36 Seizures in the Critically Ill
Seizures complicate the course of about 3% of adult intensive care unit (ICU) patients admitted for non-neurologic conditions.1 The medical and economic impact of these seizures confers importance on them out of proportion to their incidence. A seizure is often the first indication of a central nervous system (CNS) complication, and delay in recognition and treatment of seizure is associated with an increased risk of mortality2; thus, rapid diagnosis of this disorder is mandatory. In addition, since epilepsy affects 2% of the population, patients with preexisting seizures occasionally enter the ICU for treatment of other problems. Because initial treatment of these patients is the province of the intensivist, he or she must be familiar with seizure management as it affects the critically ill patient. Patients developing status epilepticus often require a critical care specialist in addition to a neurologist.
Seizures have been recognized at least since Hippocratic times, but their relatively high rate of occurrence in critically ill patients has only recently been appreciated. Seizures complicating critical care treatments (e.g., lidocaine use) are also a recent phenomenon. Early attempts at treatment included bromides3 and morphine as well as ice applications. Barbiturates were first employed in 1912, and phenytoin in 1937.4 Paraldehyde was popular in the next 2 decades.5 More recently, emphasis has shifted to the benzodiazepines, which were pioneered in the 1960s.6 Newer agents for treatment of seizures in critically ill patients include the phenytoin prodrug, fosphenytoin; the anesthetic agent, propofol; and the water-soluble benzodiazepine, midazolam.
Status epilepticus refers to prolonged seizure episodes; it may be the primary indication for admission to the ICU, or it may occur in any ICU patient with CNS disease. The definitions employed in studies of status epilepticus have varied substantially. Although conventional definitions of status epilepticus have used a cutoff of 30 or 60 minutes of sustained seizure duration, or discrete seizures without recovery, clinicians should recognize that most seizures terminate spontaneously within a few minutes. Recent data suggest that in only half of patients with seizure episodes lasting 10 to 29 minutes will the seizure self-terminate.7 Therefore, seizures that persist longer than 5 to 7 minutes should probably be treated as status epilepticus.8
 Epidemiology
Epidemiology
Limited data are available on the epidemiology of seizures in the ICU. A 10-year retrospective study of all ICU patients with seizures at the Mayo Clinic revealed that 7 patients had seizures per 1000 ICU admissions.9 Our 2-year prospective study of medical ICU patients identified 35 with seizures per 1000 admissions.1 These two studies are not exactly comparable, as the patient populations and methods of detection differed. A recent series found 8% of comatose patients without clinical signs of seizure activity to be in electrographic status epilepticus.10
Up to 34% of hospital in-patients experiencing a seizure die during their hospitalization.1 Our prospective study of neurologic complications in medical ICU patients showed that having even one seizure while in the ICU for a non-neurologic reason doubled in-hospital mortality.10 Incidence estimates for generalized convulsive status epilepticus in the United States vary from 50,000 cases per year11 to 195,000 cases per year.12 Some portion of this difference can be accounted for by different definitions; however, the latter estimate represents the only population-based data available and may be more accurate. Mortality estimates similarly vary from 1% to 2% in the former study to 22% in the latter. This disagreement follows from a conceptual discordance: the smaller number describes mortality the authors directly attribute to status epilepticus, whereas the larger figure estimates the overall mortality rate, even though death was frequently caused by the underlying disease rather than by status epilepticus itself. The elderly have an incidence of status epilepticus almost twice that of the general population and the highest associated mortality rate of any age group at 38%.13
Table 36-1 summarizes the most common causes of status epilepticus in adults in the community. Almost 50% of the cases were attributed to cerebral vascular disease.11 Garzon and colleagues14 found antiepileptic drug noncompliance as the main cause of status epilepticus in patients with a prior history of epilepsy, and CNS infection, stroke, and metabolic disturbances predominated in the group without previous seizures.
TABLE36-1 Causes of Status Epilepticus in Adults Presenting from the Community
| Prior Seizures | No Prior Seizures |
|---|---|
| Common | |
| Subtherapeutic anticonvulsant | Ethanol-related |
| Ethanol-related | Drug toxicity |
| Intractable epilepsy | CNS infection |
| Head trauma | |
| CNS tumor | |
| Less Common | |
| CNS infection | Metabolic aberration |
| Metabolic aberration | Stroke |
| Drug toxicity | |
| Stroke | |
| CNS tumor | |
| Head trauma | |
CNS, central nervous system.
Three major factors determine outcome in patients with status epilepticus: the type of status epilepticus, its cause, and its duration. Generalized convulsive status epilepticus has the worst prognosis for neurologic recovery; myoclonic status epilepticus following an anoxic episode carries a very poor prognosis for survival. Complex partial status epilepticus can produce limbic system damage, usually manifested as a memory disturbance. Causes associated with increased mortality included anoxia, intracranial hemorrhages, tumors, infections, and trauma. The mortality of patients with nonconvulsive status epilepticus has been reported as high as 33%15 and correlates with the underlying cause, severe impairment of mental status, and the development of acute complications, especially respiratory failure and infection.16 Data strongly suggest that prolonged seizure duration is a negative prognostic factor. A study of 253 adult status epilepticus patients demonstrated a 30-day mortality rate of 2.7% in patients with seizures lasting 30 to 59 minutes, compared with 32% in those with seizures of 60 minutes or longer.17
Limited data are available concerning the functional abilities of generalized convulsive status epilepticus survivors, and no data reliably permit a distinction between the effects of status epilepticus and effects of its causes. One review concluded that intellectual ability declined as a consequence of status epilepticus.18 Survivors of status epilepticus frequently seem to have memory and behavioral disorders out of proportion to the structural damage produced by the cause of their seizures. Case reports of severe memory deficits following prolonged complex partial status epilepticus have been published.19 Conversely, one prospective study of 180 children with febrile status epilepticus demonstrated no deaths and no cases of new cognitive or motor handicap.20 Experimental animal21 and human epidemiologic22 studies suggest that status epilepticus may be a risk factor in the development of future seizures. Whether treatment of prolonged seizures reduces the risk of subsequent epilepsy remains uncertain.
 Classification
Classification
The most frequently used classification scheme is that of the International League Against Epilepsy (Box 36-1).23 This scheme allows classification on clinical criteria without inferring cause. Simple partial seizures start focally in the cerebral cortex without invading other structures. The patient is aware throughout the episode and appears otherwise unchanged. Bilateral limbic dysfunction produces a complex partial seizure; awareness and ability to interact are diminished (but may not be completely abolished). Automatisms (movements a patient makes without awareness) may occur. Secondary generalization results from invasion by epileptic electrical activity of the other hemisphere or subcortical structures.
Box 36-1
International Classification of Epileptic Seizures
Adapted from Bleck TP. Status epilepticus. In: Klawans HL, Goetz CG, Tanner CM, editors. Textbook of clinical neuropharmacology. 2nd ed. New York: Raven Press; 1992, p. 65-73.
Primary generalized seizures arise from the cerebral cortex and diencephalon at the same time; no focal phenomena are visible, and consciousness is lost at the onset. Absence seizures are frequently confined to childhood; they consist of the abrupt onset of a blank stare that usually lasts 5 to 15 seconds, after which the patient abruptly returns to normal. Atypical absence seizures occur in children with the Lennox-Gastaut syndrome. Myoclonic seizures start with brief synchronous jerks without alteration of consciousness, initially followed by a generalized convulsion. They frequently occur in patients with genetic epilepsy; in the ICU, they commonly follow anoxia or metabolic disturbances.24 Tonic-clonic seizures start with tonic extension, evolve to bilaterally synchronous clonus, and conclude with a postictal phase. Clinical judgment is required to apply this system in the ICU. In patients in whom consciousness has already been altered by drugs, hypotension, sepsis, or intracranial pathologic lesion, the nature of partial seizures may be difficult to classify.
Status epilepticus is classified by a similar system that has been altered to match observable clinical phenomena (Box 36-2).25 Generalized convulsive status epilepticus is the most common type encountered in the ICU and poses the greatest risk to the patient. It may either be primarily generalized, as in the drug-intoxicated patient, or secondarily generalized, as in the brain abscess patient who develops generalized convulsive status epilepticus. Nonconvulsive status epilepticus in the ICU frequently follows partially treated generalized convulsive status epilepticus. Some practitioners use the term for all cases of status epilepticus that involve altered consciousness without convulsive movements; this blurs the distinctions among absence status epilepticus, partially treated generalized convulsive status epilepticus, and complex partial status epilepticus, which have different causes and treatments. Epilepsia partialis continua (a special form of partial status epilepticus in which repetitive movements affect a small area of the body) sometimes continues for months or years.
Box 36-2
Clinical Classification of Status Epilepticus
Adapted from Lothman EW. The biochemical basis and pathophysiology of status epilepticus. Neurology 1990;40:13-23.
The International League Against Epilepsy continues to work toward revising and updating the current classification system. The goal is a multi-axis diagnostic scheme that incorporates anatomic, etiologic, therapeutic, and prognostic implications. For the most recent information regarding this ongoing project, refer to www.epilepsy.org.26
 Pathogenesis and Pathophysiology
Pathogenesis and Pathophysiology
The cellular effects of excessive excitatory amino acid channel activity include (1) generation of toxic concentrations of intracellular free calcium; (2) activation of autolytic enzyme systems; (3) production of oxygen free radicals; (4) generation of nitric oxide, which both enhances subsequent excitation and serves as a toxin; (5) phosphorylation of enzyme and receptor systems, making seizures more likely; and (6) an increase in intracellular osmolality, which produces neuronal swelling. If adenosine triphosphate production fails, membrane ion exchange ceases, and neurons swell further. These events produce the neuronal damage associated with status epilepticus. Longer status epilepticus duration produces more profound alterations and an increasing likelihood of permanence and of becoming refractory to treatment.27 The processes involved in a single seizure and the transition to status epilepticus have been reviewed.28
Many other biophysical and biochemical alterations occur during and after status epilepticus. The intense neuronal activity activates immediate-early genes and produces heat shock proteins, providing indications of the deleterious effects of status epilepticus and insight into the mechanisms of neuronal protection.29 The mechanisms by which status epilepticus damages the nervous system have been reviewed.30 Absence status epilepticus is an exception among these conditions; it consists of rhythmically increased inhibition and does not produce clinical or pathologic abnormalities.
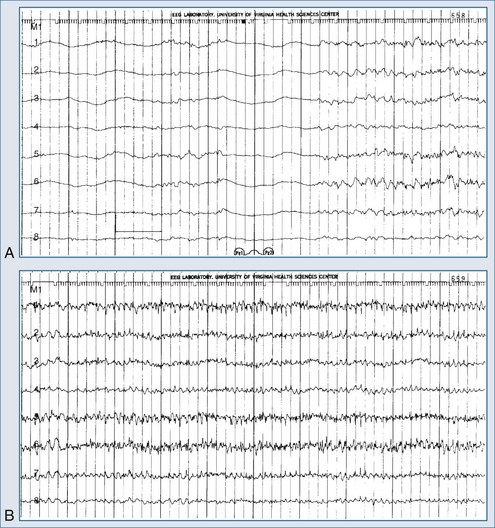
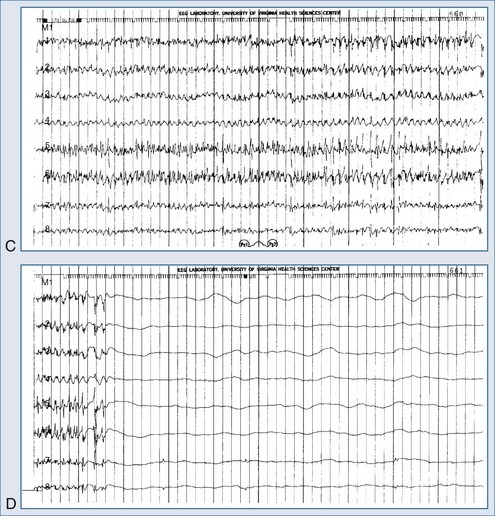
The electrical phenomena of status epilepticus at the whole brain level, as seen in the scalp electroencephalogram (EEG), reflect the seizure type that initiates status epilepticus (e.g., absence status epilepticus begins with a 3-Hz wave-and-spike pattern). During status epilepticus, this rhythm slows, but the wave-and-spike characteristic remains. Generalized convulsive status epilepticus goes through a sequence of electrographic changes (Table 36-2).31 The initial discharge becomes less well formed, implying that neuronal firing loses synchrony. The sustained depolarizations that characterize status epilepticus alter the extracellular milieu, most importantly by raising extracellular potassium. The excess potassium ejected during status epilepticus exceeds the buffering ability of astrocytes.
TABLE36-2 Electrographic-Clinical Correlations in Generalized Convulsive Status Epilepticus
| Stage | Typical Clinical Manifestations* | Electroencephalographic Features |
|---|---|---|
| 1 | Tonic-clonic convulsions; hypertension and hyperglycemia common | Discrete seizures with interictal slowing |
| 2 | Low or medium amplitude clonic activity, with rare convulsions | Waxing and waning of ictal discharges |
| 3 | Slight but frequent clonic activity, often confined to the eyes, face, or hands | Continuous ictal discharges |
| 4 | Rare episodes of slight clonic activity; hypotension and hypoglycemia become manifest | Continuous ictal discharges punctuated by flat periods |
| 5 | Coma without other manifestations of seizure activity | Periodic epileptiform discharges on a flat background |
* Clinical manifestations may vary considerably depending on the underlying neuropathophysiologic process (and its anatomy), systemic diseases, and medications. In particular, stages of the electrographic progression may be sufficiently brief to be overlooked. Partially treating status epilepticus may dissociate the clinical and electrographic features.
Data from Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia 1993;34: S2-11.
The increased cellular activity of status epilepticus elevates demand for oxygen and glucose, and cerebral blood flow initially increases. After approximately 20 minutes, however, energy supplies are exhausted, causing local catabolism to support ion pumps (in an attempt to restore the internal milieu); this is a major cause of epileptic brain damage. In addition to damaging the CNS, generalized convulsive status epilepticus produces life-threatening systemic effects.32 Excess secretion of epinephrine and cortisol cause systemic and pulmonary arterial pressures to rise dramatically at seizure onset and also produce hyperglycemia. Muscular work raises blood lactate levels. Both airway obstruction and abnormal diaphragmatic contractions impair respiration. Carbon dioxide excretion falls while its production increases markedly. Muscular work accelerates heat production, raising core body temperature.
 Clinical Manifestations
Clinical Manifestations
Three problems complicate seizure recognition: (1) the occurrence of complex partial seizures in the setting of impaired awareness, (2) the occurrence of seizures in patients receiving pharmacologically induced paralysis and/or sedation, and (3) misinterpretation of other abnormal movements as seizures. ICU patients often have depressed consciousness in the absence of seizures owing to their disease, its complications (such as hepatic33 or septic34 encephalopathy), or drug administration. A further decline in alertness may reflect a seizure; an EEG is required to confirm that one has occurred.
Patients with metabolic disturbances, anoxia, and other types of nervous system injury may demonstrate abnormal movements that can be confused with seizure. Asterixis is a brief asynchronous loss of tone at the wrist or hip joints that can appear in the setting of hepatic dysfunction. Stimulus-sensitive massive myoclonus after anoxia can be dramatic but usually self-abates in a few days. Controversy exists as to the epileptic origin of this disorder, and postanoxic myoclonus has been reported in the presence of almost total cortical suppression.35 Brain-injured patients may manifest paroxysmal episodes of sympathetic hyperactivity and associated rigidity or decerebrate posturing. These “hypothalamic seizures” can sometimes be distinguished from epileptic seizures with observation. Patients with tetanus are awake during their spasms and flex rather than extend their arms as seizure patients do. Psychiatric disturbances in the ICU occasionally resemble complex partial seizures. If doubt about the nature of abnormal movements persists, an EEG should be obtained.
The manifestations of status epilepticus depend on the type and, for partial status epilepticus, the cortical area of abnormality. Box 36-2 presents the types of status epilepticus encountered and focuses on those seen most frequently in the ICU.
Failure to recognize nonconvulsive status epilepticus is common in patients presenting with nonspecific neurobehavioral abnormalities such as delirium, lethargy, bizarre behavior, cataplexy, or mutism.36 Patients may present in nonconvulsive status epilepticus without an inciting episode of generalized convulsive status epilepticus. A high suspicion for this disorder should be maintained in patients with unexplained alteration in level of consciousness or cognition admitted to the ICU.
Partial status epilepticus in ICU patients often follows a stroke or occurs with the rapid expansion of brain masses. Clonic motor activity is most easily recognized, but the seizure takes on the characteristics of adjacent functional tissue. Therefore, somatosensory or special sensory manifestations occur, and the ICU patient may be unable to report such symptoms. Aphasic status epilepticus occurs when a seizure begins in a language area and may resemble a stroke. Epilepsia partialis continua involves repetitive movements confined to a small region of the body. It may be seen with nonketotic hyperglycemia37 or with focal brain disease; anticonvulsant treatment is seldom useful. Complex partial status epilepticus manifests with diminished awareness. The diagnosis often comes as a surprise when an EEG is obtained.
 Diagnostic Approach
Diagnostic Approach
Seizures in ICU patients have several potential causes that must be investigated. Drugs are a major cause of ICU seizures, especially in the setting of diminished renal or hepatic function or when the blood-brain barrier is breached. Theophylline frequently produces seizures or status epilepticus if it has been rapidly loaded or if high concentrations of the drug occur; occasionally, however, these complications arise at “therapeutic” levels. Imipenem-cilastatin38 and fluoroquinolones39 have substantial potential to lower the seizure threshold, especially in patients with renal dysfunction. They should be avoided if possible in patients already at risk for seizure. Other antibiotics, especially β-lactams, are occasionally implicated.40 Sevoflurane, a volatile anesthetic agent, is dose-dependently epileptogenic in patients with no predisposition to seizures.41
Recreational drugs are frequently overlooked offenders in patients presenting to the ICU. Acute cocaine or methamphetamine intoxication is characterized by a state of hypersympathetic activity followed by seizures.42 Although ethanol withdrawal is a common cause of seizures, discontinuing any hypnosedative agent may prompt convulsions 1 to 3 days later. One report suggests that narcotic withdrawal may produce seizures in the critically ill.1 In the absence of other clear causes for seizure, complete toxicologic screening should be performed.
Serum glucose, electrolyte concentrations, and serum osmolality should also be measured. Nonketotic hyperglycemia43,44 and hyponatremia can precipitate both focal and generalized seizures. Seizure activity may infrequently be the first presenting sign of diabetes mellitus. However, hypocalcemia rarely causes seizures beyond the neonatal period; its identification on analysis must not signal the end of the diagnostic workup. Hypomagnesemia has an equally unwarranted reputation as the cause of seizures in malnourished alcoholic patients.
The need for imaging studies in these patients has been an area of uncertainty. A prospective study of neurologic complications in medical ICU patients determined that 38 of 61 patients (62%) had a vascular, infectious, or neoplastic explanation for their seizures.45 Hence, head computed tomography (CT) or magnetic resonance imaging (MRI) should be performed on ICU patients with new seizures. With current technology, there are almost no patients who cannot undergo CT scanning, and MRI is particularly helpful in detecting evidence of acute ischemic stroke and encephalitis. MRI cannot be performed on patients with pacemakers. Many ICP monitor catheters are compatible with MRI provided the device is not coiled when it is secured to the scalp. Patients who need cerebrospinal fluid analysis always require imaging of the brain first. When CNS infection is suspected, empirical antibiotic treatment should be started while these studies are being performed.
Electroencephalography is a vital diagnostic tool for evaluating the seizure patient. Partial seizures usually show EEG abnormalities that begin in the area of cortex that produces seizures. Primary generalized seizures appear to start over the entire cortex simultaneously. Postictal slowing or depressed amplitude provides clues as to the focal cause of the seizures, and epileptiform activity helps classify the type of seizure and guide treatment. An emergency EEG is necessary to exclude nonconvulsive status epilepticus in those patients who do not begin to awaken soon after seizures have apparently been controlled (Figure 36-1).
Treatment for status epilepticus should not be delayed to obtain an EEG. However, a prospective evaluation of 164 patients demonstrated that nearly half manifested persistent electrographic seizures in the 24 hours after clinical control of convulsive status epilepticus.46 These data suggest that EEG monitoring after control of convulsive status epilepticus can be essential in directing the course of treatment. A variety of findings may be present on the EEG, depending on the type of status epilepticus and its duration (see Table 36-2). Complex partial status epilepticus patients are often without such organized discharges of generalized convulsive status epilepticus; instead, they have waxing and waning rhythmic activity in one or several brain regions. A diagnostic trial of intravenous (IV) benzodiazepine therapy is often necessary to diagnose complex partial status epilepticus. Patients developing refractory status epilepticus or having seizures during neuromuscular junction blockade require continuous EEG monitoring.
The availability of continuous paperless EEG monitoring allows for detection of seizure activity over a long period.47 Subclinical seizures have been observed to occur in patients receiving aggressive treatment for status epilepticus and even in patients treated with barbiturates to a burst-suppression EEG pattern. The clinical significance of these subclinical seizures, and their effect on prognosis, remains uncertain.
 Management Approach
Management Approach
Treating Isolated Seizures
Making the decision to administer anticonvulsants to an ICU patient who experiences one or a few seizures requires consideration of a provisional cause, estimation of the likelihood of recurrence, and recognition of the utility and limitations of anticonvulsants. For example, the occurrence of seizures during ethanol withdrawal does not indicate the need for chronic treatment, and giving phenytoin does not prevent further withdrawal convulsions. The patient may need prophylaxis against delirium tremens, but the few seizures themselves seldom require treatment. Patients with convulsions during barbiturate or benzodiazepine withdrawal, in contrast, should usually receive short-term treatment with lorazepam to prevent status epilepticus. Prolonged or frequent seizures caused by metabolic disturbances can be treated temporarily with benzodiazepines while the abnormality is being corrected. Seizures in these settings are notoriously resistant to treatment with phenytoin. In particular, treatment of patients with partial seizures related to nonketotic hyperglycemia should be directed at correction of the hyperglycemia and hypovolemia rather than anticonvulsant therapy.44
The ICU patient with CNS disease who has even one seizure should be given chronic anticonvulsant therapy, and this approach should be reviewed before the patient is discharged. Initiating this treatment after the first unprovoked seizure may help prevent subsequent epilepsy,48 although there is considerable difference of opinion regarding this concept.49 Starting therapy after the first seizure in a critically ill patient at risk for seizure recurrence may be even more important, especially if the patient’s condition would be seriously complicated by a convulsion.
In the ICU setting, phenytoin is frequently selected for prophylaxis or prevention of subsequent seizures, owing to its ease of administration and lack of sedative effects. Hypotension and arrhythmias may complicate IV administration and can usually be prevented by slowing the infusion to less than 25 mg/min. Because of the rare occurrence of third-degree atrioventricular block, an external cardiac pacemaker should be available when patients with conduction abnormalities receive IV phenytoin. The parenteral formulation of phenytoin is very alkaline, and this contributes to pain, burning, and redness at the injection site.50
The phenytoin prodrug, fosphenytoin, is water soluble, and its vehicle does not contain propylene glycol. Local adverse effects are less common with fosphenytoin than with IV administration of phenytoin, although cardiovascular complications are just as frequent.51,52 Fosphenytoin is dosed by phenytoin-equivalent units; therefore, no dosage adjustments are needed when converting patients from phenytoin to fosphenytoin. Fosphenytoin can be administered by intramuscular injection or by IV infusion at a rate of up to 150 mg phenytoin equivalents/min. Fosphenytoin is rapidly converted to phenytoin in vivo, and free phenytoin levels after fosphenytoin administration are not markedly different compared with phenytoin.
Phenytoin is approximately 90% protein bound in normal hosts. Patients with renal dysfunction have lower total phenytoin levels at a given dose because the drug is displaced from binding sites, but the unbound level is not affected. Thus renal failure patients, and perhaps others who are receiving highly protein-bound drugs (which compete for binding), may benefit from determination of free phenytoin level. Only the free fraction is metabolized, so the dose is not altered with changes in renal function. The clearance half-time with normal liver function varies from about 12 to 20 hours (IV form) to more than 24 hours (extended-release capsules), so a new steady-state serum concentration occurs in 3 to 6 days. Phenytoin need not be given more frequently than every 12 hours. Hepatic dysfunction mandates a decrease in the maintenance dose. Hypersensitivity is the major adverse effect of concern to the intensivist. This may manifest itself solely as fever but may include rash and eosinophilia. Adverse reactions to phenytoin and other anticonvulsants have been reviewed elsewhere.53 Levetiracetam and lacosamide are newer anticonvalsants available for IV use. The appropriate loading and maintenonce doses in critically ill patients remain to be determined.
Treating Status Epilepticus
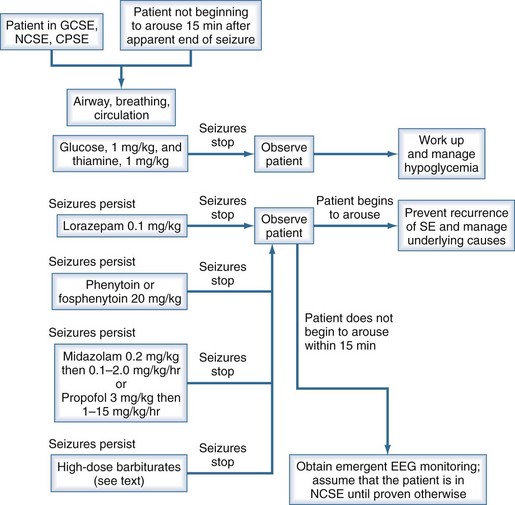
Generalized convulsive status epilepticus obviously constitutes a medical emergency; however, nonconvulsive status epilepticus and complex partial status epilepticus are also emergencies but are more difficult to recognize. In each circumstance, one must act quickly to prevent additional cerebral damage. Figure 36-2 shows a management algorithm for status epilepticus and Box 36-3 presents a sample management protocol for drug administration.54 Patients with simple partial status epilepticus or epilepsia partialis continua are at less risk for the development of widespread cerebral damage and are also less likely to respond to the aggressive approach outlined in Box 36-3. In these patients, correcting underlying problems such as nonketotic hyperosmolar hyperglycemia is crucial. Errors in terminating status epilepticus include inadequate dosing of effective drugs and continued use of drugs that are ineffective in the patient being treated.
Box 36-3
Suggested Protocol for Treating Status Epilepticus
The conventional agents used as first-line treatment of status epilepticus are the benzodiazepines (especially lorazepam, diazepam, and midazolam), phenytoin, and phenobarbital. Status epilepticus that is refractory to the traditional agents is treated with continuous infusions of the short-acting barbiturates, midazolam, or propofol. A major multicenter clinical trial55 that compared lorazepam alone, phenytoin alone, diazepam followed by phenytoin, and phenobarbital alone as initial drug treatment for generalized convulsive status epilepticus showed that the highest rate of successful treatment of “overt” generalized convulsive status epilepticus was achieved with lorazepam. There was no demonstrable difference among these four drug regimens in the initial treatment of “subtle” generalized convulsive status epilepticus. Lorazepam has been our agent of first choice for terminating status epilepticus for many years and remains so with support from this study.
Phenytoin is a less effective agent in the treatment of status epilepticus; in addition, the constraint on the rate of IV administration is of concern. Phenytoin has a long duration of action when an adequate dose is given (a 20 mg/kg dose produces a serum level above 20 µg/mL for 24 hours). Adding 5 mg/kg if the first 20 mg/kg load fails to stop status epilepticus may be useful. Fosphenytoin can be administered by a more rapid IV infusion, but the brain concentration of the phenytoin derived from it does not appear to rise faster than with the native drug. Free phenytoin levels reach a therapeutic range 10 to 20 minutes after an infusion of fosphenytoin is started.56,57 Intramuscular injection of fosphenytoin in patients with status epilepticus should not be considered acceptable therapy and should be reserved for only those rare circumstances in which IV access cannot be obtained.
Some practitioners advocate the use of phenobarbital as a first-line drug,58 but it has typically been used as a third-line agent after administration of a benzodiazepine and phenytoin.59 Although this approach has been widely accepted by the neurologic community, we rarely use phenobarbital for two reasons. First, only a small percentage of patients who have failed treatment with the first anticonvulsant drug respond to a second or third conventional agent60; second, at least an additional 20 minutes are required to obtain control in the few patients who do respond. Phenobarbital remains an important drug in the management of simple partial status epilepticus and for those patients who are being weaned from high-dose midazolam or anesthetic barbiturates.
Pentobarbital and thiopental infusions are usually reserved for refractory status epilepticus.56 Although these drugs are effective in sufficiently large doses, their side effects can limit their use and may be fatal.61 However, they are important when other modalities have failed (see Box 36-3). Endotracheal intubation and mechanical ventilation are mandatory when high-dose barbiturates are used, and both continuous EEG and invasive hemodynamic monitoring are highly recommended. Severe hypotension is the most frequent side effect of pentobarbital therapy, and its occurrence is associated with increased mortality.62 An increased occurrence of nosocomial respiratory tract infection has been reported in patients treated with pentobarbital infusion.63 An inhibitory effect on leukocyte chemotaxis and paralysis of respiratory cilia by the barbiturates have been postulated. Despite these side effects, barbiturate anesthesia should not be rapidly discontinued if it is successful in terminating refractory status epilepticus; rather, continuing therapy for at least 48 hours, gradual tapering of the infusion dose, and the administration of phenobarbital during the drug taper are recommended.64
Midazolam is a water-soluble benzodiazepine that has demonstrated high efficacy in refractory status in adults and children.65,66 At our institution, this agent is used as a second-line drug after lorazepam has failed to control status epilepticus. Clinically significant hypotension is rare even at very high doses that are often required to address tachyphylaxis. Respiratory depression is uncommon after a loading dose but should be anticipated with infusions of any duration. Sedation is quickly reversed after short-term infusions are discontinued. However, terminal half-lives of three to eight times normal have been reported with extended administration.67 In addition, prolonged elimination times have been associated with critical illness and hepatorenal dysfunction. Others have recently discussed its use in this setting.68
Propofol has been reported to be effective in the treatment of refractory status epilepticus, but direct comparisons with other agents have shown mixed results.69,70 It may offer a lower risk of ventilatory depression and promote more rapid awakening compared with other drugs when it is discontinued. Early fears of a possible proconvulsant effect appear to be unfounded, although withdrawal convulsions may occur if the drug is abruptly terminated. A dosage range of 1 to 15 mg/kg/h has been studied,71 although the actual upper limit is not known. Acidosis and oxygenation difficulties have been reported in children.72 Mortality with its use appears to be greater than with midazolam.70 Careful monitoring of creatine kinase and oxygen saturation would be prudent.73
Levetiracetam is emerging as a very commonly used IV and enteral antiseizure drug in critical care. Unfortunately, no organized dose-finding has been undertaken in critically ill patients; published series have included loading doses between 1 and 6 grams, with a wide range of maintenance doses.74 Levetiracetam has been used for prophylaxis after head trauma, but the higher mortality in the patients receiving this drug in comparison to those receiving phenytoin argues for some caution.75
IV valproate has emerged as an important drug for the treatment of several forms of status epilepticus.76 IV lacosamide is also available, but information about its use in status is limited. Topiramate may also be useful for refractory status epilepticus77 but lacks an IV form. Levetiracetam (1 gm loading dose, 1-9 gm/d maintenance) or lacosamide (300-400 mg loading dose, 300-400 mg/d maintenance) may also be useful.
Key Points
Bleck TP. Critical care of the patient in status epilepticus. In: Wasterlain C, Treiman D, editors. Status epilepticus. Boston: MIT Press; 2006:607-613.
A comprehensive review of ICU management of status epilepticus.
Fountain NB, Adams RE. Midazolam treatment of acute and refractory status epilepticus. Clin Neuropharmacol. 1999;22:261-267.
Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(suppl 2):13-23.
Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 1996;47:83-89.
Towne AR, Waterhouse EJ, Boggs JG, Garnett LK, Brown AJ, Smith JR,Jr, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340-345.
Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. N Engl J Med. 1998;339:792-798.
1 Bleck TP, Smith MC, Pierre-Louis JC, et al. Neurologic complications of critical medical illnesses. Crit Care Med. 1993;21:98-103.
2 Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: An investigation of variables associated with mortality. Neurology. 1996;47:83-89.
3 Wilks S. Bromide and iodide of potassium in epilepsy. Med Times and Gaz (Lond). 1861;2:635-636.
4 Bleck TP, Klawans HL. Mechanisms of epilepsy and anticonvulsant action. In: Klawans HL, Goetz CG, Tanner CM, editors. Textbook of Clinical Neuropharmacology. New York: Raven Press; 1992:23-30.
5 Weschler IS. Intravenous injection of paraldehyde for control of convulsions. JAMA. 1940;114:2198.
6 Gastaut H, Naquet R, Poiré R, et al. Treatment of status epilepticus with diazepam (Valium). Epilepsia. 1965;6:167-182.
7 DeLorenzo RJ, Garnett LK, Towne AR, et al. Comparison of status epilepticus with prolonged seizure episode lasting 10 to 29 minutes. Epilepsia. 1999;40:164-169.
8 Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120-122.
9 Wijdicks EFM, Sharbrough FW. New-onset seizures in critically ill patients. Neurology. 1993;43:1042-1044.
10 Aminoff MJ, Simon RP. Status epilepticus: Causes, clinical features and consequences in 98 patients. Am J Med. 1980;69:657-666.
11 Hauser WA. Status epilepticus: Epidemiologic considerations. Neurology. 1990;40(Suppl 2):9-13.
12 DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029-1035.
13 Waterhouse EJ, DeLorenzo RJ. Status epilepticus in older patients: Epidemiology and treatment options. Drugs Aging. 2001;18:133-142.
14 Garzon E, Fernandes RMF, Sakamoto AC. Analysis of clinical characteristics and risk factors for mortality in human status epilepticus. Seizure. 2003;12:337-345.
15 Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: An investigation of variables associated with mortality. Neurology. 1996;47:83-89.
16 Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61:1066-1073.
17 Towne AR, Pellock JM, Ko D, et al. Determinants of mortality in status epilepticus. Epilepsia. 1994;35:27-34.
18 Lothman EW, Bertram EH. Epileptogenic effects of status epilepticus. Epilepsia. 1993;34(Suppl 1):S59-S70.
19 Treiman DM, Delgado-Escueta AV. Complex partial status epilepticus. Adv Neurol. 1983;34:69-81.
20 Shinnar S, Pellock JM, Berg AT, et al. Short-term outcomes of children with febrile status epilepticus. Epilepsia. 2001;42:47-53.
21 Brandt C, Glien M, Potschka H, et al. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003;55:83-103.
22 Berg AT, Shinnar S, Levy SR, et al. Early development of intractable epilepsy in children: A prospective study. Neurology. 2001;56:1445-1452.
23 Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489-501.
24 Bleck TP. Metabolic encephalopathy. In: Weiner WJ, Shulman LM, editors. Emergent and Urgent Neurology. 2nd ed. Philadelphia: Lippincott; 1999:223-253.
25 Bleck TP. Status epilepticus. In: Klawans HL, Goetz CG, Tanner CM, editors. Textbook of Clinical Neuropharmacology. 2nd ed. New York: Raven Press; 1992:65-73.
26 International League Against Epilepsy. Available at: http://www.epilepsy.org/ Accessed January 24, 2004
27 Goodkin HP, Liu X, Holmes GL. Diazepam terminates brief but not prolonged seizures in young, naïve rats. Epilepsia. 2003;44:1109-1112.
28 Lothman EW. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(Suppl 2):13-23.
29 Lowenstein DH, Simon RP, Sharp FR. The pattern of 72-kDa heat shock protein-like immunoreactivity in the rat brain following flurothyl-induced status epilepticus. Brain Res. 1990;531:173-182.
30 Wasterlain CG, Fujikawa DG, Penix L, et al. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34(Suppl 1):S37-S53.
31 Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia. 1993;34(Suppl 1):S2-11.
32 Walton NY. Systemic effects of generalized convulsive status epilepticus. Epilepsia. 1993;34(Suppl 1):S54-S58.
33 Ficker DM, Westmoreland BF, Sharbrough FW. Epileptiform abnormalities in hepatic encephalopathy. J Clin Neurophys. 1997;14:230-234.
34 Bolton CF, Young GB, Zochodne DW. The neurologic complications of sepsis. Ann Neurol. 1993;33:94-100.
35 Kanemoto K, Ozawa K. A case of post-anoxic encephalopathy with initial massive myoclonic status followed by alternating Jacksonian seizures. Seizure. 2000;9:352-355.
36 Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia. 1996;37:643-650.
37 Singh BM, Strobos RJ. Epilepsia partialis continua associated with nonketotic hyperglycemia: Clinical and biochemical profile of 21 patients. Ann Neurol. 1980;8:155-160.
38 Campise M. Neurological complication during imipenem/cilastatin therapy in uraemic patients. Nephrol Dial Transplant. 1998;13:1895-1896.
39 Kushner JM, Peckman JH, Snyder CR. Seizures associated with fluoroquinolones. Ann Pharmacother. 2001;35:1194-1198.
40 Abanades S, Nolla J, Rodriguez-Campello A, et al. Reversible coma secondary to cefepime neurotoxicity. Ann Pharmacother. 2004;38:606-608.
41 Jaaskelainen SK, Kaisti K, Suni L, et al. Sevoflurane is epileptogenic in healthy subjects at surgical levels of anesthesia. Neurology. 2003;61:1073-1078.
42 Klein C, Balash Y, Pollak L, et al. Body packer: Cocaine intoxication, causing death, masked by concomitant administration of major tranquilizers. Eur J Neurol. 2000;7:555-558.
43 Morres CA, Dire DJ. Movement disorders as a manifestation of nonketotic hyperglycemia. J Emerg Med. 1989;7:359-364.
44 Hennis A, Corbin D, Fraser H. Focal seizures and nonketotic hyperglycemia. J Neurol Neurosurg Psychiatry. 1992;55:195-197.
45 Towne AR, Waterhouse EJ, Boggs JN, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340-345.
46 DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833-840.
47 Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109:506-523.
48 First Seizure Trial Group. Randomized clinical trial of the efficacy of antiepileptic drugs in reducing the risk of relapse after a first unprovoked tonic-clonic seizure. Neurology. 1993;43:478-483.
49 Musicco M, Beghi E, Solari A, et al. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology. 1997;49:991-998.
50 O’Brien TJ, Cascino GD, So EL, Hanna DR. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology. 1998;51:1034-1039.
51 Browne TR. Fosphenytoin (Cerebyx). Clin Neuropharmacol. 1997;20:1-12.
52 Fierro LS, Savulich DH, Benezra DA. Safety of fosphenytoin sodium. Am J Health Syst Pharm. 1996;53:2707-2712.
53 Smith MC, Bleck TP. Toxicity of anticonvulsants. In: Klawans HL, Goetz CG, Tanner CM, editors. Textbook of Clinical Neuropharmacology. 2nd ed. New York: Raven Press; 1992:45-64.
54 Ford G, Bleck TP. Seizures in the intensive care unit. In: Parrillo JE, editor. Current Therapy in Critical Care Medicine. 3rd ed. Toronto, B.C.: Decker; 1997:318-323.
55 Treiman DM, Meyers PD, Walton NY, et al. Treatment of generalized convulsive status epilepticus: A multicenter comparison of four drug regimens. N Engl J Med. 1998;339:792-798.
56 Osorio I, Reed RC. Treatment of refractory generalized tonic-clonic status epilepticus with pentobarbital anesthesia after high-dose phenytoin. Epilepsia. 1989;30:464-471.
57 Leppik IE, Boucher BA, Wilder BJ, et al. Pharmacokinetics and safety of a phenytoin prodrug given IV or IM. Neurology. 1990;40:456-460.
58 Shaner DM, McCurdy SA, Herring MO, et al. Treatment of status epilepticus: A prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202-206.
59 Yaffe K, Lowenstein DH. Prognostic factors of pentobarbital therapy for refractory generalized status epilepticus. Neurology. 1993;43:895-900.
60 Bleck TP. Critical care of the patient in status epilepticus. In: Wasterlain C, Treiman D, editors. Status Epilepticus. Boston: MIT Press; 2006:607-613.
61 Bleck TP. Therapy for status epilepticus. Clin Neuropharmacol. 1983;6:255-268.
62 Bleck TP. High-dose pentobarbital treatment of refractory status epilepticus: A meta-analysis of published studies. Epilepsia. 1992;33:5.
63 Sato M, Tanaka S, Suzuki K, et al. Complications associated with barbiturate therapy. Resuscitation. 1989;17:233-241.
64 Krishnamurthy KB, Drislane FW. Relapse and survival after barbiturate anesthetic treatment of refractory status epilepticus. Epilepsia. 1996;37:863-867.
65 Igartua J, Silver P, Maytal J, et al. Midazolam coma for refractory status epilepticus in children. Crit Care Med. 1999;27:1982-1989.
66 Hanley DF, Pozo M. Treatment of status epilepticus with midazolam in the critical care setting. Int J Clin Pract. 2000;54:30-35.
67 Naritoku D, Sinha S. Prolongation of midazolam half-life after sustained infusion for status epilepticus. Neurology. 2000;54:1366-1368.
68 Claassen J, Hirsch LJ, Emerson RG, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57:1036-1042.
69 Stecker MM, Kramer TH, Raps ED, et al. Treatment of refractory status epilepticus with propofol: Clinical and pharmacokinetic findings. Epilepsia. 1998;39:18-26.
70 Prasad A, Worrall BB, Bertram EB, Bleck TP. Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia. 2001;42:380-386.
71 Stecker MM, Skaar DJ, Dulaney E, et al. Treatment of refractory status epilepticus with propofol: Clinical and pharmacokinetic findings. Epilepsia. 1998;39:18-26.
72 Hanna JP, Ramundo ML. Rhabdomyolysis and hypoxia associated with prolonged propofol infusion in children. Neurology. 1998;50:301-303.
73 Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: A systematic review. Epilepsia. 2002;43:146-153.
74 Trinka E. What is the relative value of the standard anticonvulsants: Phenytoin and fosphenytoin, phenobarbital, valproate, and levetiracetam? Epilepsia. 2009;50(Suppl 12):40-43.
75 Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure phrophylaxis. Neurocrit Care. 2010;12:165-172.
76 Yu KT, Mills S, Thompson N, Cunanan C. Safety and efficacy of intravenous valproate in pediatric status epilepticus and acute repetitive seizures. Epilepsia. 2003;44:724-726.
77 Towne AR, Garnett LK, Waterhouse EJ, et al. The use of topiramate in refractory status epilepticus. Neurology. 2003;60:332-334.