Chapter 89 Scoring Systems for Comparison of Disease Severity in Intensive Care Unit Patients
Scores at ICU admission
2 Which scores are used for assessing the general severity of disease at ICU admission?
3 Why were scores to assess general disease severity at ICU admission developed?
 To assess performance of the ICU. The ICU patient is a medical or a surgical patient who has either acute failure of one major vital function or a high risk for development of such failure. Because the mortality rate of ICU populations is usually high and varies widely depending on patient admission policies, an objective assessment of the patients’ general disease severity is necessary to ensure that the mortality rate in an ICU is consistent with the overall severity of its patient population at admission. The ratio between observed and predicted mortality, called the standardized mortality ratio, is the simplest way to assess the performance of an ICU. It allows comparisons among mortality rates of various ICUs or the mortality rates documented in one ICU over time.
To assess performance of the ICU. The ICU patient is a medical or a surgical patient who has either acute failure of one major vital function or a high risk for development of such failure. Because the mortality rate of ICU populations is usually high and varies widely depending on patient admission policies, an objective assessment of the patients’ general disease severity is necessary to ensure that the mortality rate in an ICU is consistent with the overall severity of its patient population at admission. The ratio between observed and predicted mortality, called the standardized mortality ratio, is the simplest way to assess the performance of an ICU. It allows comparisons among mortality rates of various ICUs or the mortality rates documented in one ICU over time.
 To assess the patient’s risk for death. The scores give an objective evaluation that helps the clinician confirm the severity of the patient’s illness. However, these scores cannot be used to make decisions about individual patients (e.g., withdrawal of support).
To assess the patient’s risk for death. The scores give an objective evaluation that helps the clinician confirm the severity of the patient’s illness. However, these scores cannot be used to make decisions about individual patients (e.g., withdrawal of support).
 To compare or match populations in clinical studies. In randomized, controlled studies the scores have been used to confirm that the populations obtained by randomization had a similar disease severity at admission to the ICU. In case-control studies, the scores have been used to match the control to the case patients.
To compare or match populations in clinical studies. In randomized, controlled studies the scores have been used to confirm that the populations obtained by randomization had a similar disease severity at admission to the ICU. In case-control studies, the scores have been used to match the control to the case patients.
6 Which scores have been validated adequately?
 The SAPS II is well validated. The score needs to be updated with more recent ICU populations.
The SAPS II is well validated. The score needs to be updated with more recent ICU populations.
 The MPM II is well validated and has the advantage of being the only score available at ICU admission rather than at 24 hours after admission. This advantage is made possible because the score includes some therapeutic items (e.g., venous lines, drainage systems). The MPM II score also needs to be updated with more recent ICU populations.
The MPM II is well validated and has the advantage of being the only score available at ICU admission rather than at 24 hours after admission. This advantage is made possible because the score includes some therapeutic items (e.g., venous lines, drainage systems). The MPM II score also needs to be updated with more recent ICU populations.
 The APACHE III is well validated and updated regularly, but its use is limited by the fact that clinicians must pay to know and use its equation for calculating death probability.
The APACHE III is well validated and updated regularly, but its use is limited by the fact that clinicians must pay to know and use its equation for calculating death probability.
 The APACHE IV and the MPM III are well calibrated, and they have a good discrimination; they were validated in a multicentered study of 11,300 ICU patients from California showing that APACHE IV had better discrimination and longer data extraction time than MPM III. MPM III was also validated in 55,459 patients from 103 ICUs, 25 of which did not participate in the original development.
The APACHE IV and the MPM III are well calibrated, and they have a good discrimination; they were validated in a multicentered study of 11,300 ICU patients from California showing that APACHE IV had better discrimination and longer data extraction time than MPM III. MPM III was also validated in 55,459 patients from 103 ICUs, 25 of which did not participate in the original development.
 The SAPS III is well validated and updated as it was published in 2005, that is, 12 years after the most recent among the other ones. Unlike the APACHE III, its construction details were diffused to the entire scientific community. It appears to be a good candidate for an international benchmark, and its use is free of charge.
The SAPS III is well validated and updated as it was published in 2005, that is, 12 years after the most recent among the other ones. Unlike the APACHE III, its construction details were diffused to the entire scientific community. It appears to be a good candidate for an international benchmark, and its use is free of charge.
Scores over the ICU stay
11 What did these scores add to the description of ICU patients?
 The use of the OSF score initially showed that a 100% prediction of death could be made in the most severely afflicted patients after several days. However, the same score was eventually used to demonstrate that care in the ICU had improved over the years, so that published results were no longer valid 10 years later. These investigations documented that such scores are a method to assess ICU performance.
The use of the OSF score initially showed that a 100% prediction of death could be made in the most severely afflicted patients after several days. However, the same score was eventually used to demonstrate that care in the ICU had improved over the years, so that published results were no longer valid 10 years later. These investigations documented that such scores are a method to assess ICU performance.
 The mean time of occurrence of each organ failure is not the same. The peak of dysfunction for the neurologic system occurs usually before the second day; for the respiratory, cardiovascular, renal, and coagulation systems, around the third day; and for hepatic dysfunction, around the fifth day.
The mean time of occurrence of each organ failure is not the same. The peak of dysfunction for the neurologic system occurs usually before the second day; for the respiratory, cardiovascular, renal, and coagulation systems, around the third day; and for hepatic dysfunction, around the fifth day.
 The weight of each organ failure in predicting death is not the same: hematologic and hepatic failures have less effect on mortality than respiratory, cardiovascular, renal, and neurologic failures.
The weight of each organ failure in predicting death is not the same: hematologic and hepatic failures have less effect on mortality than respiratory, cardiovascular, renal, and neurologic failures.
 The weight of each organ failure in predicting death is not the same over time. The same increase in respiratory dysfunction has a worse prognosis after 1 week of ICU stay. Hepatic dysfunction has an effect on mortality only after 3 weeks of ICU stay.
The weight of each organ failure in predicting death is not the same over time. The same increase in respiratory dysfunction has a worse prognosis after 1 week of ICU stay. Hepatic dysfunction has an effect on mortality only after 3 weeks of ICU stay.
Scores at ICU discharge
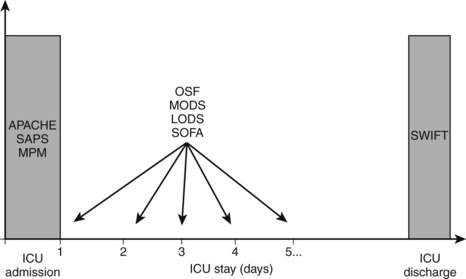
12 At what time of the ICU stay should either of these scores be used? (See Fig. 89-1.)
1 Cook R., Cook D., Tilley J., et al. Multiple organ dysfunction: baseline and serial component scores. Crit Care Med. 2001;29:2046–2050.
2 Fagon J.-Y., Chastre J., Novara A., et al. Characterization of intensive care unit patients using a model based on the presence or absence of organ dysfunctions and/or infection: the ODIN model. Intensive Care Med. 1993;19:137–144.
3 Gajic O., Malinchoc M., Comfere T.B., et al. The Stability and Workload Index for Transfer score predicts unplanned intensive care unit patient readmission: initial development and validation. Crit Care Med. 2008;36:676–682.
4 Higgins T.L., Kramer A.A., Nathanson B.H., et al. Prospective validation of the intensive care unit admission Mortality Probability Model (MPM0-III). Crit Care Med. 2009;37:1619–1623.
5 Higgins T.L., Teres D., Copes W.S., et al. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III). Crit Care Med. 2007;35:827–835.
6 Knaus W.A., Draper E.A., Wagner D.P., et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
7 Knaus W.A., Draper E.A., Wagner D.P., et al. Prognosis in acute organ-system failure. Ann Surg. 1985;202:685.
8 Knaus W.A., Wagner D.P., Draper E.A., et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636.
9 Kuzniewicz M.W., Vasilevskis E.E., Lane R., et al. Variation in ICU risk-adjusted mortality: impact of methods of assessment and potential confounders. Chest. 2008;133:1319–1327.
10 Le Gall J.R., Klar J., Lemeshow S., et al. The Logistic Organ Dysfunction system: a new way to assess organ dysfunction in the intensive care unit. JAMA. 1996;276:802–810.
11 Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963.
12 Lemeshow S., Teres D., Klar J., et al. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270:2478–2486.
13 Marshall J.C., Cook D.J., Christou N.V., et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652.
14 Metnitz P.G., Lang T., Valentin A., et al. Evaluation of the logistic organ dysfunction system for the assessment of organ dysfunction and mortality in critically ill patients. Intensive Care Med. 2001;27:992–998.
15 Moreno R.P., Metnitz P.G., Almeida E., et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2. Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355.
16 Moreno R., Vincent J.L., Matos A., et al. The use of maximum SOFA score to quantify organ failure/dysfunction in intensive care: result of a prospective multicenter study. Intensive Care Med. 1999;25:686–696.
17 Vincent J.L., de Mendonca A., Cantraine F., et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–1800.
18 Zimmerman J.E., Kramer A.A., McNair D.S., et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310.