The mainstay of treatment is adequate rehydration. The treatment of cholera and other dehydrating diarrheal diseases was revolutionized by the promotion of oral rehydration solution (ORS), the efficacy of which depends on the fact that glucose-facilitated absorption of sodium and water in the small intestine remains intact in the presence of cholera toxin. The use of ORS has reduced mortality rates for cholera from >50% (in untreated cases) to <1%. A number of ORS formulas have been used. Initial preparations were based on the treatment of patients with cholera and included a solution containing 3.5 g of sodium chloride, 2.5 g of sodium bicarbonate (or 2.9 g of sodium citrate), 1.5 g of potassium chloride, and 20 g of glucose (or 40 g of sucrose) per liter of water. Such a preparation can still be used for the treatment of severe cholera. Many causes of secretory diarrhea, however, are associated with less electrolyte loss than occurs in cholera. Beginning in 2002, the World Health Organization recommended a “reduced-osmolarity/reduced-salt” ORS that is better tolerated and more effective than classic ORS. This preparation contains 2.6 g of sodium chloride, 2.9 g of trisodium citrate, 1.5 g of potassium chloride, and 13.5 g of glucose (or 27 g of sucrose) per liter of water. ORS formulations containing rice or cereal as the carbohydrate source may be even more effective than glucose-based solutions. Patients who are severely dehydrated or in whom vomiting precludes the use of oral therapy should receive IV solutions such as Ringer’s lactate.
Although most secretory forms of traveler’s diarrhea (usually due to enterotoxigenic or enteroaggregative E. coli or to Campylobacter) can be treated effectively with rehydration, bismuth subsalicylate, or antiperistaltic agents, antimicrobial agents can shorten the duration of illness from 3–4 days to 24–36 h. Changes in diet have not been shown to have an impact on the duration of illness, while the efficacy of probiotics continues to be debated. Most individuals who present with dysentery (bloody diarrhea and fever) should be treated empirically with an antimicrobial agent (e.g., a fluoroquinolone or a macrolide) pending microbiologic analysis of stool. Individuals with shigellosis should receive a 3- to 7-day course. Individuals with Campylobacter infection often benefit from antimicrobial treatment as well. Because of widespread resistance of Campylobacter to fluoroquinolones, especially in parts of Asia, a macrolide antibiotic such as erythromycin or azithromycin may be preferred for this infection.
Treatment of salmonellosis must be tailored to the individual patient. Since administration of antimicrobial agents often prolongs intestinal colonization with Salmonella, these drugs are usually reserved for individuals at high risk of complications from disseminated salmonellosis, such as young children, patients with prosthetic devices, elderly patients, and immunocompromised persons. Antimicrobial agents should not be administered to individuals (especially children) in whom enterohemorrhagic E. coli infection is suspected. Laboratory studies of enterohemorrhagic E. coli strains have demonstrated that a number of antibiotics induce replication of Shiga toxin–producing lambdoid bacteriophages, thereby significantly increasing toxin production by these strains. Clinical studies have supported these laboratory results, and antibiotics may increase by twentyfold the risk of hemolytic-uremic syndrome and renal failure during enterohemorrhagic E. coli infection. A clinical clue in the diagnosis of the latter infection is bloody diarrhea with low fever or none at all.
PROPHYLAXIS
Improvements in hygiene to limit fecal-oral spread of enteric pathogens will be necessary if the prevalence of diarrheal diseases is to be significantly reduced in developing countries. Travelers can reduce their risk of diarrhea by eating only hot, freshly cooked food; by avoiding raw vegetables, salads, and unpeeled fruit; and by drinking only boiled or treated water and avoiding ice. Historically, few travelers to tourist destinations adhere to these dietary restrictions. Bismuth subsalicylate is an inexpensive agent for the prophylaxis of traveler’s diarrhea; it is taken at a dosage of 2 tablets (525 mg) four times a day. Treatment appears to be effective and safe for up to 3 weeks, but adverse events such as temporary darkening of the tongue and tinnitus can occur. A meta-analysis suggests that probiotics may lessen the likelihood of traveler’s diarrhea by ~15%. Prophylactic antimicrobial agents, although effective, are not generally recommended for the prevention of traveler’s diarrhea except when travelers are immunosuppressed or have other underlying illnesses that place them at high risk for morbidity from gastrointestinal infection. The risk of side effects and the possibility of developing an infection with a drug-resistant organism or with more harmful, invasive bacteria make it more reasonable to institute an empirical short course of treatment if symptoms develop. If prophylaxis is indicated, the nonabsorbed antibiotic rifaximin can be considered.
The possibility of exerting a major impact on the worldwide morbidity and mortality associated with diarrheal diseases has led to intense efforts to develop effective vaccines against the common bacterial and viral enteric pathogens. An effective rotavirus vaccine is currently available. Vaccines against S. typhi and V. cholerae also are available, although the protection they offer is incomplete and/or short lived. At present, there is no effective commercially available vaccine against Shigella, enterotoxigenic E. coli, Campylobacter, nontyphoidal Salmonella, norovirus, or intestinal parasites.
161 |
Clostridium difficile Infection, Including Pseudomembranous Colitis |
DEFINITION
Clostridium difficile infection (CDI) is a unique colonic disease that is acquired most often in association with antimicrobial use and the consequent disruption of the normal colonic microbiota. The most commonly diagnosed diarrheal illness acquired in the hospital, CDI results from the ingestion of spores of C. difficile that vegetate, multiply, and secrete toxins, causing diarrhea and pseudomembranous colitis (PMC) in the most severe cases.
ETIOLOGY AND EPIDEMIOLOGY
C. difficile is an obligately anaerobic, gram-positive, spore-forming bacillus whose spores are found widely in nature, particularly in the environment of hospitals and chronic-care facilities. CDI occurs frequently in hospitals and nursing homes (or shortly after discharge from these facilities) where the level of antimicrobial use is high and the environment is contaminated by C. difficile spores.
Clindamycin, ampicillin, and cephalosporins were the first antibiotics associated with CDI. The second- and third-generation cephalosporins, particularly cefotaxime, ceftriaxone, cefuroxime, and ceftazidime, are frequently responsible for this condition, and the fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin) are the most recent drug class to be implicated in hospital outbreaks. Penicillin/β-lactamase-inhibitor combinations such as ticarcillin/clavulanate and piperacillin/tazobactam pose significantly less risk. However, all antibiotics, including vancomycin and metronidazole (the agents most commonly used to treat CDI), carry a risk of subsequent CDI. Rare cases are reported in patients without prior antibiotic exposure.
C. difficile is acquired exogenously—most frequently in the hospital or nursing home, but also possibly in the outpatient setting—and is carried in the stool of both symptomatic and asymptomatic patients. The rate of fecal colonization is often ≥20% among adult patients hospitalized for >1 week; in contrast, the rate is 1–3% among community residents. Community-onset CDI without recent hospitalization, nursing home residence, or outpatient health-care contact probably accounts for ≤10% of all cases. The risk of C. difficile acquisition increases in proportion to the length of hospital stay. Asymptomatic fecal carriage of C. difficile in healthy neonates is very common, with repeated colonization by multiple strains in infants (<1 year old), but associated disease in these infants is extremely rare if it occurs at all. Spores of C. difficile are found on environmental surfaces (where the organism can persist for months) and on the hands of hospital personnel who fail to practice good hand hygiene. Hospital epidemics of CDI have been attributed to a single C. difficile strain and to multiple strains present simultaneously. Other identified risk factors for CDI include older age, greater severity of underlying illness, gastrointestinal surgery, use of electronic rectal thermometers, enteral tube feeding, and antacid treatment. Use of proton pump inhibitors may be a risk factor, but this risk is probably modest, and no firm data have implicated these agents in patients who are not already receiving antibiotics.
PATHOLOGY AND PATHOGENESIS
Spores of toxigenic C. difficile are ingested, survive gastric acidity, germinate in the small bowel, and colonize the lower intestinal tract, where they elaborate two large toxins: toxin A (an enterotoxin) and toxin B (a cytotoxin). These toxins initiate processes resulting in the disruption of epithelial-cell barrier function, diarrhea, and pseudomembrane formation. Toxin A is a potent neutrophil chemoattractant, and both toxins glucosylate the guanosine triphosphate (GTP)–binding proteins of the Rho subfamily that regulate the actin cell cytoskeleton. Data from studies using molecular disruption of toxin genes in isogenic mutants suggest that toxin B is the more important virulence factor. This possibility, if confirmed, might account for the occurrence of clinical disease caused by toxin A–negative strains. Disruption of the cytoskeleton results in loss of cell shape, adherence, and tight junctions, with consequent fluid leakage. A third toxin, binary toxin CDT, was previously found in only ~6% of strains but is present in all isolates of the widely recognized epidemic NAP1/BI/027 strain (see “Global Considerations,” below); this toxin is related to C. perfringens iota toxin. Its role in the pathogenesis of CDI has not yet been defined.
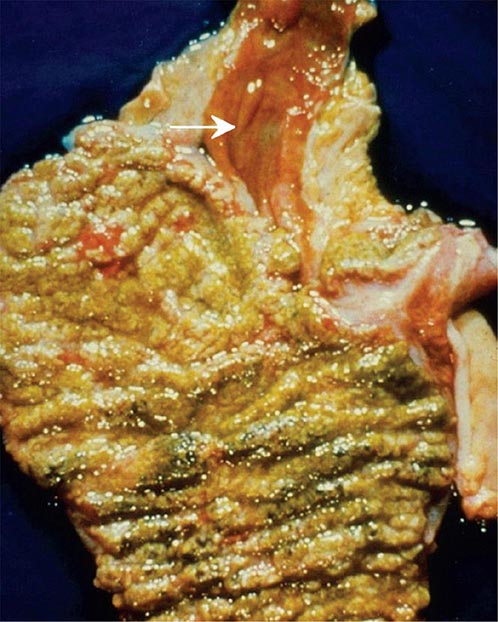
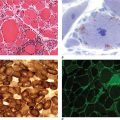
The pseudomembranes of PMC are confined to the colonic mucosa and initially appear as 1- to 2-mm whitish-yellow plaques. The intervening mucosa appears unremarkable, but, as the disease progresses, the pseudomembranes coalesce to form larger plaques and become confluent over the entire colon wall (Fig. 161-1). The whole colon is usually involved, but 10% of patients have rectal sparing. Viewed microscopically, the pseudomembranes have a mucosal attachment point and contain necrotic leukocytes, fibrin, mucus, and cellular debris. The epithelium is eroded and necrotic in focal areas, with neutrophil infiltration of the mucosa.
FIGURE 161-1 Autopsy specimen showing confluent pseudomembranes covering the cecum of a patient with pseudomembranous colitis. Note the sparing of the terminal ileum (arrow).
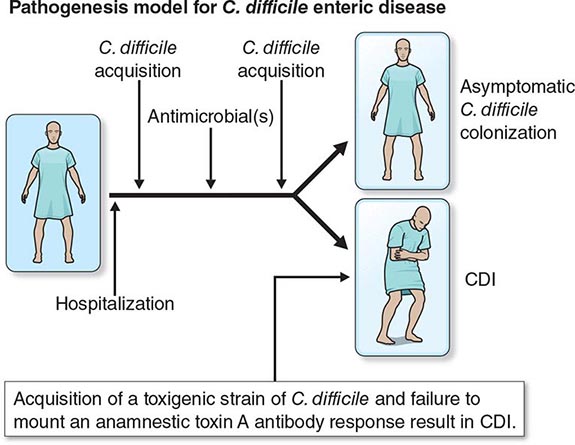
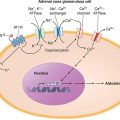
Patients colonized with C. difficile were initially thought to be at high risk for CDI. However, four prospective studies have shown that colonized patients who have not previously had CDI actually have a decreased risk of CDI. At least three events are proposed as essential for the development of CDI (Fig. 161-2). Exposure to antimicrobial agents is the first event and establishes susceptibility to C. difficile infection, most likely through disruption of the normal gastrointestinal microbiota. The second event is exposure to toxigenic C. difficile. Given that the majority of patients do not develop CDI after the first two events, a third event is clearly essential for its occurrence. Candidate third events include exposure to a C. difficile strain of particular virulence, exposure to antimicrobial agents especially likely to cause CDI, and an inadequate host immune response. The host anamnestic serum IgG antibody response to toxin A of C. difficile is the most likely third event that determines which patients develop diarrhea and which patients remain asymptomatic. In all probability, the majority of people first develop antibody to C. difficile toxins when colonized asymptomatically during the first year of life or after CDI in childhood. Infants are thought not to develop symptomatic CDI because they lack suitable mucosal toxin receptors that develop later in life. In adulthood, serum levels of IgG antibody to toxin A increase more in response to infection in individuals who become asymptomatic carriers than in those who develop CDI. For persons who develop CDI, development of increasing levels of antitoxin A during treatment correlates with a lower risk of recurrence of CDI. A clinical trial using monoclonal antibodies to both toxin A and toxin B in addition to standard therapy showed rates of recurrence significantly lower than those obtained with placebo plus standard therapy.
FIGURE 161-2 Pathogenesis model for hospital-acquired Clostridium difficile infection (CDI). At least three events are integral to C. difficile pathogenesis: (1) Exposure to antibiotics establishes susceptibility to infection. (2) Once susceptible, the patient may acquire nontoxigenic (nonpathogenic) or toxigenic strains of C. difficile as a second event. (3) Acquisition of toxigenic C. difficile may be followed by asymptomatic colonization or CDI, depending on one or more additional events (e.g., an inadequate host anamnestic IgG response to C. difficile toxin A).
GLOBAL CONSIDERATIONS
![]() Rates and severity of CDI in the United States, Canada, and Europe increased markedly after the year 2000. Rates in U.S. hospitals tripled between 2000 and 2005. In 2005, hospitals in Montreal, Quebec, reported rates four times higher than the 1997 baseline, with directly attributable mortality of 6.9% (increased from 1.5%). An epidemic strain, variously known as toxinotype III, REA type BI, polymerase chain reaction (PCR) ribotype 027, and pulsed-field type NAP1 and thus collectively designated NAP1/BI/027, is thought to account for much of the increase in incidence and has been found in North America, Europe, and Asia. It is now recognized that two clones of NAP1/BI/027 originated in the United States and Canada and spread to the United Kingdom, Europe, and Asia. The epidemic organism is characterized by (1) an ability to produce 16–23 times as much toxin A and toxin B as control strains in vitro; (2) the presence of a third toxin (binary toxin CDT); and (3) high-level resistance to all fluoroquinolones. New strains have been and probably will continue to be implicated in outbreaks, including a strain (toxinotype V, ribotype 078) commonly found in food animals that also carries binary toxin and has been associated with high mortality risk in human infections. In the past 5 years, rates of CDI in the United Kingdom have markedly decreased, and the frequency of the NAP1/BI/027 strain in the countries of the European Union has likewise decreased. However, there has been no evidence of decreased rates of CDI or a decreased incidence of NAP1/BI/027 in North America; the latter strain still causes 25–35% of all CDIs in most regions of the United States.
Rates and severity of CDI in the United States, Canada, and Europe increased markedly after the year 2000. Rates in U.S. hospitals tripled between 2000 and 2005. In 2005, hospitals in Montreal, Quebec, reported rates four times higher than the 1997 baseline, with directly attributable mortality of 6.9% (increased from 1.5%). An epidemic strain, variously known as toxinotype III, REA type BI, polymerase chain reaction (PCR) ribotype 027, and pulsed-field type NAP1 and thus collectively designated NAP1/BI/027, is thought to account for much of the increase in incidence and has been found in North America, Europe, and Asia. It is now recognized that two clones of NAP1/BI/027 originated in the United States and Canada and spread to the United Kingdom, Europe, and Asia. The epidemic organism is characterized by (1) an ability to produce 16–23 times as much toxin A and toxin B as control strains in vitro; (2) the presence of a third toxin (binary toxin CDT); and (3) high-level resistance to all fluoroquinolones. New strains have been and probably will continue to be implicated in outbreaks, including a strain (toxinotype V, ribotype 078) commonly found in food animals that also carries binary toxin and has been associated with high mortality risk in human infections. In the past 5 years, rates of CDI in the United Kingdom have markedly decreased, and the frequency of the NAP1/BI/027 strain in the countries of the European Union has likewise decreased. However, there has been no evidence of decreased rates of CDI or a decreased incidence of NAP1/BI/027 in North America; the latter strain still causes 25–35% of all CDIs in most regions of the United States.
CLINICAL MANIFESTATIONS
Diarrhea is the most common manifestation caused by C. difficile. Stools are almost never grossly bloody and range from soft and unformed to watery or mucoid in consistency, with a characteristic odor. Patients may have as many as 20 bowel movements per day. Clinical and laboratory findings include fever in 28% of cases, abdominal pain in 22%, and leukocytosis in 50%. When adynamic ileus (which is seen on x-ray in ~20% of cases) results in cessation of stool passage, the diagnosis of CDI is frequently overlooked. A clue to the presence of unsuspected CDI in these patients is unexplained leukocytosis, with ≥15,000 white blood cells (WBCs)/μL. Such patients are at high risk for complications of C. difficile infection, particularly toxic megacolon and sepsis.
C. difficile diarrhea recurs after treatment in ~15–30% of cases, and this figure may be increasing. Recurrences may represent either relapses due to the same strain or reinfections with a new strain. Susceptibility to recurrence of clinical CDI is likely a result of continued fecal-microbiota disruption caused by the antibiotic used to treat CDI.
DIAGNOSIS
The diagnosis of CDI is based on a combination of clinical criteria: (1) diarrhea (≥3 unformed stools per 24 h for ≥2 days) with no other recognized cause plus (2) toxin A or B detected in the stool, toxin-producing C. difficile detected in the stool by PCR or culture, or pseudomembranes seen in the colon. PMC is a more advanced form of CDI and is visualized at endoscopy in only ~50% of patients with diarrhea who have a positive stool culture and toxin assay for C. difficile. Endoscopy is a rapid diagnostic tool in seriously ill patients with suspected PMC and an acute abdomen, but a negative result in this examination does not rule out CDI.
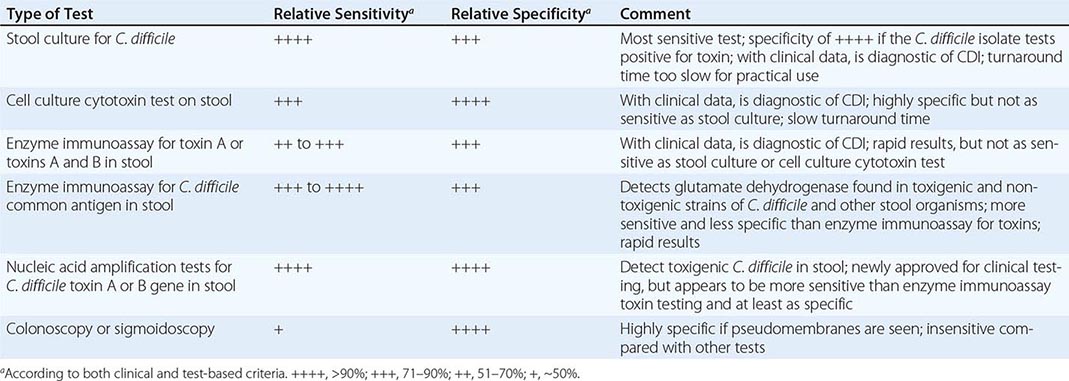
Despite the array of tests available for C. difficile and its toxins (Table 161-1), no single traditional test has high sensitivity, high specificity, and rapid turnaround. Most laboratory tests for toxins, including enzyme immunoassays, lack sensitivity. However, testing of multiple additional stool specimens is not recommended. Nucleic acid amplification tests, including PCR assays, have now been approved for diagnostic purposes and appear to be both rapid and sensitive while retaining high specificity. Testing of asymptomatic patients is not recommended except for epidemiologic study purposes. In particular, so-called tests of cure following treatment are not recommended because >50% of patients continue to harbor the organism and toxin after diarrhea has ceased and test results do not always predict recurrence of CDI. Thus these results should not be used to restrict placement of patients in long-term-care or nursing home facilities.
|
RELATIVE SENSITIVITY AND SPECIFICITY OF DIAGNOSTIC TESTS FOR CLOSTRIDIUM DIFFICILE INFECTION (CDI) |
|
TREATMENT |
CLOSTRIDIUM DIFFICILE INFECTION |
PRIMARY CDI
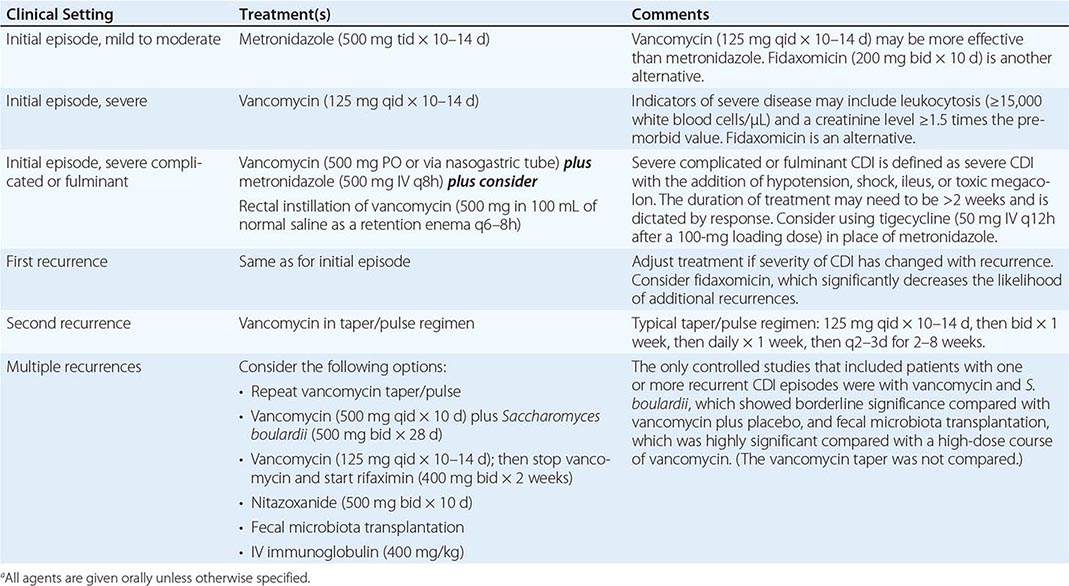
When possible, discontinuation of any ongoing antimicrobial administration is recommended as the first step in treatment of CDI. Earlier studies indicated that 15–23% of patients respond to this simple measure. However, with the advent of the current epidemic strain and the associated rapid clinical deterioration of some patients, prompt initiation of specific CDI treatment has become the standard. Empirical treatment is appropriate if CDI is strongly suspected on clinical grounds. General treatment guidelines include hydration and the avoidance of antiperistaltic agents and opiates, which may mask symptoms and possibly worsen disease. Nevertheless, antiperistaltic agents have been used safely with vancomycin or metronidazole for mild to moderate CDI.
Oral administration of vancomycin, fidaxomicin, or metronidazole is recommended for CDI treatment. IV vancomycin is ineffective for CDI, and fidaxomicin is available only for oral administration; when IV metronidazole is administered, fecal bactericidal drug concentrations are achieved during acute diarrhea; however, in the presence of adynamic ileus, IV metronidazole treatment of CDI has failed. Two large clinical trials comparing vancomycin and fidaxomicin indicated comparable resolution of diarrhea (~90% of patients) as well as significantly reduced rates of recurrent CDI with fidaxomicin from rates with vancomycin. In previous randomized trials, diarrhea response rates to oral therapy with vancomycin or metronidazole were ≥94%, but four observational studies found that response rates for metronidazole had declined to 62–78%. Although the mean time to resolution of diarrhea is 2–4 days, the response to metronidazole may be much slower. Treatment should not be deemed a failure until a drug has been given for at least 6 days. On the basis of data for shorter courses of vancomycin and the results of two large-scale clinical trials, it is recommended that vancomycin, fidaxomicin, and metronidazole be given for at least 10 days. Metronidazole is not approved for CDI by the U.S. Food and Drug Administration (FDA), but most patients with mild to moderate illness respond to 500 mg given by mouth three times a day for 10 days; extension of the treatment period may be needed for slow responders. In addition to the reports of increases in metronidazole failures, a prospective, randomized, double-blind, placebo-controlled study has demonstrated the superiority of vancomycin over metronidazole for treatment of severe CDI. The severity assessment score in that study included age as well as laboratory parameters (elevated temperature, low albumin level, or elevated WBC count), documentation of PMC by endoscopy, and treatment of CDI in the intensive care unit. Although a validated severity score is not available, it is important to initiate treatment with oral vancomycin for patients who appear seriously ill, particularly if they have a high WBC count (>15,000/μL) or a creatinine level that is ≥1.5 times higher than the premorbid value (Table 161-2). In addition, a randomized blinded trial compared a toxin-binding polymer, tolevamer, with two antibiotic regimens for treatment of CDI and showed that vancomycin was superior to metronidazole for all patients regardless of severity. Small randomized trials of nitazoxanide, bacitracin, rifaximin, and fusidic acid for treatment of CDI have been conducted. These drugs have not been extensively studied, shown to be superior, or approved by the FDA for CDI, but they provide potential alternatives to vancomycin, fidaxomicin, and metronidazole.
|
RECOMMENDATIONS FOR THE TREATMENT OF CLOSTRIDIUM DIFFICILE INFECTION (CDI)a |
RECURRENT CDI
Overall, ~15–30% of successfully treated patients experience recurrences of CDI, either as relapses caused by the original organism or as reinfections following treatment. Rates of CDI recurrence are significantly lower among patients treated with fidaxomicin rather than vancomycin. Rates of recurrence are comparable with vancomycin and metronidazole. Recurrence rates are higher among patients ≥65 years old, those who continue to take antibiotics while being treated for CDI, and those who remain in the hospital after the initial episode of CDI. Patients who have a first recurrence of CDI have a high rate of second recurrence (33–65%). In the first recurrence, re-treatment with metronidazole is comparable to treatment with vancomycin (Table 161-2), and fidaxomicin is superior to vancomycin in reducing the risk of further recurrences in patients who have had one recurrence. Recurrent CDI, once thought to be relatively mild, has now been documented to pose a significant (11%) risk of serious complications (shock, megacolon, perforation, colectomy, or death within 30 days). There is no standard treatment for multiple recurrences, but long or repeated metronidazole courses should be avoided because of potential neurotoxicity. The use of vancomycin in tapering doses or with pulse dosing every other day for 2–8 weeks may be the most practical approach to treatment of patients with multiple recurrences. Other approaches include the administration of vancomycin followed by the yeast Saccharomyces boulardii; the administration of vancomycin followed by a fecal microbiota transplant given via nasoduodenal tube, colonoscope, or enema; and the intentional colonization of the patient with a nontoxigenic strain of C. difficile. None of these biotherapeutic approaches has been approved by the FDA for use in the United States. Other non-FDA-approved antibiotic strategies include (1) sequential treatment with vancomycin (125 mg four times daily for 10–14 days) followed by rifaximin (400 mg twice daily for 14 days) and (2) treatment with nitazoxanide (500 mg twice daily for 7 days). IV immunoglobulin, which has also been used with variable success, presumably provides antibodies to C. difficile toxins.
SEVERE COMPLICATED OR FULMINANT CDI
Fulminant (rapidly progressive and severe) CDI presents the most difficult treatment challenge. Patients with fulminant disease often do not have diarrhea, and their illness mimics an acute surgical abdomen. Sepsis (hypotension, fever, tachycardia, leukocytosis) may result from severe CDI. An acute abdomen (with or without toxic megacolon) may include signs of obstruction, ileus, colon-wall thickening and ascites on abdominal CT, and peripheral-blood leukocytosis (≥20,000 WBCs/μL). With or without diarrhea, the differential diagnosis of an acute abdomen, sepsis, or toxic megacolon should include CDI if the patient has received antibiotics in the past 2 months. Cautious sigmoidoscopy or colonoscopy to visualize PMC and abdominal CT are the best diagnostic tests in patients without diarrhea.
Medical management of fulminant CDI is suboptimal because of the difficulty of delivering oral fidaxomicin, metronidazole, or vancomycin to the colon in the presence of ileus (Table 161-2). The combination of vancomycin (given via nasogastric tube and by retention enema) plus IV metronidazole has been used with some success in uncontrolled studies, as has IV tigecycline in small-scale uncontrolled studies. Surgical colectomy may be life-saving if there is no response to medical management. If possible, colectomy should be performed before the serum lactate level reaches 5 mmol/L. The incidence of fulminant CDI requiring colectomy appears to be increasing in the evolving epidemic; however, morbidity and death associated with colectomy may be reduced by performing instead a laparoscopic ileostomy followed by colon lavage with polyethylene glycol and vancomycin infusion into the colon via the ileostomy.
PROGNOSIS
The mortality rate attributed to CDI, previously found to be 0.6–3.5%, has reached 6.9% in recent outbreaks and rises progressively with increasing age. Most patients recover, but recurrences are common.
PREVENTION AND CONTROL
Strategies for the prevention of CDI are of two types: those aimed at preventing transmission of the organism to the patient and those aimed at reducing the risk of CDI if the organism is transmitted. Transmission of C. difficile in clinical practice has been prevented by gloving of personnel, elimination of the use of contaminated electronic thermometers, and use of hypochlorite (bleach) solution for environmental decontamination of patients’ rooms. Hand hygiene is critical; hand washing is recommended in CDI outbreaks because alcohol hand gels are not sporicidal. CDI outbreaks have been best controlled by restricting the use of specific antibiotics, such as clindamycin and second- and third-generation cephalosporins. Outbreaks of CDI due to clindamycin-resistant strains have resolved promptly when clindamycin use is restricted. Future preventive strategies are likely to include use of monoclonal antibodies, vaccines, and biotherapeutics containing live organisms that will restore colonization protection in the microbiota.
162 |
Urinary Tract Infections, Pyelonephritis, and Prostatitis |
Urinary tract infection (UTI) is a common and painful human illness that, fortunately, is rapidly responsive to modern antibiotic therapy. In the preantibiotic era, UTI caused significant morbidity. Hippocrates, writing about a disease that appears to have been acute cystitis, said that the illness could last for a year before either resolving or worsening to involve the kidneys. When chemotherapeutic agents used to treat UTI were introduced in the early twentieth century, they were relatively ineffective, and persistence of infection after 3 weeks of therapy was common. Nitrofurantoin, which became available in the 1950s, was the first tolerable and effective agent for the treatment of UTI.
Since the most common manifestation of UTI is acute cystitis and since acute cystitis is far more prevalent among women than among men, most clinical research on UTI has involved women. Many studies have enrolled women from college campuses or large health maintenance organizations in the United States. Therefore, when reviewing the literature and recommendations concerning UTI, clinicians must consider whether the findings are applicable to their patient populations.
DEFINITIONS
UTI may be asymptomatic (subclinical infection) or symptomatic (disease). Thus, the term urinary tract infection encompasses a variety of clinical entities, including asymptomatic bacteriuria (ASB), cystitis, prostatitis, and pyelonephritis. The distinction between symptomatic UTI and ASB has major clinical implications. Both UTI and ASB connote the presence of bacteria in the urinary tract, usually accompanied by white blood cells and inflammatory cytokines in the urine. However, ASB occurs in the absence of symptoms attributable to the bacteria in the urinary tract and does not usually require treatment, while UTI has more typically been assumed to imply symptomatic disease that warrants antimicrobial therapy. Much of the literature concerning UTI, particularly catheter-associated infection, does not differentiate between UTI and ASB. In this chapter, the term UTI denotes symptomatic disease; cystitis, symptomatic infection of the bladder; and pyelonephritis, symptomatic infection of the kidneys. Uncomplicated UTI refers to acute cystitis or pyelonephritis in nonpregnant outpatient women without anatomic abnormalities or instrumentation of the urinary tract; the term complicated UTI encompasses all other types of UTI. Recurrent UTI is not necessarily complicated; individual episodes can be uncomplicated and treated as such. Catheter-associated bacteriuria can be either symptomatic (CAUTI) or asymptomatic.
EPIDEMIOLOGY AND RISK FACTORS
Except among infants and the elderly, UTI occurs far more commonly in females than in males. During the neonatal period, the incidence of UTI is slightly higher among males than among females because male infants more commonly have congenital urinary tract anomalies. After 50 years of age, obstruction from prostatic hypertrophy becomes common in men, and the incidence of UTI is almost as high among men as among women. Between 1 year and ~50 years of age, UTI and recurrent UTI are predominantly diseases of females. The prevalence of ASB is ~5% among women between ages 20 and 40 and may be as high as 40–50% among elderly women and men.
As many as 50–80% of women in the general population acquire at least one UTI during their lifetime—uncomplicated cystitis in most cases. Recent use of a diaphragm with spermicide, frequent sexual intercourse, and a history of UTI are independent risk factors for acute cystitis. Cystitis is temporally related to recent sexual intercourse in a dose-response manner, with an increased relative risk ranging from 1.4 with one episode of intercourse to 4.8 with five episodes of intercourse in the preceding week. In healthy postmenopausal women, sexual activity, diabetes mellitus, and incontinence are risk factors for UTI.
Many factors predisposing women to cystitis also increase the risk of pyelonephritis. Factors independently associated with pyelonephritis in young healthy women include frequent sexual intercourse, a new sexual partner, a UTI in the previous 12 months, a maternal history of UTI, diabetes, and incontinence. The common risk factors for cystitis and pyelonephritis are not surprising given that pyelonephritis typically arises through the ascent of bacteria from the bladder to the upper urinary tract. However, pyelonephritis can occur without clear antecedent cystitis.
About 20–30% of women who have had one episode of UTI will have recurrent episodes. Early recurrence (within 2 weeks) is usually regarded as relapse rather than reinfection and may indicate the need to evaluate the patient for a sequestered focus. Intracellular pods of infecting organisms within the bladder epithelium have been demonstrated in animal models of UTI, but the importance of this phenomenon in humans is not yet clear. The rate of recurrence ranges from 0.3 to 7.6 infections per patient per year, with an average of 2.6 infections per year. It is not uncommon for multiple recurrences to follow an initial infection, resulting in clustering of episodes. Clustering may be related temporally to the presence of a new risk factor or to the sloughing of the protective outer bladder epithelial layer in response to bacterial attachment during acute cystitis. The likelihood of a recurrence decreases with increasing time since the last infection. A case-control study of predominantly white premenopausal women with recurrent UTI identified frequent sexual intercourse, use of spermicide, a new sexual partner, a first UTI before 15 years of age, and a maternal history of UTI as independent risk factors for recurrent UTI. The only consistently documented behavioral risk factors for recurrent UTI include frequent sexual intercourse and spermicide use. In postmenopausal women, major risk factors for recurrent UTI include a history of premenopausal UTI and anatomic factors affecting bladder emptying, such as cystoceles, urinary incontinence, and residual urine.
In pregnant women, ASB has clinical consequences, and both screening for and treatment of this condition are indicated. Specifically, ASB during pregnancy is associated with preterm birth and perinatal death of the fetus and with pyelonephritis in the mother. A Cochrane meta-analysis found that treatment of ASB in pregnant women decreased the risk of pyelonephritis by 75%.
The majority of men with UTI have a functional or anatomic abnormality of the urinary tract, most commonly urinary obstruction secondary to prostatic hypertrophy. That said, not all men with UTI have detectable urinary abnormalities; this point is particularly relevant for men ≤45 years of age. Lack of circumcision is also associated with an increased risk of UTI because Escherichia coli is more likely to colonize the glans and prepuce and subsequently migrate into the urinary tract.
Women with diabetes have been found to have a two- to threefold higher rate of ASB and UTI than women without diabetes; there is insufficient evidence to make a corresponding statement about men. Increased duration of diabetes and the use of insulin rather than oral medication are also associated with a higher risk of UTI among women with diabetes. Poor bladder function, obstruction in urinary flow, and incomplete voiding are additional factors commonly found in patients with diabetes that increase the risk of UTI. Impaired cytokine secretion may contribute to ASB in diabetic women.
ETIOLOGY
![]() The uropathogens causing UTI vary by clinical syndrome but are usually enteric gram-negative rods that have migrated to the urinary tract. The susceptibility patterns of these organisms vary by clinical syndrome and by geography. In acute uncomplicated cystitis in the United States, the etiologic agents are highly predictable: E. coli accounts for 75–90% of isolates; Staphylococcus saprophyticus for 5–15% (with particularly frequent isolation from younger women); and Klebsiella, Proteus, Enterococcus, and Citrobacter species, along with other organisms, for 5–10%. Similar etiologic agents are found in Europe and Brazil. The spectrum of agents causing uncomplicated pyelonephritis is similar, with E. coli predominating. In complicated UTI (e.g., CAUTI), E. coli remains the predominant organism, but other aerobic gram-negative rods, such as Pseudomonas aeruginosa and Klebsiella, Proteus, Citrobacter, Acinetobacter, and Morganella species, also are frequently isolated. Gram-positive bacteria (e.g., enterococci and Staphylococcus aureus) and yeasts are also important pathogens in complicated UTI. Data on etiology and resistance are generally obtained from laboratory surveys and should be understood in the context that organism identification is performed only in cases in which urine is sent for culture—i.e., typically, when complicated UTI or pyelonephritis is suspected. The available data demonstrate a worldwide increase in the resistance of E. coli to antibiotics commonly used to treat UTI. North American and European surveys from women with acute cystitis have documented resistance rates of >20% to trimethoprim-sulfamethoxazole (TMP-SMX) and to ciprofloxacin in some regions. In community-acquired infections, the increased prevalence of uropathogens producing extended-spectrum β-lactamases has left few oral options for therapy. Since resistance rates vary by local geographic region, with individual patient characteristics, and over time, it is important to use current and local data when choosing a treatment regimen.
The uropathogens causing UTI vary by clinical syndrome but are usually enteric gram-negative rods that have migrated to the urinary tract. The susceptibility patterns of these organisms vary by clinical syndrome and by geography. In acute uncomplicated cystitis in the United States, the etiologic agents are highly predictable: E. coli accounts for 75–90% of isolates; Staphylococcus saprophyticus for 5–15% (with particularly frequent isolation from younger women); and Klebsiella, Proteus, Enterococcus, and Citrobacter species, along with other organisms, for 5–10%. Similar etiologic agents are found in Europe and Brazil. The spectrum of agents causing uncomplicated pyelonephritis is similar, with E. coli predominating. In complicated UTI (e.g., CAUTI), E. coli remains the predominant organism, but other aerobic gram-negative rods, such as Pseudomonas aeruginosa and Klebsiella, Proteus, Citrobacter, Acinetobacter, and Morganella species, also are frequently isolated. Gram-positive bacteria (e.g., enterococci and Staphylococcus aureus) and yeasts are also important pathogens in complicated UTI. Data on etiology and resistance are generally obtained from laboratory surveys and should be understood in the context that organism identification is performed only in cases in which urine is sent for culture—i.e., typically, when complicated UTI or pyelonephritis is suspected. The available data demonstrate a worldwide increase in the resistance of E. coli to antibiotics commonly used to treat UTI. North American and European surveys from women with acute cystitis have documented resistance rates of >20% to trimethoprim-sulfamethoxazole (TMP-SMX) and to ciprofloxacin in some regions. In community-acquired infections, the increased prevalence of uropathogens producing extended-spectrum β-lactamases has left few oral options for therapy. Since resistance rates vary by local geographic region, with individual patient characteristics, and over time, it is important to use current and local data when choosing a treatment regimen.
PATHOGENESIS
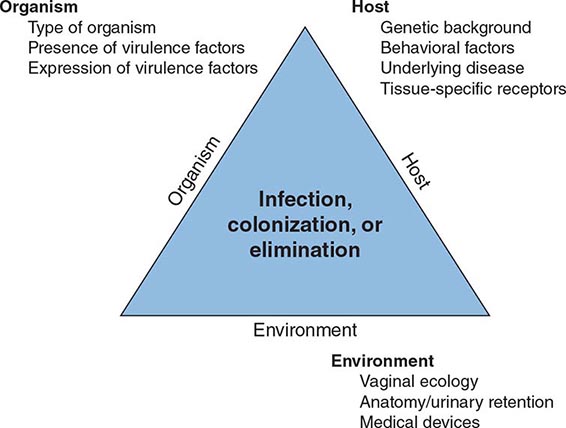
The urinary tract can be viewed as an anatomic unit united by a continuous column of urine extending from the urethra to the kidneys. In the majority of UTIs, bacteria establish infection by ascending from the urethra to the bladder. Continuing ascent up the ureter to the kidney is the pathway for most renal parenchymal infections. However, introduction of bacteria into the bladder does not inevitably lead to sustained and symptomatic infection. The interplay of host, pathogen, and environmental factors determines whether tissue invasion and symptomatic infection will ensue (Fig. 162-1). For example, bacteria often enter the bladder after sexual intercourse, but normal voiding and innate host defense mechanisms in the bladder eliminate these organisms. Any foreign body in the urinary tract, such as a urinary catheter or stone, provides an inert surface for bacterial colonization. Abnormal micturition and/or significant residual urine volume promotes true infection. In the simplest of terms, anything that increases the likelihood of bacteria entering the bladder and staying there increases the risk of UTI.
FIGURE 162-1 Pathogenesis of urinary tract infection. The relationship among specific host, pathogen, and environmental factors determines the clinical outcome.
Bacteria can also gain access to the urinary tract through the bloodstream. However, hematogenous spread accounts for <2% of documented UTIs and usually results from bacteremia caused by relatively virulent organisms, such as Salmonella and S. aureus. Indeed, the isolation of either of these pathogens from a patient without a catheter or other instrumentation warrants a search for a bloodstream source. Hematogenous infections may produce focal abscesses or areas of pyelonephritis within a kidney and result in positive urine cultures. The pathogenesis of candiduria is distinct in that the hematogenous route is common. The presence of Candida in the urine of a noninstrumented immunocompetent patient implies either genital contamination or potentially widespread visceral dissemination.
Environmental Factors • VAGINAL ECOLOGY In women, vaginal ecology is an important environmental factor affecting the risk of UTI. Colonization of the vaginal introitus and periurethral area with organisms from the intestinal flora (usually E. coli) is the critical initial step in the pathogenesis of UTI. Sexual intercourse is associated with an increased risk of vaginal colonization with E. coli and thereby increases the risk of UTI. Nonoxynol-9 in spermicide is toxic to the normal vaginal microflora and thus is likewise associated with an increased risk of E. coli vaginal colonization and bacteriuria. In postmenopausal women, the previously predominant vaginal lactobacilli are replaced with colonizing gram-negative bacteria. The use of topical estrogens to prevent UTI in postmenopausal women is controversial; given the side effects of systemic hormone replacement, oral estrogens should not be used to prevent UTI.
ANATOMIC AND FUNCTIONAL ABNORMALITIES Any condition that permits urinary stasis or obstruction predisposes the individual to UTI. Foreign bodies such as stones or urinary catheters provide an inert surface for bacterial colonization and formation of a persistent biofilm. Thus, vesicoureteral reflux, ureteral obstruction secondary to prostatic hypertrophy, neurogenic bladder, and urinary diversion surgery create an environment favorable to UTI. In persons with such conditions, E. coli strains lacking typical urinary virulence factors are often the cause of infection. Inhibition of ureteral peristalsis and decreased ureteral tone leading to vesicoureteral reflux are important in the pathogenesis of pyelonephritis in pregnant women. Anatomic factors—specifically, the distance of the urethra from the anus—are considered to be the primary reason why UTI is predominantly an illness of young women rather than of young men.
![]() Host Factors The genetic background of the host influences the individual’s susceptibility to recurrent UTI, at least among women. A familial disposition to UTI and to pyelonephritis is well documented. Women with recurrent UTI are more likely to have had their first UTI before the age of 15 years and to have a maternal history of UTI. A component of the underlying pathogenesis of this familial predisposition to recurrent UTI may be persistent vaginal colonization with E. coli, even during asymptomatic periods. Vaginal and periurethral mucosal cells from women with recurrent UTI bind threefold more uropathogenic bacteria than do mucosal cells from women without recurrent infection. Epithelial cells from women who are non-secretors of certain blood group antigens may possess specific types of receptors to which E. coli can bind, thereby facilitating colonization and invasion. Mutations in host response genes (e.g., those coding for Toll-like receptors and the interleukin 8 receptor) also have been linked to recurrent UTI and pyelonephritis. Polymorphisms in the interleukin 8–specific receptor gene CXCR1 are associated with increased susceptibility to pyelonephritis. Lower-level expression of CXCR1 on the surface of neutrophils impairs neutrophil-dependent host defense against bacterial invasion of the renal parenchyma.
Host Factors The genetic background of the host influences the individual’s susceptibility to recurrent UTI, at least among women. A familial disposition to UTI and to pyelonephritis is well documented. Women with recurrent UTI are more likely to have had their first UTI before the age of 15 years and to have a maternal history of UTI. A component of the underlying pathogenesis of this familial predisposition to recurrent UTI may be persistent vaginal colonization with E. coli, even during asymptomatic periods. Vaginal and periurethral mucosal cells from women with recurrent UTI bind threefold more uropathogenic bacteria than do mucosal cells from women without recurrent infection. Epithelial cells from women who are non-secretors of certain blood group antigens may possess specific types of receptors to which E. coli can bind, thereby facilitating colonization and invasion. Mutations in host response genes (e.g., those coding for Toll-like receptors and the interleukin 8 receptor) also have been linked to recurrent UTI and pyelonephritis. Polymorphisms in the interleukin 8–specific receptor gene CXCR1 are associated with increased susceptibility to pyelonephritis. Lower-level expression of CXCR1 on the surface of neutrophils impairs neutrophil-dependent host defense against bacterial invasion of the renal parenchyma.
![]() Microbial Factors An anatomically normal urinary tract presents a stronger barrier to infection than a compromised urinary tract. Thus, strains of E. coli that cause invasive symptomatic infection of the urinary tract in otherwise normal hosts often possess and express genetic virulence factors, including surface adhesins that mediate binding to specific receptors on the surface of uroepithelial cells. The best-studied adhesins are the P fimbriae, hairlike protein structures that interact with a specific receptor on renal epithelial cells. (The letter P denotes the ability of these fimbriae to bind to blood group antigen P, which contains a D-galactose-D-galactose residue.) P fimbriae are important in the pathogenesis of pyelonephritis and subsequent bloodstream invasion from the kidney.
Microbial Factors An anatomically normal urinary tract presents a stronger barrier to infection than a compromised urinary tract. Thus, strains of E. coli that cause invasive symptomatic infection of the urinary tract in otherwise normal hosts often possess and express genetic virulence factors, including surface adhesins that mediate binding to specific receptors on the surface of uroepithelial cells. The best-studied adhesins are the P fimbriae, hairlike protein structures that interact with a specific receptor on renal epithelial cells. (The letter P denotes the ability of these fimbriae to bind to blood group antigen P, which contains a D-galactose-D-galactose residue.) P fimbriae are important in the pathogenesis of pyelonephritis and subsequent bloodstream invasion from the kidney.
Another adhesin is the type 1 pilus (fimbria), which all E. coli strains possess but not all E. coli strains express. Type 1 pili are thought to play a key role in initiating E. coli bladder infection; they mediate binding to uroplakins on the luminal surface of bladder uroepithelial cells. The binding of type 1 fimbriae of E. coli to receptors on uroepithelial cells initiates a complex series of signaling events that leads to apoptosis and exfoliation of uroepithelial cells, with the attached E. coli organisms carried away in the urine.
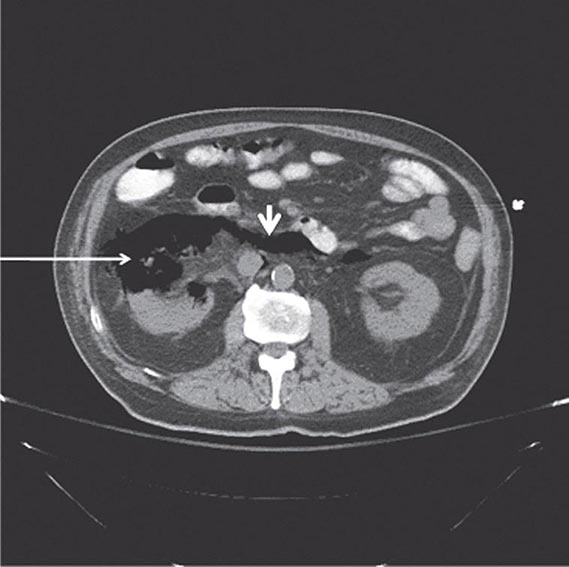
FIGURE 162-2 Emphysematous pyelonephritis. Infection of the right kidney of a diabetic man by Escherichia coli, a gas-forming, facultative anaerobic uropathogen, has led to destruction of the renal parenchyma (arrow) and tracking of gas through the retroperitoneal space (arrowhead).
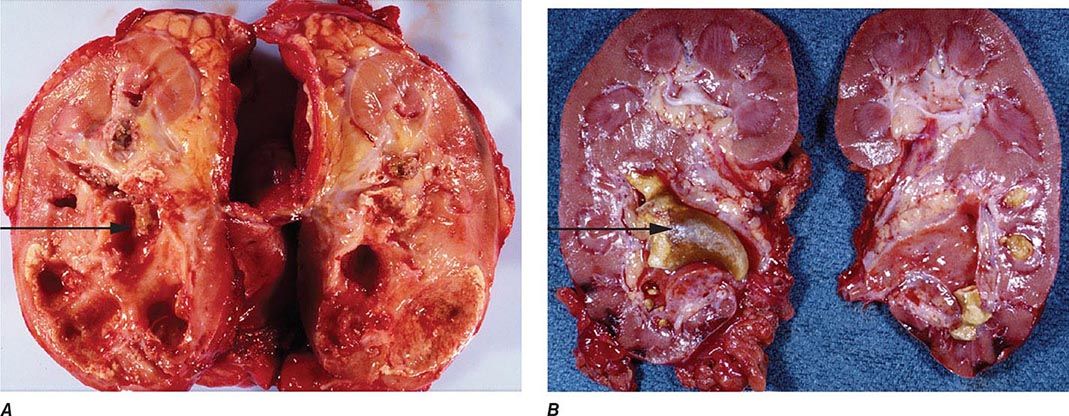
FIGURE 162-3 Xanthogranulomatous pyelonephritis. A. This photograph shows extensive destruction of renal parenchyma due to long-standing suppurative inflammation. The precipitating factor was obstruction by a staghorn calculus, which has been removed, leaving a depression (arrow). The mass effect of xanthogranulomatous pyelonephritis can mimic renal malignancy. B. A large staghorn calculus (arrow) is seen obstructing the renal pelvis and calyceal system. The lower pole of the kidney shows areas of hemorrhage and necrosis with collapse of cortical areas. (Images courtesy of Dharam M. Ramnani, MD, Virginia Urology Pathology Laboratory, Richmond, VA.)
DIAGNOSTIC TOOLS
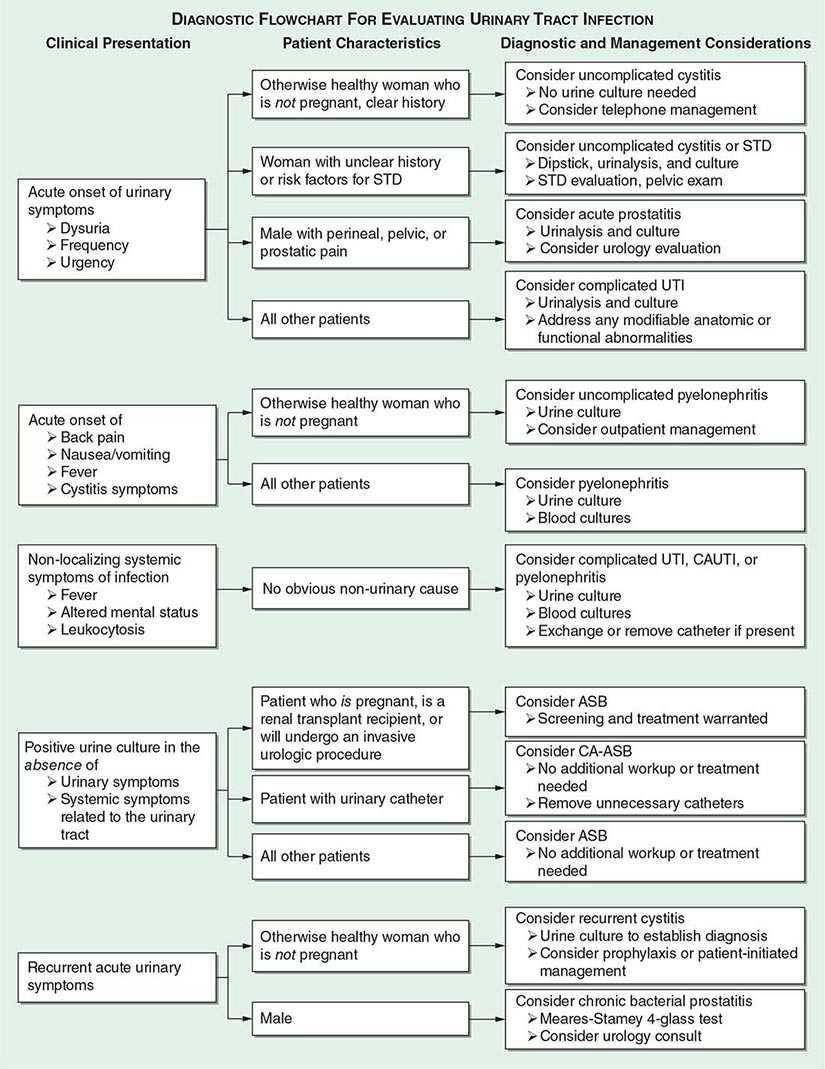
History The diagnosis of any of the UTI syndromes or ASB begins with a detailed history (Fig. 162-4). The history given by the patient has a high predictive value in uncomplicated cystitis. A meta-analysis evaluating the probability of acute UTI on the basis of history and physical findings concluded that, in women presenting with at least one symptom of UTI (dysuria, frequency, hematuria, or back pain) and without complicating factors, the probability of acute cystitis or pyelonephritis is 50%. The even higher rates of accuracy of self-diagnosis among women with recurrent UTI probably account for the success of patient-initiated treatment of recurrent cystitis. If vaginal discharge and complicating factors are absent and risk factors for UTI are present, then the probability of UTI is close to 90%, and no laboratory evaluation is needed. Similarly, a combination of dysuria and urinary frequency in the absence of vaginal discharge increases the probability of UTI to 96%. Further laboratory evaluation with dipstick testing or urine culture is not necessary in such patients before the initiation of definitive therapy.
FIGURE 162-4 Diagnostic approach to urinary tract infection (UTI). STD, sexually transmitted disease; CAUTI, catheter-associated UTI; ASB, asymptomatic bacteriuria; CA-ASB, catheter-associated ASB.
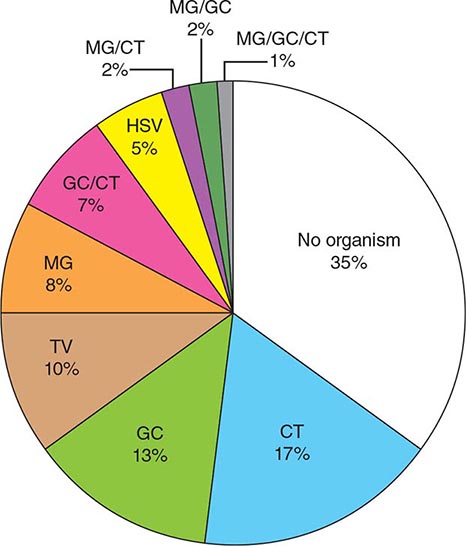
When the patient’s history is applied as a diagnostic tool, it is important to recall that the studies included in the meta-analysis cited above did not enroll children, adolescents, pregnant women, men, or patients with complicated UTI. One significant concern is that sexually transmitted disease—that caused by Chlamydia trachomatis in particular—may be inappropriately treated as UTI. This concern is particularly relevant for female patients under the age of 25. The differential diagnosis to be considered when women present with dysuria includes cervicitis (C. trachomatis, Neisseria gonorrhoeae), vaginitis (Candida albicans, Trichomonas vaginalis), herpetic urethritis, interstitial cystitis, and noninfectious vaginal or vulvar irritation. Women with more than one sexual partner and inconsistent use of condoms are at high risk for both UTI and sexually transmitted disease, and symptoms alone do not always distinguish between these conditions.
The Urine Dipstick Test, Urinalysis, and Urine Culture Useful diagnostic tools include the urine dipstick test and urinalysis, both of which provide point-of-care information, and the urine culture, which can retrospectively confirm a prior diagnosis. Understanding the parameters of the dipstick test is important in interpreting its results. Only members of the family Enterobacteriaceae convert nitrate to nitrite, and enough nitrite must accumulate in the urine to reach the threshold of detection. If a woman with acute cystitis is forcing fluids and voiding frequently, the dipstick test for nitrite is less likely to be positive, even when E. coli is present. The leukocyte esterase test detects this enzyme in the host’s polymorphonuclear leukocytes in the urine, whether the cells are intact or lysed. Many reviews have attempted to describe the diagnostic accuracy of dipstick testing. The bottom line for clinicians is that a urine dipstick test can confirm the diagnosis of uncomplicated cystitis in a patient with a reasonably high pretest probability of this disease. Either nitrite or leukocyte esterase positivity can be interpreted as a positive result. Blood in the urine also may suggest a diagnosis of UTI. A dipstick test negative for both nitrite and leukocyte esterase in the same type of patient should prompt consideration of other explanations for the patient’s symptoms and collection of urine for culture. A negative dipstick test is not sufficiently sensitive to rule out bacteriuria in pregnant women, in whom it is important to detect all episodes of bacteriuria. Performance characteristics of the dipstick test differ in men (highly specific) and in noncatheterized nursing home residents (highly sensitive).
Urine microscopy reveals pyuria in nearly all cases of cystitis and hematuria in ~30% of cases. In current practice, most hospital laboratories use an automated system rather than manual examination for urine microscopy. A machine aspirates a sample of the urine and then classifies the particles in the urine by size, shape, contrast, light scatter, volume, and other properties. These automated systems can be overwhelmed by high numbers of dysmorphic red blood cells, white blood cells, or crystals; in general, counts of bacteria are less accurate than are counts of red and white blood cells. The authors’ clinical recommendation is that the patient’s symptoms and presentation should outweigh an incongruent result on automated urinalysis.
The detection of bacteria in a urine culture is the diagnostic “gold standard” for UTI; unfortunately, however, culture results do not become available until 24 h after the patient’s presentation. Identifying specific organism(s) can require an additional 24 h. Studies of women with symptoms of cystitis have found that a colony count threshold of >102 bacteria/mL is more sensitive (95%) and specific (85%) than a threshold of 105/mL for the diagnosis of acute cystitis in women. In men, the minimal level indicating infection appears to be 103/mL. Urine specimens frequently become contaminated with the normal microbial flora of the distal urethra, vagina, or skin. These contaminants can grow to high numbers if the collected urine is allowed to stand at room temperature. In most instances, a culture that yields mixed bacterial species is contaminated except in settings of long-term catheterization, chronic urinary retention, or the presence of a fistula between the urinary tract and the gastrointestinal or genital tract.
DIAGNOSIS
The approach to diagnosis is influenced by which of the clinical UTI syndromes is suspected (Fig. 162-4).
Uncomplicated Cystitis in Women Uncomplicated cystitis in women can be treated on the basis of history alone. However, if the symptoms are not specific or if a reliable history cannot be obtained, then a urine dipstick test should be performed. A positive nitrite or leukocyte esterase result in a woman with one symptom of UTI increases the probability of UTI from 50% to ~80%, and empirical treatment can be considered without further testing. In this setting, a negative dipstick result does not rule out UTI, and a urine culture, close clinical follow-up, and possibly a pelvic examination are recommended. These recommendations are made with the caveat that no factors associated with complicated UTI, such as pregnancy, are known to be present.
Cystitis in Men The signs and symptoms of cystitis in men are similar to those in women, but this disease differs in several important ways in the male population. Collection of urine for culture is strongly recommended when a man has symptoms of UTI, as the documentation of bacteriuria can differentiate the less common syndromes of acute and chronic bacterial prostatitis from the very common entity of chronic pelvic pain syndrome, which is not associated with bacteriuria and thus is not usually responsive to antibacterial therapy. If the diagnosis is unclear, localization cultures using the two- or four-glass Meares-Stamey test (urine collection after prostate massage) should be undertaken to differentiate between bacterial and nonbacterial prostatic syndromes, and the patient should be referred to a urologist. Men with febrile UTI often have an elevated serum level of prostate-specific antigen as well as an enlarged prostate and enlarged seminal vesicles on ultrasound—findings indicative of prostate involvement. In 85 men with febrile UTI, symptoms of urinary retention, early recurrence of UTI, hematuria at follow-up, and voiding difficulties were predictive of surgically correctable disorders. Men with none of these symptoms had normal upper and lower urinary tracts on urologic workup.
Asymptomatic Bacteriuria The diagnosis of ASB involves both microbiologic and clinical criteria. The microbiologic criterion is usually ≥105 bacterial CFU/mL except in catheter-associated disease, in which ≥102 CFU/mL is the cutoff. The clinical criterion is that the person has no signs or symptoms referable to UTI.
|
TREATMENT |
URINARY TRACT INFECTIONS |
Antimicrobial therapy is warranted for any symptomatic UTI. The choice of antimicrobial agent and the dose and duration of therapy depend on the site of infection and the presence or absence of complicating conditions. Each category of UTI warrants a different approach based on the particular clinical syndrome.
![]() Antimicrobial resistance among uropathogens varies from region to region and impacts the approach to empirical treatment of UTI. E. coli ST131 is the predominant multilocus sequence type found worldwide as the cause of multidrug-resistant UTI. Recommendations for treatment must be considered in the context of local resistance patterns and national differences in some agents’ availability. For example, fosfomycin and pivmecillinam are not available in all countries but are considered first-line options where they are available because they retain activity against a majority of uropathogens that produce extended-spectrum β-lactamases. Thus, therapeutic choices should depend on local resistance, drug availability, and individual patient factors such as recent travel and antimicrobial use.
Antimicrobial resistance among uropathogens varies from region to region and impacts the approach to empirical treatment of UTI. E. coli ST131 is the predominant multilocus sequence type found worldwide as the cause of multidrug-resistant UTI. Recommendations for treatment must be considered in the context of local resistance patterns and national differences in some agents’ availability. For example, fosfomycin and pivmecillinam are not available in all countries but are considered first-line options where they are available because they retain activity against a majority of uropathogens that produce extended-spectrum β-lactamases. Thus, therapeutic choices should depend on local resistance, drug availability, and individual patient factors such as recent travel and antimicrobial use.
UNCOMPLICATED CYSTITIS IN WOMEN
Since the species and antimicrobial susceptibilities of the bacteria that cause acute uncomplicated cystitis are highly predictable, many episodes of uncomplicated cystitis can be managed over the telephone (Fig. 162-4). Most patients with other UTI syndromes require further diagnostic evaluation. Although the risk of serious complications with telephone management appears to be low, studies of telephone management algorithms generally have involved otherwise healthy white women who are at low risk for complications of UTI.
In 1999, TMP-SMX was recommended as the first-line agent for treatment of uncomplicated UTI in the published guidelines of the Infectious Diseases Society of America. Antibiotic resistance among uropathogens causing uncomplicated cystitis has since increased, appreciation of the importance of collateral damage (as defined below) has increased, and newer agents have been studied. Unfortunately, there is no longer a single best agent for acute uncomplicated cystitis.
Collateral damage refers to the adverse ecologic effects of antimicrobial therapy, including killing of the normal flora and selection of drug-resistant organisms. Outbreaks of Clostridium difficile infection offer an example of collateral damage in the hospital environment. The implication of collateral damage in this context is that a drug that is highly efficacious for the treatment of UTI is not necessarily the optimal first-line agent if it also has pronounced secondary effects on the normal flora or is likely to change resistance patterns. Drugs used for UTI that have a minimal effect on fecal flora include pivmecillinam, fosfomycin, and nitrofurantoin. In contrast, trimethoprim, TMP-SMX, quinolones, and ampicillin affect the fecal flora more significantly; these drugs are notably the agents for which rising resistance levels have been documented.
![]() Several effective therapeutic regimens are available for acute uncomplicated cystitis in women (Table 162-1). Well-studied first-line agents include TMP-SMX and nitrofurantoin. Second-line agents include fluoroquinolone and β-lactam compounds. Single-dose fosfomycin treatment for acute cystitis is widely used in Europe but has produced mixed results in randomized trials. There is increasing experience with the use of fosfomycin against UTIs (including complicated infections) caused by multidrug-resistant E. coli. Pivmecillinam is not currently available in the United States or Canada but is a popular agent in some European countries. The pros and cons of other therapies are discussed briefly below.
Several effective therapeutic regimens are available for acute uncomplicated cystitis in women (Table 162-1). Well-studied first-line agents include TMP-SMX and nitrofurantoin. Second-line agents include fluoroquinolone and β-lactam compounds. Single-dose fosfomycin treatment for acute cystitis is widely used in Europe but has produced mixed results in randomized trials. There is increasing experience with the use of fosfomycin against UTIs (including complicated infections) caused by multidrug-resistant E. coli. Pivmecillinam is not currently available in the United States or Canada but is a popular agent in some European countries. The pros and cons of other therapies are discussed briefly below.
|
TREATMENT STRATEGIES FOR ACUTE UNCOMPLICATED CYSTITIS |
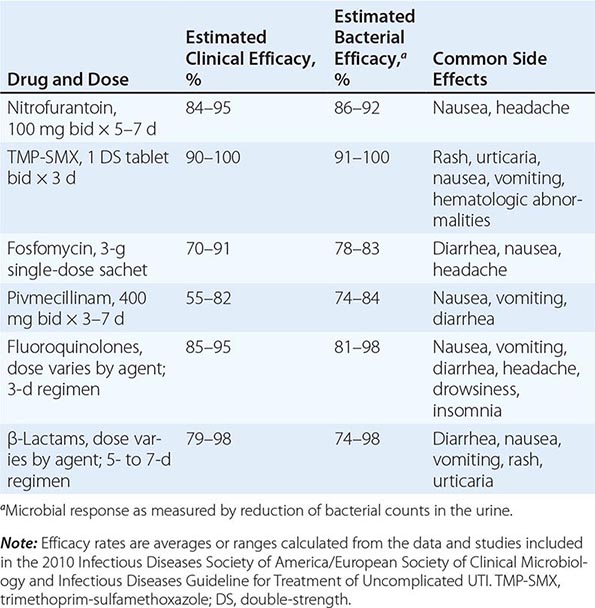
Traditionally, TMP-SMX has been recommended as first-line treatment for acute cystitis, and it remains appropriate to consider the use of this drug in regions with resistance rates not exceeding 20%. TMP-SMX resistance has clinical significance: in TMP-SMX-treated patients with resistant isolates, the time to symptom resolution is longer and rates of both clinical and microbiologic failure are higher. Individual host factors associated with an elevated risk of UTI caused by a strain of E. coli resistant to TMP-SMX include recent use of TMP-SMX or another antimicrobial agent and recent travel to an area with high rates of TMP-SMX resistance. The optimal setting for empirical use of TMP-SMX is uncomplicated UTI in a female patient who has an established relationship with the practitioner and who can thus seek further care if her symptoms do not respond promptly.
Resistance to nitrofurantoin remains low despite >60 years of use. Since this drug affects bacterial metabolism in multiple pathways, several mutational steps are required for the development of resistance. Nitrofurantoin remains highly active against E. coli and most non–E. coli isolates. Proteus, Pseudomonas, Serratia, Enterobacter, and yeasts are all intrinsically resistant to this drug. Although nitrofurantoin has traditionally been prescribed as a 7-day regimen, similar microbiologic and clinical efficacies are noted with a 5-day course of nitrofurantoin or a 3-day course of TMP-SMX for treatment of women with acute cystitis; 3-day courses of nitrofurantoin are not recommended for acute cystitis. Nitrofurantoin does not reach significant levels in tissue and cannot be used to treat pyelonephritis.
Most fluoroquinolones are highly effective as short-course therapy for cystitis; the exception is moxifloxacin, which may not reach adequate urinary levels. The fluoroquinolones commonly used for UTI include ofloxacin, ciprofloxacin, and levofloxacin. The main concern about fluoroquinolone use for acute cystitis is the propagation of fluoroquinolone resistance, not only among uropathogens but also among other organisms causing more serious and difficult-to-treat infections at other sites. Fluoroquinolone use is also a factor driving the emergence of C. difficile outbreaks in hospital settings. Most experts now call for restricting fluoroquinolones to specific instances of uncomplicated cystitis in which other antimicrobial agents are not suitable. Quinolone use in certain populations, including adults >60 years of age, has been associated with an increased risk of Achilles tendon rupture.
Except for pivmecillinam, β-lactam agents generally have not performed as well as TMP-SMX or fluoroquinolones in acute cystitis. Rates of pathogen eradication are lower and relapse rates are higher with β-lactam drugs. The generally accepted explanation is that β-lactams fail to eradicate uropathogens from the vaginal reservoir. A proposed role for intracellular biofilm communities is intriguing. Many strains of E. coli that are resistant to TMP-SMX are also resistant to amoxicillin and cephalexin; thus, these drugs should be used only for patients infected with susceptible strains.
Urinary analgesics are appropriate in certain situations to speed resolution of bladder discomfort. The urinary tract analgesic phenazopyridine is widely used but can cause significant nausea. Combination analgesics containing urinary antiseptics (methenamine, methylene blue), a urine-acidifying agent (sodium phosphate), and an antispasmodic agent (hyoscyamine) also are available.
PYELONEPHRITIS
Since patients with pyelonephritis have tissue-invasive disease, the treatment regimen chosen should have a very high likelihood of eradicating the causative organism and should reach therapeutic blood levels quickly. High rates of TMP-SMX-resistant E. coli in patients with pyelonephritis have made fluoroquinolones the first-line therapy for acute uncomplicated pyelonephritis. Whether the fluoroquinolones are given orally or parenterally depends on the patient’s tolerance for oral intake. A randomized clinical trial demonstrated that a 7-day course of therapy with oral ciprofloxacin (500 mg twice daily, with or without an initial IV 400-mg dose) was highly effective for the initial management of pyelonephritis in the outpatient setting. Oral TMP-SMX (one double-strength tablet twice daily for 14 days) also is effective for treatment of acute uncomplicated pyelonephritis if the uropathogen is known to be susceptible. If the pathogen’s susceptibility is not known and TMP-SMX is used, an initial IV 1-g dose of ceftriaxone is recommended. Oral β-lactam agents are less effective than the fluoroquinolones and should be used with caution and close follow-up. Options for parenteral therapy for uncomplicated pyelonephritis include fluoroquinolones, an extended-spectrum cephalosporin with or without an aminoglycoside, or a carbapenem. Combinations of a β-lactam and a β-lactamase inhibitor (e.g., ampicillin-sulbactam, ticarcillin-clavulanate, piperacillin-tazobactam) or imipenem-cilastatin can be used in patients with more complicated histories, previous episodes of pyelonephritis, or recent urinary tract manipulations; in general, the treatment of such patients should be guided by urine culture results. Once the patient has responded clinically, oral therapy should be substituted for parenteral therapy.
UTI IN PREGNANT WOMEN
Nitrofurantoin, ampicillin, and the cephalosporins are considered relatively safe in early pregnancy. One retrospective case-control study suggesting an association between nitrofurantoin and birth defects has not been confirmed. Sulfonamides should clearly be avoided both in the first trimester (because of possible teratogenic effects) and near term (because of a possible role in the development of kernicterus). Fluoroquinolones are avoided because of possible adverse effects on fetal cartilage development. Ampicillin and the cephalosporins have been used extensively in pregnancy and are the drugs of choice for the treatment of asymptomatic or symptomatic UTI in this group of patients. For pregnant women with overt pyelonephritis, parenteral β-lactam therapy with or without aminoglycosides is the standard of care.
UTI IN MEN
Since the prostate is involved in the majority of cases of febrile UTI in men, the goal in these patients is to eradicate the prostatic infection as well as the bladder infection. A 7- to 14-day course of a fluoroquinolone or TMP-SMX is recommended if the uropathogen is susceptible. If acute bacterial prostatitis is suspected, antimicrobial therapy should be initiated after urine and blood are obtained for cultures. Therapy can be tailored to urine culture results and should be continued for 2–4 weeks. For documented chronic bacterial prostatitis, a 4- to 6-week course of antibiotics is often necessary. Recurrences, which are not uncommon in chronic prostatitis, often warrant a 12-week course of treatment.
COMPLICATED UTI
Complicated UTI (other than that discussed above) occurs in a heterogeneous group of patients with a wide variety of structural and functional abnormalities of the urinary tract and kidneys. The range of species and their susceptibility to antimicrobial agents are likewise heterogeneous. As a consequence, therapy for complicated UTI must be individualized and guided by urine culture results. Frequently, a patient with complicated UTI will have prior urine culture data that can be used to guide empirical therapy while current culture results are awaited. Xanthogranulomatous pyelonephritis is treated with nephrectomy. Percutaneous drainage can be used as the initial therapy in emphysematous pyelonephritis and can be followed by elective nephrectomy as needed. Papillary necrosis with obstruction requires intervention to relieve the obstruction and to preserve renal function.
ASYMPTOMATIC BACTERIURIA
Treatment of ASB does not decrease the frequency of symptomatic infections or complications except in pregnant women, persons undergoing urologic surgery, and perhaps neutropenic patients and renal transplant recipients. Treatment of ASB in pregnant women and patients undergoing urologic procedures should be directed by urine culture results. In all other populations, screening for and treatment of ASB are discouraged. The majority of cases of catheter-associated bacteriuria are asymptomatic and do not warrant antimicrobial therapy.
CATHETER-ASSOCIATED UTI
Multiple institutions have released guidelines for the treatment of CAUTI, which is defined by bacteriuria and symptoms in a catheterized patient. The signs and symptoms either are localized to the urinary tract or can include otherwise unexplained systemic manifestations, such as fever. The accepted threshold for bacteriuria to meet the definition of CAUTI is ≥103 CFU/mL, while the threshold for bacteriuria to meet the definition of ASB is ≥105 CFU/mL.
The formation of biofilm—a living layer of uropathogens—on the urinary catheter is central to the pathogenesis of CAUTI and affects both therapeutic and preventive strategies. Organisms in a biofilm are relatively resistant to killing by antibiotics, and eradication of a catheter-associated biofilm is difficult without removal of the device itself. Furthermore, because catheters provide a conduit for bacteria to enter the bladder, bacteriuria is inevitable with long-term catheter use.
The typical signs and symptoms of UTI, including pain, urgency, dysuria, fever, peripheral leukocytosis, and pyuria, have less predictive value for the diagnosis of infection in catheterized patients. Furthermore, the presence of bacteria in the urine of a patient who is febrile and catheterized does not necessarily predict CAUTI, and other explanations for the fever should be considered.
The etiology of CAUTI is diverse, and urine culture results are essential to guide treatment. Fairly good evidence supports the practice of catheter change during treatment for CAUTI. The goal is to remove biofilm-associated organisms that could serve as a nidus for reinfection. Pathology studies reveal that many patients with long-term catheters have occult pyelonephritis. A randomized trial in persons with spinal cord injury who were undergoing intermittent catheterization found that relapse was more common after 3 days of therapy than after 14 days. In general, a 7- to 14-day course of antibiotics is recommended, but further studies on the optimal duration of therapy are needed.
In the setting of long-term catheter use, systemic antibiotics, bladder-acidifying agents, antimicrobial bladder washes, topical disinfectants, and antimicrobial drainage-bag solutions have all been ineffective at preventing the onset of bacteriuria and have been associated with the emergence of resistant organisms. The best strategy for prevention of CAUTI is to avoid insertion of unnecessary catheters and to remove catheters once they are no longer necessary. Evidence is insufficient to recommend suprapubic catheters and condom catheters as alternatives to indwelling urinary catheters as a means to prevent CAUTI. However, intermittent catheterization may be preferable to long-term indwelling urethral catheterization in certain populations (e.g., spinal cord–injured persons) to prevent both infectious and anatomic complications. Antimicrobial catheters impregnated with silver or nitrofurazone have not been shown to provide significant clinical benefit in terms of reducing rates of symptomatic UTI.
CANDIDURIA
The appearance of Candida in the urine is an increasingly common complication of indwelling catheterization, particularly for patients in the intensive care unit, those taking broad-spectrum antimicrobial drugs, and those with underlying diabetes mellitus. In many studies, >50% of urinary Candida isolates have been found to be non-albicans species. The clinical presentation varies from an asymptomatic laboratory finding to pyelonephritis and even sepsis. Removal of the urethral catheter results in resolution of candiduria in more than one-third of asymptomatic cases. Treatment of asymptomatic patients does not appear to decrease the frequency of recurrence of candiduria. Treatment is recommended for patients who have symptomatic cystitis or pyelonephritis and for those who are at high risk for disseminated disease. High-risk patients include those with neutropenia, those who are undergoing urologic manipulation, those who are clinically unstable, and low-birth-weight infants. Fluconazole (200–400 mg/d for 14 days) reaches high levels in urine and is the first-line regimen for Candida infections of the urinary tract. Although instances of successful eradication of candiduria by some of the newer azoles and echinocandins have been reported, these agents are characterized by only low-level urinary excretion and thus are not recommended. For Candida isolates with high levels of resistance to fluconazole, oral flucytosine and/or parenteral amphotericin B are options. Bladder irrigation with amphotericin B generally is not recommended.
PREVENTION OF RECURRENT UTI IN WOMEN
Recurrence of uncomplicated cystitis in reproductive-age women is common, and a preventive strategy is indicated if recurrent UTIs are interfering with a patient’s lifestyle. The threshold of two or more symptomatic episodes per year is not absolute; decisions about interventions should take the patient’s preferences into account.
Three prophylactic strategies are available: continuous, postcoital, and patient-initiated therapy. Continuous prophylaxis and postcoital prophylaxis usually entail low doses of TMP-SMX, a fluoroquinolone, or nitrofurantoin. These regimens are all highly effective during the period of active antibiotic intake. Typically, a prophylactic regimen is prescribed for 6 months and then discontinued, at which point the rate of recurrent UTI often returns to baseline. If bothersome infections recur, the prophylactic program can be reinstituted for a longer period. Selection of resistant strains in the fecal flora has been documented in studies of women taking prophylactic antibiotics for 12 months.
Patient-initiated therapy involves supplying the patient with materials for urine culture and with a course of antibiotics for self-medication at the first symptoms of infection. The urine culture is refrigerated and delivered to the physician’s office for confirmation of the diagnosis. When an established and reliable patient-provider relationship exists, the urine culture can be omitted as long as the symptomatic episodes respond completely to short-course therapy and are not followed by relapse.
PROGNOSIS
Cystitis is a risk factor for recurrent cystitis and pyelonephritis. ASB is common among elderly and catheterized patients but does not in itself increase the risk of death. The relationships among recurrent UTI, chronic pyelonephritis, and renal insufficiency have been widely studied. In the absence of anatomic abnormalities, recurrent infection in children and adults does not lead to chronic pyelonephritis or to renal failure. Moreover, infection does not play a primary role in chronic interstitial nephritis; the primary etiologic factors in this condition are analgesic abuse, obstruction, reflux, and toxin exposure. In the presence of underlying renal abnormalities (particularly obstructing stones), infection as a secondary factor can accelerate renal parenchymal damage. In spinal cord–injured patients, use of a long-term indwelling bladder catheter is a well-documented risk factor for bladder cancer. Chronic bacteriuria resulting in chronic inflammation is one possible explanation for this observation.
163 |
Sexually Transmitted Infections: Overview and Clinical Approach |
CLASSIFICATION AND EPIDEMIOLOGY
Worldwide, most adults acquire at least one sexually transmitted infection (STI), and many remain at risk for complications. Each year, for example, an estimated 14 million persons in the United States acquire a new genital human papillomavirus (HPV) infection, and many of these individuals are at risk for genital neoplasias. Certain STIs, such as syphilis, gonorrhea, HIV infection, hepatitis B, and chancroid, are most concentrated within “core populations” characterized by high rates of partner change, multiple concurrent partners, or “dense,” highly connected sexual networks—e.g., involving sex workers and their clients, some men who have sex with men (MSM), and persons involved in the use of illicit drugs, particularly crack cocaine and methamphetamine. Other STIs are distributed more evenly throughout societies. For example, chlamydial infections, genital infections with HPV, and genital herpes can spread widely, even in relatively low-risk populations.
In general, the product of three factors determines the initial rate of spread of any STI within a population: rate of sexual exposure of susceptible to infectious people, efficiency of transmission per exposure, and duration of infectivity of those infected. Accordingly, efforts to prevent and control STIs aim to decrease the rate of sexual exposure of susceptibles to infected persons (e.g., through individual counseling and efforts to change the norms of sexual behavior and through a variety of STI control efforts aimed at reducing the proportion of the population infected), to decrease the duration of infectivity (through early diagnosis and curative or suppressive treatment), and to decrease the efficiency of transmission (e.g., through promotion of condom use and safer sexual practices, through use of effective vaccines, and recently through male circumcision).
![]() In all societies, STIs rank among the most common of all infectious diseases, with >30 infections now classified as predominantly sexually transmitted or as frequently sexually transmissible (Table 163-1). In developing countries, with three-quarters of the world’s population and 90% of the world’s STIs, factors such as population growth (especially in adolescent and young-adult age groups), rural-to-urban migration, wars, limited or no provision of reproductive health services for women, and poverty create exceptional vulnerability to disease resulting from unprotected sex. During the 1990s in China, Russia, the other states of the former Soviet Union, and South Africa, internal social structures changed rapidly as borders opened to the West, unleashing enormous new epidemics of HIV infection and other STIs. Despite advances in the provision of highly effective antiretroviral therapy worldwide, HIV remains the leading cause of death in some developing countries, and HPV and hepatitis B virus (HBV) remain important causes of cervical and hepatocellular carcinoma, respectively—two of the most common malignancies in the developing world. Sexually transmitted herpes simplex virus (HSV) infections now cause most genital ulcer disease throughout the world and an increasing proportion of cases of genital herpes in developing countries with generalized HIV epidemics, where the positive-feedback loop between HSV and HIV transmission is a growing, intractable problem. Despite this consistent link, randomized trials evaluating the efficacy of antiviral therapy in suppressing HSV in both HIV-uninfected and HIV-infected persons have not demonstrated a protective effect against acquisition or transmission of HIV. The World Health Organization estimated that 448 million new cases of four curable STIs—gonorrhea, chlamydial infection, syphilis, and trichomoniasis—occurred in 2005. Up to 50% of women of reproductive age in developing countries have bacterial vaginosis (arguably acquired sexually). All of these curable infections have been associated with increased risk of HIV transmission or acquisition.
In all societies, STIs rank among the most common of all infectious diseases, with >30 infections now classified as predominantly sexually transmitted or as frequently sexually transmissible (Table 163-1). In developing countries, with three-quarters of the world’s population and 90% of the world’s STIs, factors such as population growth (especially in adolescent and young-adult age groups), rural-to-urban migration, wars, limited or no provision of reproductive health services for women, and poverty create exceptional vulnerability to disease resulting from unprotected sex. During the 1990s in China, Russia, the other states of the former Soviet Union, and South Africa, internal social structures changed rapidly as borders opened to the West, unleashing enormous new epidemics of HIV infection and other STIs. Despite advances in the provision of highly effective antiretroviral therapy worldwide, HIV remains the leading cause of death in some developing countries, and HPV and hepatitis B virus (HBV) remain important causes of cervical and hepatocellular carcinoma, respectively—two of the most common malignancies in the developing world. Sexually transmitted herpes simplex virus (HSV) infections now cause most genital ulcer disease throughout the world and an increasing proportion of cases of genital herpes in developing countries with generalized HIV epidemics, where the positive-feedback loop between HSV and HIV transmission is a growing, intractable problem. Despite this consistent link, randomized trials evaluating the efficacy of antiviral therapy in suppressing HSV in both HIV-uninfected and HIV-infected persons have not demonstrated a protective effect against acquisition or transmission of HIV. The World Health Organization estimated that 448 million new cases of four curable STIs—gonorrhea, chlamydial infection, syphilis, and trichomoniasis—occurred in 2005. Up to 50% of women of reproductive age in developing countries have bacterial vaginosis (arguably acquired sexually). All of these curable infections have been associated with increased risk of HIV transmission or acquisition.
|
SEXUALLY TRANSMITTED AND SEXUALLY TRANSMISSIBLE MICROORGANISMS |
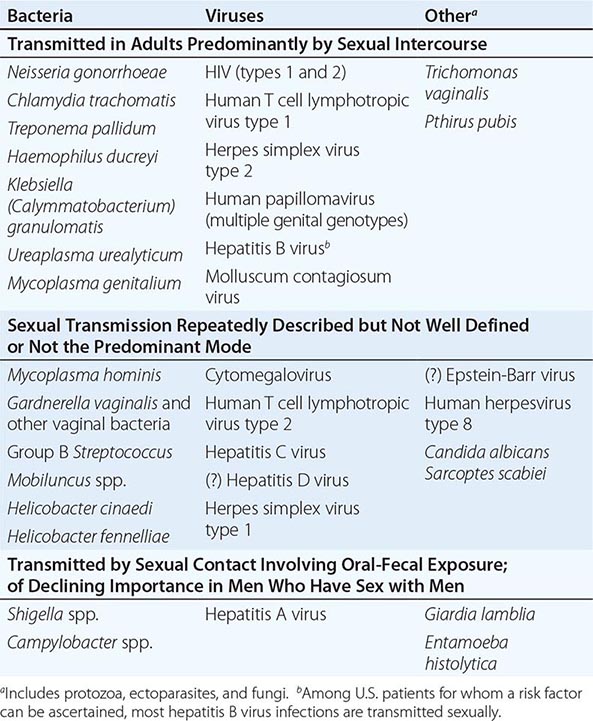
In the United States, the prevalence of antibody to HSV-2 began to fall in the late 1990s, especially among adolescents and young adults; the decline is presumably due to delayed sexual debut, increased condom use, and lower rates of multiple (four or more) sex partners, as is well documented by the U.S. Youth Risk Behavior Surveillance System. The estimated annual incidence of HBV infection has also declined dramatically since the mid-1980s; this decrease is probably attributable more to adoption of safer sexual practices and reduced needle sharing among injection drug users than to use of hepatitis B vaccine, for which coverage among young adults (including those at high risk for this infection) initially was very limited. Genital HPV remains the most common sexually transmitted pathogen in this country, infecting 60% of a cohort of initially HPV-negative, sexually active Washington state college women within 5 years in a study conducted from 1990 to 2000. The scale-up of HPV vaccine coverage among young women has already shown promise in reducing the incidence of infection with the HPV types included in the vaccines and of conditions associated with these viruses.
In industrialized countries, fear of HIV infection since the mid-1980s, coupled with widespread behavioral interventions and better-organized systems of care for the curable STIs, initially helped curb the transmission of the latter diseases. However, foci of hyperendemic transmission persist in the southeastern United States and in most large U.S. cities. Rates of gonorrhea and syphilis remain higher in the United States than in any other Western industrialized country.
In the United States, the Centers for Disease Control and Prevention (CDC) has compiled reported rates of STIs since 1941. The incidence of reported gonorrhea peaked at 468 cases per 100,000 population in the mid-1970s and fell to a low of 98 cases per 100,000 in 2012. With increased testing and more sensitive tests, the incidence of reported Chlamydia trachomatis infection has been increasing steadily since reporting began in 1984, reaching an all-time peak of 457.6 cases per 100,000 in 2011. The incidence of primary and secondary syphilis per 100,000 peaked at 71 cases in 1946, fell rapidly to 3.9 cases in 1956, ranged from ~10 to 15 cases through 1987 (with markedly increased rates among MSM and African Americans), and then fell to a nadir of 2.1 cases in 2000–2001 (with rates falling most rapidly among heterosexual African Americans). Unfortunately, since 1996, with the introduction of highly active antiretroviral therapy, the increased use of “serosorting” (i.e., the avoidance of unprotected sex with HIV-serodiscordant partners but not with HIV-seroconcordant partners, a strategy that provides no protection against STIs other than HIV infection), and an ongoing epidemic of methamphetamine use, gonorrhea, syphilis, and chlamydial infection have had a remarkable resurgence among MSM in North America and Europe, where outbreaks of a rare type of chlamydial infection (lymphogranuloma venereum [LGV]) that had virtually disappeared during the AIDS era have occurred. In 2012, ~75% of primary and secondary syphilis cases reported to the CDC were in MSM. These developments have resulted in a high degree of co-infection with HIV and other sexually transmitted pathogens (particularly syphilis and LGV), primarily among MSM.
MANAGEMENT OF COMMON SEXUALLY TRANSMITTED DISEASE (STD) SYNDROMES
Although other chapters discuss management of specific STIs, delineating treatment based on diagnosis of a specific infection, most patients are actually managed (at least initially) on the basis of presenting symptoms and signs and associated risk factors, even in industrialized countries. Table 163-2 lists some of the most common clinical STD syndromes and their microbial etiologies. Strategies for their management are outlined below. Chapters 225e and 226 address the management of infections with human retroviruses.
|
MAJOR SEXUALLY TRANSMITTED DISEASE SYNDROMES AND SEXUALLY TRANSMITTED MICROBIAL ETIOLOGIES |

STD care and management begin with risk assessment and proceed to clinical assessment, diagnostic testing or screening, treatment, and prevention. Indeed, the routine care of any patient begins with risk assessment (e.g., for risk of heart disease, cancer). STD/HIV risk assessment is important in primary care, urgent care, and emergency care settings as well as in specialty clinics providing adolescent, HIV/AIDS, prenatal, and family planning services. STD/HIV risk assessment guides detection and interpretation of symptoms that could reflect an STD; decisions on screening or prophylactic/preventive treatment; risk reduction counseling and intervention (e.g., hepatitis B vaccination); and treatment of partners of patients with known infections. Consideration of routine demographic data (e.g., gender, age, area of residence) is a simple first step in STD/HIV risk assessment. For example, national guidelines strongly recommend routine screening of sexually active females ≤25 years of age for C. trachomatis infection. Table 163-3 provides a set of 11 STD/HIV risk-assessment questions that clinicians can pose verbally or that health care systems can adapt (with yes/no responses) into a routine self-administered questionnaire for use in clinics. The initial framing statement gives permission to discuss topics that may be perceived as sensitive or socially unacceptable by providers and patients alike.
|
ELEVEN-QUESTION STD/HIV RISK ASSESSMENT |
Risk assessment is followed by clinical assessment (elicitation of information on specific current symptoms and signs of STDs). Confirmatory diagnostic tests (for persons with symptoms or signs) or screening tests (for those without symptoms or signs) may involve microscopic examination, culture, antigen detection tests, nucleic acid amplification tests (NAATs), or serology. Initial syndrome-based treatment should cover the most likely causes. For certain syndromes, results of rapid tests can narrow the spectrum of this initial therapy (e.g., saline microscopy of vaginal fluid for women with vaginal discharge, Gram’s stain of urethral discharge for men with urethral discharge, rapid plasma reagin test for genital ulcer). After the institution of treatment, STD management proceeds to the “4 Cs” of prevention and control: contact tracing (see “Prevention and Control of STIs,” below), ensuring compliance with therapy, and counseling on risk reduction, including condom promotion and provision.
Consistent with current guidelines, all adults should be screened for infection with HIV-1 at least once and more frequently if they are at elevated risk for acquisition of this infection.
URETHRITIS IN MEN
Urethritis in men produces urethral discharge, dysuria, or both, usually without frequency of urination. Causes include Neisseria gonorrhoeae, C. trachomatis, Mycoplasma genitalium, Ureaplasma urealyticum, Trichomonas vaginalis, HSV, and adenovirus.
Until recently, C. trachomatis caused ~30–40% of cases of nongonococcal urethritis (NGU), particularly in heterosexual men; however, the proportion of cases due to this organism has probably declined in some populations served by effective chlamydial-control programs, and older men with urethritis appear less likely to have chlamydial infection. HSV and T. vaginalis each cause a small proportion of NGU cases in the United States. Recently, multiple studies have consistently implicated M. genitalium as a probable cause of many Chlamydia-negative cases. Fewer studies than in the past have implicated Ureaplasma; the ureaplasmas have been differentiated into U. urealyticum and Ureaplasma parvum, and a few studies suggest that U. urealyticum—but not U. parvum—is associated with NGU. Coliform bacteria can cause urethritis in men who practice insertive anal intercourse. The initial diagnosis of urethritis in men currently includes specific tests only for N. gonorrhoeae and C. trachomatis; it does not yet include testing for Mycoplasma or Ureaplasma species. The following summarizes the approach to the patient with suspected urethritis:
1. Establish the presence of urethritis. If proximal-to-distal “milking” of the urethra does not express a purulent or mucopurulent discharge, even after the patient has not voided for several hours (or preferably overnight), a Gram’s-stained smear of an anterior urethral specimen obtained by passage of a small urethrogenital swab 2–3 cm into the urethra usually reveals ≥5 neutrophils per 1000× field in areas containing cells; in gonococcal infection, such a smear usually reveals gram-negative intracellular diplococci as well. Alternatively, the centrifuged sediment of the first 20–30 mL of voided urine—ideally collected as the first morning specimen—can be examined for inflammatory cells, either by microscopy showing ≥10 leukocytes per high-power field or by the leukocyte esterase test. Patients with symptoms who lack objective evidence of urethritis may have functional rather than organic problems and generally do not benefit from repeated courses of antibiotics.
2. Evaluate for complications or alternative diagnoses. A brief history and examination will exclude epididymitis and systemic complications, such as disseminated gonococcal infection (DGI) and reactive arthritis. Although digital examination of the prostate gland seldom contributes to the evaluation of sexually active young men with urethritis, men with dysuria who lack evidence of urethritis as well as sexually inactive men with urethritis should undergo prostate palpation, urinalysis, and urine culture to exclude bacterial prostatitis and cystitis.
3. Evaluate for gonococcal and chlamydial infection. An absence of typical gram-negative diplococci on Gram’s-stained smear of urethral exudate containing inflammatory cells warrants a preliminary diagnosis of NGU, as this test is 98% sensitive for the diagnosis of gonococcal urethral infection. However, an increasing proportion of men with symptoms and/or signs of urethritis are simultaneously assessed for infection with N. gonorrhoeae and C. trachomatis by “multiplex” NAATs of first-voided urine. The urine specimen tested should consist of the first 10–15 mL of the stream, and, if possible, patients should not have voided for the prior 2 h. Culture or NAAT for N. gonorrhoeae may yield positive results when Gram’s staining is negative; certain strains of N. gonorrhoeae can result in negative urethral Gram’s stains in up to 30% of cases of urethral infection. Results of tests for gonococcal and chlamydial infection predict the patient’s prognosis (with greater risk for recurrent NGU if neither chlamydiae nor gonococci are found than if either is detected) and can guide both the counseling given to the patient and the management of the patient’s sexual partner(s).
4. Treat urethritis promptly while test results are pending.
|
TREATMENT |
URETHRITIS IN MEN |
Table 163-4 summarizes the steps in management of sexually active men with urethral discharge and/or dysuria.
|
MANAGEMENT OF URETHRAL DISCHARGE IN MEN |
In practice, if Gram’s stain does not reveal gonococci, urethritis is treated with a regimen effective for NGU, such as azithromycin or doxycycline. Both are effective, although azithromycin may give better results in M. genitalium infection. If gonococci are demonstrated by Gram’s stain or if no diagnostic tests are performed to exclude gonorrhea definitively, treatment should include parenteral cephalosporin therapy for gonorrhea (Chap. 181) plus oral azithromycin, primarily for additive activity against N. gonorrhoeae given concerns about evolving antibiotic resistance. Azithromycin also treats C. trachomatis, which often causes urethral co-infection in men with gonococcal urethritis. Ideally, sexual partners should be tested for gonorrhea and chlamydial infection; regardless of whether they are tested for these infections, however, they should receive the same regimen given to the male index case. Patients with confirmed persistence or recurrence of urethritis after treatment should be re-treated with the initial regimen if they did not comply with the original treatment or were reexposed to an untreated partner. Otherwise, an intraurethral swab specimen and a first-voided urine sample should be tested for T. vaginalis (currently done by culture, although NAATs are more sensitive and are approved for the diagnosis of trichomoniasis in women). If compliance with initial treatment is confirmed and reexposure to an untreated sex partner is deemed unlikely, the recommended treatment is with metronidazole or tinidazole (2 g by mouth in a single dose) plus azithromycin (1 g by mouth in a single dose); the azithromycin component is especially important if this drug has not been given during initial therapy.
EPIDIDYMITIS
Acute epididymitis, almost always unilateral, produces pain, swelling, and tenderness of the epididymis, with or without symptoms or signs of urethritis. This condition must be differentiated from testicular torsion, tumor, and trauma. Torsion, a surgical emergency, usually occurs in the second or third decade of life and produces a sudden onset of pain, elevation of the testicle within the scrotal sac, rotation of the epididymis from a posterior to an anterior position, and absence of blood flow on Doppler examination or 99mTc scan. Persistence of symptoms after a course of therapy for epididymitis suggests the possibility of testicular tumor or of a chronic granulomatous disease, such as tuberculosis. In sexually active men under age 35, acute epididymitis is caused most frequently by C. trachomatis and less commonly by N. gonorrhoeae and is usually associated with overt or subclinical urethritis. Acute epididymitis occurring in older men or following urinary tract instrumentation is usually caused by urinary pathogens. Similarly, epididymitis in men who have practiced insertive rectal intercourse is often caused by Enterobacteriaceae. These older men usually have no urethritis but do have bacteriuria.
|
TREATMENT |
EPIDIDYMITIS |
Ceftriaxone (250 mg as a single dose IM) followed by doxycycline (100 mg by mouth twice daily for 10 days) constitutes effective treatment for epididymitis caused by N. gonorrhoeae or C. trachomatis. Neither oral cephalosporins nor fluoroquinolones are recommended for treatment of gonorrhea in the United States because of resistance in N. gonorrhoeae, especially (but not only) among MSM (Fig. 163-1). Oral levofloxacin (500 mg once daily for 10 days) is also effective for syndrome-based initial treatment of epididymitis when infection with Enterobacteriaceae is suspected; however, this regimen should be combined with effective therapy for possible gonococcal or chlamydial infection unless bacteriuria with Enterobacteriaceae is confirmed.
FIGURE 163-1 Proportion of Neisseria gonorrhoeae isolates with elevated ceftriaxone minimum inhibitory concentrations (MICs), United States 2008–2012. Elevated resistance is defined by ceftriaxone MICs of ≥0.125 μg/mL. *Preliminary (January–June). (From the Centers for Disease Control and Prevention: Gonococcal Isolate Surveillance Project [GISP], 2013.)
URETHRITIS AND THE URETHRAL SYNDROME IN WOMEN
C. trachomatis, N. gonorrhoeae, and occasionally HSV cause symptomatic urethritis—known as the urethral syndrome in women—that is characterized by “internal” dysuria (usually without urinary urgency or frequency), pyuria, and an absence of Escherichia coli and other uropathogens at counts of ≥102/mL in urine. In contrast, the dysuria associated with vulvar herpes or vulvovaginal candidiasis (and perhaps with trichomoniasis) is often described as “external,” being caused by painful contact of urine with the inflamed or ulcerated labia or introitus. Acute onset, association with urinary urgency or frequency, hematuria, or suprapubic bladder tenderness suggests bacterial cystitis. Among women with symptoms of acute bacterial cystitis, costovertebral pain and tenderness or fever suggests acute pyelonephritis. The management of bacterial urinary tract infection (UTI) is discussed in Chap. 162.
Signs of vulvovaginitis, coupled with symptoms of external dysuria, suggest vulvar infection (e.g., with HSV or Candida albicans). Among dysuric women without signs of vulvovaginitis, bacterial UTI must be differentiated from the urethral syndrome by assessment of risk, evaluation of the pattern of symptoms and signs, and specific microbiologic testing. An STI etiology of the urethral syndrome is suggested by young age, more than one current sexual partner, a new partner within the past month, a partner with urethritis, or coexisting mucopurulent cervicitis (see below). The finding of a single urinary pathogen, such as E. coli or Staphylococcus saprophyticus, at a concentration of ≥102/mL in a properly collected specimen of midstream urine from a dysuric woman with pyuria indicates probable bacterial UTI, whereas pyuria with <102 conventional uropathogens per milliliter of urine (“sterile” pyuria) suggests acute urethral syndrome due to C. trachomatis or N. gonorrhoeae. Gonorrhea and chlamydial infection should be sought by specific tests (e.g., NAATs of vaginal secretions collected with a swab). Among dysuric women with sterile pyuria caused by infection with N. gonorrhoeae or C. trachomatis, appropriate treatment alleviates dysuria. The role of M. genitalium in the urethral syndrome in women remains undefined.
VULVOVAGINAL INFECTIONS
Abnormal Vaginal Discharge If directly questioned about vaginal discharge during routine health checkups, many women acknowledge having nonspecific symptoms of vaginal discharge that do not correlate with objective signs of inflammation or with actual infection. However, unsolicited reporting of abnormal vaginal discharge does suggest bacterial vaginosis or trichomoniasis. Specifically, an abnormally increased amount or an abnormal odor of the discharge is associated with one or both of these conditions. Cervical infection with N. gonorrhoeae or C. trachomatis does not often cause an increased amount or abnormal odor of discharge; however, when these pathogens cause cervicitis, they—like T. vaginalis—often result in an increased number of neutrophils in vaginal fluid, which thus takes on a yellow color. Vulvar conditions such as genital herpes or vulvovaginal candidiasis can cause vulvar pruritus, burning, irritation, or lesions as well as external dysuria (as urine passes over the inflamed vulva or areas of epithelial disruption) or vulvar dyspareunia.
Certain vulvovaginal infections may have serious sequelae. Trichomoniasis, bacterial vaginosis, and vulvovaginal candidiasis have all been associated with increased risk of acquisition of HIV infection; bacterial vaginosis may promote HIV transmission from HIV-infected women to their male sex partners. Vaginal trichomoniasis and bacterial vaginosis early in pregnancy independently predict premature onset of labor. Bacterial vaginosis can also lead to anaerobic bacterial infection of the endometrium and salpinges. Vaginitis may be an early and prominent feature of toxic shock syndrome, and recurrent or chronic vulvovaginal candidiasis develops with increased frequency among women who have systemic illnesses, such as diabetes mellitus or HIV-related immunosuppression (although only a very small proportion of women with recurrent vulvovaginal candidiasis in industrialized countries actually have a serious predisposing illness).
Thus vulvovaginal symptoms or signs warrant careful evaluation, including speculum and pelvic examination, simple rapid diagnostic tests, and appropriate therapy specific for the anatomic site and type of infection. Unfortunately, a survey in the United States indicated that clinicians seldom perform the tests required to establish the cause of such symptoms. Further, comparison of telephone and office management of vulvovaginal symptoms has documented the inaccuracy of the former, and comparison of evaluations by nurse-midwives with those by physician-practitioners showed that the practitioners’ clinical evaluations correlated poorly both with the nurses’ evaluations and with diagnostic tests. The diagnosis and treatment of the three most common types of vaginal infection are summarized in Table 163-5.
|
DIAGNOSTIC FEATURES AND MANAGEMENT OF VAGINAL INFECTION |
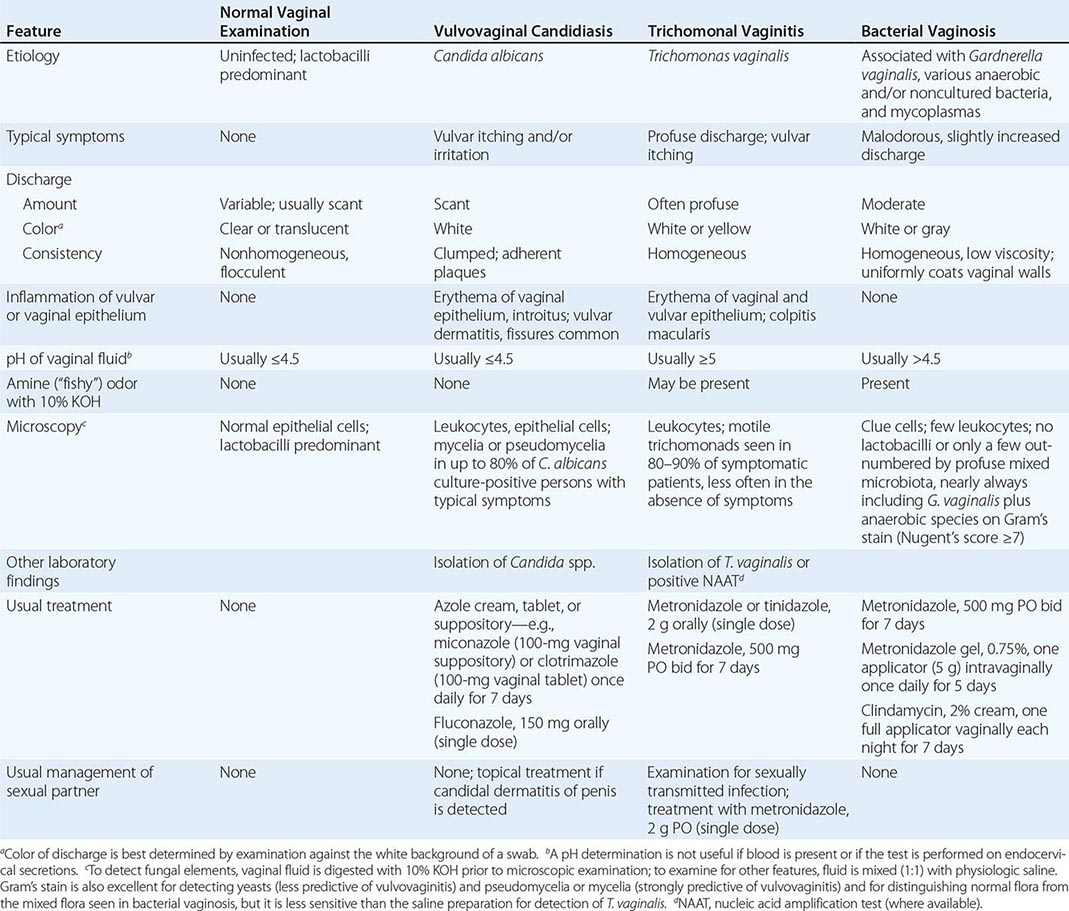
Inspection of the vulva and perineum may reveal tender genital ulcerations or fissures (typically due to HSV infection or vulvovaginal candidiasis) or discharge visible at the introitus before insertion of a speculum (suggestive of bacterial vaginosis or trichomoniasis). Speculum examination permits the clinician to discern whether the discharge in fact looks abnormal and whether any abnormal discharge in the vagina emanates from the cervical os (mucoid and, if abnormal, yellow) or from the vagina (not mucoid, since the vaginal epithelium does not produce mucus). Symptoms or signs of abnormal vaginal discharge should prompt testing of vaginal fluid for pH, for a fishy odor when mixed with 10% KOH, and for certain microscopic features when mixed with saline (motile trichomonads and/or “clue cells”) and with 10% KOH (pseudohyphae or hyphae indicative of vulvovaginal candidiasis). Additional objective laboratory tests useful for establishing the cause of abnormal vaginal discharge include rapid point-of-care tests for bacterial vaginosis and T. vaginalis, as described below; a DNA probe test (the Affirm test) to detect T. vaginalis and C. albicans as well as the increased concentrations of Gardnerella vaginalis associated with bacterial vaginosis; and a NAAT for T. vaginalis. Gram’s staining of vaginal fluid can be used to score alterations in the vaginal microbiota but is used primarily for research purposes and requires familiarity with the morphotypes and scale involved.
|
TREATMENT |
VAGINAL DISCHARGE |
Patterns of treatment for vaginal discharge vary widely. In developing countries, where clinics or pharmacies often dispense treatment based on symptoms alone without examination or testing, oral treatment with metronidazole—particularly with a 7-day regimen—provides reasonable coverage against both trichomoniasis and bacterial vaginosis, the usual causes of symptoms of vaginal discharge. Metronidazole treatment of sex partners prevents reinfection of women with trichomoniasis, even though it does not help prevent the recurrence of bacterial vaginosis. Guidelines for syndromic management promulgated by the World Health Organization suggest consideration of treatment for cervical infection and for trichomoniasis, bacterial vaginosis, and vulvovaginal candidiasis in women with symptoms of abnormal vaginal discharge. However, it is important to note that the majority of chlamydial and gonococcal cervical infections produce no symptoms.
In industrialized countries, clinicians treating symptoms and signs of abnormal vaginal discharge should, at a minimum, differentiate between bacterial vaginosis and trichomoniasis, because optimal management of patients and partners differs for these two conditions (as discussed briefly below).
Vaginal Trichomoniasis (See also Chap. 254) Symptomatic trichomoniasis characteristically produces a profuse, yellow, purulent, homogeneous vaginal discharge and vulvar irritation, sometimes with visible inflammation of the vaginal and vulvar epithelium and petechial lesions on the cervix (the so-called strawberry cervix, usually evident only by colposcopy). The pH of vaginal fluid—normally <4.7—usually rises to ≥5. In women with typical symptoms and signs of trichomoniasis, microscopic examination of vaginal discharge mixed with saline reveals motile trichomonads in most culture-positive cases. However, saline microscopy probably detects only one-half of all cases, and, especially in the absence of symptoms or signs, culture or NAAT is usually required for detection of the organism. NAAT for T. vaginalis is as sensitive as or more sensitive than culture, and NAAT of urine has disclosed surprisingly high prevalences of this pathogen among men at several STD clinics in the United States. Treatment of asymptomatic as well as symptomatic cases reduces rates of transmission and prevents later development of symptoms.
|
TREATMENT |
VAGINAL TRICHOMONIASIS |
Only nitroimidazoles (e.g., metronidazole and tinidazole) consistently cure trichomoniasis. A single 2-g oral dose of metronidazole is effective and much less expensive than the alternatives. Tinidazole has a longer half-life than metronidazole, causes fewer gastrointestinal symptoms, and may be useful in treating trichomoniasis that fails to respond to metronidazole. Treatment of sexual partners—facilitated by dispensing metronidazole to the female patient to give to her partner(s), with a warning about avoiding the concurrent use of alcohol—significantly reduces both the risk of reinfection and the reservoir of infection; treating the partner is the standard of care. Intravaginal treatment with 0.75% metronidazole gel is not reliable for vaginal trichomoniasis. Systemic use of metronidazole is recommended throughout pregnancy. In a large randomized trial, metronidazole treatment of trichomoniasis during pregnancy did not reduce—and in fact actually increased—the frequency of perinatal morbidity; thus routine screening of asymptomatic pregnant women for trichomoniasis is not recommended.
Bacterial Vaginosis Bacterial vaginosis (formerly termed nonspecific vaginitis, Haemophilus vaginitis, anaerobic vaginitis, or Gardnerella–associated vaginal discharge) is a syndrome of complex etiology that is characterized by symptoms of vaginal malodor and a slightly to moderately increased white discharge, which appears homogeneous, is low in viscosity, and evenly coats the vaginal mucosa. Bacterial vaginosis has been associated with increased risk of acquiring several other genital infections, including those caused by HIV, C. trachomatis, and N. gonorrhoeae. Other risk factors include recent unprotected vaginal intercourse, having a female sex partner, and vaginal douching. Although bacteria associated with bacterial vaginosis have been detected under the foreskin of uncircumcised men, metronidazole treatment of male partners has not reduced the rate of recurrence among affected women.
Among women with bacterial vaginosis, culture of vaginal fluid has shown markedly increased prevalences and concentrations of G. vaginalis, Mycoplasma hominis, and several anaerobic bacteria (e.g., Mobiluncus, Prevotella [formerly Bacteroides], and some Peptostreptococcus species) as well as an absence of hydrogen peroxide–producing Lactobacillus species that constitute most of the normal vaginal microbiota and help protect against certain cervical and vaginal infections. Broad-range polymerase chain reaction (PCR) amplification of 16S rDNA in vaginal fluid, with subsequent identification of specific bacterial species by various methods, has documented an even greater and unexpected bacterial diversity, including several unique species not previously cultivated (e.g., three species in the order Clostridiales that appear to be specific for bacterial vaginosis and are associated with metronidazole treatment failure [Fig. 163-2]). Also detected are DNA sequences related to Atopobium vaginae, an organism that is strongly associated with bacterial vaginosis, is resistant to metronidazole, and is also associated with recurrent bacterial vaginosis after metronidazole treatment. Other genera newly implicated in bacterial vaginosis include Megasphaera, Leptotrichia, Eggerthella, and Dialister.
FIGURE 163-2 Broad-range polymerase chain reaction amplification of 16S rDNA in vaginal fluid from a woman with bacterial vaginosis shows a field of bacteria hybridizing with probes for bacterial vaginosis–associated bacterium 1 (BVAB-1, visible as a thin, curved green rod) and for BVAB-2 (red). The inset shows that BVAB-1 has a morphology similar to that of Mobiluncus (curved rod). (Reprinted with permission from DN Fredricks et al: N Engl J Med 353:1899, 2005.)
Bacterial vaginosis is conventionally diagnosed clinically with the Amsel criteria, which include any three of the following four clinical abnormalities: (1) objective signs of increased white homogeneous vaginal discharge; (2) a vaginal discharge pH of >4.5; (3) liberation of a distinct fishy odor (attributable to volatile amines such as trimethylamine) immediately after vaginal secretions are mixed with a 10% solution of KOH; and (4) microscopic demonstration of “clue cells” (vaginal epithelial cells coated with coccobacillary organisms, which have a granular appearance and indistinct borders; Fig. 163-3) on a wet mount prepared by mixing vaginal secretions with normal saline in a ratio of ~1:1.
FIGURE 163-3 Wet mount of vaginal fluid showing typical clue cells from a woman with bacterial vaginosis. Note the obscured epithelial cell margins and the granular appearance attributable to many adherent bacteria (×400). (Photograph provided by Lorna K. Rabe, reprinted with permission from S Hillier et al, in KK Holmes et al [eds]: Sexually Transmitted Diseases, 4th ed. New York, McGraw-Hill, 2008.)
|
TREATMENT |
BACTERIAL VAGINOSIS |
The standard dosage of oral metronidazole for the treatment of bacterial vaginosis is 500 mg twice daily for 7 days. The single 2-g oral dose of metronidazole recommended for trichomoniasis produces significantly lower short-term cure rates and should not be used. Intravaginal treatment with 2% clindamycin cream (one full applicator [5 g containing 100 mg of clindamycin phosphate] each night for 7 nights) or with 0.75% metronidazole gel (one full applicator [5 g containing 37.5 mg of metronidazole] twice daily for 5 days) is also approved for use in the United States and does not elicit systemic adverse reactions; the response to both of these treatments is similar to the response to oral metronidazole. Other alternatives include oral clindamycin (300 mg twice daily for 7 days), clindamycin ovules (100 g intravaginally once at bedtime for 3 days), and oral tinidazole (1 g daily for 5 days or 2 g daily for 3 days). Unfortunately, recurrence over the long term (i.e., several months later) is distressingly common after either oral or intravaginal treatment. A randomized trial comparing intravaginal gel containing 37.5 mg of metronidazole with a suppository containing 500 mg of metronidazole plus nystatin (the latter not marketed in the United States) showed significantly higher rates of recurrence with the 37.5-mg regimen; this result suggests that higher metronidazole dosages may be important in topical intravaginal therapy. Recurrences can be significantly lessened with the twice-weekly use of suppressive intravaginal metronidazole gel. As stated above, treatment of male partners with metronidazole does not prevent recurrence of bacterial vaginosis.
Efforts to replenish numbers of vaginal lactobacilli that produce hydrogen peroxide and probably sustain vaginal health have been unsuccessful. While one randomized trial of orally ingested lactobacilli found reduced rates of recurrent bacterial vaginosis, this result has not yet been either confirmed or refuted, and a randomized multicenter trial in the United States found no benefit of repeated intravaginal inoculation of a vaginal peroxide-producing Lactobacillus species following treatment of bacterial vaginosis with metronidazole. A meta-analysis of 18 studies concluded that bacterial vaginosis during pregnancy substantially increased the risk of preterm delivery and of spontaneous abortion. However, most studies of topical intravaginal treatment of bacterial vaginosis with clindamycin during pregnancy have not reduced adverse pregnancy outcomes. Numerous trials of oral metronidazole treatment during pregnancy have given inconsistent results, and a 2013 Cochrane review concluded that antenatal treatment of women with bacterial vaginosis—even those with previous preterm delivery—did not reduce the risk of preterm delivery. The U.S. Preventive Services Task Force thus recommends against routine screening of pregnant women for bacterial vaginosis.
Vulvovaginal Pruritus, Burning, or Irritation Vulvovaginal candidiasis produces vulvar pruritus, burning, or irritation, generally without symptoms of increased vaginal discharge or malodor. Genital herpes can produce similar symptoms, with lesions sometimes difficult to distinguish from the fissures and inflammation caused by candidiasis. Signs of vulvovaginal candidiasis include vulvar erythema, edema, fissures, and tenderness. With candidiasis, a white scanty vaginal discharge sometimes takes the form of white thrush-like plaques or cottage cheese–like curds adhering loosely to the vaginal epithelium. C. albicans accounts for nearly all cases of symptomatic vulvovaginal candidiasis, which probably arise from endogenous strains of C. albicans that have colonized the vagina or the intestinal tract. Complicated vulvovaginal candidiasis includes cases that recur four or more times per year; are unusually severe; are caused by non-albicans Candida species; or occur in women with uncontrolled diabetes, debilitation, immunosuppression, or pregnancy.
In addition to compatible clinical symptoms, the diagnosis of vulvovaginal candidiasis usually involves the demonstration of pseudohyphae or hyphae by microscopic examination of vaginal fluid mixed with saline or 10% KOH or subjected to Gram’s staining. Microscopic examination is less sensitive than culture but correlates better with symptoms. Culture is typically reserved for cases that do not respond to standard first-line antimycotic agents and is undertaken to rule out imidazole or azole resistance (often associated with Candida glabrata) or before the initiation of suppressive antifungal therapy for recurrent disease.
|
TREATMENT |
VULVOVAGINAL PRURITUS, BURNING, OR IRRITATION |
Symptoms and signs of vulvovaginal candidiasis warrant treatment, usually intravaginal administration of any of several imidazole antibiotics (e.g., miconazole or clotrimazole) for 3–7 days or of a single dose of oral fluconazole (Table 163-5). Over-the-counter marketing of such preparations has reduced the cost of care and made treatment more convenient for many women with recurrent yeast vulvovaginitis. However, most women who purchase these preparations do not have vulvovaginal candidiasis, whereas many do have other vaginal infections that require different treatment. Therefore, only women with classic symptoms of vulvar pruritus and a history of previous episodes of yeast vulvovaginitis documented by an experienced clinician should self-treat. Short-course topical intravaginal azole drugs are effective for the treatment of uncomplicated vulvovaginal candidiasis (e.g., clotrimazole, two 100-mg vaginal tablets daily for 3 days; or miconazole, a 1200-mg vaginal suppository as a single dose). Single-dose oral treatment with fluconazole (150 mg) is also effective and is preferred by many patients. Management of complicated cases (see above) and those that do not respond to the usual intravaginal or single-dose oral therapy often involves prolonged or periodic oral therapy; this situation is discussed extensively in the 2010 CDC STD treatment guidelines (http://www.cdc.gov/std/treatment). Treatment of sexual partners is not routinely indicated.
Other Causes of Vaginal Discharge or Vaginitis In the ulcerative vaginitis associated with staphylococcal toxic shock syndrome, Staphylococcus aureus should be promptly identified in vaginal fluid by Gram’s stain and by culture. In desquamative inflammatory vaginitis, smears of vaginal fluid reveal neutrophils, massive vaginal epithelial-cell exfoliation with increased numbers of parabasal cells, and gram-positive cocci; this syndrome may respond to treatment with 2% clindamycin cream, often given in combination with topical steroid preparations for several weeks. Additional causes of vaginitis and vulvovaginal symptoms include retained foreign bodies (e.g., tampons), cervical caps, vaginal spermicides, vaginal antiseptic preparations or douches, vaginal epithelial atrophy (in postmenopausal women or during prolonged breast-feeding in the postpartum period), allergic reactions to latex condoms, vaginal aphthae associated with HIV infection or Behçet’s syndrome, and vestibulitis (a poorly understood syndrome).
MUCOPURULENT CERVICITIS
Mucopurulent cervicitis (MPC) refers to inflammation of the columnar epithelium and subepithelium of the endocervix and of any contiguous columnar epithelium that lies exposed in an ectopic position on the ectocervix. MPC in women represents the “silent partner” of urethritis in men, being equally common and often caused by the same agents (N. gonorrhoeae, C. trachomatis, or—as shown by case–control studies—M. genitalium); however, MPC is more difficult than urethritis to recognize. As the most common manifestation of these serious bacterial infections in women, MPC can be a harbinger or sign of upper genital tract infection, also known as pelvic inflammatory disease (PID; see below). In pregnant women, MPC can lead to obstetric complications. In a prospective study in Seattle of 167 consecutive patients with MPC (defined on the basis of yellow endocervical mucopus or ≥30 polymorphonuclear leukocytes [PMNs]/1000× microscopic field) who were seen at STD clinics during the 1980s, slightly more than one-third of cervicovaginal specimens tested for C. trachomatis, N. gonorrhoeae, M. genitalium, HSV, and T. vaginalis revealed no identifiable etiology (Fig. 163-4). More recently, a study in Baltimore using NAATs for these pathogens still failed to identify a microbiologic etiology in nearly one-half of the 133 women with MPC.
FIGURE 163-4 Organisms detected among female sexually transmitted disease clinic patients with mucopurulent cervicitis (n = 167). CT, Chlamydia trachomatis; GC, gonococcus; MG, Mycoplasma genitalium; TV, Trichomonas vaginalis; HSV, herpes simplex virus. (Courtesy of Dr. Lisa Manhart; with permission.)
The diagnosis of MPC rests on the detection of cardinal signs at the cervix, including yellow mucopurulent discharge from the cervical os, endocervical bleeding upon gentle swabbing, and edematous cervical ectopy (see below); the latter two findings are somewhat more common with MPC due to chlamydial infection, but signs alone do not allow a distinction between the causative pathogens. Unlike the endocervicitis produced by gonococcal or chlamydial infection, cervicitis caused by HSV produces ulcerative lesions on the stratified squamous epithelium of the ectocervix as well as on the columnar epithelium. Yellow cervical mucus on a white swab removed from the endocervix indicates the presence of PMNs. Gram’s staining may confirm their presence, although it adds relatively little to the diagnostic value of assessment for cervical signs. The presence of ≥20 PMNs/1000× microscopic field within strands of cervical mucus not contaminated by vaginal squamous epithelial cells or vaginal bacteria indicates endocervicitis. Detection of intracellular gram-negative diplococci in carefully collected endocervical mucus is quite specific but ≤50% sensitive for gonorrhea. Therefore, specific and sensitive tests for N. gonorrhoeae as well as for C. trachomatis (e.g., NAATs) are always indicated in the evaluation of MPC.
|
TREATMENT |
MUCOPURULENT CERVICITIS |
Although the above criteria for MPC are neither highly specific nor highly predictive of gonococcal or chlamydial infection in some settings, the 2010 CDC STD guidelines call for consideration of empirical treatment for MPC, pending test results, in most patients. Presumptive treatment with antibiotics active against C. trachomatis should be provided for women at increased risk for this common STI (risk factors: age <25 years, new or multiple sex partners, and unprotected sex), especially if follow-up cannot be ensured. Concurrent therapy for gonorrhea is indicated if the prevalence of this infection is substantial in the relevant patient population (e.g., young adults, a clinic with documented high prevalence). In this situation, therapy should include a single-dose regimen effective for gonorrhea plus treatment for chlamydial infection, as outlined in Table 163-4 for the treatment of urethritis. In settings where gonorrhea is much less common than chlamydial infection, initial therapy for chlamydial infection alone suffices, pending test results for gonorrhea. The etiology and potential benefit of treatment for endocervicitis not associated with gonorrhea or chlamydial infection have not been established. Although the antimicrobial susceptibility of M. genitalium is not yet well defined, the organism frequently persists after doxycycline therapy, and it currently seems reasonable to use azithromycin to treat possible M. genitalium infection in such cases. With resistance of M. genitalium to azithromycin now recognized, moxifloxacin may be a reasonable alternative. The sexual partner(s) of a woman with MPC should be examined and given a regimen similar to that chosen for the woman unless results of tests for gonorrhea or chlamydial infection in either partner warrant different therapy or no therapy.
CERVICAL ECTOPY
Cervical ectopy, often mislabeled “cervical erosion,” is easily confused with infectious endocervicitis. Ectopy represents the presence of the one-cell-thick columnar epithelium extending from the endocervix out onto the visible ectocervix. In ectopy, the cervical os may contain clear or slightly cloudy mucus but usually not yellow mucopus. Colposcopy shows intact epithelium. Normally found during adolescence and early adulthood, ectopy gradually recedes through the second and third decades of life, as squamous metaplasia replaces the ectopic columnar epithelium. Oral contraceptive use favors the persistence or reappearance of ectopy, while smoking apparently accelerates squamous metaplasia. Cauterization of ectopy is not warranted. Ectopy may render the cervix more susceptible to infection with N. gonorrhoeae, C. trachomatis, or HIV.
PELVIC INFLAMMATORY DISEASE
The term pelvic inflammatory disease usually refers to infection that ascends from the cervix or vagina to involve the endometrium and/or fallopian tubes. Infection can extend beyond the reproductive tract to cause pelvic peritonitis, generalized peritonitis, perihepatitis, perisplenitis, or pelvic abscess. Rarely, infection not related to specific sexually transmitted pathogens extends secondarily to the pelvic organs (1) from adjacent foci of inflammation (e.g., appendicitis, regional ileitis, or diverticulitis) or bacterial vaginosis, (2) as a result of hematogenous dissemination (e.g., of tuberculosis or staphylococcal bacteremia), or (3) as a complication of certain tropical diseases (e.g., schistosomiasis). Intrauterine infection can be primary (spontaneously occurring and usually sexually transmitted) or secondary to invasive intrauterine surgical procedures (e.g., dilation and curettage, termination of pregnancy, insertion of an intrauterine device [IUD], or hysterosalpingography) or to parturition.
Etiology The agents most often implicated in acute PID include the primary causes of endocervicitis (e.g., N. gonorrhoeae and C. trachomatis) and organisms that can be regarded as components of an altered vaginal microbiota. In general, PID is most often caused by N. gonorrhoeae where there is a high incidence of gonorrhea—e.g., in inner-city populations in the United States. In case–control studies, M. genitalium has also been significantly associated with histopathologic diagnoses of endometritis and with salpingitis.
Anaerobic and facultative organisms (especially Prevotella species, peptostreptococci, E. coli, Haemophilus influenzae, and group B streptococci) as well as genital mycoplasmas have been isolated from the peritoneal fluid or fallopian tubes in a varying proportion (typically one-fourth to one-third) of women with PID studied in the United States. The difficulty of determining the exact microbial etiology of an individual case of PID—short of using invasive procedures for specimen collection—has implications for the approach to empirical antimicrobial treatment of this infection.
Epidemiology In the United States, the estimated annual number of initial visits to physicians’ offices for PID by women 15–44 years of age fell from an average of 400,000 during the 1980s to 250,000 in 1999 and then to 90,000 in 2011. Hospitalizations for acute PID in the United States also declined steadily throughout the 1980s and early 1990s but have remained fairly constant at 70,000–100,000 per year since 1995. Important risk factors for acute PID include the presence of endocervical infection or bacterial vaginosis, a history of salpingitis or of recent vaginal douching, and recent insertion of an IUD. Certain other iatrogenic factors, such as dilation and curettage or cesarean section, can increase the risk of PID, especially among women with endocervical gonococcal or chlamydial infection or bacterial vaginosis. Symptoms of N. gonorrhoeae–associated and C. trachomatis–associated PID often begin during or soon after the menstrual period; this timing suggests that menstruation is a risk factor for ascending infection from the cervix and vagina. Experimental inoculation of the fallopian tubes of nonhuman primates has shown that repeated exposure to C. trachomatis leads to the greatest degree of tissue inflammation and damage; thus, immunopathology probably contributes to the pathogenesis of chlamydial salpingitis. Women using oral contraceptives appear to be at decreased risk of symptomatic PID, and tubal sterilization reduces the risk of salpingitis by preventing intraluminal spread of infection into the tubes.
Clinical Manifestations • ENDOMETRITIS: A CLINICAL PATHOLOGIC SYNDROME A study of women with clinically suspected PID who were undergoing both endometrial biopsy and laparoscopy showed that those with endometritis alone differed from those who also had salpingitis in significantly less often having lower-quadrant, adnexal, or cervical motion or abdominal rebound tenderness; fever; or elevated C-reactive protein levels. In addition, women with endometritis alone differed from those with neither endometritis nor salpingitis in more often having gonorrhea, chlamydial infection, and risk factors such as douching or IUD use. Thus, women with endometritis alone were intermediate between those with neither endometritis nor salpingitis and those with salpingitis with respect to risk factors, clinical manifestations, cervical infection prevalence, and elevated C-reactive protein level. Women with endometritis alone are at lower risk of subsequent tubal occlusion and resulting infertility than are those with salpingitis.
SALPINGITIS Symptoms of nontuberculous salpingitis classically evolve from a yellow or malodorous vaginal discharge caused by MPC and/or bacterial vaginosis to midline abdominal pain and abnormal vaginal bleeding caused by endometritis and then to bilateral lower abdominal and pelvic pain caused by salpingitis, with nausea, vomiting, and increased abdominal tenderness if peritonitis develops.
The abdominal pain in nontuberculous salpingitis is usually described as dull or aching. In some cases, pain is lacking or atypical, but active inflammatory changes are found in the course of an unrelated evaluation or procedure, such as a laparoscopic evaluation for infertility. Abnormal uterine bleeding precedes or coincides with the onset of pain in ~40% of women with PID, symptoms of urethritis (dysuria) occur in 20%, and symptoms of proctitis (anorectal pain, tenesmus, and rectal discharge or bleeding) are occasionally seen in women with gonococcal or chlamydial infection.
Speculum examination shows evidence of MPC (yellow endocervical discharge, easily induced endocervical bleeding) in the majority of women with gonococcal or chlamydial PID. Cervical motion tenderness is produced by stretching of the adnexal attachments on the side toward which the cervix is pushed. Bimanual examination reveals uterine fundal tenderness due to endometritis and abnormal adnexal tenderness due to salpingitis that is usually, but not necessarily, bilateral. Adnexal swelling is palpable in about one-half of women with acute salpingitis, but evaluation of the adnexae in a patient with marked tenderness is not reliable. The initial temperature is >38°C in only about one-third of patients with acute salpingitis. Laboratory findings include elevation of the erythrocyte sedimentation rate (ESR) in 75% of patients with acute salpingitis and elevation of the peripheral white blood cell count in up to 60%.
Unlike nontuberculous salpingitis, genital tuberculosis often occurs in older women, many of whom are postmenopausal. Presenting symptoms include abnormal vaginal bleeding, pain (including dysmenorrhea), and infertility. About one-quarter of these women have had adnexal masses. Endometrial biopsy shows tuberculous granulomas and provides optimal specimens for culture.
PERIHEPATITIS AND PERIAPPENDICITIS Pleuritic upper-abdominal pain and tenderness, usually localized to the right upper quadrant (RUQ), develop in 3–10% of women with acute PID. Symptoms of perihepatitis arise during or after the onset of symptoms of PID and may overshadow lower abdominal symptoms, thereby leading to a mistaken diagnosis of cholecystitis. In perhaps 5% of cases of acute salpingitis, early laparoscopy reveals perihepatic inflammation ranging from edema and erythema of the liver capsule to exudate with fibrinous adhesions between the visceral and parietal peritoneum. When treatment is delayed and laparoscopy is performed late, dense “violin-string” adhesions can be seen over the liver; chronic exertional or positional RUQ pain ensues when traction is placed on the adhesions. Although perihepatitis, also known as the Fitz-Hugh–Curtis syndrome, was for many years specifically attributed to gonococcal salpingitis, most cases are now attributed to chlamydial salpingitis. In patients with chlamydial salpingitis, serum titers of microimmunofluorescent antibody to C. trachomatis are typically much higher when perihepatitis is present than when it is absent.
Physical findings include RUQ tenderness and usually include adnexal tenderness and cervicitis, even in patients whose symptoms do not suggest salpingitis. Results of liver function tests and RUQ ultrasonography are nearly always normal. The presence of MPC and pelvic tenderness in a young woman with subacute pleuritic RUQ pain and normal ultrasonography of the gallbladder points to a diagnosis of perihepatitis.
Periappendicitis (appendiceal serositis without involvement of the intestinal mucosa) has been found in ~5% of patients undergoing appendectomy for suspected appendicitis and can occur as a complication of gonococcal or chlamydial salpingitis.
Among women with salpingitis, HIV infection is associated with increased severity of salpingitis and with tuboovarian abscess requiring hospitalization and surgical drainage. Nonetheless, among women with HIV infection and salpingitis, the clinical response to conventional antimicrobial therapy (coupled with drainage of tuboovarian abscess, when found) has usually been satisfactory.
Diagnosis Treatment appropriate for PID must not be withheld from patients who have an equivocal diagnosis; it is better to err on the side of overdiagnosis and overtreatment. On the other hand, it is essential to differentiate between salpingitis and other pelvic pathology, particularly surgical emergencies such as appendicitis and ectopic pregnancy.
Nothing short of laparoscopy definitively identifies salpingitis, but routine laparoscopy to confirm suspected salpingitis is generally impractical. Most patients with acute PID have lower abdominal pain of <3 weeks’ duration, pelvic tenderness on bimanual pelvic examination, and evidence of lower genital tract infection (e.g., MPC). Approximately 60% of such patients have salpingitis at laparoscopy, and perhaps 10–20% have endometritis alone. Among the patients with these findings, a rectal temperature >38°C, a palpable adnexal mass, and elevation of the ESR to >15 mm/h also raise the probability of salpingitis, which has been found at laparoscopy in 68% of patients with one of these additional findings, 90% of patients with two, and 96% of patients with three. However, only 17% of all patients with laparoscopy-confirmed salpingitis have had all three additional findings.
In a woman with pelvic pain and tenderness, increased numbers of PMNs (30 per 1000× microscopic field in strands of cervical mucus) or leukocytes outnumbering epithelial cells in vaginal fluid (in the absence of trichomonal vaginitis, which also produces PMNs in vaginal discharge) increase the predictive value of a clinical diagnosis of acute PID, as do onset with menses, history of recent abnormal menstrual bleeding, presence of an IUD, history of salpingitis, and sexual exposure to a male with urethritis. Appendicitis or another disorder of the gut is favored by the early onset of anorexia, nausea, or vomiting; the onset of pain later than day 14 of the menstrual cycle; or unilateral pain limited to the right or left lower quadrant. Whenever the diagnosis of PID is being considered, serum assays for human β-chorionic gonadotropin should be performed; these tests are usually positive with ectopic pregnancy. Ultrasonography and magnetic resonance imaging (MRI) can be useful for the identification of tuboovarian or pelvic abscess. MRI of the tubes can also show increased tubal diameter, intratubal fluid, or tubal wall thickening in cases of salpingitis.
The primary and uncontested value of laparoscopy in women with lower abdominal pain is for the exclusion of other surgical problems. Some of the most common or serious problems that may be confused with salpingitis (e.g., acute appendicitis, ectopic pregnancy, corpus luteum bleeding, ovarian tumor) are unilateral. Unilateral pain or pelvic mass, although not incompatible with PID, is a strong indication for laparoscopy unless the clinical picture warrants laparotomy instead. Atypical clinical findings such as the absence of lower genital tract infection, a missed menstrual period, a positive pregnancy test, or failure to respond to appropriate therapy are other common indications for laparoscopy. Endometrial biopsy is relatively sensitive and specific for the diagnosis of endometritis, which correlates well with the presence of salpingitis.
Vaginal or endocervical swab specimens should be examined by NAATs for N. gonorrhoeae and C. trachomatis. At a minimum, vaginal fluid should be evaluated for the presence of PMNs, and endocervical secretions ideally should be assessed by Gram’s staining for PMNs and gram-negative diplococci, which indicate gonococcal infection. The clinical diagnosis of PID made by expert gynecologists is confirmed by laparoscopy or endometrial biopsy in ~90% of women who also have cultures positive for N. gonorrhoeae or C. trachomatis. Even among women with no symptoms suggestive of acute PID who were attending an STD clinic or a gynecology clinic in Pittsburgh, endometritis was significantly associated with endocervical gonorrhea or chlamydial infection or with bacterial vaginosis, being detected in 26%, 27%, and 15% of women with these conditions, respectively.
|
TREATMENT |
PELVIC INFLAMMATORY DISEASE |
Recommended combination regimens for ambulatory or parenteral management of PID are presented in Table 163-6. Women managed as outpatients should receive a combined regimen with broad activity, such as ceftriaxone (to cover possible gonococcal infection) followed by doxycycline (to cover possible chlamydial infection). Metronidazole can be added, if tolerated, to enhance activity against anaerobes; this addition should be strongly considered if bacterial vaginosis is documented. Although few methodologically sound clinical trials (especially with prolonged follow-up) have been conducted, one meta-analysis suggested a benefit of providing good coverage against anaerobes.
|
COMBINATION ANTIMICROBIAL REGIMENS RECOMMENDED FOR OUTPATIENT TREATMENT OR FOR PARENTERAL TREATMENT OF PELVIC INFLAMMATORY DISEASE |
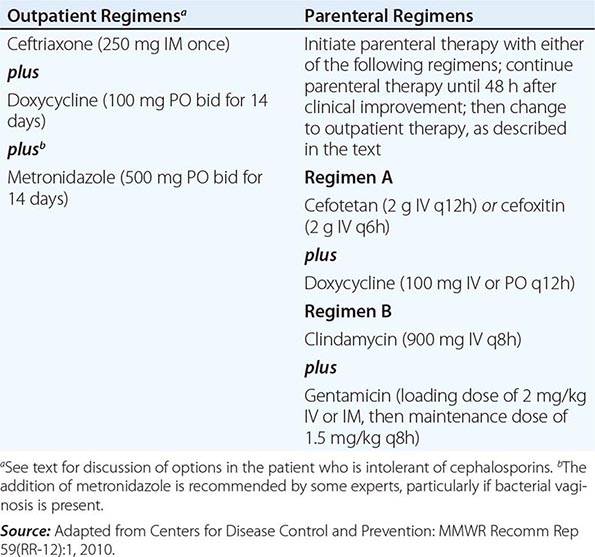
The CDC STD treatment guidelines recommend initiation of empirical treatment for PID in sexually active young women and other women at risk for PID if they are experiencing pelvic or lower abdominal pain, if no other cause for the pain can be identified, and if pelvic examination reveals one or more of the following criteria for PID: cervical motion tenderness, uterine tenderness, or adnexal tenderness. Women with suspected PID can be treated as either outpatients or inpatients. In the multicenter Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) trial, 831 women with mild to moderately severe symptoms and signs of PID were randomized to receive either inpatient treatment with IV cefoxitin and doxycycline or outpatient treatment with a single IM dose of cefoxitin plus oral doxycycline. Short-term clinical and microbiologic outcomes and long-term outcomes were equivalent in the two groups. Nonetheless, hospitalization should be considered when (1) the diagnosis is uncertain and surgical emergencies such as appendicitis and ectopic pregnancy cannot be excluded, (2) the patient is pregnant, (3) pelvic abscess is suspected, (4) severe illness or nausea and vomiting preclude outpatient management, (5) the patient has HIV infection, (6) the patient is assessed as unable to follow or tolerate an outpatient regimen, or (7) the patient has failed to respond to outpatient therapy. Some experts also prefer to hospitalize adolescents with PID for initial therapy, although younger women do as well as older women on outpatient therapy.
Currently, oral cephalosporins, doxycycline, and the fluoroquinolones do not provide reliable coverage for gonococcal infection. Thus, adequate oral treatment of women with serious intolerance to cephalosporins is a challenge. If penicillins are an option, amoxicillin/clavulanic acid combined with doxycycline has elicited a short-term clinical response in one trial. If fluoroquinolones are the only option and if the community prevalence and individual risk of gonorrhea are known to be low, oral levofloxacin (500 mg once daily) or ofloxacin (400 mg twice daily) for 14 days, with or without metronidazole, may be considered; moreover, clinical trials performed outside the United States support the effectiveness of oral moxifloxacin. In this case, it is imperative to perform a sensitive diagnostic test for gonorrhea (ideally, culture to test for antimicrobial susceptibility) before initiation of therapy. For women whose PID involves quinolone-resistant N. gonorrhoeae, treatment is uncertain but could include parenteral gentamicin or oral azithromycin, although the latter agent has not been studied for this purpose.
For hospitalized patients, the following two parenteral regimens (Table 163-6) have given nearly identical results in a multicenter randomized trial:
1. Doxycycline plus either cefotetan or cefoxitin: Administration of these drugs should be continued by the IV route for at least 48 h after the patient’s condition improves and then followed with oral doxycycline (100 mg twice daily) to complete 14 days of therapy.
2. Clindamycin plus gentamicin in patients with normal renal function: Once-daily administration of gentamicin (with combination of the total daily dose into a single daily dose) has not been evaluated in PID but has been efficacious in other serious infections and could be substituted. Treatment with these drugs should be continued for at least 48 h after the patient’s condition improves and then followed with oral doxycycline (100 mg twice daily) or clindamycin (450 mg four times daily) to complete 14 days of therapy. In cases with tuboovarian abscess, clindamycin rather than doxycycline for continued therapy provides better coverage for anaerobic infection.
FOLLOW-UP
Hospitalized patients should show substantial clinical improvement within 3–5 days. Women treated as outpatients should be clinically reevaluated within 72 h. A follow-up telephone survey of women seen in an emergency department and given a prescription for 10 days of oral doxycycline for PID found that 28% never filled the prescription and 41% stopped taking the medication early (after an average of 4.1 days), often because of persistent symptoms, lack of symptoms, or side effects. Women not responding favorably to ambulatory therapy should be hospitalized for parenteral therapy and further diagnostic evaluations, including a consideration of laparoscopy. Male sex partners should be evaluated and treated empirically for gonorrhea and chlamydial infection. After completion of treatment, tests for persistent or recurrent infection with N. gonorrhoeae or C. trachomatis should be performed if symptoms persist or recur or if the patient has not complied with therapy or has been reexposed to an untreated sex partner.
SURGERY
Surgery is necessary for the treatment of salpingitis only in the face of life-threatening infection (such as rupture or threatened rupture of a tuboovarian abscess) or for drainage of an abscess. Conservative surgical procedures are usually sufficient. Pelvic abscesses can often be drained by posterior colpotomy, and peritoneal lavage can be used for generalized peritonitis.
Prognosis Late sequelae include infertility due to bilateral tubal occlusion, ectopic pregnancy due to tubal scarring without occlusion, chronic pelvic pain, and recurrent salpingitis. The overall post-salpingitis risk of infertility due to tubal occlusion in a large study in Sweden was 11% after one episode of salpingitis, 23% after two episodes, and 54% after three or more episodes. A University of Washington study found a sevenfold increase in the risk of ectopic pregnancy and an eightfold increase in the rate of hysterectomy after PID.
Prevention A randomized controlled trial designed to determine whether selective screening for chlamydial infection reduces the risk of subsequent PID showed that women randomized to undergo screening had a 56% lower rate of PID over the following year than did women receiving the usual care without screening. This report helped prompt U.S. national guidelines for risk-based chlamydial screening of young women to reduce the incidence of PID and the prevalence of post-PID sequelae, while also reducing sexual transmission of C. trachomatis. The CDC and the U.S. Preventive Services Task Force recommend that sexually active women ≤25 years of age be screened for genital chlamydial infection annually. Despite this recommendation, screening coverage in many primary care settings remains low.
ULCERATIVE GENITAL OR PERIANAL LESIONS
Genital ulceration reflects a set of important STIs, most of which sharply increase the risk of sexual acquisition and shedding of HIV. In a 1996 study of genital ulcers in 10 of the U.S. cities with the highest rates of primary syphilis, PCR testing of ulcer specimens demonstrated HSV in 62% of patients, Treponema pallidum (the cause of syphilis) in 13%, and Haemophilus ducreyi (the cause of chancroid) in 12–20%. Today, genital herpes represents an even higher proportion of genital ulcers in the United States and other industrialized countries.
![]() In Asia and Africa, chancroid (Fig. 163-5) was once considered the most common type of genital ulcer, followed in frequency by primary syphilis and then genital herpes (Fig. 163-6). With increased efforts to control chancroid and syphilis and widespread use of broad-spectrum antibiotics to treat STI-related syndromes, together with more frequent recurrences or persistence of genital herpes attributable to HIV infection, PCR testing of genital ulcers now clearly implicates genital herpes as by far the most common cause of genital ulceration in most developing countries. LGV due to C. trachomatis (Fig. 163-7) and donovanosis (granuloma inguinale, due to Klebsiella granulomatis; see Fig. 198e-1) continue to cause genital ulceration in some developing countries. LGV virtually disappeared in industrialized countries during the first 20 years of the HIV pandemic, but outbreaks are again occurring in Europe (including the United Kingdom), in North America, and in Australia. In these outbreaks, LGV typically presents as proctitis, with or without anal lesions, in men who report unprotected receptive anal intercourse, very often in association with HIV and/or hepatitis C virus infection; the latter may be an acute infection acquired through the same exposure. Other causes of genital ulcers include (1) candidiasis and traumatized genital warts—both readily recognized; (2) lesions due to genital involvement by more widespread dermatoses; (3) cutaneous manifestations of systemic diseases such as genital mucosal ulceration in Stevens-Johnson syndrome or Behçet’s disease; (4) superinfections of lesions that may originally have been sexually acquired (for example, methicillin-resistant S. aureus complicating a genital ulcer due to HSV-2); and (5) localized drug reactions, such as the ulcers occasionally seen with topical paromomycin cream or boric acid preparations.
In Asia and Africa, chancroid (Fig. 163-5) was once considered the most common type of genital ulcer, followed in frequency by primary syphilis and then genital herpes (Fig. 163-6). With increased efforts to control chancroid and syphilis and widespread use of broad-spectrum antibiotics to treat STI-related syndromes, together with more frequent recurrences or persistence of genital herpes attributable to HIV infection, PCR testing of genital ulcers now clearly implicates genital herpes as by far the most common cause of genital ulceration in most developing countries. LGV due to C. trachomatis (Fig. 163-7) and donovanosis (granuloma inguinale, due to Klebsiella granulomatis; see Fig. 198e-1) continue to cause genital ulceration in some developing countries. LGV virtually disappeared in industrialized countries during the first 20 years of the HIV pandemic, but outbreaks are again occurring in Europe (including the United Kingdom), in North America, and in Australia. In these outbreaks, LGV typically presents as proctitis, with or without anal lesions, in men who report unprotected receptive anal intercourse, very often in association with HIV and/or hepatitis C virus infection; the latter may be an acute infection acquired through the same exposure. Other causes of genital ulcers include (1) candidiasis and traumatized genital warts—both readily recognized; (2) lesions due to genital involvement by more widespread dermatoses; (3) cutaneous manifestations of systemic diseases such as genital mucosal ulceration in Stevens-Johnson syndrome or Behçet’s disease; (4) superinfections of lesions that may originally have been sexually acquired (for example, methicillin-resistant S. aureus complicating a genital ulcer due to HSV-2); and (5) localized drug reactions, such as the ulcers occasionally seen with topical paromomycin cream or boric acid preparations.
FIGURE 163-5 Chancroid: multiple, painful, punched-out ulcers with undermined borders on the labia occurring after autoinoculation.
FIGURE 163-6 Genital herpes. A relatively mild, superficial ulcer is typically seen in episodic outbreaks. (Courtesy of Michael Remington, University of Washington Virology Research Clinic.)
FIGURE 163-7 Lymphogranuloma venereum (LGV): striking tender lymphadenopathy occurring at the femoral and inguinal lymph nodes, separated by a groove made by Poupart’s ligament. This “sign-of-the-groove” is not considered specific for LGV; for example, lymphomas may present with this sign.
Diagnosis Although most genital ulcerations cannot be diagnosed confidently on clinical grounds alone, clinical findings (Table 163-7) and epidemiologic considerations can usually guide initial management (Table 163-8) pending results of specific tests. Clinicians should order a rapid serologic test for syphilis in all cases of genital ulcer. To evaluate lesions except those highly characteristic of infection with HSV (i.e., those with herpetic vesicles), dark-field microscopy, direct immunofluorescence, and PCR for T. pallidum can be useful but are rarely available today in most countries. It is important to note that 30% of syphilitic chancres—the primary ulcer of syphilis—are associated with an initially nonreactive syphilis serology. All patients presenting with genital ulceration should be counseled and tested for HIV infection.
|
CLINICAL FEATURES OF GENITAL ULCERS |
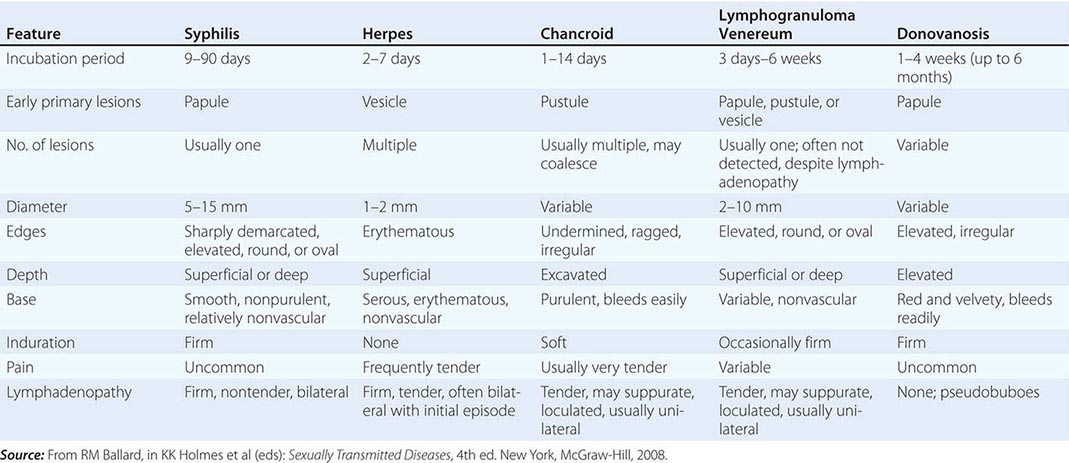
|
INITIAL MANAGEMENT OF GENITAL OR PERIANAL ULCER |
aIf results are negative but primary syphilis is suspected, treat presumptively when indicated by epidemiologic and sexual risk assessment; repeat in 1 week. bThe same treatment regimen is also effective in HIV-infected persons with early syphilis.
Abbreviations: EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; FA, fluorescent antibody; HSV, herpes simplex virus; PCR, polymerase chain reaction; RPR, rapid plasma reagin; VDRL, Venereal Disease Research Laboratory.
Typical vesicles or pustules or a cluster of painful ulcers preceded by vesiculopustular lesions suggests genital herpes. These typical clinical manifestations make detection of the virus optional; however, many patients want confirmation of the diagnosis, and differentiation of HSV-1 from HSV-2 has prognostic implications, because the latter causes more frequent genital recurrences.
Painless, nontender, indurated ulcers with firm, nontender inguinal adenopathy suggest primary syphilis. If results of dark-field examination and a rapid serologic test for syphilis are initially negative, presumptive therapy should be provided on the basis of the individual’s risk. For example, with increasing rates of syphilis among MSM in the United States, most experts would not withhold therapy for this infection pending watchful waiting and/or subsequent detection of seroconversion. Repeated serologic testing for syphilis 1 or 2 weeks after treatment of seronegative primary syphilis usually demonstrates seroconversion.
“Atypical” or clinically trivial ulcers may be more common manifestations of genital herpes than classic vesiculopustular lesions. Specific tests for HSV in such lesions are therefore indicated (Chap. 216). Commercially available type-specific serologic tests for serum antibody to HSV-2 may give negative results, especially when patients present early with the initial episode of genital herpes or when HSV-1 is the cause of genital herpes (as is often the case today). Furthermore, a positive test for antibody to HSV-2 does not prove that the current lesions are herpetic, because nearly one-fifth of the general population of the United States (and no doubt a higher proportion of those at risk for other STIs) becomes seropositive for HSV-2 during early adulthood. Although even “type-specific” tests for HSV-2 that are commercially available in the United States are not 100% specific, a positive HSV-2 serology does enable the clinician to tell the patient that he or she has probably had genital herpes, should learn to recognize symptoms, and should avoid sex during recurrences. In addition, because genital shedding and sexual transmission of HSV-2 often occur in the absence of symptoms and signs of recurrent herpetic lesions, persons who have a history of genital herpes or who are seropositive for HSV-2 should consider the use of condoms or suppressive antiviral therapy, both of which can reduce the risk of HSV-2 transmission to a sexual partner.
Demonstration of H. ducreyi by culture (or by PCR, where available) is most useful when ulcers are painful and purulent, especially if inguinal lymphadenopathy with fluctuance or overlying erythema is noted; if chancroid is prevalent in the community; or if the patient has recently had a sexual exposure elsewhere in a chancroid-endemic area (e.g., a developing country). Enlarged, fluctuant lymph nodes should be aspirated for culture or PCR to detect H. ducreyi as well as for Gram’s staining and culture to rule out the presence of other pyogenic bacteria.
When genital ulcers persist beyond the natural history of initial episodes of herpes (2–3 weeks) or of chancroid or syphilis (up to 6 weeks) and do not resolve with syndrome-based antimicrobial therapy, then—in addition to the usual tests for herpes, syphilis, and chancroid—biopsy is indicated to exclude donovanosis, carcinoma, and other nonvenereal dermatoses. If not performed previously, HIV serology should be standard because chronic, persistent genital herpes is common in AIDS.
|
TREATMENT |
ULCERATIVE GENITAL OR PERIANAL LESIONS |
Immediate syndrome-based treatment for acute genital ulcerations (after collection of all necessary diagnostic specimens at the first visit) is often appropriate before all test results become available because patients with typical initial or recurrent episodes of genital or anorectal herpes can benefit from prompt oral antiviral therapy (Chap. 216); because early treatment of sexually transmitted causes of genital ulcers decreases further transmission; and because many patients do not return for test results and treatment. A thorough assessment of the patient’s sexual-risk profile and medical history is critical in determining the course of initial management. The patient who has risk factors consistent with exposure to syphilis (e.g., a male patient who reports sex with other men or who has HIV infection) should generally receive initial treatment for syphilis. Empirical therapy for chancroid should be considered if there has been an exposure in an area of the world where chancroid occurs or if regional lymph node suppuration is evident. In resource-poor settings lacking ready access to diagnostic tests, this approach to syndromic treatment for syphilis and chancroid has helped bring these two diseases under control. Finally, empirical antimicrobial therapy may be indicated if ulcers persist and the diagnosis remains unclear after a week of observation despite attempts to diagnose herpes, syphilis, and chancroid.
PROCTITIS, PROCTOCOLITIS, ENTEROCOLITIS, AND ENTERITIS
Sexually acquired proctitis, with inflammation limited to the rectal mucosa (the distal 10–12 cm), results from direct rectal inoculation of typical STD pathogens. In contrast, inflammation extending from the rectum to the colon (proctocolitis), involving both the small and the large bowel (enterocolitis), or involving the small bowel alone (enteritis) can result from ingestion of typical intestinal pathogens through oral–anal exposure during sexual contact. Anorectal pain and mucopurulent, bloody rectal discharge suggest proctitis or protocolitis. Proctitis commonly produces tenesmus (causing frequent attempts to defecate, but not true diarrhea) and constipation, whereas proctocolitis and enterocolitis more often cause true diarrhea. In all three conditions, anoscopy usually shows mucosal exudate and easily induced mucosal bleeding (i.e., a positive “wipe test”), sometimes with petechiae or mucosal ulcers. Exudate should be sampled for Gram’s staining and other microbiologic studies. Sigmoidoscopy or colonoscopy shows inflammation limited to the rectum in proctitis or disease extending at least up into the sigmoid colon in proctocolitis.
The AIDS era brought an extraordinary shift in the clinical and etiologic spectrum of intestinal infections among MSM. The number of cases of the acute intestinal STIs described above fell as high-risk sexual behaviors became less common in this group. At the same time, the number of AIDS-related opportunistic intestinal infections increased rapidly, many associated with chronic or recurrent symptoms. The incidence of these opportunistic infections has since fallen with increasingly widespread coverage of HIV-infected persons with effective antiretroviral therapy. Two species initially isolated in association with intestinal symptoms in MSM—now known as Helicobacter cinaedi and H. fennelliae—have both been isolated from the blood of HIV-infected men and other immunosuppressed persons, often in association with a syndrome of multifocal dermatitis and arthritis.
Acquisition of HSV, N. gonorrhoeae, or C. trachomatis (including LGV strains of C. trachomatis) during receptive anorectal intercourse causes most cases of infectious proctitis in women and MSM. Primary and secondary syphilis can also produce anal or anorectal lesions, with or without symptoms. Gonococcal or chlamydial proctitis typically involves the most distal rectal mucosa and the anal crypts and is clinically mild, without systemic manifestations. In contrast, primary proctitis due to HSV and proctocolitis due to the strains of C. trachomatis that cause LGV usually produce severe anorectal pain and often cause fever. Perianal ulcers and inguinal lymphadenopathy, most commonly due to HSV, can also occur with LGV or syphilis. Sacral nerve root radiculopathies, usually presenting as urinary retention, laxity of the anal sphincter, or constipation, may complicate primary herpetic proctitis. In LGV, rectal biopsy typically shows crypt abscesses, granulomas, and giant cells—findings resembling those in Crohn’s disease; such findings should always prompt rectal culture and serology for LGV, which is a curable infection. Syphilis can also produce rectal granulomas, usually in association with infiltration by plasma cells or other mononuclear cells. Syphilis, LGV, and HSV infection involving the rectum can produce perirectal adenopathy that is sometimes mistaken for malignancy; syphilis, LGV, HSV infection, and chancroid involving the anus can produce inguinal adenopathy because anal lymphatics drain to inguinal lymph nodes.
Diarrhea and abdominal bloating or cramping pain without anorectal symptoms and with normal findings on anoscopy and sigmoidoscopy occur with inflammation of the small intestine (enteritis) or with proximal colitis. In MSM without HIV infection, enteritis is often attributable to Giardia lamblia. Sexually acquired proctocolitis is most often due to Campylobacter or Shigella species.
|
TREATMENT |
PROCTITIS, PROCTOCOLITIS, ENTEROCOLITIS, AND ENTERITIS |
Acute proctitis in persons who have practiced receptive anorectal intercourse is usually sexually acquired. Such patients should undergo anoscopy to detect rectal ulcers or vesicles and petechiae after swabbing of the rectal mucosa; to examine rectal exudates for PMNs and gram-negative diplococci; and to obtain rectal swab specimens for testing for rectal gonorrhea, chlamydial infection, herpes, and syphilis. Pending test results, patients with proctitis should receive empirical syndromic treatment—e.g., with ceftriaxone (a single IM dose of 250 mg for gonorrhea) plus doxycycline (100 mg by mouth twice daily for 7 days) for possible chlamydial infection plus treatment for herpes or syphilis if indicated. If LGV proctitis is proven or suspected, the recommended treatment is doxycycline (100 mg by mouth twice daily for 21 days); alternatively, 1 g of azithromycin once a week for 3 weeks is likely to be effective but is little studied.
PREVENTION AND CONTROL OF STIs
Prevention and control of STIs require the following:
1. Reduction of the average rate of sexual exposure to STIs through alteration of sexual risk behaviors and behavioral norms among both susceptible and infected persons in all population groups. The necessary changes include reduction in the total number of sexual partners and the number of concurrent sexual partners.
2. Reduction of the efficiency of transmission through the promotion of safer sexual practices, the use of condoms during casual or commercial sex, vaccination against HBV and HPV infection, male circumcision (which reduces risk of acquisition of HIV infection, chancroid, and perhaps other STIs), and a growing number of other approaches (e.g., early detection and treatment of other STIs to reduce the efficiency of sexual transmission of HIV). Longitudinal studies have shown that consistent condom use is associated with significant protection of both males and females against all STIs that have been examined, including HIV, HPV, and HSV infections as well as gonorrhea and chlamydial infection. The only exceptions are probably sexually transmitted Pthirus pubis and Sarcoptes scabiei infestations.
3. Shortening of the duration of infectivity of STIs through early detection and curative or suppressive treatment of patients and their sexual partners.
Financial and time constraints imposed by many clinical practices, along with the reluctance of some clinicians to ask questions about stigmatized sexual behaviors, often curtail screening and prevention services. As outlined in Fig. 163-8, the success of clinicians’ efforts to detect and treat STIs depends in part on societal efforts to teach young people how to recognize symptoms of STIs; to motivate individuals with symptoms to seek care promptly; to educate persons who are at risk but have no symptoms about what tests they should undergo routinely; and to make high-quality, appropriate care accessible, affordable, and acceptable, especially to the young indigent patients most likely to acquire an STI.
FIGURE 163-8 Critical control points for preventive and clinical interventions against sexually transmitted diseases (STDs). (Adapted from HT Waller and MA Piot: Bull World Health Organ 41:75, 1969 and 43:1, 1970; and from “Resource allocation model for public health planning—a case study of tuberculosis control,” Bull World Health Organ 48 [Suppl], 1973.)
Because many infected individuals develop no symptoms or fail to recognize and report symptoms, clinicians should routinely perform an STI risk assessment for teenagers and young adults as a guide to selective screening. As stated earlier, U.S. Preventive Services Task Force Guidelines recommend screening sexually active female patients ≤25 years of age for C. trachomatis whenever they present for health care (at least once a year); older women should be tested if they have more than one sexual partner, have begun a new sexual relationship since the previous test, or have another STI diagnosed. In women 25–29 years of age, chlamydial infection is uncommon but still may reach a prevalence of 3–5% in some settings; information provided by women in this age group on a sex partner’s concurrency (whether a male partner has had another sex partner during the time they have been together) is helpful in identifying women at increased risk. In some regions of the United States, widespread selective screening and treatment of young women for cervical C. trachomatis infection have been associated with a 50–60% drop in prevalence. Such screening and treatment also protect the individual woman from PID. Sensitive urine-based genetic amplification tests permit expansion of screening to men, teenage boys, and girls in settings where examination is not planned or is impractical (e.g., during preparticipation sports examinations or during initial medical evaluation of adolescent girls). Vaginal swabs—collected either by the health care provider at a pelvic examination or by the woman herself—are highly sensitive and specific for the diagnosis of chlamydial and gonococcal infection; they are now the preferred type of specimen for screening and diagnosis of these infections.
Although gonorrhea is now substantially less common than chlamydial infection in industrialized countries, screening tests for N. gonorrhoeae are still appropriate for women and teenage girls attending STD clinics and for sexually active teens and young women from areas of high gonorrhea prevalence. Multiplex NAATs that combine screening for N. gonorrhoeae and C. trachomatis—and, more recently, for T. vaginalis—in a single low-cost assay now facilitate the prevention and control of these infections for populations at high risk.
All patients who have newly detected STIs or are at high risk for STIs according to routine risk assessment as well as all pregnant women should be encouraged to undergo serologic testing for syphilis and HIV infection, with appropriate HIV counseling before and after testing. Randomized trials have shown that risk-reduction counseling of patients with STIs significantly lowers subsequent risk of acquiring an STI; such counseling should now be considered a standard component of STI management. Preimmunization serologic testing for antibody to HBV is indicated for unvaccinated persons who are known to be at high risk, such as MSM and people who use injection drugs. In most young persons, however, it is more cost-effective to vaccinate against HBV without serologic screening. It is important to recognize that, while immunization against HBV has contributed to marked reductions in the incidence of infection with this virus, the majority of new cases that do occur are acquired through sex. In 2006, the Advisory Committee on Immunization Practices (ACIP) of the CDC recommended the following: (1) Universal hepatitis B vaccination should be implemented for all unvaccinated adults in settings in which a high proportion of adults have risk factors for HBV infection (e.g., STD clinics, HIV testing and treatment facilities, drug-abuse treatment and prevention settings, health care settings targeting services to injection drug users or MSM, and correctional facilities). (2) In other primary care and specialty medical settings in which adults at risk for HBV infection receive care, health care providers should inform all patients about the health benefits of vaccination, the risk factors for HBV infection, and the persons for whom vaccination is recommended and should vaccinate adults who report risk factors for HBV infection as well as any adult who requests protection from HBV infection. To promote vaccination in all settings, health care providers should implement standing orders to identify adults recommended for hepatitis B vaccination, should administer hepatitis B vaccine as part of routine clinical services, should not require acknowledgment of an HBV infection risk factor for adult vaccination, and should use available reimbursement mechanisms to remove financial barriers to hepatitis B vaccination.
In 2007, the ACIP recommended routine immunization of 9- to 26-year-old girls and women with the quadrivalent HPV vaccine (against HPV types 6, 11, 16, and 18) approved by the U.S. Food and Drug Administration; the optimal age for recommended vaccination is 11–12 years because of the very high risk of HPV infection after sexual debut. In 2009, the ACIP added bivalent HPV vaccine (against types 6 and 11) as an option and expanded the groups in which immunization (with either quadrivalent or bivalent vaccine) is safe and effective to include boys and men 9–26 years old. HPV vaccines offering broader protection against additional oncogenic HPV types are anticipated. Since 2011, the ACIP has recommended routine administration of quadrivalent HPV vaccine to boys at 11 or 12 years of age and to males 13–21 years of age who have not yet been vaccinated or who have not completed the three-dose vaccine series; men 22–26 years of age may also be vaccinated.
Partner notification is the process of identifying and informing partners of infected patients about possible exposure to an STI and of examining, testing, and treating partners as appropriate. In a series of 22 reports concerning partner notification during the 1990s, index patients with gonorrhea or chlamydial infection named a mean of 0.75–1.6 partners, of whom one-fourth to one-third were infected; those with syphilis named 1.8–6.3 partners, with one-third to one-half infected; and those with HIV infection named 0.76–5.31 partners, with up to one-fourth infected. Persons who transmit infection or who have recently been infected and are still in the incubation period usually have no symptoms or only mild symptoms and seek medical attention only when notified of their exposure. Therefore, the clinician must encourage patients to participate in partner notification, must ensure that exposed persons are notified and treated, and must guarantee confidentiality to all involved. In the United States, local health departments often offer assistance in partner notification, treatment, and/or counseling. It seems both feasible and most useful to notify those partners exposed within the patient’s likely period of infectiousness, which is often considered the preceding 1 month for gonorrhea, 1–2 months for chlamydial infection, and up to 3 months for early syphilis.
Persons with a new-onset STI always have a source contact who gave them the infection; in addition, they may have a secondary (spread or exposed) contact with whom they had sex after becoming infected. The identification and treatment of these two types of contacts have different objectives. Treatment of the source contact (often a casual contact) benefits the community by preventing further transmission and benefits the source contact; treatment of the recently exposed secondary contact (typically a spouse or another steady sexual partner) prevents the development of serious complications (such as PID) in the partner, reinfection of the index patient, and further spread of infection. A survey of a random sample of U.S. physicians found that most instructed patients to abstain from sex during treatment, to use condoms, and to inform their sex partners after being diagnosed with gonorrhea, chlamydial infection, or syphilis; physicians sometimes gave the patients drugs for their partners. However, follow-up of the partners by physicians was infrequent. A randomized trial compared patients’ delivery of therapy to partners exposed to gonorrhea or chlamydial infection with conventional notification and advice to partners to seek evaluation for STD; patients’ delivery of partners’ therapy, also known as expedited partner therapy (EPT), significantly reduced combined rates of reinfection of the index patient with N. gonorrhoeae or C. trachomatis. State-by-state variations in regulations governing this approach have not been well defined, but the 2010 CDC STD treatment guidelines and the EPT final report of 2006 (http://www.cdc.gov/std/treatment/EPTFinalReport2006.pdf) describe its potential use. Currently, EPT is commonly used by many practicing physicians. Its legal status varies by state, but EPT is now permissible in 38 states and potentially allowable in another 9. (Updated information on the legal status of EPT is available at http://www.cdc.gov/std/ept.)
In summary, clinicians and public health agencies share responsibility for the prevention and control of STIs. In the current health care environment, the role of primary care clinicians has become increasingly important in STI prevention as well as in diagnosis and treatment, and the resurgence of bacterial STIs like syphilis and LGV among MSM—particularly those co-infected with HIV—emphasizes the need for risk assessment and routine screening.
164 |
Meningitis, Encephalitis, Brain Abscess, and Empyema |
Acute infections of the nervous system are among the most important problems in medicine because early recognition, efficient decision making, and rapid institution of therapy can be lifesaving. These distinct clinical syndromes include acute bacterial meningitis, viral meningitis, encephalitis, focal infections such as brain abscess and subdural empyema, and infectious thrombophlebitis. Each may present with a nonspecific prodrome of fever and headache, which in a previously healthy individual may initially be thought to be benign, until (with the exception of viral meningitis) altered consciousness, focal neurologic signs, or seizures appear. Key goals of early management are to emergently distinguish between these conditions, identify the responsible pathogen, and initiate appropriate antimicrobial therapy.
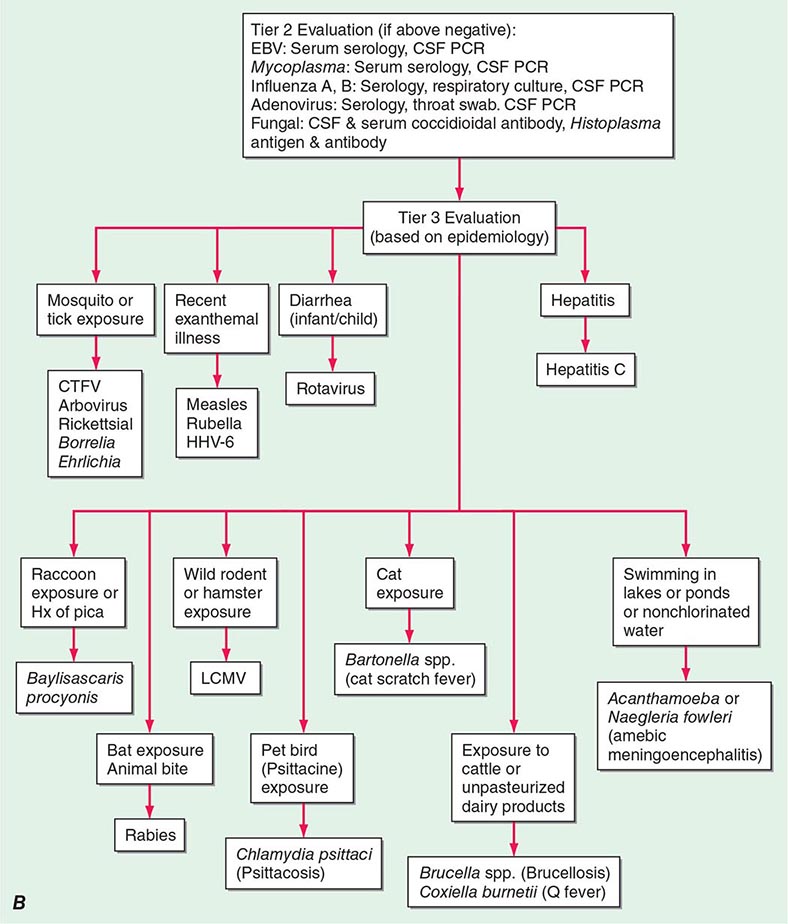
FIGURE 164-1 The management of patients with suspected central nervous system (CNS) infection. ADEM, acute disseminated encephalomyelitis; AFB, acid-fast bacillus; Ag, antigen; CSF, cerebrospinal fluid; CT, computed tomography; CTFV, Colorado tick fever virus; CXR, chest x-ray; DFA, direct fluorescent antibody; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus; LCMV, lymphocytic choriomeningitis virus; MNCs, mononuclear cells; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PMNs, polymorphonuclear leukocytes; PPD, purified protein derivative; TB, tuberculosis; VDRL, Venereal Disease Research Laboratory; VZV, varicella-zoster virus; WNV, West Nile virus.
ACUTE BACTERIAL MENINGITIS
DEFINITION
Bacterial meningitis is an acute purulent infection within the subarachnoid space. It is associated with a CNS inflammatory reaction that may result in decreased consciousness, seizures, raised intracranial pressure (ICP), and stroke. The meninges, subarachnoid space, and brain parenchyma are all frequently involved in the inflammatory reaction (meningoencephalitis).
EPIDEMIOLOGY
Bacterial meningitis is the most common form of suppurative CNS infection, with an annual incidence in the United States of >2.5 cases/100,000 population. The organisms most often responsible for community-acquired bacterial meningitis are Streptococcus pneumoniae (~50%), Neisseria meningitidis (~25%), group B streptococci (~15%), and Listeria monocytogenes (~10%). Haemophilus influenzae type b accounts for <10% of cases of bacterial meningitis in most series. N. meningitidis is the causative organism of recurring epidemics of meningitis every 8 to 12 years.
ETIOLOGY
S. pneumoniae (Chap. 173) is the most common cause of meningitis in adults >20 years of age, accounting for nearly half the reported cases (1.1 per 100,000 persons per year). There are a number of predisposing conditions that increase the risk of pneumococcal meningitis, the most important of which is pneumococcal pneumonia. Additional risk factors include coexisting acute or chronic pneumococcal sinusitis or otitis media, alcoholism, diabetes, splenectomy, hypogammaglobulinemia, complement deficiency, and head trauma with basilar skull fracture and CSF rhinorrhea. The mortality rate remains ~20% despite antibiotic therapy.
The incidence of meningitis due to N. meningitidis (Chap. 180) has decreased with the routine immunization of 11- to 18-year-olds with the quadrivalent (serogroups A, C, W-135, and Y) meningococcal glycoconjugate vaccine. The vaccine does not contain serogroup B, which is responsible for one-third of cases of meningococcal disease. The presence of petechial or purpuric skin lesions can provide an important clue to the diagnosis of meningococcal infection. In some patients the disease is fulminant, progressing to death within hours of symptom onset. Infection may be initiated by nasopharyngeal colonization, which can result in either an asymptomatic carrier state or invasive meningococcal disease. The risk of invasive disease following nasopharyngeal colonization depends on both bacterial virulence factors and host immune defense mechanisms, including the host’s capacity to produce antimeningococcal antibodies and to lyse meningococci by both classic and alternative complement pathways. Individuals with deficiencies of any of the complement components, including properdin, are highly susceptible to meningococcal infections.
Gram-negative bacilli cause meningitis in individuals with chronic and debilitating diseases such as diabetes, cirrhosis, or alcoholism and in those with chronic urinary tract infections. Gram-negative meningitis can also complicate neurosurgical procedures, particularly craniotomy, and head trauma associated with CSF rhinorrhea or otorrhea.
Otitis, mastoiditis, and sinusitis are predisposing and associated conditions for meningitis due to Streptococci sp., gram-negative anaerobes, Staphylococcus aureus, Haemophilus sp., and Enterobacteriaceae. Meningitis complicating endocarditis may be due to viridans streptococci, S. aureus, Streptococcus bovis, the HACEK group (Haemophilus sp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae), or enterococci.
Group B Streptococcus, or Streptococcus agalactiae, was previously responsible for meningitis predominantly in neonates, but it has been reported with increasing frequency in individuals >50 years of age, particularly those with underlying diseases.
L. monocytogenes (Chap. 176) is an increasingly important cause of meningitis in neonates (<1 month of age), pregnant women, individuals >60 years, and immunocompromised individuals of all ages. Infection is acquired by ingesting foods contaminated by Listeria. Foodborne human listerial infection has been reported from contaminated coleslaw, milk, soft cheeses, and several types of “ready-to-eat” foods, including delicatessen meat and uncooked hotdogs.
The frequency of H. influenzae type b (Hib) meningitis in children has declined dramatically since the introduction of the Hib conjugate vaccine, although rare cases of Hib meningitis in vaccinated children have been reported. More frequently, H. influenzae causes meningitis in unvaccinated children and older adults, and non-b H. influenzae is an emerging pathogen.
S. aureus and coagulase-negative staphylococci (Chap. 172) are important causes of meningitis that occurs following invasive neurosurgical procedures, particularly shunting procedures for hydrocephalus, or as a complication of the use of subcutaneous Ommaya reservoirs for administration of intrathecal chemotherapy.
PATHOPHYSIOLOGY
The most common bacteria that cause meningitis, S. pneumoniae and N. meningitidis, initially colonize the nasopharynx by attaching to nasopharyngeal epithelial cells. Bacteria are transported across epithelial cells in membrane-bound vacuoles to the intravascular space or invade the intravascular space by creating separations in the apical tight junctions of columnar epithelial cells. Once in the bloodstream, bacteria are able to avoid phagocytosis by neutrophils and classic complement-mediated bactericidal activity because of the presence of a polysaccharide capsule. Bloodborne bacteria can reach the intraventricular choroid plexus, directly infect choroid plexus epithelial cells, and gain access to the CSF. Some bacteria, such as S. pneumoniae, can adhere to cerebral capillary endothelial cells and subsequently migrate through or between these cells to reach the CSF. Bacteria are able to multiply rapidly within CSF because of the absence of effective host immune defenses. Normal CSF contains few white blood cells (WBCs) and relatively small amounts of complement proteins and immunoglobulins. The paucity of the latter two prevents effective opsonization of bacteria, an essential prerequisite for bacterial phagocytosis by neutrophils. Phagocytosis of bacteria is further impaired by the fluid nature of CSF, which is less conducive to phagocytosis than a solid tissue substrate.
A critical event in the pathogenesis of bacterial meningitis is the inflammatory reaction induced by the invading bacteria. Many of the neurologic manifestations and complications of bacterial meningitis result from the immune response to the invading pathogen rather than from direct bacteria-induced tissue injury. As a result, neurologic injury can progress even after the CSF has been sterilized by antibiotic therapy.
The lysis of bacteria with the subsequent release of cell-wall components into the subarachnoid space is the initial step in the induction of the inflammatory response and the formation of a purulent exudate in the subarachnoid space (Fig. 164-2). Bacterial cell-wall components, such as the lipopolysaccharide (LPS) molecules of gram-negative bacteria and teichoic acid and peptidoglycans of S. pneumoniae, induce meningeal inflammation by stimulating the production of inflammatory cytokines and chemokines by microglia, astrocytes, monocytes, microvascular endothelial cells, and CSF leukocytes. In experimental models of meningitis, cytokines including tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) are present in CSF within 1–2 h of intracisternal inoculation of LPS. This cytokine response is quickly followed by an increase in CSF protein concentration and leukocytosis. Chemokines (cytokines that induce chemotactic migration in leukocytes) and a variety of other proinflammatory cytokines are also produced and secreted by leukocytes and tissue cells that are stimulated by IL-1β and TNF-α. In addition, bacteremia and the inflammatory cytokines induce the production of excitatory amino acids, reactive oxygen and nitrogen species (free oxygen radicals, nitric oxide, and peroxynitrite), and other mediators that can induce death of brain cells, especially in the dentate gyrus of the hippocampus.
FIGURE 164-2 The pathophysiology of the neurologic complications of bacterial meningitis. CSF, cerebrospinal fluid; SAS, subarachnoid space.
Much of the pathophysiology of bacterial meningitis is a direct consequence of elevated levels of CSF cytokines and chemokines. TNF-α and IL-1β act synergistically to increase the permeability of the blood-brain barrier, resulting in induction of vasogenic edema and the leakage of serum proteins into the subarachnoid space (Fig. 164-2). The subarachnoid exudate of proteinaceous material and leukocytes obstructs the flow of CSF through the ventricular system and diminishes the resorptive capacity of the arachnoid granulations in the dural sinuses, leading to obstructive and communicating hydrocephalus and concomitant interstitial edema.
Inflammatory cytokines upregulate the expression of selectins on cerebral capillary endothelial cells and leukocytes, promoting leukocyte adherence to vascular endothelial cells and subsequent migration into the CSF. The adherence of leukocytes to capillary endothelial cells increases the permeability of blood vessels, allowing for the leakage of plasma proteins into the CSF, which adds to the inflammatory exudate. Neutrophil degranulation results in the release of toxic metabolites that contribute to cytotoxic edema, cell injury, and death. Contrary to previous beliefs, CSF leukocytes probably do little to contribute to the clearance of CSF bacterial infection.
During the very early stages of meningitis, there is an increase in cerebral blood flow, soon followed by a decrease in cerebral blood flow and a loss of cerebrovascular autoregulation (Chap. 330). Narrowing of the large arteries at the base of the brain due to encroachment by the purulent exudate in the subarachnoid space and infiltration of the arterial wall by inflammatory cells with intimal thickening (vasculitis) also occur and may result in ischemia and infarction, obstruction of branches of the middle cerebral artery by thrombosis, thrombosis of the major cerebral venous sinuses, and thrombophlebitis of the cerebral cortical veins. The combination of interstitial, vasogenic, and cytotoxic edema leads to raised ICP and coma. Cerebral herniation usually results from the effects of cerebral edema, either focal or generalized; hydrocephalus and dural sinus or cortical vein thrombosis may also play a role.
CLINICAL PRESENTATION
Meningitis can present as either an acute fulminant illness that progresses rapidly in a few hours or as a subacute infection that progressively worsens over several days. The classic clinical triad of meningitis is fever, headache, and nuchal rigidity, but the classic triad may not be present. A decreased level of consciousness occurs in >75% of patients and can vary from lethargy to coma. Fever and either headache, stiff neck, or an altered level of consciousness will be present in nearly every patient with bacterial meningitis. Nausea, vomiting, and photophobia are also common complaints.
Seizures occur as part of the initial presentation of bacterial meningitis or during the course of the illness in 20–40% of patients. Focal seizures are usually due to focal arterial ischemia or infarction, cortical venous thrombosis with hemorrhage, or focal edema. Generalized seizure activity and status epilepticus may be due to hyponatremia, cerebral anoxia, or, less commonly, the toxic effects of antimicrobial agents.
Raised ICP is an expected complication of bacterial meningitis and the major cause of obtundation and coma in this disease. More than 90% of patients will have a CSF opening pressure >180 mmH2O, and 20% have opening pressures >400 mmH2O. Signs of increased ICP include a deteriorating or reduced level of consciousness, papilledema, dilated poorly reactive pupils, sixth nerve palsies, decerebrate posturing, and the Cushing reflex (bradycardia, hypertension, and irregular respirations). The most disastrous complication of increased ICP is cerebral herniation. The incidence of herniation in patients with bacterial meningitis has been reported to occur in as few as 1% to as many as 8% of cases.
Specific clinical features may provide clues to the diagnosis of individual organisms and are discussed in more detail in specific chapters devoted to individual pathogens. The most important of these clues is the rash of meningococcemia, which begins as a diffuse erythematous maculopapular rash resembling a viral exanthem; however, the skin lesions of meningococcemia rapidly become petechial. Petechiae are found on the trunk and lower extremities, in the mucous membranes and conjunctiva, and occasionally on the palms and soles.
DIAGNOSIS
When bacterial meningitis is suspected, blood cultures should be immediately obtained and empirical antimicrobial and adjunctive dexamethasone therapy initiated without delay (Table 164-1). The diagnosis of bacterial meningitis is made by examination of the CSF (Table 164-2). The need to obtain neuroimaging studies (CT or MRI) prior to LP requires clinical judgment. In an immunocompetent patient with no known history of recent head trauma, a normal level of consciousness, and no evidence of papilledema or focal neurologic deficits, it is considered safe to perform LP without prior neuroimaging studies. If LP is delayed in order to obtain neuroimaging studies, empirical antibiotic therapy should be initiated after blood cultures are obtained. Antibiotic therapy initiated a few hours prior to LP will not significantly alter the CSF WBC count or glucose concentration, nor is it likely to prevent visualization of organisms by Gram’s stain or detection of bacterial nucleic acid by polymerase chain reaction (PCR) assay.
|
ANTIBIOTICS USED IN EMPIRICAL THERAPY OF BACTERIAL MENINGITIS AND FOCAL CENTRAL NERVOUS SYSTEM INFECTIONSa |
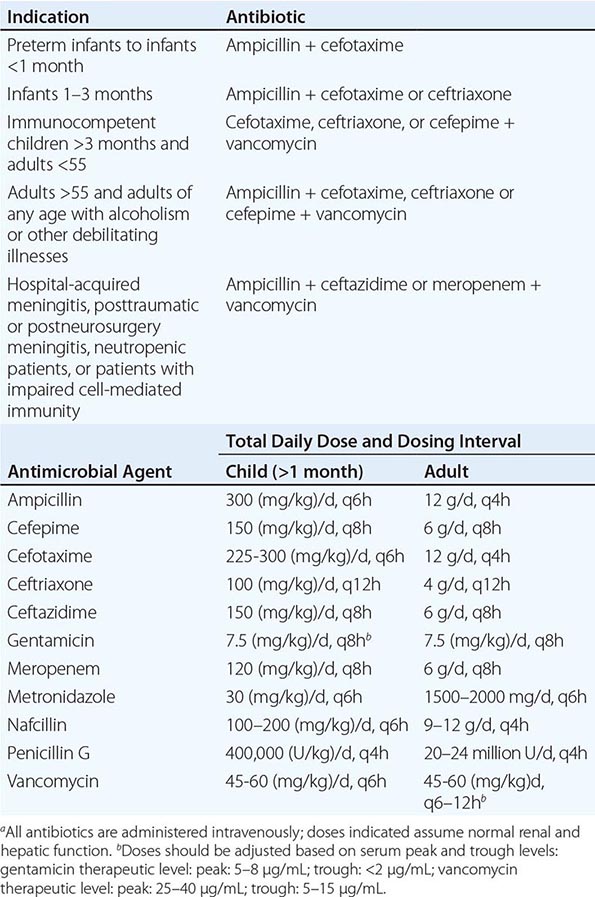
|
CEREBROSPINAL FLUID (CSF) ABNORMALITIES IN BACTERIAL MENINGITIS |
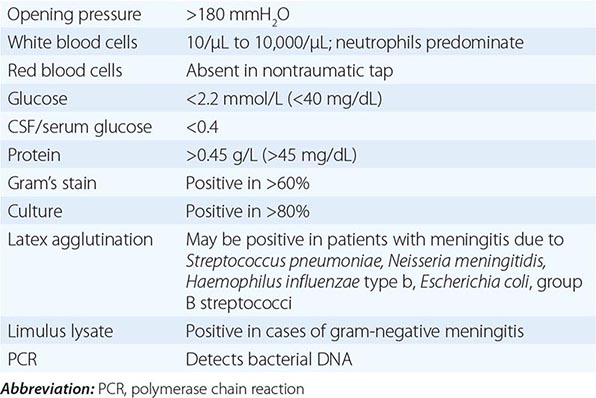
The classic CSF abnormalities in bacterial meningitis (Table 164-2) are (1) polymorphonuclear (PMN) leukocytosis (>100 cells/μL in 90%), (2) decreased glucose concentration (<2.2 mmol/L [<40 mg/dL] and/or CSF/serum glucose ratio of <0.4 in ~60%), (3) increased protein concentration (>0.45 g/L [>45 mg/dL] in 90%), and (4) increased opening pressure (>180 mmH2O in 90%). CSF bacterial cultures are positive in >80% of patients, and CSF Gram’s stain demonstrates organisms in >60%.
CSF glucose concentrations <2.2 mmol/L (<40 mg/dL) are abnormal, and a CSF glucose concentration of zero can be seen in bacterial meningitis. Use of the CSF/serum glucose ratio corrects for hyperglycemia that may mask a relative decrease in the CSF glucose concentration. The CSF glucose concentration is low when the CSF/serum glucose ratio is <0.6. A CSF/serum glucose ratio <0.4 is highly suggestive of bacterial meningitis but may also be seen in other conditions, including fungal, tuberculous, and carcinomatous meningitis. It takes from 30 min to several hours for the concentration of CSF glucose to reach equilibrium with blood glucose levels; therefore, administration of 50 mL of 50% glucose (D50) prior to LP, as commonly occurs in emergency room settings, is unlikely to alter CSF glucose concentration significantly unless more than a few hours have elapsed between glucose administration and LP.
A 16S rRNA conserved sequence broad-based bacterial PCR can detect small numbers of viable and nonviable organisms in CSF and is expected to be useful for making a diagnosis of bacterial meningitis in patients who have been pretreated with oral or parenteral antibiotics and in whom Gram’s stain and CSF culture are negative. When the broad-range PCR is positive, a PCR that uses specific bacterial primers to detect the nucleic acid of S. pneumoniae, N. meningitidis, Escherichia coli, L. monocytogenes, H. influenzae, and S. agalactiae can be obtained based on the clinical suspicion of the meningeal pathogen. The latex agglutination (LA) test for the detection of bacterial antigens of S. pneumoniae, N. meningitidis, H. influenzae type b, group B Streptococcus, and E. coli K1 strains in the CSF has been useful for making a diagnosis of bacterial meningitis but is being replaced by the CSF bacterial PCR assay. The CSF LA test has a specificity of 95–100% for S. pneumoniae and N. meningitidis, so a positive test is virtually diagnostic of bacterial meningitis caused by these organisms. However, the sensitivity of the CSF LA test is only 70–100% for detection of S. pneumoniae and 33–70% for detection of N. meningitidis antigens, so a negative test does not exclude infection by these organisms. The Limulus amebocyte lysate assay is a rapid diagnostic test for the detection of gram-negative endotoxin in CSF and thus for making a diagnosis of gram-negative bacterial meningitis. The test has a specificity of 85–100% and a sensitivity approaching 100%. Thus, a positive Limulus amebocyte lysate assay occurs in virtually all patients with gram-negative bacterial meningitis, but false positives may occur.
Almost all patients with bacterial meningitis will have neuroimaging studies performed during the course of their illness. MRI is preferred over CT because of its superiority in demonstrating areas of cerebral edema and ischemia. In patients with bacterial meningitis, diffuse meningeal enhancement is often seen after the administration of gadolinium. Meningeal enhancement is not diagnostic of meningitis but occurs in any CNS disease associated with increased blood-brain barrier permeability.
Petechial skin lesions, if present, should be biopsied. The rash of meningococcemia results from the dermal seeding of organisms with vascular endothelial damage, and biopsy may reveal the organism on Gram’s stain.
DIFFERENTIAL DIAGNOSIS
Viral meningoencephalitis, and particularly herpes simplex virus (HSV) encephalitis, can mimic the clinical presentation of bacterial meningitis (see “Viral Encephalitis,” below). HSV encephalitis typically presents with headache, fever, altered consciousness, focal neurologic deficits (e.g., dysphasia, hemiparesis), and focal or generalized seizures. The findings on CSF studies, neuroimaging, and electroencephalogram (EEG) distinguish HSV encephalitis from bacterial meningitis. The typical CSF profile with viral CNS infections is a lymphocytic pleocytosis with a normal glucose concentration, in contrast to the PMN pleocytosis and hypoglycorrhachia characteristic of bacterial meningitis. MRI abnormalities (other than meningeal enhancement) are not seen in uncomplicated bacterial meningitis. By contrast, in HSV encephalitis, on T2-weighted, fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted MRI images, high signal intensity lesions are seen in the orbitofrontal, anterior, and medial temporal lobes in the majority of patients within 48 h of symptom onset. Some patients with HSV encephalitis have a distinctive periodic pattern on EEG (see below).
Rickettsial disease can resemble bacterial meningitis (Chap. 211). Rocky Mountain spotted fever (RMSF) is transmitted by a tick bite and caused by the bacteria Rickettsia rickettsii. The disease may present acutely with high fever, prostration, myalgia, headache, nausea, and vomiting. Most patients develop a characteristic rash within 96 h of the onset of symptoms. The rash is initially a diffuse erythematous maculopapular rash that may be difficult to distinguish from that of meningococcemia. It progresses to a petechial rash, then to a purpuric rash, and if untreated, to skin necrosis or gangrene. The color of the lesions changes from bright red to very dark red, then yellowish-green to black. The rash typically begins in the wrist and ankles and then spreads distally and proximally within a matter of a few hours, involving the palms and soles. Diagnosis is made by immunofluorescent staining of skin biopsy specimens. Ehrlichioses are also transmitted by a tick bite. These are small gram-negative coccobacilli of which two species cause human disease. Anaplasma phagocytophilum causes human granulocytic ehrlichiosis (anaplasmosis), and Ehrlichia chaffeensis causes human monocytic ehrlichiosis. The clinical and laboratory manifestations of the infections are similar. Patients present with fever, headache, confusion, nausea, and vomiting. Twenty percent of patients have a maculopapular or petechial rash. There is laboratory evidence of leukopenia, thrombocytopenia, and anemia, and mild to moderate elevations in alanine aminotransferases, alkaline phosphatase, and lactate dehydrogenase. Patients with RMSF and those with ehrlichial infections may have an altered level of consciousness ranging from mild lethargy to coma, confusion, focal neurologic signs, cranial nerve palsies, hyperreflexia, and seizures.
Focal suppurative CNS infections (see below), including subdural and epidural empyema and brain abscess, should also be considered, especially when focal neurologic findings are present. MRI should be performed promptly in all patients with suspected meningitis who have focal features, both to detect the intracranial infection and to search for associated areas of infection in the sinuses or mastoid bones.
A number of noninfectious CNS disorders can mimic bacterial meningitis. Subarachnoid hemorrhage (SAH; Chap. 330) is generally the major consideration. Other possibilities include chemical meningitis due to rupture of tumor contents into the CSF (e.g., from a cystic glioma or craniopharyngioma epidermoid or dermoid cyst); drug-induced hypersensitivity meningitis; carcinomatous or lymphomatous meningitis; meningitis associated with inflammatory disorders such as sarcoid, systemic lupus erythematosus (SLE), and Behçet’s syndrome; pituitary apoplexy; and uveomeningitic syndromes (Vogt-Koyanagi-Harada syndrome).
On occasion, subacutely evolving meningitis (Chap. 165) may be considered in the differential diagnosis of acute meningitis. The principal causes include Mycobacterium tuberculosis (Chap. 202), Cryptococcus neoformans (Chap. 239), Histoplasma capsulatum (Chap. 236), Coccidioides immitis (Chap. 237), and Treponema pallidum (Chap. 206).
|
ACUTE BACTERIAL MENINGITIS |
EMPIRICAL ANTIMICROBIAL THERAPY
(Table 164-1) Bacterial meningitis is a medical emergency. The goal is to begin antibiotic therapy within 60 min of a patient’s arrival in the emergency room. Empirical antimicrobial therapy is initiated in patients with suspected bacterial meningitis before the results of CSF Gram’s stain and culture are known. S. pneumoniae (Chap. 171) and N. meningitidis (Chap. 180) are the most common etiologic organisms of community-acquired bacterial meningitis. Due to the emergence of penicillin- and cephalosporin-resistant S. pneumoniae, empirical therapy of community-acquired suspected bacterial meningitis in children and adults should include a combination of dexamethasone, a third- or fourth-generation cephalosporin (e.g., ceftriaxone, cefotaxime, or cefepime), and vancomycin, plus acyclovir, as HSV encephalitis is the leading disease in the differential diagnosis, and doxycycline during tick season to treat tick-borne bacterial infections. Ceftriaxone or cefotaxime provides good coverage for susceptible S. pneumoniae, group B streptococci, and H. influenzae and adequate coverage for N. meningitidis. Cefepime is a broad-spectrum fourth-generation cephalosporin with in vitro activity similar to that of cefotaxime or ceftriaxone against S. pneumoniae and N. meningitidis and greater activity against Enterobacter species and Pseudomonas aeruginosa. In clinical trials, cefepime has been demonstrated to be equivalent to cefotaxime in the treatment of penicillin-sensitive pneumococcal and meningococcal meningitis, and it has been used successfully in some patients with meningitis due to Enterobacter species and P. aeruginosa. Ampicillin should be added to the empirical regimen for coverage of L. monocytogenes in individuals <3 months of age, those >55, or those with suspected impaired cell-mediated immunity because of chronic illness, organ transplantation, pregnancy, malignancy, or immunosuppressive therapy. Metronidazole is added to the empirical regimen to cover gram-negative anaerobes in patients with otitis, sinusitis, or mastoiditis. In hospital-acquired meningitis, and particularly meningitis following neurosurgical procedures, staphylococci and gram-negative organisms including P. aeruginosa are the most common etiologic organisms. In these patients, empirical therapy should include a combination of vancomycin and ceftazidime, cefepime, or meropenem. Ceftazidime, cefepime, or meropenem should be substituted for ceftriaxone or cefotaxime in neurosurgical patients and in neutropenic patients, because ceftriaxone and cefotaxime do not provide adequate activity against CNS infection with P. aeruginosa. Meropenem is a carbapenem antibiotic that is highly active in vitro against L. monocytogenes, has been demonstrated to be effective in cases of meningitis caused by P. aeruginosa, and shows good activity against penicillin-resistant pneumococci. In experimental pneumococcal meningitis, meropenem was comparable to ceftriaxone and inferior to vancomycin in sterilizing CSF cultures. The number of patients with bacterial meningitis enrolled in clinical trials of meropenem has not been sufficient to definitively assess the efficacy of this antibiotic.
SPECIFIC ANTIMICROBIAL THERAPY
Meningococcal Meningitis (Table 164-3) Although ceftriaxone and cefotaxime provide adequate empirical coverage for N. meningitidis, penicillin G remains the antibiotic of choice for meningococcal meningitis caused by susceptible strains. Isolates of N. meningitidis with moderate resistance to penicillin have been identified and are increasing in incidence worldwide. CSF isolates of N. meningitidis should be tested for penicillin and ampicillin susceptibility, and if resistance is found, cefotaxime or ceftriaxone should be substituted for penicillin. A 7-day course of intravenous antibiotic therapy is adequate for uncomplicated meningococcal meningitis. The index case and all close contacts should receive chemoprophylaxis with a 2-day regimen of rifampin (600 mg every 12 h for 2 days in adults and 10 mg/kg every 12 h for 2 days in children >1 year). Rifampin is not recommended in pregnant women. Alternatively, adults can be treated with one dose of azithromycin (500 mg) or one intramuscular dose of ceftriaxone (250 mg). Close contacts are defined as those individuals who have had contact with oropharyngeal secretions, either through kissing or by sharing toys, beverages, or cigarettes.
|
ANTIMICROBIAL THERAPY OF CENTRAL NERVOUS SYSTEM BACTERIAL INFECTIONS BASED ON PATHOGENa |
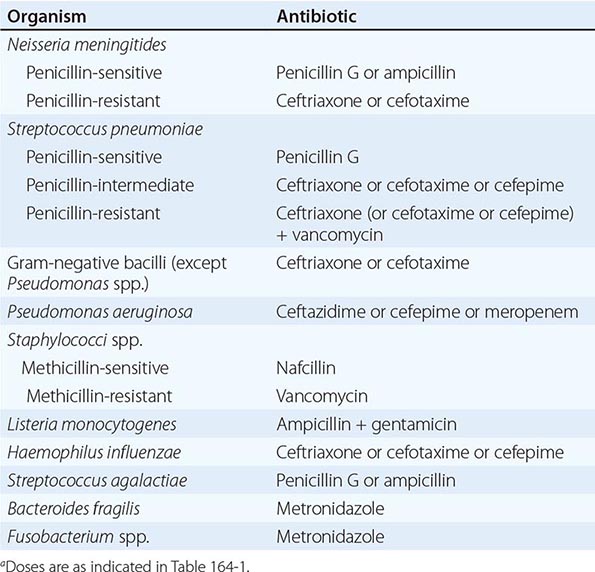
Pneumococcal Meningitis Antimicrobial therapy of pneumococcal meningitis is initiated with a cephalosporin (ceftriaxone, cefotaxime, or cefepime) and vancomycin. All CSF isolates of S. pneumoniae should be tested for sensitivity to penicillin and the cephalosporins. Once the results of antimicrobial susceptibility tests are known, therapy can be modified accordingly (Table 164-3). For S. pneumoniae meningitis, an isolate of S. pneumoniae is considered to be susceptible to penicillin with a minimal inhibitory concentration (MIC) <0.06 μg/mL and to be resistant when the MIC is >0.12 μg/mL. Isolates of S. pneumoniae that have cephalosporin MICs ≤0.5 μg/mL are considered sensitive to the cephalosporins (cefotaxime, ceftriaxone, cefepime). Those with MICs of 1 μg/mL are considered to have intermediate resistance, and those with MICs ≥2 μg/mL are considered resistant. For meningitis due to pneumococci, with cefotaxime or ceftriaxone MICs ≤0.5 μg/mL, treatment with cefotaxime or ceftriaxone is usually adequate. For MIC >1 μg/mL, vancomycin is the antibiotic of choice. Rifampin can be added to vancomycin for its synergistic effect but is inadequate as monotherapy because resistance develops rapidly when it is used alone.
A 2-week course of intravenous antimicrobial therapy is recommended for pneumococcal meningitis.
Patients with S. pneumoniae meningitis should have a repeat LP performed 24–36 h after the initiation of antimicrobial therapy to document sterilization of the CSF. Failure to sterilize the CSF after 24–36 h of antibiotic therapy should be considered presumptive evidence of antibiotic resistance. Patients with penicillin- and cephalosporin-resistant strains of S. pneumoniae who do not respond to intravenous vancomycin alone may benefit from the addition of intraventricular vancomycin. The intraventricular route of administration is preferred over the intrathecal route because adequate concentrations of vancomycin in the cerebral ventricles are not always achieved with intrathecal administration.
Listeria Meningitis Meningitis due to L. monocytogenes is treated with ampicillin for at least 3 weeks (Table 164-3). Gentamicin is added in critically ill patients (2 mg/kg loading dose, then 7.5 mg/kg per day given every 8 h and adjusted for serum levels and renal function). The combination of trimethoprim (10–20 mg/kg per day) and sulfamethoxazole (50–100 mg/kg per day) given every 6 h may provide an alternative in penicillin-allergic patients.
Staphylococcal Meningitis Meningitis due to susceptible strains of S. aureus or coagulase-negative staphylococci is treated with nafcillin (Table 164-3). Vancomycin is the drug of choice for methicillin-resistant staphylococci and for patients allergic to penicillin. In these patients, the CSF should be monitored during therapy. If the CSF is not sterilized after 48 h of intravenous vancomycin therapy, then either intraventricular or intrathecal vancomycin, 20 mg once daily, can be added.
Gram-Negative Bacillary Meningitis The third-generation cephalosporins—cefotaxime, ceftriaxone, and ceftazidime—are equally efficacious for the treatment of gram-negative bacillary meningitis, with the exception of meningitis due to P. aeruginosa, which should be treated with ceftazidime, cefepime, or meropenem (Table 164-3). A 3-week course of intravenous antibiotic therapy is recommended for meningitis due to gram-negative bacilli.
ADJUNCTIVE THERAPY
The release of bacterial cell-wall components by bactericidal antibiotics leads to the production of the inflammatory cytokines IL-1β and TNF-α in the subarachnoid space. Dexamethasone exerts its beneficial effect by inhibiting the synthesis of IL-1β and TNF-α at the level of mRNA, decreasing CSF outflow resistance, and stabilizing the blood-brain barrier. The rationale for giving dexamethasone 20 min before antibiotic therapy is that dexamethasone inhibits the production of TNF-α by macrophages and microglia only if it is administered before these cells are activated by endotoxin. Dexamethasone does not alter TNF-α production once it has been induced. The results of clinical trials of dexamethasone therapy in meningitis due to H. influenzae, S. pneumoniae, and N. meningitidis have demonstrated its efficacy in decreasing meningeal inflammation and neurologic sequelae such as the incidence of sensorineural hearing loss.
A prospective European trial of adjunctive therapy for acute bacterial meningitis in 301 adults found that dexamethasone reduced the number of unfavorable outcomes (15 vs. 25%, p = .03) including death (7 vs. 15%, p = .04). The benefits were most striking in patients with pneumococcal meningitis. Dexamethasone (10 mg intravenously) was administered 15–20 min before the first dose of an antimicrobial agent, and the same dose was repeated every 6 h for 4 days. These results were confirmed in a second trial of dexamethasone in adults with pneumococcal meningitis. Therapy with dexamethasone should ideally be started 20 min before, or not later than concurrent with, the first dose of antibiotics. It is unlikely to be of significant benefit if started >6 h after antimicrobial therapy has been initiated. Dexamethasone may decrease the penetration of vancomycin into CSF, and it delays the sterilization of CSF in experimental models of S. pneumoniae meningitis. As a result, to assure reliable penetration of vancomycin into the CSF, children and adults are treated with vancomycin in a dose of 45–60 mg/kg per day. Alternatively, vancomycin can be administered by the intraventricular route.
One of the concerns for using dexamethasone in adults with bacterial meningitis is that in experimental models of meningitis, dexamethasone therapy increased hippocampal cell injury and reduced learning capacity. This has not been the case in clinical series. The efficacy of dexamethasone therapy in preventing neurologic sequelae is different between high- and low-income countries. Three large randomized trials in low-income countries (sub-Saharan Africa, Southeast Asia) failed to show benefit in subgroups of patients. The lack of efficacy of dexamethasone in these trials has been attributed to late presentation to the hospital with more advanced disease, antibiotic pretreatment, malnutrition, infection with HIV, and treatment of patients with probable, but not microbiologically proven, bacterial meningitis. The results of these clinical trials suggest that patients in sub-Saharan Africa and those in low-income countries with negative CSF Gram’s stain and culture should not be treated with dexamethasone.
INCREASED INTRACRANIAL PRESSURE
Emergency treatment of increased ICP includes elevation of the patient’s head to 30–45°, intubation and hyperventilation (Paco2 25–30 mmHg), and mannitol. Patients with increased ICP should be managed in an intensive care unit; accurate ICP measurements are best obtained with an ICP monitoring device.
Treatment of increased intracranial pressure is discussed in detail in Chap. 330.
PROGNOSIS
Mortality rate is 3–7% for meningitis caused by H. influenzae, N. meningitidis, or group B streptococci; 15% for that due to L. monocytogenes; and 20% for S. pneumoniae. In general, the risk of death from bacterial meningitis increases with (1) decreased level of consciousness on admission, (2) onset of seizures within 24 h of admission, (3) signs of increased ICP, (4) young age (infancy) and age >50, (5) the presence of comorbid conditions including shock and/or the need for mechanical ventilation, and (6) delay in the initiation of treatment. Decreased CSF glucose concentration (<2.2 mmol/L [<40 mg/dL]) and markedly increased CSF protein concentration (>3 g/L [> 300 mg/dL]) have been predictive of increased mortality and poorer outcomes in some series. Moderate or severe sequelae occur in ~25% of survivors, although the exact incidence varies with the infecting organism. Common sequelae include decreased intellectual function, memory impairment, seizures, hearing loss and dizziness, and gait disturbances.
ACUTE VIRAL MENINGITIS
CLINICAL MANIFESTATIONS
Immunocompetent adult patients with viral meningitis usually present with headache, fever, and signs of meningeal irritation coupled with an inflammatory CSF profile (see below). Headache is almost invariably present and often characterized as frontal or retroorbital and frequently associated with photophobia and pain on moving the eyes. Nuchal rigidity is present in most cases but may be mild and present only near the limit of neck anteflexion. Constitutional signs can include malaise, myalgia, anorexia, nausea and vomiting, abdominal pain, and/or diarrhea. Patients often have mild lethargy or drowsiness; however, profound alterations in consciousness, such as stupor, coma, or marked confusion, do not occur in viral meningitis and suggest the presence of encephalitis or other alternative diagnoses. Similarly, seizures or focal neurologic signs or symptoms or neuroimaging abnormalities indicative of brain parenchymal involvement are not typical of viral meningitis and suggest the presence of encephalitis or another CNS infectious or inflammatory process.
ETIOLOGY
Using a variety of diagnostic techniques, including CSF PCR, culture, and serology, a specific viral cause can be found in 60–90% of cases of viral meningitis. The most important agents are enteroviruses (including echoviruses and coxsackieviruses in addition to numbered enteroviruses), varicella-zoster virus (VZV), HSV (HSV-2 > HSV-1), HIV, and arboviruses (Table 164-4). CSF cultures are positive in 30–70% of patients, with the frequency of isolation depending on the specific viral agent. Approximately two-thirds of culture-negative cases of “aseptic” meningitis have a specific viral etiology identified by CSF PCR testing (see below).
|
VIRUSES CAUSING ACUTE MENINGITIS AND ENCEPHALITIS IN NORTH AMERICA |
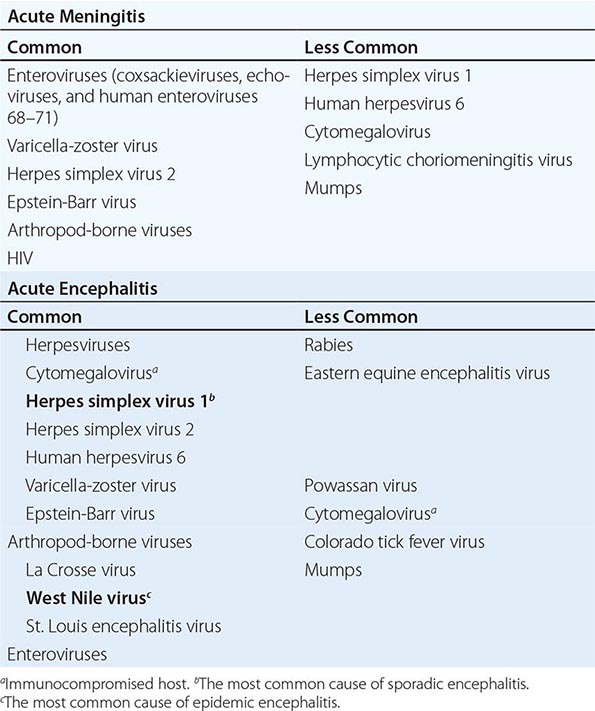
EPIDEMIOLOGY
Viral meningitis is not a nationally reportable disease; however, it has been estimated that the incidence is ~60,000–75,000 cases per year. In temperate climates, there is a substantial increase in cases during the nonwinter months, reflecting the seasonal predominance of enterovirus and arthropod-borne virus (arbovirus) infections in the summer and fall, with a peak monthly incidence of about 1 reported case per 100,000 population.
LABORATORY DIAGNOSIS
CSF Examination The most important laboratory test in the diagnosis of viral meningitis is examination of the CSF. The typical profile is a pleocytosis, a normal or slightly elevated protein concentration (0.2–0.8 g/L [20–80 mg/dL]), a normal glucose concentration, and a normal or mildly elevated opening pressure (100–350 mmH2O). Organisms are not seen on Gram’s stain of CSF. The total CSF cell count in viral meningitis is typically 25–500/μL, although cell counts of several thousand/μL are occasionally seen, especially with infections due to lymphocytic choriomeningitis virus (LCMV) and mumps virus. Lymphocytes are typically the predominant cell. Rarely, PMNs may predominate in the first 48 h of illness, especially with infections due to echovirus 9, West Nile virus, eastern equine encephalitis (EEE) virus, or mumps. A PMN pleocytosis occurs in 45% of patients with West Nile virus (WNV) meningitis and can persist for a week or longer before shifting to a lymphocytic pleocytosis. PMN pleocytosis with low glucose may also be a feature of cytomegalovirus (CMV) infections in immunocompromised hosts. Despite these exceptions, the presence of a CSF PMN pleocytosis in a patient with suspected viral meningitis in whom a specific diagnosis has not been established should prompt consideration of alternative diagnoses, including bacterial meningitis or parameningeal infections. The CSF glucose concentration is typically normal in viral infections, although it may be decreased in 10–30% of cases due to mumps or LCMV. Rare instances of decreased CSF glucose concentration occur in cases of meningitis due to echoviruses and other enteroviruses, HSV-2, and VZV. As a rule, a lymphocytic pleocytosis with a low glucose concentration should suggest fungal or tuberculous meningitis, Listeria meningoencephalitis, or noninfectious disorders (e.g., sarcoid, neoplastic meningitis).
A number of tests measuring levels of various CSF proteins, enzymes, and mediators—including C-reactive protein, lactic acid, lactate dehydrogenase, neopterin, quinolinate, IL-1β, IL-6, soluble IL-2 receptor, β2-microglobulin, and TNF—have been proposed as potential discriminators between viral and bacterial meningitis or as markers of specific types of viral infection (e.g., infection with HIV), but they remain of uncertain sensitivity and specificity and are not widely used for diagnostic purposes.
Polymerase Chain Reaction Amplification of Viral Nucleic Acid Amplification of viral-specific DNA or RNA from CSF using PCR amplification has become the single most important method for diagnosing CNS viral infections. In both enteroviral and HSV infections of the CNS, CSF PCR has become the diagnostic procedure of choice and is substantially more sensitive than viral cultures. HSV CSF PCR is also an important diagnostic test in patients with recurrent episodes of “aseptic” meningitis, many of whom have amplifiable HSV DNA in CSF despite negative viral cultures. CSF PCR is also used routinely to diagnose CNS viral infections caused by CMV, Epstein-Barr virus (EBV), VZV, and human herpesvirus 6 (HHV-6). CSF PCR tests are available for WNV but are not as sensitive as detection of WNV-specific CSF IgM. PCR is also useful in the diagnosis of CNS infection caused by Mycoplasma pneumoniae, which can mimic viral meningitis and encephalitis. PCR of throat washings may assist in diagnosis of enteroviral and mycoplasmal CNS infections. PCR of stool specimens may also assist in diagnosis of enteroviral infections (see below).
Viral Culture The sensitivity of CSF cultures for the diagnosis of viral meningitis and encephalitis, in contrast to its utility in bacterial infections, is generally poor. In addition to CSF, specific viruses may also be isolated from throat swabs, stool, blood, and urine. Enteroviruses and adenoviruses may be found in feces; arboviruses, some enteroviruses, and LCMV in blood; mumps and CMV in urine; and enteroviruses, mumps, and adenoviruses in throat washings. During enteroviral infections, viral shedding in stool may persist for several weeks. The presence of enterovirus in stool is not diagnostic and may result from residual shedding from a previous enteroviral infection; it also occurs in some asymptomatic individuals during enteroviral epidemics.
Serologic Studies For many arboviruses including WNV, serologic studies remain important diagnostic tools. Serum antibody determination is less useful for viruses with high seroprevalence rates in the general population such as HSV, VZV, CMV, and EBV. For viruses with low seroprevalence rates, diagnosis of acute viral infection can be made by documenting seroconversion between acute-phase and convalescent sera (typically obtained after 2–4 weeks) or by demonstrating the presence of virus-specific IgM antibodies. For viruses with high seroprevalence such as VZV and HSV, demonstration of synthesis of virus-specific antibodies in CSF, as shown by an increased IgG index or the presence of CSF IgM antibodies, may be useful and can provide presumptive evidence of CNS infection. Although serum and CSF IgM antibodies generally persist for only a few months after acute infection, there are exceptions to this rule. For example, WNV serum IgM has been shown to persist in some patients for >1 year following acute infection. Unfortunately, the delay between onset of infection and the host’s generation of a virus-specific antibody response often means that serologic data are useful mainly for the retrospective establishment of a specific diagnosis, rather than in aiding acute diagnosis or management. In the case of EBV, demonstration of antibody responses consistent with recent/acute infection (e.g., IgM viral capsid antibody, antibody against early antigen, absence of antibody against EBV-associated nuclear antigen) may assist in diagnosis.
CSF oligoclonal gamma globulin bands occur in association with a number of viral infections. The associated antibodies are often directed against viral proteins. Oligoclonal bands also occur commonly in certain noninfectious neurologic diseases (e.g., multiple sclerosis) and may be found in nonviral infections (e.g., neurosyphilis, Lyme neuroborreliosis).
Other Laboratory Studies All patients with suspected viral meningitis should have a complete blood count and differential, liver and renal function tests, erythrocyte sedimentation rate (ESR), and C-reactive protein, electrolytes, glucose, creatine kinase, aldolase, amylase, and lipase. Neuroimaging studies (MRI preferable to CT) are not absolutely necessary in patients with uncomplicated viral meningitis but should be performed in patients with altered consciousness, seizures, focal neurologic signs or symptoms, atypical CSF profiles, or underlying immunocompromising treatments or conditions.
DIFFERENTIAL DIAGNOSIS
The most important issue in the differential diagnosis of viral meningitis is to consider diseases that can mimic viral meningitis, including (1) untreated or partially treated bacterial meningitis; (2) early stages of meningitis caused by fungi, mycobacteria, or Treponema pallidum (neurosyphilis), in which a lymphocytic pleocytosis is common, cultures may be slow growing or negative, and hypoglycorrhachia may not be present early; (3) meningitis caused by agents such as Mycoplasma, Listeria spp., Brucella spp., Coxiella spp., Leptospira spp., and Rickettsia spp.; (4) parameningeal infections; (5) neoplastic meningitis; and (6) meningitis secondary to noninfectious inflammatory diseases, including autoimmune and hypersensitivity meningitis, SLE and other rheumatologic diseases, sarcoidosis, Behçet’s syndrome, and the uveomeningitic syndromes. Studies in children >28 days of age suggest that the presence of CSF protein >0.5 g/L (sensitivity 89%, specificity 78%) and elevated serum procalcitonin levels >0.5 ng/mL (sensitivity 89%, specificity 89%) were clues to the presence of bacterial as opposed to “aseptic” meningitis. A variety of clinical algorithms for differentiating bacterial from aseptic meningitis have been developed. One such prospectively validated system, the bacterial meningitis score, suggests that the probability of bacterial meningitis is 0.3% or less (negative predictive value 99.7%, 95% confidence interval 99.6–100%) in children with CSF pleocytosis who have (1) a negative CSF Gram’s stain, (2) CSF neutrophil count <1000 cells/μL, (3) CSF protein <80 mg/dL, (4) peripheral absolute neutrophil count of <10,000 cells/μL, and (5) no prior history or current presence of seizures.
SPECIFIC VIRAL ETIOLOGIES
Enteroviruses (EV) (Chap. 228) are the most common cause of viral meningitis, accounting for >85% of cases in which a specific etiology can be identified. Cases may either be sporadic or occur in clusters. EV71 has produced large epidemics of neurologic disease outside the United States, especially in Southeast Asia, but most recently reported cases in the United States have been sporadic. Enteroviruses are the most likely cause of viral meningitis in the summer and fall months, especially in children (<15 years), although cases occur at reduced frequency year round. Although the incidence of enteroviral meningitis declines with increasing age, some outbreaks have preferentially affected older children and adults. Meningitis outside the neonatal period is usually benign. Patients present with sudden onset of fever; headache; nuchal rigidity; and often constitutional signs, including vomiting, anorexia, diarrhea, cough, pharyngitis, and myalgias. The physical examination should include a careful search for stigmata of enterovirus infection, including exanthems, hand-foot-mouth disease, herpangina, pleurodynia, myopericarditis, and hemorrhagic conjunctivitis. The CSF profile is typically a lymphocytic pleocytosis (100–1000 cells/μL) with normal glucose and normal or mildly elevated protein concentration. However, up to 15% of patients, most commonly young infants rather than older children or adults, have a normal CSF leukocyte count. In rare cases, PMNs may predominate during the first 48 h of illness. CSF reverse transcriptase PCR (RT-PCR) is the diagnostic procedure of choice and is both sensitive (>95%) and specific (>100%). CSF PCR has the highest sensitivity if performed within 48 h of symptom onset, with sensitivity declining rapidly after day 5 of symptoms. PCR of throat washings or stool specimens may be positive for several weeks, and positive results can help support the diagnosis of an acute enteroviral infection. The sensitivity of routine enteroviral PCRs for detecting EV71 is low, and specific testing may be required. Treatment is supportive, and patients usually recover without sequelae. Chronic and severe infections can occur in neonates and in individuals with hypo- or agammaglobulinemia.
Arbovirus infections (Chap. 233) occur predominantly in the summer and early fall. Arboviral meningitis should be considered when clusters of meningitis and encephalitis cases occur in a restricted geographic region during the summer or early fall. In the United States, the most important causes of arboviral meningitis and encephalitis are WNV, St. Louis encephalitis virus, and the California encephalitis group of viruses. In WNV epidemics, avian deaths may serve as sentinel infections for subsequent human disease. A history of tick exposure or travel or residence in the appropriate geographic area should suggest the possibility of Colorado tick fever virus or Powassan virus infection, although nonviral tick-borne diseases, including RMSF and Lyme neuroborreliosis, may present similarly. Arbovirus meningoencephalitis is typically associated with a CSF lymphocytic pleocytosis, normal glucose concentration, and normal or mildly elevated protein concentration. However, ~45% of patients with WNV meningoencephalitis have CSF neutrophilia, which can persist for a week or more. The rarity of hypoglycorrhachia in WNV infection, the absence of positive Gram’s stains, and the negative cultures help distinguish these patients from those with bacterial meningitis. Definitive diagnosis of arboviral meningoencephalitis is based on demonstration of viral-specific IgM in CSF or seroconversion. The prevalence of CSF IgM increases progressively during the first week after infection, peaking at >80% in patients with neuroinvasive disease; as a result, repeat studies may be needed when disease suspicion is high and an early study is negative. CSF PCR tests are available for some viruses in selected diagnostic laboratories and at the Centers for Disease Control and Prevention (CDC), but in the case of WNV, sensitivity (~70%) of CSF PCR is less than that of CSF serology. WNV CSF PCR may be useful in immunocompromised patients who may have absent or reduced antibody responses.
HSV meningitis (Chap. 216) has been increasingly recognized as a major cause of viral meningitis in adults, and overall, it is probably second in importance to enteroviruses as a cause of viral meningitis, accounting for 5% of total cases overall and undoubtedly a higher frequency of those cases occurring in adults and/or outside of the summer-fall period when enterovirus infections are increasingly common. In adults, the majority of cases of uncomplicated meningitis are due to HSV-2, whereas HSV-1 is responsible for 90% of cases of HSV encephalitis. HSV meningitis occurs in ~25–35% of women and ~10–15% of men at the time of an initial (primary) episode of genital herpes. Of these patients, 20% go on to have recurrent attacks of meningitis. Diagnosis of HSV meningitis is usually by HSV CSF PCR because cultures may be negative, especially in patients with recurrent meningitis. Demonstration of intrathecal synthesis of HSV-specific antibody may also be useful in diagnosis, although antibody tests are less sensitive and less specific than PCR and may not become positive until after the first week of infection. Although a history of or the presence of HSV genital lesions is an important diagnostic clue, many patients with HSV meningitis give no history and have no evidence of active genital herpes at the time of presentation. Most cases of recurrent viral or “aseptic” meningitis, including cases previously diagnosed as Mollaret’s meningitis, are due to HSV.
VZV meningitis should be suspected in the presence of concurrent chickenpox or shingles. However, it is important to recognize that VZV is being increasingly identified as an important cause of both meningitis and encephalitis in patients without rash. The frequency of VZV as a cause of meningitis is extremely variable, ranging from as low as 3% to as high as 20% in different series. Diagnosis is usually based on CSF PCR, although the sensitivity of this test may not be as high as for the other herpesviruses. VZV serologic studies complement PCR testing, and the diagnosis of VZV CNS infection can be made by the demonstration of VZV-specific intrathecal antibody synthesis and/or the presence of VZV CSF IgM antibodies, or by positive CSF cultures.
EBV infections may also produce aseptic meningitis, with or without associated infectious mononucleosis. The presence of atypical lymphocytes in the CSF or peripheral blood is suggestive of EBV infection but may occasionally be seen with other viral infections. EBV is almost never cultured from CSF. Serum and CSF serology can help establish the presence of acute infection, which is characterized by IgM viral capsid antibodies (VCAs), antibodies to early antigens (EAs), and the absence of antibodies to EBV-associated nuclear antigen (EBNA). CSF PCR is another important diagnostic test, although false-positive results may reflect viral reactivation associated with other infectious or inflammatory processes or the presence of latent viral DNA in lymphocytes recruited due to other inflammatory conditions.
HIV meningitis should be suspected in any patient presenting with a viral meningitis with known or suspected risk factors for HIV infection. Meningitis may occur following primary infection with HIV in 5–10% of cases and less commonly at later stages of illness. Cranial nerve palsies, most commonly involving cranial nerves V, VII, or VIII, are more common in HIV meningitis than in other viral infections. Diagnosis can be confirmed by detection of HIV genome in blood or CSF. Seroconversion may be delayed, and patients with negative HIV serologies who are suspected of having HIV meningitis should be monitored for delayed seroconversion. For further discussion of HIV infection, see Chap. 226.
Mumps (Chap. 231e) should be considered when meningitis occurs in the late winter or early spring, especially in males (male-to-female ratio 3:1). With the widespread use of the live attenuated mumps vaccine in the United States since 1967, the incidence of mumps meningitis has fallen by >95%; however, mumps remains a potential source of infection in nonimmunized individuals and populations. Rare cases (10–100:100,000 vaccinated individuals) of vaccine-associated mumps meningitis have been described, with onset typically 2–4 weeks after vaccination. The presence of parotitis, orchitis, oophoritis, pancreatitis, or elevations in serum lipase and amylase is suggestive of mumps meningitis; however, their absence does not exclude the diagnosis. Clinical meningitis was previously estimated to occur in 10–30% of patients with mumps parotitis; however, in a recent U.S. outbreak of nearly 2600 cases of mumps, only 11 cases of meningitis were identified, suggesting the incidence may be lower than previously suspected. Mumps infection confers lifelong immunity, so a documented history of previous infection excludes this diagnosis. Patients with meningitis have a CSF pleocytosis that can exceed 1000 cells/μL in 25%. Lymphocytes predominate in 75%, although CSF neutrophilia occurs in 25%. Hypoglycorrhachia, occurs in 10–30% of patients and may be a clue to the diagnosis when present. Diagnosis is typically made by culture of virus from CSF or by detecting IgM antibodies or seroconversion. CSF PCR is available in some diagnostic and research laboratories.
LCMV infection (Chap. 233) should be considered when aseptic meningitis occurs in the late fall or winter and in individuals with a history of exposure to house mice (Mus musculus), pet or laboratory rodents (e.g., hamsters, rats, mice), or their excreta. Some patients have an associated rash, pulmonary infiltrates, alopecia, parotitis, orchitis, or myopericarditis. Laboratory clues to the diagnosis of LCMV, in addition to the clinical findings noted above, may include the presence of leukopenia, thrombocytopenia, or abnormal liver function tests. Some cases present with a marked CSF pleocytosis (>1000 cells/μL) and hypoglycorrhachia (<30%). Diagnosis is based on serology and/or culture of virus from CSF.
|
TREATMENT |
ACUTE VIRAL MENINGITIS |
Treatment of almost all cases of viral meningitis is primarily symptomatic and includes use of analgesics, antipyretics, and antiemetics. Fluid and electrolyte status should be monitored. Patients with suspected bacterial meningitis should receive appropriate empirical therapy pending culture results (see above). Hospitalization may not be required in immunocompetent patients with presumed viral meningitis and no focal signs or symptoms, no significant alteration in consciousness, and a classic CSF profile (lymphocytic pleocytosis, normal glucose, negative Gram’s stain) if adequate provision for monitoring at home and medical follow-up can be ensured. Immunocompromised patients; patients with significant alteration in consciousness, seizures, or the presence of focal signs and symptoms suggesting the possibility of encephalitis or parenchymal brain involvement; and patients who have an atypical CSF profile should be hospitalized. Oral or intravenous acyclovir may be of benefit in patients with meningitis caused by HSV-1 or -2 and in cases of severe EBV or VZV infection. Data concerning treatment of HSV, EBV, and VZV meningitis are extremely limited. Seriously ill patients should probably receive intravenous acyclovir (15–30 mg/kg per day in three divided doses), which can be followed by an oral drug such as acyclovir (800 mg five times daily), famciclovir (500 mg tid), or valacyclovir (1000 mg tid) for a total course of 7–14 days. Patients who are less ill can be treated with oral drugs alone. Patients with HIV meningitis should receive highly active antiretroviral therapy (Chap. 226). There is no specific therapy of proven benefit for patients with arboviral encephalitis, including that caused by WNV.
Patients with viral meningitis who are known to have deficient humoral immunity (e.g., X-linked agammaglobulinemia) and who are not already receiving either intramuscular gamma globulin or intravenous immunoglobulin (IVIg) should be treated with these agents. Intraventricular administration of immunoglobulin through an Ommaya reservoir has been tried in some patients with chronic enteroviral meningitis who have not responded to intramuscular or intravenous immunoglobulin.
Vaccination is an effective method of preventing the development of meningitis and other neurologic complications associated with poliovirus, mumps, measles, rubella, and varicella infection. A live attenuated VZV vaccine (Varivax) is available in the United States. Clinical studies indicate an effectiveness rate of 70–90% for this vaccine, but a booster may be required after ~10 years to maintain immunity. A related vaccine (Zostavax) is recommended for prevention of herpes zoster (shingles) in adults over the age of 60. An inactivated varicella vaccine is available for transplant recipients and others for whom live viral vaccines are contraindicated
PROGNOSIS
In adults, the prognosis for full recovery from viral meningitis is excellent. Rare patients complain of persisting headache, mild mental impairment, incoordination, or generalized asthenia for weeks to months. The outcome in infants and neonates (<1 year) is less certain; intellectual impairment, learning disabilities, hearing loss, and other lasting sequelae have been reported in some studies.
VIRAL ENCEPHALITIS
DEFINITION
In contrast to viral meningitis, where the infectious process and associated inflammatory response are limited largely to the meninges, in encephalitis the brain parenchyma is also involved. Many patients with encephalitis also have evidence of associated meningitis (meningoencephalitis) and, in some cases, involvement of the spinal cord or nerve roots (encephalomyelitis, encephalomyeloradiculitis).
CLINICAL MANIFESTATIONS
In addition to the acute febrile illness with evidence of meningeal involvement characteristic of meningitis, the patient with encephalitis commonly has an altered level of consciousness (confusion, behavioral abnormalities), or a depressed level of consciousness ranging from mild lethargy to coma, and evidence of either focal or diffuse neurologic signs and symptoms. Patients with encephalitis may have hallucinations, agitation, personality change, behavioral disorders, and, at times, a frankly psychotic state. Focal or generalized seizures occur in many patients with encephalitis. Virtually every possible type of focal neurologic disturbance has been reported in viral encephalitis; the signs and symptoms reflect the sites of infection and inflammation. The most commonly encountered focal findings are aphasia, ataxia, upper or lower motor neuron patterns of weakness, involuntary movements (e.g., myoclonic jerks, tremor), and cranial nerve deficits (e.g., ocular palsies, facial weakness). Involvement of the hypothalamic-pituitary axis may result in temperature dysregulation, diabetes insipidus, or the development of the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Even though neurotropic viruses typically cause pathologic injury in distinct regions of the CNS, variations in clinical presentations make it impossible to reliably establish the etiology of a specific case of encephalitis on clinical grounds alone (see “Differential Diagnosis,” below).
ETIOLOGY
In the United States, there are an estimated ~20,000 cases of encephalitis per year, although the actual number of cases is likely to be significantly larger. Despite comprehensive diagnostic efforts, the majority of cases of acute encephalitis of suspected viral etiology remain of unknown cause. Hundreds of viruses are capable of causing encephalitis, although only a limited subset is responsible for most cases in which a specific cause is identified (Table 164-4). The most commonly identified viruses causing sporadic cases of acute encephalitis in immunocompetent adults are herpesviruses (HSV, VZV, EBV). Epidemics of encephalitis are caused by arboviruses, which belong to several different viral taxonomic groups including Alphaviruses (e.g., EEE virus, western equine encephalitis virus), Flaviviruses (e.g., WNV, St. Louis encephalitis virus, Japanese encephalitis virus, Powassan virus), and Bunyaviruses (e.g., California encephalitis virus serogroup, La Crosse virus). Historically, the largest number of cases of arbovirus encephalitis in the United States has been due to St. Louis encephalitis virus and the California encephalitis virus serogroup. However, since 2002, WNV has been responsible for the majority of arbovirus meningitis and encephalitis cases in the United States. WNV caused 2873 confirmed cases of neuroinvasive disease (encephalitis, meningitis, or myelitis) in 2012 with 286 deaths. States reporting >200 cases included Texas (1868 cases), California (479), Louisiana (335), Illinois (290), Mississippi (249), South Dakota (203), and Michigan (202). In 2013, there were 1140 neuroinvasive cases with 100 deaths. States reporting >100 cases included California (357 cases), Colorado (315), Nebraska (213), Texas (157), South Dakota (148), North Dakota (123), and Illinois (106). It is important to recognize that WNV epidemics are unpredictable and that cases have occurred in every state in the continental United States. New causes of viral CNS infections are constantly appearing, as evidenced by the outbreak of cases of encephalitis in Southeast Asia caused by Nipah virus, a newly identified member of the Paramyxoviridae family; of meningitis in Europe caused by Toscana virus, an arbovirus belonging to the Bunyavirus family; and of neurologic disorders associated with major epidemics of Chikungunya virus, a togavirus, in Africa, India, and Southeast Asia. Parechoviruses including human parechovirus 3 (HPeV3), members of the Picornavirus family, have recently been reported as causes of fever, sepsis, and meningitis in infants (age <3 months) in the United States and abroad.
LABORATORY DIAGNOSIS
CSF Examination CSF examination should be performed in all patients with suspected viral encephalitis unless contraindicated by the presence of severely increased ICP. Ideally at least 20 mL should be collected with 5–10 mL stored frozen for later studies as needed. The characteristic CSF profile is indistinguishable from that of viral meningitis and typically consists of a lymphocytic pleocytosis, a mildly elevated protein concentration, and a normal glucose concentration. A CSF pleocytosis (>5 cells/μL) occurs in >95% of immunocompetent patients with documented viral encephalitis. In rare cases, a pleocytosis may be absent on the initial LP but present on subsequent LPs. Patients who are severely immunocompromised by HIV infection, glucocorticoid or other immunosuppressant drugs, chemotherapy, or lymphoreticular malignancies may fail to mount a CSF inflammatory response. CSF cell counts exceed 500/μL in only about 10% of patients with encephalitis. Infections with certain arboviruses (e.g., EEE virus or California encephalitis virus), mumps, and LCMV may occasionally result in cell counts >1000/μL, but this degree of pleocytosis should suggest the possibility of nonviral infections or other inflammatory processes. Atypical lymphocytes in the CSF may be seen in EBV infection and less commonly with other viruses, including CMV, HSV, and enteroviruses. Increased numbers of plasmacytoid or Mollaret-like large mononuclear cells have been reported in WNV encephalitis. Polymorphonuclear pleocytosis occurs in ~45% of patients with WNV encephalitis and is also a common feature in CMV myeloradiculitis in immunocompromised patients. Large numbers of CSF PMNs may be present in patients with encephalitis due to EEE virus, echovirus 9, and, more rarely, other enteroviruses. However, persisting CSF neutrophilia should prompt consideration of bacterial infection, leptospirosis, amebic infection, and noninfectious processes such as acute hemorrhagic leukoencephalitis. About 20% of patients with encephalitis will have a significant number of red blood cells (>500/μL) in the CSF in a nontraumatic tap. The pathologic correlate of this finding may be a hemorrhagic encephalitis of the type seen with HSV; however, CSF red blood cells occur with similar frequency and in similar numbers in patients with nonherpetic focal encephalitides. A decreased CSF glucose concentration is distinctly unusual in viral encephalitis and should suggest the possibility of bacterial, fungal, tuberculous, parasitic, leptospiral, syphilitic, sarcoid, or neoplastic meningitis. Rare patients with mumps, LCMV, or advanced HSV encephalitis and many patients with CMV myeloradiculitis have low CSF glucose concentrations.
CSF PCR
CSF PCR has become the primary diagnostic test for CNS infections caused by CMV, EBV, HHV-6, and enteroviruses (see “Viral Meningitis,” above). In the case of VZV CNS infection, CSF PCR and detection of virus-specific IgM or intrathecal antibody synthesis both provide important aids to diagnosis. The sensitivity and specificity of CSF PCRs vary with the virus being tested. The sensitivity (~96%) and specificity (~99%) of HSV CSF PCR are equivalent to or exceed those of brain biopsy. It is important to recognize that HSV CSF PCR results need to be interpreted after considering the likelihood of disease in the patient being tested, the timing of the test in relationship to onset of symptoms, and the prior use of antiviral therapy. A negative HSV CSF PCR test performed by a qualified laboratory at the appropriate time during illness in a patient with a high likelihood of HSV encephalitis based on clinical and laboratory abnormalities significantly reduces the likelihood of HSV encephalitis but does not exclude it. For example, in a patient with a pretest probability of 35% of having HSV encephalitis, a negative HSV CSF PCR reduces the posttest probability to ~2%, and for a patient with a pretest probability of 60%, a negative test reduces the posttest probability to ~6%. In both situations, a positive test makes the diagnosis almost certain (98–99%). There have been several recent reports of initially negative HSV CSF PCR tests that were obtained early (≤72 h) following symptom onset and that became positive when repeated 1–3 days later. The frequency of positive HSV CSF PCRs in patients with herpes encephalitis also decreases as a function of the duration of illness, with only ~20% of cases remaining positive after ≥14 days. PCR results are generally not affected by ≤1 week of antiviral therapy. In one study, 98% of CSF specimens remained PCR-positive during the first week of initiation of antiviral therapy, but the numbers fell to ~50% by 8–14 days and to ~21% by >15 days after initiation of antiviral therapy.
The sensitivity and specificity of CSF PCR tests for viruses other than HSV have not been definitively characterized. Enteroviral (EV) CSF PCR appears to have a sensitivity and specificity of >95%. EV PCR sensitivity for EV71 may be considerably lower (~30% in some reports). Parechoviruses are also not detected by standard EV RT-PCRs. The specificity of EBV CSF PCR has not been established. Positive EBV CSF PCRs associated with positive tests for other pathogens have been reported and may reflect reactivation of EBV latent in lymphocytes that enter the CNS as a result of an unrelated infectious or inflammatory process. In patients with CNS infection due to VZV, CSF antibody and PCR studies should be considered complementary, because patients may have evidence of intrathecal synthesis of VZV-specific antibodies and negative CSF PCRs. In the case of WNV infection, CSF PCR appears to be less sensitive (~70% sensitivity) than detection of WNV-specific CSF IgM, although PCR testing remains useful in immunocompromised patients who may not mount an effective anti-WNV antibody response.
CSF Culture CSF culture is generally of limited utility in the diagnosis of acute viral encephalitis. Culture may be insensitive (e.g., >95% of patients with HSV encephalitis have negative CSF cultures as do virtually all patients with EBV-associated CNS disease) and often takes too long to significantly affect immediate therapy.
Serologic Studies and Antigen Detection The basic approach to the serodiagnosis of viral encephalitis is identical to that discussed earlier for viral meningitis. Demonstration of WNV IgM antibodies is diagnostic of WNV encephalitis because IgM antibodies do not cross the blood-brain barrier, and their presence in CSF is therefore indicative of intrathecal synthesis. Timing of antibody collection may be important because the rate of CSF WNV IgM seropositivity increases by ~10% per day during the first week after illness onset, reaching 80% or higher on day 7 after symptom onset. In patients with HSV encephalitis, both antibodies to HSV-1 glycoproteins and glycoprotein antigens have been detected in the CSF. Optimal detection of both HSV antibodies and antigen typically occurs after the first week of illness, limiting the utility of these tests in acute diagnosis. Nonetheless, HSV CSF antibody testing is of value in selected patients whose illness is >1 week in duration and who are CSF PCR–negative for HSV. In the case of VZV infection, CSF antibody tests may be positive when PCR fails to detect viral DNA, and both tests should be considered complementary rather than mutually exclusive.
MRI, CT, and EEG Patients with suspected encephalitis almost invariably undergo neuroimaging studies and often EEG. These tests help identify or exclude alternative diagnoses and assist in the differentiation between a focal, as opposed to a diffuse, encephalitic process. Focal findings in a patient with encephalitis should always raise the possibility of HSV encephalitis. Examples of focal findings include: (1) areas of increased signal intensity in the frontotemporal, cingulate, or insular regions of the brain on T2-weighted, FLAIR, or diffusion-weighted MRI (Fig. 164-3); (2) focal areas of low absorption, mass effect, and contrast enhancement on CT; or (3) periodic focal temporal lobe spikes on a background of slow or low-amplitude (“flattened”) activity on EEG. Approximately 10% of patients with PCR-documented HSV encephalitis will have a normal MRI, although nearly 80% will have abnormalities in the temporal lobe, and an additional 10% in extratemporal regions. The lesions are typically hyperintense on T2-weighted images. The addition of FLAIR and diffusion-weighted images to the standard MRI sequences enhances sensitivity. Children with HSV encephalitis may have atypical patterns of MRI lesions and often show involvement of brain regions outside the frontotemporal areas. CT is less sensitive than MRI and is normal in up to 20–35% of patients. EEG abnormalities occur in >75% of PCR-documented cases of HSV encephalitis; they typically involve the temporal lobes but are often nonspecific. Some patients with HSV encephalitis have a distinctive EEG pattern consisting of periodic, stereotyped, sharp-and-slow complexes originating in one or both temporal lobes and repeating at regular intervals of 2–3 s. The periodic complexes are typically noted between days 2 and 15 of the illness and are present in two-thirds of pathologically proven cases of HSV encephalitis.