Screening and Surveillance Guidelines in Gastroenterology
Ansu M. Noronha
Francis A. Farraye
Introduction
This chapter focuses on clinical gastroenterology issues of interest to pathologists, including endoscopic diagnosis and management of Barrett esophagus, management of intestinal metaplasia in the setting of chronic gastritis, and surveillance in patients with inflammatory bowel disease, colonic polyps, and colon cancer.
Surveillance in Patients with Barrett Esophagus
Most authorities recommend that patients with chronic reflux symptoms lasting 5 or more years undergo an upper endoscopy to screen for Barrett esophagus (Table 2.1). The benefits of screening programs for Barrett esophagus are controversial because of a lack of sufficient evidence that such programs improve survival rates or are cost-effective.1 Furthermore, there is only indirect evidence to suggest that patients diagnosed with adenocarcinoma while undergoing surveillance have an increased chance of survival. Nevertheless, the current standard of care dictates that if Barrett esophagus is diagnosed, the patient should be entered into an endoscopic surveillance program for early detection of dysplasia and adenocarcinoma.2
Table 2.1
Screening and Surveillance Recommendations for Barrett Esophagus

Data from Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091.
In the past, endoscopic surveillance was undertaken only in patients who were medically fit to undergo esophagectomy. However, with the advent of nonsurgical ablative endoscopic techniques (e.g., photodynamic therapy, argon plasma coagulation, cryotherapy) and endoscopic mucosal resection (EMR), the number of patients eligible for surveillance has increased. Recent experience with EMR suggests that it may be the treatment of choice in patients with high-grade dysplasia (HGD) or intramucosal adenocarcinoma in the setting of Barrett esophagus, given the opportunity for histologic assessment of the area.3–6
Aggressive treatment of reflux with proton pump inhibitors is warranted before surveillance endoscopy, because active inflammation with repair can mimic dysplasia.
Currently, endoscopic surveillance is performed by obtaining four-quadrant biopsies at 2-cm intervals with the use of jumbo biopsy forceps. In addition, specific attention is paid to mucosal abnormalities such as ulcers, irregular lesions, nodules, and polyps, and these lesions should be submitted separately. In patients with known or suspected dysplasia, biopsies should be obtained at 1-cm intervals instead of the standard 2 cm. Studies have shown that endoscopy with magnification and narrow band imaging (NBI) allows for better localization of HGD and may allow more targeted and fewer biopsies.7,8
The recommended interval of surveillance for dysplasia in patients with Barrett esophagus is determined by the presence and degree of dysplasia found (Table 2.2). In the absence of dysplasia, the surveillance interval is every 3 to 5 years with four-quadrant biopsies every 2 cm. If the pathology is indeterminate, the grade of dysplasia should be clarified with an expert gastrointestinal pathologist. Antisecretory medication should be increased to reduce the presence of esophagitis, and upper endoscopy with biopsies should be repeated. The presence of low-grade dysplasia (LGD) should be confirmed with endoscopy within 6 months, followed by surveillance every 12 months.
Table 2.2
Surveillance Guidelines for Barrett Esophagus
| No dysplasia | 3-5 years |
| Indeterminate for dysplasia | Consult expert gastrointestinal pathologist Increase antisecretory medications and repeat endoscopy with biopsies |
| Low-grade dysplasia | 6-mo interval to confirm, then surveillance every 12 mo |
| High-grade dysplasia | 3 mo; consider endoscopic mucosal resection, radiofrequency ablation, or surgical resection |
Data from Evans JA, Early DS, Fukami N, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087-1094.
Patients with flat HGD confirmed by an expert gastrointestinal pathologist should undergo a repeat endoscopy within 3 months with four-quadrant biopsies every 1 cm if an eradication method is not used.9 The prevalence of cancer in resection specimens from patients who have undergone an esophagectomy for HGD ranges from 5% to 41%, and the rate of progression to cancer in patients with HGD approaches 30% at 10 years. Options for patients with flat HGD include intensive surveillance (every 3 months), esophagectomy, and ablative therapies such as BARRX or radiofrequency ablation. Patients with HGD with mucosal irregularity should undergo endoscopic mucosal or surgical resection.10
Surveillance in Patients with Chronic Gastritis and Intestinal Metaplasia or Dysplasia
The most common causes of chronic gastritis are Helicobacter pylori infection, environmental exposures including smoking, and autoimmune processes. Biopsies obtained during endoscopy from patients with chronic gastritis may reveal intestinal metaplasia (IM). A study from the United States demonstrated that 13% of patients at low risk for gastric cancer, and 50% of patients at high risk, had IM on biopsies from normal-appearing gastric mucosa.11 Although gastric IM (incomplete type) is considered a premalignant lesion, the overall risk of gastric cancer in patients with gastric IM is very low. However, patients with IM and dysplasia have an approximately 100-fold increased risk of gastric cancer.11
In the United States, where the incidence of gastric cancer is low, endoscopic surveillance of patients with gastric IM is not recommended in patients who are at low risk for gastric cancer.11 Low-risk patients include those living in developed countries, whites without any family history of gastric cancer, and individuals without dysplasia on gastric biopsy. The likelihood that endoscopic surveillance of low-risk patients with IM will increase detection of curable gastric cancer is very low, and therefore it is not likely to be cost-effective. Furthermore, IM is a histologic lesion that is not visible by the endoscopist. This makes endoscopic surveillance difficult, because numerous biopsies mapping the stomach would be needed to obtain a significant yield.
Surveillance in patients with IM who are at high risk for gastric cancer is controversial. High-risk patients include those with a family history of gastric cancer, Hispanics, blacks, and immigrants from higher-risk geographic locations. No formal recommendations or data exist to support the implementation of an endoscopic surveillance program in high-risk patients with gastric IM. The American Society of Gastrointestinal Endoscopy (ASGE) concluded that patients who are at increased risk for gastric cancer on the basis of ethnic background or family history may benefit from surveillance, but there was no specific recommendation on the frequency of endoscopy. If surveillance is performed, the ASGE recommended that endoscopic surveillance with gastric biopsies should incorporate a topographic mapping of the entire stomach histologically.12
The location and type of dysplasia found determine the surveillance guidelines for patients with IM. The histopathologic classification divides cases into the subtypes of incomplete and complete IM, a distinction best determined after assessment for and eradication of H. pylori infection. Studies have suggested that if the patient has incomplete or extensive IM, topographic mapping of the gastric mucosa should be performed at 1 year, followed by repeated surveillance endoscopy every 3 years if extensive metaplasia persists.13 There is no consensus regarding surveillance in LGD, but most studies suggest that surveillance is not warranted in average-risk patients.12 The ASGE recommends that patients with confirmed HGD on gastric biopsies be considered for gastrectomy or EMR.11 We expect that further recommendations regarding appropriate intervals for surveillance endoscopy and the use of new techniques will be formalized in the near future.
Pernicious anemia, which can occur as a result of autoimmune chronic atrophic gastritis, is another potential risk factor for gastric cancer and gastric carcinoid. A twofold to threefold increased risk of gastric cancer has been seen, depending on the location and duration of disease.14 Clear guidelines for surveillance in atrophic gastritis have not yet been established. Current guidelines from the ASGE advocate performance of a screening upper endoscopy at the time of diagnosis of pernicious anemia to identify lesions such a carcinoid or gastric cancer, but there are only weak data to support subsequent surveillance endoscopy if the initial test is negative.12
Surveillance for Colorectal Neoplasia in Patients with Inflammatory Bowel Disease
Inflammatory bowel disease (IBD), which comprises ulcerative colitis (UC) and Crohn’s disease, is the second most common inflammatory disorder after rheumatoid arthritis. There are approximately 1.4 million patients with IBD in the United States.14a Each year, between 20,000 and 25,000 new cases are diagnosed. The peak incidence occurs between the ages of 15 to 35, with males and females equally affected.
The goal of colonoscopic surveillance is to minimize the morbidity and mortality from colorectal cancer (CRC) with appropriate and timely referral for colectomy. Close interaction between endoscopist and the pathologist is crucial in the management of IBD.15 Although no prospective randomized studies have been performed to evaluate the efficacy of surveillance colonoscopy to detect dysplasia or CRC in UC patients, case-control studies suggest a reduction in mortality in those undergoing surveillance.16 Surveillance colonoscopy should optimally be performed when the patient is in clinical remission, because active inflammation may hinder the histologic diagnosis of dysplasia.
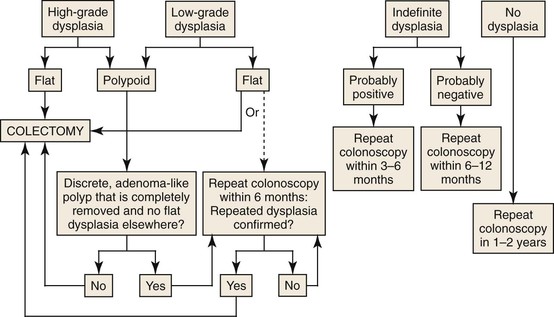
Biopsies taken during surveillance colonoscopy are graded as positive for dysplasia, negative for dysplasia, or indefinite for dysplasia. Dysplasia is further classified as LGD, HGD, or carcinoma.17 The greatest interobserver variability among pathologists lies in the interpretation of the LGD and indefinite-for-dysplasia categories. Therefore, once dysplasia is detected, a second pathologist with expertise in interpretation of gastrointestinal biopsies should confirm the diagnosis. Dysplastic mucosa may be characterized as either flat or raised. Flat dysplasia is generally considered to be endoscopically undetectable, is identified by random biopsies, and can be further classified as either multifocal or unifocal. Raised or polypoid dysplasia is referred to by the acronym DALM: dysplasia-associated lesion or mass.
Current guidelines from the American College of Gastroenterology (ACG), the American Gastroenterology Association (AGA) (Table 2.3), and the British Society of Gastroenterology(BSG) (Table 2.4) recommend that colonoscopic surveillance begin 8 to 10 years after the onset of symptoms of colitis in patients with UC or with Crohn’s colitis involving at least one third of the colon.18–20 The results of the colonoscopy determine the extent of disease, and appropriate patients are then entered into a surveillance program. Repeated surveillance colonoscopy is performed every 1 to 3 years (AGA) or 1 to 5 years (BSG).18 Patients with coexisting primary sclerosing cholangitis should begin surveillance colonoscopy at the time of diagnosis of liver disease and continue annually thereafter regardless of the extent of disease. Other risk factors associated with an increased risk of developing colorectal neoplasia include CRC in a first-degree relative, ongoing active endoscopic or histologic inflammation, and anatomic abnormalities such as a foreshortened colon, stricture, or multiple inflammatory pseudopolyps. Several studies have correlated increased severity of colonoscopic macroscopic and histologic inflammation with a higher risk of CRC.21,22 Male gender has been identified as a risk factor for development of CRC.23,24 Patients with proctitis or distal proctosigmoiditis are not at increased risk for the development of CRC and do not need to undergo surveillance.
Table 2.3
American Gastroenterology Association Surveillance Guidelines for CRC in Patients with IBD

CRC, Colorectal cancer; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis.
From Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774.e4.
Table 2.4
British Society of Gastroenterology Surveillance Colonoscopy Guidelines for Patients with IBD
| Low risk: colonoscopy at 5-yr intervals | Extensive colitis without evidence of endoscopic/microscopic inflammation at last colonoscopy, or left sided-colitis |
| Crohn’s colitis affecting <50% of the colon | |
| Intermediate risk: colonoscopy at 3-yr intervals | Extensive colitis with mild endoscopic/microscopic inflammation on previous colonoscopy |
| Family history of CRC in a first-degree relative | |
| Higher risk: yearly colonoscopy | Extensive colitis with moderate or severe endoscopic/microscopic inflammation on previous colonoscopy |
| Stricture within the past 5 years | |
| Confirmed dysplasia within the past 5 years in a patient who declines surgery | |
| Primary sclerosing cholangitis | |
| Family history of CRC in a first-degree relative before 50 years of age |
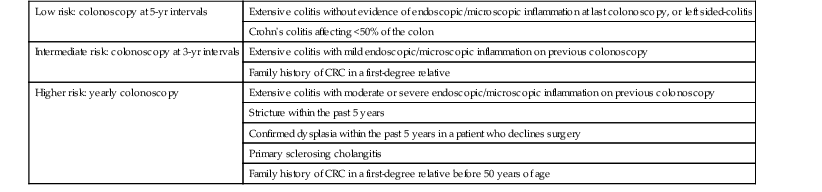
CRC, Colorectal cancer; IBD, inflammatory bowel disease.
Reproduced from Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689, with permission from BMJ Publishing Group Ltd.
There is wide variability in the practice of surveillance by gastroenterologists as well as inconsistency in the management of patients with dysplasia.25,26 Current guidelines recommend the use of chromoendoscopy and directed biopsies or four-quadrant random biopsies every 10 cm for a minimum of 33 total colonic biopsies, or 6 biopsies in 6 pathology specimen bottles.20 The recommendation for 33 biopsies is based on a retrospective analysis that revealed a 90% positive predictive value for dysplasia with 33 biopsy specimens and a 95% positive predictive value with greater than 56 specimens.27 It is also recommended that in patients with UC, four-quadrant biopsies should be taken every 5 cm in the distal sigmoid and rectum, given the increased risk of carcinoma in those areas. Other endoscopists obtain six specimens from each of the following sections: cecum and ascending colon, transverse colon, descending colon, sigmoid, rectosigmoid, and rectum. Additional biopsies should be obtained from any suspicious mucosal lesions. The BSG guidelines recommend pancolonic dye spraying and targeted biopsies of abnormal mucosa. If chromoendoscopy is not used, then two to four random biopsies every 10 cm should be taken.18
The AGA and BSG guidelines recommend proctocolectomy in cases of flat HGD.28 Most authorities also recommend proctocolectomy in patients with multifocal flat LGD or a single repetitive focus on more than one colonoscopy. Many authorities now recommend proctocolectomy in patients with even a single focus of flat LGD because this has been shown to be associated with concurrent adenocarcinoma in 20% of patients and to progress to higher grades of dysplasia in 50% of cases.28,29 A meta-analysis of 20 studies found that when raised or flat LGD is detected at surveillance, there is a ninefold increased risk for CRC and a 12-fold increased risk for any advanced lesion.30 Patients with LGD who elect against colectomy should undergo repeat surveillance colonoscopy on a 3- to 6-month basis. For patients with indefinite dysplasia, colonoscopy should be repeated at an interval of 3 to 6 months.
The management of a dysplastic “polyp” in patients with UC or Crohn’s colitis has similar guidelines. If a well-circumscribed dysplastic polyp is found proximal to histologically demonstrable colitis, it should be managed as a simple adenoma. DALMs were first identified by Blackstone and colleagues in 1981 and were associated with a high rate of CRC at colectomy.31 More recently, a raised dysplastic lesion with the appearance of sporadic adenoma has been termed an adenoma-like DALM.32 In contrast, poorly circumscribed lesions with indistinct borders and an irregular surface, or plaque like lesions, have been termed nonadenoma-like DALMs. Alternative terms are endoscopically resectable and endoscopically nonresectable polypoid dysplasia, respectively.
The endoscopist must make a distinction between an adenoma-like DALM and a nonadenoma-like DALM, because these lesions overlap histologically. Patients with UC who are found to have an area of endoscopically resectable dysplasia (adenoma-like DALM) may undergo polypectomy and continued endoscopic surveillance if no other areas of flat dysplasia are detected in the adjacent mucosa or elsewhere in the colon.33–35 It is recommended that additional biopsies be taken immediately adjacent to the polyp and elsewhere in the colon to exclude flat dysplasia. If no dysplasia is found, repeat surveillance colonoscopy should be performed within 6 to 12 months. In contrast, patients with an area of nonresectable polypoid dysplasia (nonadenoma-like DALM) are usually referred for colectomy because of the high rate of association of these lesions with synchronous or metachronous cancer. Recommendations for the management of flat and polypoid dysplasia are presented in Figure 2.1.
Studies have demonstrated that the use of chromoendoscopy can greatly increase the detection rate of dysplasia in patients with UC who have been enrolled in a surveillance program.36 Chromoendoscopy with targeted biopsies revealed significantly more dysplastic lesions than conventional colonoscopy with random biopsies. The overall sensitivity of chromoendoscopy for predicting neoplasia was 93% to 97%.37–39 Given these findings, consensus guidelines from several organizations have endorsed the use of chromoendoscopy in surveillance colonoscopy by trained endoscopists.18,20 As more data regarding chromoendoscopy become available and new imaging techniques are developed, guidelines for surveillance endoscopy in patients with IBD will be refined to reflect these advances. It is also likely that molecular biology techniques will play a more important role in the future as an adjunct to endoscopic biopsy.40
Screening and Surveillance Guidelines for Colon Polyps
The following is a review of the management of colonic polyps in patients who do not have IBD.41,42 This summary includes screening for colon polyps, surveillance after polypectomy and resection for CRC, and the approach to the patient with a malignant polyp.
CRC is the second most common cause of cancer death in the United States. The overall mortality rate approaches 60%. Approximately 5% to 6% of U.S.-born individuals will develop colon cancer in their lifetime, and 2.5% will die of the disease. The incidence does not vary significantly between men and women. It is estimated that almost 143,000 new cases of CRC will be diagnosed and almost 51,000 deaths will occur from CRC in the United States in 2013.43 CRC is a suitable disease for screening because it is a common malignancy with a long, asymptomatic preclinical phase and a high survival rate if detected in its early stage. Prevention of CRC should be achievable by application of screening programs to identify asymptomatic patients with adenomatous polyps and to remove them. In The National Polyp Study, colonoscopic polypectomy decreased the expected incidence of CRC by 53%.44 An estimated 14.2 million colonoscopies and 2.8 million flexible sigmoidoscopies were performed in 2002 in the United States.45
Definition and Clinical Considerations
Small (<1 cm) tubular adenomas are extremely common and have a low risk of becoming malignant. Only a small proportion of these develop histologic features of HGD or cancer. There has been an increasing appreciation of the concept of the “advanced adenoma” as the target for colonoscopic screening.46 Advanced adenoma is defined as any polyp larger than 1 cm in diameter or any polyp regardless of size that is villous or contains a focus of HGD. Efforts to reduce colon cancer are now shifting mainly to strategies to reliably detect and resect advanced adenomas before they become malignant rather than focusing on identifying small tubular adenomas. It should also be noted that interobserver variability with regard to diagnosis of villous components and HGD in an adenoma is high.47 Therefore, reproducible histologic criteria must be developed by pathologists so that future prospective outcome studies can accurately predict the fate of patients with advanced adenomas. Currently, 70% of polyps removed at colonoscopy are adenomas. More than 80% of these are tubular, 5% to 15% are tubulovillous, and 5% to 15% are villous adenomas.48
Initial Management of Polyps
Colonoscopy is the most accurate method for detecting polyps and allows immediate biopsy and resection. It has quickly replaced fecal occult blood testing (FOBT), flexible sigmoidoscopy, and barium enema as the primary screening modality, although flexible sigmoidoscopy and FOBT remain approved methods on screening for CRC in the asymptomatic patient. Computed tomographic colonography, another option, is still considered investigational as a screening modality. It should be reserved for those patients with incomplete optical colonoscopies, because the test has not been determined to be cost-effective for routine screening and is not reimbursed by most insurance plans.49 A complete colonoscopy should be performed at the time of every initial polypectomy to detect and resect all synchronous adenomas. Additional colonoscopic examinations may be required after resection of a large sessile adenoma, if there were multiple adenomas, or if the quality of the colonic preparation was suboptimal.
Management of Small Polyps
Small polyps (<1 cm), either sessile or pedunculated, can be resected by a number of different techniques, both with and without electrocautery. Cold snare polypectomy has replaced cold biopsy and hot biopsy techniques in removing small polyps.50 A “resect and discard” policy is being discussed by gastroenterology societies as a method to lower the cost of colonoscopy.51 The first part of this paradigm is applied to all diminutive polyps found during colonoscopy; a visual assessment is made of the polyp during colonoscopy and after removal, and the tissue is then discarded rather than submitted for pathologic review. This approach allows for reduction of costs as well as an immediate determination of the colonoscopy surveillance interval. The second aspect of the paradigm proposes not resecting multiple rectosigmoid hyperplastic polyps. This approach again leads to a reduction of costs and risks from polypectomy.52
Management of Sessile Serrated Adenomas/Polyps
There is no evidence that small, distally located hyperplastic polyps carry an increased risk for CRC, but it is now accepted that certain variants of hyperplastic-appearing or serrated polyps are precursors to CRC. For example, serrated polyps have been linked to the development of sporadic adenocarcinomas with high-level microsatellite instability (MSI-H), which are associated with the development of “interval cancers” after colonoscopy.53 Hyperplastic-appearing polyps at risk for such progression are usually large (>5 mm), sessile with a layer of yellow mucus, and found proximally in the colon on colonoscopy. These have been called atypical hyperplastic polyps, sessile serrated polyps (SSP), or sessile serrated adenomas (SSA) and sessile serrated adenomas/polyps (SSA/Ps). These SSA/Ps tend to be found later in life (median age, 61 years), with a slight female predominance.53 Initially, SSAs are not dysplastic, but over time, due to increased microsatellite instability, they develop dysplasia and are precursors to CRC, accounting for approximately 15% to 20% of these cancers.54 Dysplastic serrated adenomas are classified as sessile serrated adenomas with dysplasia (SSAD) or traditional serrated adenomas (TSA), which tend histologically to appear more like traditional adenomas.55
Because of their malignant potential, the most recent guidelines recommend that large, proximally located, hyperplastic-appearing serrated polyps be managed in the same way as adenomas of similar size. These guidelines recommend that serrated lesions proximal to the sigmoid colon and all lesions larger than 5 mm be completely resected.53,55 When lesions are removed piecemeal, a follow-up colonoscopy should be performed in 2 to 6 months to ensure that no residual polyp tissue has been left behind. SSAs smaller than 10 mm should have repeat surveillance in 5 years, whereas larger lesions and those containing any dysplasia should undergo surveillance in 3 years. See Table 2.5 for a summary of recommendations for the colonoscopic surveillance of serrated polyps.
Table 2.5
Recommendations for Surveillance and Screening Intervals in Individuals with Serrated Lesions
| Baseline Colonoscopy: Most Advanced Finding | Recommended Surveillance Interval (Yr) |
| Sessile adenomas removed piecemeal | 2-6 mo |
| Sessile serrated polyps <10 mm with no dysplasia | 5 |
| Sessile serrated polyp >10 mm | 3 |
| Sessile serrated polyp with dysplasia | 3 |
| Traditional serrated adenoma | 3 |
| Serrated polyposis syndrome | 1 |
Data from Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857.
Hyperplastic Polyposis Syndromes
Serrated polyposis syndrome (SPS), formerly known as hyperplastic polyposis, is a syndrome characterized by the presence of multiple serrated lesions, either serrated adenomas or hyperplastic polyps. The World Health Organization has defined SPS as a syndrome meeting one of the following criteria: (1) at least five serrated polyps proximal to the sigmoid colon, with two or more being larger than 10 mm; (2) any serrated polyps proximal to the sigmoid colon in any patient who has a first-degree relative with SPS; (3) more than 20 serrated polyps of any size in any location throughout the colon.56 The risk of cancer in SPS is unknown but is believed to be increased, and a retrospective study showed a 7% risk of cancer at 5 years in patients undergoing surveillance.57 Surveillance colonoscopy is recommended at 1-year intervals to clear the proximal colon of lesions larger than 5 mm; however, surgical resection is recommended if carcinoma is discovered or the number of polyps is too great to be endoscopically managed. Surgery should involve resection of the portion of the colon that contains the cancer as well as that which has the largest polyps; usually, an extended right hemicolectomy or subtotal colectomy is performed. Patients will then need annual surveillance of the remaining colon and rectum.
Management of Large Pedunculated Polyps
Endoscopic resection of large polyps can be challenging because of the risks of hemorrhage, perforation, and incomplete resection. Most endoscopists resect large pedunculated polyps using a hot snare. However, EMR has been shown to be successful for large pedunculated polyps with flat broad stalks.58
Large pedunculated polyps (≥1 cm in diameter) resected in one piece should be examined by the pathologist for adequacy of resection. The guidelines for polyp specimen processing were discussed in Chapter 1. Piecemeal resection of large pedunculated polyps impedes, but does not preclude, pathologic assessment of adequacy of resection. However, in this instance, the pathologist depends on the endoscopist to deliver a readily available stalk.
Management of Large Sessile Polyps
The prevalence of large sessile polyps is approximately 0.8% to 5.2% in patients undergoing colonoscopy. Malignancy is found in 5% to 22% of these polyps. These polyps tend to recur locally after resection, and one study quoted a rate as high as 46%.59 This same study found that the recurrence rate could be reduced to 3.8% with repeated endoscopic procedures and the use of argon plasma coagulation. Another investigation found that the use of EMR for resection of large sessile polyps was successful in 90% of cases, with size larger than 40 mm and use of argon plasma coagulation leading to lower success rates.59a
Assessment of the adequacy of excision of a large sessile polyp (≥2 cm) is problematic and depends on both the endoscopist’s assessment of whether a residual lesion is present and the pathologist’s ability to identify resection margins with confidence. This includes the issue of whether a large sessile polyp is resected intact or piecemeal. The endoscopist may tattoo the polypectomy site with India ink after endoscopic resection to facilitate visualization during a subsequent endoscopic procedure. General practice has suggested that a patient who has undergone colonoscopic excision of a large sessile polyp in piecemeal fashion should have follow-up colonoscopy in 2 to 6 months to verify complete removal. If residual polyp tissue is present, it should be resected, and the completeness of this resection should be documented within another 2- to 6-month interval. Once complete removal has been established, subsequent surveillance is recommended in 1 year. If complete resection is not possible after two or three procedures, surgical resection should be considered.41
Postpolypectomy Surveillance
Because a large number of patients with adenomas are being identified by colonoscopy, the burden placed on medical resources (i.e., the timely availability of colonoscopy) is increasing dramatically.60 The U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society recently revised the recommendations for surveillance colonoscopy after polypectomy. Updated guidelines emphasize stratification of patients into high- and low-risk groups (Table 2.6) and are based on the assumption that the initial screening colonoscopy was of optimal quality. However, certain studies have shown that approximately 17% of polyps larger than 10 mm are missed during optical colonoscopy and that these missed polyps account for the majority of interval cancers.41
Table 2.6
Risk Factors for Metachronous Advanced Adenomas
| High Risk | Low Risk |

From Lieberman DA. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857.
After an initial colonoscopy has been performed with complete polypectomy, patients who are deemed to be at low risk for metachronous advanced adenomas should have a follow-up colonoscopy performed in 5 to 10 years. The exact length of follow-up in these patients is determined by clinician judgment and patient comfort. Low-risk patients include those with only one to two small (<1 cm) tubular adenomas. If the follow-up colonoscopy is normal or shows only one or two small tubular adenomas with LGD, the interval for the next surveillance colonoscopy can be extended to 5 years. In contrast, patients with multiple adenomas, adenomas larger than 1 cm, or sessile serrated polyps larger than 1 cm need more frequent surveillance. Family history and proximal location may also predict metachronous, advanced adenomas. Currently, the data are insufficient to include these two variables as possible risk factors, and they were not included in the formulation of the Multi-Society Task Force guidelines. However, a family history of colon cancer in a first-degree relative does increase the risk of CRC, and clinicians need to individualize follow-up in these cases. A summary of surveillance intervals is shown in Table 2.7.
Table 2.7
Recommendations for Surveillance and Screening Intervals in Individuals with Baseline Average Risk
| Baseline Colonoscopy: Most Advanced Finding | Recommended Surveillance Interval (Yr) |
| No polyps | 10 |
| Small (<10 mm) hyperplastic polyps in rectum or sigmoid | 10 |
| 1-2 small (<10 mm) tubular adenomas | 5-10 |
| 3-10 tubular adenomas | 3 |
| >10 tubular adenomas | 3 |
| One or more tubular adenomas >10 mm | 3 |
| One or more villous adenomas | 3 |
| Adenoma with high-grade dysplasia | 3 |
| Sessile adenomas removed piecemeal | 2-6 mo |
| Sessile serrated polyps <10 mm with no dysplasia | 5 |
| Sessile serrated polyp >10 mm | 3 |
| Sessile serrated polyp with dysplasia | 3 |
| Traditional serrated adenoma | 3 |
| Serrated polyposis syndrome | 1 |
Data from Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857.
Management of Malignant Polyps
A malignant polyp is defined as an adenomatous polyp with cancer invading the submucosa; favorable and unfavorable histologic features are reviewed in Table 2.8. Guidelines for the management of malignant polyps are reviewed in Table 2.9.61
Table 2.8
Malignant Colonic Polyps: Favorable and Unfavorable Features
| Favorable | Unfavorable |
| Cancer is well differentiated to moderately differentiated (grade I or II) | Cancer is poorly differentiated (grade III) |
| Absence of lymphovascular invasion | Lymphovascular invasion is present |
| Carcinoma is ≥2 mm from deep margin | Cancer is ≤2 mm from deep margin |
From Winawer SJ, O’Brien MJ. Management of malignant polyps. In: Waye JD, Rex DK, Williams CB, eds. Colonoscopy: Principles and Practice. 2nd ed. Oxford: Wiley-Blackwell; 2009.
Table 2.9
Malignant Colonic Polyps: Management
| Findings | Management |

From Winawer SJ, O’Brien MJ. Management of malignant polyps. In: Waye JD, Rex DK, Williams CB, eds. Colonoscopy: Principles and Practice. 2nd ed. Oxford: Wiley-Blackwell; 2009.
No further treatment is indicated after colonoscopic resection of a malignant polyp if the endoscopic and pathologic criteria listed in Table 2.8 are fulfilled. Patients with a malignant pedunculated polyp with favorable criteria may be observed in the same way as patients with a history of advanced colonic adenomas. Patients with a malignant sessile polyp that shows favorable prognostic criteria should have follow-up colonoscopy within 3 to 6 months to check for residual neoplastic tissue at the polypectomy site. After one negative follow-up examination, the clinician may revert to a standard surveillance regimen.
When a patient’s malignant polyp has poor (unfavorable) prognostic features, the relative risk of surgical resection should be weighed against the risk of death from metastatic carcinoma. If a malignant polyp is located in a part of the lower rectum that may require an abdominal-perineal resection, local excision, rather than standard cancer resection, may be justified. In brief, the risk of local recurrence or lymph node metastasis from an invasive carcinoma in a colonoscopically resected malignant adenomatous polyp is considered to be less than the risk of death from colonic surgery if the following criteria are fulfilled:
3. The cancer is not poorly differentiated (i.e., not grade III).
4. There is no evidence of vascular or lymphatic involvement.
A study that evaluated the outcome of endoscopic polypectomy of malignant polyps versus surgery based on histologic characteristics found that in those with favorable characteristics, endoscopic polypectomy alone seemed to be sufficient.62
Colonoscopic Surveillance after Colon Cancer Resection
Patients who have undergone resection for colon cancer should be entered into a surveillance program to detect early recurrence of the initial primary cancer and to detect metachronous colorectal neoplasms. Numerous studies have found that 2% to 7% of patients with CRC have one or more synchronous cancers in the colon and rectum at the time of initial diagnosis.35 It has also been shown that the annual incidence for metachronous cancers in surveillance groups after cancer resection is 0.35% per year.63
On the basis of the available data, patients should undergo a high-quality perioperative clearing by colonoscopy in nonobstructive tumors, and by computed tomographic colonography or double-contrast barium enema in obstructing tumors. A subsequent colonoscopy should be performed within 3 to 6 months or intraoperatively in patients with obstructing tumors. Surveillance endoscopy should be performed in all patients 1 year after resection because of the high yield of detecting early metachronous cancers. If the first surveillance colonoscopy is negative, the next examination needs to be done after a 3-year interval. Recommendations for surveillance in patients after CRC resection are reviewed in Table 2.10.
Table 2.10
Colonoscopy Recommendations for Surveillance after Cancer Resection
|
|

Interaction of Gastrointestinal Endoscopists and Pathologists
An ACG review on quality improvement in colonoscopy emphasizes the great importance of the interaction between the pathologist and the gastrointestinal endoscopist in the management of these patients.64 The quality improvement targets identified to improve care provided to patients undergoing colonoscopy and polypectomy are indicated in Table 2.11.
Table 2.11
Continuous Quality Improvement Targets in Colonoscopy
| Improvement | Goal |
| Percentage of adenomas with villous elements | <10% |
| Reports using the terms “carcinoma in situ” or “intramucosal adenocarcinoma” | None |
| Designation of the degree of dysplasia in adenomas as “low grade” or “high grade” | 100% |
| Use of the terms “mild,” “moderate,” or “severe” to describe dysplasia and adenomas | None |
| Adequate characterization of malignant polyps (resection line “margin,” degree of differentiation, presence or absence of vascular [or lymphatic] invasion) | 100% |
Data from Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-1308.