic fracture.
At certain sites and with complex patterns of injury, CT is very helpful in diagnosing fractures and delineating the anatomy of fracture planes and resultant deformity. Occasionally, MRI or ultrasonography can be used to diagnose fractures in children; however, these modalities excel in delineating associated soft tissue injury rather than osseous injury. Ultrasonography, however, may be particularly helpful in infants whose epiphyses are not yet ossified. It also can be used to detect lipohemarthrosis as an indirect sign of fracture or to aid in diagnosis of an occult fracture.23 The cartilaginous epiphysis is well seen with ultrasonography, and its relationship and continuity with an adjacent metaphysis can be readily assessed. With the increasing capabilities of cross-sectional imaging, nuclear scintigraphy is less utilized than in the past. Nonetheless, scintigraphy can be a valuable tool to identify an occult fracture. Bone scans typically become positive 24 to 48 hours after a fracture.
Plastic Fractures
Etiology, Pathophysiology, and Clinical Presentation: The composition of bones in a child is different from that of bones in an adult.24 The most apparent anatomic difference in the pediatric skeleton is the presence of the physis and a thick periosteum.25 The plasticity of a child’s bones allows for substantial deformity prior to fracture (e-Fig. 143-3).2 Bone may give way and may become permanently deformed prior to a complete break. The result is an “incomplete fracture” or a “plastic fracture.”
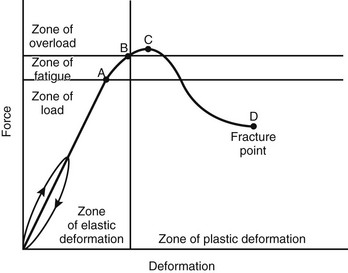
e-Figure 143-3 Graphic relation of bony deformation (bowing) and force (longitudinal compression) showing that the limit of an elastic response is not a fracture but plastic deformation.
If the force continues, a fracture results. A, reversible bowing with stress. B, Microfractures occur. C, Point of maximal strength, between C and D, bowing fractures. D, Linear fracture occurs. (Modified from Borden S IV. Roentgen recognition of acute plastic bowing of the forearm in children. Am J Roentgenol Radium Ther Nucl Med. 1975;125:524-530.)
Greenstick fractures most commonly occur in long bones, particularly the radius and ulna.2 These fractures are most common in the first decade of life, are uncommon in the second decade of life, and are not seen in normally developed and mineralized bones of adults.2 Buckle fractures most commonly affect the distal radius and ulna, tibia, and proximal first metatarsal bone.
Imaging: Radiographs are the standard method of evaluation and advanced imaging is usually not indicated. Subtypes of incomplete fractures are buckle or torus fractures (Fig. 143-4), lead pipe fractures (part transverse fracture or part buckle fracture), greenstick fractures (Fig. 143-5), and bowing deformities (e-Fig. 143-6). With incomplete fractures, the periosteum is intact wherever the cortex is intact.2
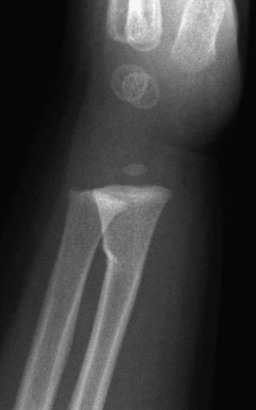
Figure 143-4 Buckle fracture of the distal radius in a 19-month-old boy.
The distal radius is slightly angulated posteriorly.
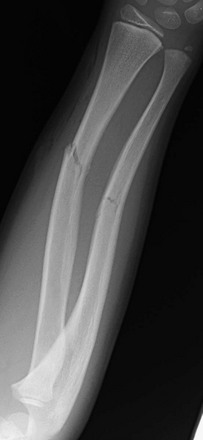
Figure 143-5 Greenstick fractures of the radial and ulnar diaphyses in an 8-year-old boy.
The fracture lines only extend through part of the cortex.
Treatment: Cast and splinting is usually performed for pain control since most of these fractures are stable. Plastic fractures usually heal completely and have a good prognosis. Rarely, it may be necessary to operatively “complete” the fracture for reduction of severe angular deformity. Mild angular deformities (<15 degrees) usually do not need to be reduced and will remodel nicely without permanent sequelae.
Physeal (Salter-Harris) Fractures
Etiology, Pathophysiology, and Clinical Presentation: For the musculoskeletal unit of the child, the physis and physeal equivalent regions are points of relative weakness and are thus predisposed to mechanical failure leading to fractures.26 The physis usually fails before ligamentous or tendinous soft tissue structures fail after biomechanical stress. This occurs more frequently during growth spurts, and affects the lower extremities more frequently than the upper extremities. Once the physis fuses, ligamentous and tendon soft tissue injuries become more frequent, as do metadiaphyseal fractures. Fracture patterns in older children are similar to fracture patterns seen in adults.
Approximately 18% of pediatric fractures involve the physes.27 Physeal fractures are classified by the system of Salter and Harris (Fig. 143-7). The Salter-Harris classification is a well-accepted classification scheme for describing physeal fractures and therefore facilitates efficient communication between clinicians and radiologists.
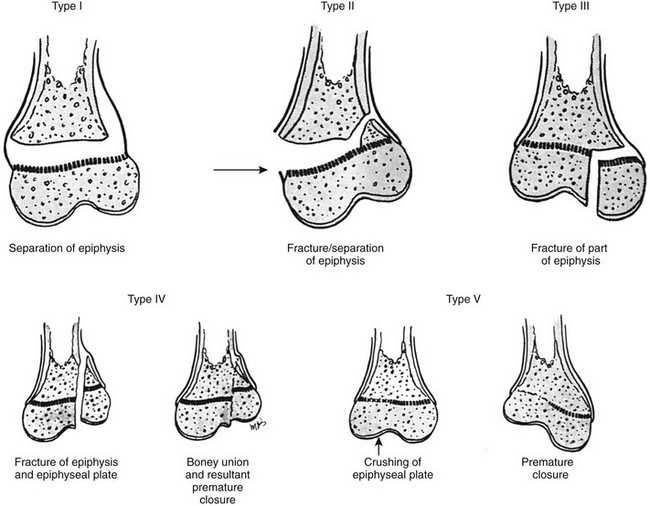
Figure 143-7 Injuries to the cartilage plate classified according to Salter and Harris.
Type I, Complete transverse laceration of the physis with longitudinal distraction and some transverse displacement of the epiphysis. The bone itself is not broken. Type II, Incomplete transverse laceration of the physis through a variable distance is associated with an oblique fracture of the contiguous metaphysis with a triangular fragment of metaphysis attached to the displaced epiphysis. The prognosis is good. Type III, Short incomplete transverse laceration of the physis with longitudinal fracture extending through the epiphyseal ossification center toward the joint. The prognosis is poor if the epiphyseal fracture is not reduced with smooth joint surfaces. Type IV, Oblique longitudinal fracture extending from the articular cartilage through the epiphyseal ossification center, across the physis, and through a short segment of the metaphysis through the cortical wall. This type is most frequently seen at the lateral condyle of the humerus. Perfect reduction is essential for a good prognosis. Type V, Segmental crushing of the physis, often followed by closure of the plate prematurely and stoppage of growth. (From Salter RE, Harris WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587-622.)
Imaging: Most physeal fractures are adequately delineated by radiography. Since these fractures are at the ends of bones, potential epiphyseal and intraarticular extent of fracture may be present and therefore, three views are necessary (frontal, lateral, oblique). The Salter-Harris classification of fractures, the degree of diastasis and angulation, if present, loose bodies, and any intraarticular involvement should be considered.
Fractures through the physis may pass solely and directly through the physis (Salter-Harris I; e-Fig. 143-8), involve the physis and a portion of the metaphysis (Salter-Harris II; Fig. 143-9), involve the physis and a portion of the epiphysis (Salter-Harris III; e-Fig. 143-10), or cross the physis in single plane involving both epiphysis and metaphysis (Salter-Harris IV; e-Fig. 143-11). Crush injury of the physis (Salter-Harris V) is rare as an isolated injury to the bone and is rarely, if ever, diagnosed prospectively. Although not in common use, additional fracture types are included in an expanded Salter-Harris classification. A Salter-Harris VI fracture occurs at the perichondral ring at the edge of the physis. A Salter-Harris VII fracture is confined to the epiphyses.2,26,28
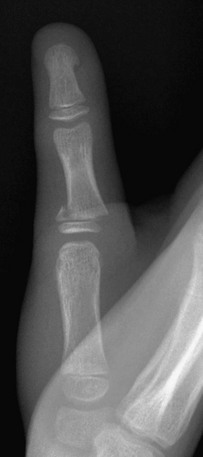
Figure 143-9 Salter-Harris II fracture of the proximal phalanx of the thumb in a 10-year-old boy.
The distal fragment is slightly displaced medially.
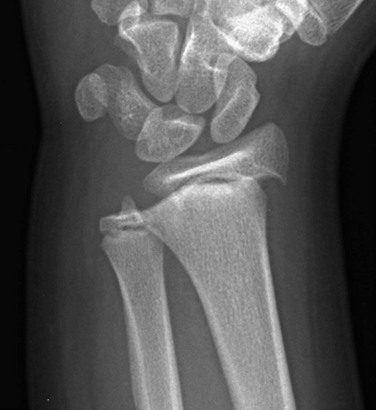
e-Figure 143-8 Salter-Harris I fracture of the distal radius in an 11-year-old girl.
The distal radial epiphysis is displaced laterally.
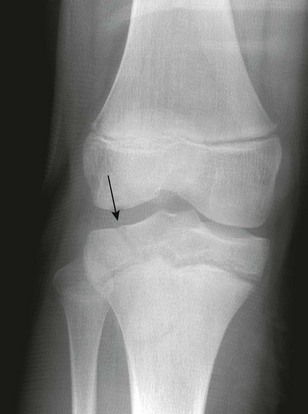
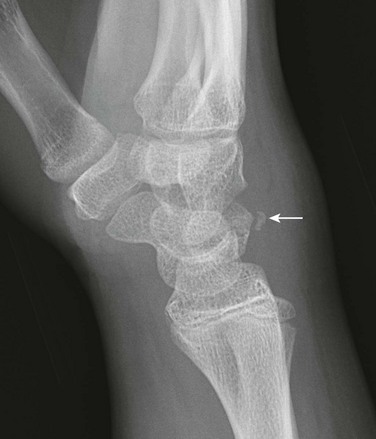
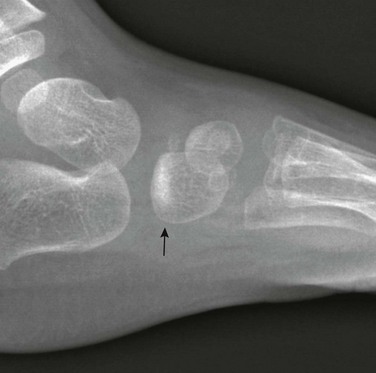
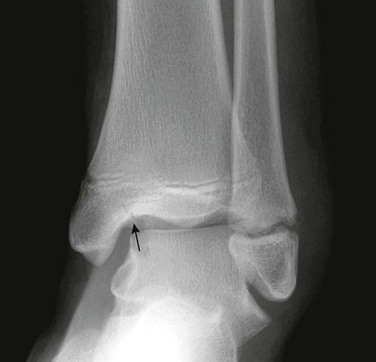
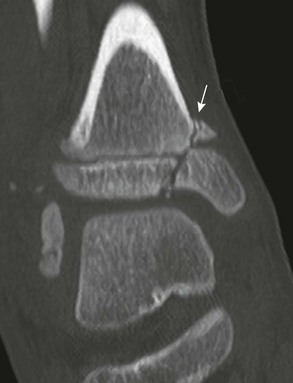
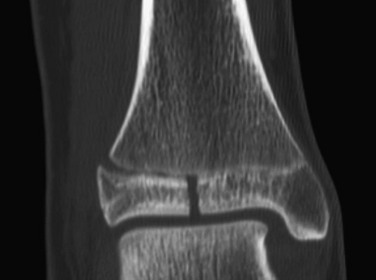
e-Figure 143-10 Salter-Harris III fracture of the proximal tibia in a 10-year-old girl.
The proximal tibial physis is wide. The arrow shows the vertical epiphyseal fracture line.
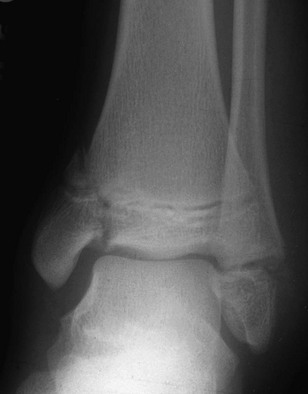
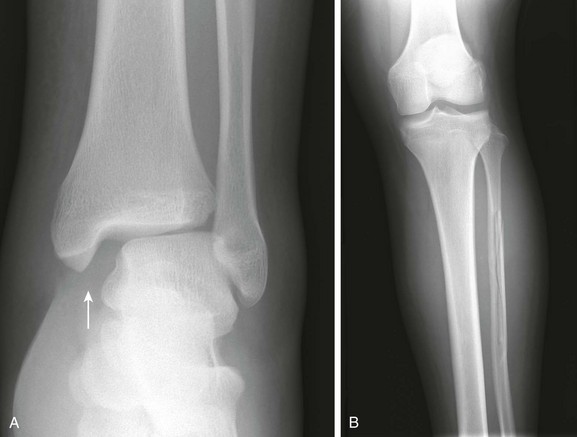
e-Figure 143-11 Salter-Harris IV fracture of the distal tibia in a 13-year-old boy who fell from a tree.
A Salter-Harris I fracture of the distal fibula with overlying soft tissue swelling is also present.
Fracture planes within the physis pass through the zone of calcified cartilage and adjacent newly formed bone, which represent the point of least resistance to fracture forces. Fracture lines that extend into the epiphysis (Salter-Harris III and IV) cross the zone of proliferating cartilage, which is more susceptible to damage leading to premature physeal fusion. The zone of proliferating cartilage is thought to be damaged by Salter-Harris V fractures. Malalignment of Salter-Harris IV fracture fragments may promote formation of a bridge across the healing fracture from metaphysis to epiphysis.28
At certain locations, CT or MRI may be used to confirm or further delineate fractures, especially for defining exact measurements of fracture diastasis and whether involvement of an articular surface exists. Defining exact measurements of fracture diastasis is important especially when an articular cartilage is involved, especially at weightbearing zones (e.g., tibial plafond). On MRI, physeal fractures are diagnosed by widening and increased T2-weighted signal within the fractured portion of the physis, adjacent bone marrow edema, associated metaphyseal (Salter-Harris II) or epiphyseal (Salter-Harris III) fracture lines, and periosteal disruption.29 The most significant complication of physeal injuries is growth arrest (see Fig. 143-2), which can lead to deformity and limb length discrepancies.30 A rare complication of physeal fracture diagnosed by MRI is entrapment of periosteum within the fracture (e-Fig. 143-12). The entrapped periosteum will prevent complete reduction of the fracture. MRI can also be used to evaluate physeal fractures that have a significant risk of complications.31
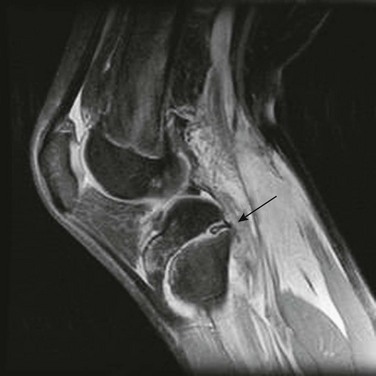
e-Figure 143-12 Proton density weighted sagittal magnetic resonance image with fat saturation of an adolescent with a Salter-Harris I fracture of the proximal tibia.
Disrupted periosteum is trapped with the physeal fracture (arrow). Abundant soft tissue edema is seen posteriorly. (Courtesy Dr. D. Grattan-Smith, Atlanta, GA.)
Treatment: Salter-Harris I and II fractures are usually treated by closed manipulation followed by casting. Salter-Harris type III and IV fractures usually require open reduction and internal fixation, as often, these types of fractures are displaced and extend to the joint. To prevent posttraumatic osteoarthritis, it is important to create a smooth articular surface; therefore, Salter-Harris fractures with epiphyseal involvement and greater than 2 mm of diastasis or significant angulation usually need the epiphyseal component surgically reduced with orthopedic fixation.
Most (85%) of growth plate injuries heal without complication; however, in a subset of patients angulation and growth arrest may occur, and this depends on the location of the physeal insult.28,32 Angular deformities tend to occur when the physeal insult is eccentric, and limb shortening without angulation may occur when the physeal insult is central. Sometimes, paradoxic overgrowth may result after a Salter-Harris fracture. This is related to regional trophic effects associated with fracture healing.
Traumatic Injuries of the Humerus
Etiology, Pathophysiology, and Clinical Presentation: Injury to the proximal humerus varies with the age of the child. Infants and toddlers are likely to have a Salter-Harris I fracture of the proximal humeral physis. From 5 to 10 years of age, buckle fractures of the proximal humeral metaphysis are most prevalent (e-Fig. 143-13). These fractures may have considerable angulation. In older children, Salter-Harris II fractures predominate (e-Fig. 143-14). Fractures of the humeral diaphysis are also common.33
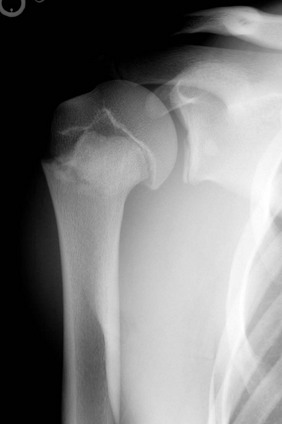
e-Figure 143-14 Salter-Harris II fracture of the proximal humerus in an adolescent.
The metaphyseal fragment is small and projects through the larger distal metaphyseal fragment. There is slight medial rotation of the humeral head due to pull of the rotator cuff tendons on the greater tuberosity.
Glenohumeral dislocation and instability is uncommon in younger children.34 This is because the physis and metaphysis are less resilient to biomechanical stress compared with the glenohumeral capsule and ligaments. Glenohumeral dislocation is common in older teens and patterns of injury are similar to those in young adults.
The proximal humeral physis can be a site of birth trauma with the middle third of the humerus most commonly fractured.35 Similar injuries can also be seen with child abuse.
Imaging: Anteroposterior and lateral radiographs of the humerus should be obtained to initially evaluate a fracture. If the fracture is near or involves the glenohumeral joint, an additional axillary view is recommended. If a Salter-Harris type I fracture is suspected in the neonate, evaluation with ultrasonography is preferable, as the proximal humeral epiphysis is cartilaginous and therefore better assessed with ultrasonography. For older patients or those with complex proximal humeral fractures, CT or MRI may be useful to evaluate for intraarticular extension and for involvement of the greater and lesser tuberosities.
Radiographically, since the humeral head is usually not ossified or only slightly ossified at birth, fracture through the physis mimics dislocation of the shoulder (e-Fig. 143-15). The humeral metaphysis may appear to align inferior to the glenoid. In the newborn, Salter-Harris I fracture of the proximal humerus is much more common than glenohumeral dislocation.36 Ultrasound may be used to diagnose the fracture by showing malalignment of the cartilaginous humeral head with the proximal humeral metaphysis and showing motion at the physis (e-Fig. 143-16).36,37
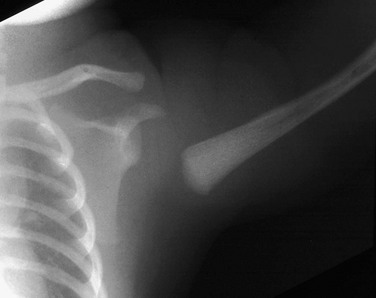
e-Figure 143-15 Proximal humeral Salter-Harris I fracture in a newborn caused by birth trauma.
The proximal humeral metaphysis appears malaligned with the glenoid. The humeral head is not ossified. In an infant, this finding is more likely from fracture than dislocation.
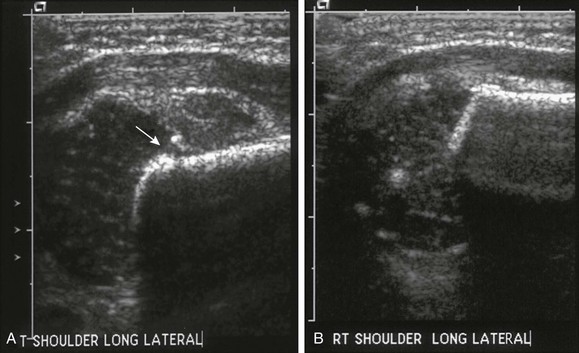
e-Figure 143-16 Proximal left humeral fracture in a 16-day-old girl with decreased arm movement and normal radiograph.
Sonographic images of the shoulders were obtained in the coronal plane with lateral transducer positioning. A, In the left shoulder, the cartilaginous epiphysis is displaced laterally from the metaphysis (arrow). There is soft tissue swelling at the fracture site. B, Normal right shoulder for comparison.
Salter-Harris fractures of the proximal humerus in older children are usually Salter-Harris II fractures, although the metaphyseal fragment is often very small. Occasionally, no metaphyseal fragment exists. Such Salter-Harris I fractures are sometimes called “slipped capital humeral epiphysis.” Typically, with a Salter-Harris I or II fracture of the proximal humerus, the epiphyseal fragment rotates medially because of unopposed pull by the rotator cuff.35 Salter-Harris III and IV fractures of the proximal humerus are extraordinarily rare. Avulsion of the greater tuberosity is a Salter-Harris III fracture. Avulsions of the lesser tuberosity caused by hyperextension with avulsion of the subscapularis tendon are rare and often delayed in diagnosis and are best delineated by an axillary view.38,39 Chronic avulsive injury of the deltoid insertion site has been reported.40
In very young children, fractures of the humeral diaphysis may be incomplete fractures; however, beyond the toddler years, most fractures of the humeral diaphysis are complete fractures. The humerus is one of the more common sites for a pathologic fracture because of its propensity to develop bone cysts.41 The positioning and alignment of the fragments of a humeral fracture are dependent on the site of fracture and its relationship to the deltoid and pectoral muscular insertions.
Management: Treatment is variable. Proximal humeral fractures are usually treated nonsurgically, including those with significant angulation and displacement. Fractures will heal and remodel without long-term orthopedic sequelae. However, some advocate operative management in certain situations.42,43 Reduction may be necessary in patients near skeletal maturity if the fracture has more than 50 to 70 degrees of angulation in the coronal or sagittal plane.44 Operative intervention also is indicated in those patients with associated neurovascular injury or who have intraarticular or open fractures.
Traumatic Injuries of the Elbow
Fractures of the elbow are one of the most common types of injuries in the pediatric population.45 The complex articulation of the elbow joint and the immaturity of the pediatric skeleton make this joint particularly susceptible to injury.46 Frequently, these fractures can be subtle. Familiarity with developmental anatomy of elbow ossification centers and assessment of fat pads and alignment aids in interpreting elbow radiography. Knowledge of common fracture patterns also assists in arriving at a correct diagnosis. Complications, although uncommon, include neurovascular injury, malunion, and compartment syndrome.47
Ossification Centers
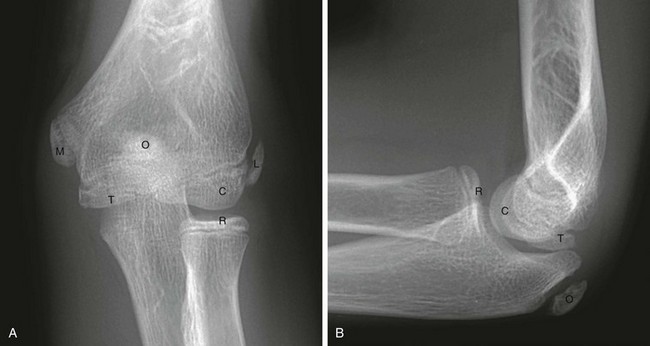
The normal, orderly progression of the appearance of the ossification of the six major ossifications centers of the elbow (Fig. 143-17) is as follows: capitellum (~1 to 2 years), radial head (~2 to 4 years), medial (internal) epicondyle (~4 to 6 years), trochlea (~9 to 10 years), olecranon (~9 to 11 years), and lateral (external) epicondyle (~9.5 to 11.5 years).48 This can be remembered by using the acronyms CRMTOL or CRITOE. With very rare exceptions, the medial epicondyle ossifies prior to the trochlea. If the trochlea is ossified, so should be the medial epicondyle. Fusion of the elbow ossification centers is less orderly, occurring after puberty. Although appearance and fusion of the elbow ossification centers are related to a range in age, they appear and fuse earlier in females compared with males.49
Joint Effusion
Acute intraarticular fractures of the elbow will usually have an elbow joint effusion.50,51 The effusion causes displacement of the anterior and posterior fat pads of the elbow (Fig. 143-18). Although the anterior fat pad is seen normally, it may appear elevated by an effusion (“sail sign”) and have an abnormal concave shape inferiorly. The normal anterior fat pad is usually convex in shape or sliverlike. The posterior fat pad normally resides within the olecranon fossa and is not seen on radiographs unless a large joint effusion is present. Ultrasonography may be useful to evaluate for the presence of effusions.52 Multidetector array computed tomography (MDCT) is a sensitive modality for evaluating radiographically occult fractures with posttraumatic elbow effusions and has a high negative predictive value.53
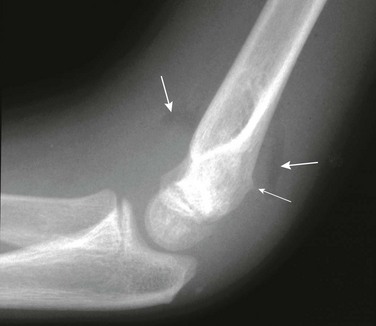
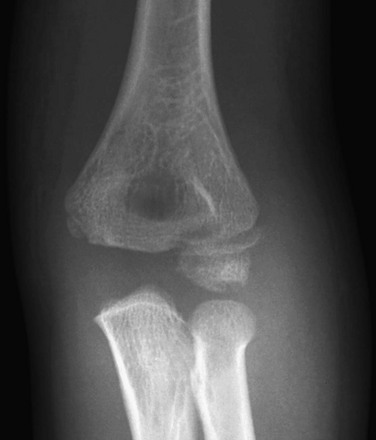
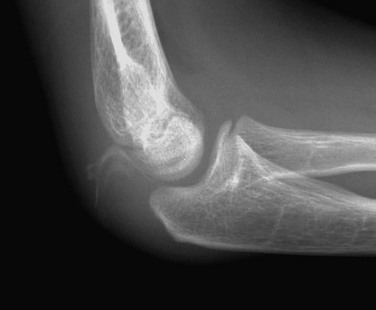
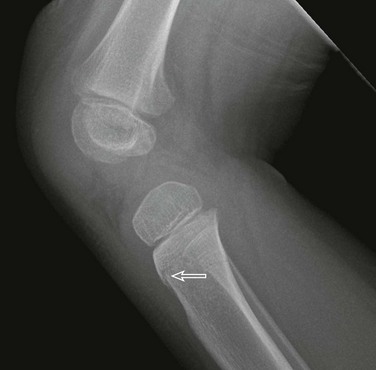
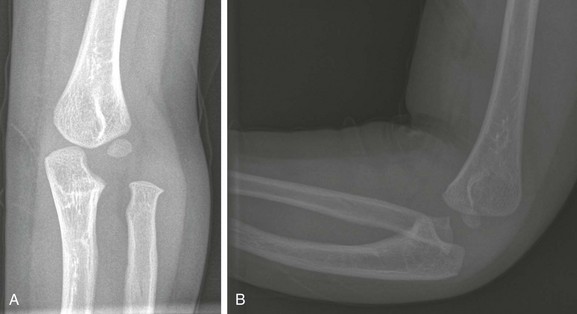
Figure 143-18 Buckle-type supracondylar fracture (small arrow) in a 6-year-old boy.
The anterior and posterior fat pads (large arrows) are displaced by an elbow joint effusion. Note that the anterior humeral cortical line passes along the anterior margin of the capitellum, indicating posterior angulation of the distal fragment.
The presence of an elbow joint effusion is strong evidence for the presence of a fracture.54,55 Usually, the fracture is obvious. In younger children, a subtle buckle or greenstick fracture of the supracondylar distal humerus may occur. In older children, a subtle fracture of the radial head or radial neck may occur. The presence of an effusion is not unequivocal evidence for a fracture.56 The prevalence of elbow fractures with a joint effusion and no other radiographic findings of a fracture range from 6% to 76%, depending on the study.55,57 A normal anterior fat pad is highly associated with absence of a fracture.58 However, as fractures are often subtle or occult, the presence of an effusion without an identifiable fracture usually prompts splinting of the arm with follow-up radiographs to assess for healing of an occult fracture. Alternatively, MRI has been used by some centers to evaluate for fracture.59–61 However, MRI may identify some subtle fractures that are probably of little clinical importance.62
Alignment Lines
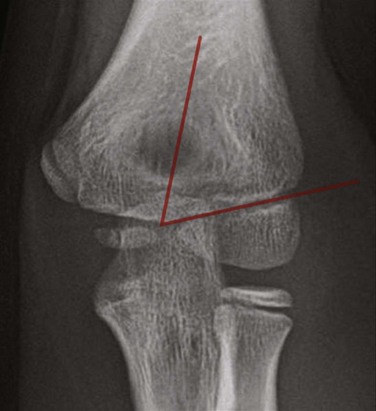
On a properly positioned lateral view, the anterior humeral cortical line is drawn along the anterior cortex of the humerus. This line should pass through the middle third of the capitellum in the majority of normal elbows; however, in children under 4 years of age, the anterior humeral line passes equally through the anterior or middle third of the capitellum.63 Disruption of this relationship aids in the detection of supracondylar fractures, which are usually posteriorly angulated and displaced. The radiocapitellar line is drawn along the axis of the radius. Regardless of patient positioning or projection, this line should pass through the capitellum (Fig. 143-19). Disruption of this relationship aids in detection of radiocapitellar dislocation.64 Other reasons for disruption of this line include lateral condylar, radial neck, and Monteggia fractures.65
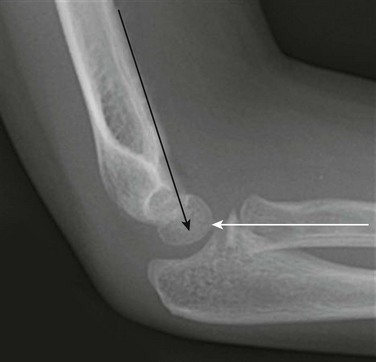
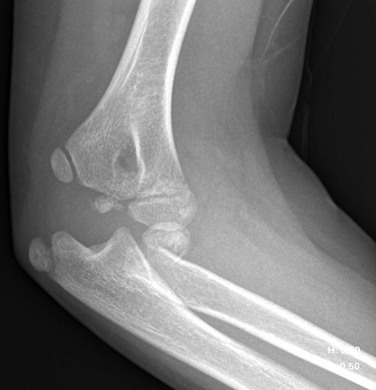
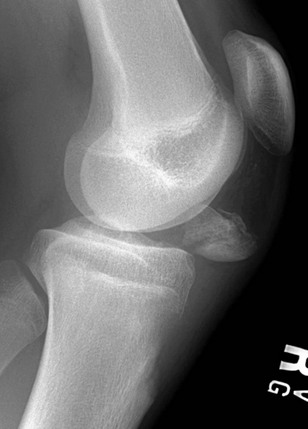
Figure 143-19 Lateral radiograph of a normal elbow demonstrating normal elbow alignment.
The anterior humeral line is drawn along the anterior cortex of the humerus and should intersect the middle third of the capitellum. The radiocapitellar line is drawn along the axis of the radius. Regardless of patient positioning or projection, this line should pass through the capitellum.
Supracondylar Fractures
Etiology, Pathophysiology, and Clinical Presentation: Supracondylar fractures of the distal humerus account for 60% of pediatric elbow fractures.66 The usual mechanism of injury is hyperextension with impingement of the olecranon on the posterior distal humerus.67 Fractures vary widely in severity from a faintly perceptible buckle fracture to a complete fracture with marked displacement and angulation. A significant percentage of patients with a supracondylar fracture will have an ipsilateral forearm fracture.68
Imaging: Anteroposterior, oblique, and lateral radiographs are required not only for diagnosis but also to guide appropriate management.47 Comparison with the contralateral elbow may be helpful in some instances but is not routinely necessary.69 The distal fragment of a supracondylar fracture is often displaced or angulated posteriorly. As a result, the anterior humeral cortical line will not bisect the capitellum. In a normal elbow, anterior angulation of the distal humeral condyles causes the anterior humeral cortical line to pass through the center of the capitellar ossification center. If this line passes through the anterior third of the capitellar ossification center or anterior to it, then a supracondylar fracture is likely present.65 It is important to assess for this finding on a properly positioned lateral view of the distal humerus. Obliquity of the distal humerus may cause the capitellar ossification center to appear falsely posterior relative to the anterior humeral cortical line.65 Supracondylar fractures invariably involve the distal humeral metaphysis but physeal involvement is unusual.
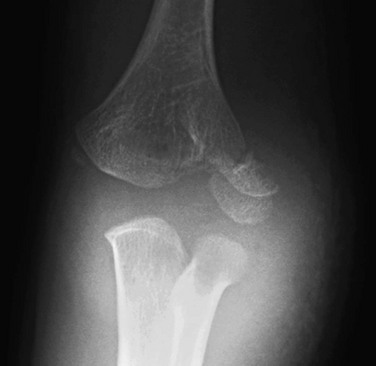
The modified Gartland classification system of supracondylar fractures is the most commonly used to succinctly describe the fracture and for treatment planning.70 Type I supracondylar fractures are nondisplaced or minimally displaced less than 2 mm and have an intact anterior humeral line (see Fig. 143-18). Type II fractures are displaced greater than 2 mm with angulation and disruption of the anterior humeral line, but with an intact posterior cortex. Type III fractures are displaced with no cortical continuity (e-Fig. 143-20). Type III fractures are subdivided into posteromedial and posterolateral fractures on the basis of displacement. Most type II and all type III fractures are treated surgically.67 The risk of developing epiphyseal osteonecrosis of the trochlea should be considered if surgery is delayed.71 Neurovascular injury may occur with displaced supracondylar fractures.
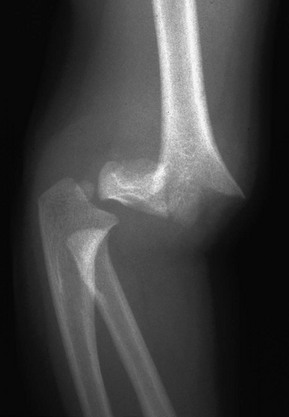
e-Figure 143-20 Supracondylar fracture in a 2-year-old boy.
The distal fragment is displaced and angulated posteriorly.
Underreduced supracondylar fractures may cause clinically significant limitations in elbow flexion.72 After reduction, supracondylar fractures often demonstrate a mild degree of posterior displacement or angulation. This will not adversely affect functional outcome; however, fusion with loss of normal cubitus valgus will potentially inhibit the range of motion of the elbow. Normally, slight lateral angulation of the radius exists relative to the humerus (cubitus valgus) and is greater in females. The Baumann angle is created by the intersection of the humeral axis with a line tangent to the physis of the lateral condyle (e-Fig. 143-21). The normal angle is approximately 75 degrees. With posttraumatic cubitus varus, the Baumann angle is greater than 83 degrees.73 Unfortunately, the Baumann angle is somewhat dependent on positioning. Cubitus varus deformity is thought to occur secondary to medial angulation of the distal fracture fragment.74 Severe deformity of the distal humerus with cubitus varus has been called “gun stock deformity.”
Treatment: Conservative versus operative management generally depends on the degree of displacement, age of the patient, location of the fracture, stability of the fracture, and associated injuries. A nondisplaced or acceptably displaced fracture will be treated conservatively; an acceptable displacement is defined as one that will be corrected by expected growth and thickness of the injured bone.75 For example, most type I or nondisplaced supracondylar fractures can be treated conservatively in a long arm cast for 3 to 4 weeks with the elbow held in 90 to 110 degrees of flexion. Most type II and III supracondylar fractures are treated operatively with closed reduction and percutaneous pinning.76,77 Most supracondylar fractures can be electively treated (i.e., next morning for a fracture identified afterhours), including type III fractures. Patients exhibiting neurovascular compromise, however, require immediate treatment.
Lateral Condylar Fractures
Etiology, Pathophysiology, and Clinical Presentation: Lateral condylar fractures are the second most common type of fracture of the pediatric elbow, accounting for 12% to 20% of pediatric elbow fractures.78 The mechanism is hyperextension with varus stress.66 Lateral condylar fractures are considered Salter-Harris IV fractures until proven otherwise.
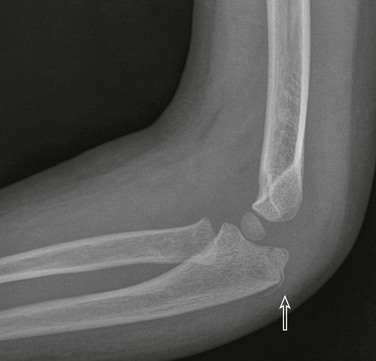
Imaging: In addition to the standard anteroposterior and lateral radiographs, some advocate obtaining an internal oblique view to better delineate the lateral condylar fracture gap.79 External oblique views tend to obscure the lateral condylar fracture but are better for delineating the radial head and neck. MDCT may be useful in certain cases to decide between surgical and nonsurgical management.80 The severity of lateral condylar fractures varies considerably. The fracture may or may not extend through the unossified portion of the distal humeral epiphysis (e-Fig. 143-22). “Stable” lateral condylar fractures (type I) do not traverse the cartilaginous epiphysis and are incomplete and thus nondisplaced or minimally displaced (Fig. 143-23). Type II lateral condylar fractures are complete and thus “unstable” but with little or no displacement. The lateral condylar fracture line may be quite subtle, often paralleling the adjacent metaphyseal margin.
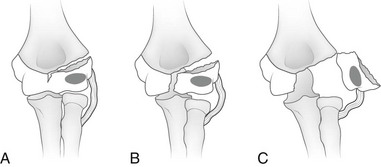
e-Figure 143-22 Classification of lateral condylar fractures according to the amount of displacement.
A, Type I (incomplete) fracture. Note that the fracture line enters the cartilaginous surface of the distal humerus between the capitellum and trochlea. However, the fracture is not complete and does not extend to the articular surface. And it is therefore nondisplaced. B, Type II (complete) fracture. The fracture extends completely through the cartilanginous distal humerus to the articular surface but is nondisplaced. C, Type III (complete, displaced) fracture. The fracture extends completely through the cartilaginous distal humerus to the articular surface and is displaced. (From Green NE, Swinotowski MF. Skeletal trauma in children. 3rd ed. Philadelphia, PA: Saunders; 2003.)
In the past, arthrography (with or without CT) was occasionally used to assess for the epiphyseal fracture line.81 Both MRI and ultrasonography have proven capable of demonstrating the fracture through epiphyseal cartilage indicating a Salter-Harris IV fracture.82 With complete or “unstable” fractures, the lateral condylar fragment is displaced and rotated (type III; e-Fig. 143-24).
Treatment: Lateral condylar fractures that are nondisplaced (stable) or are displaced 2 mm or less are managed with cast immobilization. Those that are displaced greater than 2 mm (unstable) are treated with surgical fixation with lateral entry pins.78 A lower threshold exists for surgical intervention of lateral condylar fractures compared with supracondylar fractures because these fractures involve the physis and may have an intraarticular component. Physeal growth arrest and posttraumatic osteoarthritis are more common complications of lateral condylar fractures compared with supracondylar fractures. The most common long-term deformity is relative lateral overgrowth with subsequent cubitus varus.83
Medial Epicondyle Avulsion
Etiology, Pathophysiology, and Clinical Presentation: The medial epicondyle ossifies by age 7 years and fuses by age 16 years.84 The medial epicondyle is the origin of the forearm flexor mechanism and ulnar collateral ligaments. Avulsions thus occur within this age range, although rare avulsions of the unossified medial epicondyle have been reported.85 The two chief mechanisms of acute avulsion fracture of the medial epicondyle are (1) throwing injury and (2) elbow dislocation.84,86 Acute avulsion of the medial epicondyle is but one of several injuries that can occur in the elbow of a skeletally immature throwing athlete.87 The avulsion occurs because of hyperextension, with valgus stress producing traction on the apophysis by the flexor tendons and pronators. In the setting of an acute avulsion, the child will experience sudden-onset medial elbow pain while throwing and have point tenderness over the medial epicondyle.87 Rarely, the medial epicondyle may displace into the joint mimicking a trochlear ossification center. This occurs because the valgus stress of throwing temporarily widens the joint.
Imaging: Initial assessment should include anteroposterior, oblique, and lateral radiographs of the elbow (Fig. 143-25). With elbow dislocation in the skeletally immature patient, the medial epicondyle is often avulsed from its normal location. When assessing the images of a dislocated elbow, the status of the medial epicondyle should be specifically addressed (e-Fig. 143-26). In addition, radiographs should address the type of avulsion fracture when present at the level of the medial epicondyle chondro-osseous junction, physeal equivalent, or at the juxtaphyseal metaphyseal equivalent region with displaced and fragmented bone.
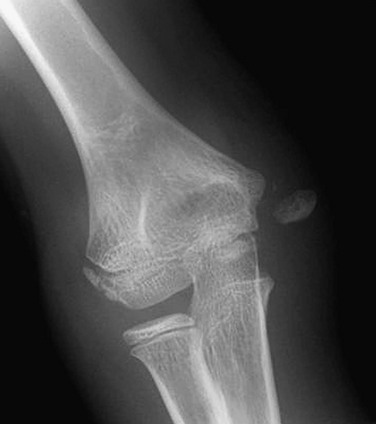
Figure 143-25 Avulsed medial epicondyle in an adolescent boy.
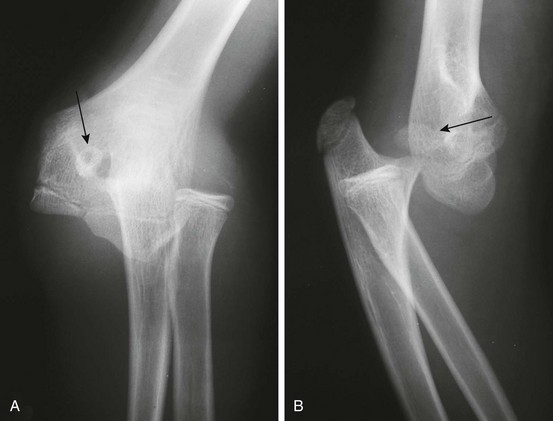
e-Figure 143-26 Anteroposterior (A) and lateral (B) radiographs of a dislocated elbow in a 12-year-old boy.
The medial epicondyle (arrows) is avulsed and displaced into the joint space.
Some cases of medial epicondyle avulsion fracture occur with a transient, unrecognized dislocation. When an elbow dislocation is reduced, the medial epicondyle may be trapped in the elbow joint (Fig. 143-27). A trapped medial epicondyle may superficially mimic a trochlear ossification center in the first decade of life. However, absence of the medial epicondyle at its normal location should prompt a search for it within the joint. If the trochlea is ossified, the medial epicondyle should be as well. Visualization of the trochlea without the medial epicondyle may be caused by displacement of the medial epicondyle or by a displaced medial epicondyle being mistaken for the trochlea.
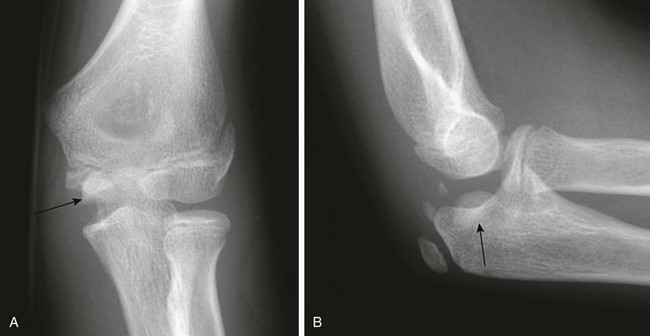
Figure 143-27 Anteroposterior (A) and lateral (B) radiographs after reduction of a dislocated elbow in a 10-year-old girl.
The avulsed medial epicondyle (arrows) is trapped in the elbow joint.
Comparison views may be helpful in confirming mildly displaced medial epicondylar avulsion injuries at the level of the medial epicondyle physeal equivalent region; however, they are usually not necessary. MRI and CT will often demonstrate associated findings such as injury to the ulnar collateral ligament and the sublime tubercle of the ulna, as well as compression-type injuries in the lateral compartment, such as radiocapitellar osteochondral injuries and bone contusions. However, these additional findings often will not change clinical management.88,89 MRI and CT are useful when radiographs do not demonstrate a clear fracture and an alternative etiology for the child’s symptoms are sought. Avulsion of the medial epicondyle prior to its ossification is distinctly rare but has been reported. Ultrasonography as well as CT or MRI can be used for fracture delineation.90
Treatment: Both surgical and nonsurgical management has been supported in the literature.65 Indication for operative treatment include entrapment of the medial epicondyle in the joint and the uncommon occurrence of an open fracture.65 Medial epicondyle avulsion fractures can be treated conservatively if the avulsed fragment is not intraarticular, if the child is less than 5 years of age or the degree of displacement is less than 4 mm. Generally, the need for intervention increases with the age of the child, degree of dislocation, and athletic activity.91
Medial Condylar Fractures
Etiology, Pathophysiology, and Clinical Presentation: Medial condylar fracture, not to be confused with medial epicondylar avulsion (see above), has an appearance similar to the more common lateral condylar fracture. Medial condylar fractures are uncommon and account for 1% to 2% of pediatric elbow fractures.66 These are typically Salter-Harris type IV fractures.
Imaging: Initial assessment should include anteroposterior, external oblique, and lateral radiographs of the elbow. The fracture line extends through the medial metaphysis separating the metaphysis and medial epicondyle from the remainder of the humerus; the fracture line extends to the trochlear articular surface. MRI can also be useful to evaluate these fractures particularly in young children in whom the diagnosis can be difficult.92
Distal Humeral Salter-Harris I Fracture (Transcondylar)
Etiology, Pathophysiology, and Clinical Presentation: Distal humeral Salter-Harris I fracture is an uncommon fracture that occurs from birth to approximately age 7 years, peaking at age 2.5 years. It is typically an injury of infants and young toddlers and is caused by child abuse in half of the cases, or by a rare birth injury.66,93
Imaging: Radiographically, the fracture may be mistaken for a dislocation of the elbow, as the bones do not appear to align. The radial axis will be normally aligned with the capitellum, but the capitellum itself will be abnormally related to the distal humeral metaphysis (e-Figs. 143-28 through 143-30). The radius, ulna and humeral epiphysis will be medially displaced. Fractures may occur prior to capitellar ossification.94
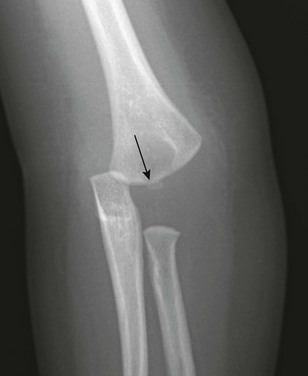
e-Figure 143-28 Salter-Harris I fracture (transcondylar fracture) of the distal humerus in a 16-month-old boy.
The capitellum (arrow) is not properly aligned with the distal humeral metaphysis, and the whole forearm is shifted medially relative to the humerus. Soft tissue swelling is present.
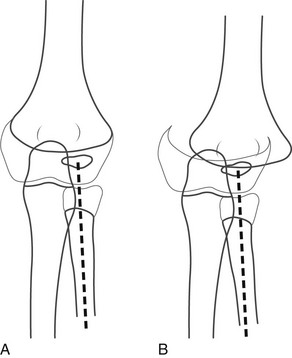
e-Figure 143-29 Separation of the entire distal humeral epiphysis.
A, Normal elbow of a 2-year-old child. Heavy lines represent cortex, and fine lines represent the margin of the cartilaginous epiphysis of the humerus and radius. The radiocapitellar line (broken line) defines the normal relationship of the radius and the capitellum. B, Salter-Harris type I separation of the entire distal humeral epiphysis. Note the medial displacement of the radius, ulna, and humeral epiphysis. The normal relationship of the radius and capitellum is maintained. (From Rogers LF. Radiology of skeletal trauma. 3rd ed. New York: Churchill Livingstone; 2002.)
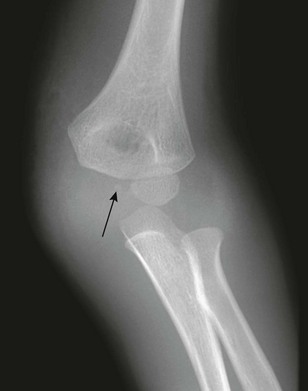
e-Figure 143-30 Dislocation of the elbow in a 5-year-old boy.
In this patient, the capitellum is normally aligned with the distal humerus; however, the radius is not properly aligned with the capitellum. Marked soft tissue swelling is seen medially. The medial epicondyle is undoubtedly avulsed and may be represented by a small ossific density (arrow).
Radial Head and Neck Fractures
Etiology, Pathophysiology, and Clinical Presentation: Fractures of the radial head and neck account for 5% of pediatric elbow fractures.66 Mechanism of injury is falling on an outstretched hand. Fractures in children often occur at or just distal to the physis; this is unlike the adult population where fractures typically involve the radial head.66 Associated injuries include fracture of the olecranon, avulsion of the medial epicondyle, or medial collateral ligament injury.66
Imaging: Radiographs are usually sufficient for diagnosis and follow-up. External oblique views best delineate the radial head and neck morphology, whereas internal oblique views are better for delineating the lateral condylar region. Radial neck fractures are usually buckle fractures followed by Salter-Harris fractures. Isolated radial neck fractures as well as fractures with extension to the radial head are unusual. The radiocapitellar joint is often preserved even when radial neck fractures are displaced and angulated (e-Fig. 143-31).
Treatment: Most pediatric radial head and neck fractures can be treated nonoperatively with closed reduction and immobilization. If there is greater than 30 degrees of residual angulation, greater than 3 to 4 mm of displacement at the fracture site or less than 45 degrees of pronation or supination, then operative intervention is suggested.95
Olecranon Fractures
Etiology, Pathophysiology, and Clinical Presentation: Olecranon fractures are relatively uncommon accounting for approximately 4% to 6% of elbow fractures in children.66 Common mechanisms include falling on an outstretched hand, twisting injury, or direct trauma.66 The olecranon is the insertion site of the triceps muscle and prone to avulsion fractures. Although most are nondisplaced, olecranon fractures commonly have associated injuries, including radial neck fractures, medial epicondylar fractures, coronoid fractures, and osteochondral injuries.66
Imaging: Radiography is usually sufficient for defining olecranon fractures. Fractures should describe the degree of displacement, if any, intraarticular involvement, involvement of the olecranon physeal equivalent zone, and distinguish any additional fractures from normal olecranon ossification centers. Olecranon fractures may be transverse, oblique, or longitudinal. Buckle, bowing, or greenstick fractures are common in younger patients.
The olecranon physis and apophyseal ossification centers vary considerably in size, location, and degree of fragmentation.96 The juxtaphyseal fragmentary appearance of the olecranon ossification center may be mistaken for fracture, and vice versa. Comparison views are helpful. Olecranon ossifications are well corticated and appear oval or round. Olecranon fractures have a thin, sliverlike appearance, are usually not corticated, and are located near and parallel the olecranon metaphyseal equivalent zone (e-Fig. 143-32). Other olecranon injuries include stress fractures and sleeve fractures. An olecranon sleeve fracture is an avulsion of the triceps tendon from the olecranon process (e-Fig. 143-33). Stress fractures of the olecranon physis occur in adolescent baseball pitchers.97 Nondisplaced stress fractures of the olecranon most commonly occur in adolescence and may only be visible on CT or MRI scans.
Nursemaid’s Elbow
Etiology, Pathophysiology, and Clinical Presentation: Nursemaid’s elbow occurs due to an upward pull on an extended elbow in pronation. The annular ligament of the proximal radius is disrupted or displaced, allowing the radial head to sublux or dislocate anteriorly relative to the capitellum. (e-Fig. 143-34).98 This injury most commonly occurs in the toddler years, up to age 5 years.99
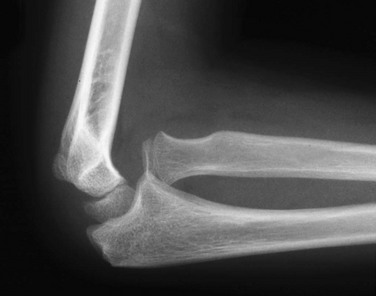
e-Figure 143-34 Dislocated radial head in a 9-year-old girl.
Imaging: An astute pediatrician will make the diagnosis clinically and reduce the radius without imaging. Often, when imaging is requested, the dislocation is reduced in the course of properly positioning the hand in supination for the anteroposterior view of the elbow. Ultrasonography may also play a role in detecting dislocation of the radial head with respect to the annular ligament.100 The utility of radiography is not to make the diagnosis of nursemaid’s elbow, but to exclude an underlying fracture. After nursemaid’s elbow is successfully reduced, the only radiographic sequelae may be a joint effusion without underlying fracture.
When radiographs demonstrate a persistent anterior dislocation despite attempts at reduction, a congenital radial head dislocation should be considered, which has characteristic findings including a convex radial head, small capitellum, and concave shaped posterior margin of the olecranon (e-Fig. 143-35).
e-Figure 143-35 A 3-year-old girl with congenital radial head dislocation.
Anteroposterior (A) and lateral (B) views demonstrate radiocapitellar dislocation, convex dysplastic radial head, and convex posterior contour of the ulna, characteristic features of congenital, rather than traumatic radiocapitellar dislocation.
Treatment: Supinating the child’s forearm with the elbow in flexion will usually reduce the dislocation. Often, the dislocation is reduced at the time of imaging as described above. A small percentage of children may have recurrent dislocations, with a higher risk the younger the first dislocation occurs.
Traumatic Injuries of the Forearm
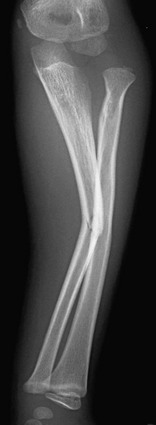
Etiology, Pathophysiology, and Clinical Presentation: Fractures of the distal radius and ulna are very common. The distal radius is the most common site of buckle fractures in children and usually occurs after a fall on an outstretched arm. Salter-Harris fractures of the distal radius and transverse fractures of the distal radial metaphysis are also very common.101 Physeal fractures of the distal radius are usually Salter-Harris II fractures. Stress injury to the distal radial physis is frequently seen in gymnasts. Fractures of the radial and ulnar shafts usually occur together. Often, the fracture in one bone is complete, whereas the other bone has an incomplete fracture.
Imaging: At least anteroposterior and lateral radiographs of the injured extremity should be obtained. Dedicated osteoarticular radiographic imaging should be performed of the elbow in the presence of a Monteggia fracture, and dedicated osteoarticular radiographic imaging of the wrist should be performed for Galeazzi fractures. CT is usually reserved for injuries that are complex and are accompanied by an associated injury of an osteoartricular joint or for defining the articular surface displacement, which is necessary for surgical planning.
In radial buckle fractures, the cortex tends to buckle dorsally. A fracture line may be present volarly. Distal radial buckle fractures may be extremely subtle and are commonly missed by inexperienced readers. It is important to look at all views for a subtle disruption of the normal smooth flared curve of the metaphyseal margin. Any extra angulation or “bump” is likely a fracture. An associated distal ulnar fracture may or may not be present. With a subtle distal radial fracture, the accompanying distal ulnar buckle fracture may be even more occult, often not identified until signs of healing are seen on follow-up radiographs. Distal radial buckle fractures may also be associated with triangular fibrocartilage injuries and fractures of the ulnar styloid process. Associated carpal injuries are uncommon.102
Several subtypes of Monteggia fractures are determined on the basis of the anatomy of the ulnar fracture and the direction of radial head dislocation.103–105 The most common direction of radial head dislocation is anterior with respect to the capitellum (Fig. 143-36). The ulnar fracture may be complete or incomplete.
Treatment: Buckle fractures, including those with mild angulation, are usually treated with a splint. Nondisplaced, complete, nonphyseal fractures are casted. Fractures that involve the distal radial physis usually require closed reduction if displaced. The amount of accepted displacement depends on the type and location of the fracture, age of the child, and direction of angular deformity. In children older than age 10 years, fractures involving the proximal one third of the radius and those with angulation appear to be at higher risk for failure when treated nonoperatively.106 Surgical reduction is performed with pin fixation across the fracture. With Monteggia fractures, it is the nature of the ulnar fracture rather than the direction of radial head dislocation that is useful in determining optimal treatment for these injuries. Stable anatomic reduction of the ulnar fractures results in stable anatomic reduction of the radial head.107 Nonsurgical management of Galeazzi fractures with anatomic reduction and immobilization in a long-arm cast has been successful in children.108
Traumatic Injuries of the Wrist
Etiology, Pathophysiology, and Clinical Presentation: Carpal bone fractures and intercarpal ligament injuries usually occur during the second decade, when children are near skeletal maturity, and follow an adult pattern of injury. Carpal fractures in the first decade are exceedingly rare because of two reasons: (1) the carpal bones have significant epiphyseal equivalent cartilage cushioning the primary carpal ossification centers from injury; and (2) the point of maximal weakness of the forearm and wrist is at the level of the radial and ulnar physis, leading to Salter-Harris fractures, as well as the level of metadiaphyseal cortex where buckle fractures occur. Therefore, in the first decade, Salter-Harris fractures and buckle fractures are far more common compared with carpal bone fractures and ligamentous injuries.
The scaphoid is the most frequently fractured carpal bone. Scaphoid fractures most commonly are transverse and extend through the scaphoid waist. Fractures of the triquetrum are the next most reported carpal bone fracture, followed by the trapezium.2
Imaging: Scaphoid views with ulnar deviation will better profile the scaphoid and can be added as a supplement to the standard three view wrist series. CT or MRI (e-Fig. 143-37) can be used to confirm the diagnosis and assess for alternative etiologies for wrist pain.109 Alternatively, if clinical suspicion persists because of mechanism or physical examination findings (snuffbox tenderness), then splints may be applied, with follow-up radiographs obtained after 10 to 14 days to reassess for fracture.
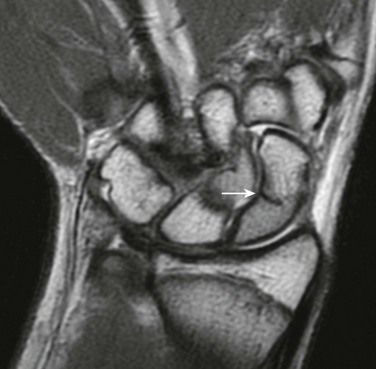
e-Figure 143-37 A 15-year-old boy with T1-weighted coronal sequence image demonstrating a nondisplaced incomplete scaphoid waist fracture (arrow).
The scaphoid artery enters from the distal pole and extends proximally to supply the proximal pole. As a result, proximal pole osteonecrosis may occur after midpole scaphoid fractures. Osteonecrosis is less frequent in the pediatric population compared with the adult population.2 The affected proximal pole of the scaphoid will appear dense relative to the other bones of the wrist (Fig. 143-38). Nonunion of the scaphoid can occur with or without epiphyseal osteonecrosis. The incidence of nonunion is increased with delay in diagnosis, although it is generally uncommon.110,111
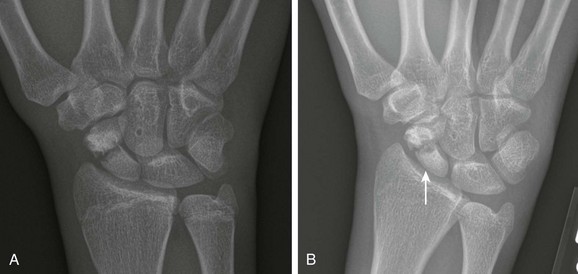
Figure 143-38 A 16-year-old boy with scaphoid waist fracture.
A, Radiograph at presentation demonstrates a mildly displaced scaphoid waist fracture. B, Repeat radiograph 6 months later shows no appreciable callus formation at the fracture site, and subtle increased proximal pole radiodensity (arrow) without collapse, suggestive of early osteonecrosis.
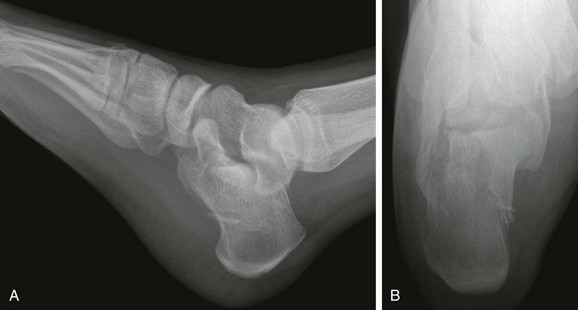
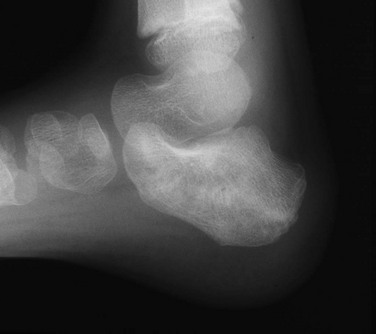
Fractures of the triquetrum (Fig. 143-39) are seen as a small bony fragment projecting dorsally. Fractures of the hook of the hamate may occur in athletes. Carpal dislocations are rare in children. As in adults, these dislocations may involve a fracture of the scaphoid. Carpal instability rarely manifests in childhood.110
Traumatic Injuries of the Hands
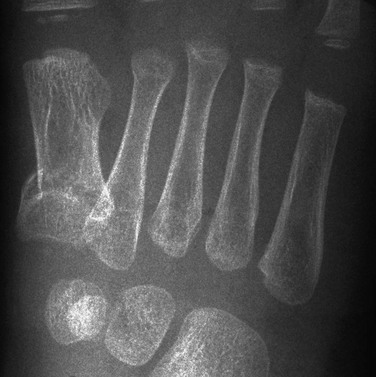
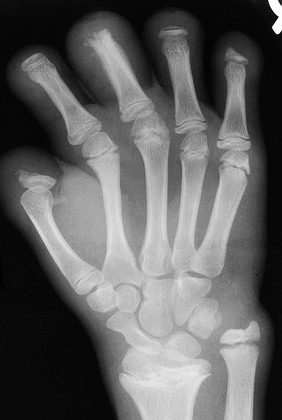
Etiology, Pathophysiology, and Clinical Presentation: Fractures of the hand are common in children. The incidence peak is bimodal, the first peak being at age 1 to 2 years and the second at age 12 years. At age 1 to 2 years, the most common hand injury is a distal phalanx fracture with soft tissue laceration. At age 12 years, the most common hand injury is fracture of the proximal phalanx of the little finger, followed by metacarpal fractures.112 In general, phalangeal fractures and interphalangeal dislocations are relatively common injuries. Fractures of the hand often involve the physis113 and Salter-Harris fractures are common. Growth disturbances in this region are relatively uncommon.2
Imaging: Radiography with at least anteroposterior, oblique, and lateral views should be obtained tailored to the specific injured digit rather than imaging the entirety of the hand. Advanced cross-sectional imaging is rarely necessary.
Avulsion injuries usually occur from hyperextension, hyperflexion, or “jamming” of the finger into an object. “Mallet finger” is the result of forced flexion of a distal interphalangeal joint leading to Salter-Harris III fracture–avulsion of the dorsal aspect of the terminal phalanx (e-Fig. 143-40).113 Volar plate Salter-Harris III avulsion fractures occur along the volar side and may occur at either the terminal phalanx or middle phalanx insertion of the flexor digitorum tendons (e-Fig. 143-41). Salter-Harris type fractures may also be related to terminal phalangeal crush injuries (e-Fig. 143-42).
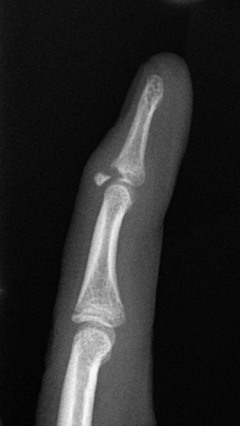
e-Figure 143-40 Mallet finger in a 13-year-old boy.
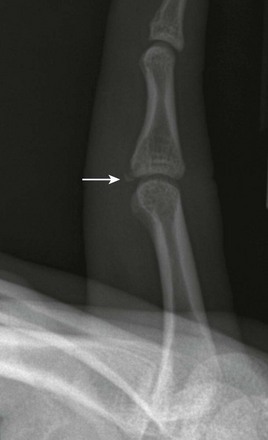
e-Figure 143-41 A 15-year-old boy with volar plate fracture (arrow).
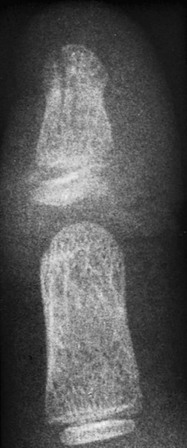
e-Figure 143-42 Comminuted crush injury to the distal phalanx of the thumb.
The distal phalanx has two long longitudinal fracture lines with abnormal physeal widening present. The cartilage plate is lacerated transversely, and the epiphyseal ossification center is impacted on one side of the shaft. An automobile door was slammed on the thumb of this 6-year-old boy.
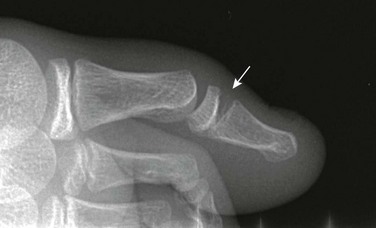
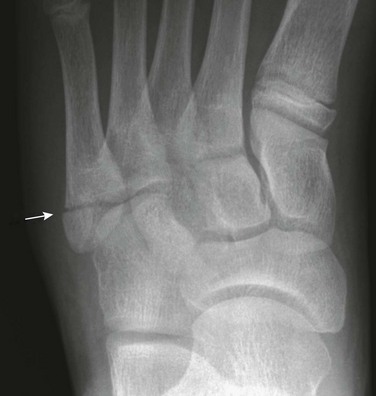
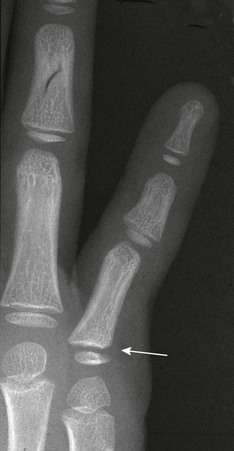
Salter-Harris II fractures are common and frequently involve the proximal phalanx of the thumb.113 Displacement is variable. The fractures may be very subtle with minimal physeal widening or a tiny metaphyseal fragment (e-Fig. 143-43). It may be difficult to distinguish between a Salter-Harris II fracture of the proximal phalanx and a bifid epiphysis, an uncommon normal variant. Salter-Harris I and III fractures occur occasionally.
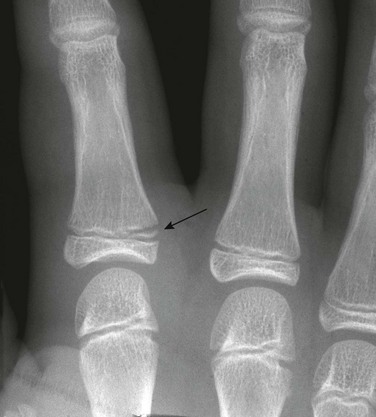
e-Figure 143-43 Salter-Harris II fracture of the proximal phalanx of the index finger (arrow) of a 9-year-old girl.
The child was hit with a ball with the finger extended.
The most common metacarpal fracture in adolescents is that of the fifth metacarpal (“boxer’s fracture”) in adolescents.112 The fracture plane is transverse or oblique through the metaphysis at the distal aspect of the fifth metacarpal. The distal fragment is angulated toward the palm (e-Fig. 143-44).
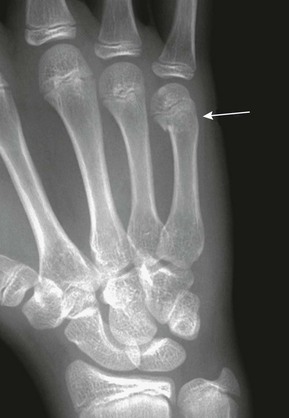
e-Figure 143-44 Boxer’s fracture of the fifth metacarpal (arrow) in a 17-year-old boy who punched a wall.
Slight palmar angulation of the distal fracture fragment is seen distally.
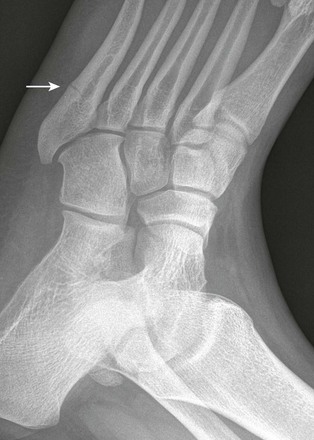
Salter-Harris III fractures of the first metacarpal bone are analogous to a Bennett fracture occurring after skeletal maturity (e-Fig. 143-45). A Bennett fracture is an intraarticular fracture at the base of the first metacarpal with first carpometacarpal subluxation. A Rolando fracture is a comminuted version of the Bennett fracture. These injuries are rare in children.
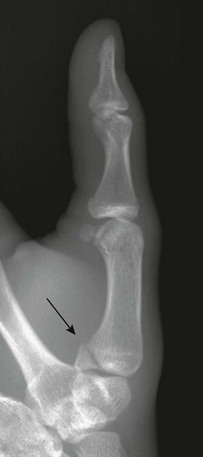
e-Figure 143-45 Salter-Harris III fracture of the base of the thumb metacarpal (arrow) in a 16-year-old boy.
The injury occurred when the boy’s thumb caught on another player’s equipment while playing football.
“Gamekeeper’s thumb” (e-Fig. 143-46) is a traumatic avulsion fracture at the proximal phalangeal insertion of the ulnar collateral ligament. Historically described in Scottish gamekeepers, this injury most commonly occurs in children from ski pole injury or breakdancing.114 An avulsion may be seen at the ulnar volar base of the proximal phalanx of the thumb, or a Salter-Harris III fracture if the physis is not yet fused. Radial subluxation of the proximal phalanx may be evident, but stress views may be required to show instability in the absence of fracture. Ulnar collateral ligament avulsion may also cause a Stener lesion with the adductor aponeurosis interposed between the torn ulnar collateral ligament and its insertion, preventing approximation.
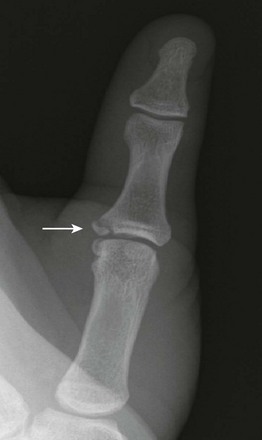
e-Figure 143-46 A 14-year-old boy with gamekeeper’s thumb fracture (arrow).
Treatment: Nearly all metacarpal and phalangeal fractures are treated nonsurgically with 3 to 4 weeks of immobilization.2 Surgical pinning is required for intraarticular fractures, particularly those involving the interphalangeal and metacarpophalangeal joints.
Fractures should probably not be immobilized greater than 6 weeks in children unless a significant delay occurs in healing or if a change in treatment is required (i.e., open reduction internal fixation).2 Nondisplaced avulsion fractures are typically treated by brace taping, or buddy taping, the injured digit to its neighbor. Stener lesions are managed operatively.113
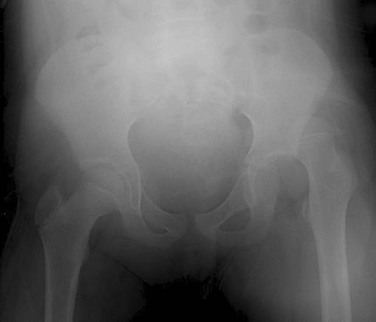
Traumatic Injuries of the Pelvis and Hip
Overview: Pelvic fractures are not common in children.115 Pedestrian–motor vehicle collisions account for the majority of cases, followed by falls, and the majority of victims are boys.115,116 The presence of a pelvic injury in a child is a marker of severe injury and should alert the clinician to search for additional injury.116,117 Apophyseal avulsion injuries of the pelvis are covered in Chapter 145.
Etiology, Pathophysiology, and Clinical Presentation: Once a patient has reached skeletal maturity, pelvic ring fracture patterns follow the adult fracture pattern and classification.118 Several classifications schemes have been applied to pediatric pelvic fractures. In the classification scheme of Torode and Zieg (e-Fig. 143-47), type I fractures are avulsions, type II are iliac wing fractures, type III are simple ring fractures with no clinical instability, and type IV are pelvic ring disruptions with instability. Type IV fractures include straddle injuries with bilateral pubic rami fractures and Malgaigne fractures with anterior and posterior disruption on the same side.119 Disruption of the sacroiliac joints or pubic symphysis may accompany pelvic fractures. Plastic or incomplete fractures may be seen in children. Usually, if a break in the obturator ring or pelvic ring is present, another break is also present. Younger children, however, may not follow this rule because of the plasticity of their bones.120,121
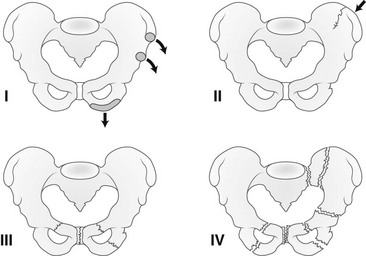
e-Figure 143-47 Pelvic fractures in children—Torode and Zieg classification.
Type I, Avulsion fracture. Type II, Iliac wing fracture. Type III, Simple ring fracture. Type IV, Ring disruption fracture. (Modified from Beaty JH, Kasser JR. Rockwood and Wilkins’ fractures in children. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.)
Fractures involving the acetabulum of young children are fortunately rare. Acetabular fractures become more common in the later teen years because of motor vehicle accidents.120 Fractures of the acetabulum in older children follow patterns similar to those in adults. Most occur in association with hip dislocations.121
Imaging: CT is the preferred modality for delineation of unstable pelvic fractures. Although most fractures are seen on radiography, CT better delineates the full extent of fracture.122 The utility of radiographs for screening for pelvic fractures has been questioned.123,124 Radiography probably is not necessary if CT will be performed to evaluate for other injuries.123
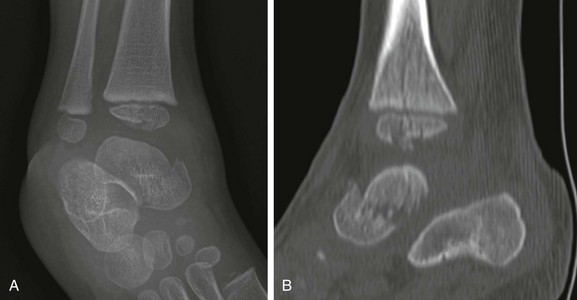
Asymmetric widening of the triradiate cartilage may be noted in acetabular fractures (e-Figs. 143-48 and 143-49). CT is used for delineation of fracture anatomy. In younger children, fractures involving the triradiate cartilage are prone to complications, particularly when displaced fragments are present. Premature fusion of the triradiate cartilage may lead to a shallow acetabulum and progressive hip instability.120 This complication is more common in children under age 10 years at the time of fracture.
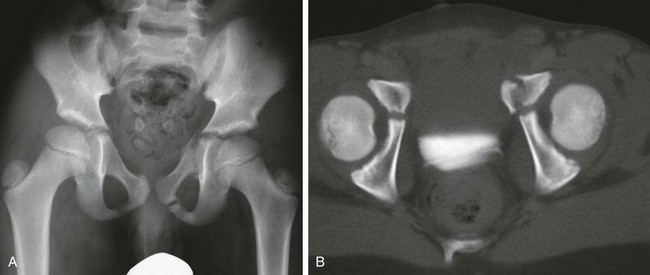
e-Figure 143-48 Acetabular fracture in an 8-year-old boy struck by an automobile.
A, Anteroposterior radiograph of the pelvis shows left pubic fractures and widening of the left triradiate cartilage. B, The axial computed tomography scan shows the superior pubic ramus fracture to extend to involve the triradiate cartilage.
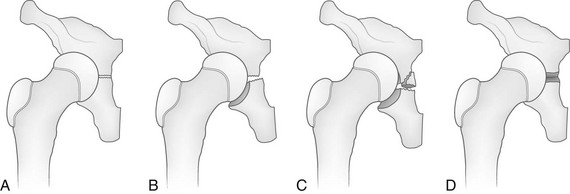
e-Figure 143-49 Types of triradiate cartilage fractures.
A, Normal triradiate cartilage. B, Salter-Harris I fracture. C, Salter-Harris II fracture. D, Salter-Harris V (compression) fracture. (From Scuderi G, Bronson MJ. Triradiate cartilage injury: report of two cases and review of the literature. Clin Orthop Relat Res. 1987;217:179-189.)
Treatment: The majority of pelvic fractures can be treated nonsurgically in children.116,125,126 Surgical intervention is most often needed for those children who require it for other injuries.118 However, in unstable or fracture-dislocations, open reduction and stabilization should be performed.125
Hip Dislocation
Overview: Traumatic hip dislocations are more common in adolescence, and the hip usually dislocates posteriorly. Unlike adult patients, these fractures are uncommonly associated with acetabular fractures.121
Etiology, Pathophysiology, and Clinical Presentation: In adolescents, hip dislocation is usually the sequela of significant trauma such as that sustained in motor vehicle accidents, whereas younger children may dislocate their hips with relatively minor trauma, usually falls.127 Fractures of the acetabulum and femoral head can occur at the same time. In children who suffer dislocations with minimal trauma, ligamentous laxity should be considered as a possible underlying condition such as that seen with Ehlers-Danlos syndrome, Larsen syndrome, and Down syndrome.
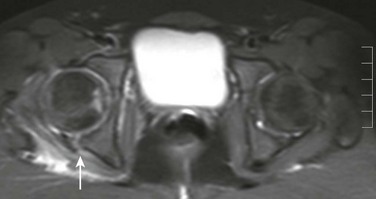
Imaging: As with most skeletal trauma, initial imaging evaluation should be radiographs (Fig. 143-50). If asymmetry of the hip joint persists after reduction, then further evaluation with either CT or MRI (e-Fig. 143-51) should be considered to evaluate for labral entrapment or interposed osteochondral fragments. Most hip dislocations are posterior related to dashboard impact in motor vehicle accidents. With a fracture of the acetabular wall or femoral head, cross-sectional imaging should also be obtained, as it could lead to alterations in patient management. Some have advocated performing a bone scan or MRI 2 weeks after reduction to look for abnormal uptake or signal in an attempt to predict epiphyseal osteonecrosis.128,129
Treatment: Most hip dislocations in children are reducible with gentle manipulation; however, closed reduction of the hip in the adolescent population carries a risk of epiphyseal osteonecrosis. Open reduction is necessary when closed reduction fails or when interposition of soft tissue or bone exists. Additional complications of hip dislocations include premature osteoarthritis and sciatic nerve injury. If the delay in reduction is greater than 6 hours, the risk of osteonecrosis is significantly increased.130,131
Slipped Capital Femoral Epiphysis
Etiology, Pathophysiology, and Clinical Presentation: Slipped capital femoral epiphysis (SCFE) is the most common hip disorder affecting the adolescent population.132 The physis is the weakest component of the proximal femur, and therefore, SCFE injuries are more common than isolated femoral neck fractures. SCFE is essentially a Salter-Harris I fracture of the proximal femoral physis. SCFE occurs more frequently in boys than in girls, tends to occur earlier in age in girls compared with boys, and occurs more commonly in African Americans. SCFE is usually idiopathic with a higher incidence in children who are clinically obese.132,133 Risk factors for SCFE include hypothyroidism, pituitary dysfunction, hypogonadism, and renal osteodystrophy.134,135 Hips within a radiation treatment field are at increased risk. When a child presents with bilateral symmetric SCFE or outside of the typical age range, an underlying disorder should be suspected.136
Approximately 25% of patients will have bilateral SCFEs, but the reported prevalence of bilateral SCFEs ranges from 20% to 80%.134,135 Approximately half of patients with bilateral SCFE present synchronously. Risk for contralateral SCFE is greatest in the first 2 years after initial diagnosis of SCFE.134
Children with SCFE usually present with hip, groin, or thigh pain. Symptoms may first occur after minor trauma. Up to one quarter of patients present with referred knee pain.136 Hip radiographs are therefore recommended in the adolescent with unexplained knee pain.
By definition, children with “acute” SCFE have had symptoms for less than 3 weeks and children with “chronic” SCFE (approximately 85% of cases) have had symptoms for greater than 3 weeks.137–139 Acute or subacute persistent hip pain in an adolescent should immediately raise suspicion for SCFE.
SCFE patients presenting with hip pain with an inability to walk are considered to have an unstable SCFE.140 SCFE patients who are able to walk are considered to have stable SCFE. This is an important clinical differentiation, since it affects timing of surgical intervention and prognosis.
Imaging: Radiography for suspected SCFE should include a pelvic anteroposterior and “frogleg” lateral view. Both hips should be included. Epiphyseal slips may occur in the medial direction, posterior direction, or both. The frogleg lateral view is helpful, since most epiphyseal slips occur in the posterior direction, which can be subtle on the anteroposterior view (Fig. 143-52). The anteroposterior view best assesses the degree of medial displacement, which is often subtle. Klein’s line is drawn parallel to the lateral margin of the femoral neck on the anteroposterior view. Normally, a small portion of the femoral head extends lateral to Klein’s line. When the femoral head does not extend lateral to Klein’s line, medial displacement should be suspected (see Fig. 143-52).141 Theoretically, Klein’s line can potentially be preserved if the SCFE is directly posterior without a medial component. The smaller the capital femoral epiphysis appears on the anteroposterior view, the greater is the degree of posterior slip.
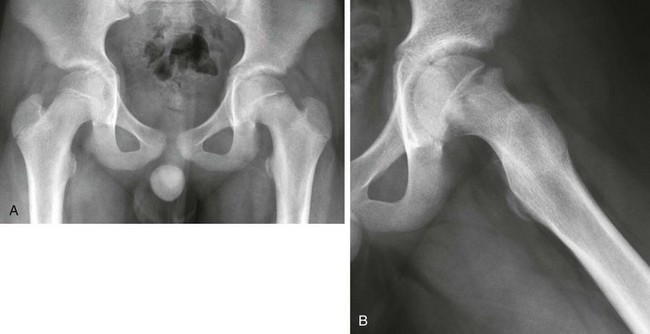
Figure 143-52 Slipped capital femoral epiphysis in an 11-year-old boy with left hip pain.
A, On the anteroposterior view, the growth plate of the left proximal femur is wide and indistinct. No portion of the left femoral head projects lateral to Klein’s line. The right proximal femur is normal. B, On the lateral view, malalignment of the femoral head and neck at the growth plate is better seen. The femoral head is displaced posteromedially relative to the femoral neck but is still in continuity.
With chronic slips, sclerosis may be seen in the medial femoral neck with bone remodeling (“buttressing”) in response to altered mechanics (e-Fig. 143-53). Physeal widening with juxtaphyseal-, epiphyseal-, and metaphyseal-based lucencies may be seen as well.
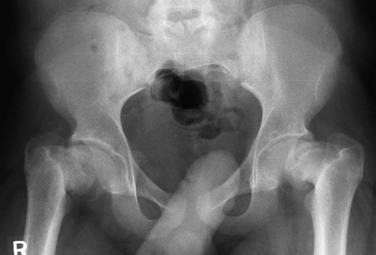
e-Figure 143-53 Advanced, chronic bilateral slipped capital femoral epiphyses at presentation in a 16-year-old boy.
The femoral heads are rotated medially with varus deformity. The femoral necks are sclerotic with buttressing medially.
CT and MRI may also be used to make the diagnosis of SCFE; however, usually radiographs suffice. Recent uses of MRI include detecting a preslip state, as evidenced by physeal widening with adjacent bone marrow edema and assessing cartilage loss.142 Joint effusions are invariably present on MRI, presumably due to posttraumatic inflammation related to altered mechanics and hip joint incongruity.
Treatment: In situ fixation with transphyseal screw is the treatment for SCFE. Prophylactic pinning of the contralateral hip may be performed in high-risk individuals. Timing of treatment is predicated on symptoms. Stable SCFEs can be electively pinned. Unstable SCFEs usually require urgent or emergent pinning, since they are at higher risk for epiphyseal osteonecrosis. Reduction of the capital femoral epiphysis is performed with extreme caution, since even minimal manipulation may disrupt the epiphyseal blood supply.
Complications after SCFE treatment include epiphyseal osteonecrosis (Fig. 143-54) and chondrolysis (e-Fig. 143-55). Epiphyseal osteonecrosis is more frequent after treatment of unstable SCFE. Chondrolysis used to complicate 5% to 10% of patients with SCFE, but the incidence has substantially lessened with newer fixation techniques.143 Chondrolysis is now rarely seen without accompanying epiphyseal osteonecrosis. Additional complication of SCFE is cam-type femoroacetabular impingement related to abnormal offset of the femoral head and neck junction (see Chapter 145).
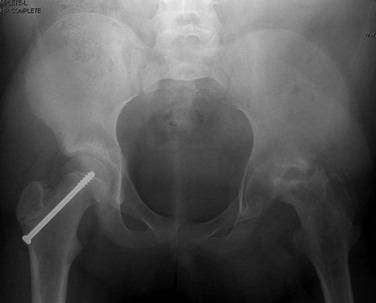
Figure 143-54 Complication of slipped capital femoral epiphysis.
Single anteroposterior radiograph of the pelvis in a 14-year-old girl demonstrates left femoral head fragmentation and collapse consistent with osteonecrosis. There are postsurgical changes of the right femur.
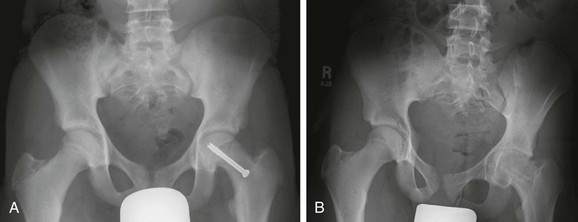
e-Figure 143-55 Chondrolysis after treatment of slipped capital femoral epiphysis (SCFE) in a 15-year-old boy.
A, Postoperative radiograph demonstrates pin fixation of left sided SCFE with normal hip joint space. B, One year later, uniform joint space narrowing of the left hip consistent with chondrolysis is seen.
Traumatic Injuries of the Knee
Etiology, Pathophysiology, and Clinical Presentation: Two types of distal femoral fractures have been described. Juvenile type injuries (age 2-10 years) are most common secondary to high energy trauma such as a motor vehicle accident, whereas adolescent type injuries are typically sports related.146 Salter-Harris type II fractures are the most common injury with usual displacement in the coronal plane, medial physeal separation, and associated medial collateral ligament sprain.146 Salter-Harris type fractures are more common in the distal femur compared with ligamentous injuries of the knee, particularly during periods of rapid growth.
Imaging: Initial evaluation begins typically with anteroposterior, lateral, and sunrise views of the knee. Sunrise views are usually not helpful in children under age 5 years, since the patella is undermineralized and patellar fractures are unusual in the first decade. Depending on the mechanism of injury, MRI or CT may be used in certain situations. MRI review of the knee in a child must include specific attention to the unfused physes as part of the search pattern.145 MRI will also be particularly useful in evaluating tendon and ligamentous injuries and bone contusions. With intraarticular fracture, lipohemarthrosis may be seen on cross-table lateral radiographs, MRI, or CT.147 This appearance is secondary to breech of the bone marrow during injury.
Most displaced Salter-Harris fractures of the distal femoral metaphysis are obvious on clinical examination and on radiographs; however, subtle distal femoral Salter-Harris injuries may be occult on radiography and not diagnosed until MRI is performed (e-Fig. 143-56).
e-Figure 143-56 Salter-Harris II fracture of the distal femur in a 12-year-old girl.
Proton density magnetic resonance imaging shows widening of the distal femoral growth plate (small black arrows) and bone marrow edema. A small metaphyseal fracture line is seen (white arrows). Periosteum is elevated (large black arrows).
Patella
Etiology, Pathophysiology, and Clinical Presentation: Fractures of the patella in skeletally immature patients are rare.148 Patellar sleeve fractures are more common and are often related to lateral patellar dislocation (see Chapter 145).149,150 Direct impaction injury is the most common cause of patellar fractures that are non-sleeve. Like the scaphoid, the patella has a recurrent blood supply. Avascular necrosis of the proximal fragment is a rare complication of transverse patellar fractures.
Imaging: Initial evaluation begins typically with anteroposterior and lateral views of the knee. The sunrise view is often challenging to obtain in children with patellar fractures, since this requires the child to flex the knee approximately 115 degrees. A Merchant view may be substituted which requires less knee flexion (45 degree flexion).
Non-sleeve patellar fractures may be simple (e-Fig. 143-57) or comminuted. Intraarticular involvement is important to define for surgical planning, and CT and MRI may be obtained for further evaluation. These fractures should be differentiated from a bipartite patella, which may fracture at the synchondrosis between the patellar body and the smaller superolateral ossification center (e-Fig. 143-58).
e-Figure 143-57 A 13-year-old boy with intra articular nondisplaced patellar fracture shown on radiography (arrow) (A) and computed tomography (B).
Computed tomography also demonstrates a medial patellar sleeve fracture.
e-Figure 143-58 A 13-year-old boy with bipartite patella (arrow).
Treatment: Patellar fractures are treated surgically, depending on several factors, including integrity of the extensor mechanism, degree of displacement, and articular extension. Patellar fractures are particularly difficult to treat conservatively because of issues with nonunion (from chronic traction related to the extensor mechanism), and premature osteoarthritis, since the articular surface is usually involved.
Tibial Tuberosity and Proximal Tibia
Etiology, Pathophysiology, and Clinical Presentation: Tibial tuberosity acute and chronic avulsion injuries, physeal equivalent Salter-Harris injuries of the tibial tuberosity, and tibial eminence anterior cruciate ligament avulsion fractures are covered in detail in Chapter 145.
The proximal tibia has an upside-down L–shaped physis and epiphysis. Both the tibial epiphysis and tuberosity are a single entity that fuses to the underlying tibia at approximately age 15 years in girls and age 17 years in boys.151,152 Therefore, any proximal tibial fracture extending to the tibial physis or tibial tuberosity physeal equivalent region should be considered a Salter-Harris fracture.
Imaging: Initial evaluation begins with anteroposterior and lateral views of the knee. Transverse fractures of the proximal tibia may appear innocuous but are technically Salter-Harris II fractures if they extend to the tibial tuberosity physeal equivalent zone (e-Fig. 143-59). Avulsion fractures that violate the tibial epiphysis or tibial tuberosity are Salter-Harris III fractures (e-Fig. 143-60). CT and MRI are useful to determine the three-dimensional orientation of the fracture and its involvement of the physis. MRI is more useful for fracture delineation when the fracture plane extends to the physeal equivalent region of the tibial tuberosity and may help evaluate if the fracture is a true Salter-Harris type fracture. Involvement of the physis and potential injury to the extensor mechanism can be assessed. MRI is preferred for tibial plateau fractures to evaluate for articular cartilage injury as well as coexisting meniscal and ligamentous injury.
Treatment: Salter-Harris fractures involving the proximal tibia weightbearing zone (tibial plateau) and Salter-Harris fractures involving the physeal equivalent region of the tibial tuberosity have different biomechanical challenges related to management. The tibial tuberosity is the insertion of the knee extensor mechanism, and therefore, tibial epiphyseal equivalent fractures in this region may be unstable with knee motion. Tibial plateau fractures are also unstable, but because these are loadbearing surfaces, when the overlying cartilage is violated, it should cause concern about posttraumatic osteoarthritis and coexisting internal derangement and meniscal tears of the knee. For these reasons, a low threshold exists for surgical fixation and immobilization for both tibial tuberosity and tibial epiphyseal Salter-Harris fractures in children.
Toddler Fractures
Etiology, Pathophysiology, and Clinical Presentation: Toddler fracture is typically seen between ages 9 months and 3 years and classically involves the distal third of the tibial diaphysis. The toddler fracture spectrum includes any fracture of the leg or foot in the toddler. The tibia is the most commonly fractured, followed by the fibula and cuboid bone.153
In the classic clinical toddler fracture history, the child’s leg is caught on an object, the leg twists, and the child falls. Some children present with pain and refusal to bear weight without a specific precipitating incident. On physical examination, the child will be point tender over the fracture.154 Toddler fractures are common once children begin to cruise and walk. The presence of a similar fracture prior to the cruising or ambulatory stage should raise greater concern for abuse.
Imaging: Toddler fractures may occur anywhere within the proximal metaphysis, diaphysis, or distal metaphysis of the tibia. The fracture line is oblique or spiral with no distraction or displacement (Fig. 143-61). Acutely, the fracture line may be very subtle. In some patients, follow-up radiographs are necessary to confirm the fracture (e-Fig. 143-62). Similarly, acute toddler fractures of the fibula and cuboid (e-Fig. 143-63) may be subtle. The healing process, with sclerosis at the margins of the fracture line and development of periosteal new bone, may increase the conspicuity of the fracture.
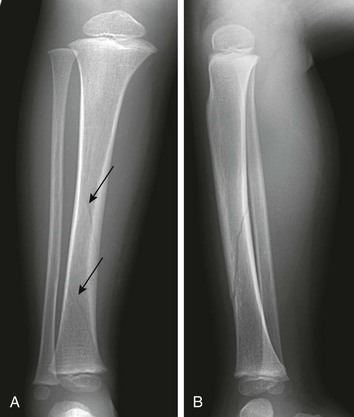
Figure 143-61 Anteroposterior (A) and lateral (B) views of a toddler fracture of the tibia in a 2-year-old girl.
The fracture line has a spiral course as shown by two apparent fracture lines on the anteroposterior view (arrows) and the intervening single fracture line on the lateral view. Slight soft tissue swelling is seen anterior to the fracture on the lateral view.
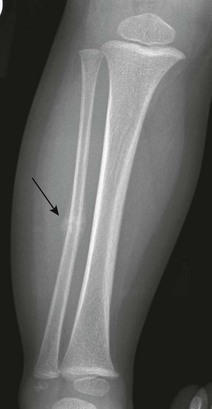
e-Figure 143-62 Fibular toddler fracture in a 19-month-old boy.
Callus is present (arrow) with a faint transverse fracture line.
Other Tibial and Fibular Fractures
Etiology, Pathophysiology, and Clinical Presentation: Most tibial fractures occur in the distal metadiaphysis.155 The distal tibial metaphysis is a common site for buckle fractures in young children. Salter-Harris fractures are also very common at the distal tibia and are covered in detail in the section on ankle fractures (see below). In older children, fractures of the tibial shaft are usually complete with a transverse or oblique fracture plane. The fibula is commonly fractured when a complete fracture of the tibial diaphysis occurs.156
Imaging: Nonphyseal and extraarticular fractures of the leg can be radiographed utilizing anteroposterior and lateral views. Once the physis of either ends of the leg are involved, dedicated osteoarticular imaging is necessary to comprehensively characterize physeal and intraarticular involvement, if present.
Whenever a medially located fracture or ligamentous disruption of the ankle occurs, radiographs of the remainder of the leg should be obtained to rule out a Maisonneuve fracture (e-Fig. 143-64).157
Treatment: Treatment of tibial fractures depends on the age of the child, type and location of the fracture, and degree of fracture displacement and angulation. Most tibial fractures can be managed nonoperatively provided that they are purely extraarticular and do not involve a physis. Fibular fractures, including those that have physeal involvement, invariably are treated nonsurgically, since the fibula is a relatively non–load-bearing bone.
Proximal tibial fractures are relatively less common compared with other sites of tibial fractures, but they are more prone to complications, including progressive varus deformity. Type III and IV Salter-Harris type fractures will require operative management. Maisonneuve fractures can be managed nonsurgically if the fracture is stable. With unstable fractures, the syndesmosis needs to be approximated and medial malleolus fixated. The associated proximal fibular fracture is treated conservatively and the deltoid ligament need not be directly repaired.158
Distal tibial Salter-Harris III fractures may be seen medially (e-Fig. 143-65) or laterally (juvenile Tillaux fracture; see below). The distal tibial metaphysis is one of the more common locations of Salter-Harris IV fractures (see e-Fig. 143-11 and e-Fig. 143-66).
Traumatic Injuries of the Ankle
Transitional Ankle Fractures (Juvenile Tillaux and Triplane)
Etiology, Pathophysiology, and Clinical Presentation: “Transitional” fractures occur in the early teen years when the distal tibial physis is nearing the time of fusion or is already partially fused. The distal tibial physis begins to fuse in the medial central physis. Fusion then proceeds medially and posteriorly (e-Fig. 143-67). The anterolateral portion of the physis is the last portion of the physis to close and thus is the plane of least resistance to fracture.159,160 The unique pattern of physeal fusion of the distal tibia leads to characteristic fracture patterns in the distal tibia.
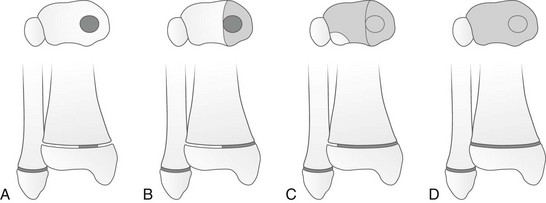
e-Figure 143-67 Diagram of the path of closure of the distal tibial physis.
Closure begins centrally (A) and proceeds medially (B) and then laterally (C) before fusion is complete (D). (Modified from Beaty JH, Kasser JR. Rockwood and Wilkins’ fractures in children. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.)
Juvenile Tillaux fracture is a Salter-Harris III fracture of the distal tibial physis and epiphysis. Juvenile Tillaux fractures probably represent an anterolateral avulsion of the distal tibial epiphysis pulled by the anterior tibiofibular ligament and anterior syndesmosis caused by forced external rotation.161 Triplane fracture is closely related to juvenile Tillaux fracture; however, there is an additional plane of fracture of the distal tibial metaphysis, usually coronal in orientation (Fig. 143-68). The three planes of a triplane fracture are thus: (1) a sagittal fracture through the epiphysis, (2) a transverse fracture through the physis, and (3) a coronal fracture through the metaphysis.162,163
Figure 143-68 Triplane fracture.
In a two-part triplane fracture, typically sagittal epiphyseal, transverse physeal, and coronal metaphyseal fracture lines are present. (Modified from Beaty JH, Kasser JR. Rockwood and Wilkins’ fractures in children. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.)
Imaging: For juvenile Tillaux fractures, the epiphyseal component of the fracture is usually sagittal or sagittal oblique, with the physeal portion of the fracture coursing through the anterolateral aspect of the distal tibial physis (Fig. 143-69). The vertical fracture line in juvenile Tillaux fractures is invariably located lateral with respect to anteromedial tibial physeal fusion, which is called “Kump’s bump” (see Fig. 143-69). This should be distinguished from epiphyseal fractures that are medially located (see e-Figs. 143-65 and 143-66). The concern with juvenile Tillaux fracture of the distal tibia is not possible premature fusion of the physis, since the physis is already fusing, but, rather, involvement of the articular surface. A significant gap or incongruity of the fracture fragments at the articular surface may portend an early progression to degenerative disease in the ankle.
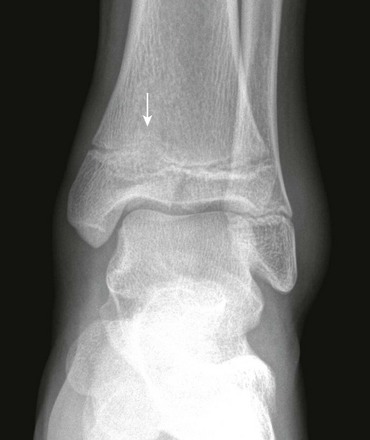
Figure 143-69 A 15-year-old boy with a juvenile Tillaux fracture.
Note the Salter-Harris III vertical fracture line is lateral to Kump’s bump (arrow), the site of initial distal tibia physeal closure.
For triplane fractures, the epiphyseal and metaphyseal fracture planes may vary somewhat from true orthogonal orientation. A classic triplane fracture has two parts; however, three-part and four-part variants occasionally occur (Fig. 143-70).164 Although the triplane fracture involves both the epiphysis and the metaphysis, it is not a true Salter-Harris IV fracture as the epiphyseal and metaphyseal fracture lines are not in continuity within the same plane. More correctly, the triplane fracture is a combination of a Salter-Harris II fracture and a Salter-Harris III fracture.
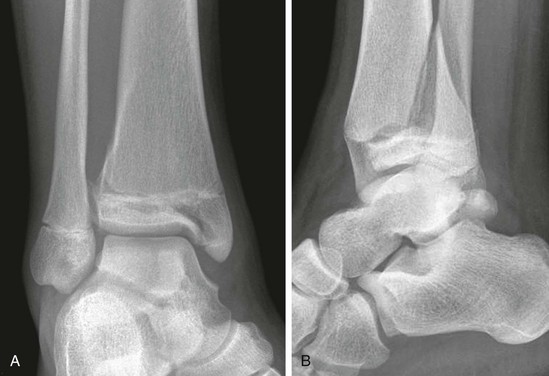
Figure 143-70 Anteroposterior (A) and lateral (B) views of a triplane fracture in an adolescent. (Courtesy Dr. B. H. Adler, Columbus, OH.)
Superficially, triplane fractures on anteroposterior view may mimic juvenile Tillaux fracture (see Fig. 143-70, A). If a Salter-Harris II fracture component is not visualized on the lateral view, CT may be helpful to distinguish these two fractures, since this will alter management.
Radiography is usually sufficient for delineating both juvenille Tillaux and triplane fractures. Sometimes, varus stress maneuvers may be necessary to delineate the epiphyseal fracture component related to juvenille Tillaux fractures. CT may be used in preoperative planning to measure displacement at the articular surface and to map the anatomy of the fracture planes (e-Fig. 143-71).165–169
Treatment: If the fracture line is wider than 2 mm, a transitional fracture will undergo operative fixation.161 Widening of the medial joint space may be a sign of fracture displacement.170 Nondisplaced fractures can be treated with immobilization.171 Juvenile Tillaux fractures are surgically fixed at the level of the epiphysis. Displaced triplane fractures will require surgical fixation of both the epiphyseal and metaphyseal fracture components.
Traumatic Injuries of the Foot
General Overview: Foot fractures account for approximately 5% to 8% of pediatric fractures.172 These fractures are generally uncommon in infants and toddlers given the significant cartilaginous composition of the foot during this age; the incidence of fractures increases with age as the foot becomes more ossified.172 The majority of pediatric foot fractures are isolated injuries.172
Etiology, Pathophysiology, and Clinical Presentation: Fractures of the talus are uncommon, compromising approximately 2% of pediatric foot fractures.172 Avulsion fractures are the most common, followed by osteochondral fractures, talar neck, then talar body fractures.173
The majority of talar neck and body fractures occur with high-energy trauma, usually from a fall or a motor vehicle accident.172 Complete fractures of the talar neck are seen in older children but are rare.
Younger children may have buckle fractures of the talar neck. The buckle is evident along the dorsal surface of the bone and may be very subtle.174 The talus is one location of the so-called tarsal toddler fracture. These fractures occur from compression of the talar neck against the anterior margin of the tibia with forced dorsiflexion.176
Peritalar dislocations, defined as dislocations of the subtalar and talonavicular joints, are uncommon and are usually the result of high-energy trauma. When a peritalar dislocation is present, additional fractures in the injured foot are common.175
Imaging: Anteroposterior, oblique, and lateral radiographs of the ankle are initially obtained. Given the complexity of some talar fractures, CT can be used to delineate fracture anatomy and articular involvement (e-Fig. 143-72). Because of the recurrent blood supply of the body of the talus, fractures may be complicated by osteonecrosis of the proximal pole. The dislocated talus is also at risk for subsequent development of osteonecrosis because of disruption of vascular supply. The presence of a subchondral lucency in the talar dome (Hawkins sign) seen approximately 2 months after injury indicates that the talar dome has an adequate blood supply, and therefore, the risk of developing osteonecrosis is low.172 Other significant complications include posttraumatic arthritis caused by malalignment.176
Calcaneal Fractures
Overview: Calcaneal fractures are uncommon, comprising only 2% of all pediatric foot fractures. Associated injuries are common.172 Toddler fractures of the calcaneus, which have been described in the literature, are uncommon.177
Etiology, Pathophysiology, and Clinical Presentation: Calcaneal fractures most commonly occur with jumps or falls from a large height and from motor vehicle accidents.172 The mechanism of injury is usually an axial loading force.178 Prior to apophyseal fusion, the posterior calcaneus may experience fractures involving the apophyseal physis and apophysis that can be classified by the Salter-Harris system.179
Imaging: Calcaneal fractures may have a variable degree of comminution and of depression of the superior margin of the calcaneus. The Böhler angle can be used to assess for calcaneal depression caused by the fracture. This angle is the posterior angle between two lines, one drawn tangent to the anterior process of the calcaneus and the highest point of the posterior subtalar articular surface, and the other drawn from the latter point to the superior margin of the posterior calcaneus. Normally, the Böhler angle measures 25 to 40 degrees in adults but is less in children.180 With depressed calcaneal fractures, the Böhler angle is decreased. Although the Essex-Lopresti classification system is widely used, it is important to differentiate between intraarticular and extraarticular fractures with reference to involvement of the subtalar joint.172 The majority of calcaneal fractures are intraarticular, and the intraarticular component is often best seen on a Harris view (Fig. 143-73).
CT is utilized for full delineation of calcaneal fracture anatomy. Subtle compression fractures of the calcaneal body are not infrequent in younger children. These injuries may be virtually impossible to diagnose acutely from radiographs unless a buckle of the margin of the bone exists. Follow-up radiographs will show an oblique band of sclerosis within the midcalcaneal body (e-Fig. 143-74).
Midfoot Injury
Overview: Isolated fractures of the cuneiform bones are rare. “Bunk bed” fractures of the cuboid have been reported (see “Bunk Bed Fracture”). Lisfranc injuries may occur throughout childhood but become more common in older children.
Etiology, Pathophysiology, and Clinical Presentation: The mechanism may be direct, from a falling object, or indirect, from forced plantar flexion, abduction, or both.172 Falls from a height account for the largest number of Lisfranc injuries in children.172 The Lisfranc joint, which courses between the distal tarsals and the metatarsals, is disrupted. Fractures may be seen within the bases of the metatarsals or within the cuneiforms. Fracture at the base of the second metatarsal is a consistent finding. Multiple metatarsal fractures and midtarsal bone injuries are more likely in children.2 These fractures may be chondro-osseous separations, which are seen as only as small slivers of bone and often occult with radiographs. Lisfranc injuries can be classified as homolateral, with the first metatarsal displaced in the same direction as the other four metatarsals, or divergent, with the first metatarsal displaced medially and the other metatarsals displaced laterally (e-Figs. 143-75 and 143-76). A fracture of the cuboid with a fracture of the second metatarsal base is compatible with significant tarsometatarsal (TMT) joint injury.
e-Figure 143-75 Lisfranc tarsometatarsal fracture–dislocations.
The two basic types are homolateral and divergent fracture–dislocations. A fracture is consistently seen at the base of the second metatarsal. Jagged lines represent additional potential sites of fracture. (From Rogers LF. Radiology of skeletal trauma. 3rd ed. New York: Churchill Livingstone; 2002.)
e-Figure 143-76 Lisfranc fracture-dislocation in a 15-year-old morbidly obese boy from a motor vehicle accident.
The injury is homolateral. The space between the first and second metatarsals is widened. The tarsal-metatarsal joint is completely disrupted. A fracture is present at the base of the second metatarsal as well as other metatarsals.
Imaging: Radiographic abnormalities may be subtle. Weightbearing views of the foot are necessary to accurately evaluate alignment of the forefoot and midfoot. Weightbearing will stress the TMT joint and may bring out subtle Lisfranc injuries. Any malalignment between the bases of the metatarsals or with their corresponding tarsal bones on any view should be regarded with suspicion. The midfoot should be closely evaluated for possibility of avulsion fractures. CT is used for confirmation of diagnosis and delineation of comorbid anatomy. Ultrasonography or MRI has been used to evaluate the Lisfranc ligament as well.183 Ultrasonography and CT are not first line studies for the investigation of TMT injuries. Their role is supplementary when initial radiographs are normal or when it is necessary to identify additional fractures or ligamentous injuries that may affect surgical planning.
Treatment: Nondisplaced or minimally displaced TMT dislocations may be treated conservatively. Patients with Lisfranc fracture–dislocations with more than minimal displacement are treated surgically.172 If closed reduction is not successful, percutaneous pin fixation may be employed for stabilization of the reduction. Stabilizing the fracture of the proximal second metatarsal is important.2
Forefoot Fractures
Overview: Metatarsal fractures are one of the most common pediatric foot injuries. Most fractures are nondisplaced or minimally displaced.178 If the fractures involve the base of the metatarsal, injury to the TMT joint must be considered. Buckle fractures of the phalanges and metatarsals are common in younger children. The most commonly injured metatarsal in younger children is the first metatarsal and in older children the fifth.184
Etiology, Pathophysiology, and Clinical Presentation: Phalangeal fractures usually occur from objects falling directly on the foot, particularly on the great toe.178 Salter-Harris fractures may affect the physis of these bones. Salter-Harris III fractures at the base of the proximal phalanx of the great toe may be mistaken for a bifid epiphysis, and vice versa. Buckle fractures of the phalanges and metatarsals are common in younger children. Often, adjacent bones are fractured together. Oblique or transverse fractures of the metatarsal shafts may be caused by dropped objects, falls, or twisting injuries. In severe twisting injuries in which the foot is caught, multiple adjacent metatarsals may be fractured.
Imaging: Dedicated radiographs in three planes of the injured phalanx is preferred rather than imaging the foot in its entirety. Fractures should be defined by its location and extent including: intraarticular or extraarticular extension, location within the phalanx (shaft, neck, or head), and the presence or absence of involvement of an open physes. True lateral radiographs of the isolated symptomatic toe are challenging to obtain because of overlap of adjacent toes but are nevertheless important to obtain for complete fracture assessment, and identifying toe dislocations that may be missed on only anteroposterior and oblique views.
Stubbed Great Toe Fracture
Overview: Stubbed great toe fractures are considered Salter-Harris I or II fractures of the base of the proximal or distal phalanx of the great toe until proven otherwise.185 These fractures of the distal phalanx of the great toe are unique given the relationship of the nail bed to the physis and potential for complications (osteomyelitis).
Etiology, Pathophysiology, and Clinical Presentation: The nail bed of the great toe is closely apposed to the physis of the distal phalanx of the digit. In fact, the skin is directly attached to the juxtaphyseal periosteum. After an injury, this relationship is thought to allow contamination of the underlying bone and physis.186 Therefore, any nail bed injury with associated physeal fracture should be considered an open fracture (Fig. 143-77).187
Imaging: Physeal widening of the terminal phalanx is the most common finding seen with stubbed toe fractures (see Fig. 143-77). Rarefaction and physeal widening can be related to the primary injury, related to superimposed osteomyelitis, or both and usually cannot be distinguished on the basis of radiography alone. If a high clinical concern for superimposed infection exists, MRI is necessary.
Bunk Bed Fracture
Etiologies, pathophysiology, and clinical presentation: A bunk bed fracture is a buckle fracture affecting the metatarsal bone of the first digit. This is most commonly seen in children 3 to 6 years of age.153,174
These fractures occur with a fall or a jump from a height onto a hard surface, with the prototypical injury occurring with a jump from the top bunk of a bunk bed onto a hardwood floor, landing in a tiptoe position.188 The child’s entire weight is placed on the first metatarsal (axial loading injury), leading to a buckle fracture. The bunk bed fracture is associated with ligamentous injury of at the TMT junction and is considered a Lisfranc variant.
Imaging: Radiography is the primary modality to evaluate these fractures (e-Fig. 143-78). Occasionally, the normal undulation of the physis of the first metatarsal may mimic a bunk bed fracture. This should not cause interruption or buckling of the metaphyseal cortex, however, as is seen with a fracture.
Fractures of the Fifth Metatarsal
Overview: Fractures at the base of the fifth metatarsal are common. Approximately 40% of all metatarsal fractures involve the fifth metatarsal.189 The types of fifth metatarsal fractures described in children include tuberosity avulsions, intraarticular, proximal metadiaphyseal, diaphyseal, and neck fractures.
Etiologies, Pathophysiology, and Clinical Presentation: The Jones fracture, originally described in the adult population, is a fracture of the proximal fifth metatarsal caused by repetitive inversion.172 The fracture is transversely oriented and approximately 1.5 cm distal to the tip of the fifth metatarsal tuberosity and extends into the adjacent intermetatarsal facet (e-Fig. 143-79). This should be differentiated from fifth metatarsal stress fractures, which are located greater than 2 cm distal with respect to the fifth metatarsal tuberosity.
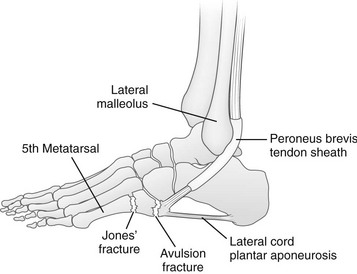
e-Figure 143-79 Fractures at the base of the fifth metatarsal.
These fractures result from inversion of the foot, which places tension on the peroneus brevis tendon and the lateral cord of the plantar aponeurosis. An avulsion fracture of the proximal bulbous tip of the metatarsal is to be distinguished from the classic Jones fracture, a transverse fracture at the base of the metatarsal located 1.5 to 2 cm distal to the tip of the metatarsal. (From Rogers LF. Radiology of skeletal trauma. 3rd ed. New York: Churchill Livingstone; 2002.)
Jones and stress fractures of the fifth metatarsal should be differentiated from fifth metatarsal tuberosity avulsion fractures. Fifth metatarsal tuberosity avulsion fractures are fifth digit TMT fractures related to forced flexion and inversion, often due to an unexpected step or falling when on stairs. Tuberosity avulsion fractures were previously thought to be caused by avulsion at the insertion of the peroneus brevis but now appear to be an avulsion from the origin of the abductor digiti minimi.172 In children, avulsions of the tuberosity are more common than true Jones fractures.
Imaging: Initial evaluation should include anteroposterior, lateral, and oblique views. A comparison view of the opposite side obtained in the same position will be confirmatory particularly when apophyseal avulsions are a consideration. The physis of the avulsed apophysis will appear widened. The key is to differentiate base of the fifth metatarsal fractures from the normal fifth metatarsal apophysis. The fifth metatarsal apophysis is longitudinally oriented (e-Fig. 143-80) whereas metatarsal stress and avulsion fractures are horizontally oriented. To add to the confusion of the growing appendicular skeleton, the apophysis of the fifth metatarsal may occasionally avulse (Fig. 143-81).
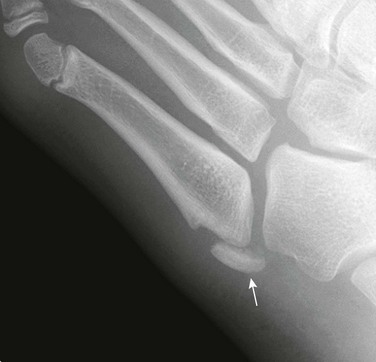
Figure 143-81 Avulsion of the fifth metatarsal apophysis (arrow) in a 13-year-old boy.
The apophysis is displaced from the underlying bone and retracted proximally.
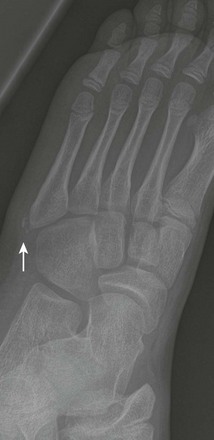
e-Figure 143-80 A 9-year-old girl with a normal longitudinally oriented fifth metatarsal apophysis (arrow).
The transverse fracture line of a tuberosity avulsion may extend through the apophysis and its physis (e-Fig. 143-82). Injuries to the base of the metatarsal can extend into the fifth metatarsal–cuboid joint or into the region between the fourth and fifth metatarsal bases. These fractures should be differentiated from more distally located Jones or stress fractures (e-Fig. 143-83) of the fifth metatarsal shaft.
Treatment: Apophyseal avulsions are treated conservatively with immobilization. With Jones fractures, instability can occur at the fracture site. As instability predisposes to delayed union or nonunion, true Jones fractures are usually surgically managed. The fracture site in Jones fracture also has a tenuous blood supply, and therefore, healing may be delayed.190
Neuropathic Injury
Etiologies, Pathophysiology, and Clinical Presentation: Causes of neuropathic injury in children include myelomeningocele, syrinx, congenital insensitivity to pain, and familial dysautonomia (Riley-Day syndrome).191–194 Superimposed infection is common.191 Neuropathic fractures and joints (“Charcot joints”) are much less common in pediatric patients than in adults.
Imaging: Neuropathic injury is characterized by the four Ds: (1) dislocation, (2) debris, (3) disorganization (deformity), and (4) density (sclerosis). Injuries fail to heal. Physeal fractures may occur and are often associated with subperiosteal hematoma and exuberant periosteal new bone. Findings may mimic tumor or infection. Repetitive trauma may lead to chronic physeal injury that may lead to premature fusion. Other sequelae include acro-osteolysis, joint deformity, joint dislocation, and epiphyseal osteonecrosis.191 Neuropathic fractures are most common in the lower extremities in the diaphysis or metaphysis (e-Fig. 143-84). Injury to the fingertips is caused by crush, thermal damage, and chewing (e-Fig. 143-85).
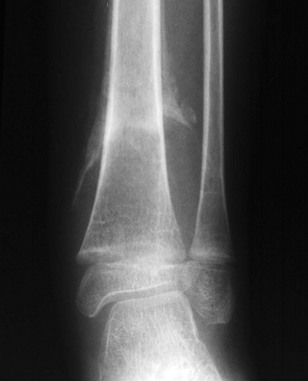
e-Figure 143-84 Neuropathic fracture of the distal tibial growth plate in an 11-year-old girl with myelomeningocele.
The patient presented with swelling. The distal tibial growth plate is widened. Periosteal new bone is elevated and interrupted because of subperiosteal hemorrhage, which was caused by delayed diagnosis and lack of immobilization.
Treatment: Treatment is variable and tailored to the underlying problem. Both nonsurgical management and surgical management are used for treating Charcot arthopathy. Nonsurgical treatment includes immobilization and reduction of stress. Examples of surgical management include removal of bony prominences, arthrodesis, plate and screw fixation, bone grafting, and reconstructive surgery.
Physical Agents
Etiologies, Pathophysiology, and Clinical Presentation: Frostbite injury is most common in children ages 5 to 10 years. The pathophysiologic mechanism of frostbite is not well defined, but a direct injury to chondrocytes may have occurred within the physis.195 The sequela in children is premature fusion of the physis and shortening of the involved digits. Bone fragmentation may be caused by ischemic damage.195 Secondary degenerative changes develop from direct articular injury and malapposition of joint surfaces in the affected digits.196
Imaging: Immediate radiographic findings occurring 1 week after exposure include soft tissue swelling and soft tissue gas, which is a poor prognostic sign.195 Radiographically identifiable osseous changes manifest weeks to months after injury. Characteristically, the thumb is spared as it is clenched at the time of exposure and covered by the other digits. Findings are most pronounced in the distal phalanges, occasionally affect the middle phalanges, and rarely involve the proximal phalanges or metacarpals (Fig. 143-86).196
Thermal and Electrical Injury
Etiologies, Pathophysiology, and Clinical Presentation: Thermal injuries are a significant cause of morbidity and mortality in children, most commonly affecting children under age 6 years.197 The hand is most commonly injured, followed by the face.197,198
Imaging: Injury from thermal burns is usually to the soft tissues, leading to contractures and ankylosis. Heterotopic bone contributes to ankylosis, as does direct thermal injury of a joint.199 Direct thermal injury to the physis may lead to premature fusion or growth disturbance (e-Fig. 143-87).199,200 Electrical injury may lead similar sequelae because of tissue heating. Osteolysis may occur.
Radiation
Etiologies, Pathophysiology, and Clinical Presentation: Irradiation of tissues may cause immediate or delayed injury to tissues.203 Radiation affects the immature skeleton by interfering with chondrogenesis and causing resorption of bone and cartilage at the physis, therefore affecting younger children who have greater growth potential.203 Therapeutic radiation doses may be sufficient to cause permanent injury to bone. With regard to growth retardation, microscopic changes are seen with a little as 300 centigrays (cGy) and growth retardation with as low as 400 cGy.203 Histologic recovery occurs with doses up to 1200 cGy; however, beyond this level, almost uniformly permanent cell damage and premature physeal fusion occur.203 Radiation-induced sarcomas are uncommon and occur years after therapy.203
Imaging: Radiation injury includes growth retardation with hypoplasia (e-Fig. 143-88), premature physeal fusion, SCFE, osteonecrosis, osteochondroma formation, and radiation-induced sarcoma.203–205
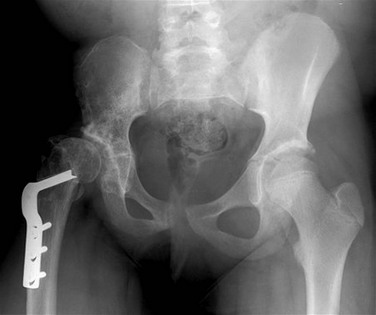
e-Figure 143-88 Sequelae of radiation therapy in an 11-year-old girl.
The patient underwent radiation therapy for Ewing sarcoma of the right ileum at age 3 years. Bones of the right hemipelvis are small. Premature fusion of the right proximal femoral growth plate and triradiate cartilage has occurred. A varus osteotomy of the proximal femur was performed to better center the right femoral head within the diminutive right acetabulum.
Metaphyseal sclerosis, fraying, and widening can be seen 1 to 2 months following long bone irradiation; this appearance resembles rickets.203 The affected bone may return to normal after 6 months. A dense metaphyseal band may also be seen shortly after therapy.203 SCFE can occur anywhere from 1 to 8 years after therapy, and therefore, long-term radiographic follow-up is warranted.203 Horizontal sclerotic lines parallel to the vertebral body endplates may be seen (bone in bone appearance) 9 to 12 months after irradiation to the spine. With higher doses of radiation (2000 to 3000 cGy), vertebral body scalloping, loss of vertebral body height, and scoliosis from asymmetric vertebral body growth have been reported.203
Ogden, JA. Skeletal injury in the child, 3rd ed. New York: Springer; 2000.
Ozonoff, MG. Pediatric orthopedic radiology, 2nd ed. Philadelphia, PA: Saunders; 1992.
Rogers, LF, Poznanski, AK. Imaging of epiphyseal injuries. Radiology. 1994;191:297–308.
Swischuk, L, Hernandez, JA. Frequently missed fractures in children (value of comparative views). Emerg Radiol. 2004;11:22–28.
Wilkins, KE. Principles of fracture remodeling in children. Injury. 2005;36(suppl 1):A3–A11.
References
1. Rogers, LF. Radiology of skeletal trauma, 3rd ed. New York, NY: Churchill Livingstone; 2002.
2. Ogden, JA. Skeletal injury in the child, 3rd ed. New York, NY: Springer; 2000.
3. Jacobsen, FS. Periosteum: its relation to pediatric fractures. J Pediatr Orthop. 1997;6:84–90.
4. Ozonoff, MB. Pediatric orthopedic radiology, 2nd ed. Philadelphia, PA: Saunders; 1992.
5. Beaty, JH, Kasser, JR. Rockwood and Wilkins’ fractures in children, 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2006.
6. Wilkins, KE. Principles of fracture remodeling in children. Injury. 2005;36(suppl 1):A3–A11.
7. Islam, O, Soboleski, D, Symons, S, et al. Development and duration of radiographic signs of bone healing in children. AJR Am J Roentgenol. 2000;175:75–78.
8. O’Conner, JF, Cohen, J. Dating fractures. In: Kleinman PK, ed. Diagnostic imaging in child abuse. Baltimore, MD: Williams & Wilkins; 1987:168–177.
9. Boles, CA, Hendrix, RW. Complications of fracture. In: Rogers LF, ed. Radiology of skeletal trauma. 3rd ed. Philadelphia, PA: Churchill Livingstone; 2002:231–271.
10. Lewallen, RP, Peterson, HA. Nonunion of long bone fractures in children: a review of 30 cases. J Pediatr Orthop. 1985;5:135–142.
11. Hensinger, RN. Complications of fractures in children. In: Ogden JA, ed. Skeletal injury in the child. 3rd ed. New York, NY: Springer; 2000:124–151.
12. Wilder, R, Berde, CB, Wolohan, M, et al. Reflex sympathetic dystrophy in children: clinical characteristics and follow-up of severity. J Bone Jone Surg Am. 1992;74:910–919.
13. Koch, E, Hofer, HO, Sialer, G, et al. Failure of MR imaging to detect reflex sympathetic dystrophy of the extremities. AJR Am J Roentgenol. 1991;156:113–115.
14. Korompilias, AV, Karantanas, AH, Lykissas, M, et al. Bone marrow edema syndrome. Skeletal Radiol. 2009;38:435–436.
15. Petje, G, Radler, C, Aigner, N, et al. Treatment of reflex sympathetic dystrophy in children using a prostacyclin analog. Clin Orthop Relat Res. 2005;433:178–182.
16. Ecklund, K, Jaramillo, D. Patterns of premature physeal arrest: MR imaging of 111 children. AJR Am J Roentgenol. 2002;178:967–972.
17. Peterson, HA. Partial growth plate arrest and its treatment. J Pediatr Orthop. 1984;4:246–258.
18. Loder, Randall, T, Swinford, A, et al. The use of helical computed tomographic scan to assess bony physeal bridges. J Pediatr Orthop. 1997;17:356–359.
19. Sailhan, F, Chotel, F, Guibal, A, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14:1600–1608.
20. Whan, A, Breidahl, W, Janes, G. MRI of trapped periosteum in a proximal tibial physeal injury of a pediatric patient. AJR Am J Roentgenol. 2003;181(5):191.
21. Khoshhal, K, Kiefer, G. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13:47–58.
23. Alison, M, Axoulay, R, Tilea, B, et al. Imaging strategies in paediatric musculoskeletal trauma. Pediatr Radiol. 2009;29:S414–S421.
22. Swischuk, L, Hernandez, JA. Frequently missed fractures in children (value of comparative views). Emerg Radiol. 2004;11:22–28.
24. Hussain, H, Barnes, C. Pediatric skeletal trauma—plain film to MRI. Appl Radiol. 2007;36:24–33.
25. Rodriguez-Merchan, EC. Pediatric skeletal trauma. Clin Orthop Relat Res. 2005;432:8–13.
27. Mizuta, T, Benson, WM, Foster, BK, et al. Statistical analysis of the incidence of physeal injuries. J Pediatr Orthop. 1987;7:518–523.
26. Salter, RB, Harris, WR. Injuries involving the epiphyseal plate. J Bone Joint Surg Am. 1963;45:587–632.
28. Rogers, LF, Poznanski, AK. Imaging of epiphyseal injuries. Radiology. 1994;191:297–308.
29. Close, BJ, Strouse, PJ. MRI of physeal fractures of the adolescent knee. Pediatr Radiol. 2000;30:756–762.
30. Jaramillo, D, Shapiro, F, Hoffer, F, et al. Posttraumatic growth-plate abnormalities: MR imaging of bony-bridge formation in rabbits. Radiology. 1990;175:767–773.
31. Jaramillo, D, Hoffer, FA, Shapiro, F, et al. MR imaging of fractures of the growth plate. AJR Am J Roentgenol. 1990;155:1261–1265.
32. Shapiro, F. Epiphyseal growth plate fracture-separations: a pathophysiologic approach. Orthopedics. 1982;5:720–736.
33. Bishop, JY. Pediatric shoulder trauma. Clin Orthop. 2005;432:41–48.
34. Bishop, JY, Flatow, EL. Pediatric shoulder trauma. Clin Orthop Relat Res. 2005;432:41–48.
35. Caviglia, H, Garrido, CP, Palazzi, FF, et al. Pediatric fractures of the humerus. Clin Orthop Rel Res. 2005;432:49–56.
36. Grissom, LE, Harcke, HT. Infant shoulder sonography: technique, anatomy and pathology. Pediatr Radiol. 2001;31:863–868.
37. Sherr-Lurie, N, Bialik, GM, Ganel, A, et al. Fractures of the humerus in the neonatal period. Isr Med Assoc J. 2011;13:363–365.
38. Levine, B, Pereira, D, Rosen, J. Avulsion fractures of the lesser tuberosity of the humerus in adolescents: review of the literature and case report. J Orthop Trauma. 2005;19:349–352.
39. Shibuya, S, Ogawa, K. Isolated avulsion fracture of the lesser tuberosity of the humerus. Clin Orthop Relat Res. 1986;211:215–218.
40. Donnelly, L, Helms, CA, Bisset, GS, III. Chronic avulsive injury of the deltoid insertion in adolescents: imaging findings in three cases. Radiology. 1999;211:233–236.
41. Shaw, BA, Murphy, KM, Shaw, A, et al. Humerus shaft fractures in young children: accident or abuse? J Pediatr Orthop. 1997;17:293–297.
42. Binder, H, Schurz, M, Aldrian, S, et al. Physeal injuries of the proximal humerus: long-term results in seventy two patients. Int Orthop. 2011;35:1497–1502.
43. Pahlavan, S, Baldwin, KD, Pandya, NK, et al. Proximal humerus fractures in the pediatric population: a systematic review. J Child Orthop. 2011;5:187–194.
44. Rab, GT, Grottkau, BG. Operative treatment of children’s fractures and injuries of the physis. In: Chapman MW, ed. Orthopedic surgery. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:4175–4194.
45. Shrader, MW. Pediatric supracondylar fractures and pediatric physeal elbow fractures. Orthop Clin North Am. 2008;39:163–171.
46. John, SD, Wherry, K, Swischuk, LE, et al. Improving detection of pediatric elbow fractures by understanding their mechanics. Radiographics. 1996;16:1443–1460.
47. Brubacher, JW, Dodds, SD. Pediatric supracondylar fractures of the distal humerus. Curr Rev Musculoskelet Med. 2008;1:190–196.
48. Girdany, BR, Golden, R. Centers of ossification of the skeleton. Am J Roentgenol Radium Ther Nucl Med. 1952;68:922–924.
49. Patel, B, Reed, M, Patel, S. Gender-specific pattern differences of the ossification centers in the pediatric elbow. Pediatr Radiol. 2009;39:226–231.
50. Norell, HG. Reontgenologic visualization of extracapsular fat: its importance in diagnosis of traumatic injuries to elbow. Acta Radiol (Diagn). 1954;42:205.
51. Bledsoe, RC, Izenstark, JL. Displacement of fat pads in diseases and injury of the elbow: a new radiographic sign. Radiology. 1959;73:717–724.
52. Zuazo, I, Bonnefoy, O, Tauzin, C, et al. Acute elbow trauma in children: role of ultrasonography. Pediatr Radiol. 2008;38:982–988.
53. Chapman, V, Grottkau, B, Albright, M, et al. MDCT of the elbow in pediatric patients with posttraumatic elbow effusions. AJR Am J Roentgenol. 2006;187:812–817.
54. Major, N, Crawford, S. Elbow effusions in trauma in adult and children: is there an occult fracture? AJR Am J Roentgenol. 2002;178:413–418.
55. Skaggs, DL, Mirzayan, R. The posterior fat pad sign in association with occult fractures of the elbow in children. J Bone Joint Surg Am. 1999;81:1429–1433.
56. Donnelly, LF, Klostermeier, TT, Klosterman, LA. Traumatic elbow effusions in pediatric patients: are occult fractures the rule? AJR Am J Roentgenol. 1998;171:243–245.
57. de Beaux, AC, Beattie, T, Gilbert, F. Elbow fat pad sign: implications for clinical management. J R Coll Surg Edinb. 1992;37:205–206.
58. Blumberg, SM, Kunkov, S, Crain, EF, et al. The predictive value of a normal radiographic anterior fat pad sign following elbow trauma in children. Pediatr Emerg Care. 2011;27:596–600.
59. Anderson, SE, Otsuka, NY, Steinbach, LS. MR imaging of pediatric elbow trauma. Semin Musculoskelet Radiol. 1998;2:185–198.
60. Beltran, J, Rosenberg, ZS, Kawelblum, M, et al. Pediatric elbow fractures: MRI evaluation. Skeletal Radiol. 1994;23:277–281.
61. Pudas, T, Hurme, T, Mattlia, K, et al. Magnetic resonance imaging in pediatric elbow fractures. Acta Radiol. 2005;46:636–644.
62. Griffith, JF, Roebuck, DJ, Cheng, JCY, et al. Acute elbow trauma in children: Spectrum of injury revealed by MR imaging not apparent on radiographs. AJR Am J Roentgenol. 2001;176:53–60.
63. Herman, MJ, Boardman, MJ, Hoover, JR, et al. Relationship of the anterior humeral line to the capitellar ossific nucleus: variability with age. J Bone Joint Surg Am. 2009;91:2188–2193.
64. Miles, KA, Finlay, DB. Disruption of the radiocapitellar line in the normal elbow. Injury. 1989;20:365–367.
65. Skaggs, DL. Elbow fractures in children: diagnosis and management. J Am Acad Orthop Surg. 1997;5:303–312.
66. Lins, RE, Simovitch, RW, Waters, PM. Pediatric elbow trauma. Orthop Clin North Am. 1999;30:119–132.
67. Omid, R, Choi, PD, Skaggs, DL. Supracondylar humeral fractures in children. J Bone Joint Surg Am. 2008;90:1121–1132.
68. Taylor, KA, Junewick, JJ. Simultaneous ipsilateral elbow and forearm fractures in children: a retrospective review. Emerg Radiol. 2002;9:314–316.
69. Kissoon, N, Galpin, R, Gayle, M, et al. Evaluation of the role of comparison radiographs in the diagnosis of traumatic elbow injuries. J Pediatr Orthop. 1982;15:449–453.
70. Gartland, JJ. Management of supracondylar fractures of the humerus in children. Surg Gynecol Obstet. 1959;109:145–154.
71. Silva, M, Wong, TC, Bernthal, NM. Outcomes of reduction more than 7 days after injury in supracondylar humeral fractures in children. J Pediatr Orthop. 2011;31:751–756.
72. Simanovsky, N, Lamdan, R, Mosheiff, R, et al. Underreduced supracondylar fracture of the humerus in children: clinical significance at skeletal maturity. J Pediatr Orthop. 2007;27:739–742.
73. Keenan, WN, Clegg, J. Variation of Baumann’s angle with age, sex, and side: implications for its use in radiological monitoring of supracondylar fracture of the humerus in children. J Pediatr Orthop. 1996;16:97–98.
74. Labelle, H, Bunnell, WP, Duhaime, M, et al. Cubitus varus deformity following supracondylar fractures of the distal humerus in children. J Pediatr Orthop. 1982;2:539–546.
75. Kraus, R, Wessel, L. The treatment of upper limb fractures in children and adolescents. Dtsch Arztebl Int. 2010;107:903–910.
76. Lawson, C, Midbrandt, T. Common elbow injuries in children: evaluation, treatment and clinical outcomes. Curr Opin Orthopaed. 2004;14:286–294.
77. Abzug, JM, Herman, MJ. Management of supracondylar fractures in children: current concepts. J Am Acad Orthop Surg. 2012;20:69–77.
78. Tejwani, N, Phillips, D, Goldstein, RY. Management of lateral humeral condylar fracture in children. J Am Acad Orthop Surg. 2011;19:350–358.
79. Song, KS, Kang, CH, Min, BW, et al. Internal oblique radiographs for diagnosis of nondisplaced or minimally displaced lateral condylar fractures of the humerus in children. J Bone Joint Surg Am. 2007;89:58–63.
80. Chapman, VM, Grottkau, BE, Albright, M, et al. Multidetector computed tomography of pediatric lateral condylar fractures. J Comput Assist Tomogr. 2005;29:842–846.
81. Blane, CE, King, TF, Jr., Andrew, JC, et al. Arthrography in the posttraumatic elbow in children. AJR Am J Roentgenol. 1984;143:17–21.
82. Kamegaya, M, Shinohara, Y, Kurokawa, M, et al. Assessment of stability in children’s minimally displaced lateral humeral condyle fracture by magnetic resonance imaging. J Pediatr Orthop. 1999;19:570–572.
83. Koh, KH, Seo, SW, Kim, KM, et al. Clinical and radiographic results of lateral condylar fracture of distal humerus in children. J Pediatr Orthop. 2010;30:425–429.
84. Klingele, KE, Kocher, MS. Little league elbow: valgus overload injury in the paediatric athlete. Sports Med. 2002;32:1005–1015.
85. Harrison, RB, Keats, TE, Frankel, CJ, et al. Radiographic clues to fractures of the unossified medial humeral condyle in young children. Skeletal Radiol. 1984;11:209–212.
86. Chambers, HG, Wilkens, KE. Fractures involving the medial epicondylar apophysis. In: Rockwood CA, Jr., Wildens KE, Beaty JH, eds. Fractures in children. 4th ed. Philadelphia, PA: JB Lippincott; 1998:801–819.
87. Osbahr, DC, Chalmers, PN, Frank, JS, et al. Acute, avulsion fractures of the medial epicondyle while throwing in youth baseball players: a variant of Little league elbow. J Shoulder Elbow Surg. 2010;19:951–957.
88. Wei, AS, Khana, S, Limpisvasti, O, et al. Clinical and magnetic resonance imaging findings associated with little league elbow. J Pediatr Orthop. 2010;30:715–719.
89. Kijowski, R, Tuite, MJ. Pediatric throwing injuries of the elbow. Semin Musculoskelet Radiol. 2010;14:419–429.
90. May, DA, Disler, DG, Jones, EA, et al. Using sonography to diagnose an unossified medial epicondyle avulsion in a child. AJR Am J Roentgenol. 2000;174:1115–1117.
91. Haxhija, EQ, Mayr, JM, Grechenig, W, et al. Treatment of medial epicondyle apophyseal avulsion injury in children. Oper Orthop Traumatol. 2006;18:120–134.
92. Leet, AI, Young, C, Hoffer, MM. Medial condyle fractures of the humerus in children. J Pediatr Orthop. 2002;22:2–7.
93. Jacobsen, S, Hansson, G, Nathorst-Westfelt, J. Traumatic separation of the distal epiphysis of the humerus sustained at birth. J Bone Joint Surg. 2009;91-B:797–802.
94. Rogers, LF, Rockwood, CA, Jr. Separation of the entire distal humeral epiphysis. Radiology. 1973;106:393–400.
95. Dormans, JP, Rang, M. Fractures of the olecranon and radial neck in children. Orthop Clin North Am. 1990;21(2):257–268.
96. Evans, MC, Graham, MK, Kerr, H. Olecranon fractures in children: Part 1: a clinical review: Part 2: a new classification system and management algorithm. J Pediatr Orthop. 1999;19:559–569.
97. Retting, AC, Wurth, TR, Mieling, P. Nonunion of olecranon stress fractures in adolescent baseball pitchers. Am J Sports Med. 2006;34:653–656.
98. Salter, RB, Zaltz, C. Anatomic investigations of the mechanism of injury and pathologic anatomy of “pulled elbow” in young children. Clin Orthop Relat Res. 1971;77:134–143.
99. Bretland, PM. Pulled elbow in childhood. Br Radiol. 1994;67:1176–1185.
100. Kim, MC, Eckhardt, BP, Craig, C, et al. Ultrasonography of the annular ligament partial tear and recurrent “pulled elbow. Pediatr Radiol. 2004;34:999–1004.
101. Rodriguez-Merchan, EC. Pediatric skeletal trauma: a review and historical perspective. Clin Orthop Relat Res. 2005;432:8–13.
102. Pretell-Mazzini, J, Carrigan, RB. Simultaneous distal radial fractures and carpal bone injuries in children: a review article. J Pediatr Orthop Bull. 2011;20:330–333.
103. Kay, RM, Skaggs, DL. The pediatric Monteggia fracture. Am J Orthop. 1998;27:606–609.
104. Wiley, JJ, Galey, JP. Monteggia injuries in children. J Bone Joint Surg Br. 1985;67:728–731.
105. Reckling, FW, Cordell, LD. Unstable fracture-dislocations of the forearm: the Monteggia and Galeazzi lesions. Arch Surg. 1968;96:999–1007.
106. Bowman, EN, Mehlman, CT, Lindsell, CJ, et al. Nonoperative treatment of both bone forearm shaft fractures in children: predictors of early radiographic failure. J Pediatr Orthop. 2011;31:23–32.
107. Ring, D, Jupiter, JB, Waters, PM. Monteggia fractures in children and adults. J Am Acad Orthop Surg. 1998;6:215–224.
108. Atesok, KL, Jupiter, JB, Weiss, AP. Galeazzi fracture. J Am Acad Orthop Surg. 2011;19:622–633.
109. Johnson, KL, Haigh, SF, Symonds, KE. MRI in the management of scaphoid fractures in skeletally immature patients. Pediatr Radiol. 2000;30:685–688.
110. Goddard, N. Carpal fractures in children. Clin Orthop Relat Res. 2005;432:73–76.
111. Huckstadt, T, Klitscher, D, Weltzien, A, et al. Pediatric fractures of the carpal scaphoid: a retrospective clinical and radiological study. J Pediatr Orthop. 2007;27:447–450.
112. Valencia, J, Leyva, F, Gomez-Bajo, GJ. Pediatric hand trauma. Clin Orthop Relat Res. 2005;432:77–86.
113. Nofsinger, CC, Wolfe, SF. Common pediatric hand fractures. Curr Opin Pediatr. 2002;14:42–45.
114. Deibert, MC, Aronsson, DD, Johnson, RJ, et al. Skiing injuries in children, adolescents and adults. J Bone Joint Surg Am. 1998;80:25–32.
115. Momiy, JP, Clayton, JL, Villalba, H, et al. Pelvic fractures in children. Am Surg. 2006;72:962–965.
116. Chia, JP, Holland, AJ, Little, D, et al. Pelvic fractures and associated injuries in children. J Trauma. 2004;56:83–88.
117. Leonard, M, Ibrahim, M, Mckenna, P, et al. Paediatric pelvic ring fractures and associated injuries. Injury. 2011;42:1027–1030.
118. Silber, JS, Flynn, JM. Changing patterns of pediatric pelvic fractures with skeletal maturation: Implications for classification and management. J Pediatr Orthop. 2002;22:22–26.
119. Silber, JS, Flynn, JM, Koffler, KM, et al. Analysis of the cause, classification, and associated injuries of 166 consecutive pediatric pelvic fractures. J Pediatr Orthop. 2001;21:446–450.
120. Schlickwei, W, Keck, T. Pelvic and acetabular fractures in childhood. Injury. 2005;36:A57–A63.
121. Quick, TJ, Eastwood, DM. Pediatric fractures and dislocations of the hip and pelvis. Clin Orthop Relat Res. 2005;432:87–96.
122. Guillamondegui, OD, Mahboubi, S, Stafford, PW, et al. The utility of the pelvic radiograph in the assessment of pediatric pelvic fractures. J Trauma. 2003;55:236–239.
123. Stewart, BG, Rhea, JT, Sheridan, RL, et al. Is the screening portable pelvis film clinically useful in multiple trauma patients who will be examined by abdominopelvic CT? Experience with 397 patients. Emerg Radiol. 2002;9:266–271.
124. Rees, MJ, Aickin, R, Kolbe, A, et al. The screening pelvic radiograph in pediatric trauma. Pediatr Radiol. 2001;31:497–500.
125. Schneidmueller, D, Wutzler, S, Kelm, A, et al. Pelvis injuries in childhood and adolescence: Retrospective analysis of 5 year data from a national trauma centre. Unfallchirurg. 2011;116:510–516.
126. Holden, CP, Holman, J, Herman, MJ. Pediatric pelvic fractures. J Am Acad Orthop Surg. 2007;15:172–177.
127. Schlonsky, J, Miller, P. Traumatic hip dislocations in children. J Bone Joint Surg Am. 1973;55:1057–1063.
128. Salisbury, RD, Eastwood, DM. Traumatic dislocation of the hip in children. Clin Orthop Relat Res. 2000;377:106–111.
129. Canale, ST. Fractures of the hip in children and adolescents. Orthop Clin North Am. 1990;21:341–352.
130. Herrera-Soto, J, Price, CT. Traumatic hip dislocations in children and adolescents: pitfalls and complications. J Am Acad Orthop Surg. 2009;17:15–21.
131. Mehlman, CT, Hubbard, GW, Crawford, AH, et al. Traumatic hip dislocations in children: Long-term follow-up of 42 patients. Clin Orthop. 2000;376:68–79.
132. Restrepo, R, Reed, MH. Impact of obesity in the diagnosis of SCFE and knee problems in obese children. Pediatr Radiol. 2009;39:S220–S225.
133. Brenkel, IJ, Dias, JJ, Davies, TG, et al. Hormone status in patients with slipped capital femoral epiphysis. J Bone Joint Surg Br. 1989;71:33–38.
134. Loder, RT, Aronson, DD, Greenfield, ML. The epidemiology of bilateral slipped capital femoral epiphysis: a study of children in Michigan. J Bone Joint Surg Am. 1993;75:1141–1147.
135. Loder, RT, Wittenberg, B, DeSilva, G. Slipped capital femoral epiphysis associated with endocrine disorders. J Pediatr Orthop. 1995;15:349–356.
136. Boles, CA, El-Khoury, GY. Slipped capital femoral epiphysis. Radiographics. 1997;17:809–823.
137. Mannoff, EM, Banffy, MB, Winnell, JJ. Relationship between body mass index and slipped capital femoral epiphysis. J Pediatr Orthop. 2005;25:744–746.
138. Loder, RT, Aronson, D, Dobbs, MB, et al. Slipped capital femoral epiphysis. J Bone Joint Surg Am. 2000;82:1170–1188.
139. Tins, B, Cassar-Pullicino, V, Mcall, I. The role of pretreatment MRI in established cases of slipped capital femoral epiphysis. Eur J Radiol. 2009;70:570–578.
140. Loder, RT. Unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2001;21:694–699.
141. Klein, A, Joplin, RJ, Reidy, JA, et al. Roentgenographic features of slipped capital femoral epiphysis. AJR Am J Roentgenol. 1951;66:361–374.
142. Miese, FR, Zilkens, C, Holstein, A, et al. Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol. 2011;52:106–110.
143. Vrettos, BC, Hoffman, EB. Chondrolysis in slipped upper femoral epiphysis: long-term study of the aetiology and natural history. J Bone Joint Surg Br. 1993;75:956–961.
144. Strouse, PJ. MRI of the knee: key points in the pediatric population. Pediatr Radiol. 2010;40:447–452.
145. Pai, DR, Strouse, PJ. MRI of the pediatric knee. AJR Am J Roentgenol. 2011;196:1019–1027.
146. Edwards, PH, Jr., Grana, WA. Physeal fractures about the knee. J Am Acad Orthop Surg. 1995;3:63–69.
147. Lee, JH, Weissman, BN, Nikpoor, N, et al. Lipohemarthrosis of the knee: a review of recent experiences. Radiology. 1989;173:189–191.
148. Khanna, G, El-Khoury, GY. Sleeve fracture at the superior pole of the patella. Pediatr Radiol. 2007;37:720–723.
149. Ray, JM, Hendrix, J. Incidence, mechanism of injury, and treatment of fractures of the patella in children. J Trauma. 1992;32:464–467.
150. Dai, LY, Zhang, WM. Fractures of the patella in children. Knee Surg Sports Traumatol Arthosc. 1999;7:243–245.
151. McKoy, BE, Stanitski, CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34:397–403.
152. Dupuis, CS, Westra, SJ, Makris, J, et al. Injuries and conditions of the extensor mechanism of the pediatric knee. Radiographics. 2009;29:877–886.
153. Blumberg, K, Patterson, RJ. The toddler’s cuboid fracture. Radiology. 1991;179:93–94.
154. Dunbar, JS, Owen, HF, Nogrady, MB, et al. Obscure tibial fracture of infants—the toddler’s fracture. J Can Assoc Radiol. 1964;15:136–144.
155. Heinrick, S. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ fractures in children. Philadelphia: Lippincott Williams & Wilkins; 2011:1077–1118.
156. Hart, ES, Luther, B, Grottkau, BE. Broken bones: common pediatric lower extremity fractures—part III. Orthop Nurs. 2006;25:390–407.
157. Kalyani, BS, Roberts, CS, Giannoudis, PV. The Masionneuve injury: a comprehensive review. Orthopedics. 2010;33:196–197.
158. Stufkens, SA, van den Bekrom, MP, Doornberg, JN, et al. Evidence-based treatment of maisonneuve fractures. J Foot Ankle Surg. 2011;50:62–67.
159. Kleiger, B, Mankin, HJ. Fracture of the lateral portion of the distal tibial epiphysis. J Bone Joint Surg. 1964;46:25–32.
160. Stefanich, RJ, Lozman, J. The juvenile Tillaux fracture. Clin Orthop Relat Res. 1986;210:219–227.
161. Kay, RM, Matthys, GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9:268–278.
162. Benson M, Fixsen J, Macnicol M, et al, eds. Children’s orthopedics and fractures, 3rd ed, London, UK: Springer-Verlag, 2010.
163. Ho-Fung, V, Pollock, A. Triplane fracture. Pediatr Emerg Care. 2011;27:70–72.
164. Spiegel, PG, Mast, JW, Cooperman, DR, et al. Triplane fractures of the distal tibial epiphysis. Clin Orthop Relat Res. 1984;188:74–89.
165. Horn, BD, Crisci, K, Krug, M, et al. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21:162–164.
166. Cutler, L, Molloy, A, Dhukuram, V, et al. Do CT scans aid assessment of distal tibial physeal fractures? J Bone Joint Surg Br. 2004;86:239–243.
167. Brown, SD, Kasser, JR, Zurkowski, D, et al. Analysis of 50 tibial triplane fractures using CT with multiplanar reconstruction. AJR Am J Roentgenol. 2004;183:1489–1495.
168. Kim, JR, Song, KH, Song, KJ, et al. Treatment outcomes of triplane and Tillaux fractures of the ankle in adolescence. Clin Orthop Surg. 2010;2:34–38.
169. Happamaki, W, Kiuru, MJ, Koskinen, SK. Ankle and foot injuries: analysis of MDCT findings. AJR Am J Roentgenol. 2004;183:615–622.
170. Gourineni, P, Gupta, A. Medial joint space widening of the ankle in displaced Tillaux and triplane fractures in children. J Orthop Trauma. 2011;25:608–611.
171. Schnetzler, KA, Hoerschemeyer, D. The pediatric triplane ankle fracture. J Am Acad Orthop Surg. 2007;15:738–747.
172. Kay, RM, Tang, CW. Pediatric foot fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9:308–319.
173. Jensen, I, Wester, JU, Rasmussen, F, et al. Prognosis of fracture of the talus in children. 20 (7-34)-year follow-up of 14 cases. Acta Orthop Scand. 1994;65:398–400.
174. John, SD, Moorthy, CS, Swischuk, LE. Expanding the concepts of the toddler’s fracture. Radiographics. 1997;17:367–376.
175. Dimentberg, R, Rosman, M. Peritalar dislocations in children. J Pediatr Orthop. 1993;13:89–93.
176. Eberl, R, Singer, G, Schalamon, J, et al. Fractures of the talus—differences between children and adolescents. J Trauma. 2010;68:126–130.
177. Laliotis, N, Pennie, BH, Carty, H, et al. Toddler’s fracture of the calcaneum. Injury. 1993;24:169–170.
178. Ribbans, WJ, Natarajan, R, Alavala, S. Pediatric foot fractures. Clin Orthop Relat Res. 2005;432:107–115.
179. Walling, AK, Grogan, DP, Carty, CT, et al. Fractures of the calcaneal apophysis. J Orthop Trauma. 1990;4:349–355.
180. Gross, RH. Fractures and dislocations of the foot. In: Rockwood CA, Jr., Wilkins KE, Beaty JH, eds. Fractures in children. 4th ed. Philadelphia: Lippincott-Raven; 1996:1429–1497.
181. Summers, H, Kramer, AP, Benirschke, SK. Pediatric calcaneal fractures. Orthop Rev (Pavia). 2009;30:e9.
182. Petit, CJ, Lee, BM, Kasser, JR, et al. Operative treatment of intraarticular calcaneal fractures in the pediatric population. J Pediatr Orthop. 2007;27:856–882.
183. Woodward, S, Jacobson, JA, Femino, JE, et al. Sonographic evaluation of Lisfranc ligament injuries. J Ultrasound Med. 2009;28:351–357.
184. Singer, G, Cichocki, M, Schalamon, J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am. 2008;90:772–776.
185. Swischuk, LE, Musculoskeletal: what is different in children? Tripped and jammed my toe. Emerg Radiol, 2010;17:1–2.
186. Pinckney, LE, Currarino, G, Kennedy, LA. The stubbed great toe: a cause of occult compound fracture and infection. Radiology. 1981;138:375–377.
187. Kensinger, DR, Guille, JT, Horn, BD, et al. The stubbed great toe: importance of early recognition and treatment of open fractures of the distal phalanx. J Pediatr Orthop. 2001;21:31–34.
188. Swischuk, LE. Musculoskeletal: what is different in children? Jumped off bed; foot pain. Emerg Radiol. 2009;16:171–174.
189. Arsal, Swiontkowski MF, eds. Skeletal trauma in children. WB Saunders: Philadelphia, PA, 1994:449–516.
190. Herrera-Soto, JA, Scherb, M, Duffy, MF, et al. Fractures of the fifth metatarsal in children and adolescents. J Pediatr Orthop. 2007;27:427–431.
191. Schulman, H, Tsodikow, V, Einhorn, M, et al. Congenital insensitivity to pain with anhidrosis (CIPA): the spectrum of radiological findings. Pediatr Radiol. 2001;31:701–705.
192. Laplaza, FJ, Turajane, T, Axelrod, FB, et al. Nonspinal orthopaedic problems in familial dysautonomia (Riley-Day Syndrome). J Pediatr Orthop. 2001;21:229–232.
193. Zimmerman, A, Law, C, Blount, J, et al. Neuropathic arthropathy of the elbow in a paediatric patient with myelomeningocele. Dev Neurorehabil. 2007;10:261–264.
194. Mobini, M, Javadzadeh, A, Forghanizadeh, J. Neuropathic osteoarthropathy in a patient with congenital insensitivity to pain. Arch Iran Med. 2009;12:599–602.
195. Crouch, C, Smith, WL. Long term sequelae of frostbite. Pediatr Radiol. 1990;20:365–366.
196. Carrera, GF, Kozin, F, Flaherty, L, et al. Radiographic changes in the hands following childhood frostbite injury. Skeletal Radiol. 1981;6:33–37.
197. D’Souza, AL, Nelson, NG, McKenzie, LB. Pediatric burn injuries treated in US emergency departments between 1990 and 2006. Pediatrics. 2009;124:1424–1430.
198. Choi, M, Armstrong, MB, Panthaki, ZJ. Pediatric hand burns: thermal, electrical, chemical. J Craniofac Surg. 2009;20:1045–1048.
199. Balen, PF, Helms, CA. Bony ankylosis following thermal and electrical injury. Skeletal Radiol. 2001;30:393–397.
200. Kumar, D, Papini, R, Tillman, RM. Partial growth plate fusion caused by burn. Burns. 2001;27:664–667.
201. Cox, SG, Rode, H, Darani, AN, et al. Thermal injury within the first 4 months of life. Burns. 2011;37:828–834.
202. Bingham, H. Electrical burns. Clin Plast Surg. 1986;13:75–85.
203. Williams, HJ, Davies, AM. The effect of x-rays on bone: a pictorial review. Eur Radiol. 2006;16:619–633.
204. Jaffe, N, Ried, HL, Cohen, M, et al. Radiation induced osteochondroma in long-term survivors of childhood cancer. Int J Radiat Oncol Biol Phys. 1983;9:665–670.
205. Fletcher, BD, Crom, DB, Krance, RA, et al. Radiation-induced bone abnormalities after bone marrow transplantation for childhood leukemia. Radiology. 1994;191:231–235.