CHAPTER 35 Shunting*
Hydrocephalus is a commonly encountered disorder that occurs either as a primary condition or as the sequela to an intracranial hemorrhage, a space-occupying lesion, or meningitis. For more than a half-century, a cerebrospinal fluid (CSF) shunt has been the mainstay for treatment of hydrocephalus. Although many consider shunting a relatively simple procedure, problems with CSF shunts are common, costly, and sometimes debilitating. Within the first year, shunts fail at extraordinary rates of up to 40% and show nearly a 10% infection rate.1–4 Thus, the shunt operation has one of the highest associated complication rates in neurosurgery. Furthermore, cases of hydrocephalus can be some of the most complex and challenging clinical scenarios facing a neurosurgeon.5,6
The aim of this chapter is to help neurosurgeons choose the type of shunt, valve setting, and shunt location that will offer the highest probability of a good outcome while avoiding complications and revisions. Unfortunately, there are scant class I and class II evidentiary data on which to base guidelines pertaining to shunting methods and materials for adult hydrocephalus patients. Our recommendations are therefore derived from personal experience (more than 6000 outpatient encounters and 700 surgical procedures on adult hydrocephalus patients during a 14-year-period), insight drawn from our clinical studies,7–9 and information gleaned from the literature.
Although this chapter is entitled Shunting, neurosurgeons should reflexively consider endoscopic third ventriculostomy an alternative when appropriate.7,10 The “knee-jerk” response to proceed automatically with a shunt operation, particularly in patients presenting with shunt failure, robs the patient of an opportunity to live shunt free. Clinicians should investigate the etiology and ventricular anatomy in every case of hydrocephalus. In some cases, even patients whose physicians previously said that they had “communicating” hydrocephalus in fact have a ventricular obstruction that clinicians can readily visualize by modern high-resolution magnetic resonance imaging (MRI) technology (such as the CISS [constructive interference in steady state] or FIESTA [fast imaging employing steady-state acquisition] sagittal sequences7,11,12). Adult patients shunted in early childhood have a particularly high incidence of noncommunicating (intraventricular) hydrocephalus in our experience.
Valve Design and Terminology
Probably the most important component of a shunt system is the valve. Neurosurgeons can choose from more than 125 commercially available valves.13 During the past 50 years, the predominant theme in the evolution of valve design has been the goal of preventing CSF overdrainage. This includes the introduction of anti-siphon devices, flow-restricting elements, multistage valves, and adjustable valves. It is important to understand that manufacturers have little or no direct in vivo intracranial pressure or CSF flow data to back up advertised claims, such as “preventing excessive flow while allowing constant physiological drainage” or “regulates flow through the valve at a rate close to that of CSF secretion, therefore minimizing the risks of underdrainage or overdrainage.” Our studies8 demonstrate that the in vivo behavior of even the simplest shunt, the ventriculoperitoneal shunt with a standard differential pressure valve, is poorly predicted by the first-order, steady-flow equations that are the basis of the many valve designs.
Differential Pressure Valve
The basic building block of most shunt valves is a differential pressure “check valve” mechanism. The basic design of John Holter continues in some form more than half a century after its development.14 In most current valve designs, it consists of a tiny ball situated on a ring, with a spring pushing the ball downward on the ring. CSF passes through the ring, elevating the ball if the pressure exceeds the pressure exerted by the spring. This creates a one-way flow mechanism because reverse flow will not occur as the ball sits down onto the ring.
A common misconception is that the valve opening pressure must be lower than the ventricular pressure (as measured at the time of surgery) for CSF to flow down the shunt. Our studies demonstrate that this is clearly an invalid assumption. In a study of patients with normal-pressure hydrocephalus (NPH), intracranial pressure was statistically lower at all head-of-bed elevations compared with preoperative values, even with the valve set at 200 mm H2O opening pressure. For example, despite a mean preoperative intracranial pressure of 164 ± 64 mm H2O, the mean postoperative intracranial pressure was 125 ± 69 mm H2O (P = .04).8
The finding that an intracranial pressure reduction occurs even with a very high valve opening pressure might appear counterintuitive and physiologically untenable, but this misconception arises from a perpetuated oversimplification of intracranial pressure and CSF flow hydrodynamics. The concepts of CSF opening pressure (which, by default, is a mean pressure) and bulk CSF flow have been the standards of hydrocephalus pathophysiology teaching for decades. In reality, the intracranial pressure waveform is pulsatile, with significant elevations of intracranial pressure occurring because of coughing and Valsalva maneuvers as well as intrinsic vasomotor changes. The interaction between pulsatile intracranial pressure and the one-way valve mechanism (inherent to differential pressure valves) is poorly studied. Our continuous intracranial pressure recordings demonstrate that peak intracranial pressures often exceed 200 mm H2O among patients with a mean intracranial pressure of 164 mm H2O.7 Even taking into account distal intra-abdominal pressure, one-way CSF egress occurs during these peaks, thereby lowering the mean intracranial pressure. The one-way flow check-valve phenomenon results in the shunt’s draining CSF even with opening pressures exceeding the mean intracranial pressure. This demonstrates that use of a low-pressure valve setting is not necessary and results in excessive CSF drainage in many patients.
Key Point
Adjustable (“Programmable”) Valves
A “programmable” or adjustable valve is created by adding a mechanism that enables precise changes of the spring tension of a differential pressure valve. There are several competing designs enabling this—all incorporating a magnetic actuation of a rotor. Strictly speaking, these valves are not truly programmable and are better considered as merely adjustable valves. Adjustable valves arose from the realization that fixed-pressure differential pressure valves result in either overdrainage or underdrainage in a significant number of adult patients. The overdrainage side of this argument is supported by data from the Dutch Normal-Pressure Hydrocephalus Study,15 one of the few prospective, randomized studies performed in adult hydrocephalus. This study demonstrated that subdural hygromas occurred in 71% of patients with low-pressure valve shunts versus 34% of patients randomized to medium-pressure shunts. Given the likelihood that expanding or large subdural hygromas are a risk for subdural hematoma, this is one example that there is clearly a risk of selecting too low of an opening pressure. The analysis of our series of 114 consecutive idiopathic NPH patients, each treated with an initial valve opening pressure of 200 mm H2O, revealed a subdural hygroma incidence of 4%.7 As shown in Figure 35-1, combining the results of the Dutch Normal-Pressure Hydrocephalus Study with our experience suggests a direct relationship between subdural hygromas and valve opening pressure.
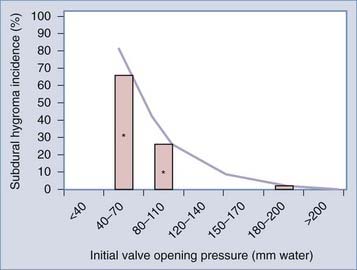
FIGURE 35-1 Estimated risk of subdural hygroma formation with idiopathic NPH. The Dutch Normal-Pressure Hydrocephalus Study15 documented a subdural hygroma (effusion) incidence of approximately 70% and approximately 30% with low- and medium-pressure differential pressure valves, respectively (data signified with an asterisk). We encountered a 4% incidence among patients with an initial valve setting of 200 mm H2O. Combining these data sets results in a direct relationship between valve opening pressure and subdural hygroma incidence. The hygroma incidence for other valve designs and arrangements has not been well documented.
(From Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery. 2008;62:SHC643-660.)
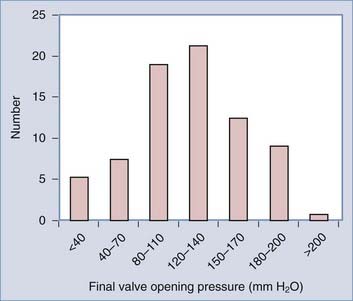
Another justification for the routine use of adjustable valves is based on the range of “final” valve opening pressures when these valves are used. In our retrospective evaluation of 114 consecutive NPH patients surgically treated with a CSF shunt, the histogram distribution of the final valve opening pressure revealed a roughly gaussian distribution, with most patients in the range of 120 to 140 mm H2O (Fig. 35-2).7 This finding closely agrees with that of other large NPH studies.16 With the wide distribution of final valve pressures shown in Figure 35-2 (from <40 to >200 mm H2O), it is difficult to fathom how a fixed-pressure valve could adequately serve this population unless there is a way of selecting the appropriate valve pressure preoperatively. Although some have suggested algorithms to do so,16,17 none has been independently evaluated or validated.
Some neurosurgeons remain reluctant to use adjustable valves on a routine basis (or at all). On their side are the results of a prospective, randomized trial comparing the Codman Hakim adjustable valve and a standard differential pressure valve that failed to demonstrate a difference in shunt failure rates.4 This study, however, was primarily a pediatric study and, in our opinion, not conclusive with regard to adult hydrocephalus. Arguments that these valves are unreliable, or malfunction more frequently than fixed valves do, are not supported by any clinical study (or our clinical experience with the implantation of more than 400 of these devices). There is a fear that in certain patients, particularly in patients with chronic headache or with particular psychosocial issues, the clinician will be plagued with continued requests for valve adjustments. In our experience, this has not materialized to any significant degree. Perhaps the biggest drawback is cost. Currently, adjustable valves are two to three times more costly compared with fixed-pressure valves, and there is no clinical study comparing cost-effectiveness. A direct comparison of cost utilization would have to factor in the morbidity associated with repeated operations and associated operative risks when fixed-pressure valves are used.
Both the Aesculap proGAV and Codman Hakim adjustable valve have multiple, smaller discrete settings (from 0 to 200 mm H2O or 30 to 200 mm H2O, respectively). Both the Medtronic Strata and Sophysa Polaris valves have only five settings, thereby necessitating a larger jump between steps. We are not aware of any clinical study demonstrating an advantage of smaller steps, although changes as small as 10 mm H2O can result in clinical responses.16 Our current management algorithm typically involves making valve adjustments of 30 mm H2O; only in uncommon scenarios are smaller adjustments apparently beneficial.
Siphon-Control and Anti-Siphon Devices
We refer to these collectively as anti-siphon devices (ASDs), although there are mechanical and marketing differences between them. ASDs are add-on devices, meaning that they are used in conjunction with (immediately distal to) a differential pressure valve mechanism. These devices have been used clinically for more than 30 years.18
In general, the device is based on a membrane that is mechanically coupled to the subcutaneous tissue overlying it.18 The pressure differential between the internal valve lumen and the atmosphere, transmitted across the skin and ASD membrane, determines the flow-pressure characteristics of the ASD device. When the intraluminal pressure becomes significantly negative (relative to atmospheric pressure), the membrane is drawn inward—interacting with other fixed components of the ASD and thereby creating an increased pressure gradient. The original ASD was a separate component (Heyer-Schulte) that had to be inserted into the shunt. The Heyer-Schulte ASD fell into disfavor because of a variety of reasons, typically underdrainage, and has been largely supplanted by a more advanced design marketed by Medtronic.19 The Medtronic Delta chamber is found in the Delta valve (fixed-pressure apposing membrane differential pressure valve with integral Delta chamber) and Strata valve (adjustable ball-ring differential pressure valve with integral Delta chamber).
ASDs were developed on the basis of the premise that “siphoning” is the etiology of shunt-related CSF overdrainage. Shunt overdrainage has existed since the inception of the shunt.13,20–22 This phenomenon, better termed gravity-dependent drainage, occurs as the result of gravity-driven CSF flow down the distal catheter when the patient is in the upright position. Early studies20 documented significantly negative intracranial pressures in shunted patients in the upright position. At the time, it was natural to assume that overdrainage complications (such as subdural hematomas) were due to this gravity-dependent drainage.
Our intracranial pressure studies in idiopathic NPH patients,8 as well as those of others,15 suggest that gravity-dependent drainage is likely to play a lesser role in the etiology of overdrainage complications. As any person assumes an upright position, intracranial pressure decreases whether they have a shunt or not. As a matter of fact, in the standing position, most people have a slightly subatmospheric intracranial pressure. When you place a shunt with a differential pressure valve, the curve of intracranial pressure versus head-of-bed elevation in shunted patients nearly parallels that of the pre-shunt state (Fig. 35-3).8 In other words, a shunt with a differential pressure valve essentially lowers the intracranial pressure nearly equally across the head-of-bed angulation range. The degree of intracranial pressure reduction is largely a function of the valve opening pressure.
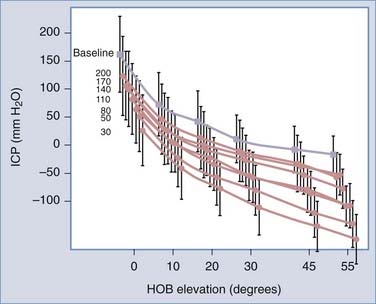
FIGURE 35-3 Intracranial pressure (ICP, mean ± standard deviation) versus head-of-bed (HOB) elevation curves through the full range of differential pressure opening pressures (200, 170, 140, and so on) measured in idiopathic NPH patients treated with a ventriculoperitoneal shunt.8 The pre-shunt baseline curve (gray line, filled gray square) was obtained from the same group of patients. Note that the preoperative and postoperative curves roughly parallel one another, demonstrating the limited role of “siphoning” as the cause of overdrainage in idiopathic NPH patients.
Key Point
There is little clinical evidence to support the contention that ASDs prevent overdrainage. A large, prospective, randomized study comparing a standard differential pressure valve, the Medtronic Delta valve, and the Orbis-Sigma valve found no statistical difference in the rate of ventricular reduction, the final ventricle size, or the incidence of clinical shunt failure.1,23 A follow-up single-armed prospective study3 to the prospective, randomized trial comparing the Codman Hakim adjustable valve with a standard differential pressure valve4 similarly revealed that the programmable Strata valve also failed to show any benefit in pediatric patients.
For some hydrocephalus patients, the presence of an ASD is detrimental.24–29 This so-called low-pressure hydrocephalus syndrome, of which the incidence has not been quantified but is presumed to be less than 5%, occurs both in childhood and in adults. Given the low incidence of low-pressure hydrocephalus, we do not think that this “risk” constitutes a contraindication to the general use of products with ASDs. For clinicians who routinely use ASD devices, however, it is important that they become familiar with the signs and symptoms of low-pressure hydrocephalus.7,24 In our experience as well as that of others,30 the addition of an ASD can be effective in patients with clinically symptomatic overdrainage.
Key Point
A patient who fails to improve clinically (or deteriorates), a patient who remains with significant ventriculomegaly that did not change, a patient who has low measured intracranial pressure, or a patient with an ASD should not be written off as a nonresponder. The diagnosis of low-pressure hydrocephalus should be considered.24
Flow Restriction Devices
There is scarce in vivo clinical evidence, however, to support these manufacturer claims. A large, prospective, randomized study comparing a standard differential pressure valve, the Medtronic Delta valve, and the Orbis-Sigma valve (the original design, predating the Orbis-Sigma II) found no statistical difference in the rate of ventricular reduction, the final ventricle size, or the incidence of clinical shunt failure.1,23 In a retrospective study comparing the Orbis-Sigma valve with a standard differential pressure valve in NPH, Weiner and colleagues31 found no significant difference in the time to initial malfunction (shunt survival) between the Orbis-Sigma valve and the differential pressure valve shunts. There were three subdural hematomas and one infection in the Orbis-Sigma valve group compared with no complications in the differential pressure valve group (P = .11). Nearly 90% of all patients experienced improvement in gait after shunting, regardless of the valve system that was used.
Remarkably, there exist some in vivo data of measured CSF flow rate through shunts. Miyake and associates17 created an externalized loop connected to an indwelling ventriculoperitoneal shunt and measured CSF flow rates in patients with NPH. They assessed the Codman adjustable (differential pressure valve) and Orbis-Sigma valves. They demonstrated that shunt flow differed across patients, but in general, flow increased as the adjustable valve setting was lowered regardless of whether the patient was recumbent or sitting. At higher opening pressures of the adjustable valve (140 to 200 mm H2O) in the recumbent position, the flow was intermittent, whereas at the lowest setting of 30 mm H2O, the flow rate was 100 to 200 µL/min. In the sitting position, the shunt flow rates were higher, ranging from 200 and 600 µL/min. For the Orbis-Sigma valve, the flow rates were very similar to the adjustable valve set at 200 mm H2O in both the recumbent and sitting positions. This actual in vivo flow data would appear to contradict the Orbis-Sigma manufacturer’s concept that in stage I, it functions as a low-pressure differential pressure valve. There are no in vivo data available either to confirm or to refute the manufacturer’s claims regarding stage II and stage III activity.
The Orbis-Sigma II valve was studied in a single-armed, prospective, multicenter clinical study that included 270 adult hydrocephalic patients.32 Shunt obstruction occurred in 14% of patients. The probability of having experienced a shunt failure–free interval was 71% at 1 year and 67% at 2 years; no difference was observed in shunt survival in pediatric versus adult groups. According to the authors, “overdrainage” occurred in only 2% of patients, although their definition of overdrainage was very narrowly defined. Clinical underdrainage was not assessed.
Another approach to flow restriction is the incorporation of a high-resistance element. The Codman Siphonguard is a coiled helical device that is placed immediately distal to a differential pressure valve (adjustable or fixed pressure). Unlike some ASDs, the Siphonguard device is unaffected by scar tissue encapsulation or external pressure. According to the manufacturer, the mechanical design “detects the difference between the normal and excessive flow and closes the primary pathway only when excessive flow occurs. The secondary pathway is always open and allows for the slow release of CSF when the primary pathway is closed.” To our knowledge, to date, there are no published clinical studies evaluating the Siphonguard device. In vitro bench-top testing from an independent laboratory33 demonstrates that switching between the primary and secondary pathways was initiated at a fluid flow rate between 700 and 1800 µL/min. On the basis of the data of Miyake and associates17 presented earlier, in which measured flow rates did not exceed 600 µL/min, it is unclear whether the flow-restricting circuit would be activated at all in NPH. Similar flow data do not exist for younger hydrocephalus patients to our knowledge, although presumably the flow rates may be higher than in patients with NPH. Unlike gravity-dependent devices, both the Orbis-Sigma and Siphonguard designs potentially mitigate overdrainage that may occur in the recumbent position.
Medtronic manufactures a peritoneal catheter with a smaller internal diameter, which also achieves a fixed added flow resistance. This catheter is intended to be used in conjunction with a valve. To date, there have been no published clinical studies addressing the clinical efficacy or pitfalls of this approach. Interestingly, Sotelo and coworkers34 reported the use of a valveless shunt that instead incorporated a peritoneal catheter with a highly precise cross-sectional internal diameter of 0.51 mm. At the end of the observation period of 44 ± 17 months, the failure rate of the shunting device was 14% for the high-resistance valveless shunt compared with 46% for controls (P < .0002). Shunt endurance was 88% for patients with the valveless shunt and 60% for patients with conventional valve shunts. Signs of overdrainage developed in 40% of patients treated with valved shunts but apparently were not observed in patients with the high-resistance valveless shunt.
Gravitational Devices
This approach is not new (the Integra horizontal-vertical valve has been marketed for more than two decades), but recent improved designs have offered a graded transition (Aesculap proGav and shunt-assist valves) as well as a wider selection of the low- and high-pressure (fixed) valve settings. If used alone without a series adjustable differential pressure valve, gravitational devices do not prevent overdrainage or underdrainage clinical conditions.35 It was subsequently recommended that these gravitational devices be used in series distal to an adjustable differential pressure valve, although this too has been beset with technical problems.36 Our preliminary experience with the add-on Aesculap shunt-assist valve is that like the Delta ASD device, it is effective in alleviating overdrainage headaches.
Shunt Overdrainage and Underdrainage Defined
Overdrainage
Overdrainage symptoms are equivalent to a post–lumbar puncture or “spinal” headache. We know from the lumbar puncture literature that depending on needle size and design, the incidence of post–dural puncture headaches is 1% to 30%.37 Presumably, most subjects after lumbar puncture experience some period of intracranial hypotension, but only a minority are sensitive to the state. This means that the mere presence of negative intracranial pressure is not pathognomonic of overdrainage. In fact, our studies and those of others8,20 document that some degree of intracranial hypotension is the norm in shunted patients (data exist primarily for differential pressure valve shunts), but only a small percentage complain of postural headaches.
Key Point
The development of subdural fluid collections is a second possible manifestation of shunt overdrainage. Subdural hygroma (also known as effusion) formation is relatively common in the shunted NPH population. Small subdural hygromas (<5 mm) are usually asymptomatic15 and are often associated with improvement in NPH symptoms because they occur only in conjunction with reduction of the ventricular system. As a result, the presence of a subdural hygroma is not by itself diagnostic of shunt overdrainage. Expanding or large subdural hygromas are more worrisome and, many would agree, are risk factors for the development of acute hemorrhage (subdural hematoma). A non–trauma-related subdural hematoma in a shunted patient is obviously an overdrainage presentation.
“Slit” or collapsed ventricles are typically a manifestation of chronic overdrainage. Clearly, not all patients with slit (or unilateral slit) ventricles are symptomatic, but it is generally agreed that this state increases the risk of ventricular shunt obstruction. The apposition of the ventricular catheter to the ventricular wall increases the chance of ingrowth of ependymal cells or choroid plexus. The adult slit ventricle syndrome is an ill-defined disorder, but the key components are “slit” or “collapsed” ventricles seen on computed tomography or MRI in a symptomatic, shunted patient. The incidence is unknown but represents about 5% of the non-NPH evaluations in our clinic.7 Although relatively few in number, these patients represent a disproportionate amount of clinical effort expended with frequent emergency department visits and requests for office visits. The syndrome occurs more commonly in patients who have been shunted for many years, either as an adult or in childhood. In addition, it is our observation that a significant proportion of patients with adult slit ventricle syndrome have previously unrecognized noncommunicating hydrocephalus.
Underdrainage
For example, what if there is no clinical improvement in a patient with suspected NPH despite the valve’s being brought down to its lowest setting? After confirming shunt patency, many might consider such a patient a “nonresponder” and therefore by inference misdiagnosed. For patients in this scenario who remain with significant ventriculomegaly, the low-pressure hydrocephalus state should be considered.25,29 For these patients, clinical improvement strongly coincides with reduction in the ventricular size, and only with significant negative intracranial pressure does reduction in the ventricular size occur. Not surprisingly, this state occurs with higher incidence in patients with ASDs.24–29
If underdrainage is suspected, shunt obstruction is always a consideration. Based largely on the experience with pediatric hydrocephalus, many neurosurgeons use nuclear medicine isotope studies to determine shunt patency.38–41 At our center, however, we have found fewer indications for this study, and it is now rarely ordered. The primary reason is that the results of the study seldom alter the clinical decision tree. As noted before, many cases of shunt malfunction are readily identified on the basis of the history or imaging findings, and a “confirmatory” patency study is not needed and perhaps is relatively contraindicated. In more clinically challenging cases, the question of shunt patency arises in association with the possible nonresponder. If the patient remains with unchanged (large) ventricle size and has not improved clinically (with a reasonable suspicion of clinical hydrocephalus), the results of a nuclear medicine study will unlikely alter the management plan. If no flow is found (which could be a false-positive finding because CSF drainage may occur only if the patient is allowed to assume the upright position for some time), the patient needs a shunt revision. Even if shunt flow is documented, one should pursue other interventions. For example, if there is an ASD, remove it. If the patient has a fixed-pressure valve or a flow-restricting valve, change it to an adjustable differential pressure valve (no ASD). It is our observation that ventriculoatrial shunts provide more drainage than ventriculoperitoneal shunts do, and therefore we offer a shunt revision to a ventriculoatrial shunt as well. It is only the case in which the patient has a ventriculoatrial shunt with a differential pressure valve set to 30 mm H2O or less that an operative intervention is not recommended. Therefore, the results of a nuclear medicine study (positive or negative) likely would not obviate a shunt revision (for these selected patients).
Valve Selection
There are no evidenced-based guidelines to support any recommendations. If the prospective pediatric hydrocephalus valve studies1,3,4,23,42 are extrapolated to the adult population, no one valve design would appear to hold an advantage. Most would agree that NPH is clearly a distinct entity from pediatric hydrocephalus forms, and therefore the relevance of these studies can be challenged. On the basis of peer-reviewed published clinical studies and our large experience, we see no reasonable justification for not using an adjustable valve for NPH. Although adjustable valves are not the panacea, the use of a nonadjustable valve for the treatment of NPH exposes the patient to an unacceptable underdrainage or overdrainage risk.
The second decision in valve selection is choosing the initial opening pressure. Our experience during the past decade is largely limited to the use of an adjustable, stand-alone differential pressure valve. For NPH, it is our experience that approximately 96% of patients are aptly treated with a valve pressure range between 30 and 200 mm H2O. About 2% will have clinical overdrainage despite a valve pressure setting of 200 mm H2O, and the other 2% will have underdrainage at a setting of 30 mm H2O. An adjustable valve with a range from 10 to 240 mm H2O would meet the needs of more than 99% of NPH patients on the basis of our experience. In our practice,7,24 the valve pressure for all NPH patients is initially set at 200 mm H2O, and the opening pressure is then sequentially lowered to effect.
Shunt Configuration
Cerebrospinal Fluid Access
For routine shunt placement, we prefer a frontal (precoronal) ventricular puncture shunt rather than a posterior or occipital shunt. A retrospective analysis of shunt operations from the U.K. Registry study demonstrated that frontal catheters were adequately placed in 67% of cases, whereas occipital catheters were adequate in 52%.43 Moreover, we typically use frameless stereotaxis for the shunt ventricular catheter placement in patients with a bifrontal distance (maximum distance of lateral frontal horns) of less than 40 mm to increase the chances of optimal catheter placement. The tip of the catheter should reside just anterior to the ipsilateral foramen of Monro to keep it away from the choroid plexus. If the ventricles are large, the catheter is first positioned orthogonal to the skull, then angled slightly about 5 degrees anteriorly before the freehand insertion. The ideal depth is typically 6 cm of catheter at the dura.
Some neurosurgeons routinely use ventricular endoscopy to assist ventricular shunt placement. A multicenter trial demonstrated no benefit from this strategy.44 In our opinion, endoscopy is not a substitute for stereotaxis. A poor initial trajectory may not be remediable by attempted endoscopic catheter placement.
Distal Site
During the past decade, our center has performed a similar number of ventriculoperitoneal and ventriculoatrial shunts. We have found a nearly identical complication rate between the two techniques.7 We use a pragmatic decision-making process. If the patient is not obese and has no history (or probability) of peritoneal adhesions, a ventriculoperitoneal shunt is offered. Otherwise, a ventriculoatrial shunt is recommended. There is a growing literature on laparoscopy-assisted peritoneal catheter placement for obese patients and patients with peritoneal adhesions.45–49 For the very cases in which laparoscopy is indicated, a ventriculoatrial shunt can usually be performed instead. As a result, we have used laparoscopic assistance in only two cases during a 14-year period.
Ventriculoatrial Shunt Technique
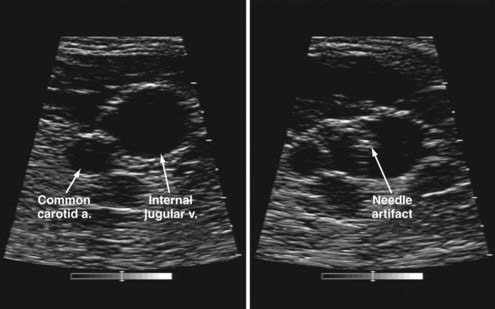
We routinely use a modified percutaneous technique.50–52 With use of a sterile intraoperative ultrasound unit53 to visualize the needle cannulation of the internal jugular vein (Fig. 35-4), only a 5-mm incision is needed. We use an 8 French peel-away vascular access kit and fluoroscopic visualization to place the tip of the catheter at the distal superior vena cava (we still use the term ventriculoatrial shunt for simplicity). We avoid placement of the catheter in the atrium to minimize the risk of sinus arrhythmias.
There continues to be reluctance for ventriculoatrial shunt placement. The perception that the infection rate is higher with ventriculoatrial shunts in comparison to ventriculoperitoneal shunts is not supported by the literature.7,54 One concern is shunt nephritis, an immune complex–mediated glomerulonephritis that results from long-term, subacute bacteremia (typically an indolent species, such as Staphylococcus epidermidis).55 During the last 15 years, with placement of more than 250 ventriculoatrial shunts, we have seen one case of documented shunt nephritis. This condition is not unique to ventriculoatrial shunts, having been reported in ventriculoperitoneal shunts as well.56,57 Patients present with fever of unknown origin and microscopic hematuria. It underscores the importance of a shunt tap in shunted patients in whom another obvious source of infection cannot be identified. Shunt cultures should be kept in the incubator for at least 5 days to identify indolent bacterial forms.
Ventriculoperitoneal Shunt Technique
As noted before, our center has extremely limited experience with laparoscopy-assisted placement of peritoneal catheters. It is our experience that with the approach described, the incision is small and not a cosmetic issue. Therefore, the rationale of using laparoscopy for cosmetic purposes is difficult to justify in our opinion. One of the known complications of ventriculoperitoneal shunts is retraction of the peritoneal catheter into the subcutaneous pocket underlying the wound. In this case, a laparoscopic technique proposed by Nfonsam and coworkers58 is appealing in that the shunt tunneler penetrates the peritoneum under laparoscopic visualization away from the open incision sites.
Infection Avoidance
Nearly every prospective pediatric population shunt study has reported an infection rate of approximately 8%.1,3,4,44 Less information is available about infection rates for adults. There are clearly multiple contributing factors for shunt infections, but given the highest incidence within the first month of surgery, the “contamination” most likely occurs at the time of the shunt surgery. A study by Kulkarni and associates59 suggested that many infections are iatrogenic, identifying surgical glove breaks as one of the likely and common culprits.60
Key Point
Meta-analyses support the routine use of perioperative intravenous antibiotics.61,62 We routinely use cephazolin (Ancef), although an argument could be made for an antibiotic with better central nervous system penetration.
Whether antibiotic-impregnated catheters reduce the incidence of shunt infections remains undetermined. A small prospective, randomized study assessing a catheter impregnated with rifampin-clindamycin found a reduced infection rate, although the control arm had a high rate of 16%.63 Arguments pertaining to creation of resistant organisms may have some merit, although this has not been documented to date with the use of special catheters. An analysis by Eymann and colleagues64 suggests that despite the incremental implant costs associated with the use of antibiotic-impregnated catheters, the overall reduction in infection-related costs made their use cost-beneficial. We routinely use antibiotic-impregnated catheters for all higher risk shunt operations (as defined earlier).
A third tier of antimicrobials is the instillation of intrathecal (intraventricular) antibiotics at the time of the shunt operation.65 This better addresses the possibility of CSF “contamination” at the time of surgery. Many of the common skin bacteria associated with shunt infections, such as coagulase-negative staphylococci and Propionibacterium acnes, are highly sensitive to antibiotics; therefore, giving a high concentration of intraventricular antibiotics at the time of the presumed contamination sounds reasonable to us. During the last 2 years, we have routinely used intrathecal antibiotics for all higher risk shunt operations (as defined earlier). The antibiotic solution (tobramycin, 8 mg, and vancomycin, 10 mg, in 6 mL of saline) is prepared by the hospital pharmacy in a sterile hood.
A wound breakdown or CSF leak increases the risk of (or is a sign of) shunt infection.59 The importance of planning the incision sites and configurations so as not to overlie any shunt hardware cannot be overemphasized. We do not use monopolar electrocautery (and avoid any coagulation) on skin incisions. Meticulous closure of the wounds is also important. For scalp wounds, following an interrupted layer of absorbable galeal sutures, we routinely close the skin with a 3-0 (or 4-0) running vertical mattress suture to best appose and align the skin edges.
Key Point
Shunt Allergies
True shunt allergies are rare. CSF often demonstrates persistent eosinophilia (3% to 36%), with negative cultures. Recurrent shunt failure is a common presentation. Pathologic examination of the ventricular catheter often demonstrates mechanical obstruction by inflammatory debris consisting of eosinophils and multinucleated giant cells. There are documented cases of immune responses to unpolymerized silicone in the literature.66–69 There are several management strategies. One is to consider an endoscopic third ventriculostomy and to remove the offending shunt. A second is to use a shunt system devoid of silicone, such as a polyurethane shunt system (Medtronic, Goleta, CA). We favor the use of hyperextruded silicone components (Medtronic). According to the manufacturer, many shunt allergies arise from a reaction to the oils used during the silicone manufacturing. A second extrusion cycle apparently effectively removes these oils that are otherwise present in trace amounts.
Recommendations for Specific Challenging Scenarios
High Protein Concentration or Cell Count in the Cerebrospinal Fluid
High CSF protein concentration alone does not appear to increase the incidence of shunt obstruction.69 It is the cell count (which is often associated with a high protein concentration) that is more problematic. Ideally, the fewer the total cells (specifically white blood cells) the better. Issues such as pleocytosis chronicity must be considered; therefore, it is not possible to assign an arbitrary cutoff point for cell number. For example, in a patient with coccidioidomycosis meningitis, you may have to accept CSF cell counts in the hundreds.
Patient Undergoing Anticoagulation or Antiplatelet Therapy
We make every effort to normalize the clotting profile before shunt implantation. Aspirin is stopped at least 8 days before surgery, whereas clopidogrel (Plavix) is stopped 14 days before surgery. Warfarin (Coumadin) therapy is reversed and, if necessary, enoxaparin (Lovenox) is temporarily prescribed and then discontinued 24 hours before surgery. A normal partial thromboplastin time and international normalized ratio are documented before skin incision. After surgery, warfarin can be restarted as early as 3 to 5 days postoperatively.70 It is critical to maintain a tightly controlled international normalized ratio. Aspirin or clopidogrel is restarted 7 to 10 days after surgery. In our opinion, combined aspirin-clopidogrel is contraindicated after shunt surgery because of the high risk of subdural hematoma.
Drake JM. Does double gloving prevent cerebrospinal fluid shunt infection? J Neurosurg. 2006;104:3-4.
Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294-303.
Dusick JR, McArthur DL, Bergsneider M. Success and complication rates of endoscopic third ventriculostomy for adult hydrocephalus: a series of 108 patients. Surg Neurol. 2008;69:5-15.
Ellegaard L, Mogensen S, Juhler M. Ultrasound-guided percutaneous placement of ventriculoatrial shunts. Childs Nerv Syst. 2007;23:857-862.
Eymann R, Chehab S, Strowitzki M, et al. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr. 2008;1:444-450.
Goodwin CR, Kharkar S, Wang P, et al. Evaluation and treatment of patients with suspected normal pressure hydrocephalus on long-term warfarin anticoagulation therapy. Neurosurgery. 2007;60:497-501.
1 Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294-303.
2 Kestle JR, Garton HJ, Whitehead WE, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105:177-181.
3 Kestle JR, Walker ML. A multicenter prospective cohort study of the Strata valve for the management of hydrocephalus in pediatric patients. J Neurosurg. 2005;102:141-145.
4 Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery. 1999;45:1399-1408.
5 Bergsneider M, Egnor MR, Johnston M, et al. What we don’t (but should) know about hydrocephalus. J Neurosurg. 2006;104:157-159.
6 Chahlavi A, El-Babaa SK, Luciano MG. Adult-onset hydrocephalus. Neurosurg Clin North Am. 2001;12:753-760. ix
7 Bergsneider M, Miller C, Vespa PM, Hu X. Surgical management of adult hydrocephalus. Neurosurgery. 2008;62:SHC643-660.
8 Bergsneider M, Yang I, Hu X, et al. Relationship between valve opening pressure, body position, and intracranial pressure in normal pressure hydrocephalus: paradigm for selection of programmable valve pressure setting. Neurosurgery. 2004;55:851-858.
9 Cook SW, Bergsneider M. Why valve opening pressure plays a relatively minor role in the postural ICP response to ventricular shunts in normal pressure hydrocephalus: modeling and implications. Acta Neurochir Suppl. 2002;81:15-17.
10 Dusick JR, McArthur DL, Bergsneider M. Success and complication rates of endoscopic third ventriculostomy for adult hydrocephalus: a series of 108 patients. Surg Neurol. 2008;69:5-15.
11 Aleman J, Jokura H, Higano S, et al. Value of constructive interference in steady-state three-dimensional, Fourier transformation magnetic resonance imaging for the neuroendoscopic treatment of hydrocephalus and intracranial cysts. Neurosurgery. 2001;48:1291-1295.
12 Kurihara N, Takahashi S, Tamura H, et al. Investigation of hydrocephalus with three-dimensional constructive interference in steady state MRI. Neuroradiology. 2000;42:634-638.
13 Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22:67-93.
14 Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from the ventricle to jugular vein. Surg Forum. 1952;2:399-403.
15 Boon AJ, Tans JT, Delwel EJ, et al. Dutch Normal-Pressure Hydrocephalus Study: randomized comparison of low- and medium-pressure shunts. J Neurosurg. 1998;88:490-495.
16 Zemack G, Romner B. Adjustable valves in normal-pressure hydrocephalus: a retrospective study of 218 patients. Neurosurgery. 2002;51:1392-1400.
17 Miyake H, Ohta T, Kajimoto Y, Nagao K. New concept for the pressure setting of a programmable pressure valve and measurement of in vivo shunt flow performed using a microflowmeter. Technical note. J Neurosurg. 2000;92:181-187.
18 Portnoy HD, Schulte RR, Fox JL, et al. Anti-siphon and reversible occlusion valves for shunting in hydrocephalus and preventing post-shunt subdural hematomas. J Neurosurg. 1973;38:729-738.
19 Watson DA. The Delta Valve: a physiologic shunt system. Childs Nerv Syst. 1994;10:224-230.
20 Chapman PH, Cosman ER, Arnold MA. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts: a telemetric study. Neurosurgery. 1990;26:181-189.
21 Portnoy HD. The merits of the antisiphon device. Neurosurgery. 1991;28:167.
22 Portnoy HD, Tripp L, Croissant PD. Hydrodynamics of shunt valves. Childs Brain. 1976;2:242-256.
23 Kestle J, Drake J, Milner R, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230-236.
24 Bergsneider M, Black PM, Klinge P, et al. Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:S29-S39.
25 Bergsneider M, Peacock WJ, Mazziotta JC, Becker DP. Beneficial effect of siphoning in treatment of adult hydrocephalus. Arch Neurol. 1999;56:1224-1229.
26 da Silva MC, Drake JM. Complications of cerebrospinal fluid shunt antisiphon devices. Pediatr Neurosurg. 1991;17:304-309.
27 McCullough D, Wells M. Complications with antisiphon devices in hydrocephalics with ventriculoperitoneal shunts. In: Epstein F, Raimondi A, editors. Concepts in Pediatric Neurosurgery. Basel: Karger; 1982:63-75.
28 McCullough DC. Symptomatic progressive ventriculomegaly in hydrocephalics with patent shunts and antisiphon devices. Neurosurgery. 1986;19:617-621.
29 Pang D, Altschuler E. Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery (Baltimore). 1994;35:643-656.
30 Kondageski C, Thompson D, Reynolds M, Hayward RD. Experience with the Strata valve in the management of shunt overdrainage. J Neurosurg. 2007;106:95-102.
31 Weiner HL, Constantini S, Cohen H, Wisoff JH. Current treatment of normal-pressure hydrocephalus: comparison of flow-regulated and differential-pressure shunt valves. Neurosurgery. 1995;37:877-884.
32 Hanlo PW, Cinalli G, Vandertop WP, et al. Treatment of hydrocephalus determined by the European Orbis Sigma Valve II survey: a multicenter prospective 5-year shunt survival study in children and adults in whom a flow-regulating shunt was used. J Neurosurg. 2003;99:52-57.
33 Czosnyka Z, Czosnyka M, Pickard JD. Hydrodynamic performance of a new siphon preventing device: the SiphonGuard. J Neurol Neurosurg Psychiatry. 1999;66:408-409.
34 Sotelo J, Arriada N, Lopez MA. Ventriculoperitoneal shunt of continuous flow vs valvular shunt for treatment of hydrocephalus in adults. Surg Neurol. 2005;63:197-203.
35 Sprung C, Miethke C, Schlosser HG, Brock M. The enigma of underdrainage in shunting with hydrostatic valves and possible solutions. Acta Neurochir Suppl. 2005;95:229-235.
36 Meier U, Lemcke J. First clinical experiences in patients with idiopathic normal-pressure hydrocephalus with the adjustable gravity valve manufactured by Aesculap (proGAV(Aesculap)). Acta Neurochir Suppl. 2006;96:368-372.
37 Turnbull DK, Shepherd DB. Post–dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718-729.
38 May CH, Aurisch R, Kornrumpf D, Vogel S. Evaluation of shunt function in hydrocephalic patients with the radionuclide 99mTc-pertechnetate. Childs Nerv Syst. 1999;15:239-244.
39 O’Brien DF, Taylor M, Park TS, Ojemann JG. A critical analysis of “normal” radionucleotide shuntograms in patients subsequently requiring surgery. Childs Nerv Syst. 2003;19:337-341.
40 Vernet O, Farmer JP, Lambert R, Montes JL. Radionuclide shuntogram: adjunct to manage hydrocephalic patients. J Nucl Med. 1996;37:406-410.
41 Williams MA, Razumovsky AY, Hanley DF. Evaluation of shunt function in patients who are never better, or better than worse after shunt surgery for NPH. Acta Neurochir Suppl. 1998;71:368-370.
42 Drake J. The surgical management of pediatric hydrocephalus. Neurosurgery. 2008;62:SHC633-642.
43 Price S, Santarius T, Richards HK, et al. The accuracy of ventricular catheter placement: does it influence shunt revision rates? Cerebrospinal Fluid Res. 2006;3:S8.
44 Kestle JR, Drake JM, Cochrane DD, et al. Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg. 2003;98:284-290.
45 Al-Mufarrej F, Nolan C, Sookhai S, Broe P. Laparoscopic procedures in adults with ventriculoperitoneal shunts. Surg Laparosc Endosc Percutan Tech. 2005;15:28-29.
46 Bani A, Telker D, Hassler W, Grundlach M. Minimally invasive implantation of the peritoneal catheter in ventriculoperitoneal shunt placement for hydrocephalus: analysis of data in 151 consecutive adult patients. J Neurosurg. 2006;105:869-872.
47 Kavic SM, Segan RD, Taylor MD, Roth JS. Laparoscopic management of ventriculoperitoneal and lumboperitoneal shunt complications. JSLS. 2007;11:14-19.
48 Roth J, Sagie B, Szold A, Elran H. Laparoscopic versus non–laparoscopic-assisted ventriculoperitoneal shunt placement in adults. A retrospective analysis. Surg Neurol. 2007;68:177-184.
49 Schubert F, Fijen BP, Krauss JK. Laparoscopically assisted peritoneal shunt insertion in hydrocephalus: a prospective controlled study. Surg Endosc. 2005;19:1588-1591.
50 Britz GW, Avellino AM, Schaller R, Loeser JD. Percutaneous placement of ventriculoatrial shunts in the pediatric population. Pediatr Neurosurg. 1998;29:161-163.
51 Carol M, Robinson W, Harris BS. Percutaneous placement of ventriculoatrial shunts: four-year case experience. Neurosurgery. 1986;18:348-349.
52 Harrison MJ, Welling BG, DuBois JJ. A new method for inserting the atrial end of a ventriculoatrial shunt. Technical note. J Neurosurg. 1996;84:705-707.
53 Ellegaard L, Mogensen S, Juhler M. Ultrasound-guided percutaneous placement of ventriculoatrial shunts. Childs Nerv Syst. 2007;23:857-862.
54 Lam CH, Villemure JG. Comparison between ventriculoatrial and ventriculoperitoneal shunting in the adult population. Br J Neurosurg. 1997;11:43-48.
55 Haffner D, Schindera F, Aschoff A, et al. The clinical spectrum of shunt nephritis. Nephrol Dial Transplant. 1997;12:1143-1148.
56 Okoro BA, Ohaegbulam SC. Experience with ventriculo peritoneal shunts at the University of Nigeria Teaching Hospital, Enugu. East Afr Med J. 1995;72:322-324.
57 Vernet O, Rilliet B. Late complications of ventriculoatrial or ventriculoperitoneal shunts. Lancet. 2001;358:1569-1570.
58 Nfonsam V, Chand B, Rosenblatt S, et al. Laparoscopic management of distal ventriculoperitoneal shunt complications. Surg Endosc. 2008;22:1866-1870.
59 Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195-201.
60 Drake JM. Does double gloving prevent cerebrospinal fluid shunt infection? J Neurosurg. 2006;104:3-4.
61 Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34:87-92.
62 Langley JM, LeBlanc JC, Drake J, Milner R. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta-analysis. Clin Infect Dis. 1993;17:98-103.
63 Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 2003;99:831-839.
64 Eymann R, Chehab S, Strowitzki M, et al. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr. 2008;1:444-450.
65 Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105:242-247.
66 Hashimoto M, Yokota A, Urasaki E, et al. A case of abdominal CSF pseudocyst associated with silicone allergy. Childs Nerv Syst. 2004;20:761-764.
67 Hussain NS, Wang PP, James C, et al. Distal ventriculoperitoneal shunt failure caused by silicone allergy. Case report. J Neurosurg. 2005;102:536-539.
68 Jimenez DF, Keating R, Goodrich JT. Silicone allergy in ventriculoperitoneal shunts. Childs Nerv Syst. 1994;10:59-63.
69 Brydon HL, Hayward R, Harkness W, Bayston R. Does the cerebrospinal fluid protein concentration increase the risk of shunt complications? Br J Neurosurg. 1996;10:267-273.
70 Goodwin CR, Kharkar S, Wang P, et al. Evaluation and treatment of patients with suspected normal pressure hydrocephalus on long-term warfarin anticoagulation therapy. Neurosurgery. 2007;60:497-501.
* The senior author (M. B.) has received travel stipends from Codman & Shurtleff, Medtronic, and Sophysa. The senior author has served on Advisory Boards for Codman & Shurtleff and Medtronic. Clinically, the UCLA Adult Hydrocephalus Center uses Codman, Medtronic, Sophysa, Aesculap, and Integra products.