Chapter 39 Sinus Node Dysfunction
In the early 1900s, Keith and Flack identified the sinus node as the region responsible for the activation of the heart.1 Laslett first suggested sinus node dysfunction as a cause of bradycardia in 1909, and during the 1950s and 1960s, Short, Ferrer, Lown, and others described the clinical spectrum of sinus node dysfunction commonly called sick sinus syndrome resting sinus bradycardia.2–5 Sinus node dysfunction can have multiple electrocardiographic manifestations, including sinus pauses, bradycardia-tachycardia syndrome, and inappropriate sinus node response to exercise (chronotropic incompetence).
Epidemiology
It can be difficult to differentiate sinus node dysfunction from physiological sinus bradycardia in a specific population. In a study of 50 young adult males, 24-hour ambulatory electrocardiographic monitoring revealed that 24% of the study group had transient heart rates less than 40 beats/min, pauses up to 1.7 seconds while awake, and 2.1-second pauses while asleep.6 Similarly, in a study of 50 young adult women, pauses from 1.6 to 1.9 seconds were observed.7 In older asymptomatic individuals, transient heart rates less than 40 beats/min and pauses of 1.5 to 2.0 seconds were observed in less than 2%.8 The decreased incidence of nocturnal bradycardia in this normal older adult population is probably caused by decrease in vagal tone that occurs with increasing age.
Sinus node dysfunction should be suspected when a patient describes symptoms of fatigue, syncope or presyncope, or exercise intolerance and is noted to have sinus bradycardia or pauses on the 12-lead electrocardiogram (ECG) or during Holter monitoring. In general, symptomatic sinus node dysfunction increases with age, with the incidence doubling between the fifth and sixth decades of life. In one study of approximately 9000 patients visiting a regional cardiac center in Belgium, Kulbertus et al estimated that the incidence of sinus node dysfunction is less than 5 per 3000 people older than 50 years of age.9 However, sinus node dysfunction is more commonly identified today because of an increased older adult population and increased physician awareness. In recent epidemiologic studies, sinus node dysfunction accounted for approximately 50% of the 300,000 new pacemakers implanted in the United States and 20% to 30% of the 900,000 new pacemakers implanted in Europe.10–12
Although more common in older adult patients, it is important to note that several specific younger patient groups can also have sinus node dysfunction. First, patients with congenital heart disease can have sinus node dysfunction. In 39 patients younger than 40 years of age who underwent pacemaker implantation for sinus node dysfunction at the Mayo Clinic, 64% had associated congenital heart disease.13 The most common condition was transposition of the great arteries corrected by a Mustard operation, since this procedure requires extensive atriotomies. Second, sinus node dysfunction is commonly observed after heart transplantation. Third, several familial forms of sinus node dysfunction have been identified and account for approximately 2% of patients who present with sinus node dysfunction.14–16 Bharati et al described a family with congenital absence of sinus rhythm. Several other investigators have described different forms of sinus node dysfunction that appeared to be genetically transmitted.14 Finally, sinus node dysfunction is observed in approximately 4% of patients after cardiac transplantation.17,18 Some, but not all, studies have found that a heart from a donor older than 40 years of age is associated with a higher incidence of sinus node dysfunction, necessitating permanent cardiac pacing.17,18 It appears that the incidence of permanent pacing after transplantation has decreased significantly with the widespread use of bicaval anastomoses rather than bi-atrial anastomoses.19
Anatomy
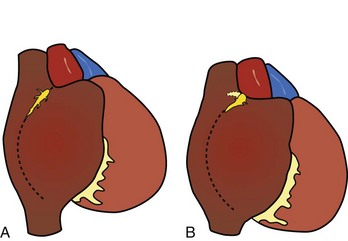
As described by Keith and Flack, the sinus node was shown to be lying in the terminal groove (sulcus terminalis), in the lateral part of the junction between the superior caval vein and the right atrium. This lateral position was endorsed by Koch and by most subsequent investigators.1,20–23 The horseshoe-shaped arrangement, with the node situated anteriorly and draped over the crest of the atrial appendage, as described by Hudson, is found in approximately 10% of hearts (Figure 39-1).24,25
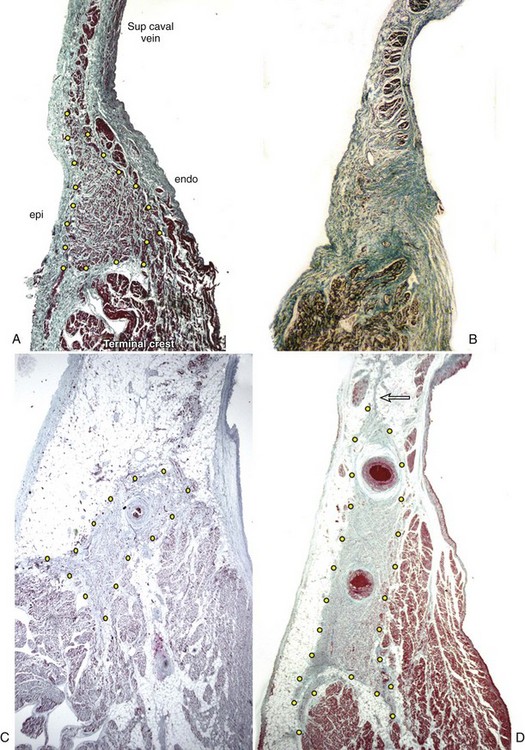
The shape of the node, most commonly, is like that of a tadpole, with the head section situated anterosuperiorly and the tapering tail extending for a variable distance inferiorly toward the entrance of the inferior vena cava.25,26 The fatty tissues of the terminal groove serve as the epicardial landmark, whereas the terminal crest (crista terminalis) in the anterolateral quadrant of the entrance of the superior vena cava is the endocardial landmark for the nodal head. In the adult heart, the nodal body is approximately 1 to 2 cm long, but the tail portion can extend considerably longer. In the subepicardium, the long axis of the node is parallel to the terminal groove, but the body and tail then gradually penetrate intramyocardially toward the subendocardium when traced inferiorly. Thus, while the nodal head is in the subepicardium of the terminal crest, the tail portion is close to the subendocardium.26
On histology, the node appears as a dense aggregation. The nodal cells are specialized myocytes that are histologically distinct from the ordinary myocardium of the atrial walls (Figure 39-2). They are small interlacing cells, less darkly stained, and with fewer myofibrils than in the neighboring ordinary myocytes. The specialized cells are associated with a fibrous tissue matrix. Ordinary myocardial cells are sometimes found scattered within the node. The nodal margins may be discrete, with fibrous separation from the atrial myocardium, or interdigitate through a short transitional zone. In the latter, tongues of nodal and transitional cells extend into the atrial myocardium. Prongs radiating from the nodal body are common. An anatomic study demonstrated 1 to 10 tongues of nodal tissue radiating mostly from the nodal body.26 The radiations penetrated the terminal crest in all directions, and some even extended into the muscular sleeve around the superior caval vein (see Figure 39-2). Another interesting observation from this study was the fragmentation of the tail portion of the node. In nearly two thirds of the nodes examined, the distal tail was traced to islands of nodal tissues among the fibro-fatty tissues and atrial myocytes in the subendocardium.
Innervation
The sinus node is the most densely innervated component of the cardiac conduction system.27,28 In the epicardium and in the environs of the node are collections of ganglion cells, which contribute vagal postganglionated nerve fibers to the node. The central part of the node is more densely populated with nerve fibers and bundles than are the peripheral regions.27
Arterial Supply
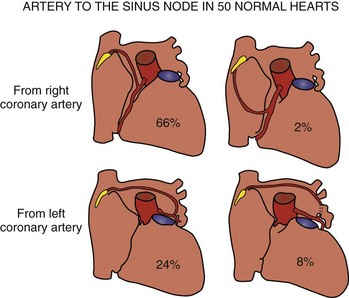
The artery supplying the node is a branch from the right coronary artery in 55% of hearts and from the left coronary artery in the remainder (Figure 39-3).29 When arising from the right coronary artery, it is usually the first atrial artery and ascends in the inter-atrial groove. Rarely, the artery arises near the acute margin from the lateral atrial artery to ascend the lateral wall of the right atrial appendage. The nodal artery is often a branch of the left anterior atrial artery when it arises from the left coronary system. In some hearts, it is a branch of the left lateral atrial artery. In both settings, it traverses the anterior wall of the left atrium and crosses the anterosuperior wall to reach the sinus node. The nodal artery approaches the node from an anterior direction in the majority of hearts but can also approach from a posterior direction or form an arterial circle around the cavoatrial junction.29 Typically, a prominent nodal artery passes centrally through the length of the nodal body and gives rise to small side branches along the way. In some hearts, the nodal artery branches as it approaches the node and several branches penetrate the nodal body. Occasionally, the artery passes along the side of the node and sends branches into the node. When the node is supplied by an arterial circle, the arteries enter the node at both ends.
Age-Related Changes
The sinus node displays marked histologic differences in infancy and in adulthood. In infants and in children, nodal cells predominate and the node appears relatively large compared with the bulk of the terminal crest. In the young individual, the node comprises nodal cells and fibrous tissue in nearly equal proportions.30 From about 30 years of age, fibrous tissue becomes more prominent, and the node appears smaller (see Figure 39-2).31 In the older adult, nodal cells decline, with increase in fibrosis. From 70 years onward, nodal cells may make up only 10% of the sinus node in patients who were in sinus rhythm up to death.31 However, as Shirashi and colleagues noted, the average volume of nodal cells in adults is 2.4 times more than in infants.32 This is mainly due to cellular hypertrophy. The same group found a 7.4 times increase in the volume of interstitial connective tissue in adult sinus nodes compared with that in infants. Another study using stains that are more specific for collagen tissue found an increase in collagen from infancy to adulthood, but the amount remains constant throughout adulthood.33 Contrary to general concepts, a detailed study has found that the loss of nodal cells is independent of the degree of arteriosclerosis.34 Fibrosis causing loss of continuity with the neighboring myocardium and increased fibrosis in the nodal approaches and internodal myocardium occur with increasing age. Using semi-quantitative assessment, Chow et al found dominance of sympathetic nerves initially in infancy, which gradually changed to co-dominance of sympathetic and parasympathetic nerves with increasing age.28
Sinus Node in Congenital Heart Malformations
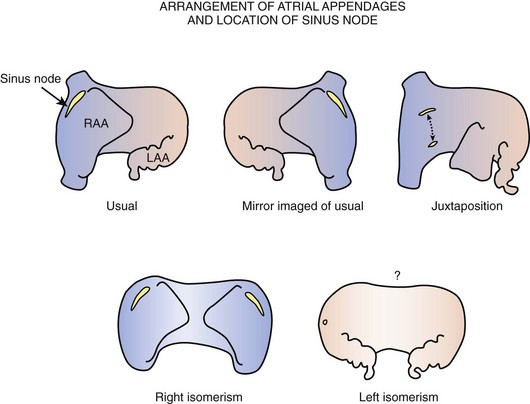
The majority of malformed hearts have the atrial chambers in their usual positions and the sinus node in its regular position. Abnormal positions of the sinus node have been found to occur in hearts with juxtaposition of the right appendage and in hearts with an atrial arrangement other than the usual type (Figure 39-4). Left juxtaposition exists when both atrial appendages lie to the left side of the arterial trunks rather than to either side at the base of the heart. In this malformation, the right appendage passes between the front of the atrial chambers and the great arteries. While the orifice of the superior vena cava remains in its usual position, the terminal crest is distorted, and the location of the sinus node is deviated. A study of six hearts with left juxtaposition showed a variable displacement of the node anteriorly (see Figure 39-4).35
Other malformations with abnormal positions of the sinus node are those that affect the arrangement of the atrial chambers. When atrial chambers are arranged in mirror-image fashion, the morphologically right atrium containing the sinus node is in the left-sided position. When bilateral right atrial appendages are present, as in right atrial isomerism, the sinus node is duplicated and is situated in both right and left positions.36,37 By contrast, when both atrial chambers are of left morphology, as in left atrial isomerism, the terminal crests are absent.37 Although, in this group, bilateral superior venae cavae can be present, the sinus node is not found in its usual position relative to either vena cava. When present, it is inferiorly displaced and smaller than usual. In some hearts, a remnant of specialized tissue is found in the posterior atrial wall near the atrioventricular (AV) junction, and in other hearts no nodal tissue can be identified.
Pathology
Many factors have been implicated in the etiology of sinus node dysfunction. Amyloid deposition within the sinus node and adjacent musculature is well recognized, as are anatomic findings of diffuse fibrosis or fibro-fatty replacement. Senile amyloidosis in older adults affects the atrial myocardium, including the approaches to the sinus node and the node itself. Marked loss of nodal cells associated with increased fibrosis or fatty changes of the atrial myocardium has been reported in sick sinus syndrome.33,38 Although usually seen in the older patient, these changes can also be seen in the young. Hypoplastic or atrophic sinus node may exist as a congenital anomaly that, with age-related loss of specialized cells, may progress to nodal dysfunction in later life or may be present in infancy (see Figure 39-2).39–41
Basic Electrophysiology of the Sinus Node
The structural and functional organization of the sinus node is quite complex, and significant differences are seen in the organization of the sinus node among species.42,43 The most extensive studies of the sinus node have been performed in rabbits. The rabbit node contains prototypical, small, structurally primitive pacemaker cells that are concentrated in the center; larger transitional latent pacemaker cells that are concentrated more peripherally; and intermingled, nonpacing atrial cells extending into the node from the atrial margins of the node in strands that are more prominent in the periphery. The cells within the node are relatively poorly coupled by gap junctions, and substantial interstitial tissue is interspersed among the fascicles of nodal cells.44 The resultant relatively poor intercellular communication slows the propagation of impulses from the central pacemaking regions toward the periphery of the node. In addition, the coupling of the transitional cells near the margins of the node with atrial myocardial cells is not a smooth continuum but consists of irregular junctions of interweaving strands of transitional cells and atrial cells extending into the interior of the node. Mapping of electrograms in and around the node has disclosed an apparent “multicentric” initiation of activation that likely represents irregular propagation to the atrial myocardium rather than simultaneous generation of impulses at separate sites. The node preferentially connects to the atrium in the superior aspects of the crista terminalis, and conduction block seems to exit the node in the direction of the atrial septum.
A dense representation of sympathetic and parasympathetic nerves and ganglia in the node ensures a sensitive autonomic responsiveness.45 With increasing vagal influence, the primary pacemaking site near the superior aspect of the node tends to migrate inferiorly, whereas an increasing adrenergic influence produces return to the primary dominant pacemaker sites in the superior region of the node. Detailed and sensitive analyses of P wave morphology and mapping of the sinus node indicate a dynamic shifting of pacemaker sites as well as changes in heart rate.
Action Potentials
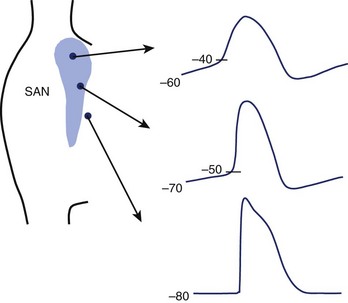
Small, primitive pacemaker cells in the interior of the node generate the dominant pacemaker potentials and show the least polarized maximum diastolic potentials (–60 to –40 mV), the most rapid rates of diastolic depolarization with smooth transition from end-diastole to the upstroke, and the slowest upstroke velocities (≈1 to 10 V/sec) (Figure 39-5).46 Latent pacemakers are concentrated more peripherally in the node. These cells are more polarized and show less rapid diastolic depolarization, a more abrupt transition from diastole to upstroke, and more rapid upstrokes. A continuous gradation of the properties of transitional cells—from the characteristics of primary pacemaker cells to the properties of highly polarized surrounding atrial cells with stable resting potentials close to –80 mV and upstroke velocities approaching 100 V/sec—can be seen. This gradation of properties is conditioned by cell coupling and electrotonic interaction as well as by the differing intrinsic properties of the myocytes within the node. Because of the electrotonic influence of atrial cells, the pacemaker capabilities of transitional cells are muted, whereas the more remotely connected interior cells are shielded from nonpacing atrial myocytes, allowing them to maintain pacemaker dominance.47 Separation of latent pacemaker cells near the atrial margins from the atrium and from the centrally located dominant pacemakers results in a faster intrinsic rate in latent pacemakers freed from the influence of nonpacemaking atrial cells. Cells in other parts of the atrium can also act as backup pacemakers, especially those in the inferior portions of the node near the coronary sinus. These pacemaker cells can respond appropriately to autonomic influence and, under abnormal conditions, can usurp control of the heart. The electrophysiological properties of these subsidiary pacemakers have not been as well characterized as those of the sinus node.
Recent studies indicate an influence of the fibroblasts within the node on pacemaker function and conduction.48 Inexcitable fibroblasts can couple with sinus node cells through gap junctions and depolarize the cells, reduce the rate of pacemaking, and impair conduction. Loss of sinus node myocytes associated with an increase in fibroblasts, collagen, and elastin has been implicated in sinus slowing and increased incidence of sinus node dysfunction and sinus node block with aging.
Detailed mapping by electrical and optical methods suggests that the sinus node is functionally connected to the atrial myocardium in discrete, relatively narrow sites, rather than diffusely along the margins of the node.49,50 At these sites, the propagating wavefront encounters a larger mass of the myocardium and source–sink mismatch that diminishes the safety factor for conduction. With certain conditions, including aging, that cause fibrosis or reduction of excitatory current, sinus node block may occur.
Currents
Sinus node pacemaker cells are relatively depolarized because of an absence or paucity of channels for the IK1 current.51,52 These channels are plentiful and open at negative membrane potentials in atrial and ventricular myocytes. They establish a dominance of K+ permeability in the resting state, thereby determining a resting potential approximating the K+
permeability in the resting state, thereby determining a resting potential approximating the K+ equilibrium potential (≈ –90 mV). The absence of these channels is most complete in the small, central pacemaker cells operating at diastolic potentials between –60 mV and –30 mV. In larger, more polarized transitional cells, IK1 may be present but reduced to varying degrees. The low upstroke velocities of these cells are related to a lack of operating sodium (Na+) channels, those channels that transmit the intense excitatory Na+ current in atrial and ventricular cells.52 The absence or paucity of this excitatory current is caused by a deficiency of the channels in individual myocytes and the depolarization of the myocytes during diastole to levels of membrane potential at which Na+ channels, if present, would be inactivated. The smallest cells may lack Na+ channels entirely. Larger transitional cells may contain Na+ channels and may operate at diastolic potentials at which Na+ channels can be activated to provide some excitatory Na+ current and more rapid upstrokes. The slow and diminutive upstrokes in the primary pacemaker cells are generated by the L-type Ca2+ current (ICaL), which serves as the trigger for release of Ca2+ by the sarcoplasmic reticulum and therefore the trigger for contraction in all cardiac cells but is the primary excitatory current in depolarized sinus nodal cells. This current is slower and far less intense than the Na+ current, accounting for the poor upstrokes and slow conduction within the node. The currents producing diastolic depolarization, which is the fundamental pacemaker potential, comprise a multitude of candidates, but no uniform consensus about this exists at present.53–57 The “funny” current If, encoded by HCN4, is a nonspecific cation current that is mainly an inward Na+ current activating relatively slowly at negative membrane potentials in the range of diastolic potentials.58 It becomes more intense at more negative membrane potentials. This current is well expressed in sinus nodal cells, responds appropriately to adrenergic and cholinergic stimulation, and thus is a plausible candidate to be an important pacemaker current. However, some studies have shown activation at more negative levels than the diastolic potentials of the primary pacemakers (below –60 mV) and a greater representation in peripheral latent pacemakers.
equilibrium potential (≈ –90 mV). The absence of these channels is most complete in the small, central pacemaker cells operating at diastolic potentials between –60 mV and –30 mV. In larger, more polarized transitional cells, IK1 may be present but reduced to varying degrees. The low upstroke velocities of these cells are related to a lack of operating sodium (Na+) channels, those channels that transmit the intense excitatory Na+ current in atrial and ventricular cells.52 The absence or paucity of this excitatory current is caused by a deficiency of the channels in individual myocytes and the depolarization of the myocytes during diastole to levels of membrane potential at which Na+ channels, if present, would be inactivated. The smallest cells may lack Na+ channels entirely. Larger transitional cells may contain Na+ channels and may operate at diastolic potentials at which Na+ channels can be activated to provide some excitatory Na+ current and more rapid upstrokes. The slow and diminutive upstrokes in the primary pacemaker cells are generated by the L-type Ca2+ current (ICaL), which serves as the trigger for release of Ca2+ by the sarcoplasmic reticulum and therefore the trigger for contraction in all cardiac cells but is the primary excitatory current in depolarized sinus nodal cells. This current is slower and far less intense than the Na+ current, accounting for the poor upstrokes and slow conduction within the node. The currents producing diastolic depolarization, which is the fundamental pacemaker potential, comprise a multitude of candidates, but no uniform consensus about this exists at present.53–57 The “funny” current If, encoded by HCN4, is a nonspecific cation current that is mainly an inward Na+ current activating relatively slowly at negative membrane potentials in the range of diastolic potentials.58 It becomes more intense at more negative membrane potentials. This current is well expressed in sinus nodal cells, responds appropriately to adrenergic and cholinergic stimulation, and thus is a plausible candidate to be an important pacemaker current. However, some studies have shown activation at more negative levels than the diastolic potentials of the primary pacemakers (below –60 mV) and a greater representation in peripheral latent pacemakers.
The If current is enhanced in the diastolic potential range of sinus node cells by activation of adenylate cyclase, for example, with adrenergic (sympathetic) stimulation, increasing cyclic adenosine monophosphate (cAMP) in the cytosol. Binding of cAMP to the channel increases the probability of the channel opening in the voltage range of –40 mV to –100 mV. Cholinergic (vagal) stimulation reduces cAMP, the If current, and the rate of pacemaking. Downregulation of HCN4 and If with prolonged atrial tachycardia may account for the impairment of sinus node function in atrial fibrillation and tachycardia.59,60
The importance of If in pacemaking in human sinus node had been validated by recent discoveries. Mutations in HCN4 impair sinus node pacemaking.56,61 Selective blockers of If have been demonstrated to slow sinus rate in human subjects.58 If has been demonstrated in sinus node cells from excised human sinus node.62
In the absence of IK1, the delayed rectifier K+ currents IKr and IKs, which are activated during the action potential, can play a role in the attainment of the maximum diastolic potential by providing the K+ permeability that would bring the transmembrane potential close to the K+ equilibrium potential at the end of the action potential when they are fully activated. As these currents deactivate in diastole, the membrane potential would drift toward the more positive equilibrium potentials of other major ions such a Na+, Ca2+, and Cl–. It has been argued that one or another of the delayed rectifier currents is the major pacemaker current.54 However, although IKs has an appropriate autonomic sensitivity, IKr does not. IKs is not prominent in the sinus nodes of all species.
It has recently been proposed that inward Na+ /Ca2+ exchange current (NCX) activated by spontaneous release of Ca2+ from sarcoplasmic reticulum is a major depolarizing current in late diastole in sinus node cells. This postulated “internal Ca2+ clock” is driven by high levels of phosphorylation of Ca2+-cycling proteins, modulated by adrenergic and cholinergic stimulation.57
Etiology
Sinus node dysfunction results from various conditions that have in common the capability to depress automaticity in and electrical conduction from the sinus node and perinodal and atrial tissues. These conditions can be intrinsic, resulting from structural damage to the sinus node, or extrinsic, caused by medications or systemic illnesses. The clinical and electrocardiographic manifestation of sinus node dysfunction is inappropriate sinus or atrial bradycardia.63–66 Some patients may also experience episodes of supraventricular tachycardias (tachycardia-bradycardia syndrome). Because the clinical manifestations of sinus node dysfunction can mimic normal physiological conditions (bradycardia) or can be caused by diseases that do not affect the sinus node (supraventricular tachycardias), the assessment and management of patients with suspected sinus node dysfunction can be challenging.67–69
Intrinsic Causes of Sinus Node Dysfunction
Intrinsic sinus node dysfunction is usually caused by degenerative processes involving the sinus node and the sinus node area. The syndrome is usually acquired but can rarely be familial.8 Sinus node dysfunction is present when inappropriate sinus bradycardia, pauses in sinus rhythm (sinus arrest), sinus node block, or a combination of these exist.70 The degenerative process and associated fibrosis may also involve the AV node and intraventricular conduction system; as many as 17% of patients with sick sinus syndrome have evidence of AV block and bundle branch block.71
Natural History
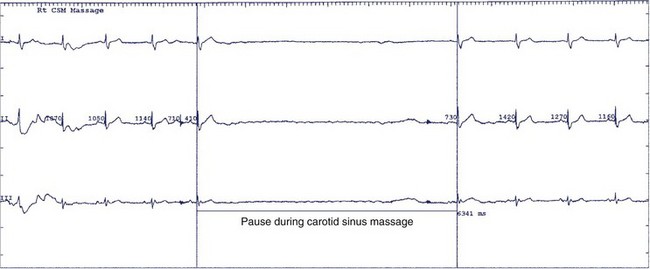
The natural history of sick sinus syndrome is one of spontaneous clinical improvement alternating with periods of clinical deterioration. Patients generally seek medical attention when they are symptomatic from bradycardia. In the majority of cases, the heart rate spontaneously increases, and symptoms diminish.72–74 However, the clinical course is not predictable. Even patients with more severe symptoms such as syncope may remain free of symptom recurrence for years, and slightly more than half do not experience another syncopal episode over a 4-year follow-up.74 No clear explanation exists for this erratic course of the syndrome. The autonomic nervous system may play an important role in the genesis of symptoms, especially in the trigger of syncope.74 The prevalence of abnormal responses to carotid sinus massage (pauses exceeding 3 seconds) and tilt table testing is significantly higher in patients who experience syncope than in those who do not, highlighting the contribution of abnormal neural reflexes in the pathophysiology (Figure 39-6).74,75
Although symptoms are common in patients with sick sinus syndrome, survival is usually not affected, even in patients who develop syncope.76 Death related directly to dysfunction of the sinus node occurs in less than 2% of patients over 6 to 7 years of follow-up.77,78 However, patients with sick sinus syndrome often have comorbid conditions that can shorten their lifespan. Coronary atherosclerosis is the most prevalent among these conditions, although myocardial ischemia and infarction, congestive heart failure, and advanced age are also common.79,80 In one report, patients with sick sinus syndrome had a 4% to 5% excess annual mortality in the first 5 years of follow-up compared with an age-matched and sex-matched population.81 However, the mortality in patients without other coexisting diseases at the time of diagnosis of sinus node dysfunction did not differ significantly from that observed in controls. Thus, although medical intervention and permanent cardiac pacing may be required to improve symptoms, no data exist supporting the claim that these management strategies improve survival.
Clinical Manifestations
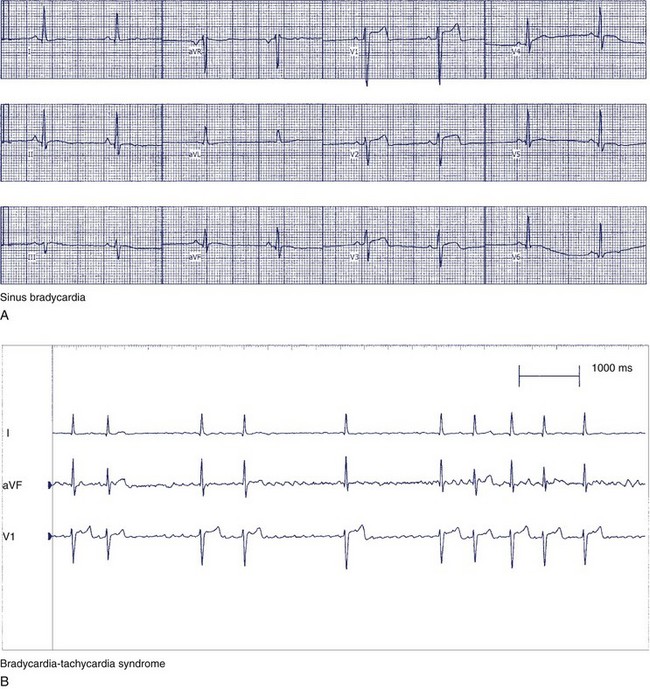
The clinical manifestations of sick sinus syndrome are caused by both bradycardia and tachycardia. In bradycardia-tachycardia syndrome, patients experience paroxysmal episodes of supraventricular tachycardia, which can be atrial tachycardia, atrial flutter or fibrillation, or re-entry tachycardia (Figure 39-7). More than one type of tachycardia may occur in the same patient. Episodes of rapid heart rate can lead to palpitations, angina, and syncope. Conversely, slowing of the heart rate without a compensatory increase in stroke volume leads to a reduction in cardiac output, which results in fatigue, weakness, lightheadedness, and dizziness.
Failure of the heart rate to increase appropriately with exercise (chronotropic incompetence) is a manifestation of sinus node dysfunction. Proposed definitions for chronotropic incompetence include failure to reach 85% of age-predicted maximum heart rate at peak exercise, failure to achieve a heart rate above 100 beats/min, or a maximal heart rate during exercise greater than two standard deviations below that of a control population.82–84 In patients with chronotropic incompetence, symptoms may be present only during activity.
In about 17% of patients with sick sinus syndrome, overt congestive heart failure may develop, which may be related or contributed to by the slow heart rate, loss of the atrial contribution to left ventricular filling in patients who develop atrial fibrillation, or loss of AV synchrony in patients with implanted ventricular pacing systems.73
Syncope or severe presyncope is one of the classical manifestations of sick sinus syndrome and occurs in about 25% of patients. In one prospective trial, the actuarial rates of syncope were 16% and 31% after 1 and 4 years of follow-up, respectively.73,74 Syncope is usually caused by excessive slowing of or transient pauses in sinus rhythm or by sinus exit block with an inadequate escape rhythm (Figure 39-8). In patients with bradycardia-tachycardia syndrome, overdrive suppression of the sinus node may occur when the tachycardia terminates, resulting in prolonged pauses and syncope. Use of antiarrhythmic medications in these patients, while successful in controlling the tachycardia, frequently leads to worsening of the bradycardia episodes with exacerbation of symptoms, and permanent cardiac pacing is required for rate support.
About one third to one half of patients with sick sinus syndrome experience episodes of supraventricular tachycardia.73,78 Published reports indicate that chronic atrial fibrillation occurs in about 11% of cases after a mean follow-up of 19 months, increases to 16% at 5 years, and to 28% at 10 years.73,74,84 Variables that have been associated with the development of chronic atrial fibrillation are age; left ventricular end-diastolic diameter; presence of valvular heart disease; and ventricular, rather than physiological, atrial or dual-chamber, pacing.84–86 Chronic atrial fibrillation is also more common in patients with tachycardia-bradycardia syndrome who have had paroxysmal atrial fibrillation. When atrial fibrillation develops, many patients previously symptomatic from bradyarrhythmia experience a substantial improvement, likely due to the increase in ventricular rate.87
Thromboembolic events, which occur in 3% to 9% of patients, may be a manifestation of sick sinus syndrome.73,74 In patients with bradycardia-tachycardia syndrome, the incidence increases to 24%.56 Ventricular pacing (as opposed to atrial or dual-chamber pacing) and the presence of pre-existing cerebrovascular disease are also associated with a higher thromboembolic event rate during follow-up.84–86 High-risk patients should be carefully identified and placed on long-term anticoagulation therapy. However, not all cerebrovascular accidents in these patients are caused by embolic events. Older adults with atherosclerotic cerebrovascular disease may have transient ischemic attacks or frank cerebral infarction if bradyarrhythmia or tachyarrhythmia is associated with a fall in cardiac output and reduction in cerebrovascular perfusion.
Atrioventricular Conduction in Sinus Node Dysfunction
At the time of diagnosis, up to 17% of patients with sick sinus syndrome have evidence of AV conduction system disease; although high-degree AV block is unusual, it is reported in only 5% to 10% of cases.88 The presence of AV conduction disease will affect therapeutic decisions, for example, those related to safety of concomitant antiarrhythmic drug use and pacemaker mode choice. Electrocardiographic and electrophysiological findings suggestive of significant AV conduction system involvement include a P-R interval greater than 240 ms, complete bundle branch block, development of type I second-degree AV block during atrial pacing at rates of 120 beats/min or less, H-V interval prolongation, and second-degree or third-degree AV block. During follow-up, AV conduction in patients with sick sinus syndrome usually remains stable.89–91 In one literature survey of 28 studies of atrial pacing, an annual incidence of second-degree and third-degree AV blocks of 0.6% per year was reported.89 A similarly low incidence (4 of 110 patients) was reported in a prospective trial of atrial versus ventricular pacing in patients with sick sinus syndrome (including those 70 years of age or younger with P-Q intervals ≤220 ms and those older than 70 years of age with P-Q intervals ≤260 ms); in addition, all patients had normal AV conduction at an atrial pacing rate of 100 beats/min at pacemaker implantation.86
The progression of AV conduction disease is thus usually slow and can be detected by careful clinical and electrocardiographic monitoring of these patients. Extrinsic influences such as exposure to antiarrhythmic agents or drugs that can block conduction in the AV node are more frequently responsible for worsening of AV conduction than is progressive degeneration within the conduction system.92–94
Sinus Node Dysfunction in Acute Myocardial Infarction
Sinus bradycardia occurs commonly in patients with acute myocardial infarction, especially those with inferior and posterior infarctions.95,96 The bradycardia is usually due to stimulation of the afferent vagus nerve terminals, which are more common in the inferior and posterior ventricular walls. This vagal response can be potentiated by pain and by the use of vagotonic medications such as morphine sulfate and can be associated with a vasodepressor response resulting in systemic hypotension. Intravenous atropine usually reverses the vagal effects associated with myocardial infarction.
In addition to autonomic nervous system influences, sinus bradycardia may also be caused by ischemia of the sinus node or atrial tissue, although this diagnosis is rarely made clinically. Sinus node ischemia is also more common in inferior wall myocardial infarction, since the sinus node is usually supplied by the right coronary artery. The clinical manifestations are sinus bradycardia in the majority of cases; however, bradycardia alternating with episodes of supraventricular tachycardia has been reported in up to 35% of patients.97 In the majority of cases, the sinus node dysfunction is temporary, and normal sinus rhythm returns during the hospitalization.97,98 Pacemaker implantation is rarely indicated. However, patients who experience alternation of bradycardia and tachycardia occasionally may require long-term antiarrhythmic therapy.97
Diagnostic Evaluation
Noninvasive Testing
The diagnosis of sinus nodal dysfunction is rarely made from a random ECG. If symptoms suggestive of sinus node dysfunction are frequent, Holter monitoring may be useful.77,99,100 It is important to have the patient wear a Holter monitor and document the symptoms in a diary for correlation of symptoms with the heart rhythm recorded at the time. In many cases, a Holter monitor can exclude sinus nodal dysfunction as the cause of symptoms if normal sinus rhythm is documented during dizziness, presyncope, or syncope. However, sinus bradycardia and sinus pauses may be recorded in asymptomatic individuals, reducing the specificity of these findings for the diagnosis.6,101
Event recorders are more useful than Holter monitors in patients with infrequent symptoms. Patient-activated models exist but are limited to patients who have symptoms prolonged enough to record the rhythm during an event. For patients who have little to no warning, a loop recorder event monitor can be used. These recorders can be activated as soon as symptoms occur or after the fact, since the last 45 seconds of ECG recording are “frozen.” Newer models that can be automatically triggered by bradycardia or tachycardia are useful in some patients. One study has demonstrated mobile cardiac outpatient telemetry (MCOT) to be even more useful than external event loop recorders in making an arrhythmic diagnosis.102 When an ECG diagnosis cannot be recorded by less invasive means, implantable loop recorders are useful in patients with recurrent symptoms suggestive of a bradyarrhythmia.
Exercise testing is of limited value in diagnosing sinus node dysfunction.70 However, it is useful in differentiating patients with chronotropic incompetence from those with resting bradycardia who are able to demonstrate a normal heart rate increase with exercise. Patients with sinus nodal dysfunction and chronotropic incompetence exhibit abnormal heart rate responses to exercise. The increase in heart rate at each stage of exercise may be less than normal, with a plateau seen below the maximum age-predicted heart rate. Other patients may achieve an appropriate peak heart rate during exercise but have slow heart rate acceleration in the initial stage of exercise or a rapid deceleration of heart rate in the recovery stage. These abnormal chronotropic responses can help identify the cause of exercise intolerance in some patients with sinus nodal dysfunction and help determine their pacemaker prescription.103
Autonomic testing of the sinus node includes various pharmacologic interventions and maneuvers to test reflex responses. An abnormal response to carotid sinus massage (pause greater than 3 seconds) indicates carotid sinus hypersensitivity and may suggest the presence of carotid sinus syndrome. This response may also occur in asymptomatic older adults.104 Heart rate response to the Valsalva maneuver (normally decreased) or upright tilt (normally increased) can also be used to verify that the autonomic nervous system is itself intact.
The most commonly used pharmacologic intervention in the evaluation of sinus node dysfunction is the determination of the intrinsic heart rate.105 A low intrinsic heart rate is consistent with abnormal intrinsic sinus nodal function. A normal intrinsic heart rate in a patient with known clinical sinus nodal dysfunction suggests abnormal autonomic regulation.
Invasive Testing
Pacing the atrium at rates faster than the inherent sinus rate is used to record the sinus node recovery time. A delay in the return of spontaneous pacemaker activity (overdrive suppression) is a normal finding immediately after cessation of rapid atrial pacing. In patients with sinus nodal dysfunction, however, the sinus node generally takes longer to recover. A better measurement is the corrected sinus nodal recovery time, which is obtained by subtracting the spontaneous sinus cycle length before pacing from the sinus nodal recovery time. Thus, a patient with an abnormally long sinus nodal recovery time could have a normal corrected sinus nodal recovery time if the resting heart rate is slow.106 The indirect measurement of the corrected sinus nodal recovery time reflects both sinus node conduction time and sinus automaticity and thus has some limitations. Indirect sinus nodal recovery time measurements can be confounded by sinus nodal entrance block during rapid atrial pacing with resultant shortening of the sinus nodal recovery time and by sinus nodal exit block after pacing, thereby prolonging the measured sinus nodal recovery time. An abnormal sinus nodal recovery time is not found in all patients with sinus nodal dysfunction, partly because of sinus nodal dysfunction not being a homogenous entity from the standpoint of pathology. Despite these limitations, the indirect corrected sinus nodal recovery time is employed frequently in the evaluation of sinus nodal function.
The sinus node conduction time, another commonly used invasive pacing test, is traditionally measured indirectly from the high right atrium.107,108 Several assumptions are used in the calculation, including sinus nodal automaticity not being affected by the premature beat, conduction in and out of the sinus node being equal, and the premature atrial beat not causing a shift in the principal pacemaker site. An additional limitation of the sinus node conduction time test is the need for a regular cycle length in sinus rhythm. Sinus arrhythmia may make the calculation of the sinus node conduction time by this method impossible.
Effectiveness of Pacing Therapy
Only a single published study, the THEOPACE (theophylline and permanent pacemaker) study, has evaluated the effectiveness of pacing therapy versus no therapy or pharmacologic therapy for preventing symptoms in patients with sinus node dysfunction. In this study, 107 patients with presumed sinus node dysfunction (>45 years of age, with a mean resting sinus rate <50 beats/min or intermittent sinus node block, or both, noted on diurnal ECG on two separate occasions, and symptoms thought to be secondary to sinus node dysfunction) were randomized to no treatment, oral theophylline, or dual-chamber rate-adaptive pacing.72 Patients with severe sinus node dysfunction, defined as symptomatic heart rates less than 30 beats/min or sinus pauses greater than 3 seconds, were excluded. After an average 18-month follow-up, syncope had occurred in 6% of patients who received pacing therapy and 17% and 23% in the theophylline and control arms, respectively. In all three groups, the incidence of atrial tachycardias was similar (26% to 28%). The THEOPACE study demonstrated that pacing therapy provides symptomatic benefit in patients with sinus node dysfunction. However, it is unlikely that pacing therapy confers a survival benefit, since natural history studies suggest that sinus node dysfunction by itself does not appear to be associated with an increased risk of death.76
Pacing Mode Choice
In patients with sinus node dysfunction, bradycardia can be prevented by single-chamber ventricular pacing (VVI mode), single-chamber atrial pacing (AAI mode), or dual-chamber pacing (DDD mode). Several randomized studies have evaluated the effects of pacing mode in patients with sinus node dysfunction. The first prospective study, initially published in 1994 with follow-up data presented in 1997 and 1998, evaluated 225 patients with sinus node dysfunction, who were randomized to single-chamber atrial pacing or single-chamber ventricular pacing.86 After a mean follow-up of 3.3 years, atrial pacing was associated with a significant decrease in thromboembolic events (atrial pacing: 5.5%; ventricular pacing: 17.4%) and a nonsignificant reduction in atrial fibrillation (atrial pacing: 14%; ventricular pacing: 23%). In addition, progression of heart failure symptoms was observed in 9% of patients in the atrial pacing group and 31% of the ventricular pacing group. In the Pacemaker Selection in the Elderly (PASE) trial 407 older adults (mean age, 76 years) were randomized to either the VVIR mode or the DDDR pacing mode, with a mean follow-up of 30 months.109 In 175 patients with sinus node dysfunction, the DDDR pacing mode was associated with improved cardiovascular functional status and better quality-of-life scores in the role physical, role emotional, and social function categories of the SF-36 questionnaire. The DDDR pacing mode was associated with insignificant reductions in mortality (DDDR, 12%; VVIR, 20%) and incidence of atrial fibrillation (DDDR, 19%; VVIR, 28%). Of the entire study group, 26% of patients crossed over from the VVIR pacing mode to the DDDR pacing mode because of symptoms related to pacemaker syndrome.
The Canadian Trial of Physiologic Pacing (CTOPP) evaluated the effects of pacing mode choice in patients with symptomatic bradycardia.110 The 2568 patients in the study were randomized to single-chamber ventricular pacing or a “physiologic” pacing mode that preserved AV synchrony (AAI mode or DDD mode). While no significant differences in the annual rate of stroke or death were detected (ventricular pacing, 5.5%; physiological pacing, 4.9%), the annual rate of atrial fibrillation was significantly lower in the physiological pacing group (ventricular pacing, 6.6%; physiological pacing, 5.3%). The reduction in atrial fibrillation became more apparent 2 years after initial randomization. Subgroup analysis suggested that the indication for permanent pacing (sinus node dysfunction or AV block) did not have a significant effect on the annual rate of stroke or death. An important caveat with regard to this study is that after 5 years of follow-up, 95% of patients randomized to ventricular pacing were still programmed to ventricular pacing, whereas only 75% of patients randomized to physiological pacing were actually still in a physiological pacing mode.
In the latest large randomized study, the Mode Selection Trial (MOST), 2010 patients with sinus node dysfunction were randomized to dual-chamber pacing or single-chamber ventricular pacing.111 After a 5-year follow-up, no differences in mortality or stroke were detected, but a marked reduction in progression to atrial fibrillation was noted, particularly in patients without a prior history of the arrhythmia; however, the rate of crossover to dual-chamber pacing because of symptoms of pacemaker syndrome was 31%, and dual-chamber pacing was associated with improved quality of life.
Recently, a large meta-analysis that combined data from all of the large prospective trials with a total of 35,000 patient years of follow-up was published.112 Atrial-based pacing was not associated with improved survival or reduction in cardiovascular death or heart failure. However, atrial-based pacing was associated with a modest 20% reduction in atrial fibrillation and stroke. No subgroup appeared to receive special benefit from atrial pacing.
Management
Guidelines for Management of Patients with Intrinsic Sinus Node Dysfunction
When intrinsic sinus node dysfunction is suspected, it is important to try to correlate the symptoms with documentation of the arrhythmia, since sinus node dysfunction is common, especially in older adults, and may not cause symptoms. The intermittency of symptoms and ECG features characteristic of the syndrome may make it difficult to establish a cause/effect relationship. A Holter monitor, event recorder, or implantable loop recorder may be useful to establish the diagnosis, and prolonged monitoring may be required. In selected cases, it is best to use the implantable loop recorder that continuously acquires electrocardiographic signals.111
Krahn et al placed implantable loop recorders in 16 patients with syncope and negative electrophysiological and tilt table tests. Of the 16 patients, 15 had recurrent syncope, and sinus arrest was documented in 5 patients.111 As indicated above, invasive electrophysiological studies are usually not required to specifically evaluate sinus node function, as they have significant limitations.
Therapy is aimed at improving symptoms, as no evidence that medical therapy or pacemaker implantation improves survival exists; this is partly because of the low mortality rate related to bradyarrhythmia per se. However, it is important to acknowledge the possibility of other causes besides bradycardia for a patient’s symptoms. In a post hoc analysis of 177 patients from the MOST study with an ejection fraction of 35% or less, the 4-year sudden cardiac death rate was 15.5%, or 3.9% annually.113
Indications for Permanent Pacing
Pharmacologic therapy for bradycardia caused by sinus node dysfunction is generally ineffective; pacemaker implantation is therefore the optimal therapy. In the United States, sinus node dysfunction is the most common indication for pacemaker implantation.10 The benefit to be expected from permanent pacing depends largely on the appropriateness of the indication.
Guidelines for pacemaker implantation in sinus node dysfunction have been published.114 Indications for permanent pacing in sinus node disease are summarized in Table 39-1. The guidelines emphasize the importance of correlating symptoms and bradycardia, whenever possible. The principal benefits of pacing therapy are prevention of syncope, improvement of symptoms caused by poor tissue perfusion, and prevention of congestive heart failure caused by decreased cardiac output, which, in turn, is caused by slow heart rates. In a 1970s observational series of severely symptomatic patients, pacemaker therapy was shown to improve symptoms of fatigue, lightheadedness, and near-syncope.115 In a randomized trial from the 1990s, which was conducted to assess the efficacy of pacemakers in patients with sick sinus syndrome, the occurrence of syncope was lower in the paced group over a mean follow-up duration of 18 months (6% in the pacemaker group vs. 23% in controls; P = .02).72 Heart failure also occurred less often in patients assigned to pacemaker therapy (3% vs. 17%; P = .05), whereas the incidence of sustained paroxysmal tachyarrhythmias, chronic atrial fibrillation, and thromboembolic events was not different between the groups. Compared with observational series and retrospective studies, pacemaker implantation in this randomized trial did not demonstrate different effects on “minor” symptoms such as fatigue, dizziness, palpitation, and New York Heart Association class in the pacemaker group compared with the “no treatment” group; this was due to subjective improvement occurring in the placebo group as early as 3 months following randomization.72
Table 39-1 Indications for Permanent Pacing in Sinus Node Dysfunction
| Class | Indications |
|---|---|
| Class I | Sinus node dysfunction with documented symptomatic bradycardia, including frequent sinus pauses that produce symptoms. In some patients, bradycardia is iatrogenic and will occur as a consequence of essential long-term drug therapy of a type and dose for which there are no acceptable alternatives. |
| Symptomatic chronotropic incompetence | |
| Class IIa | Sinus node dysfunction occurring spontaneously or as a result of necessary drug therapy with a heart rate <40 beats/min when a clear association between symptoms consistent with bradycardia and actual presence of bradycardia has not been documented |
| Syncope of unexplained origin when major abnormalities of sinus node function are discovered or provoked in electrophysiological studies | |
| Class IIb | Minimally symptomatic patients, chronic heart rate <40 beats/min while awake |
| Class III | Sinus node dysfunction in asymptomatic patients, including those in whom substantial sinus bradycardia (heart rate <40 beats/min) is a consequence of long-term drug treatment |
| Sinus node dysfunction in patients with symptoms suggestive of bradycardia that are clearly documented as not associated with a slow heart rate | |
| Sinus node dysfunction with symptomatic bradycardia due to nonessential drug therapy |
Class I: Conditions for which there is evidence or general agreement, or both, that a given procedure or treatment is beneficial, useful, and effective.
Class II: Conditions for which there is conflicting evidence or a divergence of opinion, or both, about the usefulness/efficacy of a procedure or treatment.
Class IIa: Weight of evidence/opinion is in favor of usefulness/efficacy.
Class IIb: Usefulness/efficacy is less well established by evidence/opinion.
Class III: Conditions for which there is evidence or general agreement, or both, that a procedure/treatment is not useful/effective and, in some cases, may be harmful.
(From Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons, Circulation 117:e350–e408, 2008.)
The published guidelines also take into consideration the erratic course of the disease and the difficulty in establishing the cause/effect relationship between symptoms and arrhythmia.114 Accordingly, pacing is considered useful in patients with heart rates less than 40 beats/min in whom symptoms are consistent with bradycardia but the correlation between the two cannot be clearly established. Pacing is not indicated in asymptomatic patients, in patients with bradycardia caused by nonessential medical therapy, and when symptoms are clearly documented to be not caused by bradycardia.
Permanent pacing is indicated in patients with chronotropic incompetence and who become symptomatic during activity because of inability to increase heart rate and cardiac output. These patients experience an improvement in symptoms and exercise tolerance with rate-responsive pacing.9,116
Pacing Mode Selection
Single-chamber atrial pacemakers, single-chamber ventricular pacemakers, and dual-chamber pacemakers will all prevent bradycardia in the patient with sinus node disease. Each pacemaker type is associated with inherent advantages and disadvantages. Single-chamber atrial pacemakers are simple, relatively inexpensive (approximately $3000 to $4000), and maintain AV synchrony. However, they will not prevent ventricular bradycardia if AV block develops. Up to 20% of patients will have abnormal AV conduction at the time of diagnosis of sinus node dysfunction; these patients are not candidates for single-chamber atrial pacing.71 In a prospective study of 225 patients with 1 : 1 conduction at heart rates less than 100 beats/min. Andersen et al found that AV conduction was unchanged from initial evaluation after a mean 5.5-year follow-up.90 The annual incidence of second-degree and third-degree AV block that required implantation of a dual-chamber pacing system was only 0.6% per year.90
Single-chamber ventricular pacemakers are also simple and inexpensive. They prevent bradycardia in the presence of AV block but do not maintain AV synchrony. Loss of AV synchrony is associated with a 20% to 30% decrease in cardiac output and is associated with pacemaker syndrome.117 Pacemaker syndrome is a constellation of symptoms that can include dizziness, chest pain, weakness, effort intolerance, presyncope, and syncope. The mechanism of pacemaker syndrome is complex but appears to be caused by decreased cardiac output from loss of AV contraction and retrograde conduction through the His-Purkinje-AV node axis. Reduced cardiac output leads to reflex sympathetic activation. Atrial contraction when the mitral and tricuspid valves are closed also leads to an increase in atrial pressure and release of atrial natriuretic peptide and peripheral venous and arterial dilation. The reported incidence of pacemaker syndrome has varied widely among studies (1% to 80%), which probably reflects variability in definition rather than a true variability in incidence.118,119 In large randomized trials such as PASE and MOST, crossover from single-chamber ventricular pacing to dual-chamber pacing because of pacemaker syndrome was approximately 25% to 30%.110,120,121
Dual-chamber pacemakers maintain AV synchrony and prevent bradycardia from all causes. However, dual-chamber pacemakers are more complex and relatively expensive ($5000 to $7000). In addition, since two intracardiac leads are required, the incidence of lead dislodgment is higher with dual-chamber systems (dual-chamber, 6%; single-chamber, 2%). Dual-chamber pacing requires specific programming in patients with sinus node dysfunction to avoid ventricular pacing, which can be hemodynamically detrimental in many patients. In the SAVE PACe (Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction) trial, 1065 patients with sinus node dysfunction were randomized to standard dual-chamber pacing or to use of a pacing algorithm that minimizes ventricular pacing.122 The risk of developing persistent atrial fibrillation was significantly reduced by 40% by minimizing ventricular pacing (conventional, 12.7% vs. special algorithm to minimize ventricular pacing, 7.9%).
Currently available pacing systems have a rate-adaptation feature. When rate adaptation is programmed “on,” the pacing system employs a sensor such as body motion, minute ventilation, Q-T interval changes, measures of myocardial contractility, or combinations of these to estimate metabolic need. The pacemaker will change the pacing rate on the basis of input from the sensor. This feature is particularly useful for patients with sinus node dysfunction associated with chronotropic incompetence. However, in the randomized, controlled Advanced Elements of Pacing Trial Trial (ADEPT), 872 patients with a blunted heart rate response (<80% maximum predicted heart rate) were randomized to rate adaptive pacing “on” or “off.”123 Rate adaption was not associated with improved exercise capacity or improved quality of life.
Sinus Node Dysfunction in Specific Conditions
Acute Myocardial Infarction
Sinus bradycardia is common in acute myocardial infarction, especially in inferior and posterior wall infarction, where it is usually caused by increased vagal tone or ischemia of the sinus node tissue.17,18 Increased vagal tone may also result in transient AV block and hypotension from peripheral vasodilation. This arrhythmia usually does not require treatment unless the patient is symptomatic (hypotension, ischemia, or bradycardia-related ventricular arrhythmia). It usually responds well to intravenous atropine. In symptomatic patients who are unresponsive to atropine or who have recurrences requiring multiple doses of atropine, temporary transvenous pacing may be required. Pacing is usually performed from the right ventricular apex; however, where it is important to maintain AV synchrony (such as refractory hypotension), an additional J-shaped electrode can be placed in the right atrial appendage for dual-chamber pacing. Alternatively, atrial pacing can be achieved using a temporary electrode positioned in the proximal coronary sinus. Sinus node dysfunction occurring during acute myocardial infarction is usually temporary, and permanent pacing is rarely required.
Carotid Sinus Hypersensitivity and Carotid Sinus Syndrome
An abnormal response to carotid sinus massage (>3 seconds of asystole) may occur in asymptomatic patients and does not constitute an indication for therapy; correlation with symptoms is essential (Table 39-2). Up to 64% of patients with syncope caused by carotid sinus syndrome can remain asymptomatic during follow-up; therapy should be reserved for patients with recurrent presyncope or syncope.124 Drugs that can enhance the hypersensitive response to carotid sinus massage, such as digoxin and sympatholytic agents (clonidine, methyldopa), should be discontinued, if possible.
Table 39-2 Indications for Permanent Pacing In Hypersensitive Carotid Syndrome
| Class | Indications |
|---|---|
| Class I | Recurrent syncope caused by carotid sinus stimulation; minimal carotid sinus pressure induces ventricular asystole of >3 seconds’ duration |
| Class IIa | Syncope without clear, provocative events and with a hypersensitive cardio-inhibitory response |
| Class IIb | Significantly symptomatic neurocardiogenic syncope associated with bradycardia documented spontaneously or at the time of tilt table testing |
| Class III | Hypersensitive cardio-inhibitory response to carotid sinus stimulation without symptoms or with vague symptoms |
| Recurrent syncope, lightheadedness, or dizziness in the absence of a hyperactive cardio-inhibitory response |
Class I: Conditions for which there is evidence or general agreement, or both, that a given procedure or treatment is beneficial, useful, and effective.
Class II: Conditions for which there is conflicting evidence or a divergence of opinion, or both, about the usefulness/efficacy of a procedure or treatment.
Class IIa: Weight of evidence/opinion is in favor of usefulness/efficacy.
Class IIb: Usefulness/efficacy is less well established by evidence/opinion.
Class III: Conditions for which there is evidence or general agreement, or both, that a procedure/treatment is not useful/effective and, in some cases, may be harmful.
(From Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons, Circulation 117:e350–e408, 2008.)
The type of therapy (pacing vs. pharmacologic) and success are based on the mechanism of syncope. Pacing is efficacious in the cardio-inhibitory response to carotid sinus massage.125–128 In patients with a predominant vasodepressor response, neither pacing nor anticholinergic therapy prevents the fall in blood pressure, since this is caused by inhibition of sympathetic vasoconstrictor nerves as well as by activation of cholinergic sympathetic vasodilator fibers.128 Elastic support stockings and volume expansion with sodium-retaining drugs may be useful in alleviating the symptoms.
Initial data suggested that unexplained falls in older adults might be caused by carotid sinus hypersensitivity and that, in some cases, pacing therapy may be beneficial.129 A selected group of 175 patients with a history of nonaccidental falls and significant bradycardia (asystole >3 seconds) in response to carotid sinus massage were randomized into pacing therapy and no pacing therapy groups; after a 1-year follow-up, injurious events were reduced by 70% in the pacing therapy group. However, in a recently published double-blind, placebo-controlled, crossover trial, pacing therapy did not reduce the number of falls in a cohort of older patients with carotid sinus hypersensitivity.130
The indications for permanent pacing in carotid sinus syndrome are summarized in Table 41-2. Single-chamber atrial pacing is contraindicated, since vagal activation frequently results in AV block and absence of a ventricular escape rhythm. Although single-chamber ventricular pacing prevents bradycardia, it can potentially exacerbate symptoms caused by neurohormonal effects associated with pacemaker syndrome. Dual-chamber pacing is therefore preferred, since it maintains AV synchrony regardless of the cause of bradycardia. In addition, the current generation of dual-chamber pacing systems allows programming of different heart rates after sensed and paced ventricular beats. By programming a pacemaker to pace at a relatively fast rate on initiation of pacing, symptoms associated with carotid sinus syndrome can be ameliorated even in the presence of a significant vasodepressor response.131
Vasovagal Syncope
Vasovagal syncope is the most common cause of syncope in young people. In 1932, Lewis used the term vasovagal to emphasize the combination of arterial vasodilation and bradycardia associated with this syndrome.132 While the exact mechanism of vasovagal syncope is not known, it does appear that activation of cardiac mechanoreceptors leads to activation of higher neural centers and reflex withdrawal of sympathetic tone and increased vagal tone.
The most common cause for bradycardia in vasovagal syncope is sinus bradycardia or sinus arrest. For this reason, although pacemakers do not affect the vasodepressor component of this syndrome, they have been used to treat the subset of patients with particularly severe symptoms unresponsive to drug therapy. Two relatively large randomized but unblinded studies have evaluated the efficacy of pacing therapy for the treatment of vasovagal syncope. In the North American Vasovagal Pacemaker Study (VPS-I), 54 patients with severe vasovagal syncope and relative bradycardia (trough heart rate <60 beats/min during tilt table testing) were randomized to pacing or no pacing.133 The study was terminated after 2 years when analysis showed that pacing was associated with an 85% decrease in syncopal episodes. Similarly, in the Vasovagal Syncope International Study (VASIS-I), 42 patients with severe drug-refractory vasovagal syncope were randomized to pacing or no pacing.134 Only one patient in the pacing group experienced syncope, while 14 patients in the no-pacing group experienced syncope during a mean 3.7-year follow-up. More recently, the first randomized, double-blind trial (VPS-II) found a nonsignificant 30% risk reduction in time to syncope with DDD pacing (P = .14), which was considerably lower than in previous studies.135–138 Significant procedural complications, including lead dislodgement, venous thrombosis, infection, and pericardial tamponade, occurred in 10% of patients. Given the limited efficacy of pacing therapy, pacing should be reserved only for severe drug-refractory cases of vasovagal syncope that are associated with a prominent bradycardia component. Patients who require pacing therapy for drug-resistant vasovagal syncope should receive a dual-chamber pacemaker because transient AV block can be observed during bradycardia episodes. Special programming features that provide an initial higher pacing rate when pacing is initiated have been developed to optimize pacing therapy for patients with vasovagal syncope.136,139
Key References
Anderson RH, Ho SY. The architecture of the sinus node, the atrioventricular conduction axis, and the internal atrial myocardium. J Cardiovasc Electrophysiol. 1998;9:1233-1248.
Andersen HR, Nielsen JC, Thomsen PEB, et al. Long-term follow-up of patients from a randomized trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216.
Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomized trial of atrial versus ventricular pacing in sick sinus syndrome. Lancet. 1994;344:1523-1528.
Chandler NJ, Greener ID, Tellez JO, et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation. 2009;119:1562-1575.
Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. N Engl J Med. 2000;342:1385-1391.
Davies MJ, Pomerance A. Quantitative study of ageing changes in the human sinoatrial node and internodal tracts. Br Heart J. 1972;34:150-152.
Demoulin JC, Kulbertus HE. Histopathological correlates of sinoatrial disease. Br Heart J. 1978;40:1384-1389.
Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921-1932.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350-e408.
Healey JS, Toff WD, Lamas GA, et al. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: Meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114(1):11-17. 4
Holden W, McAnulty JH, Rahimtoola SH. Characterization of heart rate response to exercise in the sick sinus syndrome. Br Heart J. 1978;40:923-930.
James TN. Anatomy of the human sinus node. Anat Rec. 1961;141:109-116.
Josephson ME. Sinus node function. Clinical cardiac electrophysiology, ed 2. Lea & Febiger, Philadelphia, 1993.
Lamas GA, Lee K, Sweeney MO, et al. Ventricular pacing or dual chamber pacing for sinus node dysfunction. N Engl J Med. 2002;346:1854-1862.
Lev M, Bharati S. Lesions of the conduction system and their functional significance. Pathol Annu. 1974;8:157-160.
Link MS, Helkamp AS, Estes NAIII, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol. 2004;43:2066-2071.
Mazuz M, Friedman HS. Significance of prolonged electrocardiographic pauses in sinoatrial disease: Sick sinus syndrome. Am J Cardiol. 1983;52:485-489.
Mortensen PT. Atrioventricular conduction during long-term follow-up of patients with sick sinus syndrome. Circulation. 1998;98:1515-1521.
Nenozzi C, Brignole M, Albini P, et al. The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol. 1998;82:1205-1209.
Parry SW, Steen N, Bexton RS, Tynan M, Kenny RA. Pacing in elderly recurrent fallers with carotid sinus hypersensitivity: A randomised, double-blind, placebo controlled crossover trial. Heart. 2009;95(5):405-409.
Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18:1-7.
Rubenstein JJ, Schulman CL, Yurchak PM, DeSanctis RW. Clinical spectrum of the sick sinus syndrome. Circulation. 1972;46:5-13.
Schussler RB. Abnormal sinus node function in clinical arrhythmias. J Cardiovasc Electrophysiol. 2003;14:215-217.
Shaw DB, Holman RR, Gowers JI. Survival in sinoatrial disorder (sick sinus syndrome). Br Med J. 1980;280:139-141.
Sutton R, Kenny R. The natural history of sick sinus syndrome. Pacing Clin Electrophysiol. 1986;9:1110-1114.
Sweeney MO, Bank AJ, Nsah E, et al. Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial: Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000-1008.
1 Keith A, Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J Anat Physiol. 1907;41:172-189.
2 Laslett EE. Syncopal attacks associated with prolonged arrest of the whole heart. Quart J Med. 2, 1909. 347–253
3 Ferrer MI. The sick sinus syndrome in atrial disease. JAMA. 1968;206:645.
4 Lown B. Electrical reversion of cardiac arrhythmias. Br Heart J. 1967;29:469-489.
5 Short DS. The syndrome of alternating bradycardia and tachycardia. Br Heart J. 1954;16:208-214.
6 Brodsky M, Wu D, Denses P, et al. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol. 1977;39:390-395.
7 Sobotka PA, Mayer JH, Bauernfeind RA, et al. Arrhythmias documented by 24 hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J. 1981;101:753-759.
8 Fleg JL, Kennedy HL. Cardiac arrhythmias in a healthy elderly population: Detection by 24-hour ambulatory electrocardiography. Chest. 1982;81:301-307.
9 Kulbertus HE, De Leval-Rutten F, Mary L, et al. Sinus node recovery time in the elderly. Br Heart J. 1975;37:420-425.
10 Bernstein AD, Parsonnet V. Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol. 2001;24:842-855.
11 Ector H, Rickards AF, Kappenberger L, et al. The World Survey of cardiac pacing and implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2001;24:842-855.
12 Greagaratos G. Indications and recommendations for pacemaker therapy. Am Fam Physician. 2005;71:1563-1570.
13 Albin G, Hayes DL, Holmes DR. Sinus node dysfunction in pediatric and young adult patients: Treatment by implantation of a permanent pacemaker in 39 cases. Mayo Clin Proc. 1985;60:667-672.
14 Bharati S, Surawicz B, Vidaillet HJ, Lew M. Familial congenital sinus rhythm abnormalities: Clinical and pathological correlations. Pacing Clin Electrophysiol. 1992;15:1720-1729.
15 Mehta AV, Chidambaram B, Garrett A. Familial symptomatic sinus bradycardia: Autosomal dominant inheritance. Pediatr Cardiol. 1995;16:231-234.
16 Olson TM, Keating MT. Mapping a cardiomyopathy locus to chromosome 3p22-p25. J Clin Invest. 1996;97:528-532.
17 Chau EM, McGregor CG, Rodeheffer RJ, et al. Increased incidence of chronotropic incompetence in older donor hearts. J Heart Lung Transplant. 1995;14:743-748.
18 Heinz G, Ohner T, Laufer G, et al. Demographic and perioperative factors associated with initial and prolonged sinus node dysfunction after orthotopic heart transplantation. The impact of ischemic time. Transplantation. 1991;51:1217-1224.
19 Meyer SR, Modry DL, Bainey K, et al. Declining need for permanent pacemaker insertion with the bicaval technique of orthotopic heart transplantation. Can J Cardiol. 2005;21:159-163.
20 Koch W. Der funktionelle Bau des menschlichen Herzens. Berlin: Urban und Schwarzenburg; 1922.
21 James TN. Anatomy of the human sinus node. Anat Rec. 1961;141:109-116.
22 Truex RC, Smythe MQ, Taylor MJ. Reconstruction of the human sinoatrial node. Anat Rec. 1967;159:371-378.
23 Lev M, Bharati S. Lesions of the conduction system and their functional significance. Pathol Annu. 1974;8:157-160.
24 Hudson REB. The human pacemaker and its pathology. Br Heart J. 1960;22:153-156.
25 Anderson KR, Ho SY, Anderson RH. Location and vascular supply of sinus node in human heart. Br Heart J. 1979;41:28-32.
26 Sanchez-Quintana D, Cabrera C, Farre J, et al. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart. 2005;91:189-194.
27 Crick SJ, Sheppard MN, Ho SY, Anderson RH. Localisation and quantitation of autonomic innervation in the porcine heart I: Conduction system. J Anat. 1999;195:341-357.
28 Chow LT, Chow SS, Anderson RH, Gosling JA. Autonomic innervation of the human cardiac conduction system: changes from infancy to senility—an immunohistochemical and histochemical analysis. Anat Rec. 2001;264:169-182.
29 Busquet J, Fontan F, Anderson RH, et al. The surgical significance of the atrial branches of the coronary arteries. Int J Cardiol. 1984;6:223-234.
30 James TN. Pathogenesis of arrhythmias in acute myocardial infarction. Am J Pathol. 1969;24:791-799.
31 Davies MJ, Pomerance A. Quantitative study of ageing changes in the human sinoatrial node and internodal tracts. Br Heart J. 1972;34:150-152.
32 Shiraishi I, Takamatsu T, Minamikawa T, et al. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85:2176-2184.
33 Alings AM, Abbas RF, Bouman LN. Age-related changes in structure and relative collagen content of the human and feline sinoatrial node. A comparative study. Eur Heart J. 1993;14:1278-1288.
34 Thery C, Gosselin B, Lekieffre J, Warembourg H. Pathology of sinoatrial node. Correlations with electrocardiographic findings in 111 patients. Am Heart J. 1977;93:735-740.
35 Ho SY, Monro JL, Anderson RH. The disposition of the sinus node in left-sided juxtaposition of the atrial appendage. Br Heart J. 1979;41:129-132.
36 Dickinson DF, Wilkinson JL, Anderson KR, et al. The cardiac conduction system in situs ambiguous. Circulation. 1979;59:879-885.
37 Ho SY, Seo JW, Brown NA, et al. Morphology of the sinus node in human and mouse hearts with isomerism of the atrial appendages. Br Heart J. 1995;74:437-442.
38 Demoulin JC, Kulbertus HE. Histopathological correlates of sinoatrial disease. Br Heart J. 1978;40:1384-1389.
39 Evans R, Shaw DB. Pathological studies in sinoatrial disorder (sick sinus syndrome). Br Heart J. 1977;39:778-786.
40 Lev M, Bharati S. Lesions of the conduction system and their functional significance. Pathol Annu. 1974;9:157-207.
41 Fox KM, Anderson RH, Hallidie-Smith KA. Hypoplastic and fibrotic sinus node associated with intractable tachycardia in a neonate. Circulation. 1980;61:1048-1052.
42 Schuessler RB, Boineau JP, Brombert BI. Origin of the sinus impulse. J Cardiovasc Electrophysiol. 1996;7:263-274.
43 Anderson RH, Ho SY. The architecture of the sinus node, the atrioventricular conduction axis, and the internal atrial myocardium. J Cardiovasc Electrophysiol. 1998;9:1233-1248.
44 Anumonwo JMB, Jalife J. Cellular and subcellular mechanisms of pacemaker activity initiation and synchronization in the heart. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From cell to bedside. Philadelphia: Saunders, 1995.
45 Crick SJ, Wharton J, Sheppard MN, et al. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation. 1994;89:1697-1708.
46 Bleeker WK, MacKaay AJC, Masson-Pevet M, et al. Functional and morphologic organization of the rabbit sinus node. Circ Res. 1980;46:11-22.
47 Watanabe E, Honjo H, Anno T, et al. Modulation of pacemaker activity of sinoatrial node cells by electrical load imposed by an atrial cell model. Am J Physiol. 1995;269:H1735-H1742.
48 Fahrenback J, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol. 2007;585(2):565-578.
49 Schussler RB. Abnormal sinus node function in clinical arrhythmias. J Cardiovasc Electrophysiol. 2003;14:215-217.
50 Fedorov VV, Schussler RB, Hemphill M, et al. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res. 2009;104:915-923.
51 Shibata EF, Giles WR. Ionic currents that generate the spontaneous diastolic depolarization in individual cardiac pacemaker cells. Proc Natl Acad Sci USA. 1985;82:7796-7800.
52 Honjo H, Boyett MR, Kodama I, Toyama J. Correlation between electrical activity and the size of rabbit sino-atrial node cells. J Physiol. 1996;496:795-808.
53 Robinson RB, Difrancesco D. Sinoatrial node and impulse initiation. In: Spooner P, Rosen M, editors. Foundations of cardiac arrhythmias: Basic concepts and clinical approaches. New York: Marcel Dekker, 2001.
54 Vassalle M, Yu H, Cohen IS. Pacemaker channels and cardiac automaticity. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From cell to bedside. Philadelphia: Saunders, 1995.
55 Chandler NJ, Greener ID, Tellez JO, et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation. 2009;119:1562-1575.
56 Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921-1932.
57 Maltsev VA, Lakatta EG. Normal heart rhythm is initiated and regulated by an intracellular calcium clock within pacemaker cells. Heart Lung Circ. 2007;16(5):335-348.
58 DiFrancesco D, Borer J. The funny current: Cellular basis for the control of heart rate. Drugs. 2007;67(Suppl 2):15-24.
59 Elvan A, Wylie K, Zipes DP. Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs: electrophysiological remodeling. Circulation. 1996;94:2953-2960.
60 Yeh YH, Burstein B, Qi XY, et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: A molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576-1585.
61 Nof E, Luria D, Brass D, et al. Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation. 2007;116:463-470.
62 Verkerk A, Wilders R, van Borren MM, et al. Pacemaker current (If) in the human sinoatrial node. Eur Heart J. 2007;28:2472-2478.
63 Thiene G, editor. Cardiac arrhythmias: Recent progress in investigation and management. Amsterdam: Elsevier, 1988.
64 Spodick DH, Raju P, Bishop RL, Rifkin RD. Operational definition of normal sinus heart rate. Am J Cardiol. 1992;69:1245-1246.
65 Spodick DH. Survey of selected cardiologists for an operational definition of normal sinus heart rate. Am J Cardiol. 1993;72:487-488.
66 Spodick DH. Normal sinus heart rate: Appropriate rate thresholds for sinus tachycardia and bradycardia. South Med J. 1996;89:666-667.
67 Viitasalo MT, Kala R, Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Br Heart J. 1982;47:213-220.
68 Kantelip JP, Sage E, Duchene-Marullaz P. Findings on ambulatory monitoring in subjects older than 80 years. Am J Cardiol. 1986;57:398-401.
69 Northcote RJ, Canning GP, Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br Heart J. 1989;61:155-160.
70 Lorber A, Maisuls E, Palant A. Autosomal dominant inheritance of sinus node disease. Int J Cardiol. 1986;15:252-256.
71 Sutton R, Kenny R. The natural history of sick sinus syndrome. Pacing Clin Electrophysiol. 1986;9:1110-1114.
72 Alboni P, Menozzi C, Brignole M, et al. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome. The THEOPACE Study: A randomized controlled trial. Circulation. 1997;96:260-266.
73 Nenozzi C, Brignole M, Albini P, et al. The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol. 1998;82:1205-1209.
74 Alboni P, Menozzi C, Brignoli M, et al. An abnormal neural reflex plays a role in causing syncope in sinus bradycardia. J Am Coll Cardiol. 1993;22:1130-1134.
75 Brignole M, Menozzi C, Gianfranchi L, et al. Neurally mediated syncope detected by carotid sinus massage and head-up tilt test in sick sinus syndrome. Am J Cardiol. 1991;68:1032-1036.
76 Shaw DB, Holman RR, Gowers JI. Survival in sinoatrial disorder (sick sinus syndrome). Br Med J. 1980;280:139-141.
77 Rubenstein JJ, Schulman CL, Yurchak PM, DeSanctis RW. Clinical spectrum of the sick sinus syndrome. Circulation. 1972;46:5-13.
78 Lien WP, Lee YS, Chang FZ, et al. The sick sinus syndrome. Natural history of dysfunction of the sinoatrial node. Chest. 1977;72:628-634.
79 Bigger JTJr, Reiffel JA. Sick sinus syndrome. Ann Rev Med. 1979;30:91-118.
80 Chung EK. Sick sinus syndrome: Current views. Mod Concepts Cardiovasc Dis. 1980;49:61-66.
81 Skagen K, Hansen JF. The long-term prognosis for patients with sinoatrial block treated with permanent pacemaker. Acta Med Scand. 1975;199:13-15.
82 Ellestad MH, Wan MK. Predicted implications of stress testing: Follow-up of 2700 subjects after maximum treadmill stress testing. Circulation. 1975;51:363-369.
83 Wiens R, Lafia P, Marder CM, et al. Chronotropic incompetence in clinical exercise testing. Am J Cardiol. 1984;54:74-82.
84 Sgarbossa EB, Pinski SL, Maloney JD, et al. Chronic atrial fibrillation and stroke in paced patients with sick sinus syndrome. Relevance of clinical characteristics and pacing modalities. Circulation. 1993;88:1045-1053.
85 Andersen HR, Thuesen L, Bagger JP, et al. Prospective randomized trial of atrial versus ventricular pacing in sick sinus syndrome. Lancet. 1994;344:1523-1528.
86 Andersen HR, Nielsen JC, Thomsen PEB, et al. Long-term follow-up of patients from a randomized trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216.
87 Vera Z, Mason DT, Awan NA, et al. Improvement of symptoms in patients with sick sinus syndrome by spontaneous development of stable atrial fibrillation. Br Heart J. 1977;39:160-167.
88 Gann D, Tolentino R, Samet P. Electrophysiologic evaluation of elderly patients with sinus bradycardia: A long-term follow-up study. Ann Intern Med. 1979;24:29.
89 Rosenqvist M, Obel IW. Atrial pacing and the risk for AV block: Is there a time for change in attitude? Pacing Clin Electrophysiol. 1989;12:97-101.
90 Narula OS. Atrioventricular conduction defects in patients with sinus bradycardia. Analysis by His bundle recordings. Circulation. 1971;44:1096-1110.
91 Mortensen PT. Atrioventricular conduction during long-term follow-up of patients with sick sinus syndrome. Circulation. 1998;98:1515-1521.
92 Van Mechelen R, Segers A, Hagemeijer F. Serial electrophysiologic studies after single chamber atrial pacemaker implantation in patients with symptomatic sinus node dysfunction. Eur Heart J. 1984;5:628-636.
93 Stangl K, Wirtzfeld A, Seitz K, et al. Atrial stimulation: Long- term follow-up of 110 patients. In: Belhassen B, Feldman S, Copperman Y, editors. Cardiac pacing and electrophysiology. Proceedings of the VIII World Symposium on Cardiac Pacing and Electrophysiology. Jerusalem: R&L Creative Communications, 1987.
94 Santini M, Alexidou G, Ansalone G, et al. Relation of prognosis in sick sinus syndrome at age, conduction defects and modes of permanent cardiac pacing. Am J Cardiol. 1990;565:729-735.
95 Grauer LE, Gershen BJ, Orlando MM, Epstein SE. Bradycardia and its complications in the pre-hospital phase of acute myocardial infarction. Am J Cardiol. 1973;32:607-611.
96 Rotman M, Wagner GS, Wallace AGP. Bradyarrhythmias in acute myocardial infarction. Circulation. 1972;45:703-722.
97 Parameswaran R, Ohe T, Goldberg H. Sinus node dysfunction in acute myocardial infarction. Br Heart J. 1976;38:93-96.
98 Hatle L, Bathen J, Rokseth R. Sinoatrial disease in acute myocardial infarction. Long-term prognosis. Br Heart J. 1976;38:410-414.
99 Mangrum JM, Dimarco JP. The evaluation and management of bradycardia. N Engl J Med. 2000;342:703-709.
100 Kaplan BM, Langendorf R, Lev M, Pick A. Tachycardia-bradycardia syndrome (so-called “sick sinus syndrome”). Pathology, mechanisms and treatment. Am J Cardiol. 1973;31:497-508.
101 Mazuz M, Friedman HS. Significance of prolonged electrocardiographic pauses in sinoatrial disease: Sick sinus syndrome. Am J Cardiol. 1983;52:485-489.
102 Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18:1-7.
103 Holden W, McAnulty JH, Rahimtoola SH. Characterization of heart rate response to exercise in the sick sinus syndrome. Br Heart J. 1978;40:923-930.
104 Mandel WJ, Hayakawa H, Allen HN, et al. Assessment of sinus node function in patients with sick sinus syndrome. Circulation. 1972;46:761-769.
105 Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4:160-167.
106 Josephson ME. Sinus node function. Clinical cardiac electrophysiology, ed 2. Philadelphia: Lea & Febiger; 1993.
107 Strauss HC, Saroff AL, Bigger JTJr, Giardina EG. Premature atrial stimulation as a key to the understanding of sinoatrial conduction in man. Presentation of data and critical review of literature. Circulation. 1973;47:86-93.
108 Narula OS, Shantha N, Vasquez M, et al. A new method for measurement of sinoatrial conduction time. Circulation. 1978;58:706-714.
109 Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. N Engl J Med. 1998;338:1097-1104.
110 Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. N Engl J Med. 2000;342:1385-1391.
111 Lamas GA, Lee K, Sweeney MO, et al. Ventricular pacing or dual chamber pacing for sinus node dysfunction. N Engl J Med. 2002;346:1854-1862.
112 Healey JS, Toff WD, Lamas GA, et al. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: Meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114(1):11-17.
113 Sweeney MO, Hellkamp AS, Ellenbogen KA, Lamas GA. Reduced ejection fraction, sudden cardiac death, and heart failure death in the mode selection trial (MOST): Implications for device selection in elderly patients with sinus node disease. J Cardiovasc Electrophysiol. 2008;19(11):1160-1166.
114 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350-e408.
115 Breivik K, Ohm OJ, Segadal L. Sick sinus syndrome treated with permanent pacemaker in 109 patients. A follow-up study. Acta Med Scand. 1979;206:153-159.
116 Gomes JAC, Kang PS, El-Sherif N. The sinus node electrogram in patients with and without sick sinus syndrome: Techniques and correlation between directly measured and indirectly estimated sinoatrial conduction time. Circulation. 1982;66:864.
117 Ausubel K, Furman S. The pacemaker syndrome. Ann Intern Med. 1985;103:420-429.
118 Batey L, Sweesy MW, Scala G, Forney RC. Comparison of low rate dual chamber pacing to activity responsive rate variable ventricular pacing. Pacing Clin Electrophysiol. 1990;13:646-652.
119 Sulke N, Dritsas A, Bostock J, et al. “Subclinical” pacemaker syndrome: A randomized study of symptom free patients with ventricular demand (VVI) pacemakers upgraded to dual chamber devices. Br Heart J. 1992;67:57-64.
120 Heldman D, Mulvihill D, Nguyen H, et al. True incidence of pacemaker syndrome. Pacing Clin Electrophysiol. 1990;13:1742-1750.
121 Link MS, Helkamp AS, Estes NAIII, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol. 2004;43:2066-2071.
122 Sweeney MO, Bank AJ, Nsah E, et al. Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial: Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000-1008.
123 Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity—evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007;4(9):1125-1132.
124 Strasberg B, Sagie A, Erdman S, et al. Carotid sinus hypersensitivity and the carotid sinus syndrome. Prog Cardiovasc Dis. 1989;31:379-391.
125 Richardson DA, Bexton RS, Shaw FE, Kenny RA. Prevalence of cardioinhibitory carotid sinus hypersensitivity in patients 50 years or over presenting to the accident and emergency department with “unexplained” or “recurrent” falls. Pacing Clin Electrophysiol. 1997;20:820-823.
126 Huang SK, Ezri MD, Hauser RG, et al. Carotid sinus hypersensitivity in patients with unexplained syncope: Conical, electrophysiologic, and long-term follow-up observations. Am Heart J. 1988;116:989-996.
127 Brignole M, Menozzi C, Lolli G, et al. Long-term outcome of paced and non-paced patients with severe carotid sinus syndrome. Am J Cardiol. 1992;69:1039-1043.
128 Sugrue DD, Gersh BJ, Holmes DR, et al. Symptomatic “isolated” carotid sinus hypersensitivity: Natural history and results of treatment with anticholinergic drugs or pacemakers. J Am Coll Cardiol. 1986;7:158-162.
129 Kenny RA, Richardson DA, Steen N, et al. Carotid sinus syndrome: A modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol. 2001;38:1491-1496.
130 Parry SW, Steen N, Bexton RS, et al. Pacing in elderly recurrent fallers with carotid sinus hypersensitivity: A randomised, double-blind, placebo controlled crossover trial. Heart. 2009;95(5):405-409.
131 Katritsis D, Ward DE, Camm AJ. Can we treat carotid sinus syndrome? Pacing Clin Electrophysiol. 1991;14:1367-1369.
132 Lewis T. Vasovagal syncope and the carotid sinus mechanism. BMJ. 1932;1:873-876.
133 Connolly SJ, Sheldon R, Roberts R, et al. The North American vasovagal pacemaker study: A randomized trial of permanent cardiac pacing for the prevention of vasovagal syncope. J Am Coll Cardiol. 1999;33:16-20.
134 Sutton R, Brignole M, Menozzi C, et al. Dual chamber pacing in the treatment of neurally-mediated tilt positive cardioinhibitory syncope: Pacemaker versus no therapy: A multicentre randomized study. The Vasovagal Syncope International Study. Circulation. 2000;102:294-299.
135 Connolly SJ, Sheldon R, Thorpe KE, et al. The Second Vasovagal Pacemaker Study (VPSII). Pacing Clin Electrophysiol. 2003;26:1565.
136 Sutton R. Has cardiac pacing a role in vasovagal syncope? J Intervent Cardiac Electrophysiol. 2003;9:145-149.
137 Connolly SJ, Sheldon R, Thorpe KE, et alfor VPS Investigators. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope. Second vasovagal pacemaker study (VPS-2). A randomized trial. JAMA. 2003;209:2224-2229.
138 Ammirati F, Colivicchi F, Toscano S, et al. DDD pacing with rate drop response function versus DDI pacing with rate hysteresis pacing for cardioinhibitory vasovagal syncope. Pacing Clin Electrophysiol. 1998;21:2178-2181.
139 Ammirati F, Colivicchi F, Santini M. Permanent cardiac pacing versus medical treatment for the prevention of recurrent vasovagal syncope: A multicenter, randomized, controlled trial. Circulation. 2001;104:52-57.