Chapter 96 Scoliotic Cervical Deformity
Spinal deformities are more often encountered in the thoracic and lumbar spine. However, the cervical spine may also develop structural deformities secondary to congenital disorders,1 neuromuscular diseases,2,3 trauma,4 neoplastic disease,5 or previous spine surgery.6 These deformities may also occur in patients with systemic arthritides such as ankylosing spondylitis and rheumatoid arthritis. This chapter reviews the normal subaxial cervical anatomy, alignment, biomechanical properties, etiologies of scoliotic deformity, and treatment strategies.
Anatomy of the Cervical Spine
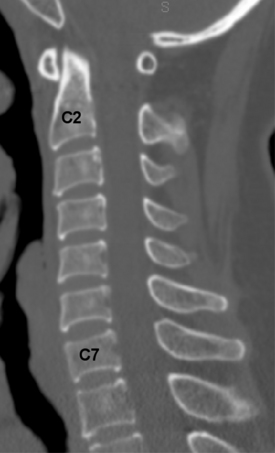
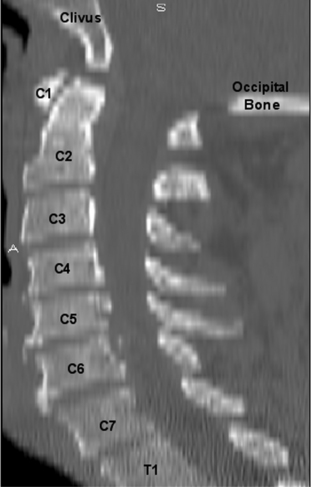
The cervical spine consists of seven vertebrae (C1-7) (Fig. 96-1).7 C1 and C2 are anatomic and functionally unique, which allows for the transition and attachment of the cervical spine to the cranium. These vertebrae (C1-2) are not considered when discussing the subaxial cervical spine (C3-7).
Vertebral Body and Disc
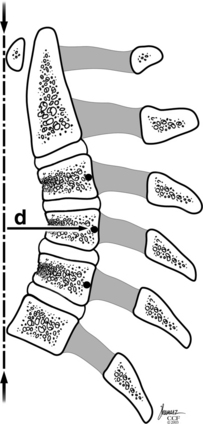
The size of the subaxial cervical vertebral bodies (C3-7) generally increases from rostral to caudal (see Fig. 96-1). The exception is the C6 body, which is slightly decreased in size compared with C5.8–10 This increase in the vertebral body’s width, depth, and total end-plate cross-sectional area allows greater loads to be supported and forces to be dispersed.11 The majority of these physiologic loads are carried through the ventral cervical vertebral body in a flexion posture, whereas the loads are carried through the dorsal elements (articular columns) in extension.10
The intervertebral disc connects the vertebral end plates and is composed of the cartilaginous end plate, anulus fibrosus, and nucleus pulposus. The disc heights are not symmetrical, with the ventral height being greater than the dorsal height (see Fig. 96-1). This contributes to the lordotic curvature of the cervical spine.9,12
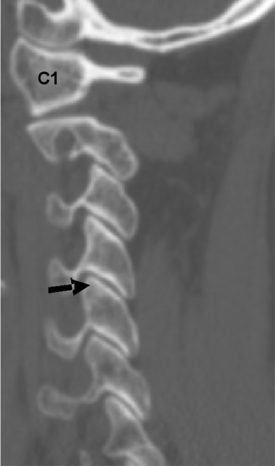
The pedicles are horizontal columns of bone that connect the vertebral body to the dorsal elements (Fig. 96-2). The cervical pedicles have an elliptical shape, with the height being greater than the width.11,13 The cortical bone surface of the cervical pedicles is similar on the rostral and caudal portion, but the lateral wall thickness is significantly less than the medial wall thickness.14 These pedicles insert into the vertebral body with transverse angle ranges from −8 to +11 degrees from the horizontal and sagittal angles ranging from 40 to 29 degrees.11
Articulations
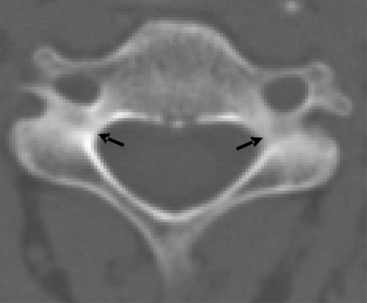
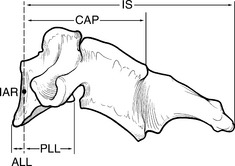
There are four articular surfaces between adjacent vertebral segments in the subaxial cervical spine, one set located on the vertebral body and another set involving the dorsal elements. The articulations located on the rostral, lateral dorsal aspect of the vertebral body are termed the uncovertebral or Luschka joints. These “joints” articulate with the caudal, dorsal, lateral aspect of the rostral vertebral body (Fig. 96-3).8 The heights of the uncinate process gradually increase as one descends the spine at each segment from C3-7, whereas the length and width remained relatively constant.13 These joints are actually believed to be degenerative clefts and not true joints because they are not present at birth and develop during adolescence.15,16
The dorsal elements of the cervical spine allow a large degree of mobility due to a pair of segmental articulations in the form of facet joints (see Fig. 96-3). These are apophyseal joints and are composed of a loose but strong capsule and synovial lining. The cervical facet joints are oriented at approximately 45 degrees in the coronal plane and 80 to 90 degrees in the sagittal plane.8,17 This facet orientation permits a large degree of sagittal plane motion, flexion, and extension but limits or restricts translation and lateral movements or bending.8,18
Ligaments
The anterior longitudinal ligament (ALL) and the posterior longitudinal ligament (PLL) are the two major ligaments in the cervical spine and are attached directly to the vertebral body. The ALL is a fibrous band that attaches to the edges of the vertebral bodies (C2 to sacrum) and that is diminished in width at the disc spaces. It provides significant support and resists cervical extension.19 The PLL also is continuous from C2 to the sacrum but differs from the ALL in that it narrows over the vertebral bodies and then widens at the disc interspace where it is interwoven with the anulus fibrosus. This ligament resists flexion and has half the strength of the ALL.19
Normal Cervical Alignment
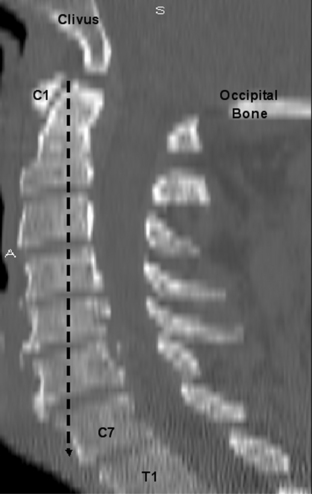
The cervical spine permits head rotation, flexion, and extension to maintain the line of sight while also placing the cranium over the pelvis and supporting a balanced upright posture. Hardacker et al. demonstrated that a plumb line dropped from the tip of the odontoid process would fall ventral to the seventh cervical vertebra, hence sagittal balance20 (Fig. 96-4).
It is difficult to define spine deformity based on clinical observation alone. Gross deformities may be determined through observation of the relationship of the tragus to the spinous process of C7 in the sagittal plane. However, these physical examination findings have been found to be inaccurate in most circumstances. In general, surface contour has not been shown to correlate with vertebral body location and position.21,22 Therefore, radiographs are essential to attempt to understand and document spinal alignment objectively.
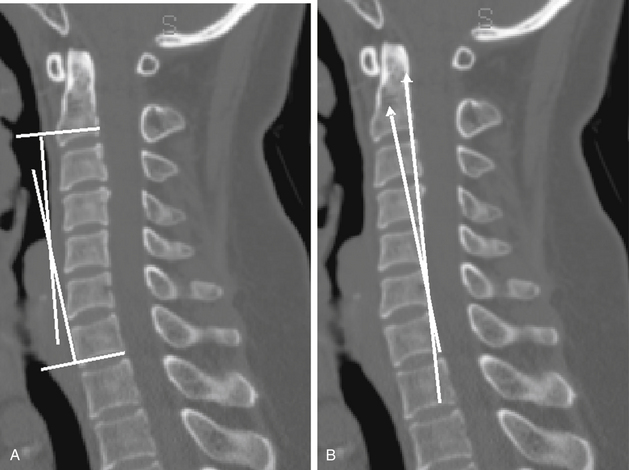
At present, there is no accepted standard measurement algorithm for sagittal or coronal cervical spinal curvature. The most commonly used method is the Cobb angle technique that places parallel lines from the caudal and rostral aspects of the vertebral bodies and then measures the intersection angle of these perpendicular lines23 (Fig. 96-5A). Inaccuracy of the Cobb method has been reported because it is based on the noncuboidal shape of the vertebral bodies, where the vertebral body end plate–to–dorsal cortical angle is greater than 90 degrees.24 Harrison used tangential lines to the dorsal vertebral bodies (Fig. 96-5B) and calculated the “normal” cervical (C2-7) lordosis to be 26 degrees (C2 and C7).24 Based on these results and engineering principles, he concluded that the tangential technique is more accurate at assessing the cervical angle than the Cobb method, which from C1-7, overestimated, and from C2-7, underestimated the lordosis.24
Cervical Alignment—Neutral
The length of the cervical spinal canal measured in the sagittal plane during flexion (kyphotic posture) is greater than during extension (lordotic posture).25 Therefore, the normal cervical lordosis allows the neural elements to traverse the spinal canal through a shorter course without ventral compression. The lordotic curvature may also protect against neural injury because axial loads are dispersed dorsally onto the facet joints and large articular pillars, rather than the vertebral body (as seen in kyphosis).
A number of disease processes affect the spine, in particular the thoracic and lumbar spine’s sagittal balance, which in turn affects the entire spine’s sagittal balance. The flexibility of the cervical spine allows it the ability to compensate for misalignment of the thoracic and lumbar spine. Therefore, an increased lordotic cervical posture has been observed when there was a concurrent exaggerated thoracic kyphosis.14,20,26–28 This compensation permits the maintenance of the overall sagittal balance (i.e., head over the pelvis).
Hardacker et al. measured the cervical curvature of 100 volunteers and recorded the mean total cervical lordosis alignment (foramen magnum to C7 inferior end plate) as 40 degrees (standard deviation of 9.7 degrees) where the majority of the lordotic curve was at the C1-2 junction and only 6 degrees was present from C4 to C7.20 Despite no patient having an overall cervical kyphotic posture, 39% had a segmental kyphotic angle greater than 5 degrees, typically at C4-5 and C5-6, based on individual segmental angles analysis.20 Gore et al.29 also measured mean lordotic angles in the cervical spine of osteoarthritis patients from perpendicular lines of the C2 and C7 bodies and observed a 16- to 22-degree lordosis for men and a 15- to 25-degree lordosis in women. Others have measured the C2-7 lordotic curvature to be approximately 14 degrees.30,31 Although numerous studies have shown a wide range for cervical lordosis, slight head extension (0–13.9 degrees) has been shown not to affect the cervical spine alignment.32
Overall, there is not an accepted range defined as “normal” for cervical posture. Although definitive angles have not been calculated, studies have shown that due to aging and degenerative changes, the cervical spine has an increase in the lordotic angle.20,29,33 This lordotic angle increases with aging,20 from approximately 15 degrees in the third decade to 22 to 25 degrees in the seventh decade.29
Cervical Alignment—Dynamic Movement
The cervical spine, also, allows a great degree of flexibility, and dynamic images (flexion and extension radiographs) permit an assessment of the intersegmental motion related to this flexibility. Flexion and extension radiographs of normal individuals have shown that the greatest motion occurs at C4-5 and C5-6 and the least at C2-3.25,33–35 Lin et al.35 further demonstrated that the spine moved from a lordotic position in extension to a nearly parallel position with flexion, such that all intervertebral differences in angular displacement were less than 7 degrees and translation was less than 0.6 mm. The total range of motion (ROM) from C2 to C7 was reported from 50 degrees to greater than 90 degrees with a normal gaussian distribution and a mean of 67 degrees.35 This ROM is affected by the stiffening of the spine, which occurs throughout the normal aging process.36
Degenerative Changes
Cervical spondylotic myelopathy is a pathologic process that affects the aging spine. Gore et al. showed that 90% of asymptomatic males, age 60 to 65 years, had degenerative changes on cervical roentgenographic studies.29 As a result of aging, the vertebral discs dehydrate, and the vertebral column loses height (Fig. 96-6). This loss of height results in a decreased tension of the ligamentum flavum. The loss of tension on the ligament causes it to shorten and results in buckling of the ligament into the spinal canal, with possible spinal cord compression. Also, degenerative and osteophytic changes take place in the facet joints, along with the vertebral disc space junction. The result is a stiffening or rigidity of the cervical spine and a decreased ROM. Holmes illustrated this by showing that the spine segments with the greatest motion migrate from C5-6 to C4-5 with aging.33
The overall alignment of the cervical spine should not assume a kyphotic posture due to the normal aging process (see Fig. 96-6). Gore et al. showed that, despite the decrease in the intervertebral distance, the overall lordotic curve (C2-7) increased with aging in 200 asymptomatic adults.29
Biomechanical Principles of the Cervical Spine
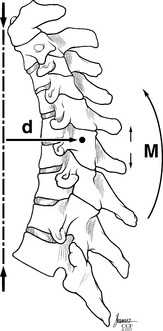
The point or position in the vertebral body that all other points rotate about when a movement occurs is termed the instantaneous axis of rotation (IAR). This is not a static point but rather is dynamic and changes with position, posture, and direction of movements. Also, each segment has a unique IAR for every movement; it is influenced by spine alignment, anatomy, muscle, and loads exerted. White and Panjabi10 theorized that in the sagittal plane for flexion and extension, the IAR is located in the ventral portion of the vertebral body. Therefore, each vertebral body has its own IAR for each directional movement. The summation of all the IAR movements dictates spinal column orientation and motion (Fig. 96-7).
These forces that act on the spinal column have both a direction and a magnitude and are therefore referred to as force vectors. The perpendicular distance between a force vector and the IAR is defined as the lever or moment arm. The combination of the force vector and the lever arm results in a bending moment about the IAR. Therefore, the IAR can be thought of as a fulcrum, such that with flexion all points ventral to it come together and all points dorsal spread apart8 (see Fig. 96-7).
The muscle and ligament complexes of the cervical spine have a significant effect on the support, motion, and stability of the cervical spine. The effectiveness of each ligament is not only related to the strength of that ligament but also to the moment arm through which the ligament acts8,37 (Fig. 96-8). A weaker ligament with a longer level arm might provide more support and strength to the spine than a very strong ligament located on the spinal column, with a short lever arm.
Multiple studies have illustrated the importance of intact and functioning cervical musculature to support and maintain the cervical lordosis. Nolan and Sherk performed a biomechanical analysis of the extensor musculature of the cervical spine and demonstrated that the semispinalis muscle acted as a dynamic stabilizer and the removal of its attachments resulted in the loss of cervical lordosis.38 Iizuka et al.39 reattached the semispinalis cervicis muscle after laminoplasty and found that attachment of the extensor musculature on serial MRIs correlated with a maintained cervical lordosis in the postoperative period.
The majority of the cervical spine’s ligamentous and muscle complexes support and attach to the dorsal aspect of the spine. These structures, along with the laminae, provide a lever arm, which allows the cervical spine to maintain a lordotic curve. Panjabi showed that without muscular support, the osteoligamentous cervical spine would buckle and fail at only one fifth the weight of the human head.40,41 These cervical spine specimens (C0-T1) failed with increasing loads of only 11 N,40 whereas in vivo load testing has shown load ranges from 53 to 1175 N.42,43 These data have to be taken in the context that in vitro models comparing segmental vertebral body motions do not correlate well with in vivo data.41
The human body’s center of gravity is located approximately 4 cm ventral to the sacrum. Therefore, in the sagittal plane, this center of gravity is ventral to the vertebral body. In the standing individual, a plumb line dropped from the tip of the odontoid process falls slightly ventral (0–2 cm) to the ventral surface of the C7 vertebral body (see Fig. 96-4). The cervical IAR is also located ventral to the vertebral bodies. Therefore, there is a constant attraction or force drawing or pulling the spine toward the center of gravity. Newton’s first law states that an object at rest tends to stay at rest while an object in motion tends to stay in motion with the same speed and in the same direction unless acted on by an unbalanced force. Therefore, as long as the spine has the ability to resist against these gravitational forces through a strong tension band in the form of the dorsal musculoligamentous complex, the spine will maintain a lordotic curvature. Otherwise, the cervical spine will gravitate to its lowest energy state or a kyphotic posture, which implies that the cervical spine, without resisting forces, would prefer to be located at the center of gravity or zero energy state.
The strong muscles and ligaments of the cervical spine are able to maintain this lordotic posture against the gravitational forces due to their increased distance or greater lever arm relative to the IAR (see Fig. 96-8). This increased distance creates a mechanical advantage that the muscles and ligaments use to maintain a lordotic curvature. If the muscles and ligaments are weakened either due to congenital diseases or iatrogenic causes (postsurgical), such a dorsal “anchor” has a less substantial effect. Then, despite the mechanical advantage provided by the increased lever arm, the cervical spine will migrate further ventrally and the result will be a loss of the lordotic curvature. The ventral migration toward the IAR causes the lever arm that the compromised dorsal muscles and ligaments are acting through to be shorter (Fig. 96-9). The shorter lever arm and the weakened dorsal tension band, ligaments, and muscles fail to provide ample support. This in turn results in further progression of the kyphotic deformity.
This cycle of impaired muscles and shorter lever arm continues until a significant deformity with possible neurologic findings results. This sequence may be illustrated in individuals with Marfan syndrome. In this disorder, the affected patients have an increased laxity of their ligamentous structures and therefore are at an increased risk for abnormal cervical alignment. Hobbs et al.27 confirmed this by showing that 36% of patients with this disorder had absence of the normal cervical lordosis.
Etiologies of Cervical Deformity
Subaxial cervical deformities can develop in any plane or direction but most commonly occur in the sagittal plane, primarily as a kyphotic deformity, and rarely as coronal plane abnormalities (i.e., scoliosis). Cervical and cervicothoracic scoliosis are extremely rare disorders and only sparse reports of their occurrences are available in the literature. However, there are multiple causes for kyphotic deformities, resulting either through congenital or acquired disorders. Congenital disorders (Klippel-Feil syndrome, hemivertebrae),44–46 systemic disorders (dystrophic dystonia, Larsen syndrome),47–51 and neuromuscular disorders (Marfan syndrome, Prader-Willi syndrome)27,52 can all result in cervical deformities. Acquired kyphotic cervical spine disorders may be secondary to spinal tumors (neurofibromatosis),53 occupational exposures,54 iatrogenic causes (postlaminectomy, pseudarthrosis),55,56 and traumatic injuries.
This discussion focuses on cervical scoliotic deformities, their etiologies, and their treatment.
Cervical and Cervicothoracic Scoliosis
Smith defined cervical and cervicothoracic scoliosis as a structural curvature of the cervical or cervicothoracic region resulting from an osseous abnormality such as a block vertebra or unilateral bar that is visible on either anteroposterior or lateral radiographs (Fig. 96-10).44,45 Other etiologies include a failure of segmentation, block vertebrae, a failure of formation, hemivertebrae, or a combination of these anomalies.44,45 Cervical scoliosis is occasionally associated with unilateral congenital nerve defects in the upper extremity.46 This disorder typically occurs in children, and they present early due to cosmetic concerns of malpositioning of the head. Cervical spine alignment abnormalities do not have the ability to form a rostral compensatory curve because the head is the rostral terminus of the spine.
Congenital Scoliosis
The origin of congenital scoliosis may be environmental or genetic, or the condition may result from a combination of factors. Environmental factors, genetics, vitamin deficiency, chemicals, and drugs, singly or in combination, have all been implicated in the development of vertebral abnormalities. Whatever the cause, the physiologic injury occurs early in the embryologic period, well before the development of cartilage and bone. The resulting defects can lead to full or partial fusion or lack of development of the vertebrae, which, in turn, can cause a curvature that may be progressive during the growth of the child.57
The body of literature describing congenital cervical scoliosis is found in the form of case reports. Winter and House46 describe two cases of congenital cervical scoliosis associated with a unilateral progressive nerve deficit in the upper extremity. A unilateral spina bifida was present in both cases. The patients were treated with a fusion at 2 and 5 years. Poole and Briggs58 describe a “cranio-facio-cervical scoliosis complex” in six cases, all presenting with facial asymmetry, vertical orbital dystopia, and torticollis. Five of the six patients had hemifacial microsomia. Four of the six had symptomatic cervical scoliosis.
Ruf et al.59 evaluated three patients treated with hemivertebra resection for congenital cervical scoliosis presenting with torticollis. Surgery was performed by the dorsal-ventral-dorsal approach at a mean age of 9 years. The mean Cobb angle was 29 degrees prior to operating, 5 degrees postoperatively, and 6 degrees on follow-up. Torticollis improved by 16 degrees to an average of 1 degree postoperatively and 3 degrees on follow-up examination.
Svantesson et al.60 analyzed 320 patients in Sweden with juvenile rheumatoid arthritis and found that 17 (5.3%) had scoliosis predominating in the thoracic and lumbar spine, with minimal cervical involvement. Twelve of the 17 had a curvature greater than 20 degrees. Torticollis was seen in 3 of the 17 patients and was associated with increased cervical curvature.
Marfan Syndrome
The incidence of scoliosis in patients with Marfan syndrome ranges from 55% to 64% among reported case series.61 There is a predilection for thoracic and lumbar spine involvement, and cervical scoliosis is a rare entity in Marfan syndrome. It is proposed that connective tissue laxity in conjunction with early puberty in this population causes earlier peak growth velocity that results in pelvic malpositioning or rotation. The resulting malpositioned pelvis may predispose to scoliosis.61
The coronal spinal deformity or scoliosis associated with Marfan syndrome has a clinical presentation similar to idiopathic scoliosis, with the exception of a higher prevalence of triple major curvature and double thoracic curvature. Sponsellar et al. noted that an adolescent curvature exceeding 40 to 50 degrees had a higher likelihood to progress into maturity, suggesting that treatment goals should be to prevent curvature of adolescence from exceeding 40 degrees.62
Sponsellar et al. further monitored 14 patients with coronal curves less than 45 degrees, treated for an average of 21 hours daily with a brace for at least 2 years or until skeletal maturity. He reported a 17% “success rate” where success was defined as prevention of curvature from exceeding 45 degrees or progression of curvature below 5 degrees. This “success rate” drastically contrasts to the 45% to 63% success rate for bracing patients with idiopathic scoliosis.63 Progression of curvature averaged 6 to 8 degrees per year with a final average curvature of 49 degrees.62 These data suggest that for adolescent patients with curvature greater than 40 degrees, bracing would be ineffective at preventing disease progression. However, bracing may still be an option for those with curvature ranging from 15 to 25 degrees.
In an additional retrospective review, 39 patients with Marfan syndrome who underwent deformity correction surgery at two institutions demonstrated the technical difficulties with instrumentation and inherent comorbidity due to cardiopulmonary insufficiency.63 Pseudarthrosis was noted in 10%, and there was an additional instrumentation fixation failure rate of 21%. Infection was as high as 8%. Experience with operating on this rare population demonstrates the need for preoperative CT evaluation of the bony anatomy for assessment of its adequacy for fixation.63
Halo traction is a well-recognized adjunct in addition to ventral and dorsal release and fusion for correcting severe, complex, rigid scoliotic curves. However, caution should be applied when using halo traction in patients with Marfan or other connective tissue disorders. Halo traction creates more tension in the cervical spine than the thoracolumbar spine. This, in addition to the laxity of the connective tissue in Marfan, can cause worsening of existing cervical kyphosis after the traction.64
Jeune Syndrome
Jeune syndrome, also known as asphyxiating thoracic dystrophy, is characterized by a small, narrow chest with varying degrees of limb shortness. The prognosis is grim due to neonatal respiratory distress.65 In a case series involving 13 patients, thoracic malformations tended to become less severe with age, although the majority of patients’ height was found to be below the third percentile by adulthood.65 A variety of pelvic abnormalities, a high rate of dwarfism, and predominantly thoracic skeletal dysplasia all are thought to contribute to scoliosis.
Patients with Jeune syndrome have a high rate of proximal cervical stenosis and should undergo screening with cervical spine films at birth.66 Significant stenosis or instability may require decompression and cervico-occipital fusion.
Larsen Syndrome
Larsen syndrome is a rare congenital disease typically presenting with multiple congenital hip dislocations, “dish” facies (a term for a saddle nose and hypertelorism), and less commonly cervical scoliosis and kyphosis.67 Larsen syndrome may have early-onset scoliosis that is very rigid and requires early intervention. Cervical kyphosis and subluxation may be lethal in affected patients, and screening radiographs are important. Upper airway abnormalities are an anesthesia concern.
Literature pertaining to coronal spinal deformity correction is entirely retrospective by way of case reports. One case report by Hosoe et al.67 describes a 12-year-old girl who presented with bilaterally dislocated hips and right thoracic curvature with a Cobb angle of 77 degrees. Abnormal thoracic lordosis and lumbar hyperlordosis were present. Minimal cervical scoliosis also was evident radiographically. A dorsal spinal fusion from T4 to L2 resulted in improved thoracic scoliosis from 77 to 28 degrees and improvement of deformity in the sagittal plane as well. On 15-year follow-up, the patient was asymptomatic in her spine and hips. The significance of hip dislocations is that the associated flexion contracture of the hips is thought to have an influence on spinal sagittal alignment. In this case, spinal surgery preceded hip surgery. The position of the pelvis after spinal fusion is unpredictable. Secondly, the effect of abnormal muscle forces to the hips due to spinal deformity makes hip surgery technically difficult.68
Jarcho-Levin Syndrome
Jarcho-Levin syndrome is a congenital disorder occasionally marked by scoliosis. It is characterized most commonly by the presence of rib and vertebral defects at birth. This syndrome most commonly presents in infancy and is characterized by identification of a short neck, trunk, and stature. Skeletal survey characterizes multiple vertebral anomalies at different levels of the spine, including “butterfly vertebrae,” hemivertebrae, and fused hypoplastic vertebrae. Unfortunately, the associated small size of the thorax in newborns frequently leads to respiratory compromise and death in infancy.69
Jarcho-Levin syndrome results in a thoracic volume depletion deformity due to shortness of the thoracic cavity, due to either a spondylocostal dysostosis variant or spondylothoracic dysplasia. The former has chaotic congenital scoliosis with varied combination of missing and fused ribs. Although spondylocostal dysostosis has a benign reputation in the literature for respiratory complications, respiratory insufficiency is nevertheless common and one death is known from respiratory failure. Spondylothoracic dysplasia seldom has significant scoliosis but has a mortality rate approaching 50% from respiratory complications due to thoracic insufficiency syndrome. In spite of severe restrictive respiratory disease, adult survivors of spondylothoracic dysplasia appear to do well clinically for unknown reasons. Cerebrocostomandibular syndrome is characterized by scoliosis, micrognathia, and thoracic insufficiency syndrome, due to an “implosion” deformity of the thorax from congenital pseudarthrosis of the dorsal ribs.66
Klippel-Feil Syndrome
Klippel-Feil syndrome is a congenital disorder of spinal segmentation distinguished by the bony fusion of ventral cervical vertebrae. Feil subclassified patients presenting with Klippel-Feil syndrome into three groups. Group I included patients with fusion of cervical and upper thoracic vertebrae with synostosis. Group II included patients with congenital fusion of the vertebrae in the cervical spine only. Group III included patients with cervical vertebral fusions as well as associated lower thoracic or upper lumbar fusion.70 Scoliosis, mirror movements, and otolaryngologic, kidney, ocular, cranial, limb, and/or digit anomalies are often associated. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome.71
Wildervanck syndrome, or cervico-oculo-acoustic syndrome, refers to Klippel-Feil syndrome with the additional findings of congenital sensorineural deafness and Duane retraction syndrome (characterized by deficits with abduction and adduction of the eye).72 Wildervanck syndrome is the most common abnormality to overlap with Duane retraction syndrome. The fact that Klippel-Feil syndrome has been shown to overlap with Noonan, Turner, and Wildervanck syndromes should warrant a comprehensive examination by the clinician to rule out alternative diagnoses.72
Thomsen et al. reviewed data from 57 patients with Klippel-Feil syndrome treated over 25 years. He found that scoliosis in patients with group I had a Cobb angle of 31 degrees at the level of deformity. Patients in groups II and III had Cobb angles of 9 and 23 degrees, respectively. With isolated cervical spine involvement, there was the least risk of scoliosis. Still, 70% of the patient population demonstrated scoliosis.70
Cervical Spine Dysmorphisms
Cervical spine dysmorphisms (CSDs) occur in a heterogeneous group of patients unified by the presence of congenital defects that result from malalignment, formation, or segmentation of the cervical spine, thus generating disability. This problem requires comprehensive evaluation of patients with a diagnosis of scoliosis, correlating clinical and radiologic findings and the presence of numerous abnormalities of other systems to give an appropriate syndrome diagnosis and multidisciplinary management of these patients with the aim to give them an integral rehabilitation treatment increasing their quality of life. Santillan Chapa et al. described clinical and radiologic findings in children with diagnosed CSD. They studied 47 consecutive outpatients of the Pediatric Rehabilitation Division in Instituto Nacional de Rehabilitacion with diagnosed scoliosis. Sixteen patients (34%) had diagnosed CSD. The most frequently seen syndromes were Klippel-Feil (19%), Wildervanck (4.3%), neurofibromatosis (4.3%), Morquio (2.1%), Stickler (2.1%), and Williams (2.1%). The researchers found CSD in 34% of the group studied, greater than in the medical literature.73
Morquio syndrome, or mucopolysaccharidosis IVA, is a genetic deficiency of N-acetylgalactosamine-6-sulfate sulfatase that results in accumulation of lysosomal mucopolysaccharides. This accumulation occasionally manifests as cervical scoliosis among a varied presentation of skeletal dysplasias.74 Stickler syndrome is an autosomal dominant connective tissue disorder marked by ocular involvement. Obvious skeletal and facial deformities are present. Cervical dysmorphism is highly likely by adulthood.75
Neuromuscular Scoliosis
Scoliosis is seen in a variety of neurologic disorders at different levels of the nervous system, from peripheral nerves to the CNS.76 As seen in poliomyelitis, spina bifida, cerebral palsy, spinal muscular atrophy as well as rarer conditions, disorders of the segmental efferent nervous system result in the development of scoliosis secondarily.76 The goal of deformity correction is to stabilize the spine without the loss of motor or sensory function, with the torso balanced over a level spine.77
Surgical stabilization is the standard treatment of neuromuscular spinal deformities. The debate persists today regarding the necessity for extending the construct and arthrodesis to the sacrum. Studies suggest a similarity initially between patients with fusions distally to either the lumbar spine or sacrum. One view is that with higher grade curvature and pelvic obliquity, suprapelvic fusion results in an increased loss of correction on long-term follow-up.78 On the other hand, McCall and Hayes demonstrate in long-term follow-up of 55 patients who underwent instrumentation and fusion to L5 only that a comparable degree of deformity correction is maintained.77
Idiopathic Scoliosis
Adolescent idiopathic scoliosis (AIS) is the most common form of scoliosis because of its exclusionary diagnosis. AIS is diagnosed as a coronal curvature of the spine greater than 10 degrees with a rotational component and no other etiologic explanation in a child 9 years of age or older. If the child is younger than 9 years of age, the diagnosis is referred to as early-onset scoliosis.69 Despite years of research, the etiology of AIS is poorly understood.76 Indeed, the pathogenesis is multifactorial. Wynne-Davies79 screened 114 first-, second-, and third-degree relatives of patients with AIS to indicate a dominant or multiple gene inheritance pattern. Work by Robin and Cohen80 suggested autosomal inheritance.
Historically, treatment options for AIS included exercise, in-patient rehabilitation, braces, and surgery. Evidence suggests that the use of scoliosis intensive rehabilitation and braces can alter the natural history of the condition. However, no prospective controlled studies compare the natural history with surgical treatment.81 Genome-wide association studies have successfully identified single nucleotide polymorphisms related to the severity of curve progression.69
Traumatic Scoliosis
Scoliosis can occur in the acute setting of trauma. Shen and Samartzis82 describe a 7-year-old boy with the development of scoliosis following a motor vehicle crash. The patient was without motor or sensory deficits on examination and had intact rectal tone and normal volition. An upright radiograph demonstrated a traumatic scoliosis with widening of the dorsal elements, a so-called “Chance fracture” or “flexion-distraction” injury. The patient underwent a dorsal spinal fusion and stabilization with realignment. Follow-up examination and radiographs demonstrated a solid fusion, full ROM, and absence of pain.
Purely ligamentous flexion-distraction injuries, as seen in the previously described case, can be difficult to heal with bracing alone and can result in a dramatic acute traumatic scoliosis.82 Surgical fixation is needed to achieve stabilization through arthrodesis. Unilateral cervical jumped fractures can also present with scoliosis. Treatment is closed (via traction) or open (via surgery) reduction of the fracture, depending on the neurologic status of the patient and a review of appropriate imaging, followed by halo or instrumented fixation.
Treatment Strategies for Cervical Scoliosis
Treatment options vary depending on the degree of curvature, the anatomic abnormalities, and the age and medical condition of the patient. Bracing is the least invasive technique but unfortunately is not an optimal treatment strategy, because most deformities are due to segmental, formation, and developmental disorders, and, therefore, are usually associated with a large curve. In the few patients with a scoliotic angle less than 30 degrees that passively correct past the neutral plane, bracing is the best treatment option.44,45 These patients must be able to wear their brace for extended periods and be followed clinically with serial imaging and clinical examinations for signs of curve progression.
Unfortunately, most patients present with severe curves (i.e., >40 degrees), rigidity, severe torticollis, and lateral tilting that does not respond to bracing.44,45 Smith et al.44,45 advocated operative treatment strategy for these patients via a dorsal fusion of the structural portion of the curve. Surgical dissection must be meticulous because the incidence of bone abnormalities and absent lamina has been reported to be as high as 30%.45 Fortunately, these curves are usually flexible due to the young age of the patient population and can be manipulated intraoperatively. Smith,45 using this dorsal arthrodesis technique, had a solid fusion in 20 of 21 patients, with an average follow-up of 17 years. If the curves are stiff and rigid, the use of traction preoperatively increases the mobility of the spine. Winter83 had two cases of cervical scoliosis with associated arm paralysis and advocated early surgical intervention for progressive spinal deformity. Deburge84 reported one patient with a cervical scoliosis secondary to a hemivertebra and Klippel-Feil syndrome that was treated with a staged ventral and dorsal procedure.
With the advent of segmental pedicle screw fixation that enables more powerful corrective forces, Suk et al. reported that an additional ventral procedure may be unnecessary even in severe cervical scoliotic deformities. Thirty-five scoliosis patients treated by pedicle screw fixation and rod derotation were retrospectively analyzed after a minimum follow-up of 2 years. Residual coronal decompensation was observed in only three patients postoperatively. The authors concluded that dorsal segmental pedicle screw fixation without ventral release in severe scoliosis had satisfactory deformity correction without significant loss of curve correction.85
Hilibrand A.S., Tannenbaum D.A., Graziano G.P., et al. The sagittal alignment of the cervical spine in adolescent idiopathic scoliosis. J Pediatr Orthop. 1995;15:627-632.
Hobbs W.R., Sponseller P.D., Weiss A.P., Pyeritz R.E. The cervical spine in Marfan syndrome. Spine (Phila Pa 1976). 1997;22:983-989.
Smith M.D. Congenital scoliosis of the cervical or cervicothoracic spine. Orthop Clin North Am. 1994;25:301-310.
Smith M.D., Lonstein J.E., Winter R.B. Congenital cervicothoraic scoliosis. A long term follow-up study. Orthop Trans. 1992;16:165-170.
Weiss H.R. Is there a body of evidence for the treatment of patients with adolescent idiopathic scoliosis (AIS)? Scoliosis. 2007;2:19.
White A.A.III, Johnson R.M., Panjabi M.M., et al. Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res. 1975;109:85-96.
Wynne-Davies R. Genetic aspects of idiopathic scoliosis. Dev Med Child Neurol. 1973;15:809-811.
1. Bethem D., Winter R.B., Lutter L. Disorders of the spine in diastrophic dwarfism. J Bone Joint Surg [Am]. 1980;62:529-536.
2. Abumi K., Shono Y., Taneichi H., et al. Correction of cervical kyphosis using pedicle screw fixation systems. Spine (Phila Pa 1976). 1999;24:2389-2396.
3. Piccirilli C.B., Chadduck W.M. Cervical kyphotic myelopathy in a child with Morquio syndrome. Childs Nerv Syst. 1996;12:114-116.
4. Polly D.W., Klemme W.R., Shawen S. Management options for the treatment of posttraumatic thoracic kyphosis. Semin Spine Surg. 2000;12:110-116.
5. Asazuma T., Yamagishi M., Nemoto K., et al. Spinal fusion using a vascularized fibular bone graft for a patient with cervical kyphosis due to neurofibromatosis. J Spinal Disord. 1997;10:537-540.
6. Johnston F.G., Crockard H.A. One-stage internal fixation and anterior fusion in complex cervical spinal disorders. J Neurosurg. 1995;82:234-238.
7. Gray H. Anatomy, descriptive and surgical. New York: Bounty Books; 1977.
8. Benzel E.C. Biomechanics of spine stabilization. Rolling Meadows, IL: American Association of Neurological Surgeons; 2001.
9. Lu J., Ebraheim N.A., Yang H., Rollins J., et al. Anatomic bases for anterior spinal surgery: surgical anatomy of the cervical vertebral body and disc space. Surg Radiol Anat. 1999;21:235-239.
10. White A.A.III, Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: Lippincott Williams & Wilkins; 1990.
11. Panjabi M.M., Duranceau J., Goel V., et al. Cervical human vertebrae. Quantitative three-dimensional anatomy of the middle and lower regions. Spine (Phila Pa 1976). 1991;16:861-869.
12. De Palma A.F., Rothman R.H. The intervertebral disc. Philadelphia: WB Saunders; 1970.
13. Ugur H.C., Attar A., Uz A., et al. Surgical anatomic evaluation of the cervical pedicle and adjacent neural structures. Neurosurgery. 2000;47:1162-1168. discussion 1168–1169
14. Panjabi M.M., Shin E.K., Chen N.C., Wang J.L. Internal morphology of human cervical pedicles. Spine (Phila Pa 1976). 2000;25:1197-1205.
15. Bland J.H., Boushey D.R. Anatomy and physiology of the cervical spine. Semin Arthritis Rheum. 1990;20:1-20.
16. Heller J.G., Silcox D.H.III, Sutterlin C.E.III. Complications of posterior cervical plating. Spine (Phila Pa 1976). 1995;20:2442-2448.
17. Panjabi M.M., Oxland T., Takata K., et al. Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1993;18:1298-1310.
18. Raynor R.B., Moskovich R., Zidel P., Pugh J. Alterations in primary and coupled neck motions after facetectomy. Neurosurgery. 1987;21:681-687.
19. White A.A.III, Johnson R.M., Panjabi M.M., Southwick W.O. Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res. 1975;109:85-96.
20. Hardacker J.W., Shuford R.F., Capicotto P.N., Pryor P.W. Radiographic standing cervical segmental alignment in adult volunteers without neck symptoms. Spine (Phila Pa 1976). 1997;22:1472-1480. discussion 1480
21. Johnson G.M. The correlation between surface measurement of head and neck posture and the anatomic position of the upper cervical vertebrae. Spine. 1998;23:921-927.
22. Refshauge K.M., Goodsell M., Lee M. The relationship between surface contour and vertebral body measures of upper spine curvature. Spine (Phila Pa 1976). 1994;19:2180-2185.
23. Cobb J.R. Chapter outlines for the study of scoliosis. In: Edwards J.W., editor. Outlines for the study of scoliosis: instructional course lecture. Ann Arbor, MI: American Academy of Orthopedic Surgeons; 1948:261-275.
24. Harrison D.E., Harrison D.D., Cailliet R., et al. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976). 2000;25:2072-2078.
25. Penning L. Normal movements of the cervical spine. AJR Am J Roentgenol. 1978;130:317-326.
26. Hilibrand A.S., Tannenbaum D.A., Graziano G.P., et al. The sagittal alignment of the cervical spine in adolescent idiopathic scoliosis. J Pediatr Orthop. 1995;15:627-632.
27. Hobbs W.R., Sponseller P.D., Weiss A.P., Pyeritz R.E. The cervical spine in Marfan syndrome. Spine (Phila Pa 1976). 1997;22:983-989.
28. Loder R.T. The sagittal profile of the cervical and lumbosacral spine in Scheuermann thoracic kyphosis. J Spinal Disord. 2001;14:226-231.
29. Gore D.R., Sepic S.B., Gardner G.M. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976). 1986;11:521-524.
30. Pal G.P., Sherk H.H. The vertical stability of the cervical spine. Spine (Phila Pa 1976). 1988;13:447-449.
31. Zdeblick T.A., Zou D., Warden K.E., et al. Cervical stability after foraminotomy. A biomechanical in vitro analysis. J Bone Joint Surg [Am]. 1992;74:22-27.
32. Harrison D.E., Harrison D.D., Janik T.J., et al. Slight head extension: does it change the sagittal cervical curve? Eur Spine J. 2001;10:149-153.
33. Holmes A., Wang C., Han Z.H., Dang G.T. The range and nature of flexion-extension motion in the cervical spine. Spine (Phila Pa 1976). 1994;19:2505-2510.
34. Johnson R.M., Hart D.L., Simmons E.F., et al. Cervical orthoses. A study comparing their effectiveness in restricting cervical motion in normal subjects. J Bone Joint Surg [Am]. 1977;59:332-339.
35. Lin R.M., Tsai K.H., Chu L.P., Chang P.Q. Characteristics of sagittal vertebral alignment in flexion determined by dynamic radiographs of the cervical spine. Spine (Phila Pa 1976). 2001;26:256-261.
36. Ten Have H.A., Eulderink F. Degenerative changes in the cervical spine and their relationship to its mobility. J Pathol. 1980;132:133-159.
37. Panjabi M.M., Greenstein G., Duranceau J., Nolte L.P. Three-dimensional quantitative morphology of lumbar spinal ligaments. J Spinal Disord. 1991;4:54-62.
38. Nolan J.P.Jr., Sherk H.H. Biomechanical evaluation of the extensor musculature of the cervical spine. Spine (Phila Pa 1976). 1988;13:9-11.
39. Iizuka H., Shimizu T., Tateno K., et al. Extensor musculature of the cervical spine after laminoplasty: morphologic evaluation by coronal view of the magnetic resonance image. Spine (Phila Pa 1976). 2001;26:2220-2226.
40. Panjabi M.M., Cholewicki J., Nibu K., et al. Critical load of the human cervical spine: an in vitro experimental study. Clin Biomech (Bristol, Avon). 1998;13:11-17.
41. Panjabi M.M., Miura T., Cripton P.A., et al. Development of a system for in vitro neck muscle force replication in whole cervical spine experiments. Spine (Phila Pa 1976). 2001;26:2214-2219.
42. Hattori S., Oda H., Kawaii S. Cervical intradiscal pressure in movements and traction of the cervical spine. Z Orthop. 1998;119:568-569.
43. Moroney S.P., Schultz A.B., Miller J.A. Analysis and measurement of neck loads. J Orthop Res. 1988;6:713-720.
44. Smith M.D. Congenital scoliosis of the cervical or cervicothoracic spine. Orthop Clin North Am. 1994;25:301-310.
45. Smith M.D., Lonstein J.E., Winter R.B. Congenital cervicothoraic scoliosis. A long term follow-up study. Orthop Trans. 1992;16:165-170.
46. Winter R.B., House J.H. Congenital cervical scoliosis with unilateral congenital nerve deficit in the upper extremity. Report of two cases. Spine (Phila Pa 1976). 1981;6:341-346.
47. Goto S., Kobayashi Y., Saisu T., Moriya H. Cervical myelopathy caused by destructive kyphotic spine in Cushing’s disease. J Bone Miner Metab. 1999;17:301-307.
48. Larsen L.J., Schottstaedt E.R., Bost F.C. Multiple congenital dislocations associated with characteristic facial abnormality. J Pediatr. 1950;37:574-581.
49. Micheli L.J., Hall J.E., Watts H.G. Spinal instability in Larsen’s syndrome: report of three cases. J Bone Joint Surg [Am]. 1976;58:562-565.
50. Remes V., Marttinen E., Poussa M., et al. Cervical kyphosis in diastrophic dysplasia. Spine (Phila Pa 1976). 1999;24:1990-1995.
51. Remes V., Tervahartiala P., Poussa M., Peltonen J. Cervical spine in diastrophic dysplasia: an MRI analysis. J Pediatr Orthop. 2000;20:48-53.
52. Tsuji M., Kurihara A., Uratsuji M., Shoda E. Cervical myelopathy with Prader-Willi syndrome in a 13-year-old boy. A case report. Spine (Phila Pa 1976). 1991;16:1342-1344.
53. Nemoto K., Asazuma T., Amako M., et al. Vascularized fibula graft for spinal fusion in severe cervical kyphosis due to neurofibromatosis. J Reconstr Microsurg. 1997;13:559-562.
54. Kelkar P., O’Callaghan B., Lovblad K.O. Asymptomatic grotesque deformities of the cervical spine. An occupational hazard in railway porters. Spine (Phila Pa 1976). 1998;23:737-740.
55. Albert T.J., Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976). 1998;23:2738-2745.
56. Fager C.A. Laminectomy and kyphotic deformity. J Neurosurg. 2001;95:157-158.
57. Batra S., Ahuja S. Congenital scoliosis: management and future directions. Acta Orthop Belg. 2008;74:147-160.
58. Poole M.D., Briggs M. The cranio-facio-cervical scoliosis complex. Br J Plast Surg. 1990;43:670-675.
59. Ruf M., Jensen R., Harms J. Hemivertebra resection in the cervical spine. Spine (Phila Pa 1976). 2005;30:380-385.
60. Svantesson H., Marhaug G., Haeffner F. Scoliosis in children with juvenile rheumatoid arthritis. Scand J Rheumatol. 1981;10:65-68.
61. Garreau de Loubresse C., Mullins M.M., et al. Spinal and pelvic parameters in Marfan’s syndrome and their relevance to surgical planning. J Bone Joint Surg [Br]. 2006;88:515-519.
62. Sponseller P.D., Bhimani M., Solacoff D., Dormans J.P. Results of brace treatment of scoliosis in Marfan syndrome. Spine (Phila Pa 1976). 2000;25:2350-2354.
63. Jones K.B., Erkula G., Sponseller P.D., Dormans J.P. Spine deformity correction in Marfan syndrome. Spine (Phila Pa 1976). 2002;27:2003-2012.
64. Yang J.S., Sponseller P.D. Severe cervical kyphosis complicating halo traction in a patient with Marfan syndrome. Spine (Phila Pa 1976). 2009;34:E66-E69.
65. de Vries J., Yntema J.L., van Die C.E., et al. Jeune syndrome: description of 13 cases and a proposal for follow-up protocol. Eur J Pediatr. 2010;169:77-88.
66. Campbell R.M. Jr: Spine deformities in rare congenital syndromes: clinical issues. Spine (Phila Pa 1976). 2009;34:1815-1827.
67. Hosoe H., Miyamoto K., Wada E. Surgical treatment of scoliosis in Larsen syndrome with bilateral hip dislocation. Spine (Phila Pa 1976). 2006;31:E302-E306.
68. Asher M.A. Orthopedic screening: especially congenital dislocation of the hip and spinal deformity. Pediatr Clin North Am. 1977;24:713-721.
69. Ogilvie J. Adolescent idiopathic scoliosis and genetic testing. Curr Opin Pediatr. 2010;22:67-70.
70. Thomsen M.N., Schneider U., Weber M., et al. Scoliosis and congenital anomalies associated with Klippel-Feil syndrome types I-III. Spine (Phila Pa 1976). 1997;22:396-401.
71. Tassabehji M., Fang Z.M., Hilton E.N., et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Hum Mutat. 2008;29:1017-1027.
72. Hughes P.J., Davies P.T., Roche S.W., et al. Wildervanck or cervico-oculo-acoustic syndrome and MRI findings. J Neurol Neurosurg Psychiatry. 1991;54:503-504.
73. Santillan Chapa C.G., Martinez Coria E., et al. [Congenital cervical vertebral dysmorphism. Syndromatic integration through radiological clinical correlation]. Acta Ortop Mex. 2007;21:133-138.
74. Bouzidi H., Khedhiri S., Laradi S., et al. [Mucopolysaccharidosis IVA (Morquio A syndrome): clinical, biological and therapeutic aspects]. Ann Biol Clin (Paris). 2007;65:5-11.
75. Bowling E.L., Brown M.D., Trundle T.V. The Stickler syndrome: case reports and literature review. Optometry. 2000;71:177-182.
76. Kouwenhoven J.W., Castelein R.M. The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine (Phila Pa 1976). 2008;33:2898-2908.
77. McCall R.E., Hayes B. Long-term outcome in neuromuscular scoliosis fused only to lumbar 5. Spine (Phila Pa 1976). 2005;30:2056-2060.
78. Bell D.F., Moseley C.F., Koreska J. Unit rod segmental spinal instrumentation in the management of patients with progressive neuromuscular spinal deformity. Spine (Phila Pa 1976). 1989;14:1301-1307.
79. Wynne-Davies R. Genetic aspects of idiopathic scoliosis. Dev Med Child Neurol. 1973;15:809-811.
80. Robin G.C., Cohen T. Familial scoliosis. A clinical report. J Bone Joint Surg [Br]. 1975;57:146-148.
81. Weiss H.R. Is there a body of evidence for the treatment of patients with adolescent idiopathic scoliosis (AIS)? Scoliosis. 2007;2:19.
82. Shen F.H., Samartzis D. Traumatic scoliosis. Lancet. 2005;365:910.
83. Winter R.B., Moe J.H., Lonstein J.E. The incidence of Klippel-Feil syndrome in patients with congenital scoliosis and kyphosis. Spine (Phila Pa 1976). 1984;9:363-366.
84. Deburge A., Briard J.L. Cervical hemivertebra excision. J Bone Joint Surg [Am]. 1981;63:1335-1338.
85. Suk S.I., Kim J.H., Cho K.J., et al. Is anterior release necessary in severe scoliosis treated by posterior segmental pedicle screw fixation? Eur Spine J. 2007;16:1359-1365.