CHAPTER 103 Short Bowel Syndrome
ETIOLOGY
The major causes of SBS in adults are Crohn’s disease for which multiple intestinal resections have been performed; mesenteric infarction from venous or arterial thrombosis, arterial embolism, or midgut volvulus; massive enterectomy performed to manage traumatic injuries or tumor resection, and radiation injury (Table 103-1). The causes of SBS in the pediatric population are congenital abnormalities (see Chapter 96), including gastroschisis, intestinal atresia, malrotation, aganglionosis, and necrotizing enterocolitis. More than 90% of infants now survive the extensive intestinal resections required for these conditions, and these patients need careful follow-up for their SBS as they mature to adulthood. Intestinal failure also can result from chronic intestinal pseudo-obstruction syndrome in both adults and children (see Chapter 120), as well as from unclassified sprue in adults (see Chapter 104) and congenital villus atrophy in children.
Table 103-1 Causes of Short Bowel Syndrome and Intestinal Failure
| In Adults |
* Functional short bowel syndrome also can occur in conditions associated with severe malabsorption, in which the bowel length often is intact.
INCIDENCE AND PREVALENCE
The incidence of SBS is difficult to assess in the United States, because of a lack of a national registry for affected persons and a lack of prospective studies in defined populations of patients who have undergone extensive intestinal resections. The incidence of severe SBS necessitating long-term parenteral nutrition is estimated to be 2 to 4 cases per 1 million persons per year, based on multinational European data.1 It is estimated that between 10,000 and 20,000 patients in the United States follow a home parenteral nutrition regimen for SBS. Approximately 50% to 70% of patients with SBS who initially require parenteral nutrition can be weaned from this therapy and therefore might not be reflected in the prevalence estimates.2,3 Such patients often still require aggressive nutritional monitoring. The incidence and prevalence of SBS associated with Crohn’s disease are decreasing now that infliximab and strictureplasty have become commonplace.
PATHOPHYSIOLOGY
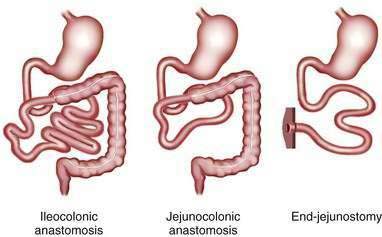
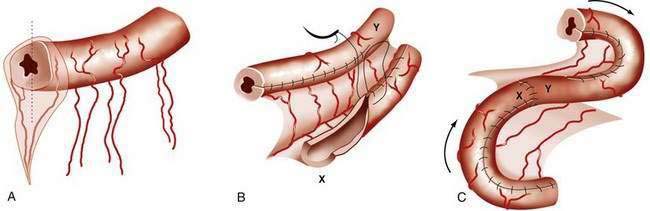
The major consequence of extensive intestinal resection is loss of absorptive surface area, which results in malabsorption of macronutrients, micronutrients, electrolytes, and water.4 The degree of malabsorption is determined by the length of the remnant intestine; the specific portions of small and large intestine resected, along with their site-specific transport processes and endocrine cells; and the adequacy of adaptive processes in the residual intestine over time. Three types of intestinal resections typically are encountered: limited ileal resection for Crohn’s disease, often with cecectomy or right hemicolectomy; extensive ileal resection with or without partial colectomy and with jejunocolonic anastomosis; and extensive small intestinal resection and total colectomy resulting in proximal jejunostomy (Fig. 103-1). Patients in the two latter groups commonly suffer from Crohn’s disease or had mesenteric infarction.
LOSS OF ABSORPTIVE SURFACE AREA
Nutrient Malabsorption
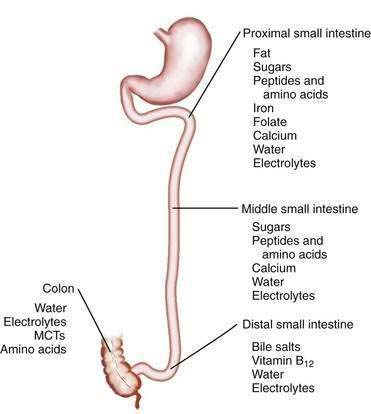
The length of the small intestine is estimated at 3 to 8 meters, and nutrient absorption is preserved until more than one half of the small intestine is resected.5–9 Most macronutrients (carbohydrate, fat, and nitrogen) are absorbed in the proximal 100 to 150 cm of intestine.10 Specific areas of absorption in the small intestine of nutrients, minerals, vitamins, electrolytes, and trace elements are discussed in Chapters 99 to 101 and are illustrated in Figure 103-2. Enterocytes lining the small intestine appear uniform from the duodenum to the ileocecal valve, but a distinct proximal-to-distal gradient exists in both morphology and function.11 Villi are taller and crypts are deeper in the jejunum than in the ileum, and the activity of microvillus enzymes and nutrient absorptive capacity per unit length of intestine are several-fold higher in the proximal than in the distal small intestine; loss of part of the jejunum initially compromises nutrient absorption more than does loss of an ileal segment of similar length, because of these morphologic and functional differences. The ileum, however, eventually is able to compensate for jejunal loss, whereas the jejunum is unable to compensate for ileal absorption of bile salts and vitamin B12.
Normal digestion and absorption depend on the gradual gastric emptying of partially digested nutrients, mixing of these nutrients with bile and pancreatic enzymes in the duodenum, and rapid digestion and absorption of the digestive products in the proximal small intestine. Patients with a proximal jejunostomy have rapid gastric emptying of liquids and rapid intestinal transit, which can compromise the gastric phase of digestion and result in inadequate mixing with biliary and pancreatic secretions, insufficient enzymatic digestion, and nutrient maldigestion. Rapid intestinal transit decreases nutrient-enterocyte contact time, and therefore, segmental absorption is decreased. Patients with a high jejunostomy are net secretors of salt and fluid, because jejunal fluid secretion is stimulated by oral intake and subsequent gastric emptying of nutrients; these patients excrete more fluid than they ingest, and accordingly, their fluid management may be challenging.12
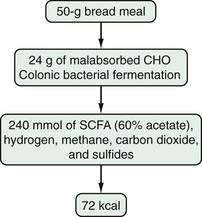
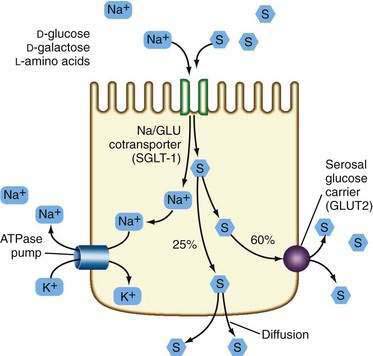
Most patients whose jejunum is shorter than 100 cm and who have no colon require long-term parenteral nutrition. Preservation of even some colon at surgery is highly beneficial for nutrient absorption. The ileocecal valve acts as a brake to slow intestinal transit, thereby increasing nutrient-enterocyte contact time and enhancing absorption. In addition, malabsorbed carbohydrates are fermented by bacterial enzymes in the colon to short-chain fatty acids (SCFAs), which are readily absorbed and used by colonocytes (Fig. 103-3). It has been estimated that this intracolonic digestive process can generate up to 1000 kcal (4.2 MJ) per day; in energy supply (Fig. 103-4), 1.0 MJ equals 238.8 kcal.13–15 Small intestine should be anastomosed to colon as soon as the patient is stable.
Water and Electrolyte Malabsorption
Loss of intestinal absorptive surface area can result in significant stomal or fecal losses of electrolytes, water, minerals, and trace elements (Table 103-2). The proximal small bowel receives approximately 7 to 9 L daily of water and electrolytes from food and secretions each day, of which 6 to 8 L are reabsorbed (see Chapter 99). On unrestricted diets, patients with a proximal jejunostomy cannot reabsorb such large volumes, a consequence of which is that voluminous diarrhea develops, often complicated by hypovolemia, hyponatremia, and hypokalemia. For example, in one study,12 the diarrheal volume in six jejunostomy patients with a mean jejunal length of 50 cm ranged from 3.2 to 8.3 L/day when they were allowed free access to food and water. All six patients were in negative sodium and water balance, four of the six were in negative potassium balance, and all six required parenteral nutrition with electrolyte replacement and restriction of oral intake of food and water to avoid unacceptable stomal losses. In the same study,12 seven of nine other jejunostomy patients who had a mean jejunal length of 120 cm were able to maintain positive water and sodium balance under the same conditions; absorption of water, sodium, and potassium in these 15 jejunostomy patients was correlated with jejunal length. At least 100 cm of intact jejunum is required to maintain positive water and electrolyte balance, similar to the length of jejunum required for nutrient absorption.
Table 103-2 Daily Stomal or Fecal Losses of Electrolytes, Minerals, and Trace Elements in Severe Short Bowel Syndrome*
| COMPONENT | AMOUNT LOST |
|---|---|
| Sodium | 90-100 mEq/L |
| Potassium | 10-20 mEq/L |
| Calcium | 772 (591-950) mg/day |
| Magnesium | 328 (263-419) mg/day |
| Iron | 11 (7-15) mg/day |
| Zinc | 12 (10-14) mg/day |
| Copper | 1.5 (0.5-2.3) mg/day |
* For sodium and potassium, the average concentration per liter of stomal effluent is given. The values for minerals and trace elements are mean 24-hour losses, with the range in parentheses. See text for details.
In general, patients with a proximal jejunostomy lose 90 to 100 mEq sodium and 10 to 20 mEq potassium per liter of stomal effluent (see Table 103-2).16 Some of these patients require long-term parenteral electrolyte and water supplements, often administered overnight, but others can maintain a positive balance by sipping a glucose-saline oral rehydration solution (ORS) throughout the day. The tight junctions of the jejunum are relatively leaky compared with the tight junctions of the ileum and colon, and therefore, a high NaCl concentration (greater than 90 mmol/L) is required in the glucose-saline solution to achieve net sodium and water absorption.17,18 Actively absorbed solutes (e.g., glucose, glucose polymers, galactose, oligopeptides, or l-amino acids) promote intestinal ion transport, although solutes also may be absorbed passively by means of solvent drag once active electrogenic Na+ absorption occurs.
Water transport into the enterocyte is directly proportional to Na+ transport. Na+ also is absorbed by means of an active electrogenic mechanism coupled with Cl− and H+ exchange and solvent drag. Absorptive and secretory processes occur simultaneously. Active Na+ secretion occurs against a concentration gradient from the enterocyte by means of the sodium pump, activated by Na+K+-ATPase (adenosine triphosphatase) in the basolateral membrane (Fig. 103-5). A mixture of 90 to 120 mmol/L NaCl and 50 mmol/L glucose is recommended, although such a solution might not be palatable. This mixture takes advantage of the coupled active transport of sodium with glucose and amino acids in the jejunum (see Chapter 99). Electrolyte and water absorption continue in the colon, and in normal humans only 100 to 150 mL of water is lost in the stool each day. The colon has a large reserve absorptive capacity for electrolytes and water, estimated to be 3 to 4 L of isotonic salt solution per day. Preservation of even part of the colon can reduce fecal electrolyte and water losses significantly in patients with SBS. A comparison of two groups of patients with similar jejunal length and jejunum either ending in a jejunostomy or anastomosis to colon showed that patients in the latter group were less likely to require oral or intravenous supplements.19
LOSS OF SITE-SPECIFIC TRANSPORT PROCESSES
Nutrient absorption potentially takes place at any level of the small intestine, albeit at different rates, owing to the proximal-to-distal gradient in functional activity of microvillus enzymes and transporters. The absorption of some compounds is restricted to certain areas of the small intestine (see Fig. 103-2); thus, calcium, magnesium, phosphorus, iron, and the water- and fat-soluble vitamins are absorbed predominantly in the duodenum and proximal jejunum (see Chapters 99 and 100).
Most patients with SBS have an intact duodenum and a variable length of jejunum, so the development of iron, phosphorus, or water-soluble vitamin deficiency, even in patients with a proximal jejunostomy, is relatively uncommon. Calcium absorption was found to be highly variable in a large study of patients with small intestinal resections.20 The net absorption of calcium (intake minus fecal loss) ranged from +573 to −268 mg/day, with a median of +65 mg/day; 64% of the patients, however, were in a negative calcium balance (intake minus fecal and urinary loss). In a study of 25 patients with a mean jejunal length of 128 cm, large-volume diarrhea (2 to 6 L/day), and steatorrhea,21 hypocalcemia and hypomagnesemia developed in 13 and 18 patients, respectively, during a trial of enteral hyperalimentation—despite supplementation with calcium, magnesium, and vitamin D. Malabsorption of calcium and magnesium is a consequence of fat malabsorption, because these minerals are precipitated intraluminally by unabsorbed long-chain fatty acids. Calcium and magnesium absorption improve on a low-fat diet in patients with small intestinal resections.22
The active absorption of vitamin B12 and bile acids is restricted to the ileum. The B12-intrinsic factor complexes and bile acids are taken up by specific transport proteins in ileal enterocytes (see Chapters 64 and 100). Most patients with SBS have lost part or all of the ileum, as a result of which vitamin B12 and bile acid malabsorption develops. The degree of malabsorption depends on the length of resected ileum. Vitamin B12 malabsorption usually is demonstrable when more than 60 cm of ileum has been resected.4 Resection of less than 100 cm of ileum causes moderate bile acid malabsorption and increased bile acid loss to the colon or in stomal effluents.23 The increased loss of bile acids to the colon induces electrolyte and water secretion and can exacerbate diarrhea. More-extensive ileal resections (greater than 100 cm) cause severe bile acid malabsorption, which, if bile acid loss exceeds hepatic synthesis, can result in a reduced bile acid pool size, with insufficient micellar solubilization of lipolytic products and resultant steatorrhea. Following extensive ileal resection, fat malabsorption develops, as can fat-soluble vitamin deficiency; essential fatty acid (linoleic acid) deficiency is rare. Loss of unabsorbed long-chain fatty acids to the colon can exacerbate diarrhea if the fatty acids are hydroxylated by colonic bacteria, because hydroxylated fatty acids stimulate colonic electrolyte and water secretion.24
LOSS OF SITE-SPECIFIC ENDOCRINE CELLS AND GASTROINTESTINAL HORMONES
The synthesis of gastrointestinal hormones in the intestinal mucosa is distributed in a site-specific manner along the gastrointestinal tract (see Chapter 1). Gastrin, cholecystokinin (CCK), secretin, gastric inhibitory polypeptide, and motilin are produced by endocrine cells in the proximal gastrointestinal tract and regulate secretory processes and motility. The area within which these hormones are synthesized usually is intact in patients with SBS, and hormonal profiles are normal. In approximately 50% of patients with extensive intestinal resections, however, hypergastrinemia and increased gastric acid secretion temporarily develop in the early postoperative phase.25,26 The cause of this postoperative hypergastrinemia is not known but could be loss of inhibitory signals, because it resolves spontaneously.
Glucagon-like peptides 1 and 2 (GLP-1 and GLP-2), neurotensin, and peptide YY (PYY) are produced in the ileum and proximal colon, and these intestinal segments are often lost in SBS patients.27 GLP-1 and GLP-2 and PYY are released by intraluminal fat and carbohydrates, cause a delay in gastric emptying, and slow intestinal transit (the ileal brake).28,29 Jejunostomy patients demonstrate impaired release of these hormones in response to a meal, rapid gastric emptying, and rapid intestinal transit of liquids.30,31 Patients with SBS and preserved colon have increased GLP-1 and GLP-2 concentrations and demonstrate normal gastric emptying.32 GLP-1 and GLP-2 and PYY also have been shown to inhibit gastric acid secretion and to promote intestinal growth in animal models.
LOSS OF THE ILEOCECAL VALVE
The primary functions of the ileocecal valve are to separate ileal and colonic contents, thereby minimizing bacterial colonization of the small intestine, and to regulate emptying of ileal contents into the colon. The ileocecal valve is removed in most ileal resections, as a consequence of which intestinal transit time decreases, and bacterial overgrowth is risked if the ileum is anastomosed to the colon. Bacterial overgrowth can worsen nutrient and cobalamin malabsorption (see Chapters 100 and 102), because bacteria compete with enterocytes for nutrient assimilation. Rapid intestinal transit in these patients, however, can counteract the risk of bacterial colonization. Studies are lacking to document the role of bacterial overgrowth in malabsorption in patients with SBS.
INTESTINAL ADAPTATION TO RESECTION
Adaptive changes in the remaining intestine after intestinal resection have been studied extensively in animal models and to a limited extent in humans33,34; adaptive changes are more pronounced in the ileum than in the jejunum. After jejunectomy and duodenoileal anastomosis, the ileum attains the morphologic characteristics of the jejunum, with taller villi and deeper crypts35; with time, an increase in ileal diameter and length also occurs. A prospective study of seven patients with jejunoileal bypass operation (20 cm of jejunum anastomosed to 25 cm of ileum) showed an increase in the length and diameter of the jejunum (80% and 40%, respectively) and ileum (128% and 50%, respectively) after 18 months of observation.36 An increase in absorptive capacity was demonstrated in another study of 41 patients with SBS (mean jejunal length, 119 cm) in whom the mean stool volume decreased from 2.5 to 0.9 L per day over a period of 3 months with continuous oral intake37; patients gained weight, and nitrogen balance increased from +3.2 g in the first month to +7.8 g in the second month postoperatively. The same study also demonstrated a gradual increase in intestinal transit time, which was most pronounced for ileal transit. The result of all of these changes is an increase in intestinal absorptive surface area, with an increase in microvillus enzyme activity and absorptive capacity per unit length of intestine.38 An improvement in mineral absorption with time also has been observed in a series of 30 patients with SBS (mean jejunal length, 81 cm) in whom fractional calcium absorption was correlated with time after surgery.39
In humans, these adaptive changes can take 1 to 2 years to develop fully. The younger the patient, the more profound the adaptive response. Adaptive changes depend on the presence of food and biliary and pancreatic secretions in the intestinal lumen40; adaptive hyperplasia of the ileum failed to develop in jejunectomized animals fed only by parenteral alimentation.41 To induce these adaptive processes, patients with SBS are encouraged to start oral intake as early as possible in the postoperative phase. Patients with SBS whose colon is in continuity demonstrate qualitative and quantitative changes in colonic flora that result in an increased capacity to metabolize carbohydrate and in an increased fecal bacterial mass.42
Adaptive hyperplasia is the result of an increase in crypt cell production rate, presumably mediated by growth factors released by the presence of food and secretions in the intestinal lumen. Vascular endothelial growth factor (VEGF), CCK, gastrin, insulin, neurotensin, GLP-2, and l-glutamine have been shown to stimulate intestinal growth in experimental animals;43–45 studies in humans have not indicated any value of supplemental glutamine to enhance intestinal adaptation.46,47 These extracellular growth factors stimulate polyamine synthesis in crypt cells, which in turn induces increased DNA synthesis and mitotic activity.48 Inhibition of polyamine synthesis in jejunectomized animals prevents adaptive changes in the ileum.49 Elucidation of the mediators regulating enterocyte proliferation eventually can lead to development of pharmacologic interventions that can accelerate intestinal adaptation in patients with SBS. The presence of comorbid conditions and the health of the residual bowel and its blood flow are important factors in the prognosis for patients who have undergone massive enterectomy.
MEDICAL MANAGEMENT
The initial management of the patient with SBS involves primarily supportive care designed to enhance the potential for survival. This care includes achievement of hemodynamic stability and appropriate fluid and electrolyte management. In the immediate postoperative phase, most patients with extensive intestinal resections are kept fasting and are supported with total parenteral nutrition (TPN). Weight and volume status are carefully monitored and stomal, fecal, and urinary losses of water, sodium, and potassium are measured to ensure optimal electrolyte and water balance. Histamine H2 receptor blockers or proton pump inhibitors are given intravenously to suppress hypergastrinemia-induced gastric acid hypersecretion and to limit volume losses.50,51 Patients with jejunostomies have stomal effluents up to several liters per day in this early phase, with obligatory losses of sodium, potassium, and possibly magnesium. Enteral tube feeding, followed by oral feeding, is begun in the late postoperative phase once the patient is hemodynamically stable, adequate intestinal blood flow has been restored, and postoperative ileus has resolved. Patients with extensive resections are kept fasting up to 5 to 10 days to allow a second-look operation at 24 to 48 hours, for the healing of enteric anastomoses, and to assess basal losses of water and electrolytes.
LIMITED ILEAL RESECTION
Patients with a limited ileal resection (less than 100 cm), with or without right hemicolectomy, may resume intake of solid food in the late postoperative phase. The response to solid food is determined mainly by the length of ileum removed and whether or not the right colon was resected; patients can develop diarrhea or steatorrhea with consumption of a regular diet. Secretory diarrhea without steatorrhea is the typical finding in limited ileal resections. Treatment with a bile acid-binding resin, such as cholestyramine (2-4 g with meals) or colestipol (1-2 g with meals) often ameliorates diarrhea if bile acid malabsorption is the main cause. Colestipol often is tolerated better than is cholestyramine. The diarrhea of some patients with limited ileal resection and right hemicolectomy does not respond to cholestyramine or colestipol despite documented bile acid malabsorption and presumably is due to loss of intestinal absorptive capacity for sodium chloride.52
Patients with documented fat malabsorption on a regular diet might have less severe steatorrhea while on a low-fat (40 g), high-carbohydrate diet; however, oral energy intake also will be reduced, because fat is calorically dense (9 kcal/g). Patients maintained on such a diet experience a decrease in diarrhea and steatorrhea and improve their net absorption of calcium, magnesium, and zinc.4 If necessary, medium-chain triglycerides (MCTs), which do not require micellar solubilization, can be added as a source of fat calories. The possibility of vitamin B12 malabsorption should be assessed with a Schilling test, and if the malabsorption is documented, parenteral B12, usually in a dose of 1 mg intramuscularly monthly, is required for life.
Malabsorption of fat-soluble vitamins, calcium, and magnesium is a risk in patients with fat malabsorption. Fourteen of 27 patients with ileal resections of 50 to 150 cm and an intact colon were in negative calcium balance when studied on a fixed daily calcium intake of 800 mg supplemented with 400 to 800 IU of vitamin D daily.21 Supplementation with vitamins, calcium, and possibly magnesium should be initiated before overt signs of vitamin deficiency or hypocalcemia and hypomagnesemia develop. Magnesium supplementation by mouth may be unrewarding, because magnesium is a cathartic. Although magnesium gluconate is water soluble and therefore may be the most readily absorbed magnesium salt, some patients still require periodic parenteral replacement. Magnesium deficiency can occur despite a normal serum concentration, because most Mg2+ is present in the intracellular space. Therefore, measurement of 24-hour urine Mg2+ concentration is prudent in patients who have suspected magnesium deficiency but normal serum Mg2+ concentration. Magnesium deficiency can result in calcium deficiency, because the release of parathyroid hormone is impaired in the presence of hypomagnesemia.53
Most patients with SBS already are in a negative calcium balance54 and therefore an oral supplement of calcium at a daily dose of 800 to 1500 mg is recommended. The tests to assess vitamin and mineral balance and recommended dosages in patients with malabsorption are discussed in Chapters 99 and 100. Absorption of water-soluble vitamins, carbohydrates, and proteins is, in general, not compromised in patients with limited ileal resections.
EXTENSIVE SMALL INTESTINAL RESECTION AND PARTIAL COLECTOMY
Management of Fluid and Electrolytes
Massive enterectomy is associated with gastric hypersecretion for approximately the first six months postoperatively. These patients benefit from the use of intravenous H2 antagonists or oral or intravenous proton pump inhibitors; absorption of orally ingested medications may be impaired, and more than the usual doses of these agents may be required (Table 103-3). Rapid intestinal transit contributes to malabsorption and diarrhea, and use of antidiarrheal drugs is common (see Table 103-3). These medications should be taken one hour before meals, and their effect on volume of diarrhea should be evaluated before long-term treatment is recommended.
Table 103-3 Therapeutic Agents Used to Decrease Intestinal Transit and Diarrheal Volume
| AGENT | DOSAGE |
|---|---|
| Loperamide* | 4-6 mg four times daily |
| Diphenoxylate-atropine* | 2.5-5 mg four times daily |
| Codeine phosphate* | 15-30 mg two to four times daily |
| Tincture of opium | 0.6 mL (2.5 mg) two to four times daily |
| Ranitidine† | 300 mg twice daily |
| Omeprazole‡ | 40 mg twice daily |
| Octreotide | 50-100 µg SC twice daily |
| Clonidine | 0.3 mg transcutaneous patch once weekly |
SC, subcutaneously.
* The antidiarrheal agents loperamide, diphenoxylate-atropine, and codeine are given 1 hour before meals and at bedtime. Dosages may be increased over those recommended, because of incomplete absorption in patients with short bowel syndrome.
† Cimetidine, famotidine, and nizatidine are alternatives.
‡ Esomeprazole, lansoprazole, rabeprazole, and pantoprazole are alternatives.
Use of antimotility agents is important to control fluid losses; such agents include loperamide hydrochloride (16 to 24 mg/day) and diphenoxylate (10 to 20 mg/day), codeine (30 to 240 mg/day), tincture of opium, and the somatostatin analog octreotide (50 to 100 µg two or three times a day). Most studies have shown that these agents reduce stomal output by up to 50%, but a positive water and electrolyte balance rarely is achieved. Octreotide usually is not necessary except for some patients with a proximal jejunostomy. Octreotide can slow intestinal transit and increase sodium and water absorption,55–58 but it also decreases splanchnic protein synthesis, thereby inhibiting postresectional intestinal adaptation57; the risk of cholelithiasis also is increased with octreotide.58 The α2-adrenergic agonist clonidine also may be useful to decrease diarrhea by its effects on chloride absorption. Transdermal administration avoids the potential for medication malabsorption.59 Glucose polymer-based ORSs should be provided to patients to improve hydration and thereby reduce TPN requirements.
Glucose and sodium are absorbed by the same active transport mechanism and stimulate absorption of each other. In addition, glucose promotes sodium and water absorption by means of solvent drag (see Fig. 103-5).60 Therefore, because the jejunum is permeable to both sodium and chloride, passively absorbed solutions that have a high sodium chloride concentration are absorbed to a significant degree; sodium is not as readily absorbed from isotonic or hypotonic solutions. A simple solution developed by the World Health Organization (WHO) can be formulated by dissolving 2.5 g of table salt, 1.5 g of KCl (requires a prescription), 2.5 g of sodium bicarbonate (NaHCO3), and 1.5 g of table sugar (sucrose) in 1 L of water. This solution provides a sodium concentration of approximately 90 mmol/L. Additional salt may be added to increase the osmolarity as tolerated, to 100 to 120 mmol/L or more, which can increase effectiveness.61 Sodium losses actually increase when solutions are consumed that contain less sodium than is in the small bowel effluent (90 mmol/L). The use of ORS is not as critical in patients in whom the colon is intact, provided that sufficient dietary sodium is present, because of the colon’s ability to absorb sodium and water. For patients who have had significant jejunal resections, the addition of glucose to the ORS is not critical, because glucose does not enhance ileal water absorption.62 In addition to sodium losses, significant quantities of bicarbonate and magnesium are lost in feces.
Management with Diet
Patients with SBS should be encouraged to eat substantially more than usual (a hyperphagia diet) to compensate for malabsorption; they might need to consume two to three times as much energy as that normally ingested before their abdominal catastrophe. This may be the single most important dietary intervention to reduce parenteral nutrition requirements. It has been suggested that patients may counterbalance the discomfort associated with increased fecal volume by the satisfaction of recovering relatively normal eating habits and requiring less parenteral nutrition.21 Patients also should be encouraged to eat small portions throughout the day, rather than at defined meal times. Separation of liquid and solid portions of meals is impractical and not associated with decreased fecal wet weight loss.
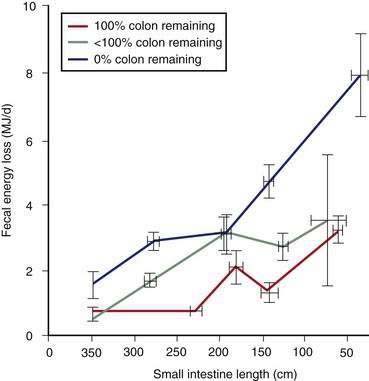
Patients with SBS whose colon is in continuity should be provided a high complex-carbohydrate diet that includes starch, nonstarch polysaccharides, and soluble fiber (Table 103-4). These foodstuffs typically are not absorbed by the human small intestine63; however, when they pass undigested into the colon, colonic bacteria ferment these foodstuffs into SCFAs such as butyrate, acetate, and propionate. Approximately 75 mmol of SCFAs are produced from 10 g of unabsorbed carbohydrate (see Fig. 103-3). Butyrate is the preferred fuel for the colonocyte.64 A person with an intact colon can absorb a wide range of up to 310 to 740 kcal (1.3 to 3.1 MJ) daily when fed a 60% carbohydrate diet.15 Other studies have indicated that up to 525 to 1170 kcal (2.2 to 4.9 MJ) daily can be absorbed by an intact colon from fermentation of unabsorbed carbohydrate and soluble fiber.13 The amount of energy absorbed is proportional to the amount of residual colon15,19 (see Fig. 103-4) and can increase as part of the adaptive response to enterectomy.43,65 During this adaptive period, colonic bacteria increase and β-galactosidase and other enzymes appear to increase in concentration or activity.42 Sodium and water absorption are stimulated by SCFAs as well, although decreased fecal fluid and sodium losses have not been documented clinically.15
Table 103-4 Macronutrient Requirements in Patients with Short Bowel Syndrome
| COLON PRESENT | COLON ABSENT |
|---|---|
| Carbohydrate | |
| Complex carbohydrates/starches | Variable types |
| 30-35 kcal/kg/day | 30-35 kcal/kg/day |
| Soluble fiber | |
| Fat | |
| MCT/LCT | LCT |
| 20%-30% of caloric intake | 20%-30% of caloric intake |
| Protein | |
| Intact protein | Intact protein |
| 1.0-1.5 g/kg/day | 1.0-1.5 g/kg/day |
LCT, long-chain triglycerides; MCT, medium-chain triglycerides.
When more than 100 cm of terminal ileum has been resected, fat maldigestion can develop, because bile salt malabsorption leads to decreased micelle formation, which results in poor fat solubilization. Use of bile salt replacement therapy with ox bile or a synthetic conjugated bile acid (cholylsarcosine) has been reported only in a few patients,66,67 decreasing fecal fat in most, but leaving fecal volume either unchanged or increased. The bile acid-sequestering agent cholestyramine may be useful to decrease bile salt-related diarrhea in patients with less than 100 cm of terminal ileum resected, but it can worsen steatorrhea in patients who have undergone a more-significant resection; because of its binding to dietary lipid,68 fat-soluble vitamin deficiency also can develop. In addition, cholestyramine binds to many medications, including warfarin, antibiotics, beta-blockers, diuretics, oral hypoglycemia agents, and others.
Limited data are available to support the use of low-fat diets in patients with massive enterectomy,69 although fat restriction often does lead to decreased steatorrhea in patients with limited terminal ileal resections. Because fat is concentrated energy, however, dietary fat restriction results in decreased energy intake and can worsen the patient’s energy balance; a low-fat diet also may be unpalatable. A high-fat diet, although having greater energy content than that of a high-carbohydrate diet, is associated with increased loss of divalent cations (Ca2+, Mg2+, and Zn2+),70 slows gastric emptying, and can induce early satiety, leading to reduced total energy intake. Diets high in fat also can lead to water secretion from the colon.
Because MCTs (C8 to C10, 8.3 kcal/g) are absorbed in the colon, dietary supplementation with MCTs can lead to increased energy absorption.71 MCT supplementation is of much more limited benefit in patients with an end-jejunostomy. MCTs also do not supply essential fatty acids, and excessive MCT intake is associated with nausea, vomiting, and ketosis.
Experience with long-term parenteral nutrition mainly has been gained in patients with severe SBS (see Chapter 5). Despite the limited adaptive capacity of the jejunum, approximately 50% of patients on a home parenteral nutrition regimen can discontinue TPN and resume oral intake after one to two years.72 The diet composition for patients with a jejunostomy on oral intake can be more liberal, because the percentages of energy absorption are similar for a low-fat, high-carbohydrate and a high-fat, low-carbohydrate diet.73,74 The average daily stomal losses of electrolytes, minerals, and trace elements in severe SBS are listed in Table 103-2.22,70,73,75,76
Water-soluble vitamins, except for vitamin B12, are absorbed in the proximal jejunum, as are macronutrients. It is unusual for deficiencies of these vitamins to develop, except in patients with a proximal jejunostomy or duodenostomy, and these patients invariably require TPN with vitamin supplementation. Loss of the ileum results in bile acid and vitamin B12 malabsorption, but patients who suffer such a loss as well as those with extensive jejunal resection also are at risk for more pronounced malabsorption of nutrients, minerals, vitamins, and electrolytes and water than is seen in patients with limited ileal resections, because of the greater loss of absorptive surface area and rapid intestinal transit. Fat-soluble vitamin (A, D, E, K) deficiency is encountered much more commonly as a result of concurrent fat malabsorption than as a result of loss of absorptive surface area. Most of the human daily vitamin K requirement is synthesized by colonic bacterial flora,77 so patients with any residual colon are a lower risk for developing vitamin K deficiency than are those whose colon has been resected. Conversely, patients who have received broad-spectrum antibiotics also are at risk for vitamin K deficiency. Zinc and selenium are lost in significant concentrations in the feces. The concentration of zinc is 12 mg/L in small bowel effluent and 16 mg/L in stool.78 Oral supplementation of vitamins, minerals, and trace elements generally is required for patients who do not require TPN (Table 103-5).
Table 103-5 Vitamin and Mineral Requirements* in Patients with Short Bowel Syndrome
| MICRONUTRIENT | REQUIREMENT |
|---|---|
| Vitamin A | 10,000-50,000 units daily* |
| Vitamin B12 | 1000 µg subcutaneously monthly for patients with terminal ileal resection or disease |
| Vitamin C | 200 mg daily |
| Vitamin D | 50,000 units 1,25(OH2)-D3 twice weekly to twice daily |
| Vitamin E | 30 International Units daily |
| Vitamin K | 10 mg weekly |
| Calcium | 1000-1500 mg daily |
| Magnesium | See text |
| Iron | As needed |
| Selenium | 60-150 µg daily |
| Zinc | 220-440 mg daily (sulfate or gluconate form) |
| Bicarbonate | As needed |
Note: The table lists rough guidelines only. Vitamin and mineral supplementation must be monitored routinely and tailored to the individual patient, because relative absorption and requirements can vary. Supplements may be taken orally unless otherwise indicated.
* Use cautiously in patients with cholestatic liver disease, because of the potential for liver toxicity.
Malabsorption of medication also occurs in patients with SBS.79,80 Many medications are absorbed in the jejunum, but medication malabsorption still can occur in patients who have undergone ileal resections alone because their intestinal transit time is decreased.
The loss of the ileocecal valve increases the risk of bacterial overgrowth in the small intestine, which can worsen nutrient absorption and make management more difficult. The ultimate goal is to ensure a stable condition in which all nutritional needs are met, preferably by oral intake alone. In a series of 38 patients with a jejunum shorter than 200 cm and in continuity with the colon, all those with a jejunal length of more than 100 cm could be managed on oral intake alone.19
Nitrogen is the least affected nutrient in SBS. Because absorption of dietary protein in the form of dipeptides and tripeptides occurs in the very proximal bowel, patients with only short segments of residual jejunum can benefit from the use of hydrolyzed protein or free amino acid-based enteral formulas. McIntyre and colleagues compared energy, nitrogen, and fat absorption in seven patients, each of whom had an end-jejunostomy, when they were provided with either a polymeric formula or a peptide-based formula. The length of residual jejunum in these patients ranged between 6 and 150 cm; no differences in nutrient absorption were observed.73 Similar uncontrolled observations were reported by Levy and coworkers.81 Contrary to these results, however, were those of Cosnes and associates, who reported modest improvement in six end-jejunostomy patients (mean residual small bowel length, 90 to 150 cm) who received a peptide-based diet, although energy absorption was unaffected.82 The patient populations in these studies were somewhat heterogeneous, and the peptide chain length and relative concentrations varied among formulas, making it difficult to compare the two studies.
Lactose malabsorption due to substantial loss of jejunal length can worsen diarrhea, but a study of 14 patients with SBS on either a lactose-free diet or a diet with 20 g of lactose per day showed no significant differences in stool volumes.83 Patients with SBS whose colon is in continuity should receive an oxalate-restricted diet (see “Calcium Oxalate Kidney Stones” under “Complications”) (Table 103-6).
Table 103-6 Dietary Recommendations for Patients Who Require an Oxalate-Restricted Diet
| FOODS CLASSIFIED BY OXALATE CONTENT | ||
|---|---|---|
| Little* or None (<2 mg per Serving) Eat as Desired |
Moderate (2-10 mg per Serving) Limit: Two 1/2-cup Servings per day |
High (>10 mg per Serving) Avoid Completely |
| Beverages | ||
HOME PARENTERAL NUTRITION
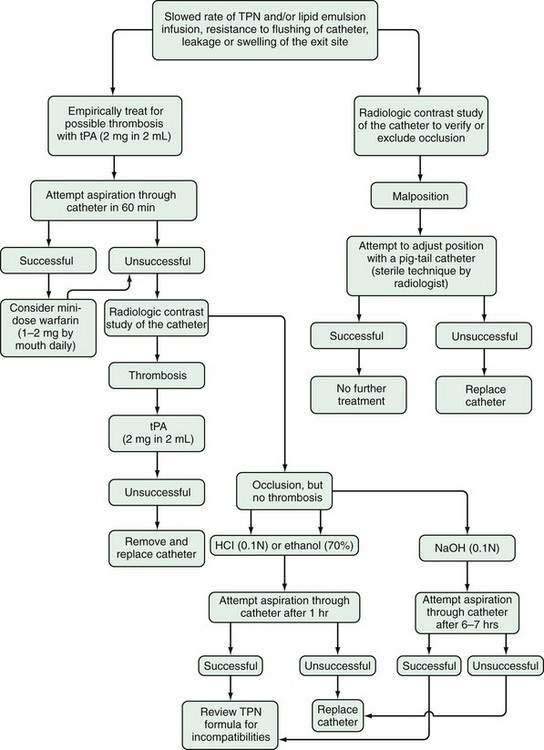
To prepare the patient for home TPN, the TPN regimen should be compressed gradually in two- to four-hour daily increments so that the total volume can be infused over a 10- to 12-hour period, typically overnight. Some patients with hyperglycemia or renal or congestive heart failure require a more prolonged infusion. The TPN infusion generally is tapered off over a 30- to 60-minute period to avoid hypoglycemia. Patients with a proximal jejunostomy might require additional fluid before or following completion of home TPN and, in some cases, during the day as well. TPN solutions are hypertonic and therefore must be infused into a central vein, such as the superior or inferior vena cava, through a tunneled catheter, to decrease the risks of infection and thrombosis.84,85 Percutaneously inserted central catheters (PICCs) should be reserved for short-term use (less than six months). For the patient to qualify for Medicare benefits, home TPN must be required for at least three months, and fat malabsorption as well as failed enteral nutritional support must be documented.
Patients often find it helpful to contact a local support group of the Oley Foundation (1-800-776-OLEY or www.oley.org). This independent, nonprofit organization includes patients and their families, as well as health care providers, and provides information, outreach services, emotional support, and conference activities. Physicians caring for patients on a home TPN regimen also should be familiar with TPN- and catheter-related complications and their recognition and treatment. These topics are beyond the scope of this chapter but have been reviewed in Chapter 5 and elsewhere (Figs. 103-6 and 103-7).86,87
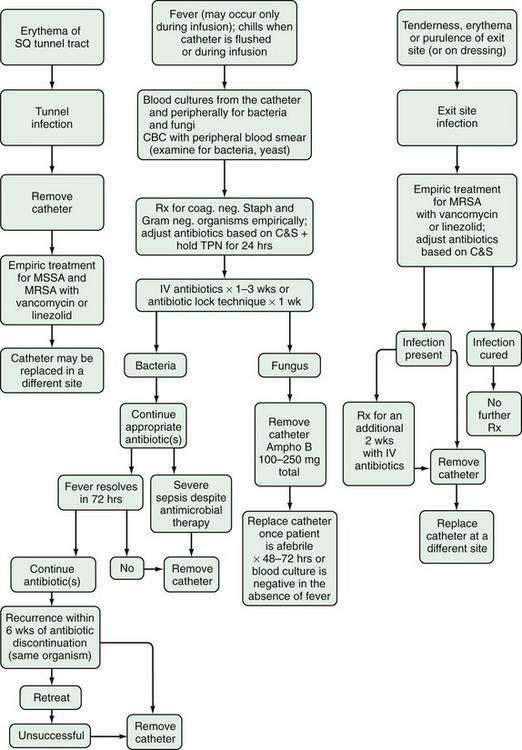
Patients in whom the frequency of TPN infusions can be reduced to fewer than five nights per week should have their micronutrient status monitored two to three times yearly to detect deficiencies. At the clinical visit, particular attention should be paid to the catheter exit site for evidence of erythema, purulent discharge, warmth, or tenderness. A catheter may remain in place indefinitely if it is properly maintained.88
COMPLICATIONS
GALLSTONES
Interruption of the enterohepatic circulation of bile acids by ileal resection results in decreased hepatic bile acid secretion and altered composition of hepatic bile in terms of its organic components: bile acid, cholesterol, and phospholipids (see Chapters 64 and 72). Hepatic bile becomes supersaturated with cholesterol, with subsequent formation of cholesterol crystals and gallstones in gallbladder bile (see Chapter 65). Most gallstones in patients with SBS, however, are composed of calcium bilirubinate; the pathophysiology is unclear. A prevalence of 44% of asymptomatic gallstones was documented in a study of 84 patients with severe SBS who required TPN.19 Formation of biliary sludge and gallbladder hypomotility probably contribute to the high prevalence of these stones, because many of these patients are on long-term parenteral nutrition.89 Postprandial CCK concentration is decreased in some patients with SBS,90 and injections of CCK have been used experimentally to induce gallbladder contraction, although this therapy is not always successful and results in nausea, vomiting, and abdominal pain in some patients.91,92
LIVER DISEASE
Liver disease often develops in patients who require long-term TPN. After five years of TPN, more than 50% of these patients will be found to have severe liver disease defined as grade 2 fibrosis, cirrhosis, or one of the following: total serum bilirubin greater than 3.5 mg/dL for longer than one month, ascites, portal hypertension, hepatic encephalopathy, or liver failure with a factor V concentration less than 50% of normal.93 Liver failure develops in approximately 15% of all TPN-dependent patients.94 The incidence, prevalence, and severity of liver disease in young children and infants, in particular, are much greater than in adults.95 The incidence and prevalence of liver disease and liver failure specifically in patients with SBS requiring TPN are unknown. Although these disorders often are referred to as TPN-associated liver disease, the pathogenesis probably is related to malabsorption of nutrients such as choline96 and to the route of nutrient assimilation, namely, through the central axis, rather than the portal circulation.97 Patients with the least amount of residual intestine are at greatest risk for developing liver disease.98,99
Diagnosis of liver disease related to intestinal failure in the patient with SBS requires the exclusion of other potential causative disorders. SBS-related liver disease can manifest as cholestasis, steatosis, or steatohepatitis; cholestasis is more common in infants. Studies have suggested benefit from oral lecithin, although it is poorly absorbed, and from intravenous choline (investigational) and, to a lesser extent, ursodeoxycholic acid.100–105 A recent uncontrolled case series of 18 infants described the substitution of a fish oil-based lipid emulsion for the conventional long-chain triglyceride-based emulsion. Compared with historic controls from a different era, reversal of cholestasis occurred sooner (9.4 vs. 44.1 weeks in the fish oil-supplemented group.106 Dextrose overfeeding (greater than 40 kcal/kg/ day) and excessive fat emulsion infusion (2.5 g/kg/day, possibly only 1.0 g/kg/day) should be avoided.93 A minimum of 2% to 4% of total calories, however, should be provided as linoleic fatty acid (50% of most lipid emulsions), to prevent essential fatty acid deficiency. Carnitine supplementation is not useful.107
CALCIUM OXALATE KIDNEY STONES
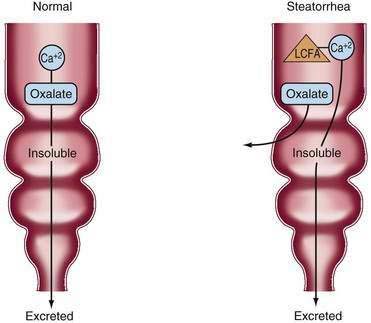
Fat malabsorption secondary to bile acid deficiency in patients with extensive ileal resection is associated with an increased risk of oxalate kidney stones if the colon is preserved. Oxalate in food usually precipitates as calcium oxalate in the intestinal lumen and is lost in the stool. Lipolysis in patients with SBS and fat malabsorption is normal, and unabsorbed long-chain fatty acids compete with oxalate for available luminal calcium. Consequently, a larger amount of free oxalate is lost to the colon, where it is absorbed and ultimately excreted by the kidney (Fig. 103-8), manifesting as just hyperoxaluria or with calcium oxalate stone formation. Patients with SBS who do not have a colon in continuity are not at increased risk. In one study, symptomatic kidney stones developed within two years of enterectomy in 9 of 38 patients (24%) with SBS and an intact colon.19 Urinary oxalate excretion should be monitored regularly in these patients. Treatment of hyperoxaluria consists of restriction of oxalate-containing food products (see Table 103-6). If hyperoxaluria persists, then oral administration of calcium citrate should be tried; the extra calcium precipitates dietary oxalate, and the citrate prevents stone growth in the urine. A single case report describes the use of conjugated bile acid supplementation to reduce hyperoxaluria.108 Hyperoxaluria also may be related to the metabolism of the vitamin C in TPN solution in the presence of light.109
D-LACTIC ACIDOSIS
d-Lactic acidosis is a rare complication of SBS and in this setting is observed only in patients with a preserved colon. The episodes of acidosis usually are precipitated by increased oral intake of refined carbohydrates and can be induced in the patient with SBS by carbohydrate overfeeding.110 Malabsorbed carbohydrate is metabolized by colonic bacteria to SCFAs and lactate, which lower the intracolonic pH. A lower pH inhibits the growth of the predominant Bacteroides species and promotes the growth of acid-resistant, Gram-positive anaerobes (Bifidobacterium, Lactobacillus, and Eubacterium), which have the capacity to produce d-lactate. d-Lactate is absorbed from the colon and is metabolized to only a limited extent in humans because of our lack of d-lactate dehydrogenase. The main excretory route for d-lactate is the kidney.111
Treatment consists of correcting the acidosis with sodium bicarbonate and stopping oral intake, which usually results in rapid abatement of the neurologic symptoms. The potential benefit of antibiotic treatment to change the colonic flora is debated. Substitution of refined carbohydrates for starch has prevented recurrent d-lactic acidosis in a few patients.112 The mediator of the neurologic symptoms still is unknown, and infusion of d-lactic acid in normal subjects to achieve blood levels commonly observed in patients with d-lactic acidosis does not cause any neurologic symptoms. The neurologic symptoms have a striking resemblance to those of Wernicke’s encephalopathy, and in one patient with SBS, recurrent d-lactic acidosis was prevented by thiamine supplementation.113
SURGICAL MANAGEMENT
INTESTINAL LENGTHENING PROCEDURES
The most important surgical procedure is reanastomosis of the residual small bowel to the residual colon. This procedure carries relatively low mortality and morbidity rates and allows enhanced energy absorption from SCFAs produced from the bacterial fermentation of unabsorbed carbohydrate. A number of other surgical procedures, such as tapering enteroplasty, construction of intestinal valves, creation of recirculating loops, reversal of a short intestinal segment, or colonic interposition, have been attempted to increase intestinal transit time. These procedures are considered experimental, the experience with each is limited, and outcomes generally are not optimal.1
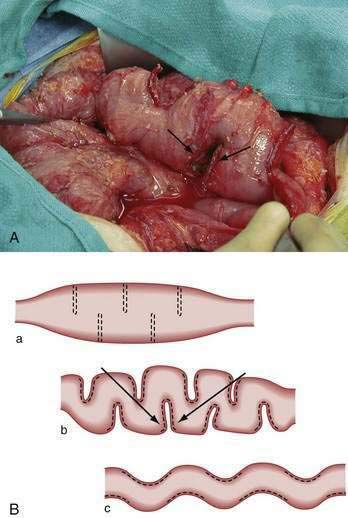
Longitudinal intestinal lengthening and tailoring (Bianchi procedure) (Fig. 103-9) may be useful in patients who have segmental dilation and nonfunctional intestine due to dysmotility and bacterial overgrowth. In this procedure, the surgeon divides the dilated bowel, creates two hemiloops, and anastomoses the hemiloops in an end-to-end fashion, thereby doubling the bowel length (Fig. 103-10).118 Although the surface area is not truly increased, bowel function can improve, allowing reduction or elimination of TPN. Nearly all of the approximately 100 operations reported have been undertaken in children. This procedure should be attempted only as a last resort before intestinal transplantation, and it should be performed only in centers with significant experience in this area. To date, no studies have been conducted to compare medical and surgical therapies.
A less complex procedure, the serial transverse enteroplasty (STEP), developed by Kim, is a novel technique during which a linear surgical stapler is applied from alternating and opposite directions along the intestine’s mesenteric border to incompletely staple and divide the dilated intestine (Fig. 103-11).119 This procedure leads to the tapering of the intestine in a zig-zag pattern, which results in nutrients being channeled along a narrower but longer intestine. Rather than an intestinal lengthening procedure, this technique is better described as an intestinal tapering procedure. Results reported from an international registry comprising 38 patients from 19 centers have indicated the procedure increases intestinal length by almost 50%, and it has resulted in close to a 100% increase in nutrient absorption.120,121 These improvements in some patients, however, may have been due in part to increased segmental absorption, observed as part of the natural postenterectomy adaptation process. Nevertheless, the tapering of a dilated, essentially nonfunctional loop of bowel might decrease bacterial overgrowth and improve nutrient absorption. Furthermore, the STEP might have an advantage over the Bianchi procedure in addition to being less technically demanding, in that by avoiding intestinal transection, it may be easier to preserve the blood supply of the intestine.
INTESTINAL TRANSPLANTATION
Intestinal transplantation is being performed in an increasing number of centers worldwide. The main indication for transplantation in children and adults is TPN-dependent SBS complicated by progressive liver disease. Combined intestine-liver transplantation is the only alternative for patients in whom end-stage liver disease has developed. Isolated intestinal transplantation may be considered for patients with clinically significant liver disease that has not yet progressed to cirrhosis.122 Patients who have significant fluid losses and who experience frequent episodes of severe dehydration despite appropriate medical management also may be candidates for isolated intestinal transplantation. Medicare has approved other indications, including two-major-vessel thrombosis, a single episode of fungemia, a single episode of bacterial sepsis with shock, and two lifetime episodes of catheter sepsis, although the preponderance of evidence does not support these as appropriate indications for transplantation.
Survival has improved considerably since intestinal transplantation was initiated, with reported survival and nutritional autonomy of up to 18 years.123 As of May 31, 2007 (most recent data from the International Small Bowel Transplant Registry), 1720 transplantation procedures had been performed worldwide in 1608 patients, 909 of whom were still alive. This experience included 746 isolated intestine, 594 intestine-liver, and 380 multivisceral transplants. Patients who have undergone transplantation more recently, generally have had better survival because of improved technique and optimized immunosuppressive regimens. Mean hospitalization was 56 days for isolated intestine, 76 days for intestine-liver, and 62 days for multivisceral transplant recipients. Additional information can be found on the International Intestinal Transplant Registry website, http://intestinaltransplant.org, which is updated every 2 years (last time in 2007). Current (2008) patient and graft survival data for the United States are presented in Table 103-7.
Table 103-7 Patient and Graft Survival Rates (%) for Transplants Performed for Short Bowel Syndrome from January 1988 to March 30, 2007, in the United States
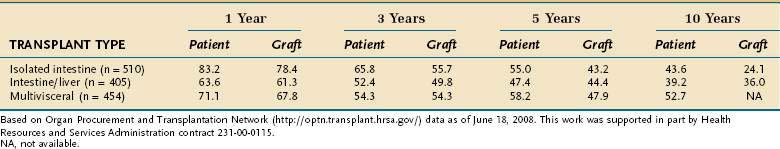
The mortality rate for patients waiting for an intestinal-liver transplant is significantly greater than for those waiting for an isolated liver transplant.1 Therefore, early referral to an intestinal transplantation center at the first sign of liver disease is recommended, even if a transplant does not ultimately become necessary. Intestinal and multiorgan transplantations are expensive and generally cost between $250,000 and $3 million per case. Post-transplantation complications, and the most common causes of death afterward, include acute rejection, chronic rejection, cytomegalovirus infection, sepsis (often complicating rejection), and post-transplantation lymphoproliferative disease (PTLD).124 Antirejection medications amount to another $10,000 yearly, in addition to repeated hospitalizations for infection and rejection. For patients who do well, however, nearly all are successfully weaned from TPN, although a few require some maintenance intravenous fluids. This expense compares with a charge of $100,000 to $150,000 per year for home TPN, in addition to the costs of hospitalization for complications. The actual costs of TPN (including pharmacists’ time) however, are closer to $20 to $30 per day. Intestinal transplantation has reached a stage at which it is a feasible, but not yet practical, alternative to conservative treatment of the patient with SBS.
One of the greatest dilemmas facing intestinal transplantation is balancing the avoidance of premature transplantation with late referral for transplantation; the latter often requires addition of a liver graft and often results in a less-optimal outcome.125 High-risk patients likely to develop complications on home TPN need to be identified early, and every attempt must be made to enhance nutrient and fluid absorption and decrease the need for TPN. Both the Model for End-stage Liver Disease (MELD) score and C-reactive protein (CRP) can be used to predict mortality in patients who require long-term TPN. In a study by Putchakayala and colleagues,126 each point of increase in the MELD (to more than 15) was associated with an increased death risk of 12%, and MELD scores were significantly elevated up to 180 days before death, although they were less reliable when obtained 90 days or more before death in a group of 133 patients with intestinal failure; mortality was 50% at 328 days patients with a MELD score between 15 and 25. Increased CRP was also an independent predictor of death within 90 days before death, and a CRP of four or greater universally predicted mortality.126 One unit increase in CRP was associated with a 20% increased risk of immediate death. Further evaluation of such predictors of poor outcome will be necessary, however, before they can be used reliably to support early intestinal transplantation.
PHARMACOLOGIC ENHANCEMENT OF BOWEL ADAPTATION
The growing knowledge about growth factors has stimulated several clinical studies in patients with SBS. The promising results with the use of growth hormone and dietary l-glutamine in a large uncontrolled study of TPN-dependent patients with SBS127 raised hopes that intestinal mucosal growth could be enhanced beyond the adaptive period.6 Two placebo-controlled studies of identical growth hormone and l-glutamine supplementation failed to show any beneficial effect on absorption,46,47 however, and two other studies showed only marginal improvements in fluid and nutrient retention.128,129
GLP-2 is an intestinotrophic enteric hormone that initially was evaluated in a small uncontrolled study of eight patients with SBS, who received native GLP-2 400 mg subcutaneously twice a day for 35 days.130 The treatment resulted in an increase in several absorptive parameters, body weight, and mucosal growth. Use of a synthetic analog of GLP-2 (teduglutide) was associated with increased villus height and increased fluid absorption, with more modest improvements in energy and nitrogen absorption, that regressed once the medication was discontinued.131 A double-blind, randomized, multicenter study indicated administration of the GLP-II analog resulted in a significant decrease in the requirement for parenteral nutrition, although only a few subjects were able to be fully weaned.132 Increased fluid retention was associated with less chronic dehydration,133 a primary factor in the development of nephropathy in patients who require long-term TPN.134 The rapid advance in our knowledge of epithelial growth factors undoubtedly will lead to discovery of still other growth factors that can stimulate intestinal epithelial growth and thus benefit these patients.
A double-blind, randomized, controlled trial of growth hormone (0.1 mg/kg/day for 4 weeks) in 41 TPN-dependent patients showed that TPN requirements in treated patients could be reduced by an additional 2 L per week (or one night weekly) over the reduction with standard therapy described earlier in this chapter.135 It is unclear whether these effects were related to improved absorption or appetite stimulation. This study led to the FDA approval of growth hormone injections for treating TPN-dependent SBS. The benefit from this therapy lasted nearly four months following completion of three weeks of daily growth hormone injections; it is unclear whether booster injections will be required. The benefits of this therapy must be weighed against the potential side effects, which include fluid retention, edema, arthralgias, and carpal tunnel syndrome; it also is unknown whether any of the potential growth factor therapies would be more effective if administered during the adaptive phase following enterectomy.
SURVIVAL AND QUALITY OF LIFE
The probability of survival and TPN dependence has been assessed in a prospective study of 124 patients with SBS.72 Most of these patients had intestinal resection for either mesenteric infarction or radiation enteritis. The probability of survival was 86% at 2 years and 75% at 5 years. TPN dependence rates were 49% at two years and 45% at five years, suggesting that most patients requiring long-term TPN can be weaned successfully within two years using conventional techniques. In a multivariate analysis, survival was related negatively to high jejunostomy, small bowel length less than 50 cm, and mesenteric infarction as a cause for intestinal resection. TPN dependence was related primarily to small bowel length. Remnant bowel length less than 100 cm was highly predictive of permanent intestinal failure and lifelong TPN dependence. Similar results were reported in a study of 225 patients from the Mayo Clinic.136
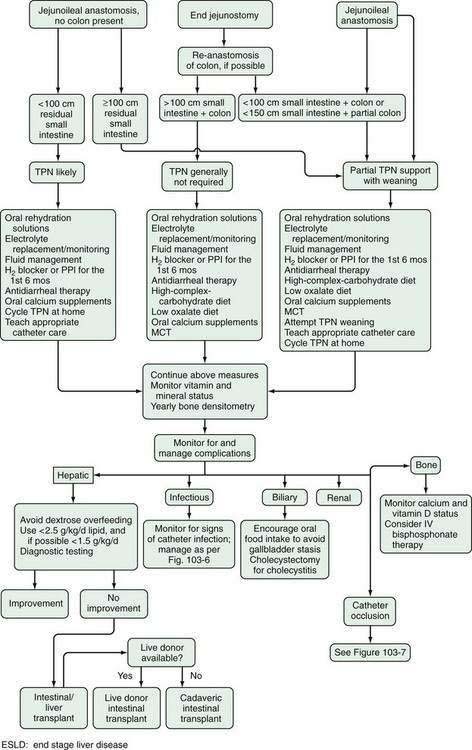
Most patients with SBS have a good quality of life and can work full time. Figure 103-12 depicts an algorithm for managing the patient with SBS.
Buchman AL. Complications of long-term home total parenteral nutrition: Their identification, prevention and treatment. Dig Dis Sci. 2001;46:1-18. (Ref 86.)
Buchman AL. Practical Nutrition Support Techniques. Thorofare, NJ: Slack; 2003. (Ref 87.)
Buchman AL, Iyer K, Fryer J. Parenteral nutrition–associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006;43:9-19. (Ref 96.)
Buchman AL, Moukarzel A. Metabolic bone disease associated with total parenteral nutrition. Clin Nutr. 2000;19:217-31. (Ref 115.)
Buchman AL, Scolapio J, Fryer J. AGA technical review on SBS and intestinal transplantation. Gastroenterology. 2003;124:1111-34. (Ref 1.)
Buchman AL, Sohel M, Dubin M, et al. Choline deficiency causes reversible hepatic abnormalities in patients during parenteral nutrition: Proof of a human choline requirement; a placebo-controlled trial. JPEN J Parenter Enteral Nutr. 2001;25:260-8. (Ref 102.)
Cavicchi M, Beau P, Crenn P, et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132:525-32. (Ref 93.)
Fryer JP. Intestinal transplantation: Current status. Gastroenterol Clin N Am. 2007;36:145-59. (Ref 125.)
Jeppesen PB, Hartmann B, Hansen BS, et al. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45:559-63. (Ref 31.)
Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with SBS. Gastroenterology. 1999;117:1043-50. (Ref 2.)
Nordgaard I, Hansen BS, Mortensen PB. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am J Clin Nutr. 1996;64:222-31. (Ref 13.)
Putchakayala K, Polensky S, Fitzhugh J, et al. An evaluation of the model for end-stage liver disease and serum C-reactive protein as prognostic markers in intestinal failure patients on parenteral nutrition. JPEN J Parenter Enteral Nutr. 2009;33(1):55-61. (Ref 126.)
Roslyn JJ, Pitt HA, Mann LL, et al. Gallbladder disease in patients on long-term parenteral nutrition. Gastroenterology. 1983;84:148-54. (Ref 89.)
Scolapio JS, Camilleri M, Fleming CR, et al. Effect of growth hormone, glutamine, and diet on adaptation in short-bowel syndrome: A randomized, controlled trial. Gastroenterology. 1997;113:1074-81. (Ref 46.)
Solhaug JH, Tvete S. Adaptive changes in the small intestine following bypass operation for obesity. Scand J Gastroenterol. 1978;13:401-8. (Ref 36.)
Woolf GM, Miller C, Kurian R, Jeejeebhoy KN. Diet for patients with a short bowel: High fat or high carbohydrate? Gastroenterology. 1983;84:823-8. (Ref 69.)
1. Buchman AL, Scolapio J, Fryer J. AGA technical review on SBS and intestinal transplantation. Gastroenterology. 2003;124:1111-34.
2. Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with SBS. Gastroenterology. 1999;117:1043-50.
3. Carbonnel F, Cosnes J, Chevret S, et al. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. JPEN J Parenter Enteral Nutr. 1996;20:275-80.
4. Andersson H, Bosaeus I, Brummer R-J, et al. Nutritional and metabolic consequences of extensive bowel resection. Dig Dis. 1986;4:193-201.
5. Crenn P, Haniche M, Valleur P, et al. Surgical versus radiological evaluation of remaining small bowel length (RSBL) in short bowel syndrome (SBS). Gastroenterology. 1996;110:A321.
6. Bryant J. Observations upon the growth and length of the human intestine. Am J Med Sci. 1924;167:499-520.
7. Slater G, Aufses AHJr. Small-bowel length in Crohn’s disease. Am J Gastroenterol. 1991;8:1037-40.
8. Fanucci A, Cerro P, Fraracci L, Letto F. Small-bowel length measured by radiology. Gastrointestinal Radiol. 1984;9:349-51.
9. Nightingale JMD, Bartram CI, Lennard-Jones JE. Length of residual small bowel after partial resection: Correlation between radiographic and surgical measurements. Gastrointestinal Radiol. 1991;16:305-6.
10. Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957;36:1521-36.
11. Clarke RM. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970;107:519-29.
12. Nightingale JM, Lennard-Jones JE, Walker ER, Farthing MJ. Jejunal efflux in SBS. Lancet. 1990;336-8:765-8.
13. Nordgaard I, Hansen BS, Mortensen PB. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am J Clin Nutr. 1996;64:222-31.
14. Royall D, Wolever TMS, Jeejeebhoy KN. Evidence for colonic conservation of malabsorbed carbohydrate in SBS. Am J Gastroenterol. 1992;87:751-6.
15. Nordgaard I, Hansen BS, Mortensen PB. Colon as a digestive organ in patients with short bowel. Lancet. 1994;343:373-76.
16. Ladefoged K, Olgaard K. Fluid and electrolyte absorption and renin-angiotensin-aldosterone axis in patients with severe short-bowel syndrome. Scand J Gastroenterol. 1979;14:729-35.
17. Spiller RC, Jones BJM, Silk DBA. Jejunal water and electrolyte absorption from two proprietary enteral feeds in man: Importance of sodium content. Gut. 1987;28:681-7.
18. Lennard-Jones JE. Oral rehydration solutions in SBS. Clin Ther. 1990;12:129-37.
19. Nightingale JMD, Lennard-Jones JE, Gertner DJ, et al. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33-7:1493.
20. Hylander E, Ladefoged K, Madsen S. Calcium balance and bone mineral content following small-intestinal resection. Scand J Gastroenterol. 1981;16:167-76.
21. Cosnes J, Gendre J-P, Evard D, et al. Compensatory enteral hyperalimentation for management of patients with severe SBS. Am J Clin Nutr. 1985;41:1002-9.
22. Hessov I, Andersson H, Isaksson B. Effects of a low-fat diet on mineral absorption in small-bowel disease. Scand J Gastroenterol. 1983;18:551-4.
23. Andersson H. Effects of a fat-reduced diet on the faecal excretion of radioactivity following administration of 14C-cholic acid and on the duodenal concentration of bile salts in patients with ileal disease. Nutr Metab. 1976;20:254-63.
24. Bright-Asare P, Binder H. Stimulation of colonic secretion of water and electrolytes by hydroxy fatty acids. Gastroenterology. 1973;64:81-8.
25. Williams NS, Evans P, King RF. Gastric acid secretion and gastrin production in the SBS. Gut. 1985;26:914-19.
26. Hyman PE, Everett SL, Harada T. Gastric acid hypersecretion in SBS in infants: Association with extent of resection and enteral feeding. J Pediatr Gastroenterol Nutr. 1986;5:191-7.
27. Nightingale JMD, Kamm MA, van der Sijp JR. Gastrointestinal hormones in SBS. Peptide YY may be the colonic brake to gastric emptying. Gut. 1996;39:267-72.
28. Spiller RC, Trotman IF, Higgins BE. The ileal brake—inhibition of jejunal motility after ileal perfusion in man. Gastroenterology. 1984;25:365-74.
29. Holgate AM, Read NW. Effect of ileal infusion of intralipid on gastrointestinal transit, ileal flow rate and carbohydrate absorption in humans after ingestion of a liquid meal. Gastroenterology. 1985;88:1005-11.
30. Nightingale JMD, Kamm MA, van der Sijp JR, et al. Disturbed gastric emptying in the SBS: Evidence for a “colonic brake.”. Gut. 1993;34:1171-6.
31. Jeppesen PB, Hartmann B, Hansen BS, et al. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45:559-63.
32. Jeppesen PB, Hartmann B, Thulesen J, et al. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut. 2000;47:370-6.
33. Williamson RCN, Chir M. Intestinal adaptation. I. Structural, functional and cytokinetic changes. N Engl J Med. 1978;298:1393-1402.
34. Williamson RCN, Chir M. Intestinal adaptation. II. Mechanisms of control. N Engl J Med. 1978;298:1444-50.
35. Appleton GVN, Bristol JB, Williamson RCN. Proximal enterectomy provides a stronger systemic stimulus to intestinal adaptation than distal enterectomy. Gut. 1987;28:165-8.
36. Solhaug JH, Tvete S. Adaptive changes in the small intestine following bypass operation for obesity. Scand J Gastroenterol. 1978;13:401-8.
37. Levy E, Frileux P, Sandrucci S, et al. Continuous enteral nutrition during the early adaptive stage of the SBS. Br J Surg. 1988;75:549-53.
38. Chaves M, Smith MW, Williamson RCN. Increased activity of digestive enzymes in ileal enterocytes adapting to proximal small bowel resection. Gut. 1987;28:981-7.
39. Gouttebel MC, Saint Aubert B, Colette C, et al. Intestinal adaptation in patients with SBS. Dig Dis Sci. 1989;34:709-15.
40. Dowling RH. Small bowel adaptation and its regulation. Scand J Gastroenterol. 1982;17:53.
41. Johnson LR, Copeland EM, Diedrich SJ. Structural and hormonal alterations in the gastrointestinal tract of parenterally fed rats. Gastroenterology. 1975;68:1177-83.
42. Briet F, Flourie B, Achour L, et al. Bacterial adaptation in patients with short bowel and colon in continuity. Gastroenterology. 1995;109:1446-53.
43. Drucker DJ, Ehrlich P, Asa SL, et al. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911-16.
44. Rhoads JM, Argenzio RA, Chen W, et al. l-Glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943-53.
45. Sham J, Martin G, Meddings JB, Sigalet DL. Epidermal growth factor improves nutritional outcome in a rat model of SBS. J Pediatric Surg. 2002;37:765-9.
46. Scolapio JS, Camilleri M, Fleming CR, et al. Effect of growth hormone, glutamine, and diet on adaptation in short-bowel syndrome: a randomized, controlled trial. Gastroenterology. 1997;113:1074-81.
47. Szkudlarek J, Jeppesen PB, Mortensen PB. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: A randomised, double blind, crossover, placebo controlled study. Gut. 2000;47:199-205.
48. Luk G, Baylin SB. Polyamines and intestinal growth-increased polyamine biosynthesis after jejunectomy. Am J Physiol. 1983;245:G656-60.
49. Luk G, Baylin SB. Inhibition of intestinal epithelial DNA synthesis and adaptive hyperplasia after jejunectomy in the rat by suppression of polyamine biosynthesis. J Clin Invest. 1984;74:698-704.
50. Nightingdale JMD, Walker ER, Farthing MJG, Lennard-Jones JE. Effect of omeprazole on intestinal output in the SBS. Aliment Pharmacol Therap. 1991;5:405-12.
51. Jeppesen PB, Staun M, Tjellesen L, Mortensen PB. Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut. 1998;43:763-9.
52. Arrambide KA, Santa Ana CA, Schiller LR, et al. Loss of absorptive capacity for sodium chloride as a cause of diarrhea following partial ileal and right colon resection. Dig Dis Sci. 1989;34:193-201.
53. Anast CS, Winnacker JL, Forte LR, Burns TW. Impaired release of parathyroid hormone in magnesium deficiency. Clin Endocrinol Metab. 1976;42:707-17.
54. Hylander E, Ladefoged K, Madsen S. Calcium balance and bone mineral content following small-intestinal resection. Scand J Gastroenterol. 1981;16:167-76.
55. O’Keefe SJD, Peterson ME, Fleming CR. Octreotide as an adjunct to home parenteral nutrition in the management of permanent end-jejunostomy syndrome. J Parenter Enter Nutr. 1994;18:26-34.
56. Ladefofed K, Christensen KC, Hegnhoj J, Jarnum S. Effect of a long-acting somatostatin analogue SMS 201-995 on jejunostomy effluents in patients with severe SBS. Gut. 1989;30:943-9.
57. Niv Y, Charash B, Sperber AD, Oren M. Effect of octreotide on gastrostomy, duodenostomy, and cholecystostomy effluents: A physiologic study of fluid and electrolyte balance. Am J Gastroenterol. 1997;92:2107-11.
58. Catnach SM, Anderson JV, Fairclough PD, et al. Effect of octreotide on gallstone prevalence and gallbladder motility in acromegaly. Gut. 1993;34:270-3.
59. Buchman AL, Fryer J, Wallin A, et al. Clonidine reduces diarrhea and sodium loss in patients with proximal jejunostomy: A controlled study. J Parenter Enter Nutr. 2006;30:487-91.
60. Fortran JS. Stimulation of active and passive sodium absorption by sugars in the human jejunum. J Clin Invest. 1975;55:728-37.
61. Pfeiffer A, Schmidt T, Kaess H. The role of osmolality in the absorption of a nutrient solution. Aliment Pharmacol Ther. 1998;12:281-6.
62. Davis GR, Santa Ana CA, Morawski SG, Fortran JC. Permeability characteristics of human jejunum, ileum, proximal colon, and distal colon: Results of potential difference measurements and unidirectional fluxes. Gastroenterology. 1982;83:844-50.
63. Englyst HN, Trowell H, Southgate DAT, Cummings JH. Dietary fibre and resistant starch. Ann J Clin Nutr. 1987;46:873-4.
64. Bond JH, Currier BE, Buchwald H, Levitt MD. Colonic conservation of malabsorbed carbohydrate. Gastroenterology. 1980;78:444.
65. Florent C, Flourie B, Leblond A, et al. Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study). J Clin Invest. 1985;75:608-13.
66. Little KH, Schiller LR, Bilhartz LE, Fortran JS. Treatment of severe steatorrhea with ox bile in an ileectomy patient with residual colon. Dig Dis Sci. 1992;37:929-33.
67. Fordtran JS, Bunch F, Davis GR. Ox bile treatment of severe steatorrhea in an ileectomy-ileostomy patient. Gastroenterology. 1982;82:564-8.
68. Hoffman AF, Poley R. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. Gastroenterology. 1972;62:918-34.
69. Woolf GM, Miller C, Kurian R, Jeejeebhoy KN. Diet for patients with a short bowel: High fat or high carbohydrate? Gastroenterology. 1983;84:823-8.
70. Ovesen L, Chu R, Howard L. The influence of dietary fat on jejunostomy output in patients with severe SBS. Am J Clin Nutr. 1983;38:270-7.
71. Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut. 1998;43:478-83.
72. Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the SBS. Gastroenterology. 1999;117:1043-50.
73. McIntyre PB, Fitchew M, Lennard-Jones JE. Patients with a high jejunostomy do not need a special diet. Gastroenterology. 1986;91:25-33.
74. Woolf GM, Miller C, Kurian R, et al. Nutritional absorption in SBS. Dig Dis Sci. 1987;32:8-15.
75. Ladefoged K. Intestinal and renal loss of infused minerals in patients with severe SBS. Am J Clin Nutr. 1982;36:59-67.
76. Engels LGJ, van den Hamer CJA, van Tongeren JHM. Iron, zinc, and copper balance in short bowel patients on oral nutrition. Am J Clin Nutr. 1984;40:1038-41.
77. Conly JM, Stein K, Worobetz L, et al. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol. 1994;89:915-23.
78. Wolman SL, Anderson GH, Marliss EB, Jeejeebhoy KN. Zinc in total parenteral nutrition: Requirements and metabolic effects. Gastroenterology. 1979;76:458-67.
79. McFadden MA, Delegge MH, Kirby DF. Medication delivery in the SBS. JPEN J Parenter Enteral Nutr. 1993;17:180-6.
80. Ehrenpreis ED, Guerriero S, Nogueras JJ, Carroll MA. Malabsorption of digoxin tablets, gel tabs, and elixir in a patient with an end jejunostomy. Ann Pharmacother. 1994;28:1239-40.
81. Levy E, Frileux P, Sandrucci S, et al. Continuous enteral nutrition during the early adaptive stage of the SBS. Br J Surg. 1988;75:549-53.
82. Cosnes J, Evard D, Beaugerie L, et al. Improvement in protein absorption with a small peptide-base diet in patients with high jejunostomy. Nutrition. 1992;8:406-11.
83. Marteau P, Messing B, Arrigoni E, et al. Do patients with short-bowel syndrome need a lactose-free diet? Nutrition. 1997;13:13-6.
84. Buchman AL, Moukarzel A, Goodson B, et al. Catheter-related infections associated with home parenteral nutrition and predictive factors for the need for catheter removal in their treatment. JPEN J Parenter Enteral Nutr. 1994;18:297-302.
85. Buchman AL, Goodson B, Herzog F, Ament ME. Catheter thrombosis and superior/inferior vena cava syndrome are rare complications of long-term parenteral nutrition. Clin Nutr. 1994;13:356-60.
86. Buchman AL. Complications of long-term home total parenteral nutrition: Their identification, prevention and treatment. Dig Dis Sci. 2001;46:1-18.
87. Buchman AL. Practical nutrition support techniques. Thorofare, NJ: Slack; 2003.
88. Buchman AL, Ament ME. Fifteen-year survival of a Broviac catheter used for home parenteral nutrition. JPEN J Parenter Enteral Nutr. 1993;17(5):489.
89. Roslyn JJ, Pitt HA, Mann LL, et al. Gallbladder disease in patients on long-term parenteral nutrition. Gastroenterology. 1983;84:148-54.
90. Ling PR, Sheikh M, Boyce P, et al. Cholecystokinin (CCK) secretion in patients with severe SBS (SSBS). Dig Dis Sci. 2001;46:859-64.
91. Sitzman JV, Pitt HA, Steinborn PA, et al. Cholecystokinin prevents parenteral nutrition induced biliary sludge in humans. Surg Gynecol Obstet. 1990;170:25-31.
92. Apelgren KN, Willard DA, Vargish T. TPN alters gallbladder responsivity to cholecystokinin. JPEN J Parenter Enteral Nutr. 1988;12:11S.
93. Cavicchi M, Beau P, Crenn P, et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132:525-32.
94. Chan S, McCowen KC, Bistrian BR, et al. Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery. 1999;126:28-34.
95. Kelly DA. Liver complications of pediatric parenteral nutrition—epidemiology. Nutrition. 1998;14:153-7.
96. Buchman AL, Iyer K, Fryer J. Parenteral nutrition–associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006;43:9-19.
97. Stegnick LD, Besten LD. Synthesis of cysteine from methionine in normal adult subjects: Effect of route of alimentation. Science. 1972;178:514-16.
98. Stanko RT, Nathan G, Mendelow H, Adibi SA. Development of hepatic cholestasis and fibrosis in patients with massive loss of intestine supported by prolonged parenteral nutrition. Gastroenterology. 1987;92:197-202.
99. Bowyer BA, Fleming CR, Ludwig J, et al. Does long-term parenteral nutrition in adult patients cause chronic liver disease? JPEN J Parenter Enteral Nutr. 1985;9:11-17.
100. Buchman AL, Dubin M, Jenden D, et al. Lecithin supplementation causes a decrease in hepatic steatosis in patients receiving long term parenteral nutrition. Gastroenterology. 1992;102:1363-70.
101. Buchman AL, Dubin MD, Moukarzel AA, et al. Choline deficiency: A cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399-403.
102. Buchman AL, Sohel M, Dubin M, et al. Choline deficiency causes reversible hepatic abnormalities in patients during parenteral nutrition: Proof of a human choline requirement; a placebo-controlled trial. JPEN J Parenter Enteral Nutr. 2001;25:260-8.
103. Spagnuolo MI, Iorio R, Vegnente A, Guarino A. Ursodeoxycholic acid for treatment of cholestasis in children on long-term total parenteral nutrition: A pilot study. Gastroenterology. 1996;111:716-19.
104. Lindor KD, Burnes J. Ursodeoxycholic acid for the treatment of home parenteral nutrition–associated cholestasis. Gastroenterology. 1991;101:250-3.
105. De Marco G, Sordino D, Bruzzese E, et al. Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Aliment Pharmacol Ther. 2006;24:387-94.
106. Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatr. 2008;121:e678-86.
107. Bowyer BA, Miles JM, Haymond MW, Fleming CR. l-Carnitine therapy in home parenteral nutrition patients with abnormal liver tests and low plasma carnitine concentration. Gastroenterology. 1988;94:434-8.
108. Emmett M, Guirl MJ, Santa Ana CA, et al. Conjugated bile acid replacement therapy reduces urinary oxalate excretion in SBS. Am J Kidney Dis. 2003;41:230-7.
109. Rockwell GF, Campfield T, Nelson BC, Uden PC. Oxalogenesis in parenteral nutrition solution components. Nutrition. 1998;14:836-9.
110. Dahlquist NR, Perrault J, Callaway CW, et al. d-Lactic acidosis and encephalopathy after jejunoileostomy: Response to overfeeding and to fasting in humans. Mayo Clin Proc. 1984;59:141-6.
111. Oh MS, Uribarri J, Alveranga D, et al. Metabolic utilization and renal handling of d-lactate in men. Metabolism. 1985;34:621-25.
112. Mayne AJ, Handy DJ, Preece MA, et al. Dietary management of d-lactic acidosis in SBS. Arch Dis Child. 1990;65:229-31.
113. Hudson M, Pocknee R, Mowat AG. d-Lactic acidosis in SBS—an examination of possible mechanisms. Q J Med. 1990;74:157-63.
114. Buchman AL, Moukarzel A, Ament ME, et al. Serious renal impairment is associated with long-term parenteral nutrition. J Parenter Enteral Nutr. 1993;17:438-44.
115. Buchman AL, Moukarzel A. Metabolic bone disease associated with total parenteral nutrition. Clin Nutr. 2000;19:217-31.
116. Buchman AL, Sohel M, Brown M, et al. Verbal and visual memory improve after choline supplementation in long-term total parenteral nutrition: A pilot study. JPEN J Parenter Enteral Nutr. 2001;25:30-5.
117. Idoate MA, Martinez AJ, Bueno J, et al. The neuropathology of intestinal failure and small bowel transplantation. Acta Neuropathol. 1999;97:502-8.
118. Bianchi A. Intestinal loop lengthening—a technique for increasing small intestinal length. J Pediatr Surg. 1980;15:145-51.
119. Kim HB, Fauza D, Garza J, et al. Serial transverse enteroplasty (STEP): A novel bowel lengthening procedure. J Pediatr Surg. 2003;38:425-9.
120. Modi BP, Javid PJ, Jaksic T, et al. First report of the international serial transverse enteroplasty data registry: Indications, efficacy, and complications. J Am Coll Surg. 2007;204:365-71.
121. Wale PW, de Silva N, Langer JC, Fecteau A. Intermediate outcomes after serial transverse enteroplasty in children with short bowel syndrome. J Pediatric Surg. 2007;42:1804-10.
122. Fishbein TM, Kaufman SS, Florman SS, et al. Isolated intestinal transplantation: Proof of clinical efficacy. Transplantation. 2003;76:636-40.
123. Lacaille F, Vass N, Sauvat F, et al. Long-term outcome, growth and digestive function in children 2 to 18 years after intestinal transplantation. Gut. 2008;57:455-61.
124. Grant D. Intestinal transplantation: 1997 report of the international registry. Transplantation. 1999;67:1061-4.
125. Fryer JP. Intestinal transplantation: Current status. Gastroenterol Clin N Am. 2007;36:145-59.
126. Putchakayala K, Polensky S, Fitzhugh J, et al. An evaluation of the model for end-stage liver disease and serum C-reactive protein as prognostic markers in intestinal failure patients on parenteral nutrition. JPEN J Parenter Enteral Nutr. 2009;33(1):55-61.
127. Byrne TA, Persinger RL, Young LS, et al. A new treatment for patients with short-bowel syndrome—growth hormone, glutamine, and a modified diet. Ann Surg. 1995;222:243-54.
128. Ellegard L, Bosaeus I, Nordgren S, Bengtsson BA. Low-dose recombinant growth hormone increases body weight and lean body mass in patients with SBS. Ann Surg. 1997;225:88-96.
129. Seguy D, Vahedi K, Kapel N, et al. Low dose growth hormone in adult home parenteral nutrition-dependent SBS patients: A positive study. Gastroenterology. 2003;124:293-302.
130. Jeppesen PB, Hartmann B, Thulesen J, et al. Treatment of short bowel patients with glucagon-like peptide 2 (GLP-2), a newly discovered intestinotrophic, anti-secretory, and transit modulating peptide. Gastroenterology. 2000;118:A178.
131. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224-31.
132. O’Keefe SJ, Gilroy R, Jeppesen PB, et al. Teduglutide, a novel GLP-2 analog in the management of short bowel syndrome (SBS) in patients dependent on parenteral nutrition: A multicenter, multinational placebo-controlled clinical trial [abstract]. Gastroenterology. 2008;134:A37.
133. Jeppesen PB, Gottschalck I, Holst JJ, Mortensen PB. Improvements in renal function in short bowel syndrome (SBS) patients treated for two years with subcutaneous native glucagon-like peptide 2 (GLP-2) [abstract]. Gastroenterology. 2008.
134. Lauverjat M, Hadj AA, Vanhems P, et al. Chronic dehydration may impair renal function in patients with chronic intestinal failure on long-term parenteral nutrition. Clin Nutr. 2006;25:75-81.
135. Byrne TA, Wilmore DW, Iyer K, et al. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: A prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655-61.
136. Scolapio JS, Fleming CR, Kelly DG, et al. Survival of home parenteral nutrition–treated patients: 20 years of experience at the Mayo Clinic. Mayo Clin Proc. 1999;74:217-22.