CHAPTER 193 Shunt Infections and Their Treatment
Hydrocephalus is a common neurological condition in children and accounts for approximately 2% of all pediatric hospital admissions.1 The real costs of these admissions are substantial, with total hospital charges estimated to be between $1.4 billion and $2 billion annually.1 Although shunt insertion greatly mitigates the long-term disability caused by hydrocephalus, the complications associated with shunt insertion are a challenge in the long-term management of these patients. One shunt-related complication is the postoperative occurence of infection, which leads to increased hospital stays, additional negative impacts on the patient’s developmental progress, and increased mortality.2 This chapter summarizes the clinical features of shunt infection, its treatment, the benefits of preventive measures, and the long-term outcomes of shunt infection in the pediatric population.
Infection Rates
General
Shunt infection rates differ among reported studies.2,3 Borgbjerg and associates3 studied 884 individuals (440 of whom were younger than 14 years) who underwent placement of a new shunt from 1958 to 1989. The infection rate in this group was 6.2% for the first postoperative month, with an overall rate of 7.4%. Robust data are available from randomized clinical trials evaluating shunt designs in patients recruited from several medical centers. In one study, 344 patients were prospectively randomized to receive three different shunt designs.4 Nearly 40% of newly inserted shunts failed in the first year, with a shunt infection rate of 8.1% over the follow-up period of 1 to 3 years. These data reflect the shunt infection rate following a single shunt procedure. If patients are followed for longer periods, the cumulative likelihood of shunt infection related to multiple shunt procedures ranges from 19% to 38%.5,6 Despite the presence of a self-reporting bias, the Hydrocephalus Association database demonstrated that nearly 40% of patients who had hydrocephalus for at least 10 years experienced at least one shunt infection.6 Although preventive measures (see later) may reduce shunt infection rates, a reasonable conclusion is that the overall infection rate for new shunts is between 3% and 8%. Compared to historical data, it seems that there has been a gradual decline in shunt infection rates.7 It is not entirelly clear what factors have contributed to this decline, although greater attention to sterile technique, preoperative antibiotics, and improved surgical technique may all play a role.8
Timing of Infection
Among pediatric patients, the majority of shunt infections occur relatively soon after operative placement of the shunt. In one of the larger series of pediatric patients with extended follow-up, Casey and colleagues5 reported that among children with shunted hydrocephalus who underwent a first shunt revision for infection, 92% of infections occurred within 3 months of the initial shunt placement. Similarly, in infants, who appear to have higher shunt infection rates, the majority of infections occurred in the first 3 months after surgery.9 However, in a longer term analysis from a randomized trial, there appeared to be delayed shunt infections that occurred 2 to 3 years after shunt insertion.10
Risk Factors
Although many variables are proposed to affect shunt infection rates, the most consistent factor is patient age, with neonates and very young children at greatest risk.5,11 In one cohort study, children 6 months or younger had a 19% rate of infection, versus 7% among older children; this finding is similar to the reports of other groups.5
A variety of explanations accounts for the increased shunt infection rate in very young children, including the presence of age-related changes in the density and identity of bacterial populations on the skin of neonates, as well as increased susceptibility to pathogens due to the relative deficiency of the neonatal immune system. Although maternal breast-feeding has been associated with the maintenance of immunoglobulin G levels in neonates, no data exist on the potential role of breast-feeding in reducing the risk of shunt infection. Some data suggest that more highly adherent strains of coagulase-negative Staphylococcus, the most prevalent organism in shunt infections, occur in neonates.12
Along with age, numerous other factors have been examined for their role in shunt infection, including the timing of shunt placement, educational level of the surgeon, length and time of surgery, use of antibiotics before and after surgery, surgical method for placement of the distal catheter, type of shunt, reason for shunting (e.g., posthemorrhagic versus congenital hydrocephalus), previous shunt history, spinal dysraphism, number of early revisions, and concurrent infection. In some studies, the reason for shunt placement5,9 and the presence of spinal dysraphism were associated with increased rates of infection.11 Two studies reported that patients with congenital hydrocephalus had lower rates of infection than did patients with either postinfectious or posthemorrhagic hydrocephalus. One study reported that half the children in the postinfectious and posthemorrhagic group had at least one episode of shunt infection by the end of 1 year.9 Although earlier studies suggested that the type of shunt affected infection rates, more recent randomized studies examining shunt types have not confirmed this finding. A caveat with regard to these results is that the findings are not consistent across individual studies, suggesting that other variables are leading to an observed bias.
With respect to surgical factors, there is a paucity of convincing data. Shunt infection requires the exposure of shunt hardware to an infectious agent, usually bacteria, that subsequently leads to an inflammatory response of varying severity. An infection can result from several sources: colonization at the time of surgery, skin breakdown and subsequent colonization, hematogenous spread, and retrograde infection involving either a perforated viscus or coincident peritoneal infection. The timing of shunt infection and the predominance of skin organisms suggest that colonization from the patient’s skin is the likely cause of most shunt infections. However, some data suggest that the patient’s own skin flora may not be the primary source of these bacteria.13,14 A postoperative cerebrospinal fluid (CSF) leak leads to a very high risk of shunt infection (odds ratio of 19), presumably by allowing a direct path from the patient’s skin to the shunt hardware.15
Clinical Evaluation
Imaging Studies
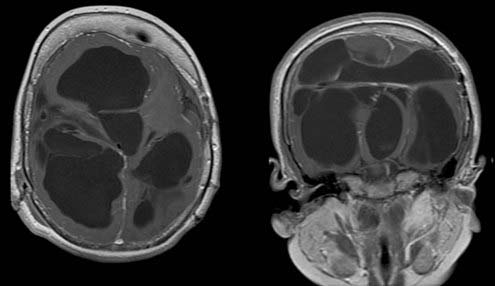
In many cases shunt infection causes some degree of shunt obstruction and results in findings consistent with that diagnosis, such as increased ventricular size. This is usually easily determined on an ultrasound study if the fontanelle is open or with computed tomography (CT) or magnetic resonance imaging (MRI). Complex shunt infections, associated with multicompartmental hydrocephalus, severe ventriculitis, or resistant or virulent organisms, result in dramatic findings (Fig. 193-1). Imaging studies are needed in these situations to determine whether cystic collections should be fenestrated or multiple catethers are required for effective CSF drainage.
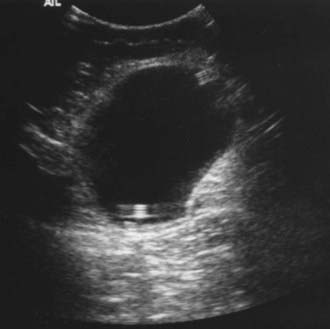
Peritoneal infections usually result in the formation of loculated fluid collections that can be detected by sonography or CT. Formation of an abdominal “pseudocyst” is usually caused by a localized reaction of the omentum and progressive accumulation of fluid within that space (Fig. 193-2). Drainage of the pseudocyst is rarely needed unless there is strong suspicion of a true abscess or the collection fails to resolve after treatment. Antibiotic treatment usually results in rapid improvement. It is our practice to confirm resolution of the fluid collection with sonography or CT before placement of a new shunt.
Laboratory and Bacteriologic Studies
CSF ventricular shunt catheter infections occur via three routes: contamination of the shunt material with skin organisms at the time of surgery, contamination from the bloodstream, and contamination along the shunt tubing from an abdominal source (generally associated with bowel perforation, although in some cases, no gross disruption of the bowel is observed). The source of the infection is reflected in the causative bacterial agent. The most common organisms found in shunt infections are typical skin flora such as coagulase-negative S. epidermidis,5,9,16 followed by S. aureus in a roughly 2 : 1 ratio. Interestingly, data suggest that in addition to its easy access by virtue of being a skin contaminant, S. epidermidis secretes a mucoid material that enhances its ability to adhere to foreign bodies such as shunt material.17 Shunt infections in which enteric organisms are isolated, including gram-negative bacteria such as E. coli, Proteus, and Klebsiella, are also common. Delayed infections with anaerobic diphtheroids such as Propionibacterium are particularly difficult to assess and treat because cultures may remain negative for more than a week.18 In the setting of repeated shunt failure, an indolent infection with Propionibacterium acnes should be considered, and CSF cultures should be followed for at least 1 week. Fungal infections are reported but are less common than bacterial infections. These often lead to repeated shunt failures and are notoriously difficult to treat, often requiring prolonged administration of antifungal agents.
Treatment
Most shunt infections caused by staphylococcal species do not cause significant tissue damage or a severe inflammatory response. Intravenous antibiotic treatment usually results in rapid bacteriogic clearance, with resolution of the CSF pleocytosis. Persistence of viable bacteria in or around the shunt hardware usually precludes the use of antibiotics as the sole treatment for shunt infections. The majority of shunt infections require surgical removal of the shunt, placement of an external CSF drain, and several days of intravenous antibiotics. A new shunt is usually not placed until CSF cultures are negative for bacterial growth. The recommended interval between shunt removal and reinsertion averages approximately 10 to 14 days, with at least 48 hours between the final negative CSF culture and reinsertion. A survey of centers to determine variations in practice patterns noted that the treatment time for shunt infection varied widely from 4 to 47 days.19
An alternative to surgical replacement of the infected shunt is the use of antibiotics alone. Although there have been cases of shunt infection eradicated by this method,20,21 purely medical treatment, administered either intravenously or by a combination of intravenous and intrathecal routes, appears to be less effective than surgical treatment.2,17,22 In the sole randomized trial to compare medical versus surgical treatment of shunt infection, 30 patients with ventriculoperitoneal shunts were randomized to one of three treatment arms: (1) removal of the shunt, accompanied by the use of systemic antibiotics and an interim period of external ventricular drainage or ventricular taps; (2) immediate surgical replacement of the infected shunt, along with antibiotic treatment; and (3) antibiotic treatment only. All study subjects were treated with intravenous and intraventricular antibiotics. The cure rates, as measured by negative CSF cultures 48 hours and 1 to 4 months after treatment, were 100%, 90%, and 30% for the three groups, respectively. In addition to lower rates of cure, medically treated patients had a mean hospital stay of 47 days, versus 33 and 25 days for the two surgical groups, respectively.17
In general, studies reporting high rates of cure from the use of antibiotics alone are limited by small sample sizes or the failure to randomize patients in treatment groups. Although it is likely that some shunt infections can be treated by antibiotic therapy alone, there is often doubt about the organism’s identity at the time of diagnosis and a desire to avoid progression of a CSF infection. Prudence usually prompts the surgeon to proceed with shunt removal and broad-spectrum antibiotic coverage. Clearly, for resistant or recurrent infections, the intrathecal administration of antibiotics leads to increased antibiotic levels within the ventricular system; however, the role of routine intrathecal antibiotics in addition to intravenous antibiotics is unclear.23
Outcome
A primary concern is the success of treatment. Kestle and colleagues19 noted that the reinfection rate in their study was 26%. Two thirds of the reinfections were caused by the same organism, suggesting a failure to completely eradicate the original infection. Surprisingly, the reinfection rate was not affected by the duration of antibiotic therapy. This suggests that other factors such as resistance or lack of antibiotic penetration may account for reinfection.
An important question is whether patients with shunt infections have a poorer health-related quality of life as well as higher overall medical morbidity and mortality. Although some studies report no difference in the rate of death in children with infected versus noninfected shunts,5 the data from other studies suggest an increased mortality risk in the former. In a series of 108 infants presenting with hydrocephalus at birth and operated on from 1971 to 1981, the 10-year mortality rate was 71% in children who had a shunt infection, compared with 51% in children who did not.24 Similarly, Walters and colleagues2 reported a mortality rate of 34% among infected patients, versus 18% in a group of shunted patients without infection.
Few studies include data on the long-term outcomes for patients with shunts, and those that do provide conflicting results. Casey and colleagues5 followed 155 shunted hydrocephalic children for 10 years and found no evidence that the number of infection-related episodes affected the final intellectual outcome, with a good outcome defined as the child’s ability to attend school. Renier and associates,24 however, found that shunt infection was associated with poor functional results; the mean “late” postoperative IQ was 40 among children who had at least one shunt infection, versus an IQ of 60 among children without infection. There has been no examination of the possible correlation between intellectual outcome and clinical or demographic patient characteristics, such as the severity of infection or the age at which a child suffers an infection. Future studies are needed to examine these relationships in greater detail.
Prevention
The overwhelming preponderance of skin flora as the causative organisms in shunt infections suggests that minimizing contact between shunt equipment and the skin and strict adherence to sterile technique may reduce colonization by organisms. Specific intraoperative factors contributing to shunt infection were analyzed, with only postoperative CSF leak, prematurity, and a possible breach in the surgeons’ gloves reaching statistical significance.15
Data regarding the use of prophylactic antibiotics are strongly suggestive, but confirmation is lacking. Meta-analyses from pooled data suggest a 50% reduction in infection rates when prophylactic antiobiotics are used.25,26 In one study, the benefit of prophylactic antibiotics was lost if the baseline infection rate was below 5%. A recent systematic review noted that although there was a trend toward a reduction in infection rates with the use of antibiotics, there was no support for continuting antibiotics beyond 24 hours after surgery.27 All these analyses are limited by variations in technique and treatment of shunt infections.19
Recently, antibiotic-impregnated shunt (AIS) components have been introduced for use as temporary external ventricular catheters and implanted shunts.28 The antibiotics found in AISs include rifampin, clindamycin, and minocycline. A number of studies have shown a reduction in infection rates, but these studies are usually observational or cohort studies without the benefit of randomization to control for other changes in surgical technique. Nevertheless, some of these results are particularly compelling. For example, using AIS components in a population of very young infants, Sciubba and associates29 reported a shunt infection rate of 4.6% (5 of 74 patients) with at least 9 months’ follow-up. This represents a substantial reduction when compared with historical controls. A similar finding was noted by other investigators who compared groups of patients receiving AIS components with their own historical cohorts.30,31 A particular benefit was noted in the neonatal subgroup receiving first-time shunts. In contrast, an observational study of mainly adult patients did not find any reduction of shunt infection rates using AIS components.32
Arnell K, Enblad P, Wester T, et al. Treatment of cerebrospinal fluid shunt infections in children using systemic and intraventricular antibiotic therapy in combination with externalization of the ventricular catheter: efficacy in 34 consecutively treated infections. J Neurosurg. 2007;107:213.
Borgbjerg BM, Gjerris F, Albeck MJ, et al. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien). 1995;136:1.
Casey AT, Kimmings EJ, Kleinlugtebeld AD, et al. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27:63.
Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294.
Duhaime AC, Bonner K, McGowan KL, et al. Distribution of bacteria in the operating room environment and its relation to ventricular shunt infections: a prospective study. Childs Nerv Syst. 1991;7:211.
Gupta N, Park J, Solomon C, et al. Long-term outcomes in patients with treated childhood hydrocephalus. J Neurosurg. 2007;106:334.
Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34:87.
James HE, Walsh JW, Wilson HD, et al. Prospective randomized study of therapy in cerebrospinal fluid shunt infection. Neurosurgery. 1980;7:459.
Kestle J, Drake J, Milner R, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230.
Kestle JR, Garton HJ, Whitehead WE, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105:177.
Kestle JR, Hoffman HJ, Soloniuk D, et al. A concerted effort to prevent shunt infection. Childs Nerv Syst. 1993;9:163.
Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195.
Langley JM, LeBlanc JC, Drake J, et al. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta-analysis. Clin Infect Dis. 1993;17:98.
Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg. 1992;77:29.
Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr. 2008;1:48.
Renier D, Sainte-Rose C, Pierre-Kahn A, et al. Prenatal hydrocephalus: outcome and prognosis. Childs Nerv Syst. 1988;4:213.
Simon TD, Riva-Cambrin J, Srivastava R, et al. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131.
Walters BC, Hoffman HJ, Hendrick EB, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60:1014.
Zabramski JM, Whiting D, Darouiche RO, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98:725.
1 Simon TD, Riva-Cambrin J, Srivastava R, et al. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131.
2 Walters BC, Hoffman HJ, Hendrick EB, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60:1014.
3 Borgbjerg BM, Gjerris F, Albeck MJ, et al. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien). 1995;136:1.
4 Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43:294.
5 Casey AT, Kimmings EJ, Kleinlugtebeld AD, et al. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27:63.
6 Gupta N, Park J, Solomon C, et al. Long-term outcomes in patients with treated childhood hydrocephalus. J Neurosurg. 2007;106:334.
7 Schoenbaum SC, Gardner P, Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975;131:543.
8 Kestle JR, Hoffman HJ, Soloniuk D, et al. A concerted effort to prevent shunt infection. Childs Nerv Syst. 1993;9:163.
9 Dallacasa P, Dappozzo A, Galassi E, et al. Cerebrospinal fluid shunt infections in infants. Childs Nerv Syst. 1995;11:643.
10 Kestle J, Drake J, Milner R, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33:230.
11 Ammirati M, Raimondi AJ. Cerebrospinal fluid shunt infections in children. A study on the relationship between the etiology of hydrocephalus, age at the time of shunt placement, and infection rate. Childs Nerv Syst. 1987;3:106.
12 Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg. 1992;77:29.
13 Duhaime AC, Bonner K, McGowan KL, et al. Distribution of bacteria in the operating room environment and its relation to ventricular shunt infections: a prospective study. Childs Nerv Syst. 1991;7:211.
14 Shapiro S, Boaz J, Kleiman M, et al. Origin of organisms infecting ventricular shunts. Neurosurgery. 1988;22:868.
15 Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195.
16 Rotim K, Miklic P, Paladino J, et al. Reducing the incidence of infection in pediatric cerebrospinal fluid shunt operations. Childs Nerv Syst. 1997;13:584.
17 James HE, Walsh JW, Wilson HD, et al. Prospective randomized study of therapy in cerebrospinal fluid shunt infection. Neurosurgery. 1980;7:459.
18 Arnell K, Cesarini K, Lagerqvist-Widh A, et al. Cerebrospinal fluid shunt infections in children over a 13-year period: anaerobic cultures and comparison of clinical signs of infection with Propionibacterium acnes and with other bacteria. J Neurosurg Pediatr. 2008;1:366.
19 Kestle JR, Garton HJ, Whitehead WE, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105:177.
20 Brown EM, Edwards RJ, Pople IK. Conservative management of patients with cerebrospinal fluid shunt infections. Neurosurgery. 2006;58:657.
21 Lerman SJ. Haemophilus influenzae infections of cerebrospinal fluid shunts. Report of two cases. J Neurosurg. 1981;54:261.
22 Arnell K, Enblad P, Wester T, et al. Treatment of cerebrospinal fluid shunt infections in children using systemic and intraventricular antibiotic therapy in combination with externalization of the ventricular catheter: efficacy in 34 consecutively treated infections. J Neurosurg. 2007;107:213.
23 Varelas PN, Rehman M, Pierce W, et al. Vancomycin-resistant enterococcal meningitis treated with intrathecal streptomycin. Clin Neurol Neurosurg. 2008;110:376.
24 Renier D, Sainte-Rose C, Pierre-Kahn A, et al. Prenatal hydrocephalus: outcome and prognosis. Childs Nerv Syst. 1988;4:213.
25 Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34:87.
26 Langley JM, LeBlanc JC, Drake J, et al. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta-analysis. Clin Infect Dis. 1993;17:98.
27 Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr. 2008;1:48.
28 Zabramski JM, Whiting D, Darouiche RO, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. 2003;98:725.
29 Sciubba DM, Noggle JC, Carson BS, et al. Antibiotic-impregnated shunt catheters for the treatment of infantile hydrocephalus. Pediatr Neurosurg. 2008;44:91.
30 Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 2003;99:831.
31 Hayhurst C, Cooke R, Williams D, et al. The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst. 2008;24:557.
32 Ritz R, Roser F, Morgalla M, et al. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007;7:38.