Scoliosis
The term scoliosis is defined as a structural lateral curvature of the spine in a coronal plane greater than 10 degrees, as measured by the Cobb method on a standing radiograph.1 Curves less than 10 degrees are termed spinal asymmetry. The lateral curvature is often accompanied by abnormalities in the axial and the sagittal planes rendering it a three-dimensional abnormality, an important concept that influences assessment and management. Scoliosis is classified into various categories, according to etiology, curve location, age at onset, and curve type. The Scoliosis Research Society has classified scoliosis into the following broad subcategories:2
1. Idiopathic (infantile, juvenile, and adolescent)
2. Congenital (osteogenic and neuropathic)
3. Neuromuscular (neuropathic and myopathic)
Biomechanics and Pathogenesis
Scoliosis is a three-dimensional deformity involving the coronal, sagittal, and axial planes. The initiation and progression of the scoliotic curve is commonly thought to result from the effect of Hueter-Volkmann law, which states that epiphyseal growth (ring apophysis of the vertebral body) in the skeletally immature is inhibited when a compressive force acts on it and stimulated when a distraction force is applied.1,3 An initial abnormality in the axial plane leads to more compressive forces on the ventral aspect of vertebral body or disc and less on the posterior aspect. This discrepant growth of the anterior part versus the posterior part of the spine is accentuated over time, particularly during rapid skeletal growth, leading to eventual differential growth of the left and right sides of the spine, with suppression of growth on the concave side and excessive growth on the convex side eventually leading to scoliosis.3 This asymmetric growth not only forms the genesis of the curve but may also have significant surgical implications, as the pedicles on the concave side become asymmetrically smaller, posing a surgical challenge in pedicle screw for spinal fixation.4
Terminology
An understanding of the nomenclature and methods of measurement used to describe scoliosis on radiographs is essential for radiologists (Fig. 135-1). Identification of the curve apex or the apical vertebra, significant vertebrae, and central sacral vertebral line (CSVL) is not only crucial for denoting the curve type, assessing curve stability, and selecting the surgical approach and instrumentation system but also for determining the optimal level for fusion.5,6
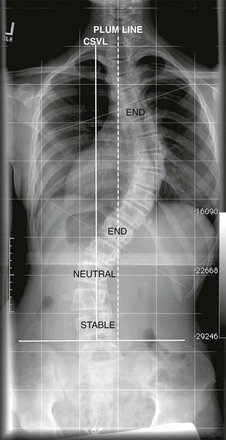
Figure 135-1 Nomenclature.
Standing posteroanterior radiograph from a patient with scoliosis: central sacral vertebral line (CSVL) (solid line) is a line that is perpendicular to a tangent across the iliac crest, bisecting the sacrum. The stable vertebra is the vertebra that is bisected or nearly bisected by the CSVL. The end vertebrae are the vertebra that are most tilted along the curve. Tangents (thin dotted-dash line) are drawn along the superior end plate of superior end vertebra and inferior end plate of inferior end vertebra to calculate the Cobb angle. A neutral vertebra is one that is not rotated. The plumb line is a line that is drawn from the center of the C7 vertebral body and is parallel to the edge of the image. If the distance between the CSVL and the plumb line is 2 cm or greater, it indicates coronal imbalance.
• CSVL: CSVL is a vertical line that is drawn perpendicular to an imaginary tangential line drawn across the top of the iliac crests bisecting the sacrum. This is used to identify the stable vertebrae, evaluate coronal balance, and determine the curve type, independent of the curve classification system applied.5–7
• Apex: Apex is defined as the vertebra or the disc that is farthest from the CSVL and the most rotated and horizontal vertebra.
• End vertebrae: The vertebrae with the maximum tilt or angulation toward the apex or concavity of the curve. They usually demarcate the proximal and distal vertebral bodies of the curve and are used to measure the Cobb angle.
• Stable vertebra: The vertebral body at the distal and proximal aspect of the curve that is bisected or nearly bisected by the CSVL.
• Neutral vertebrae: These represent the most nonrotated vertebrae and may be at the same level as end vertebrae or proximal or distally within the curve.
• Plumb line: This represents the vertical line drawn downward on standing radiographs from the center of the C7 vertebral body to assess coronal and sagittal balance. Measuring the distance between the CSVL and the plumb line assesses coronal balance. Sagittal balance is evaluated by measuring the distance between the posterosuperior aspect of the S1 vertebral body and the plumb line. An abnormal coronal and sagittal balance is defined if the distance is greater than 2 cm. A plumb line located to the right of the CSVL reflects positive coronal balance and a line located to the left of the CSVL reflects negative coronal balance. Similarly, a plumb line that is anterior to the posterosuperior aspect of the S1 body is considered positive sagittal balance, whereas a plumb line that is posterior to the posterosuperior aspect of the S1 body is considered negative sagittal balance.5–8
• Sagittal balance: The primary sagittal curves, which are present since birth, include the thoracic and sacral kyphoses. The secondary curves, which are acquired to assume bipedal stance, include the cervical and lumbar lordoses. This normal mature pattern of sagittal alignment is established by age 6 years.9,10 Thoracic kyphosis ranges between 10 and 50 degrees and is measured from T5 to T12 with apex usually at T6-T8. Lumbar lordosis ranges from 35 to 80 degrees when measured from L1 to L5, with apex between L3 and L4.1,11,12 In scoliosis, the thoracic kyphosis is mainly influenced by the spinal deformity, whereas lumbar lordosis is mainly influenced by pelvic configuration.13 Most thoracic idiopathic scoliosis is associated with a decrease in normal thoracic kyphosis. A true kyphotic component may indicate a congenital or neuromuscular origin; however, some idiopathic cases may have a true kyphotic component.1,6,13 Positive sagittal balance is more significantly associated with pain and disability than curve magnitude, curve location, or coronal imbalance.13,14
• Curve location: This is defined on the basis of the location of the apical vertebra and is classified as cervical (apex between C2 and C6), cervicothoracic (C7–T1), thoracic (T2–T11), thoracolumbar (T12–L1), lumbar (L2–L4), or lumbosacral (L5 and below).1,6,13
• Curve types: The curve is classified broadly as primary (major) or secondary (minor) curves, or structural or nonstructural curves. Primary curves are the first to appear and are the largest abnormal curves. Secondary curves usually develop later to stabilize the position of the head and pelvis.6,13–15 A structural curve is a curve that is not correctable on side-bending views or on traction and is accompanied by vertebral rotational abnormalities. A nonstructural curve usually corrects on bending views and may be secondary curves or functional curves (curves related to posture, secondary to leg length discrepancy, muscle spasm, etc.).13 Curves that are 25 degrees or greater on the standing anteroposterior radiograph and do not decrease to less than 25 degrees on the side-bending radiographs should be considered structural.16 A curve less than 25 degrees on the standing anteroposterior radiograph may be deemed structural if the regional sagittal profile reveals a kyphosis +20 degrees or greater.16 If a curve is considered structural, it should be included in the fusion surgery.
• Classification of curves: Various different classification systems exist describing the curve types preoperatively. The importance of classification systems is that they help guide the surgical approach and help compare the efficacy of different treatment methods.6,13 The Lenke classification system is the most recent and widely used classification system, replacing the established King-Moe classification system. The Lenke system is considered more comprehensive, complete, and reliable and takes into account the three-dimensional nature of scoliosis as opposed to the two-dimensional approach of the King-Moe classification.6,13,16,17 The Lenke system divides curves on the basis of location and type, differentiates structural from nonstructural curves, includes lumbar and sagittal modifiers, and proposes that only major curves and minor structural curves should be included in spinal arthrodesis.16,17 For unknown reasons, the typical curve in adolescent idiopathic scoliosis is usually a thoracic curve with right-sided convexity.
Idiopathic Scoliosis
Idiopathic scoliosis constitutes 80% of the patients with spinal deformity and is a diagnosis of exclusion.1 A judicious use of various imaging is a necessary prerequisite to exclude other underlying causes of scoliosis before labeling any case as idiopathic.
Etiologies, Pathophysiology, and Clinical Presentation: The reported prevalence of idiopathic scoliosis in children and adolescents ranges from 0.5 to 3 per 100.1,18,19 In the mild curves (<20 degrees), the female-to-male ratio is 1 : 1, but, when greater and progressive curves are evaluated (>30 degrees), females predominate with a 5 : 1 to 7 : 1 ratio. Only 0.2% of children have severe curves (>30 degrees).1,19,20
Idiopathic scoliosis is further divided into the following types based on age at diagnosis: infantile (0-3 years); juvenile (4-10 years); adolescent (11-17 years); and adult (≥18 years). Age at onset has prognostic significance both in terms of underlying neuraxis abnormality as a cause of scoliosis and the natural history of the curve. Whether juvenile scoliosis is truly a separate entity, is debatable because of the high prevalence of associated abnormalities in preadolescent scoliosis by magnetic resonance imaging (MRI).2 Moreover, the demarcation is further obscured by the possibility of early existence of the established adolescent curve. Hence the terms early onset and late onset are increasingly being used to classify scoliosis after the age of 5 years.2,21 The rationale behind this distinction is the increased risk of cardiopulmonary compromise in children before age 5 years with larger curves.2,22
Imaging: The role of imaging in scoliosis is early detection and characterization of the type of curve and its severity, assessment of disease progression, definition of the need and timing of the surgery, monitoring of changes related to treatment, and identification of those cases in which an underlying structural anomaly is present.23 The extent and type of imaging is also governed by the category of scoliosis. Cross-sectional imaging is extensively used in congenital scoliosis to identify and characterize structural anomalies, whereas radiography is principally utilized in idiopathic scoliosis to monitor curve progression.2
Screening: Upright, standing, posteroanterior radiograph of the entire spine is the standard initial screening examination, once the deformity is suspected clinically. The radiograph should include the cervicothoracic junction, enough of the pelvis to show the iliac crest in full extent, and the triradiate cartilage to enable assessment of skeletal maturity. Although standing radiography is preferred, sitting or supine radiography may be the only alternative in young patients, patients with congenital scoliosis, or patients with severe neuromuscular disorders. The rationale behind standing radiography is that treatment methods are based on standing views and the magnitude of the curve is greatest in the standing position. This is an important consideration in congenital and infantile scoliosis when young patients with scoliosis start to ambulate. It is easy to mistake change in curvature as curve progression if the upright radiograph is compared with a prior supine radiograph.1 The patient should stand with the feet placed shoulder width apart, looking straight ahead with the elbows bent and knuckles in the supraclavicular fossa bilaterally, allowing visualization of the upper thoracic region on the lateral radiographs.24 A lateral radiograph is not required at the time of screening but should be obtained in patients with documented scoliosis to assess sagittal balance. Radiographic techniques should minimize radiation exposure, especially to sensitive organs (e.g., breast, thyroid, and lens of eyes). A posteroanterior technique involves less radiation to the breast compared with an anteroposterior technique. The image may have a grid that helps identify deviations of the spine from the plumb line. When surgical treatment is considered, side-bending radiographs are acquired to differentiate between structural and nonstructural minor curves to help guide the optimal level of fusion. Various techniques used to obtain side-bending radiographs include supine bending, standing bending, and bending over a bolster.
Curve Magnitude: The Cobb angle is the accepted standard for assessing the magnitude of the scoliotic curve. It is defined as the angle formed by the intersection of two lines, one parallel to the end plate of the superior end vertebra and the other parallel to the end plate of the inferior end vertebra. Alternatively, pedicles may be used to calculate the Cobb angle when the end plates are poorly defined because of radiographic technique or obscured because of the severity of the curve. Although the Cobb angle provides limited assessment of the deformity and does not take rotation into account, it is still the foundation for diagnosis, follow-up, and treatment.1 The angle may be plotted manually or digitally with equal reliability.25 It is important to note that multiple factors, including patient positioning, radiographic techniques, known diurnal variation of 5 degrees, and interobserver and intraobserver variability, may affect the reproducibility of the angle.26–30 A progressive curve is usually defined by a Cobb angle increase of 5 degrees or more between consecutive radiographic examinations.1,2,6 The Cobb angle may decrease because of prone positioning and anesthesia during surgery, which sometimes leads to a postoperative rebound effect, with loss of correction when the patient returns to the standing position.6,13 Despite multiple caveats, measurements of the Cobb angle are usually considered reproducible, particularly when measuring end plates, patient positioning and techniques are kept constant.
Assessing Curve Progression: Prognosis and management in scoliosis is highly specific to the case and the surgeon but is usually governed by three important factors: (1) etiology, (2) magnitude and the type of the curve at presentation, and (3) speed of curve progression. The risk of progression is directly related to the spinal growth remaining, the severity of the curve at presentation, and the gender of the patient.1,10,19,31,32 Various clinical tools to assess residual growth potential include chronologic age, menarche in girls, serial height measurements, and Tanner staging.10,19,33–37 In general, the onset of the adolescent growth spurt occurs at 13 years in boys and 11 years in girls, with menarche indicating the declining phase of the growing peak and a low risk of progression for idiopathic curves under 30 degrees.1,10,33,35 Various radiographic parameters may indicate remaining potential growth. Development of the iliac bone apophysis, quantified by Risser, has become a universally accepted sign (Fig. 135-2). Despite the wide use of the Risser grade as a measure of maturity, the appearance of the iliac apophysis generally occurs after the most important period of rapid growth.1,33,34 Given the inaccuracies related to the Risser grade, other radiographic parameters are used to assess bone maturation. Bone age may be assessed from a hand and wrist radiograph; however, use of bone age becomes less accurate in the juvenile age group secondary to the larger standard deviation and suffers from interobserver variability.1,38 Assessment of pelvic triradiate cartilage is considered more accurate than the Risser grade. The triradiate cartilage closes around age 11 years in girls at the time of peak growth velocity.1,23 The physis of the olecranon of the elbow closes at age 13 years, shortly before menarche and may also be used to assess remaining skeletal growth.23 Unfortunately, many of the above-mentioned markers of maturity are quite variable and may be difficult to put in proper context without an accurate record of prior growth performance. Sometime, more than one imaging parameter is taken into consideration for accurately assessing skeletal maturation.
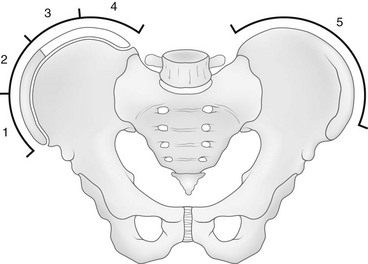
Figure 135-2 Risser grade.
The Risser grade proceeds from grade 0 (no ossification) to grade 4 (all four quadrants show ossification of the iliac apophysis). When the ossified apophysis fuses completely to the ilium (Risser grade 5), the patient is skeletally mature. (From Herring JA, ed. Scoliosis. In: Herring JA, ed. Tachdjian’s pediatric orthopedics. vol 1. 3rd ed. Philadelphia: WB Saunders; 2002:213-321.)
With regard to curve patterns, curves with an apex above T-12 are more likely to progress compared with isolated lumbar curves.1 With regard to curve magnitude, Risser stage 0 or 1 patients with an initial curve of 5 to 19 degrees progressed in 22% of cases, compared with curve progression in 68% of cases with initial curve magnitude of 20 to 29 degrees.32 The rate of curve progression increased to 90% when the initial curve was 30 to 59 degrees.1
Most idiopathic scoliosis cases do not require imaging beyond radiography. Evidence does not support the usefulness of MRI in an otherwise normal adolescent with scoliosis.1,6 The use of MRI in idiopathic scoliosis is restricted to unusual curve patterns, alarming clinical manifestations, or both.1,6 The prevalence of central nervous system abnormalities in patients with presumed adolescent idiopathic scoliosis is very low, ranging from 2% to 4%.39,40 Hence, the use of MRI for presurgical screening of neural axis abnormality in patients who have presumed idiopathic scoliosis with a typical curve pattern but no pain or a neurologic deficit is still controversial with no clear consensus.1,2,6,41
Indications for using MRI in scoliosis include infantile group; juvenile group; left thoracic curve; abnormal neurologic examination, including abnormal sensory evoked potentials; painful scoliosis; hyperkyphotic sagittal alignment; and rapidly progressing curves.1,2,6,39,40,42
Treatment and Follow-up: Treatment in idiopathic scoliosis varies on a case-by-case basis and is highly surgeon specific. The majority of patients with idiopathic scoliosis present with curves less than 20 degrees, with very minimal risk of progression even without treatment. These patients usually are monitored with clinical and radiographic follow-up only.1,6
Bracing: Bracing (e-Fig. 135-3) is generally indicated when a scoliotic curve progresses from 25 to 30 degrees with a documented annual curve progression of at least 5 degrees, with the upper limit of curve magnitude being 45 degrees.1,43 Bracing modifies spinal growth by applying an external force and is usually effective in patients with substantial spinal growth remaining (Risser 2 to 3 or less).1,2,6 The use of bracing may be altered on the basis of the clinical circumstance, with early bracing being used by surgeons when a strong positive family history for progressive scoliosis is present. Bracing may be useful for limiting curve progression but usually does not stop or improve the curve.
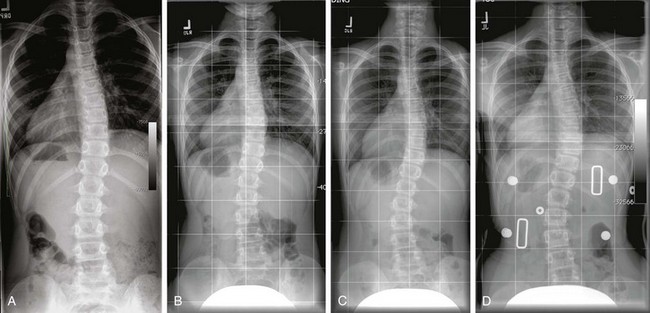
e-Figure 135-3 Juvenile idiopathic scoliosis.
A to C, Multiple posteroanterior standing spine radiographs (A, age 9; B, age 10; C, age 11) demonstrates progression of the right thoracic and left lumbar curve in a patient with idiopathic scoliosis (juvenile) with remaining skeletal growth. D, A brace was finally applied, and it shows reduction of both the curves. In general, bracing is indicated in patients with remaining skeletal growth and curves between 25 degrees and 45 degrees. Usually, a definite progression of 5 degrees is used as a threshold to use a brace in patients with a curve of less than 30 degrees.
Risser Casting: In some cases, a Risser cast or molded cast is applied as an alternative to bracing to provide more corrective force than can be applied by bracing alone (e-Fig. 135-4). The primary indication for Risser casting is in patients who have progressive scoliosis and are too small for rigid instrumentation prior to fusion or in those who have severe deformities and are too young to undergo fusion. The idea behind casting is to avoid repeated surgery as much as possible that may follow application of a growing rod instrumentation system.1 Similar to bracing, Risser casting only limits curve progression but does not stop or reduce the curve.
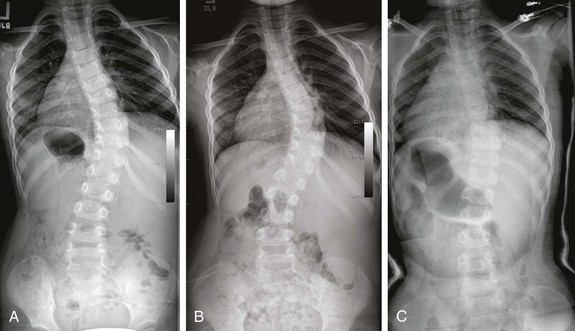
e-Figure 135-4 Infantile scoliosis.
Posteroanterior spine radiographs at 2 years of age (A) and 4 years of age (B) demonstrate progressive infantile scoliosis. A Risser body cast was finally placed, and it shows decreased thoracic and lumbar curves (C).
Surgery: The primary goal of surgical treatment is stabilization of curve progression, the secondary goals being improvement in the spinal alignment and balance of the body.1,6 The indications for surgical correction are multifaceted and include the degree of curve magnitude, risk for progression, skeletal maturity, and curve pattern. Moreover, the cosmetic perception of the deformity by the patient or the patient’s family may also greatly influence the decision for surgery.1
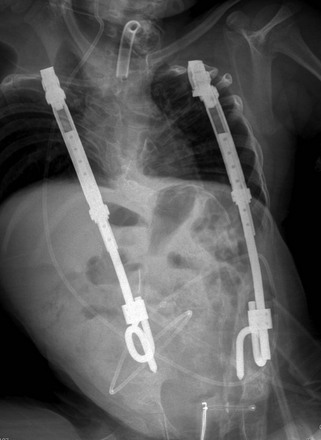
Thoracic curves of the Cobb angle greater than 40 to 50 degrees in skeletally immature patients are usually surgically corrected; in skeletally mature patients, surgical correction is reserved for curves of 50 degrees or more.1 However, a wide range of Cobb angles may act as a threshold for surgery secondary to the reasons mentioned above. The surgical treatment includes corrective instrumentation plus anterior or posterior fusion of the spine, with the aim of correcting both the coronal and sagittal deformities, which include decreased thoracic kyphosis and lumbar lordosis in most cases. The surgical goal is to achieve a stable bony fusion mass to maintain the correction over time, with the hardware serving as internal struts while fusion proceeds (Fig. 135-5). Earlier hardware for spinal fusion included Harrington rods, which corrected coronal plane imbalance at the expense of decreased thoracic kyphosis and flattening of the lumbar spine. As most idiopathic scoliosis patients have a hypokyphotic component, placement of Harrington rods led to a flat back deformity.1 Because of these reasons, Harrington rods are no longer used and have been replaced by multiple hooks and rod systems addressing the coronal imbalance while maintaining the sagittal balance.1,44 Instrumentation without fusion (as with growing rods) is sometime used as a temporary method in young children with rapidly progressing curves. The vertical expandable prosthetic titanium rib (VEPTR) is one such method, which is more commonly used in the treatment of infantile and juvenile types of idiopathic scoliosis.
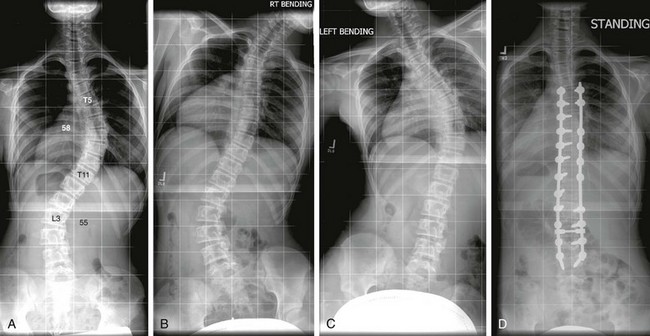
Figure 135-5 Idiopathic scoliosis.
A posteroanterior radiograph (A) shows a right main thoracic curve with the largest Cobb angle of 58 degrees with its apex at the level of the T8-T9 disk and its end vertebrae at T5 and T11. Rightward-bending anteroposterior (AP) radiograph (B) shows some improvement of the main thoracic curve; however, the Cobb angle remains more than 25 degrees, a finding consistent with structural curve based on the Lenke classification. Leftward-bending AP radiograph (C) shows that the Cobb angles of the proximal thoracic and lumbar curves do not exceed 25 degrees, indicating nonstructural curves. A postoperative radiograph (D) shows posterior spinal fusion extending from T5 through L3 level.
The length of fusion varies according to the curve type and the associated lumbar modifiers.17 In broad perspective, to improve surgical outcomes, the segment to be fused should be as short as possible but long enough to minimize residual disease. Usually, the stable vertebra, that is, the point where the CSVL bisects the spine, broadly defines the lower limit of fusion.13 Efforts are made to avoid nonstructural curves and to spare as many mobile segments of the lower lumbar spine.6
Follow-up Imaging: No standard guidelines are available to monitor patients with idiopathic scoliosis. It is generally recommended that patients with idiopathic scoliosis be monitored every 4 to 12 months, depending on age and growth rate. Those in the rapid phases of growth are seen at more frequent intervals (every 4 to 6 months).1,6 After the cessation of spinal growth, only curves with Cobb angles greater than 30 degrees should be monitored for progression. Follow-up imaging usually is performed every 5 years, although the follow-up interval depends on the patient’s symptoms and the severity of the curvature.1
Follow-up usually involves not only monitoring curve progression but also the efficacy of nonsurgical treatment such as bracing. Effective bracing should achieve about 30% correction of the Cobb angle.45 Subsequent to fixation, patients are usually followed up until the fusion has matured to ensure that the fixation is secure, to exclude the development of pseudoarthrosis, and to exclude recurrence of the deformity.45
Congenital Scoliosis
Congenital scoliosis results from disordered embryologic or intrauterine spine development, presumably during somitogenesis between weeks 5 and 8 of gestation.46 Vertebral malformations are often associated with coexistent nonspinal, musculoskeletal malformations. Multiple recognized syndromes are associated with congenital scoliosis. The two most common known coexisting conditions are VACTERL (vertebral anomaly, anorectal atresia, cardiac lesion, tracheoesophageal fistula, renal anomaly, limb defect) association and Klippel-Feil syndrome.46 Scoliosis as a spectrum of skeletal dysplasia is considered separate from congenital scoliosis. Anomalies in skeletal dysplasia result from failure of normal bone growth and development and are not caused by early failures of vertebral segmentation or formation as in congenital scoliosis. Although labeled as congenital, it is important to note that the clinical deformity may not be apparent at birth and usually develops with spinal growth presenting later in childhood.46,47
Etiologies, Pathophysiology, and Clinical Presentation: Congenital scoliosis is caused by failures of vertebral segmentation, formation, or a combination of the two. Classifications by Winter et al. and McMaster (Fig. 135-6) are helpful in describing anomalies and predicting which will be progressive.47–50 If the failure of formation or of segmentation occurs on the right or left side of the vertebral body, scoliosis results. If the segmentation anomaly occurs along the anterior or posterior vertebral body, then congenital kyphosis or lordosis results, respectively.46 Most cases are combinations of both coronal and sagittal plane deformities, emphasizing the three-dimensional nature of the deformity.
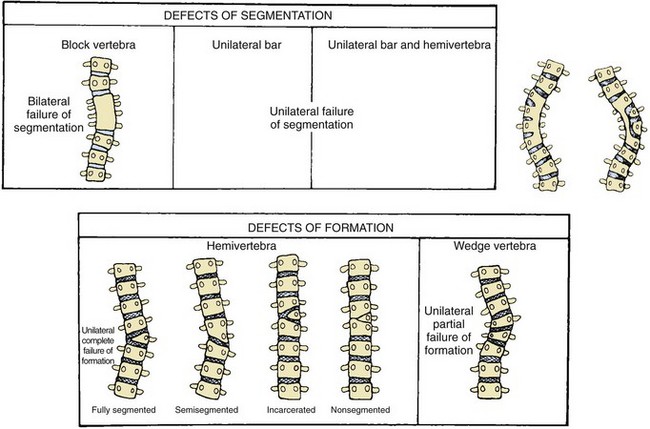
Figure 135-6 Forms of congenital scoliosis.
Various types of congenital scoliosis based on defects of segmentation versus defects of formation. (From McMaster MJ. Congenital scoliosis. In: Weinstein SL, ed. The pediatric spine. Philadelphia: Lippincott Williams & Wilkins; 2001:161-177.)
The most common underlying spinal abnormality is a hemivertebra, seen in about 40% of cases, usually resulting from unilateral complete failure of formation and by the absence of a contralateral-paired pedicle.46,51 Hemivertebrae may be further classified as fully segmented, semisegmented, or nonsegmented on the basis of the relationship to the cranial and caudal vertebrae. Wedged vertebrae result from a unilateral partial failure of formation with presence of two pedicles. “Butterfly vertebrae” are presumed to originate from failure of anterior midline fusion between somite pairs. Complete anterior and posterior failure of segmentation creates a block vertebra, whereas unilateral anterior and posterior failure of segmentation causes a unilateral bar.46
Natural history and the risk of progression in congenital scoliosis are related to multiple elements: (1) type of anomaly, (2) site, (3) age at presentation, (4) single versus multiple curves, and (5) associated nonspinal abnormalities.52 Curve progression has been strongly related to the type of vertebral abnormality (Table 135-1), with unilateral unsegmented bars with contralateral hemivertebrae having the poorest prognosis, a less severe progression with hemivertebrae, and the least severe progression with block and wedge vertebrae (Fig. 135-7).
Table 135-1
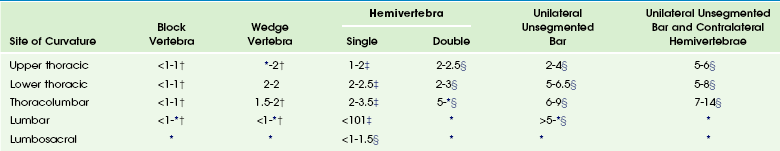
*, Too few or no curves; †, no treatment required; ‡, may require spinal surgery; §, requires spinal fusion. Ranges represent the degree of deterioration before and after 10 years of age.
Modified from McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis: A study of 251 patients. J Bone Joint Surg Am. 1982;66:588-601. McMaster MJ. Congenital scoliosis caused by a unilateral failure of vertebrae segmentation with contralateral hemivertebrae. Spine. 1998;23:998-1005, and McMaster MJ. Congenital scoliosis. In: Weinstein SL, ed. The pediatric spine. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:161-177.
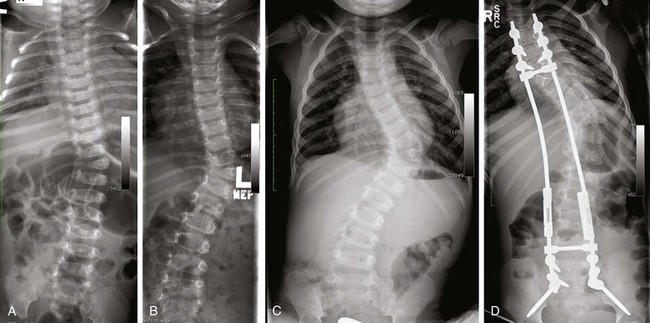
Figure 135-7 Congenital scoliosis.
Rapid progression of curve in a patient with fully segmented hemivertebra from 32 months (A) to 3 years of age (B) to 4 years of age (C). A postoperative radiograph (D) shows placement of expandable posterior spinal fusion hardware, which still allows some continued growth of the abnormal spine with maintaining or controlling the deformity. Curve progression in congenital scoliosis is strongly related to the type of underlying vertebral abnormality, with a poor prognosis associated with unilateral unsegmented bars with contralateral hemivertebrae and fully segmented hemivertebrae, as in this case.
Imaging: Fetal imaging modes, including ultrasonography and MRI, allow early and more comprehensive detection of fetal spinal anomalies, but the significance of earlier detection and prognosis is still being debated.46 The postnatal diagnosis of congenital scoliosis is usually made with the use of radiography. Findings suggestive of congenital scoliosis include absence of spinous processes, rib fusions, joined pedicles, or absent or narrowed disc spaces.46 In the majority of cases, identification of the type of anomaly is possible with radiography. Absolute Cobb angle magnitude is of much less significance than the overall deformity and the change in that deformity over time with growth. Documentation of radiographic change with growth is the mainstay of observation and surgical decision making.46
Radiography is very useful if both anterior and posterior structures of anomalous vertebrae correspond to each other. However, it is not uncommon to have differences between the anterior and posterior structures often leading to a complex unclassified pattern of abnormality.46,51 These characteristics make independent evaluation of the malformation in each vertebra extremely difficult with radiography, which highlights the role of three-dimensional computed tomography (CT) in the evaluation of complex malformations, particularly the posterior elements. Radiography has difficulty in accurately identifying the deformities that will be progressive, which has called for a newer three-dimensional classification of congenital scoliosis.51 CT is currently restricted as a preoperative evaluation tool. CT may be especially helpful when planning the surgical excision of an anomalous vertebra, as CT may depict unexpected osseous anomalies not clearly depicted on radiography. Preoperative CT angiography may also be useful for determining coexistent vascular anomalies and to assess the relationship of the aorta to the spine.
Imaging in congenital scoliosis not only demands detailed evaluation of the spine but also exclusion of extraspinal anomalies, mainly cardiovascular and genitourinary anomalies.46 Associated intraspinal or neural axis abnormalities with congenital scoliosis may occur in 20% to 37% of patients, mandating screening (Fig. 135-8).46,53,54 The most common associated abnormalities include syrinx, Chiari I malformation, cord or spinal canal tumors, diastematomyelia, and low conus medullaris.46 Spinal ultrasonography may be effective for initial screening. The role of MRI as a screening tool in all congenital scoliosis cases is controversial, with some experts believing that MRI should be restricted to presurgical evaluation. If not addressed preoperatively, major associated intraspinal abnormalities, including cord tethering, may lead to progressive deformity and progressive or acute neurologic loss.46,54
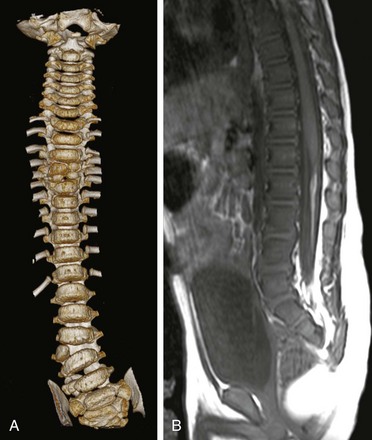
Figure 135-8 Neural axis abnormality in congenial scoliosis.
A coronal three-dimensional, surface shaded reformatted image of the spine (A) clearly demonstrates a lumbar hemivertebra with butterfly vertebrae involving the midthoracic spine. A sagittal T1 magnetic resonance image of the lumbar spine (B) in the same patient shows a low-lying cord with an associated filum lipoma. Intraspinal abnormalities in congenital scoliosis are not uncommon, and magnetic resonance imaging of the neural axis is a routine prerequisite before any treatment is contemplated.
Treatment: Surgery is the mainstay in the treatment of congenital scoliosis, as nonoperative measures provide no definite efficacy; early surgical treatment is preferred to late treatment. If an underlying spinal cord abnormality is found, it should be corrected prior to scoliosis surgery. The various surgical options are complex and beyond the scope of this discussion; however, the traditional approach still includes early fusion to avoid the development of more severe deformities.46 In some patients, expansion thoracoplasty with the use of the VEPTR device (Fig. 135-9) is used instead of early fusion.55 This technique is usually utilized in patients with shortened spines, which would necessitate fusion of a long portion of spine at an early age, especially in those with associated chest wall anomalies. The VEPTR technique allows some continued growth of the abnormal spinal elements while still controlling the deformity. A major disadvantage of this treatment is the need for repetitive lengthening procedures, but it may be an appropriate temporary choice in certain children.46
Neuromuscular Scoliosis
Etiologies, Pathophysiology, and Clinical Presentation: The Scoliosis Research Society further classifies neuromuscular scoliosis into neuropathic and myopathic scoliosis.10 The first group includes include neurologic disorders such as cerebral palsy or spinomuscular dystrophies, and the second group includes muscular disorders such as the muscular dystrophies.
Imaging: The characteristic curve pattern in neuromuscular scoliosis is an early onset long C-shaped curve (e-Fig. 135-10) with rapid progression, which continues beyond skeletal maturity. Curve patterns similar to adolescent idiopathic scoliosis may also be seen. Affected children also often present with severe kyphosis or lordosis in the sagittal plane, with multiple additional comorbidities related to their primary disease.10 Because of non-weight bearing status, the vertebral bodies are often tall and slender. Additional findings include diminished lumbar lordosis with a single, broad-based thoracic kyphosis. In addition, because of the underlying disease entity, these children often have coexisting hip dislocation caused by pelvic muscle imbalance and non–weight bearing status.
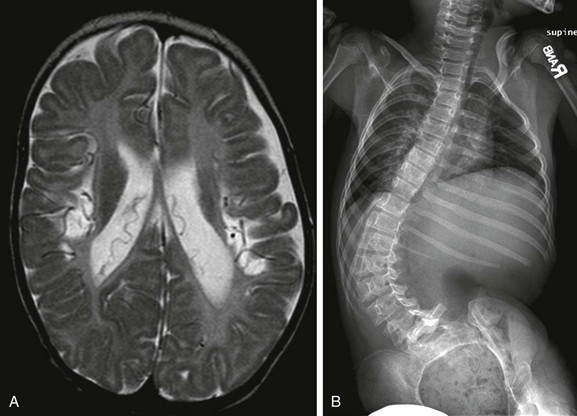
e-Figure 135-10 Neuromuscular scoliosis.
An axial T2-weighted magnetic resonance image (A) in a patient with neuromuscular scoliosis shows cystic encephalomalacia and volume loss involving the perirolandic regions bilaterally. A supine radiograph of the spine (B) shows a long C-shaped curve with convexity to the left side and significant pelvic obliquity, a curve pattern often seen in patients with neuromuscular scoliosis.
Treatment: Bracing is usually reserved for nonsevere flexible curves, immature children, and ambulatory patients, and usually does not prevent curve progression.56 Surgical fusion is the mainstay of the treatment. The objectives of curve correction in neuromuscular scoliosis are to restore seating balance, to facilitate wheelchair use, to control pain, and to support the trunk so as to reinforce respiratory function.10 Postoperative complications occur more frequently in patients with neuromuscular scoliosis than in those with idiopathic scoliosis, ranging from 44% to 62%, likely related to the presence of multiple comorbidities.57
Scoliosis and Radiation
The radiographic examination of children with scoliosis has historically involved a relatively high radiation dose, particularly to the breast, and may increase the risk of subsequent breast cancer.58 The issues are twofold: (1) Initially, scoliosis radiographs were done in the anteroposterior projection, exposing the adolescent breast to maximum dose; and (2) many patients with scoliosis are adolescent girls with pubertal growth spurt and rapid breast development. Radiography for scoliosis must entail optimization to minimize radiation risk. This includes the use of posteroanterior positioning, high tube voltage, high-speed screen film combinations, contoured filtration, shielding of breasts (if anteroposterior positioning is used, that is, in nonambulatory children who have sitting anteroposterior scoliosis studies), replacement of antiscatter grids with air gap techniques, and appropriate techniques to balance the need for adequate visualization and the use of the lowest possible radiation dosage. The radiation dose may be further reduced by using CT and digital imaging systems.59,60
Cassar-Pullicino, VN, Eisenstein, SM. Imaging in scoliosis: what, why and how? Clin Radiol. 2002;57(7):543–562.
Goethem, JV, Campenhout, AV, Hauwe, LVD, et al. Scoliosis. Neuroimag Clin North Am. 2007;17:105–115.
Kim, H, Kim, HS, Moon, ES, et al. Scoliosis imaging: What radiologists should know. RadioGraphics. 2010;30:1823–1842.
Lenke, LG, Betz, RR, Harms, J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83-A(8):1169–1181.
Lenke, LG, Edwards, CC, II., Bridwell, KH. The Lenke classification of adolescent idiopathic scoliosis: how it organizes curve patterns as a template to perform selective fusions of the spine. Spine. 2003;28(20):S199–S207.
References
1. Newton, P, Wenger, D. Idiopathic Scoliosis. In: Morrissy R, Weinstein S, eds. Pediatric orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:693–762.
2. Cassar-Pullicino, VN, Eisenstein, SM. Imaging in scoliosis: what, why and how? Clin Radiol. 2002;57(7):543–562.
3. Stokes, IA. Mechanical effects on skeletal growth. J Musculoskelet Neuronal Interact. 2002;2(3):277–280.
4. Parent, S, Labelle, H, Skalli, W, et al. Thoracic pedicle morphometry in vertebrae from scoliotic spines. Spine (Phila Pa 1976). 2004;29(3):239–248.
5. Potter, BK, Rosner, MK, Lehman, RA, Jr., et al. Reliability of end, neutral, and stable vertebrae identification in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2005;30(14):239–248.
6. Kim, H, Kim, HS, M, ES, et al. Scoliosis imaging: What radiologists should know. RadioGraphics. 2010;30:1823–1842.
7. King, HA, Moe, JH, Bradford, DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65(9):1302–1313.
8. Malfair, D, Flemming, AK, Dvorak, MF, et al. Radiographic evaluation of scoliosis: review. AJR Am J Roentgenol. 2010;194(3 suppl):S8–S22.
9. Bernhardt, M, Normal spinal anatomy: normal sagittal plane alignment. The textbook of spinal surgery, 2nd ed. Bridwell, KH, Dewald, RL, eds. The textbook of spinal surgery, Philadelphia, PA, Lippincott-Raven, 1997;vol. 1:185–191.
10. Campos, MA, Weinstein, SL. Pediatric scoliosis and kyphosis. Neurosurg Clin North Am. 2007;18:515–529.
11. Bernhardt, M, Bridwell, KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine. 1989;14:717–721.
12. Propst-Proctor, SL, Bleck, EE. Radiographic determination of lordosis and kyphosis in normal and scoliotic children. J Pediatr Orthop. 1983;3:344–346.
13. Goethem, JV, Campenhout, AV, Hauwe, LVD, et al. Scoliosis. Neuroimag Clin North Am. 2007;17:105–115.
14. Glassman, SD, Bridwell, K, Dimar, JR. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30(18):2024–2029.
15. Malfair, D, Flemming, AK, Dvorak, MF, et al. Radiographic evaluation of scoliosis: review. AJR Am J Roentgenol. 2010;194(3 suppl):S8–S22.
16. Lenke, LG, Betz, RR, Harms, J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83-A(8):1169–1181.
17. Lenke, LG, Edwards, CC, II., Bridwell, KH. The Lenke classification of adolescent idiopathic scoliosis: how it organizes curve patterns as a template to perform selective fusions of the spine. Spine. 2003;28(20):S199–S207.
18. Dickson, RA. Scoliosis in the community. Br Med J (Clin Res Ed). 1983;286:1745.
19. Miller, NH. Cause and natural history of adolescent idiopathic scoliosis. Orthop Clin North Am. 1999;30:343–352.
20. Bunnell, WP. The natural history of idiopathic scoliosis. Clin Orthop. 1988;229:20–25.
21. Dickson, RA, Lawton, JO, Archer, IA, et al. The pathogenesis of idiopathic scoliosis: biplanar spinal asymmetry. J Bone Joint Surg. 1984;66B:8.
22. Weinstein, SL, Zavala, DC, Ponseti, IV. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63(5):702–712.
23. Kotwicki, T. Evaluation of scoliosis today: examination, X-rays and beyond. Disabil Rehabilitat. 2008;30(10):742–751.
24. O’Brien, MF, Kuklo, TR, Blanke, KM. Spinal deformity: radiographic measurement manual. Memphis, TN: Medtronic Sofamor Danek; 2004.
25. Kuklo, TR, Potter, BK, O’Brien, MF, et al. Reliability analysis for digital adolescent idiopathic scoliosis measurements. J Spinal Disord Tech. 2005;18(2):152–159.
26. Pruijs, JE, Hageman, MA, Keessen, W, et al. Variation in Cobb angle measurements in scoliosis. Skeletal Radiol. 1994;23(7):517–520.
27. Beauchamp, M, Labelle, H, Grimard, G, et al. Diurnal variation of Cobb angle measurement in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 1993;18(12):1581–1583.
28. Carman, DL, Browne, RH, Birch, JG. Measurement of scoliosis and kyphosis radiographs. Intraobserver and interobserver variation. J Bone Joint Surg Am. 1990;72:328–333.
29. Morrissy, RT, Goldsmith, GS, Hall, EC, et al. Measurement of the Cobb angle on radiographs of patients who have scoliosis. Evaluation of intrinsic error. J Bone Joint Surg Am. 1990;72:320–327.
30. Loder, RT, Urquhart, A, Steen, H, et al. Variability in Cobb angle measurements in children with congenital scoliosis. J Bone Joint Surg Br. 1995;77:768–770.
31. Ganey, TM, Ogden, JA. Development and maturation of the axial skeleton. In: Weinstein SL, ed. The pediatric spine: principles and practice. 2nd ed. New York: Lippincott Williams & Wilkins; 2001:3–54.
32. Lonstein, JE, Carlson, JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66(7):1061–1071.
33. Dimeglio, A. Growth in pediatric orthopaedics. J Pediatr Orthop. 2001;21(4):549–555.
34. Little, DG, Song, KM, Katz, D, et al. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am. 2000;82(5):685–693.
35. Lonstein, JE. Scoliosis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:248–259.
36. McCarthy, RE. Evaluation of the patient with deformity. In: Weinstein SL, ed. The pediatric spine: principles and practice. 2nd ed. New York: Lippincott Williams & Wilkins; 2001:133–157.
37. Song, KM, Little, DG. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop. 2000;20(3):286–288.
38. Greulich, W, Pyle, S. Radiographic atlas of skeletal development of the hand and wrist, 2nd ed. Stanford, CA: Stanford University Press; 1959.
39. Do, T, Fras, C, Burke, S, et al. Clinical value of routine preoperative magnetic resonance imaging in adolescent idiopathic scoliosis: a prospective study of three hundred and twenty seven patients. J Bone Joint Surg Am. 2001;83-A(4):577–579.
40. Davids, JR, Chamberlin, E, Blackhurst, DW. Indications for magnetic resonance imaging in presumed adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2004;86-A(10):2187–2195.
41. Jaramillo, D, Poussaint, TY, Grottkau, BE. Scoliosis: evidence-based diagnostic evaluation. Neuroimag Clin North Am. 2003;13:335–341.
42. Cheng, JC, Guo, X, Sher, AH, et al. Correlation between curve severity, somatosensory evoked potentials, and magnetic resonance imaging in adolescent idiopathic scoliosis. Spine. 1999;24:1679–1684.
43. Nachemson, AL, Peterson, LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am. 1995;77:815–822.
44. Cotrel, Y, Dubousset, J, Guillaumat, M. New universal instrumentation in spinal surgery. Clin Orthop. 1988;227:10–23.
45. Thomsen, M, Rainer, A. Imaging in scoliosis from the orthopedic surgeon’s point of view. Eur J Radiol. 2008;58:41–47.
46. Emans, JB. Congenital scoliosis. In: Morrissy R, Weinstein S, eds. Pediatric orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:764–795.
47. McMaster, MJ. Spinal growth and congenital deformity of the spine. Spine. 2006;31:2284–2287.
48. Winter, RB. Congenital deformities of the spine. New York: Thieme-Stratton; 1983.
49. Winter, RB, Moe, JH, Wang, JF. Congenital kyphosis—its natural history and treatment as observed in a study of one hundred thirty patients. J Bone Joint Surg Am. 1973;55:223–256.
50. Kawakami, N, Tsuji, T, Imagama, S, et al. Classification of congenital scoliosis and kyphosis: A new approach to the three-dimensional classification for progressive vertebral anomalies requiring operative treatment. Spine. 2009;34:1756–1765.
51. Shahcheraghi, GH, Hobbi, MH. Patterns and progression in congenital scoliosis. J Pediatr Orthop. 1999;19:766–775.
52. Marks, DS, Qaimkhani, SA. The natural history of congenital scoliosis and kyphosis. Spine. 2009;34:1751–1755.
53. Basu, PS, Elsebaie, H, Noordeen, MH. Congenital spinal deformity: a comprehensive assessment at presentation. Spine. 2002;27(20):2255–2259.
54. Prahinski, JR, Polly, DW, Jr., McHale, KA, et al. Occult intraspinal anomalies in congenital scoliosis. J Pediatr Orthop. 2000;20(1):59–63.
55. Campbell, RM, Jr., Smith, MD, Hell-Vocke, AK. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am. 2004;86-A(suppl 1):51–64.
56. Miller, A, Temple, T, Miller, F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16(3):332–335.
57. Murphy, NA, Firth, S, Jorgensen, T, et al. Spinal surgery in children with idiopathic and neuromuscular scoliosis. What’s the difference? J Pediatr Orthop. 2006;26(2):216–220.
58. Ron, E. Ionizing radiation and cancer risks: evidence from epidemiology. Pediatr Radiol. 2002;32:232–237.
59. Geijer, H, Verdonck, B, Beckman, KW, et al. Digital radiography of scoliosis with a scanning method: radiation dose optimization. Eur Radiol. 2003;13:543–551.
60. Kluba, T, Schafer, J, Hahnfeldt, T, et al. Prospective randomized comparison of radiation exposure from full spine radiographs obtained in three different techniques. Eur Spine J. 2006;15(6):752–756.