Chapter 12 Scientific Basis of Rehabilitation after Anterior Cruciate Ligament Autogenous Reconstruction
FACTORS THAT AFFECT POSTOPERATIVE REHABILITATION AND OUTCOMES
Considerable advances have been made in the treatment of complete anterior cruciate ligament (ACL) ruptures and reconstruction methods since the mid 1980s. These include the appropriate selection of patient candidates and criteria that should be achieved before surgery, such as resolving limitations of knee motion, muscle atrophy, gait abnormalities, pain, and joint effusion. Appropriate graft selection, harvest, implantation, tensioning, and fixation are all paramount to achieving a reliable and desirable outcome, and considerable attention has been devoted to these principles in the orthopaedic literature.18,19,62
Appropriate postoperative rehabilitation after ACL reconstruction is critical to achieve normal knee function and prevent complications such as arthrofibrosis and reinjury. The goals of postoperative therapy are to regain normal knee motion, gait mechanics, lower extremity muscle strength, coordination, proprioception, and neuromuscular indices using exercises and modalities that are not deleterious to the healing graft. Dye introduced the concept of restoring normal knee osseous and soft tissue homeostasis after ACL injury and reconstruction using an appropriate combination of medical and therapeutic measures.45,46 The exercise program should not produce undue forces on the patellofemoral or tibiofemoral compartments or result in chronic joint effusions. Unfortunately, few investigations have studied the effect of specific exercises and treatment modalities commonly used after ACL reconstruction. In fact, entire protocols have appeared and been used extensively based on clinical observations and retrospective analyses instead of prospective, randomized, controlled clinical trials.83,179
One of the difficulties in conducting rehabilitation investigations is the multitude of factors that may affect both the initial and the long-term recovery after ACL reconstruction. In addition, few ACL ruptures are truly isolated in nature, because approximately 80% of patients sustain concomitant bone bruises52,93 and 60% suffer meniscus tears.130,136 The factors that affect recovery include
Critical Points FACTORS THAT AFFECT POSTOPERATIVE REHABILITATION AND OUTCOMES
Goals of Postoperative Therapy
Authors have noted no benefit28,117 and, in some cases, deleterious outcomes120,176,182,205 when ACL reconstruction is performed early after injury before the resolution of limitations in knee motion, muscle atrophy, swelling, and pain. Meighan and coworkers117 conducted one of the few prospective, randomized investigations regarding this issue. These investigators followed 31 patients who received either an early ACL hamstring reconstruction (within 2 wk of the injury) or a delayed reconstruction (8–12 wk) to determine outcomes up to 1 year postoperatively. The early reconstruction group had significantly less knee extension and flexion at 2 weeks postoperatively, and greater deficits in quadriceps isokinetic work and power at 12 weeks postoperatively, than the delayed group. No other differences were noted in outcome throughout the study period. The authors concluded that early reconstruction did not provide any benefit to athletic individuals. Shelbourne and associates182 noted an increased rate of arthrofibrosis in patients who underwent acute ACL reconstruction (within 1 wk of the injury) compared with those in whom the reconstruction was delayed for 21 days or more. Shelbourne and colleagues166,177 have heavily emphasized the need to restore normal knee motion when possible before surgical intervention. The exception is the presence of a mechanical block to extension, such as a bucket-handle meniscus tear or ruptured ACL that is impinged in the intercondylar notch. In these cases, early surgical intervention is warranted to repair the meniscus tear or remove the ACL, regain full knee extension and flexion, and then proceed later with ACL reconstruction.
Modern studies of ACL bone–patellar tendon–bone (B-PT-B) autograft reconstruction typically report low failure rates of approximately 5% to 10%.10,67,129,143,181 Failure is defined as an increase in anteroposterior (AP) displacement of 6 mm or greater compared with the normal contralateral limb or a fully positive (grade II or III) pivot shift test. However, the percentage of grafts that undergo some amount of elongation, resulting in 3 to 5 mm of increased AP displacement or a mildly positive (grade I) pivot shift test, is quite variable and ranges from 5% to 50%.1,7,72,129,143,173,174,206 Therefore, although the overall rate of failure is low, some authors express concern that any amount of abnormal anterior tibial translation may be detrimental to the knee joint over the long term.20,148 Whether graft elongation occurs as a result of technical aspects of the operative procedure, inconsistencies in maturation of the collagenous and bony components of the construct, the rehabilitation program, or a combination of these controllable and uncontrollable factors is unknown. Only one study has been conducted, to our knowledge, that followed knees reconstructed with ACL B-PT-B autografts with serial KT-2000 testing throughout the postoperative period (2 yr), discussed in detail later in this chapter.10
The purpose of this chapter is to review the current knowledge surrounding rehabilitation after ACL autogenous reconstruction. The scientific basis for immediate knee motion, early weight-bearing, specific exercises, and evaluation criteria for return to activity are reviewed. The authors’ ACL postoperative rehabilitation programs formulated from the scientific literature and nearly 3 decades of empirical clinical experience are detailed in Chapter 13, Rehabilitation of Primary and Revision Anterior Cruciate Ligament Reconstruction.
AUTOGENOUS ACL GRAFT MATURATION IN HUMANS
More than 100,000 ACL reconstructions are performed in the United States each year.146 Few studies have been conducted on the maturation process of ligament grafts in humans; only a minuscule sampling (<1%) of B-PT-B and semitendinosus-gracilis (STG) grafts have undergone histologic analysis after implantation. The strong potential for sampling error prevents conclusions on when graft maturation is complete, indicated by when the transplanted tissue resembles a normal ACL’s histologic, structural, biomechanical, and material properties.
Rougraff and coworkers164 performed biopsies on 23 B-PT-B autografts 3 weeks to 6.5 years after implantation. The authors reported that all patients had “clinically stable” knees; however, Lachman, pivot shift, and knee arthrometer data were not provided. The patients were allowed full weight-bearing immediately postoperatively and returned to sports between 3 and 6 months after surgery. The authors described four stages of ligamentization. The first stage occurred during the first 2 postoperative months and was characterized by an increased number of fibroblasts compared with time zero, preservation of mature collagen, and early neovascular ingrowth. The second stage, composed of rapid remodeling, occurred from 2 to 10 months postoperatively. A rapid increase in the number of metabolically active fibroblasts and replacement of approximately two thirds of mature collagen with immature matrix was noted. The third stage, in which the fibroblast count slowly decreased, collagen matrix matured, and vascularity decreased, occurred from 1 to 3 years postoperatively. In the final stage, the grafts were noted to be less cellular and vascular and appeared similar to those of control ACLs. The study did not include electron microscopy to evaluate the pattern of collagen fibril diameters in the grafts.
Critical Points AUTOGENOUS ACL GRAFT MATURATION IN HUMANS
B-PT-B Autografts: Four Stages of Ligamentization
B-PT-B and STG Autografts
B-PT-B, bone–patellar tendon–bone; STG, semitendinosus-gracilis.
Rougraff and Shelbourne165 performed biopises on nine B-PT-B autografts between 3 and 8 weeks after implantation. All patients followed an accelerated rehabilitation protocol179 and agreed to the biopsy for investigational purposes. All specimens showed evidence of survival of portions of the original tendon. Vascular invasion was present at 3 weeks postoperative, with increased cell counts compared with controls found in all samples. All specimens had areas of acellularity and degeneration; however, these were small (no more than 30% of the biopsied area) in comparison with the areas of vascularity and normal-appearing tendon tissue.
Petersen and Laprell153 examined the insertion of B-PT-B and STG autografts to bone in 14 knees that required revision ACL reconstruction. The time from the primary ACL reconstruction to revision ranged from 6 to 37 months. The hamstring specimens revealed a fibrous insertion of the graft into the periphery of the tibial bone tunnel. The patellar tendon (PT) specimens taken from the femoral tunnel showed fibrocartilage at the bone-tendon interface and resembled the chondral insertion of a normal ACL.
Johnson94 performed biopsies on 20 STG ACL grafts between 20 days and 44 months postoperative. All patients required follow-up arthroscopy for various symptoms, at which time biopsy samples were taken from the tendon graft and surrounding fibrous tissue. The reconstructed ACL was a composite of these two distinctly different tissues, which had diverse histologic properties. The 3-month postoperative tendon samples appeared normal histologically, with no signs of inflammation and organized collagen bundles. Specimens taken beyond 3 months showed continued tissue maturation and crimping of collagen bundles similar to those of the native ACL. Specimens taken from the fibrous tissue had a disorganized cellular pattern and hypervascularity at 3 months postoperative and, although with time they developed increasing amounts of collagen, never resembled a normal tendon.
Beynnon and associates23 examined the knees of a B-PT-B autograft recipient 8 months after implantation after the patient had died of causes unrelated to the knee joint. The patient’s rehabilitation program included immediate continuous passive motion (CPM) from 0° to 90°; however, he underwent a prolonged period of partial weight-bearing for 12 weeks for unknown reasons. At 4 months postoperative, he was examined clinically and had a stable reconstruction. The postmortem examination revealed 6 mm of increased AP displacement, a 13% reduction in ultimate failure loads, and a 53% reduction in energy absorbed at failure compared with the contralateral native ACL. Histologic analyses were not conducted. Interestingly, the ultimate failure load of the patient’s contralateral native ACL of 1015.6 N was well below the expected value of 1725 ± 269 N.132
Delay and colleagues40 inspected a retrieved whole B-PT-B autograft 18 months after implantation in a patient who died from a traumatic injury. Whereas the graft was mainly cellular and resembled a normal ACL morphologically, there were areas of acellularity deep within the tendinosis portion and also in the femoral intra-articular region where the autograft emerged from the tibial tunnel. The graft had been placed in an over-the-top position that the authors speculated could have caused increased stresses, resulting in inconsistent remodeling.
Falconiero and coworkers53 obtained biopsies of 35 B-PT-B and 8 STG autografts in 48 patients from 3 to 120 months after implantation. The rehabilitation program the patients followed was not described. Significant differences were noted in vascularity, cellularity, and fiber pattern between grafts that were examined 3 to 6 months postoperative and those that were observed greater than 12 months after implantation. Grafts that were biopsied 7 to 12 months postoperatively were described as transitional in terms of vascular maturity. No difference was found between fiber patterns and vascularity in grafts that had been implanted 7 to 12 months before the biopsy and those that had been implanted more than 12 months before the biopsy. The authors concluded that whereas graft maturity generally occurs over a 12-month period, some autografts may reach full maturation even earlier.
Although it appears from the literature reviewed that ACL autografts undergo increasing maturation postoperatively, the small sample size of examined grafts and the host of both intrinsic and extrinsic variables affects this process and prevents definitive conclusions. Questions remain regarding whether the healing process produces a graft that has histologic, ultrastructural, and biomechanical characteristics equivalent to those of a native ACL. It is probable that the delicate nature and microgeometry of native ligament fibers is never regained, but that the ligament functions more as a checkrein to resist gross knee displacements rather than replicating normal kinematics.89 Recent studies have demonstrated that even though B-PT-B and STG autograft reconstructions frequently restore normal AP displacements as measured with a KT-2000 arthrometer (<3 mm side-to-side difference), normal knee kinematics may not be restored, causing abnormal joint motions when the knee is subjected to weight-bearing activities.148 The delay in maturation of ACL allografts and the potential effects on postoperative recovery are discussed in Chapter 5, Biology of ACL Graft Healing.
IMMEDIATE KNEE MOTION
The scientific basis supporting immediate knee motion after ACL reconstruction is well established.18,78,87,131,134,135,158,163 Early knee joint motion decreases pain and postoperative joint effusions, aids in the prevention of scar tissue formation and capsular contractions that can limit normal knee flexion and extension, decreases muscle disuse effects (Fig. 12-1), maintains articular cartilage nutrition, and benefits the healing ACL graft.* There is a consensus in the medical community that immobilization is detrimental to the knee joint structures and may result in a permanent limitation of knee motion, prolonged muscle atrophy, patella infera, and articular cartilage deterioration.33,70,95,139–141,150,170
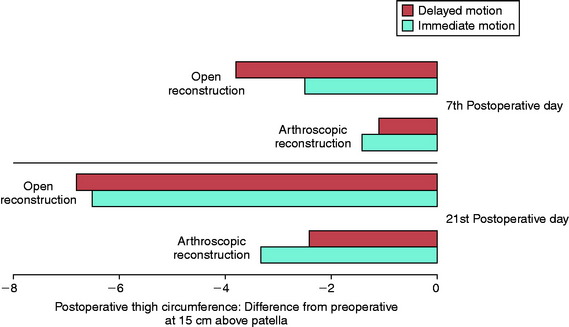
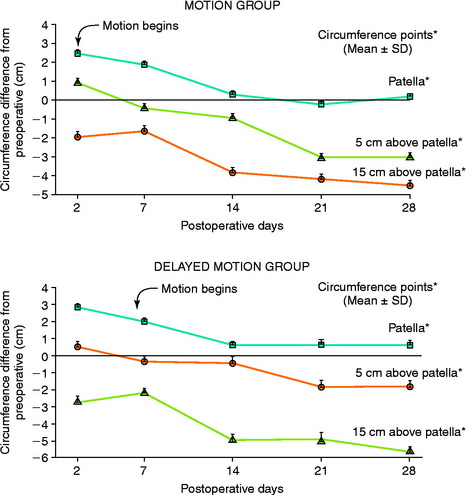
In 1983, Noyes and associates133 first published recommendations for immediate knee motion after ACL B-PT-B autograft reconstruction after a successful 4-year experience with this program. Advances in knee ligament reconstructive and fixation techniques allowed the controlled movement of the knee from 0° to 90° and did not disrupt the healing graft. In 1987, these authors134 published one of the first clinical studies that assessed joint effusion, joint motion, muscle atrophy, and the integrity of ACL grafts in a series of 18 patients who underwent ACL allograft reconstruction. Patients were randomly placed into either an immediate motion group that began CPM on the 2nd postoperative day or a delayed motion group that initiated passive and active-assisted flexion and extension exercises on the 7th postoperative day. All other parameters of the postoperative rehabilitation program were identical for the two groups, including exercises, bracing, and modalities. The results showed no differences between groups for knee joint effusion, the amount of knee flexion and extension achieved by 3 months postoperative, decrease in thigh circumference loss (Fig. 12-2), and frequency and type of pain medication used while in the hospital. There was no difference between groups in AP displacements at 12 months postoperative. Patients in whom the ACL graft had been implanted through an arthrotomy had significantly greater thigh circumference loss compared with those who had an arthroscopic-assisted technique, noted by the 7th postoperative day.
Richmond and colleagues in 1991158 compared the effectiveness of CPM when used for the first 4 postoperative days to utilization during the first 14 days in 19 patients who underwent ACL B-PT-B autograft reconstruction. CPM was used for at least 6 hours a day. All patients also performed passive motion exercises from 0° to 90° three times daily. There were no differences between the groups at 6 weeks postoperative for swelling, thigh girth measurements, knee flexion and extension, time to regain full motion, or AP displacements. Immediate motion was concluded to be safe and not deleterious to the healing grafts in the initial postoperative period. No long-term benefit of CPM was demonstrated.
Rosen and coworkers163 followed 75 patients who had ACL B-PT-B autogenous reconstruction and were sorted into three groups based on a knee motion and rehabilitation program. Patients who began active-assisted early knee motion exercises had similar results in regard to knee motion achieved at week 1 and at monthly intervals for the first 6 postoperative months as those who used CPM an average of 7 hours a day for 4 weeks postoperatively. There were no deleterious effects of early motion on AP displacements at the final follow-up evaluation, 6 months postoperative. The authors concluded that the CPM device offered no advantage and increased the cost of physical therapy by $1,800.
Beynnon and associates21 conducted in vivo measurements of the elongation of B-PT-B autografts in 20 patients immediately after implantation. In 11 knees, the length of the graft increased after 14 knee flexion-extension motion cycles, and in 9 patients, the graft length decreased. The predicted maximum increase in anterior tibial translation after 20 knee motion cycles was only 1 mm.
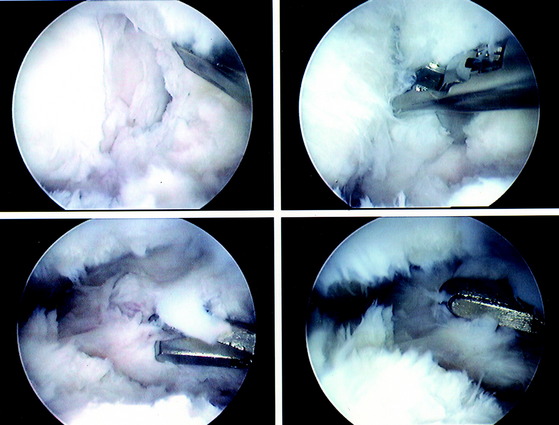
The current authors135 followed 207 knees that underwent arthroscopically assisted ACL allograft reconstruction and immediate knee motion. All patients began CPM for 8 to 10 hours a day immediately after surgery. In addition, passive and active-assisted motion exercises were initiated on the 2nd postoperative day. Partial weight-bearing was allowed immediately postoperative and was gradually progressed to full by 4 to 6 weeks based on resumption of normal gait mechanics. Any patient who demonstrated a limitation of knee flexion or extension from predetermined goals was placed into a specific treatment program as early as the 7th postoperative day. The program included overpressure exercises and modalities initially (Fig. 12-3), followed, if required, by a serial extension cast program (Fig. 12-4) and in rare instances, arthroscopic débridement of scar tissues (Fig. 12-5). Of the 207 knees, 189 (91%) regained at least 0° to 135° of motion without additional intervention. Eighteen knees were placed into the phased treatment program, of which 14 regained normal motion and 2 lacked 5° of full extension. Two other patients who had not complied with the rehabilitation program had a permanent significant limitation of motion. The incidence of postoperative motion problems was related to concomitant surgical procedures: medial collateral ligament (MCL), 23%; meniscus repair, 12%; iliotibial band extra-articular procedure, 10%; and isolated ACL, 4%.
In a second study from the authors’ center,131 443 knees that had an arthroscopic-assisted ACL B-PT-B autograft reconstruction were followed to determine the effectiveness of an immediate knee motion program and treatment protocol for limitations of flexion and extension. A CPM device was not used in this group of patients. A normal range of motion (ROM) was achieved in 436 knees (98%) and a mild limitation of extension of 5° was detected in 7. Treatment intervention was required in 23 knees, all of which achieved normal knee motion. The 7 patients whose knees had mild losses of extension refused additional treatment. Only 3 knees (<1%) required an arthroscopic lysis of adhesions that, combined with an in-patient epidural program, resulted in successful resolution of the motion limitations. The incidence of knee motion problems based on concomitant surgical procedures was 22% for MCL repairs, 18% for patellar realignment procedures, 8% for meniscus repairs, and 6% for isolated ACL reconstructions.
In 2002, Henriksson and coworkers78 compared the outcome of 5 weeks of immobilization with early knee ROM exercises in 45 patients after ACL B-PT-B autograft reconstruction. Patients in the early ROM group began motion exercises from 0° to 90° from the 7th postoperative day. The early ROM group demonstrated greater extension and flexion for the first 20 weeks. Patients in the immobilization group generally required more physical therapy visits to achieve full knee motion.
EARLY WEIGHT-BEARING
Whereas many authors have recommended early partial or full weight-bearing immediately after ACL reconstruction, few studies have examined this factor and its potential effect on both short- and long-term recovery. The effect of immediate full weight-bearing on the outcome in knees with noteworthy articular cartilage damage or those in which major concomitant operative procedures were required is unknown. In addition, gait abnormalities that may ensue from immediate full weight-bearing due to pain, knee joint effusion, and muscle weakness have not been examined. It does appear from the literature and the authors’ experience that immediate partial weight-bearing is safe and not deleterious to the healing graft.10
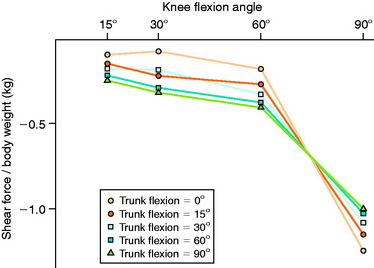
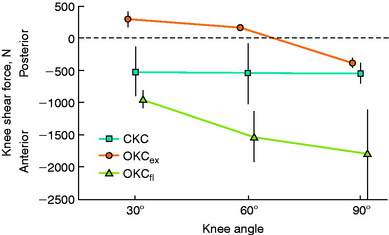
In 1991, Ohkoshi and associates144 recommended early weight-bearing exercises after ACL reconstruction based on results of a study in which shear forces on the tibia during standing were predicted from an analytical model at various trunk and knee flexion angles (Fig. 12-6). Although weight-bearing was normally prohibited in the initial postoperative period at the time of this publication, these investigators reported that the calculated shear forces were negative in all positions, with increasing posterior drawer forces found as trunk flexion angles increased (at knee flexion angles of 30° and 60°). Co- contraction of the quadriceps and hamstrings was observed at all knee and trunk flexion angle positions, with increasing hamstrings activity measured with increasing trunk flexion angles. Exercises performed in the standing position with the knees flexed and the trunk anteriorly flexed, such as half-squatting, were believed not only to be safe but also to have the potential advantages of increasing muscle strength, endurance, and proprioception and decreasing bone atrophy.
Critical Points EARLY WEIGHT-BEARING
Ohkoshi et al.144
Tyler and colleagues in 1998203 evaluated the effect of immediate full weight-bearing after ACL B-PT-B autograft reconstruction on ROM, patellofemoral pain, and AP displacement in a randomized, controlled trial. The results of 25 patients who were allowed immediate full weight-bearing were compared with those of 20 patients in whom no weight-bearing was allowed for 2 weeks postoperative. All patients performed immediate ROM exercises and followed an identical rehabilitation protocol (which was not detailed). There was no difference between groups for AP displacement, ROM, and vastus medialis obliquus (VMO) electromyographic activity at follow-up an average of 7 months postoperatively. Patients in the delayed weight-bearing group had a higher incidence of anterior knee pain than those in the immediate weight-bearing group (35% and 8%, respectively). Extension deficits of 5° or greater were reported in 14% of patients in the delayed weight-bearing group and in 20% in the immediate weight-bearing group. There was no correlation between loss of knee extension and anterior knee pain. Patients in the immediate weight-bearing group had significantly greater VMO electromyographic activity at 2 weeks postoperative compared with those in the delayed weight-bearing group, and the authors hypothesized that this aided in lowering the incidence of anterior knee pain by preventing the reflex inhibition of the VMO. Approximately 15% in each group had greater than 5 mm of increased AP displacement at follow-up.
POSTOPERATIVE BRACING
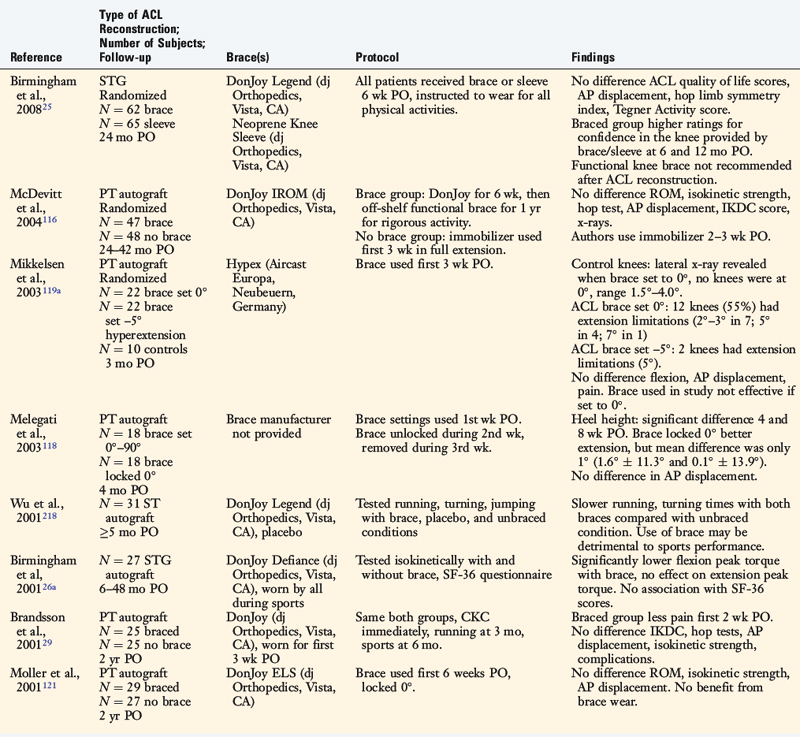
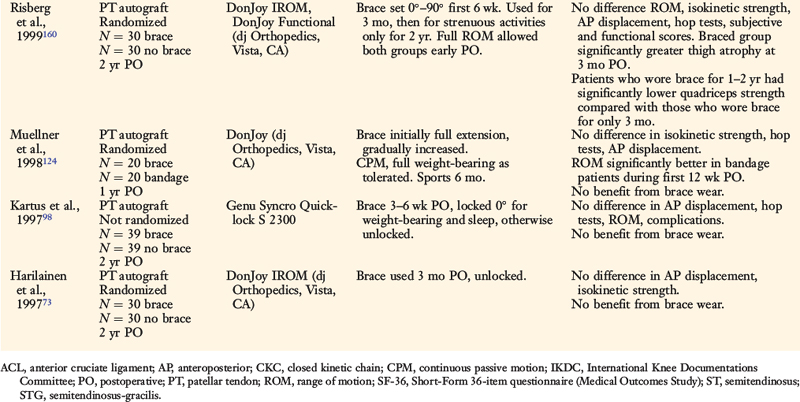
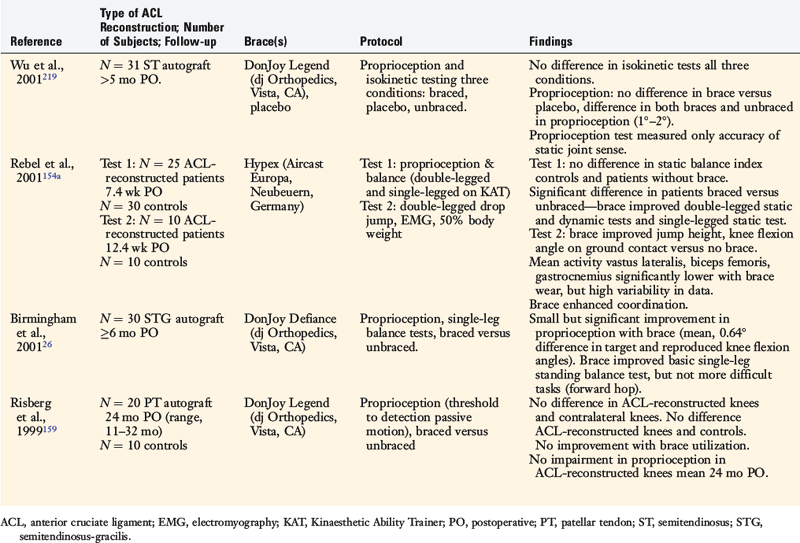
The effects of postoperative and functional braces on pain, return of normal knee motion, complications, sports activity performance, balance, and proprioception have been assessed by many investigators (Table 12-1). Most studies report that postoperative braces do not provide significant benefit in the amount of time postoperatively that normal range of knee motion is achieved, isokinetic lower extremity muscle strength, AP displacements, lower limb symmetry assessed by single-leg hop tests, and functional knee rating and activity level scores.* Negative effects of braces have been reported regarding quadriceps strength,160 running and turning times,218 and hamstrings isokinetic peak torque.26 One study reported that a functional brace improved standing balance, but not more difficult tasks such as balance after a forward hop.26 In regard to proprioception, two investigations reported significant, although small, improvements in static joint sense of 1° to 2° with brace utilization (Table 12-2).26,219 The clinical impact of these improvements remains questionable. Risberg and coworkers159 reported no improvement in the threshold to detect passive motion with application of a functional brace an average of 24 months after ACL B-PT-B autograft reconstruction. These investigators also found no impairment in proprioception in the ACL-reconstructed knees compared with those of controls.
Critical Points POSTOPERATIVE BRACING
Most studies: postoperative braces do not provide significant benefit:
Significant variation among surgeons surveyed on the use of braces postoperatively.
Authors recommend postoperative braces in complex multiligament reconstructions.
Marx and associates114 published a survey of data collected from 1998 to 1999 conducted by the American Academy of Orthopaedic Surgeons. Significant variation was reported among surgeons on the use of braces in the postoperative period, with half of the surgeons indicating they prescribed a brace for the first 6 postoperative weeks and the remaining half indicating no brace utilization. Surgeons who performed more ACL reconstructions reported less brace use. Approximately 60% of all surgeons responded that they recommended a brace for sports participation.
Beynnon and colleagues18 concluded from a review of this topic that there “appears to be a consensus among investigators that, during the early phase of recovery, the use of a rehabilitation brace results in fewer problems with swelling, lower prevalence of hemarthrosis and wound drainage, and less pain compared to rehabilitation without a brace; however, at longer-term follow-up, rehabilitation bracing does not appear to have an effect on clinical outcome.” The authors recommended postoperative bracing in complex multiligament reconstructions for protection of healing grafts and unloading braces in medial and posterolateral reconstructions to allow early partial weight-bearing.
LOWER EXTREMITY MUSCLE STRENGTH ATROPHY AND RECOVERY AFTER SURGERY
Lower extremity muscle weakness represents an unresolved problem after ACL reconstruction.* Studies have reported that the magnitude of quadriceps atrophy and strength loss exceeds 20% and 30%, respectively, in the first few months postoperatively.54,55,68,117,160 Deficits in semitendinosus and gracilis muscle volume of 10% and 30%, respectively, have been noted after STG reconstructions.86,211 Conflicting data have been published regarding the return of normal quadriceps and hamstrings muscle strength 6 months or longer postoperatively.
Critical Points LOWER EXTREMITY MUSCLE STRENGTH RECOVERY AFTER SURGERY
Lower extremity muscle weakness is an unresolved problem after ACL reconstruction.
Pathophysiology of the loss of muscle size and strength after major knee surgery is unknown.
The pathophysiology of the loss of muscle size and strength after major knee surgery remains speculative. Konishi and coworkers101 proposed abnormal gamma loop function in the quadriceps muscles due to the lack of normal sensory function in the reconstructed ACL as a potential explanation. Other authors advocated that the loss of native ACL mechanoreceptors, which have an important role in enhancing normal activity of gamma motoneurons, was a potential cause of this problem.90–92,187,192,193 Young and associates224 attributed quadriceps atrophy to a reduction in muscle fiber size from histologic findings in 14 patients. Indeed, some clinical investigators have suggested that fast-twitch muscle fibers (accounting for ~60% of the rectus femoris muscle187) become hypotrophic after ACL injury and reconstruction.13,48 Residual abnormal anterior tibial displacement after reconstruction may play a role in persistent quadriceps weakness.84 Finally, the magnitude of preoperative muscle weakness may inhibit the ability of a patient to regain normal strength and endurance after surgery.
The question of whether differential atrophy exists between type I and II muscle fibers in the quadriceps remains unclear. Haggmark and colleagues71 obtained muscle biopsies before surgery and after 5 weeks of postoperative immobilization in nine patients who underwent ACL reconstruction. A decrease in the cross-sectional area (CSA) of type 1 fibers and a reduction in the oxidative capacity of quadriceps muscle were noted, indicating a rapid fall in aerobic capacity. The authors concluded that selective atrophy of type 1 fibers occurred and that type 2 fibers were not significantly affected. In contrast, Gerber and coworkers63 studied the changes in the quadriceps muscles of 41 chronic ACL-deficient knees and reported that neither histologic nor electron microscopy showed any selective loss of muscle fiber type. These authors concluded that there was no scientific rationale for selective rehabilitation of type 1 or type 2 fibers and that vigorous training of quadriceps muscles was essential after ACL injuries. Lorentzon and associates109 studied the morphology of muscle types with biopsy and isokinetic testing of 18 male subjects with chronic ACL deficiency. These authors did not find any evidence of selective type 1 or type 2 fiber atrophy. In addition, there was no correlation between isokinetic test data and muscle size or morphologic changes. These authors concluded that decreases in muscle strength cannot be explained by muscle atrophy or structural change. They speculated that nonoptimal activation of muscles during voluntary contractions is the most causative mechanism of strength decrease found in patients with chronic ACL deficiency.
A comparison of high- and low-intensity electrical muscle stimulation (EMS), combined with an intensive rehabilitation program, on recovery of quadriceps strength and gait mechanics was conducted in two investigations.189,191 A variety of ACL grafts implanted among 110 patients were included in these studies, including Achilles tendon allografts, B-PT-B allografts, STG autografts, and B-PT-B autografts. Although high-intensity EMS significantly increased rectus femoris strength recovery (compared with the opposite limb) compared with the low-intensity EMS protocol, this effect was not as pronounced for patients who underwent ACL PT autogenous reconstruction. The strength of the quadriceps correlated with flexion and extension excursions of the knee during stance. Patients who were treated with high-intensity EMS walked with more normal excursions, which the authors attributed to the gains in quadriceps strength. Low-intensity EMS, delivered via portable units for home use, was ineffective in promoting return of normal quadriceps strength.
Fitzgerald and colleagues58 reported modest (effect size, 0.48) improvements in quadriceps isometric peak torque 16 weeks after ACL reconstruction when a “modified” EMS-training protocol was incorporated into the postoperative rehabilitation program of 21 patients. EMS was delivered with the patient positioned supine, lying passively with the knee in full extension. This protocol was modified from previously reported training methods, in which high-intensity EMS was delivered with the patient seated in an isokinetic dynamometer, the knee positioned in approximately 60° of flexion, and the patient actively contracting the quadriceps during application of the electrical current.41,189,190 The EMS protocol was modified for patients who experienced patellofemoral pain during the training sessions. The protocol involved 10 contractions to maximum patient tolerance, conducted twice weekly for 16 weeks. At 12 weeks postoperative, the experimental group demonstrated significantly greater quadriceps isometric peak torque compared with that of a matched group of patients who did not receive EMS training (75.9 ± 16.8 and 67.0 ± 19.9, respectively; P < .05). However, no significant differences were measured at 16 weeks postoperative between these groups. A greater proportion of the EMS-trained subjects initiated agility training at 16 weeks compared with the control group (62% and 32%, respectively; P < .05). Still, the authors concluded that the original high-intensity EMS-training protocol was preferred when possible based on patient tolerance and equipment availability.
Investigators and clinicians have recently questioned whether rehabilitation programs should focus more efforts on eccentric training to reduce the early postoperative loss of muscle CSA, volume, and strength following ACL reconstruction.64–66,105 In an exhaustive review of the literature on the effectiveness of concentric, eccentric, or combined concentric-eccentric training in normal, uninjured subjects, Wernbom and coworkers207 concluded that there was no evidence to support one mode of training over another with regard to achieving superior muscle hypertrophy. These authors assessed published training programs according to frequency, intensity, and duration of work on CSA and volume of the quadriceps and elbow flexor muscle groups. The observation was made that training protocols to induce muscle hypertrophy may differ from those designed to produce maximum strength.
Eccentric training is believed by some authors to be superior to concentric training owing to its potential to overload the muscle and produce greater increases in muscle size and strength.64,66 There are concerns with high-force eccentric training after ACL reconstruction, including the potential for inducing damage to the muscle and healing graft. A repeated, gradual, and progressive exposure to this type of training was successfully accomplished by Gerber and associates64 who conducted eccentric training in patients who had either ACL B-PT-B autograft (N = 20) or STG autograft (N = 20) reconstruction. The patients were randomly assigned to either a 12-week program of eccentric exercises or a standard rehabilitation program. All patients followed a similar protocol for the first 3 postoperative weeks that emphasized regaining full knee motion and basic quadriceps function. Then, patients in the eccentric exercise group initiated progressive exercises using a recumbent eccentric ergometer. The duration and intensity of the negative-work training gradually increased throughout the 12-week period. The patients underwent magnetic resonance imaging (MRI) 3 and 15 weeks postoperative.
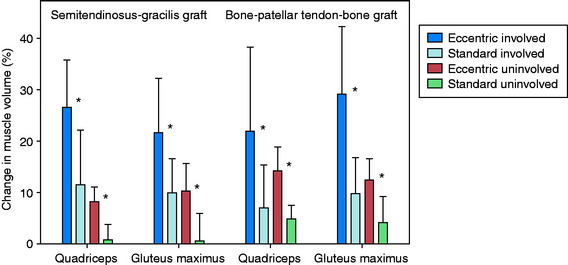
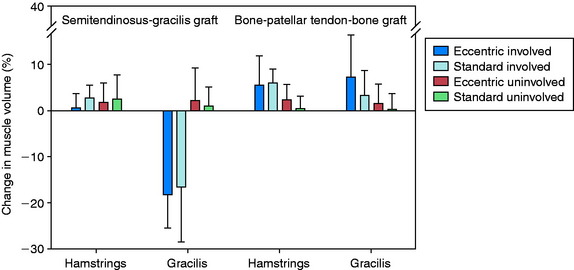
The volume and CSA measurements of the quadriceps in the reconstructed knees improved significantly (P < .001) in both groups (Fig. 12-7) between the pre- and the post-training time periods. The increases in these measurements were significantly greater (P < .001), by more than twofold, in the eccentrically trained patients than in the standard rehabilitation group. In the eccentric group, quadriceps volume increased 23.1 ± 12.9% and peak CSA increased 24.2 ± 12.6%. In comparison, in the standard rehabilitation group, quadriceps volume increased 8.8 ± 9.3% and peak CSA increased 9.3 ± 9.4%. The increase was not related to the type of graft used for the ACL reconstruction. Significant increases in these measurements were also found in the quadriceps in the noninvolved side.
There were no significant differences between the training groups in the improvement in volume and CSA of the hamstring muscles (Fig. 12-8). When analyzed according to graft type, significant improvements were noted in these measurements in the B-PT-B autograft patients (P ≤ .006), but not in the STG autograft patients. A reduction in gracilis muscle volume of nearly 20% was found 3 weeks postoperative in the STG group, which increased to a deficit of 35% by 15 weeks postoperative. The authors concluded that the gradual progressive eccentric exercise program safely and effectively improved quadriceps structure in comparison with a standard rehabilitation program. Whether the short-term results of this investigation will demonstrate longer-term benefits are unknown.
Shaw and colleagues175 conducted a prospective, blinded, randomized trial to determine the effectiveness of instituting quadriceps exercises immediately after ACL reconstruction on a number of parameters. A total of 91 patients who underwent either a B-PT-B or an STG autograft reconstruction were randomized into either the quadriceps exercise–training group or the no-quadriceps exercise–training group. The quadriceps training included straight leg raises and isometric quadriceps contractions performed three times daily for the first 2 postoperative weeks. After 2 weeks, all patients were entered into a similar rehabilitation program. At 6 months postoperative, no difference was found between groups for isokinetic quadriceps strength or lower limb symmetry on functional hop testing.
It is important to note that several authors have documented quadriceps muscle inhibition from an experimentally induced knee joint effusion.147,194,199,200 This problem has been documented in separate studies during walking, jogging, and landing from a jump. Therefore, it is imperative that knee joint effusion be avoided and, if noted, treated immediately to lessen its deleterious impact on quadriceps function.
OPEN VERSUS CLOSED KINETIC CHAIN EXERCISES: BIOMECHANICAL, IN VIVO, AND CLINICAL STUDIES
The process of graft maturation and healing is assumed to be influenced by strains and forces applied to the ACL during weight-bearing and exercises. Whereas a general consensus exists that some strain is necessary to promote the process of ligamentization,20 questions remain regarding the amount of load that is safe and the amount that may produce graft elongation. In addition, ligamentization (the return to normal native ACL characteristics) has never actually been shown to occur with graft remodeling and healing. Since the mid 1980s, investigations have attempted to measure, through either analytical or direct means, force and strain incurred on the ACL during common exercises used in rehabilitation. These exercises are typically referred to as either closed kinetic chain (CKC) or open kinetic chain (OKC). During CKC exercises, the foot is fixed to a platform or surface, the motion at the knee joint is accompanied by predictable motions at the hip and ankle joints, and the entire limb is loaded such as during a leg press or squat. In OKC exercises, the foot is mobile and not fixed to a surface, and motion at the knee joint occurs independent of motion at the hip and ankle joints. Common examples include the leg extension and hamstring curl exercises.
Cadaver Studies
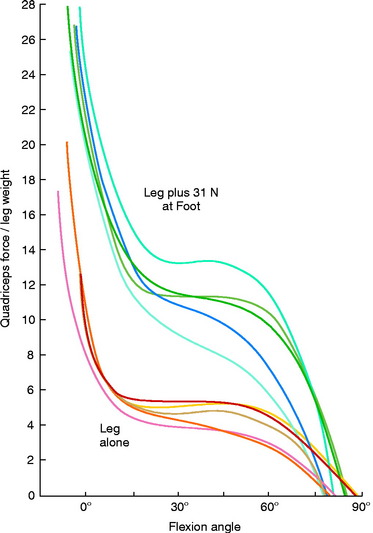
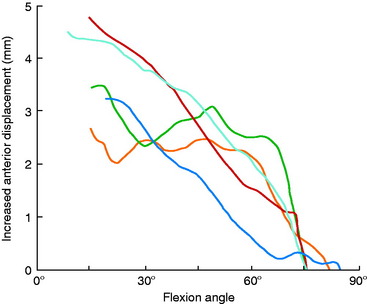
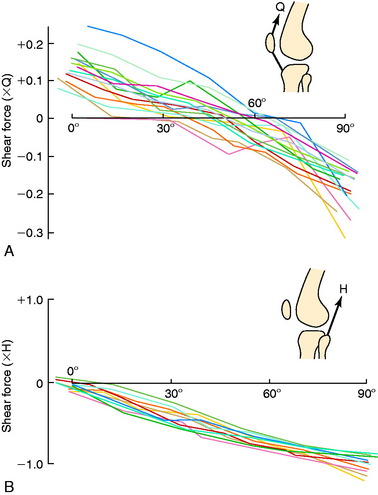
Grood and coworkers69 biomechanically examined the knee extension exercise in cadavers to determine the conditions in which the ACL was loaded, the quadriceps force that developed during knee flexion-extension, and the forces that were incurred on the extensor mechanism as the knee was extended. Both ACL-intact and ACL-sectioned conditions were created, and the OKC knee extension exercise was simulated both without resistance and with 31 N (7 lb) of resistance applied with an ankle weight at the foot. Although the quadriceps force required to extend the knee remained at a constant value of 177 N between 50° and 15°, it rose rapidly to 350 N at 0° (Fig. 12-9). The addition of resistance doubled the quadriceps force that was required to extend the knee, resulting in large muscle forces on the patellofemoral joint. There was no change in the quadriceps force required to extend the knee when the ACL was removed. However, loss of the ACL resulted in an increase in anterior tibial displacement from 0° to 30° of extension without resistance. The addition of 31 N of resistance resulted in further increases in anterior tibial displacement throughout the range of extension, with a mean increase of 3.8 mm at 15° (Fig. 12-10). The authors concluded that the range of 0° to 30° of flexion should be avoided during rehabilitation of ACL-deficient or ACL-reconstructed knees and in patients with patellofemoral symptoms.
Critical Points OPEN VERSUS CLOSED KINETIC CHAIN EXERCISES
Cadaver Studies
Effect of Open and Closed Kinetic Chain Exercises on Anterior Tibial Displacement in Intact and ACL-deficient Knees
Calculated ACL Forces, Tibiofemoral Compressive Forces, and Muscle Forces in Human Subjects
Measurement of In Vivo ACL Strain in ACL-deficient and Intact Knees during Common Open and Closed Kinetic Chain Exercises
Muscle Recruitment Patterns during Common Open and Closed Kinetic Chain Exercises
Comparative Clinical Studies
Bynum et al.31: Prospective, Randomized Study: OKC or CKC Program after ACL B-PT-B Autograft
Mikkelsen et al.119: Prospective, Randomized Study: CKC or Combined CKC-OKC Program (Began OKC Exercises Week 6) after ACL B-PT-B Autograft
Three investigations same laboratory: No benefit from OKC short-term postoperative period.
Beynnon et al.24: Prospective, Randomized Double-Blind Study of Two Rehabilitation Programs (“Accelerated” Weight-Bearing, OKC, and Traditional) after ACL B-PT-B Autograft
Renstrom and associates157 measured the strain of cadaver ACL specimens under simulated isometric contractions of various muscle groups. The authors reported that isolated hamstring contractions decreased ACL strain relative to normal passive strain at all knee flexion angles tested (0°–120°). Isolated quadriceps isometric and isotonic contractions increased ACL strain from 0° to 45° of flexion (Fig. 12-11); the greatest magnitude (5% above the normal passive strain) was measured at full knee extension. Isometric co-contractions of the quadriceps and hamstrings also increased ACL strain from 0° to 30°, with the maximum increase of 5% measured at 0° and 15° of flexion. The authors concluded that isolated quadriceps isometric and isotonic exercises, and isometric co-contraction exercises, could produce potentially harmful forces on healing ACL grafts.
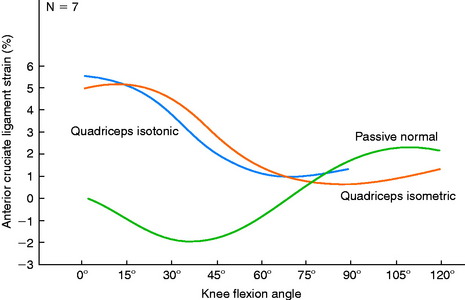
FIGURE 12-11 Mean passive normal, simulated quadriceps isometric, and simulated quadriceps isotonic strain patterns.
(From Renstrom, P.; Arms, S. W.; Stanwyck, T. S.; et al.: Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med 14:83–87, 1986.)
More and colleagues122 used a cadaver model that incorporated quadriceps and hamstrings muscle loads to examine knee kinematics and ACL loads during the squat exercise. In the intact knees, the addition of a hamstrings load resulted in a significant reduction of anterior tibial translation and internal tibial rotation during flexion. After the ACL was sectioned, the amount of anterior tibial translation increased significantly between 15° and 45° during the squat compared with the intact knee. After ACL reconstruction, maximal graft tension was measured at full extension. During the squat exercise, the addition of a hamstrings load caused a significant decrease in graft load that was most evident between 15° and 45°. The authors concluded that the squat exercise may be safe in the early postoperative period after ACL reconstruction.
Effect of OKC and CKC Exercises on Anterior Tibial Displacement in Intact and ACL-deficient Knees
Several investigators measured AP tibial displacements in ACL-intact and ACL-deficient knees during various OKC and CKC exercises, including active knee extension and squatting.97,104,220 It is important to note that the amount of AP displacement is influenced by many factors including muscle activation, joint compression forces, geometry, and ligament restraints, primarily the ACL. Overall, significantly greater anterior tibial displacements were found in the ACL-deficient knees during OKC activities than during CKC exercises.
Yack and coworkers220 measured the amount of anterior tibial displacement in 11 patients with unilateral ACL-deficient knees during an OKC exercise (resisted knee extension) and a CKC exercise (parallel squat). The average anterior tibial displacement during the squatting exercise (~7.5 mm) was significantly less in the ACL-deficient knees than that measured in the knee extension exercise from 66° to 10° (~14 mm). There was no difference in anterior tibial displacement between these exercises in the contralateral normal knees. The conclusion was reached that CKC exercises produced significantly less stress to the ruptured ACL.
In a second study, Yack and associates221 measured anterior tibial displacement in 14 patients with unilateral ACL-deficient knees during an OKC position and during progressive weight-bearing CKC positions. The progressive weight-bearing protocol included 25%, 50%, 75%, and 100% of each subject’s body weight (BW) placed on the foot during a squat held at 20° of flexion. Hamstring muscle activation was controlled to be less than 10% of its maximum activation level during all test conditions. The amount of anterior tibial translation induced during an 89-N Lachman test was measured and compared with that produced during the weight-bearing and non–weight-bearing conditions. The authors reported that anterior tibial translation was significantly less during all weight-bearing conditions than that measured during the Lachman test and the non–weight-bearing conditions. The clinical implication was that rehabilitation after ACL reconstruction could institute weight-bearing exercises without causing excessive strain to the passive restraints. Progressive loading during non–weight-bearing activities should be delayed until the later stages of rehabilitation.
Similar findings were reported by Kvist and Gillquist,104 who measured anterior tibial translation and lower limb muscle activation in patients with ACL-intact and ACL-ruptured knees during active knee extension (with and without resistance) and squatting exercises in which the center of gravity was placed either over, behind, or in front of the feet. The CKC squat exercises produced less anterior tibial translation than the knee extension in the ACL-deficient knees. In the normal knees, anterior tibial translation increased with increasing loads in all of the exercises except during the squats with the center of gravity behind the feet. This activity produced less translation than did all of the other exercises in both the normal and the ACL-deficient knees. Overall, hamstring muscle activity was low in all knees during both the OKC and the CKC exercises, but co-activation of the gastrocnemius and quadriceps muscles was noted. The authors concluded that the CKC exercises caused increased joint compression forces and co-activation of the gastrocnemius and quadriceps muscles, thereby resulting in decreased anterior tibial displacements in ACL-deficient knees.
Jurist and Otis97 measured anterior and posterior tibial displacement in five knees with intact ACLs to determine the effects of knee flexion angle (30°, 60°, and 90°) and the position of external resistance (proximal, middle, or distal on the long axis of the tibia) during isometric contractions. The results demonstrated that a proximal position of external load produced posterior displacement of the tibia. The authors concluded that quadriceps muscle strengthening could be safely initiated between 90° and 30° of flexion in ACL-reconstructed knees early postoperatively.
Calculated ACL Forces, Tibiofemoral Compressive Forces, and Muscle Forces in Human Subjects
Several investigators used two-dimensional mathematical models from in-vivo experimental measures to calculate muscle forces, tibiofemoral shear, and compressive forces during isometric and isokinetic exercises.* The analytical models did not allow for the authors to determine the magnitude of the ligament forces incurred during various exercises.
Yasuda and Sasaki222 calculated anterior and posterior drawer forces exerted on the tibia in 20 healthy adult males during isometric contractions of the quadriceps and hamstrings at knee flexion angles ranging from 5° to 90° (Fig. 12-12). During the quadriceps isometric contractions, maximum anterior shear forces were measured at 5° of knee flexion. As the angle of knee flexion increased, the calculated anterior shear force decreased. The mean knee flexion angle at which the anterior drawer force changed to a posterior drawer force during this exercise was 45.3° ± 12.5°. During isometric hamstring contractions, posterior shear forces were measured at all knee flexion angles. The authors concluded that early postoperative rehabilitation should include quadriceps isometrics exercises with knee flexion greater than 70° and hamstrings isometric exercises at all flexion angles. The question was raised of the efficacy of quadriceps isometric training at high knee flexion angles for adequate muscle training.
Lutz and colleagues110 analyzed forces at the tibiofemoral joint during OKC and CKC exercises in five healthy subjects. A two-dimensional model was used to calculate tibiofemoral shear and compression forces during maximal isometric contractions at 30°, 60°, and 90° of knee flexion. The OKC knee extension contraction produced the greatest amount of anterior shear force (Fig. 12-13; 285 ± 120 N at 30° of flexion). CKC exercises produced significantly less anterior shear force at all flexion angles. In addition, CKC exercises produced significantly greater compressive forces and muscular co-contraction at the same knee flexion angles at which the OKC exercises produced maximum shear forces and minimum muscular co-contraction. The authors recommended CKC exercises after ACL injury or reconstruction.
Ohkoshi and associates144 used a two-dimensional model derived from radiographs and electromyographic analyses to predict shear forces on the tibia during standing at various trunk and knee flexion angles in 21 normal male subjects. Co-contraction of the quadriceps and hamstrings was observed at all knee and trunk flexion angle positions. Hamstrings activity increased with increasing trunk flexion angles. The calculated shear forces were negative in all positions, with increasing posterior drawer forces found as trunk flexion angles increased (at knee flexion angles of 30° and 60°). The authors concluded that exercises done in the standing position with the knees flexed and the trunk anteriorly flexed (such as half-squatting) could be performed safely in the early stages after ACL reconstruction.
Other investigators used three-dimensional models to predict internal muscle forces, tibiofemoral compressive forces, tension in the ACL and posterior cruciate ligament, and patellofemoral compressive forces during OKC and CKC exercises.51,99,201,210 Kaufman and coworkers99 reported that an isokinetic OKC knee extension exercise produced mean tibiofemoral compressive forces of 4.0 ± 0.7 times BW at 60°/sec and 3.8 ± 0.9 BW at 180°/sec; however, these were calculated at 55° of knee flexion. Anterior shear forces existed between 40° and full extension, potentially loading the ACL.
Escamilla and coworkers51 and Wilk and associates210 reported that greater tibiofemoral compressive forces occurred during CKC exercises than during OKC knee extension (Fig. 12-14) and that these compressive forces were greatest when the knee was fully flexed. The calculated joint compression forces were dependent on the position of the trunk relative to the knee and ankle joints, with greater forces generated when the body was positioned directly over the knee. Peak ACL tensile forces occurred only during OKC exercises near full extension and were calculated as 0.20 × BW. CKC exercises produced greater co-contraction between the quadriceps and the hamstrings compared with knee extension; however, the magnitude was dependent on the trunk position and knee flexion angle. In addition, the squat produced approximately double the amount of hamstring activity as the leg press and knee extension exercises. Knee extension produced greater quadriceps activity, leading the investigators to conclude that the ACL could be loaded from 0° to 60° during this exercise.
Toutoungi and colleagues201 determined cruciate ligament forces from analytical modeling in normal knees during OKC isokinetic and isometric exercises and during CKC double-leg and single-leg squatting. During isokinetic and isometric extension, peak ACL forces occurred from 35° to 40° of nearly 400 N, or 0.55 × BW. However, during isokinetic extension, ACL forces decreased significantly with increasing dynamometer speed, from 349 N at 60°/sec to 254 N at 180°/sec. Small forces were incurred on the ACL during squats at knee flexion angles less than 50°. The authors concluded that isokinetic flexion and squats were safe to perform in the early postoperative period after ACL reconstruction, but that isokinetic knee extension should be avoided until graft healing is well advanced.
Shields and coworkers183 examined the single-leg squat in normal subjects to determine the effect of resistance to both flexion and extension and knee flexion angle on lower extremity muscle activity. Resistance to flexion and extension was set as a percentage of BW, being either 0%, 4%, or 8%. The results revealed that co-contraction of the quadriceps and hamstrings occurred throughout the squatting exercise (0°–40°). Although the quadriceps had greater activity than the hamstrings at all levels of resistance, the quadriceps-to-hamstrings ratio decreased with higher levels of resistance. Biceps femoris activity increased during knee flexion with resistance from approximately 12% of that of a maximum voluntary isometric contraction (MVIC) during low resistance (0% BW) to 27% of that of a MVIC during high resistance (8% BW). The authors suggested that this CKC exercise done under controlled conditions with resistance to both flexion and extension was effective in increasing the dynamic control of the knee joint.
Measurement of In Vivo ACL Strain in ACL-deficient and Intact Knees during Common OKC and CKC Exercises
Henning and associates77 were the first to measure the in vivo ACL strain in human subjects. The authors applied a strain gauge to two subjects with partially disrupted ACLs and measured ACL strain and elongation during OKC and CKC activities, which were compared with loads induced by an 356-N (80-lb) Lachman test. The sample size did not allow for statistical comparisons or analyses. The authors noted that activities such as partial weight-bearing with crutches and stationary bicycling produced only 7% as much elongation as the Lachman test. Knee extension exercises with 89 N (20 lb) of resistance produced 87% to 121% from 22° to 0°, but produced only 50% as much elongation as the Lachman test. The authors recommended avoidance of quadriceps exercises and testing by knee extension for the first year after ACL injury or reconstruction.
Beynnon and colleagues17,22,59,61,76 conducted a series of studies in which a Hall effect transducer was arthroscopically implanted into the anteromedial fibers of the normal ACL in volunteers undergoing surgical procedures under local anesthesia. Patients performed a variety of OKC and CKC exercises at different knee flexion angles, including isometric contractions,17 squatting,22 bicycling,60 stair climbing,59 and lunging.76 The mean peak ACL strains reported in these studies are shown in Table 12-3.20 The magnitude of ACL strain produced by the exercises assessed in these studies was lowest in those that involved isometric contractions of the hamstring muscles; simultaneous contractions of the quadriceps and hamstrings at 30°, 60°, and 90° of knee flexion; isometric quadriceps contractions at 60° and 90° of knee flexion; and passive flexion and extension of the knee. The activities that produced the highest ACL strain included isometric quadriceps contractions at 15° of knee flexion with 30 Nm of extension torque, squatting without and with resistance, and active flexion and extension of the knee with a 45-N weight boot. The authors emphasized that the limits of ACL strain that are safe and not deleterious to healing ACL grafts remain unknown. Although excessive loading must be avoided early postoperatively, controlled loading is required to enhance graft healing and ligamentization.
TABLE 12-3 Rank Comparison of Mean Peak Anterior Cruciate Ligament Strain Values Measured In Vivo in Subjects with Uninjured Knees
| Exercise | OKC or CKC | Peak ACL Strain (%) |
|---|---|---|
| Isometric quadriceps contraction at 15° (30 Nm extension torque) | OKC | 4.4 |
| Squat with Sport Cord | CKC | 4.0 |
| Active flexion and extension (45-N weight boot) | OKC | 3.8 |
| Lachman test at 30° (150 N anterior shear load) | 3.7 | |
| Squat, no resistance | CKC | 3.6 |
| Isometric gastrocnemius contraction at 15° (15 Nm plantar flexion torque) | OKC | 3.5 |
| Active flexion and extension, no resistance | OKC | 2.8 |
| Co-contraction quadriceps and hamstrings at 15° | OKC | 2.8 |
| Isometric gastrocnemius contraction at 5° (15 Nm plantar flexion torque) | OKC | 2.8 |
| Single-legged sit-to-stand exercise | CKC | 2.8 |
| Isometric quadriceps contraction at 30° (30 Nm extension torque) | OKC | 2.7 |
| Stair climbing | CKC | 2.7 |
| Step-up and step-down | CKC | 2.5 |
| Weight-bearing at 20° | 2.1 | |
| Leg press at 20° (40% body weight) | CKC | 2.1 |
| Anterior drawer test at 90° (150 N anterior shear load) | 1.8 | |
| Lunge | CKC | 1.8 |
| Stationary bicycling | CKC | 1.7 |
| Isometric hamstrings contraction at 15° (10 Nm flexion torque) | OKC | <1.0 |
| Co-contraction quadriceps and hamstrings at 30° | OKC | <1.0 |
| Isometric gastrocnemius contraction at 30° (15 Nm plantar flexion torque) | OKC | <1.0 |
| Passive flexion and extension | OKC | <1.0 |
| Isometric quadriceps contraction at 60° and 90° (30 Nm extension torque) | OKC | 0 |
| Isometric gastrocnemius contraction at 45° (15 Nm plantar flexion torque) | OKC | 0 |
| Co-contraction quadriceps and hamstrings at 60° and 90° | OKC | 0 |
| Isometric hamstrings contraction at 30°, 60°, and 90° (10 Nm flexion torque) | OKC | 0 |
ACL, anterior cruciate ligament; CKC, closed kinetic chain; OKC, open kinetic chain.
Muscle Recruitment Patterns during Common OKC and CKC Exercises
Stensdotter and coworkers197 studied onset time and amplitude of the quadriceps muscles during a knee extension and simulated leg press isometric contraction in 10 healthy subjects. The onset of activity for all of the quadriceps muscles was simultaneous during the CKC activity (Fig. 12-15). During the OKC task, the onset of vastus lateralis, vastus medialis longus, and rectus femoris occurred before that of VMO. The mean amplitude for rectus femoris was significantly greater during the OKC exercise, whereas the mean amplitude for VMO was significantly larger during the CKC exercise.
Beutler and associates16 measured quadriceps and hamstring activation in healthy subjects during two CKC activities. The peak levels of quadriceps activation were 201% and 207% of a MVIC for single-leg squats and step-ups, respectively. Hamstring activity (biceps femoris muscle only) was approximately 20% to 40% MVIC, which could have been influenced by trunk flexion angle, which was not controlled for in this study. The authors concluded that both of these CKC exercises were effective for males and females for achieving maximal quadriceps contraction for strength training or rehabilitation.
Salem and colleagues168 evaluated the kinematics and kinetics of the ankle, knee, and hip in eight ACL-reconstructed and contralateral knees during a two-legged squat. The patients were tested a mean of 30 ± 12 weeks postoperative; all had implemented the two-legged squat into their rehabilitation at least 6 weeks prior to testing. Two distinctly different strategies were noted for generating the joint torques required to perform the exercise. In the noninvolved limb, equal distribution of hip and knee muscular effort was noted. However, in the reconstructed limb, patients used a more hip-dominant strategy, thereby reducing the knee extension peak torque effort. The authors cautioned that patients may use substitution methods during bilateral CKC activities by either shifting the effort from the reconstructed limb to the contralateral limb or adopting a hip-dominant strategy, limiting the potential effectiveness of these exercises.
Comparative Rehabilitation Studies after ACL Reconstruction
Few investigators have prospectively compared the outcome of rehabilitation programs of OKC and CKC exercises. Bynum and coworkers31 were the first to report a prospective, randomized study in which patients were placed into either an OKC or a CKC program after ACL B-PT-B autogenous reconstruction. Forty-seven patients performed OKC exercises beginning with hamstring isotonics immediately postoperatively, straight leg raises at 3 weeks, and quadriceps isotonics with low resistance at 6 weeks that progressed to unrestricted at 12 weeks. Knee motion restrictions for the OKC exercises were not detailed. Fifty patients performed CKC exercises including double-leg partial squats, leg press, and stationary bicycling initially. All 97 patients were entered into an immediate knee motion program (0°–90°) and allowed partial weight-bearing, progressing to full as tolerated. Only 64 patients (66%) returned for evaluation from 12 to 36 months postoperatively; 85 completed a subjective analysis by phone interview. At follow-up, significantly lower values were reported in mean anterior tibial translation and patellofemoral pain restricting activities in the CKC group. Significantly higher values were found in the patient rating of the end result of the operation in the CKC group as well. The authors recommended the exclusive use of CKC protocols after ACL reconstruction.
Mikkelsen and associates119 followed 44 patients who underwent ACL B-PT-B autogenous reconstruction and who were randomized into either a CKC program or a combined CKC-OKC program that initiated OKC exercises at postoperative week 6. The OKC exercises consisted of quadriceps isokinetic training done under supervised conditions in the range of 90° to 40°, which gradually progressed during the next 6 weeks to 90° to 10°. The patients were reviewed at 6 months postoperative with knee arthrometer and muscle strength testing and at an average of 31 months postoperative with a questionnaire. There was no difference between groups in the mean anterior tibial displacement. Patients in the combined OKC-CKC group had significantly greater quadriceps peak torque values than those in the CKC group at 6 months postoperative; however, this difference was not observed after this time period. In addition, the peak torque values were not normalized for BW, so direct comparisons are susceptible to error. A higher percentage of patients (55%) in the combined OKC-CKC group returned to preinjury sports activities levels than those in the CKC group (23%). The authors recommended the combined protocol under carefully supervised conditions.
Three prospective trials were conducted from a group of surgeons in London, U.K., that compared OKC and CKC for hip and knee extensor training after ACL reconstruction.81,123,152 The first study comprised 36 patients who underwent ACL B-PT-B autogenous reconstruction; in 9, a ligament augmentation device (LAD) was implanted along with a small strip of the PT, and in 27, the central third of the PT was harvested.123 At 2 weeks postoperative, patients were randomized into either a CKC program or an OKC program; the patients were trained and followed for the next 4 weeks. The CKC patients performed unilateral leg press exercises in the range of 90° to 0°, and the OKC patients performed hip and knee extension isotonic exercises through the range of 90° to 0° three times a week (for a total of 60 cycles of concentric and eccentric contractions). Neither group performed squats or step-ups, but all were allowed to use the stationary bicycle. The only outcome measure in this study was anterior tibial displacement, which was not significantly different at the conclusion of the 4-week training period. The authors concluded that because the OKC offered no benefit, only CKC should be used for rehabilitation after ACL reconstruction.
In the second investigation, gait analyses were conducted on the population described by Morrissey and colleagues before123 and after training.81 No statistically significant differences were found after training between the OKC and the CKC groups for 16 variables assessed during level walking and ascending and descending stairs. The authors were unable to provide a definitive recommendation for rehabilitation based on the study’s findings.
The third study involved 49 patients from 12 surgeons who performed a variety of ACL reconstructive methods, including B-PT-B autograft and LAD, arthroscopic-assisted or open central third PT, and semitendinosus and/or gracilis tendons.152 CKC and OKC training was initiated 8 weeks postoperative for hip and knee extensors as described by Morrissey and colleagues.123 Training was conducted three times a week for 6 weeks, and all patients also performed stationary bicycling, lunges, single-leg exercises on a minitrampoline and a balance board, lateral plyometric hopping, and hamstring isotonic exercises. Post-training testing performed at 14 weeks postoperative revealed no significant differences between the groups for anterior tibial displacement, subjective function as measured by visual analog scales, or single-leg hop tests. The authors recommended only CKC after ACL reconstruction, citing no benefit of OKC.
Beynnon and coworkers24 conducted a prospective, randomized, double-blind study of two rehabilitation programs after ACL arthroscopic-assisted B-PT-B autogenous reconstruction. The programs differed according to the time of supervised physical therapy patients were requested to participate in postoperatively and the amount of strain believed to be incurred on the graft based on the authors’ prior in vivo studies. The “accelerated” program lasted 19 weeks and allowed exercises that produced high ACL strain to be initiated sooner than the nonaccelerated program, which lasted 32 weeks. For example, OKC exercises were initiated as early as week 2 (straight leg raises) in the accelerated group, as well as allowance of full weight-bearing without crutch support. Knee extensions from 0° to 90° were begun at week 6 in the accelerated group, compared with week 12 in the nonaccelerated group. The patients (10 in the accelerated group and 12 in the nonaccelerated group) were followed for 2 years postoperatively.
PATELLOFEMORAL JOINT CONSIDERATIONS
As previously discussed, the quadriceps force that develops during knee flexion-extension and the forces incurred on the extensor mechanism as the knee extends were experimentally measured in the authors’ laboratory.69 The investigation demonstrated that the quadriceps force required to extend the knee remained at a constant value of 177 N between 50° and 15° of knee flexion, and then rose rapidly to 350 N at 0°. The addition of a small amount of resistance (31 N) doubled the quadriceps force that was required to extend the knee, resulting in large forces on the patellofemoral joint. This was the first investigation to recommend avoidance of quadriceps exercises in the range of 0° to 30° of flexion during rehabilitation of the ACL-deficient or ACL-reconstructed knee and in knees with patellofemoral symptoms.
Critical Points PATELLOFEMORAL JOINT CONSIDERATIONS
Doucette and Child43 used computed tomography to measure the patellar congruence angle in patients with symptomatic lateral patellar compression syndrome under three conditions: with the lower extremity relaxed, holding an OKC (knee extension) position, and holding a CKC exercise position. Measurements were made in 10° increments from 0° to 40° of flexion. There were significant differences at 0°, 10°, and 20° of flexion between the OKC position and both the CKC and the relaxed conditions. At each flexion angle, significantly greater lateral patellar tracking occurred during the OKC exercise. During all three conditions, patellar congruence progressively improved from 0° to 40° of flexion. The authors concluded that a quadriceps contraction has less influence on patellar tracking at 30° of flexion than at 0° of flexion owing to the increased stability of the patella as it moves into the intercondylar groove as the knee is flexed. In low, functional knee ROMs, CKC exercises were recommended owing to the improved patellar positioning and decreased joint irritation in symptomatic patellofemoral patients.
Steinkamp and associates196 calculated knee moments, patellofemoral joint reaction forces, and patellofemoral joint stresses in 20 normal subjects at four knee flexion angles (0°, 30°, 60°, and 90°) during the leg press and knee extension exercises. All three parameters were significantly greater at 0° and 30° in the leg extension exercises than during the leg press exercise (Fig. 12-16). The opposite was true at the high knee flexion angles, for which all parameters were significantly greater in the leg press exercise. The authors concluded that the leg press placed minimal stress on the patellofemoral joint in the functional ROM (low knee flexion angles) and noted empirically that patients with patellofemoral disorders frequently complained of pain with the knee extension exercise. During the leg press exercise, compressive forces were higher but distributed over a larger contact area, whereas during the leg extension exercise, compressive forces were lower but concentrated over a smaller contact area.
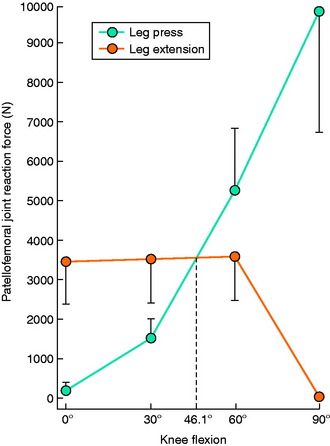
FIGURE 12-16 Mean ± standard deviation of patellofemoral joint reaction force at four flexion angles.
(From Steinkamp, L. A.; Dillingham, M. F.; Markel, M. D.; et al.: Biomechanical considerations in patellofemoral joint rehabilitation. Am J Sports Med 21:438–444, 1993.)
Witvrouw and colleagues212,213 conducted investigations on 51 patients with isolated patellofemoral pain who were randomly assigned to either an OKC or a CKC 5-week exercise program. The OKC exercises consisted of maximal static quadriceps contractions with the knee in full extension, straight leg raises in the supine position, leg adduction exercises in the lateral decubitus position, and short arc movements from 10° of flexion to terminal extension. The CKC exercises included seated leg press, double or single one third knee bend, stationary biking, rowing machine, step-up and step-down exercises, and jumping on a minitrampoline. The patients were evaluated upon completion of the program and then 5 years later. There were no significant differences between the two treatment groups for the majority of parameters evaluated. At the 5-year evaluation, 92% of the OKC group was participating in sports compared with 60% of those in the CKC group. The OKC group demonstrated, on visual analog scales, less swelling, pain on descending stairs, and pain at night compared with the CKC group. Owing to the overall lack of differences between the groups in the majority of parameters studied, the authors recommended a combination of both OKC and CKC exercises in patients with patellofemoral symptoms.
Dye45 reviewed the role of loading in patellofemoral pain, including the amount sustained by a single blow (such as during a fall onto the pavement) and the amount sustained with repeated smaller loads that disrupt normal tissue homeostasis (such as climbing up or down stairs, kneeling, squatting, or jumping). The stress on the patellofemoral joint depends not only on the load applied but also on the surface areas of the patella and femur that may be in contact at any given point in time. Estimated loads on the patellofemoral joint with weight-bearing activities ranged from 3.3 times BW with stair climbing to 7.6 times BW with squatting to up to 20 times BW with jumping.156,186 The ability of the entire knee joint to absorb and distribute these forces is dependent on what Dye45 termed the envelope of function, or “that range of loading applied across the joint that is compatible with and probably inductive of maintenance of tissue homeostasis.” The envelope includes three zones. The first is the zone of subphysiologic underload, or diminished loads (e.g., prolonged bedrest), that cause muscle atrophy or calcium loss. The second zone, that of homeostatic loading, represents the range of acceptable loading in which tissue homeostasis is maintained. This zone is highly variable between individuals, because knee joints accept loads that range from less than 1 to nearly 8 times BW.45 The third zone, supraphysiologic overload, represents loads that exceed the knee joint’s ability to accept and distribute forces in a manner that maintains homeostasis. The author expressed that patellofemoral pain could frequently be diminished by simply lowering the forces to the patient’s asymptomatic envelope of function.
ALTERATIONS IN GAIT, NEUROMUSCULAR FUNCTION, AND PROPRIOCEPTION AFTER ACL RECONSTRUCTION
Chronic ACL deficiency produces marked alterations in gait during a variety of activities.4,5,15,137,149,208 During level walking, ACL-deficient subjects demonstrate significantly decreased external knee flexion moments and increased external knee extension moments compared with healthy control subjects.208 The resultant quadriceps avoidance gait pattern has been identified in these knees in several investigations: in 16 of 32 knees that also had varus malalignment in the authors’ laboratory,137 in 7 of 8 subjects who were greater than 7 years postinjury by Wexler and coworkers,208 and in 12 of 16 (75%) subjects by Berchuck and associates.15 Wexler and coworkers208 found that changes in sagittal plane knee moments were more pronounced as the amount of time after the injury increased. Berchuck and associates15 reported that gait adaptations were present in both the injured and the contralateral limbs owing to the symmetrical function required for weight-bearing activities. Patel and colleagues149 found that patients with ACL deficiency had a significantly reduced peak external flexion moment during jogging and stair climbing that correlated with significantly reduced quadriceps strength. Andriacchi and coworkers5 used a finite-element model from three-dimensional cartilage volumes created from MRI to predict progression of osteoarthritis (OA) in normal and ACL-deficient knees. The model predicted a more rapid rate of cartilage thinning in the ACL-deficient knees, especially in the medial tibiofemoral compartment. The investigators concluded that this was due to a shift in the normal load-bearing regions of the knee joint during weight-bearing activities and stressed the importance of restoring proper gait mechanics after ACL reconstruction.
Several investigations have documented altered gait biomechanics, neuromuscular function, and proprioception many months or years after ACL reconstruction.27,30,42,50,106,214 Unfortunately, the majority of these investigations did not provide detailed information regarding the postoperative rehabilitation program. Timoney and associates198 were among the first investigators to document altered gait kinematics in a study conducted on 10 male patients an average of 10 months (range, 9–12 mo) after ACL B-PT-B autograft reconstruction. Compared with a control group, the patients had a significantly lower mean external knee flexion moment at midstance (3.74% and 2.02%, respectively) and a significantly lower mean heel-strike transient value (66.6 and 44.3 BW/sec, respectively). The patients’ reconstructed limb had a significantly lower mean midstance external knee flexion moment than the uninvolved limb (2.02% and 3.10%, respectively). The patients did not demonstrate a quadriceps-avoidance gait, because a net external flexion moment was present throughout most of the stance phase.
Devita and colleagues42 conducted gait analysis testing in eight patients who had a B-PT-B autograft ACL reconstruction 3 weeks and 6 months after surgery and an “accelerated” rehabilitation program. The data were compared with those collected from 22 healthy subjects. Although the ACL-reconstructed patients walked with normal kinematic patterns 6 months postoperatively, they demonstrated altered joint torque and power patterns at the hip and knee. The hip extensors provided more vertical support and forward progression during the first half of stance compared with the contribution measured in the normal subjects. There was also a decrease in the magnitude of extensor torque at the knee in early stance compared with that in the healthy subjects.
Altered Gait Biomechanics
Altered Neuromuscular Function
Altered Proprioception
Kowalk and coworkers102 conducted gait analyses on 7 patients who had a B-PT-B autograft reconstruction (mean, 6 mo postoperative; range, 3.2–11.3 mo) and on 10 healthy subjects. The analysis consisted of ascending three steps that were attached to two force plates. The authors reported statistically significant reductions for peak moment, power, and work in the reconstructed knees along with significant increases in excursion, moment, and power at the contralateral ankle joint. The patients compensated during the stair ascent task by generating increased power at the contralateral ankle.
Ernst and associates50 measured lower extremity kinematics in 20 patients who underwent a B-PT-B autograft reconstruction (mean, 9.8 mo postoperative; range, 8–15 mo) and in 20 matched normal subjects. The subjects performed a single-leg vertical jump and a lateral step-up. The knee extension moment of the ACL-reconstructed subjects was less than that of their contralateral lower extremity and those of the lower extremities of the controls during take-off and landing on the vertical jump and the lateral step-up exercise. The authors suggested that this finding was related to either weakness of the quadriceps femoris muscle or some alteration of the neuromuscular system in which hip or ankle extensors were recruited. Patients may compensate during these activities and not adequately recruit the quadriceps musculature, thereby reducing the potential effectiveness of the exercises.
Bush-Joseph and colleagues30 measured knee kinematics and kinetics in 22 patients who had ACL B-PT-B autograft reconstruction 22 ± 12 months postoperative. The data were compared with a control group of 22 subjects. All patients were satisfied with the results of the reconstruction and all had negative Lachman and pivot shift tests. Quadriceps and hamstrings isokinetic peak torques were similar to those of the control group and the contralateral limbs. Peak external moments were similar between the ACL and the control subjects for light activities such as walking and stair climbing. However, a decrease was noted in the peak external flexion moment (net quadriceps moment) in the ACL-reconstructed knees compared with that of controls during jogging and a jog-and-cut maneuver (13.3 ± 3.9% BW × height [Ht] and 16.1 ± 4.2% BW × Ht, respectively; P = .024). The decrease in the peak external flexion moment during jogging significantly correlated with quadriceps muscle strength at 60°/sec (R2 = 0.464, P < .001) and at 180°/sec and 240°/sec (P < .02). This correlation between quadriceps muscle strength and decreased external flexion moment was not found during the jog-and-cut task. Subjects in the ACL-reconstructed group with the weakest quadriceps muscles had the greatest reductions in the peak flexion moment during jogging. The investigators concluded that functional adaptations during more demanding athletic activities were present in patients who had well-functioning ACL reconstructions and minimal decreases in quadriceps strength compared with controls. The authors speculated that further improvements in lower extremity strength may improve these adaptations and reinforced the value of ensuring comprehensive quadriceps training after ACL reconstruction.
Wojtys and Huston214 measured lower extremity muscle strength, endurance, reaction time, and time to reach peak torque in a group of 25 patients who received a B-PT-B autograft reconstruction at 6, 12, and 18 months postoperative. A group of 40 healthy subjects served as controls. At 18 months postoperative, 88% of the reconstructed knees were within 3 mm of increased AP displacement compared with the opposite limb and 80% of the patients believed they had regained their preinjury sports activity level. However, quadriceps peak torque of the reconstructed limbs was equal to that of the opposite limbs in only 72% (Fig. 12-17). Quadriceps endurance failed to reach the level of the opposite limb throughout the study period. Time to reach peak torque of both the quadriceps (Fig. 12-18) and the hamstrings (Fig. 12-19) was significantly slower than the uninvolved limb at all test sessions.
FIGURE 12-17 Comparison of quadriceps peak torque over time.
(Redrawn from Wojtys, E. M.; Huston, L. J.: Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med 28:336–344, 2000.)
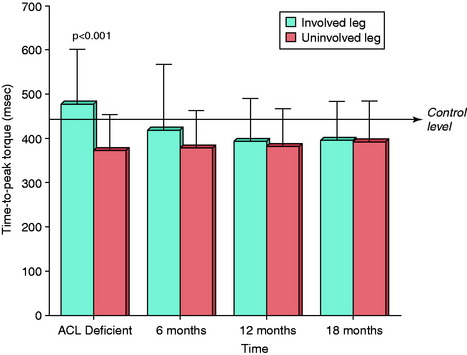
FIGURE 12-18 Comparison of the time to peak torque of the quadriceps over time.
(Redrawn from Wojtys, E. M.; Huston, L. J.: Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med 28:336–344, 2000.)
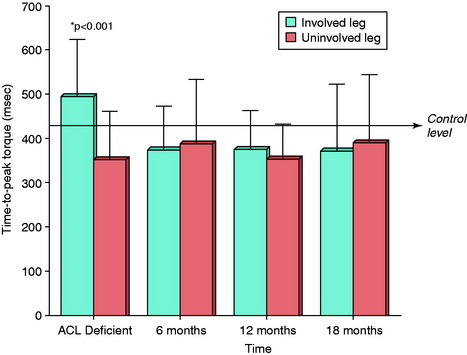
FIGURE 12-19 Comparison of the time to peak torque of the hamstring muscles over time.
(Redrawn from Wojtys, E. M.; Huston, L. J.: Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med 28:336–344, 2000.)
Although deficits in proprioception after ACL reconstruction have been documented by several investigators,12,27,106,112 others have shown that no significant difference exists after surgery.32,82,88,155,159 The differences in findings are based on variability in test procedures, time from injury to ACL reconstruction, the presence of associated injuries to the menisci and articular cartilage, the rehabilitation program, and the use of internal versus external control limbs.155 It is also difficult to understand whether statistically significant differences reported by authors represent clinically relevant findings. The two most common tests for proprioception are threshold for detection of passive motion (TDPM) and joint position sense (JPS). Differences in the magnitude of error in the patients’ ability to reproduce a specific joint position (flexion angle) postoperatively during these tests are frequently less than 1° to 2° compared with either internal or external control data.
Lephart and coworkers106 reported significantly decreased kinesthetic awareness in 12 ACL-reconstructed knees compared with the uninvolved limb near the terminal end of knee motion. The ACL-reconstructed knees demonstrated a longer TDPM at 15° of knee flexion (mean difference, ~1.5°, moving from 15° of flexion to full extension). However, no significant difference was found between limbs in kinesthetic awareness at higher knee flexion angles (≥45°). MacDonald and associates112 reported a significant difference in the mean TDPM in ACL-reconstructed (0.17°–0.22°) as well as ACL-deficient (0.14°) knees when the mean values were compared with those of internal controls (uninvolved knees). However, this study found no difference in mean TDPM values when ACL-deficient and ACL-reconstructed knee data were compared with external control knee values. Neither of these studies conducted JPS testing.
Risberg and coworkers159 found no significant difference in mean TDPM between ACL-reconstructed knees and internal or external controls. The patients were tested a mean of 24 months (range, 11–32 mo) after B-PT-B autogenous ACL reconstruction and a supervised rehabilitation program. No effects were found in TDPM values when a functional knee brace was applied to the reconstructed knees. Fischer-Rasmussen and Jensen57 also found no difference in mean TDPM between ACL-reconstructed and ACL-deficient knees compared with external controls. These authors did find a significant difference in JPS at 60° of flexion between ACL-reconstructed and external controls and between ACL-deficient and external controls. No difference was found between the three groups in JPS at full extension.
Co and coworkers32 measured TDPM and JPS in 10 patients a mean of 31.6 months after ACL B-PT-B autogenous reconstruction. All of the knees had less than 3 mm of increased AP displacement and a significantly decreased quadriceps isokinetic peak torque of the reconstructed limb compared with the opposite limb (84 ± 24 Nm and 98 ± 24 Nm, respectively; P = .005). An external control group of 10 subjects was included in the investigation. No significant differences were found between the ACL-reconstructed limbs and both the internal control (contralateral) and the external control limbs for JPS. However, a significant difference was found for TDPM between the ACL-reconstructed limbs and both the internal control (contralateral) and the external control limbs. The ACL-reconstructed limbs had a more accurate response than the external controls. The authors concluded that, in successfully ACL reconstructed knees (demonstrating <3 mm of increased AP displacement), proper rehabilitation and training may help overcome loss of proprioception usually demonstrated in chronic ACL-deficient knees. The authors did not measure proprioception in ACL-reconstructed knees with less optimal results for AP displacement or poorer rehabilitation training methods.
Roberts and associates161 reported that bilateral proprioception deficits were found in 20 patients who had a B-PT-B autogenous ACL reconstruction for chronic ruptures compared with an external control group. The patients were tested an average of 2 years postoperatively; however, no data were given regarding AP displacement or their overall functional status. The patients had a significantly higher TDPM in the reconstructed limb compared with external controls from starting positions of 20° and 40° for both extension (1.0° and 0.75°, respectively) and flexion. The differences between the reconstructed and the external control limbs for flexion were 1.0° at the starting position of 20° and 0.5° at 40°. Patients also had a higher TDPM in their contralateral limb compared with external controls from starting positions of 20° and 40° for both extension and flexion. However, there was high variability in the data, which could influence the significance of these findings. For instance, the range of values for threshold toward extension at the 20° flexion starting position in the patients’ reconstructed limbs was 1.0° to 6.0°, with a median of 1.0°. The range of values for this test in the external control group was 0.5° to 2.25°, with a median of 0.75°. Further, the authors indicated that although some patients in their series demonstrated a marked “decrease in proprioception ability,” others had small deviations from the group median. The number of patients with the marked proprioception deficits was not provided.
Bonfim and colleagues27 conducted an investigation to detect sensory and motor deficits in 10 controls and 10 patients who had B-PT-B autogenous ACL reconstruction. The authors measured JPS, TDPM, latency of hamstring muscles, and maintenance of an upright stance position. The patients were tested an average of 18 months (range, 12–30 mo) postoperatively. The ACL-reconstructed knees demonstrated significantly decreased JPS (at 0°, 15°, 30°, 45°, and 60° of flexion) and significantly higher TDPM for both flexion and extension compared with the noninvolved side. The patients also showed longer latency of the hamstring muscles, and increased body sway during single-leg stance, in the reconstructed limb compared with the noninvolved side. The authors concluded that these findings were due to the disrupted ACL mechanoreceptors that were not restored by the reconstruction.
Hopper and coworkers82 reported no significant differences in JPS measured during full weight-bearing in nine patients who underwent STG ACL reconstruction. No external control group was incorporated into this investigation. Reider and associates155 found no significant differences in JPS or TDPM between ACL-reconstructed knees and external controls at 3 weeks, 6 weeks, and 3 months after surgery. At 6 months postoperative, the ACL-reconstructed knees had significantly better JPS mean values compared with the external control group (5.67° and 7.53°, respectively; mean difference, –1.86°). There was no difference between ACL-reconstructed knees and external controls in TDPM mean values 6 months postoperatively.
CLINICAL STUDIES AND OUTCOME OF ACL REHABILITATION PROGRAMS
Effect of Postoperative Exercises on AP Knee Displacements
The authors conducted a study to determine the effect of rehabilitation exercises and time elapsed postoperatively on AP knee displacements after ACL B-PT-B autograft reconstruction performed by a single surgeon.10 A total of 142 patients were followed a minimum of 2 years postoperatively; 90 had the operation for chronic ACL ruptures and 52, for acute ruptures (reconstruction performed within 12 wk of the injury). One experienced examiner conducted KT-2000 testing throughout the study period (134 N) at 8, 12, 16, 20, 24, 52, and 128 weeks after surgery. A total of 938 arthrometer measurements were collected, for an average of 7 per patient.
Critical Points CLINICAL STUDIES AND OUTCOME OF ACL REHABILITATION PROGRAMS
Effect of Postoperative Exercises on Anteroposterior Knee Displacements
Barber-Westin et al.10: Studied effect of rehabilitation exercises and time elapsed postoperatively on AP knee displacements after ACL B-PT-B autograft reconstruction performed by a single surgeon.
Home- versus Clinically Based Supervised Physical Therapy Programs
There was also no association between the initial onset of abnormal displacements and the phase of rehabilitation in which they were detected. The percentage of knees with less than 3 mm, 3 to 5 mm, and greater than 5 mm during each phase of rehabilitation is shown in Figure 12-20. In 8 patients, the abnormal displacements were first detected during the early strength-training phase; in 6 patients, during the intensive strength-training phase; and in 7 patients, after return to sports. There was no relationship between the type of sports activity patients had returned to and the presence of abnormal AP displacements.
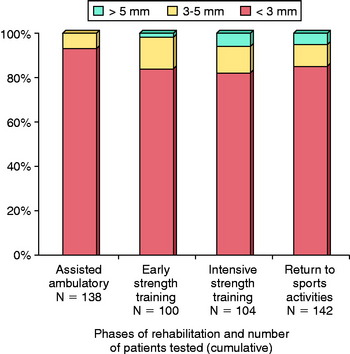
FIGURE 12-20 The percentage of knees distributed in the three arthrometer categories according to the phases of rehabilitation.
(Redrawn from Barber-Westin, S. D.; Noyes, F. R.; Heckmann, T. P.; Shaffer, B. L.: The effect of exercise and rehabilitation on anterior-posterior knee displacements after anterior cruciate ligament autograft reconstruction. Am J Sports Med 27:84–93, 1999.)
Home- versus Clinically Based Supervised Physical Therapy Programs
Four randomized investigations14,56,68,172 have been published to date that compared home-based rehabilitation to supervised clinically based physical therapy programs. All essentially concluded that similar outcomes are feasible between these programs. The authors described the essential components required for a successful home-based program: preoperative patient education sessions, a comprehensive written description of the postoperative program, and periodic monitoring of the patient’s progress by a therapist.
Schenck and colleagues172 conducted a randomized study in which the outcomes of a clinic-based program (average, 14.2 physical therapy clinic visits) were compared with those of a home-based rehabilitation program (average, 2.85 clinic visits) in a series of 37 patients who were 18 to 32 years old. The home-based program was monitored by a physical therapist and involved periodic postoperative visits to assess for potential problems and provide instruction on progression of activities. All patients also had preoperative instruction before the B-PT-B autograft reconstruction. Return to full activities was allowed at approximately 4 to 5 months postoperatively and was based on functional testing and symptoms.
Beard and Dodd14 conducted a randomized trial to compare the short-term outcome of a home-based rehabilitation program with that of a protocol that combined home exercises with a formal supervised program. Patients in the supervised program were asked to attend therapy classes twice a week from weeks 4 to 6 to weeks 16 to 18. Patients in the home-based program were evaluated and progressed based on the therapist’s discretion. A total of 13 patients who all had a B-PT-B autogenous ACL reconstruction were enrolled in each group and followed for 6 months. There were no significant differences between the groups for all parameters examined, including IKDC grade and isokinetic quadriceps and hamstrings peak torque. Although a difference was found between groups for AP displacement (mean home-based, 3.3 ± 3.2 mm; mean supervised, 0.8 ± 4.3 mm), the sample size precluded statistical significance. In addition, although no difference was noted in IKDC grades, there was also insufficient statistical power to avoid a type II error. Attempts at measuring patient compliance were unsuccessful. The authors concluded that a home-based program was appropriate, but the recommendation was stressed for “regular physical therapy outpatient assessment.”
Fischer and coworkers56 randomly assigned 54 patients who underwent a B-PT-B autogenous ACL reconstruction to a home-based or a supervised therapy program. The patients in the home-based group averaged 5 therapy visits (range, 3–7) within the first 12 weeks, and those in the supervised group averaged 20 visits (range, 10–28). The home-based group was provided with formal written descriptions and diagrams of exercises and timing of progression. At 6 months postoperative, there were no significant differences between the groups for range of knee motion, thigh circumference, AP displacement, functional hop tests, and Lysholm scores. No data were provided regarding return to athletics. The authors stressed that in order to be successful, a home-based program must include careful patient selection for compliance, periodic monitoring of patient progress by a therapist, and formal written instructions.
A randomized investigation was performed by Grant and associates68 to determine the initial (12-wk) outcome of home-based and supervised physical therapy programs. All patients underwent a B-PT-B autogenous ACL reconstruction; 66 patients in the supervised and 63 patients in the home-based programs were followed to the study endpoint assessment. The home-based group averaged 3 formal physical therapy sessions (range, 0–8) and the supervised group attended an average of 14 sessions (range, 2–20). All had preoperative patient education and received detailed written instructions regarding the four-phase therapy program. No differences were reported between the groups for AP displacement (mean 2.0 side-to-side difference in both groups) and isokinetic strength (mean quadriceps peak torque compared with the contralateral side, 61% home-based group and 60% supervised group). The home-based patients regained significantly more motion; however, the authors noted that the median differences between the groups (–2° and –3° for extension; 3° and 6° for flexion) were of questionable clinical relevance. The study concluded that the home-based program was effective in recreational athletes undergoing nonacute ACL reconstruction.
Retrospective studies were conducted by DeCarlo and Sell37 and Treacy and colleagues202 to determine whether the number of supervised physical therapy visits affected outcome after ACL reconstruction. In one investigation, patients were randomly selected and grouped based on the number of miles they lived from the authors’ clinic and only those with “consistent follow-up” were included.37 There was no difference in isokinetic muscle strength or subjective measures 12 months postoperative between those who lived in close proximity to the clinic (average, 7 therapy visits) and those who attended therapy elsewhere (average, 20 therapy visits).
ACCELERATED REHABILITATION: INDICATIONS, CONTRAINDICATIONS, AND OUTCOME
The phrase accelerated rehabilitation was introduced by Shelbourne and Nitz in 1990179 to describe a program that incorporated rapid allotments for strength and functional training and return to full activities after B-PT-B autogenous ACL reconstruction. Light sports were permitted by 2 months and full activity by 4 to 6 months postoperative. Since then, many authors have used this term to describe other facets of rehabilitation and the authors agree with Beynnon and colleagues’ assessment18 that “there is little consensus in the literature about what composes an accelerated versus a more conservative rehabilitation program.” Shelbourne and Nitz’s report179 provided outcome data on 73 of 237 (30%) patients who participated in the accelerated program. The results of these patients were compared retrospectively with those of others who had participated in a more conservative program. The authors reported that the accelerated program was more effective in restoring knee motion and preventing complications related to arthrofibrosis. There was no difference between programs in quadriceps strength at 1 year postoperative, patient perception of the results of surgery, or AP displacements on KT-1000 testing.
Critical Points ACCELERATED REHABILITATION: INDICATIONS, CONTRAINDICATIONS, AND OUTCOME
One author reported a significant increase in the incidence of joint effusions in knees in accelerated program.113
Subsequent reports by Shelbourne and coworkers176,177 emphasized the need for preoperative rehabilitation to restore full ROM, decrease swelling, and resume normal gait and leg control. The longest postoperative follow-up of this program reported by these investigators to date (mean, 4 yr; range, 2–9.1) showed a low failure rate of 3%, a low arthrofibrosis rate of 1%, and an average time to return to sports of 6.2 ± 2.3 months. The authors did not report what types of athletic activities patients participated in before their injury or at the most recent follow-up evaluation. As well, the authors noted that some patients had problems with the accelerated program in terms of pain and chronic joint effusion, especially those whose quadriceps strength was less than 65% of that of the opposite limb. The long-term effects of the return to strenuous training in the early postoperative period (5 wk on average) on articular cartilage and future joint arthrosis are unclear.
Majima and associates113 reported that accelerated rehabilitation (as described by Shelbourne179) produced a significant increase in the incidence of joint effusions in 32 patients who underwent STG reconstruction compared with 18 patients who were treated with a conservative protocol. Within the first 8 postoperative weeks, a total of 15 aspirations (in 3 patients) had been done in the conservative group compared with a total of 60 aspirations (in 13 patients) in the accelerated group (P < .01). No difference was found between the two protocols in regard to return of muscle strength (after 9 mo postoperative), IKDC scores, or knee function. Patients in the accelerated group had a trend toward greater AP displacement, as 20% had greater than 3 mm of increased displacement compared with 13% in the conservative group.
Roi and colleagues162 published a detailed accelerated rehabilitation program that was used to return a professional soccer player to his sport 90 days after an ACL STG reconstruction. The athlete did not sustain concomitant ligament or meniscus injuries and had minimal pain and limitations with daily activities after the injury, all of which allowed surgery to be performed just 4 days later. Although the treatment protocol was successful in this case, the authors qualified their aggressive program as one that “may only be possible with an individual who has the resources (time and money) to invest in unlimited access to rehabilitation facilities and personnel.”162 The authors used objective criteria to advance the program including ROM, KT-2000, functional, isokinetic, aerobic, and anaerobic testing.
Wilk209 questioned the applicability and indications of accelerated rehabilitation. Citing the ultimate goal of ACL reconstruction and postoperative rehabilitation as the return of normal homeostasis to the knee joint47 over the long term, he cautioned that aggressive rehabilitation may have more risks than benefits, especially in regard to its impact on preexisting articular cartilage damage and bone bruising.
Yu and Paessler225 investigated the influence of an aggressive rehabilitation protocol on tunnel widening after four-strand STG reconstruction. At 6 months postoperative, patients who followed the aggressive protocol (immediate full weight-bearing, OKC exercises begun at the 6th week, running allowed from the 8th to the 10th week) had significantly greater tibial tunnel widening on posteroanterior (PA) and lateral radiographs compared with a group of patients who followed a more conservative protocol. There was no difference between groups in mean KT-1000 arthrometer values.
CRITERIA FOR PATIENT RELEASE AND RETURN TO SPORTS ACTIVITIES
Critical Points CRITERIA FOR PATIENT RELEASE AND RETURN TO SPORTS ACTIVITIES
Kvist103 detailed multiple criteria to be fulfilled before release to full sports activities that included rehabilitation, surgical, and other factors. The assessment included objective muscle strength and performance, pain, effusion, ROM, functional knee stability, static knee stability, associated injuries, and psychological and social aspects. The patient had to demonstrate less than 15% deficit on isokinetic and single-leg hop tests, no pain or effusion, full ROM, functional knee stability, and static knee stability on KT-1000 testing. The influence of associated meniscal, cartilage, or other ligamentous injuries; psychological issues such as motivation or fear of reinjury; and social factors such as lost time owing to occupational demands could not be predicted on the ability to return to sports successfully after ACL reconstruction.
Discharge criteria at the authors’ center after ACL reconstruction based on patient goals for athletics and occupations, the rating of symptoms, KT-1000 testing, muscle strength testing, and function testing have been previously described (Table 12-4).75 First, patients complete the Cincinnati Sports Activity Scale11 and the Occupational Rating Scale11 in order to document sports and occupational levels that are desired after surgery (see Chapter 44, The Cincinnati Knee Rating System). Upon completion of the rehabilitation program, pain, swelling, and giving-way are rated on the Cincinnati Symptom Rating Scale.11 The patient must not experience symptoms at the level of activity that she or he wishes to participate in prior to discharge. KT-2000 testing is performed at 134 N of total ‘AP force and must be within normal limits (<3 mm difference between limbs) prior to allowance of return to strenuous activities. Muscle strength testing is performed with an isokinetic dynamometer to ensure that adequate strength exists prior to the initiation of the running and cutting programs. At least two single-leg hop function tests are completed and limb symmetry calculated as described previously.9,128 Patients desiring to return to high-risk activities complete a neuromuscular-retraining program before release to full competition. Recommendations for the types of activities that patients should consider resuming are made based on the results of these criteria and the condition of the articular cartilage and menisci. Patients in whom articular cartilage damage was visualized during the ACL reconstruction (especially those with grade 2B or 3; see Chapter 47138), or those in whom the majority of function of one or both menisci has been compromised are encouraged to return to low-impact activities to preserve the knee joint for as long as possible.
RISKS FOR REINJURY AND FUTURE JOINT ARTHROSIS
An important component of the surgery and rehabilitation decision making process is an understanding of the risks of reinjury to either the reconstructed or the contralateral knee and the chance of developing future joint arthrosis. A few studies have reported high rates of reinjury upon return to sports.145,154,169,204 Salmon and coworkers169 followed 67 patients for 7 to 13 years postoperatively after ACL B-PT-B autogenous reconstruction and a conservative rehabilitation program. Patients were kept non–weight-bearing for the first 4 postoperative weeks, with ROM permitted only from 30° to 90°. Competitive sports were not allowed until 9 months after surgery. A total of 23 patients (34%) sustained an ACL injury after return to sports; 9 (13%) patients reinjured the reconstructed ACL and 15 (22%) ruptured the contralateral ACL. The authors related that their patients had not participated in a neuromuscular-retraining program, which may have been at least partially responsible for the high reinjury rate. At 13 years postoperative, 37% of the patients reported swelling after moderate activity and 44% had a limitation in knee extension.
Orchard and associates145 followed Australian football players over eight seasons to determine the occurrence of ACL injuries. The overall ACL injury incidence was 0.82/1000 athlete exposures. The incidence for noncontact ACL injuries was 0.62/1000 athlete exposures. All ACL injuries in this study were treated with reconstruction. The players who sustained an ACL injury had approximately a 10 times greater incidence of sustaining further ACL injury within the first 12 months of surgery compared with uninjured players. After 12 months, these players still had a greater than 4 times increased risk of sustaining a noncontact ACL injury, which was evenly divided between the reconstructed and the contralateral knee.
Critical Points RISKS FOR REINJURY AND FUTURE JOINT ARTHROSIS
Pinczewski et al.154
Published rates of radiographically documented osteoarthritis (OA) after ACL reconstruction vary.
Pinczewski and colleagues154 presented high reinjury rates after return to activity in a cohort of patients who received either a B-PT-B (90 patients) or an STG autogenous (90 patients) ACL reconstruction and who were observed for 10 years. The rehabilitation program included no brace, immediate partial weight-bearing, jogging after 5 weeks, and return to sports 6 months postoperatively if knee stability was confirmed. The overall ACL reinjury rate, including both the reconstructed and the contralateral sides, was 27%; 22% for STG grafts and 30% for B-PT-B grafts. A total of 19 patients (11%) ruptured the ACL graft: 8% (7/90) in the B-PT-B group and 13% (12/90) in the STG group. A total of 29 patients (16%) sustained contralateral ACL ruptures: 22% (20/90) in the B-PT-B group and 10% (9/90) in the STG group.
Wright and coworkers216 reported early outcomes of the Multicenter Orthopaedic Outcome Network (MOON) investigation that documented reinjury rates in both ACL-reconstructed and contralateral knees in 235 patients followed for 2 years after autograft or allograft reconstruction. The reinjury rates included only patients who underwent ACL revision or primary reconstruction in the contralateral knee. The authors acknowledged the limitations of the study of no on-site physician examination of the patients who had not undergone further surgery and a short-term follow-up. It is feasible that other patients sustained reinjuries that were not detected. There were seven tears of the intact ACL in the contralateral knee and seven ruptures of ACL grafts, for an overall incidence rate of 3% for each knee. No information was provided regarding the type of postoperative rehabilitation programs the patients participated in or functional outcome.
Published rates of radiographically documented OA after ACL reconstruction vary tremendously (Table 12-5).* It is important to note that some studies report an increased incidence of mild OA (IKDC grade nearly normal, Ahlback grade 1) changes in the knee and not moderate or severe degeneration. Whether mild OA changes on standing radiographs represent actual articular cartilage deterioration is questionable. Two investigations concluded that standing radiographs have low sensitivity rates in detecting mild articular cartilage damage. Wright and associates215 determined the sensitivity and specificity of standing AP and weight-bearing PA radiographs in detecting Outerbridge grade 2 (fragmentation and fissuring, <½ inch in diameter) chondral lesions in a group of 349 patients. Although both radiographic techniques had specificity rates greater than 90%, both had poor sensitivity rates for detecting grade 2 changes in the medial (3% AP, 6% PA) and lateral (16% AP, 6% PA) compartments. Lysholm and colleagues111 reported a similar poor correlation between mild OA (Outerbridge grade 2) and the Ahlback classification2 rating of standing radiographs.
TABLE 12-5 Radiographic Documentation of Osteoarthritis after ACL Autogenous Reconstruction: Mid- to Long-Term Investigations
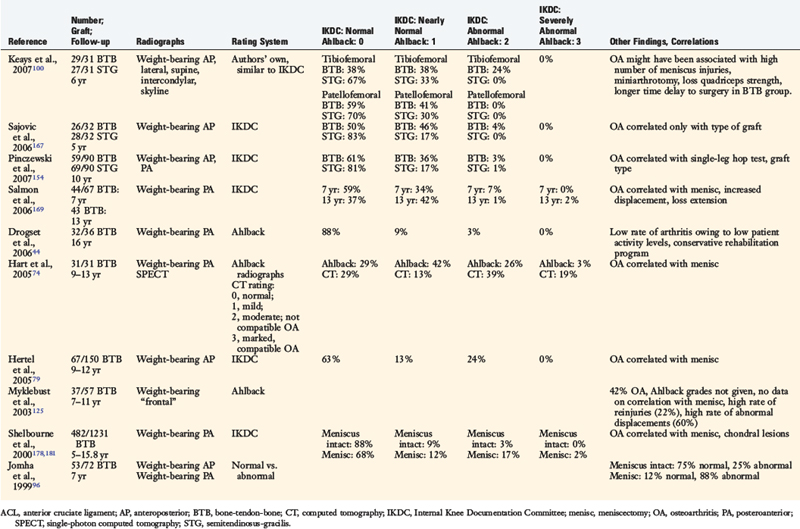
Determining the risk of developing future OA after ACL reconstruction is difficult for many reasons. Many factors may influence the development of articular cartilage deterioration, including the effects of the initial impact injury on the subchondral bone (bone bruising34,52,80,195), loss of meniscus tissue (either before, during, or after the ACL reconstruction), patient age, time from injury to reconstruction, number of reinjuries prior to reconstruction, failure of the reconstruction to restore normal AP displacement and knee kinematics, the rehabilitation program, complications (such as infection or arthrofibrosis), final knee motion achieved, and the activity level the patient resumes after surgery. In many studies, these variables are not controlled for, which when combined with the high incidence of the detection of only mild radiographic OA changes, makes reaching conclusions on this topic difficult. In addition, most long-term studies have an unfortunately high rate of transfer bias (patients lost to follow-up) and do not compare the radiographic findings of the reconstructed knee with the contralateral normal knee.
One conclusive association with future joint OA is loss of meniscus tissue. Nearly every long-term study reports a statistically significant correlation between prior meniscectomy and radiographic OA. Hart and coworkers74 conducted a 9- to 13-year follow-up study on 31 patients who underwent ACL B-PT-B reconstruction. Both weight-bearing PA radiographs and single-photon emission computed tomography (SPECT) were used to detect OA. When the population was sorted according to meniscectomy (15 meniscus intact, 16 meniscectomy), the SPECT scans detected OA in 31% of the patients compared with just 7% in whom meniscus tissue had been preserved.
Salmon and coworkers169 reported data on 43 patients who underwent radiographic assessment 13 years postoperatively in which the IKDC radiographic scores were 37% normal; 42% nearly normal; 19% abnormal; and 2% severely abnormal. In comparison, at 7 years postoperative, 44 patients underwent radiographic assessment and 59% were rated as normal; 34% nearly normal; and 7% abnormal. The difference in the proportion of patients with an abnormal radiographic score between evaluations was significant (P = .01) and correlated with meniscectomy and greater amount of anterior tibial displacement on Lachman testing. The majority of moderate to severe degeneration occurred in the medial tibiofemoral compartment. No associations were found between radiographic and IKDC symptom and function scores.
Pinczewski and colleagues154 reported 10-year radiographic postoperative data on 59 B-PT-B patients in whom the IKDC scores were normal in 61%, nearly normal in 36%, and abnormal in 3%. Sixty-nine of the STG patients in this series were radiographically assessed and the IKDC scores were normal in 81%, nearly normal in 17%, and abnormal in 1%. The IKDC scores correlated with the single-leg hop test and graft type.
Shelbourne and Gray178 followed 482 patients who received a B-PT-B autogenous ACL reconstruction between 5 and 15.8 years postoperatively. The patients were sorted according to prior meniscectomy and articular cartilage damage noted at the time of the ACL reconstruction. A clear association was found between meniscectomy and radiographic OA. A normal or nearly normal radiographic IKDC score was reported in 97% of patients who had intact menisci compared with just 64% of patients who had one or both menisci removed and articular cartilage damage. The authors concluded that ACL reconstruction offered a protective effect in knees that had normal menisci and no articular cartilage damage. Most authors agree that early knee joint stabilization procedures should be considered prior to reinjuries and subsequent meniscus and articular cartilage damage.
In a review of literature on the long-term consequence of ACL injuries, Lohmander and associates108 proposed an overall long-term mean value of greater than 50% of patients who sustained this injury will develop OA. The authors combined data from ACL-injured and ACL-reconstructed studies and were not able to account for the effect of meniscectomy or reinjuries. Many problems in study design and loss of follow-up (transfer bias) were cited; even so, the authors concluded that there is a lack of evidence to support that ACL reconstruction reliably plays a protective role against the development of OA.
As additional well-designed long-term studies on the outcome of ACL reconstruction become available, the issue of the operation’s ability to provide chondroprotective effects should become clearer. Studies should document the chronicity of the ACL rupture and whether reinjuries occurred before the reconstruction, the status of the menisci during the entire observation period, the condition of the articular cartilage noted at the reconstruction (rated by a validated system, Chapter 47), athletic activity levels patients returned to, and radiographic findings of both knees.
1 Aglietti P., Buzzi R., Giron F., et al. Arthroscopic-assisted anterior cruciate ligament reconstruction with the central third patellar tendon. A 5-8 year follow-up. Knee Surg Sports Traumatol Arthrosc. 1997;5:138-144.
2 Ahlback S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiol Diagn (Stockh) Suppl. 1968;277:7-72.
3 Alm A., Liljedahl S.-O., Stromberg B. Clinical and experimental experience in reconstruction of the anterior cruciate ligament. Orthop Clin North Am. 1976;7:181-189.
4 Andriacchi T.P., Birac D. Functional testing in the anterior cruciate ligament–deficient knee. Clin Orthop Relat Res. 1993;288:40-47.
5 Andriacchi T.P., Dyrby C.O., Johnson T.S. The use of functional analysis in evaluating knee kinematics. Clin Orthop Relat Res. 2003;410:44-53.
6 Arangio G.A., Chen C., Kalady M., Reed J.F.3rd. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26:238-243.
7 Arciero R.A., Scoville C.R., Snyder R.J., et al. Single- versus two-incision arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:462-469.
8 Baltzopolous V. Muscular and tibiofemoral joint forces during isokinetic concentric knee extension. Clin Biomech. 1995;10:208-214.
9 Barber S.D., Noyes F.R., Mangine R.E., et al. Quantitative assessment of functional limitations in normal and anterior cruciate ligament–deficient knees. Clin Orthop. 1990;255:204-214.
10 Barber-Westin S.D., Noyes F.R., Heckmann T.P., Shaffer B.L. The effect of exercise and rehabilitation on anterior-posterior knee displacements after anterior cruciate ligament autograft reconstruction. Am J Sports Med. 1999;27:84-93.
11 Barber-Westin S.D., Noyes F.R., McCloskey J.W. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27:402-416.
12 Barrett D.S. Proprioception and function after anterior cruciate reconstruction. J Bone Joint Surg Br. 1991;73:833-837.
13 Baugher W.H., Warren R.F., Marshall J.L., Joseph A. Quadriceps atrophy in the anterior cruciate insufficient knee. Am J Sports Med. 1984;12:192-195.
14 Beard D.J., Dodd C.A. Home or supervised rehabilitation following anterior cruciate ligament reconstruction: a randomized controlled trial. J Orthop Sports Phys Ther. 1998;27:134-143.
15 Berchuck M., Andriacchi T.P., Bach B.R., Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72:871-877.
16 Beutler A.I., Cooper L.W., Kirkendall D.T., Garrett W.E.Jr. Electromyographic analysis of single-leg, closed chain exercises: implications for rehabilitation after anterior cruciate ligament reconstruction. J Athl Train. 2002;37:13-18.
17 Beynnon B.D., Fleming B.C., Johnson R.J., et al. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24-34.
18 Beynnon B.D., Johnson R.J., Abate J.A., et al. Treatment of anterior cruciate ligament injuries, part 2. Am J Sports Med. 2005;33:1751-1767.
19 Beynnon B.D., Johnson R.J., Abate J.A., et al. Treatment of anterior cruciate ligament injuries, part 1. Am J Sports Med. 2005;33:1579-1602.
20 Beynnon B.D., Johnson R.J., Fleming B.C. The science of anterior cruciate ligament rehabilitation. Clin Orthop Relat Res. 2002;402:9-20.
21 Beynnon B.D., Johnson R.J., Fleming B.C., et al. The measurement of elongation of anterior cruciate ligament grafts in vivo. J Bone Joint Surg Am. 1994;76:520-531.
22 Beynnon B.D., Johnson R.J., Fleming B.C., et al. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension. A comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25:823-829.
23 Beynnon B.D., Risberg M.A., Tjomsland O., et al. Evaluation of knee joint laxity and the structural properties of the anterior cruciate ligament graft in the human. A case report. Am J Sports Med. 1997;25:203-206.
24 Beynnon B.D., Uh B.S., Johnson R.J., et al. Rehabilitation after anterior cruciate ligament reconstruction. A prospective, randomized, double-blind comparison of programs administered over 2 different time periods. Am J Sports Med. 2005;33:347-359.
25 Birmingham T.B., Bryant D.M., Giffin J.R., et al. A randomized controlled trial comparing the effectiveness of functional knee brace and neoprene sleeve use after anterior cruciate ligament reconstruction. Am J Sports Med. 2008;36:648-655.
26 Birmingham T.B., Kramer J.F., Kirkley A., et al. Knee bracing after ACL reconstruction: effects on postural control and proprioception. Med Sci Sports Exerc. 2001;33:1253-1258.
26a Birmingham T.B., Kramer J.F., Kirkley A. Effect of a functional knee brace on knee flexion and extension strength after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2002;83:1472-1475.
27 Bonfim T.R., Paccola C.A., Barela J.A. Proprioceptive and behavior impairments in individuals with anterior cruciate ligament reconstructed knees. Arch Phys Med Rehabil. 2003;84:1217-1223.
28 Bottoni C.R., Liddell T.R., Trainor T.J., et al. Postoperative range of motion following anterior cruciate ligament reconstruction using autograft hamstrings: a prospective, randomized clinical trial of early versus delayed reconstructions. Am J Sports Med. 2008;36:656-662.
29 Brandsson S., Faxen E., Kartus J., et al. Is a knee brace advantageous after anterior cruciate ligament surgery? A prospective, randomised study with a two-year follow-up. Scand J Med Sci Sports. 2001;11:110-114.
30 Bush-Joseph C.A., Hurwitz D.E., Patel R.R., et al. Dynamic function after anterior cruciate ligament reconstruction with autologous patellar tendon. Am J Sports Med. 2001;29:36-41.
31 Bynum E.B., Barrack R.L., Alexander A.H. Open versus closed chain kinetic exercises after anterior cruciate ligament reconstruction. A prospective randomized study. Am J Sports Med. 1995;23:401-406.
32 Co F.H., Skinner H.B., Cannon W.D. Effect of reconstruction of the anterior cruciate ligament on proprioception of the knee and the heel strike transient. J Orthop Res. 1993;11:696-704.
33 Cosgarea A.J., Sebastianelli W.J., DeHaven K.E. Prevention of arthrofibrosis after anterior cruciate ligament reconstruction using the central third patellar tendon autograft. Am J Sports Med. 1995;23:87-92.
34 Costa-Paz M., Muscolo D.L., Ayerza M., et al. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17:445-449.
35 Coutts R., Rothe C., Kaita J. The role of continuous passive motion in the rehabilitation of the total knee patient. Clin Orthop. 1981;159:126-132.
36 Dahlkvist N.J., Mayo P., Seedhom B.B. Forces during squatting and rising from a deep squat. Eng Med. 1982;11:69-76.
37 De Carlo M.S., Sell K.E. The effects of the number and frequency of physical therapy treatments on selected outcomes of treatment in patients with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1997;26:332-339.
38 de Jong S.N., van Caspel D.R., van Haeff M.J., Saris D.B. Functional assessment and muscle strength before and after reconstruction of chronic anterior cruciate ligament lesions. Arthroscopy. 2007;23:21-28. 28.e1–3
39 Dehne E., Torp R.P. Treatment of joint injuries by immediate mobilization. Based upon the spinal adaptation concept. Clin Orthop Relat Res. 1971;77:218-232.
40 Delay B.S., McGrath B.E., Mindell E.R. Observations on a retrieved patellar tendon autograft used to reconstruct the anterior cruciate ligament. A case report. J Bone Joint Surg Am. 2002;84:1433-1438.
41 Delitto A., Rose S.J., McKowen J.M., et al. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys Ther. 1988;68:660-663.
42 DeVita P., Hortobagyi T., Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc. 1998;30:1481-1488.
43 Doucette S.A., Child D.D. The effect of open and closed chain exercise and knee joint position on patellar tracking in lateral patellar compression syndrome. J Orthop Sports Phys Ther. 1996;23:104-110.
44 Drogset J.O., Grontvedt T., Robak O.R., et al. A sixteen-year follow-up of three operative techniques for the treatment of acute ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 2006;88:944-952.
45 Dye S.F. The pathophysiology of patellofemoral pain: a tissue homeostasis perspective. Clin Orthop Relat Res. 2005;436:100-110.
46 Dye S.F., Chew M.H. Restoration of osseous homeostasis after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:748-750.
47 Dye S.F., Wojtys E.M., Fu F.H., et al. Factors contributing to function of the knee joint after injury or reconstruction of the anterior cruciate ligament. Instr Course Lect. 1999;48:185-198.
48 Elmqvist L.G., Lorentzon R., Johansson C., Fugl-Meyer A.R. Does a torn anterior cruciate ligament lead to change in the central nervous drive of the knee extensors? Eur J Appl Physiol Occup Physiol. 1988;58:203-207.
49 Eriksson E. Rehabilitation of muscle function after sport injury—major problem in sports medicine. Int J Sports Med. 1981;2:1-6.
50 Ernst G.P., Saliba E., Diduch D.R., et al. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000;80:251-260.
51 Escamilla R.F., Fleisig G.S., Zheng N., et al. Biomechanics of the knee during closed kinetic chain and open kinetic chain exercises. Med Sci Sports Exerc. 1998;30:556-569.
52 Faber K.J., Dill J.R., Amendola A., et al. Occult osteochondral lesions after anterior cruciate ligament rupture. Six-year magnetic resonance imaging follow-up study. Am J Sports Med. 1999;27:489-494.
53 Falconiero R.P., DiStefano V.J., Cook T.M. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197-205.
54 Feller J.A., Webster K.E. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:564-573.
55 Feller J.A., Webster K.E., Gavin B. Early post-operative morbidity following anterior cruciate ligament reconstruction: patellar tendon versus hamstring graft. Knee Surg Sports Traumatol Arthrosc. 2001;9:260-266.
56 Fischer D.A., Tewes D.P., Boyd J.L., et al. Home based rehabilitation for anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 1998;347:194-199.
57 Fischer-Rasmussen T., Jensen P.E. Proprioceptive sensitivity and performance in anterior cruciate ligament–deficient knee joints. Scand J Med Sci Sports. 2000;10:85-89.
58 Fitzgerald G.K., Piva S.R., Irrgang J.J. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2003;33:492-501.
59 Fleming B.C., Beynnon B.D., Renstrom P.A., et al. The strain behavior of the anterior cruciate ligament during stair climbing: an in vivo study. Arthroscopy. 1999;15:185-191.
60 Fleming B.C., Beynnon B.D., Renstrom P.A., et al. The strain behavior of the anterior cruciate ligament during bicycling. An in vivo study. Am J Sports Med. 1998;26:109-118.
61 Fleming B.C., Ohlen G., Renstrom P.A., et al. The effects of compressive load and knee joint torque on peak anterior cruciate ligament strains. Am J Sports Med. 2003;31:701-707.
62 Frank C.B., Jackson D.W. Current concepts review. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79:1556-1576.
63 Gerber C., Hoppeler H., Claassen H., et al. The lower-extremity musculature in chronic symptomatic instability of the anterior cruciate ligament. J Bone Joint Surg Am. 1985;67:1034-1043.
64 Gerber J.P., Marcus R.L., Dibble L.E., et al. Effects of early progressive eccentric exercise on muscle structure after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89:559-570.
65 Gerber J.P., Marcus R.L., Dibble L.E., et al. Safety, feasibility, and efficacy of negative work exercise via eccentric muscle activity following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2007;37:10-18.
66 Gerber J.P., Marcus R.L., Dibble L.E., et al. Early application of negative work via eccentric ergometry following anterior cruciate ligament reconstruction: a case report. J Orthop Sports Phys Ther. 2006;36:298-307.
67 Goldblatt J.P., Fitzsimmons S.E., Balk E., Richmond J.C. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21:791-803.
68 Grant J.A., Mohtadi N.G., Maitland M.E., Zernicke R.F. Comparison of home- versus physical therapy–supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33:1288-1297.
69 Grood E.S., Suntay W.J., Noyes F.R., Butler D.L. Biomechanics of the knee-extension exercise. Effect of cutting the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66:725-734.
70 Haggmark T., Eriksson E. Cylinder or mobile cast brace after knee ligament surgery: a clinical analysis and morphologic and enzymatic studies of changes in the quadriceps muscle. Am J Sports Med. 1979;7:48-56.
71 Haggmark T., Jansson E., Eriksson E. Fiber type area and metabolic potential of the thigh muscle in man after knee surgery and immobilization. Int J Sports Med. 1981;2:12-17.
72 Hanypsiak B.T., Spindler K.P., Rothrock C.R., et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med. 2008;36:671-677.
73 Harilainen A., Sandelin J., Vanhanen I., Kivinen A. Knee brace after bone-tendon-bone anterior cruciate ligament reconstruction. Randomized, prospective study with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1997;5:10-13.
74 Hart A.J., Buscombe J., Malone A., Dowd G.S. Assessment of osteoarthritis after reconstruction of the anterior cruciate ligament: a study using single-photon emission computed tomography at ten years. J Bone Joint Surg Br. 2005;87:1483-1487.
75 Heckmann T.P., Noyes F.R., Barber-Westin S.D. Autogeneic and allogeneic anterior cruciate ligament rehabilitation. In: Ellenbecker T.S., editor. Knee Ligament Rehabilitation. Philadelphia: Churchill Livingstone; 2000:132-150.
76 Heijne A., Fleming B.C., Renstrom P.A., et al. Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc. 2004;36:935-941.
77 Henning C.E., Lynch M.A., Glick K.R.Jr. An in vivo strain gauge study of elongation of the anterior cruciate ligament. Am J Sports Med. 1985;13:22-26.
78 Henriksson M., Rockborn P., Good L. Range of motion training in brace vs. plaster immobilization after anterior cruciate ligament reconstruction: a prospective randomized comparison with a 2-year follow-up. Scand J Med Sci Sports. 2002;12:73-80.
79 Hertel P., Behrend H., Cierpinski T., et al. ACL reconstruction using bone–patellar tendon–bone press-fit fixation: 10-year clinical results. Knee Surg Sports Traumatol Arthrosc. 2005;13:248-255.
80 Hogervorst T., Pels Rijcken T.H., Rucker D., et al. Changes in bone scans after anterior cruciate ligament reconstruction: a prospective study. Am J Sports Med. 2002;30:823-833.
81 Hooper D.M., Morrissey M.C., Drechsler W., et al. Open and closed kinetic chain exercises in the early period after anterior cruciate ligament reconstruction. Improvements in level walking, stair ascent, and stair descent. Am J Sports Med. 2001;29:167-174.
82 Hopper D.M., Creagh M.J., Formby P.A., et al. Functional measurement of knee joint position sense after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84:868-872.
83 Howell S.M., Taylor M.A. Brace-free rehabilitation, with early return to activity, for knees reconstructed with a double-looped semitendinosus and gracilis graft. J Bone Joint Surg Am. 1996;78:814-825.
84 Ikeda H. Isokinetic torque of quadriceps in patients with untreated anterior cruciate ligament injury of the knee joint. J Jpn Orthop Assoc. 1993;67:826-835.
85 Imran A., O’Connor J.J. Control of knee stability after ACL injury or repair: interaction between hamstrings contraction and tibial translation. Clin Biomech (Bristol, Avon). 1998;13:153-162.
86 Irie K., Tomatsu T. Atrophy of semitendinosus and gracilis and flexor mechanism function after hamstring tendon harvest for anterior cruciate ligament reconstruction. Orthopedics. 2002;25:491-495.
87 Isberg J., Faxen E., Brandsson S., et al. Early active extension after anterior cruciate ligament reconstruction does not result in increased laxity of the knee. Knee Surg Sports Traumatol Arthrosc. 2006;14:1108-1115.
88 Iwasa J., Ochi M., Adachi N., et al. Proprioceptive improvement in knees with anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2000;381:168-176.
89 Jackson D.W., Grood E.S., Goldstein J.D., et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-185.
90 Johansson H., Sjolander P., Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res. 1991;268:161-178.
91 Johansson H., Sjolander P., Sojka P. Activity in receptor afferents from the anterior cruciate ligament evokes reflex effects on fusimotor neurones. Neurosci Res. 1990;8:54-59.
92 Johansson H., Sjolander P., Sojka P. Receptors in the knee joint ligaments and their role in the biomechanics of the joint. Crit Rev Biomed Eng. 1991;18:341-368.
93 Johnson D.L., Urban W.P., Caborn D.N.M., et al. Articular cartilage changes seen with magnetic resonance imaging–detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26:409-414.
94 Johnson L.L. The outcome of a free autogenous semitendinosus tendon graft in human anterior cruciate reconstructive surgery: a histological study. Arthroscopy. 1993;9:131-142.
95 Johnson R.J., Eriksson E., Haggmark T., Pope M.H. Five- to ten-year follow-up evaluation after reconstruction of the anterior cruciate ligament. Clin Orthop Relat Res. 1984;183:122-140.
96 Jomha N.M., Borton D.C., Clingeleffer A.J., Pinczewski L.A. Long-term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop Relat Res. 1999;358:188-193.
97 Jurist K.A., Otis J.C. Anteroposterior tibiofemoral displacements during isometric extension efforts. The roles of external load and knee flexion angle. Am J Sports Med. 1985;13:254-258.
98 Kartus J., Stener S., Kohler K., et al. Is bracing after anterior cruciate ligament reconstruction necessary? A 2-year follow-up of 78 consecutive patients rehabilitated with or without a brace. Knee Surgery Sports Traumatol Arthrosc. 1997;5:157-161.
99 Kaufman K.R., An K.N., Litchy W.J., et al. Dynamic joint forces during knee isokinetic exercise. Am J Sports Med. 1991;19:305-316.
100 Keays S.L., Bullock-Saxton J.E., Keays A.C., et al. A 6-year follow-up of the effect of graft site on strength, stability, range of motion, function, and joint degeneration after anterior cruciate ligament reconstruction: patellar tendon versus semitendinosus and gracilis tendon graft. Am J Sports Med. 2007;35:729-739.
101 Konishi Y., Fukubayashi T., Takeshita D. Mechanism of quadriceps femoris muscle weakness in patients with anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2002;12:371-375.
102 Kowalk D.L., Duncan J.A., McCue F.C.3rd, Vaughan C.L. Anterior cruciate ligament reconstruction and joint dynamics during stair climbing. Med Sci Sports Exerc. 1997;29:1406-1413.
103 Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34:269-280.
104 Kvist J., Gillquist J. Sagittal plane knee translation and electromyographic activity during closed and open kinetic chain exercises in anterior cruciate ligment–deficient patients and control subjects. Am J Sports Med. 2001;29:72-82.
105 LaStayo P.C., Woolf J.M., Lewek M.D., et al. Eccentric muscle contractions: their contribution to injury, prevention, rehabilitation, and sport. J Orthop Sports Phys Ther. 2003;33:557-571.
106 Lephart S., Kocher M.S., Fu F., et al. Proprioception following anterior cruciate ligament reconstruction. J Sport Rehabil. 1992;1:188-196.
107 Lephart S.M., Kocher M.S., Harner C.D., Fu F.H. Quadriceps strength and functional capacity after anterior cruciate ligament reconstruction. Patellar tendon autograft versus allograft. Am J Sports Med. 1993;21:738-743.
108 Lohmander L.S., Englund P.M., Dahl L.L., Roos E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756-1769.
109 Lorentzon R., Elmqvist L.G., Sjostrom M., et al. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989;17:423-429.
110 Lutz G.E., Palmitier R.A., An K.N., Chao E.Y. Comparison of tibiofemoral joint forces during open-kinetic-chain and closed-kinetic-chain exercises. J Bone Joint Surg Am. 1993;75:732-739.
111 Lysholm J., Hamberg P., Gillquist J. The correlation between osteoarthrosis as seen on radiographs and on arthroscopy. Arthroscopy. 1987;3:161-165.
112 MacDonald P.B., Heeden D., Pacin O., Sutherland K. Proprioception in anterior cruciate ligament–deficient and reconstructed knees. Am J Sports Med. 1996;24:774-778.
113 Majima T., Yasuda K., Tago H., et al. Rehabilitation after hamstring anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2002;397:370-380.
114 Marx R.G., Jones E.C., Angel M., et al. Beliefs and attitudes of members of the American Academy of Orthopaedic Surgeons regarding the treatment of anterior cruciate ligament injury. Arthroscopy. 2003;19:762-770.
115 Mattacola C.G., Perrin D.H., Gansneder B.M., et al. Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37:262-268.
116 McDevitt E.R., Taylor D.C., Miller M.D., et al. Functional bracing after anterior cruciate ligament reconstruction: a prospective, randomized, multicenter study. Am J Sports Med. 2004;32:1887-1892.
117 Meighan A.A., Keating J.F., Will E. Outcome after reconstruction of the anterior cruciate ligament in athletic patients. A comparison of early versus delayed surgery. J Bone Joint Surg Br. 2003;85:521-524.
118 Melegati G., Tornese D., Bandi M., et al. The role of the rehabilitation brace in restoring knee extension after anterior cruciate ligament reconstruction: a prospective controlled study. Knee Surg Sports Traumatol Arthrosc. 2003;11:322-326.
119 Mikkelsen C., Werner S., Eriksson E. Closed kinetic chain alone compared to combined open and closed kinetic chain exercises for quadriceps strengthening after anterior cruciate ligament reconstruction with respect to return to sports: a prospective matched follow-up study. Knee Surg Sports Traumatol Arthrosc. 2000;8:337-342.
119a Mikkelsen C., Cerulli G., Lorenzini M. Can a postoperative brace in slight hyperextension prevent extension deficit after anterior cruciate ligament reconstruction? A prospective randomised study. Knee Surg Sports Traumatol Arthrosc. 2003;11:318-321.
120 Mohtadi N.G., Webster-Bogaert S., Fowler P.J. Limitation of motion following anterior cruciate ligament reconstruction. A case-control study. Am J Sports Med. 1991;19:620-624. discussion 624–625
121 Moller E., Forssblad M., Hansson L., et al. Bracing versus nonbracing in rehabilitation after anterior cruciate ligament reconstruction: a randomized prospective study with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2001;9:102-108.
122 More R.C., Karras B.T., Neiman F., et al. Hamstrings—an anterior cruciate ligament protagonist: an in vitro study. Am J Sports Med. 1993;21:231-237.
123 Morrissey M.C., Hudson Z.L., Drechsler W.I., et al. Effects of open versus closed kinetic chain training on knee laxity in the early period after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2000;8:343-348.
124 Muellner T., Alacamlioglu Y., Nikolic A., Schabus R. No benefit of bracing on the early outcome after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6:88-92.
125 Myklebust G., Holm I., Maehlum S., et al. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: a follow-up study. Am J Sports Med. 2003;31:981-989.
126 Nisell R., Ericson M.O., Nemeth G., Ekholm J. Tibiofemoral joint forces during isokinetic knee extension. Am J Sports Med. 1989;17:49-54.
127 Nisell R., Nemeth G., Ohlsen H. Joint forces in extension of the knee. Analysis of a mechanical model. Acta Orthop Scand. 1986;57:41-46.
128 Noyes F.R., Barber S.D., Mangine R.E. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19:513-518.
129 Noyes F.R., Barber-Westin S.D. A comparison of results in acute and chronic anterior cruciate ligament ruptures of arthroscopically assisted autogenous patellar tendon reconstruction. Am J Sports Med. 1997;25:460-471.
130 Noyes F.R., Bassett R.W., Grood E.S., Butler D.L. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62:687-695. 757
131 Noyes F.R., Berrios-Torres S., Barber-Westin S.D., Heckmann T.P. Prevention of permanent arthrofibrosis after anterior cruciate ligament reconstruction alone or combined with associated procedures: a prospective study in 443 knees. Knee Surg Sports Traumatol Arthrosc. 2000;8:196-206.
132 Noyes F.R., Butler D.L., Grood E.S., et al. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66:344-352.
133 Noyes F.R., Butler D.L., Paulos L.E., Grood E.S. Intra-articular cruciate reconstruction. I: perspectives on graft strength, vascularization, and immediate motion after replacement. Clin Orthop. 1983;172:71-77.
134 Noyes F.R., Mangine R.E., Barber S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:149-160.
135 Noyes F.R., Mangine R.E., Barber S.D. The early treatment of motion complications after reconstruction of the anterior cruciate ligament. Clin Orthop. 1992;277:217-228.
136 Noyes F.R., Mooar P.A., Matthews D.S., Butler D.L. The symptomatic anterior cruciate–deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65:154-162.
137 Noyes F.R., Schipplein O.D., Andriacchi T.P., et al. The anterior cruciate ligament–deficient knee with varus alignment. An analysis of gait adaptations and dynamic joint loadings. Am J Sports Med. 1992;20:707-716.
138 Noyes F.R., Stabler C.L. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505-513.
139 Noyes F.R., Torvik P.J., Hyde W.B., DeLucas J.L. Biomechanics of ligament failure. II. An analysis of immobilization, exercise, and reconditioning effects in primates. J Bone Joint Surg Am. 1974;56:1406-1418.
140 Noyes F.R., Wojtys E.M. The early recognition, diagnosis and treatment of the patella infera syndrome. In: Tullos H.S., editor. Instructional Course Lectures. Rosemont, IL: AAOS; 1991:233-247.
141 Noyes F.R., Wojtys E.M., Marshall M.T. The early diagnosis and treatment of developmental patella infera syndrome. Clin Orthop. 1991;265:241-252.
142 O’Driscoll S.W., Kumar A., Salter R.B. The effect of continuous passive motion on the clearance of a hemarthrosis from a synovial joint. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1983;176:305-311.
143 O’Neill D.B. Arthroscopically assisted reconstruction of the anterior cruciate ligament. A prospective randomized analysis of three techniques. J Bone Joint Surg Am. 1996;78:803-813.
144 Ohkoshi Y., Yasuda K., Kaneda K., et al. Biomechanical analysis of rehabilitation in the standing position. Am J Sports Med. 1991;19:605-611.
145 Orchard J., Seward H., McGivern J., Hood S. Intrinsic and extrinsic risk factors for anterior cruciate ligament injury in Australian footballers. Am J Sports Med. 2001;29:196-200.
146 Owings M.F., Kozak L.J. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119.
147 Palmieri-Smith R.M., Kreinbrink J., Ashton-Miller J.A., Wojtys E.M. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35:1269-1275.
148 Papannagari R., Gill T.J., Defrate L.E., et al. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34:2006-2012.
149 Patel R.R., Hurwitz D.E., Bush-Joseph C.A., et al. Comparison of clinical and dynamic knee function in patients with anterior cruciate ligament deficiency. Am J Sports Med. 2003;31:68-74.
150 Paulos L.E., Wnorowski D.C., Greenwald A.E. Infrapatellar contracture syndrome. Diagnosis, treatment, and long-term follow-up. Am J Sports Med. 1994;22:440-449.
151 Perkins G. Rest and movement. J Bone Joint Surg Br. 1953;35:521-539.
152 Perry M.C., Morrissey M.C., King J.B., et al. Effects of closed versus open kinetic chain knee extensor resistance training on knee laxity and leg function in patients during the 8- to 14-week post-operative period after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2005;13:357-369.
153 Petersen W., Laprell H. Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc. 2000;8:26-31.
154 Pinczewski L.A., Lyman J., Salmon L.J., et al. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35:564-574.
154a Rebel M., Paessler H.H. The effect of knee brace on coordination and neuronal leg muscle control: an early postoperative functional study in anterior cruciate ligament reconstructed patients. Knee Surg Sports Traumatol Arthrosc. 2001;9:272-281.
155 Reider B., Arcand M.A., Diehl L.H., et al. Proprioception of the knee before and after anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:2-12.
156 Reilly D.T., Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43:126-137.
157 Renstrom P., Arms S.W., Stanwyck T.S., et al. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986;14:83-87.
158 Richmond J.C., Gladstone J., MacGillivray J. Continuous passive motion after arthroscopically assisted anterior cruciate ligament reconstruction: comparison of short- versus long-term use. Arthroscopy. 1991;7:39-44.
159 Risberg M.A., Beynnon B.D., Peura G.D., Uh B.S. Proprioception after anterior cruciate ligament reconstruction with and without bracing. Knee Surg Sports Traumatol Arthrosc. 1999;7:303-309.
160 Risberg M.A., Holm I., Steen H., et al. The effect of knee bracing after anterior cruciate ligament reconstruction. A prospective, randomized study with two years’ follow-up. Am J Sports Med. 1999;27:76-83.
161 Roberts D., Friden T., Stomberg A., et al. Bilateral proprioceptive defects in patients with a unilateral anterior cruciate ligament reconstruction: a comparison between patients and healthy individuals. J Orthop Res. 2000;18:565-571.
162 Roi G.S., Creta D., Nanni G., et al. Return to official Italian first division soccer games within 90 days after anterior cruciate ligament reconstruction: a case report. J Orthop Sports Phys Ther. 2005;35:52-66.
163 Rosen M.A., Jackson D.W., Atwell E.A. The efficacy of continuous passive motion in the rehabilitation of anterior cruciate ligament reconstructions. Am J Sports Med. 1992;20:122-127.
164 Rougraff B., Shelbourne K.D., Gerth P.K., Warner J. Arthroscopic and histologic analysis of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:277-284.
165 Rougraff B.T., Shelbourne K.D. Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1999;7:9-14.
166 Rubinstein R.A.Jr., Shelbourne K.D., VanMeter C.D., et al. Effect on knee stability if full hyperextension is restored immediately after autogenous bone–patellar tendon–bone anterior cruciate ligament reconstruction. Am J Sports Med. 1995;23:365-368.
167 Sajovic M., Vengust V., Komadina R., et al. A prospective, randomized comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: five-year follow-up. Am J Sports Med. 2006;34:1933-1940.
168 Salem G.J., Salinas R., Harding F.V. Bilateral kinematic and kinetic analysis of the squat exercise after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84:1211-1216.
169 Salmon L.J., Russell V.J., Refshauge K., et al. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am J Sports Med. 2006;34:721-732.
170 Salter R.B. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989;242:12-25.
171 Salter R.B., Simmonds D.F., Malcolm B.W., et al. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62:1232-1251.
172 Schenck R.C.Jr., Blaschak M.J., Lance E.D., et al. A prospective outcome study of rehabilitation programs and anterior cruciate ligament reconstruction. Arthroscopy. 1997;13:285-290.
173 Sgaglione N.A., Schwartz R.E. Arthroscopically assisted reconstruction of the anterior cruciate ligament: initial clinical experience and minimal 2-year follow-up comparing endoscopic transtibial and two-incision techniques. Arthroscopy. 1997;13:156-165.
174 Shaieb M.D., Kan D.M., Chang S.K., et al. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:214-220.
175 Shaw T., Williams M.T., Chipchase L.S. Do early quadriceps exercises affect the outcome of ACL reconstruction? A randomised controlled trial. Aust J Physiother. 2005;51:9-17.
176 Shelbourne K.D., Foulk D.A. Timing of surgery in acute anterior cruciate ligament tears on the return of quadriceps muscle strength after reconstruction using an autogenous patellar tendon graft. Am J Sports Med. 1995;23:686-689.
177 Shelbourne K.D., Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year follow-up. Am J Sports Med. 1997;25:786-795.
178 Shelbourne K.D., Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28:446-452.
179 Shelbourne K.D., Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18:292-299.
180 Shelbourne K.D., Rubinstein R.A.Jr., VanMeter C.D., et al. Correlation of remaining patellar tendon width with quadriceps strength after autogenous bone–patellar tendon–bone anterior cruciate ligament reconstruction. Am J Sports Med. 1994;22:774-777. discussion 777–778
181 Shelbourne K.D., Urch S.E. Primary anterior cruciate ligament reconstruction using the contralateral autogenous patellar tendon. Am J Sports Med. 2000;28:651-658.
182 Shelbourne K.D., Wilckens J.H., Mollabashy A., DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19:332-336.
183 Shields R.K., Madhavan S., Gregg E., et al. Neuromuscular control of the knee during a resisted single-limb squat exercise. Am J Sports Med. 2005;33:1520-1526.
184 Skyhar M.J., Danzig L.A., Hargens A.R., Akeson W.H. Nutrition of the anterior cruciate ligament. Effects of continuous passive motion. Am J Sports Med. 1985;13:415-418.
185 Smidt G.L. Biomechanical analysis of knee flexion and extension. J Biomech. 1973;6:79-92.
186 Smith A.J. Estimates of muscle and joint force at the knee and ankle during jumping activities. J Hum Movement Stud. 1975;1:78-86.
187 Snyder-Mackler L., Binder-Macleod S.A., Williams P.R. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 1994;25:783-789.
188 Snyder-Mackler L., De Luca P.F., Williams P.R., et al. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555-560.
189 Snyder-Mackler L., Delitto A., Bailey S.L., Stralka S.W. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg. 1995;77:1166-1173.
190 Snyder-Mackler L., Delitto A., Stralka S.W., Bailey S.L. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901-907.
191 Snyder-Mackler L., Ladin Z., Schepsis A.A., et al. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1991;73:1025-1036.
192 Sojka P., Sjolander P., Johansson H., Djupsjobacka M. Influence from stretch-sensitive receptors in the collateral ligaments of the knee joint on the gamma-muscle-spindle systems of flexor and extensor muscles. Neurosci Res. 1991;11:55-62.
193 Solomonow M., Baratta R., Zhou B.H., et al. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15:207-213.
194 Spencer J.D., Hayes K.C., Alexander I.J. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171-177.
195 Stein L.N., Fischer D.A., Fritts H.M., Quick D.C. Occult osseous lesions associated with anterior cruciate ligament tears. Clin Orthop Relat Res. 1995;313:187-193.
196 Steinkamp L.A., Dillingham M.F., Markel M.D., et al. Biomechanical considerations in patellofemoral joint rehabilitation. Am J Sports Med. 1993;21:438-444.
197 Stensdotter A.K., Hodges P.W., Mellor R., et al. Quadriceps activation in closed and in open kinetic chain exercise. Med Sci Sports Exerc. 2003;35:2043-2047.
198 Timoney J.M., Inman W.S., Quesada P.M., et al. Return of normal gait patterns after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:887-889.
199 Torry M.R., Decker M.J., Millett P.J., et al. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4:1-8.
200 Torry M.R., Decker M.J., Viola R.W., et al. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon). 2000;15:147-159.
201 Toutoungi D.E., Lu T.W., Leardini A., et al. Cruciate ligament forces in the human knee during rehabilitation exercises. Clin Biomech (Bristol, Avon). 2000;15:176-187.
202 Treacy S.H., Barron O.A., Brunet M.E., Barrack R.L. Assessing the need for extensive supervised rehabilitation following arthroscopic ACL reconstruction. Am J Orthop. 1997;26:25-29.
203 Tyler T.F., McHugh M.P., Gleim G.W., Nicholas S.J. The effect of immediate weight-bearing after anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 1998;357:141-148.
204 Walden M., Hagglund M., Ekstrand J. High risk of new knee injury in elite footballers with previous anterior cruciate ligament injury. Br J Sports Med. 2006;40:158-162. discussion 162
205 Wasilewski S.A., Covall D.J., Cohen S. Effect of surgical timing on recovery and associated injuries after anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:338-342.
206 Webb J.M., Corry I.S., Clingeleffer A.J., Pinczewski L.A. Endoscopic reconstruction for isolated anterior cruciate ligament ruptures. J Bone Joint Surg Br. 1998;80:288-294.
207 Wernbom M., Augustsson J., Thomee R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37:225-264.
208 Wexler G., Hurwitz D.E., Bush-Joseph C.A., et al. Functional gait adaptations in patients with anterior cruciate ligament deficiency over time. Clin Orthop Relat Res. 1998;348:166-175.
209 Wilk K.E. Are there speed limits in rehabilitation? J Orthop Sports Phys Ther. 2005;35:50-51.
210 Wilk K.E., Escamilla R.F., Fleisig G.S., et al. A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. Am J Sports Med. 1996;24:518-527.
211 Williams G.N., Snyder-Mackler L., Barrance P.J., et al. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus-gracilis graft. J Bone Joint Surg Am. 2004;86:1936-1946.
212 Witvrouw E., Danneels L., Van Tiggelen D., et al. Open versus closed kinetic chain exercises in patellofemoral pain: a 5-year prospective randomized study. Am J Sports Med. 2004;32:1122-1130.
213 Witvrouw E., Lysens R., Bellemans J., et al. Open versus closed kinetic chain exercises for patellofemoral pain. A prospective, randomized study. Am J Sports Med. 2000;28:687-694.
214 Wojtys E.M., Huston L.J. Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med. 2000;28:336-344.
215 Wright R.W., Boyce R.H., Michener T., et al. Radiographs are not useful in detecting arthroscopically confirmed mild chondral damage. Clin Orthop Relat Res. 2006;442:245-251.
216 Wright R.W., Dunn W.R., Amendola A., et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007;35:1131-1134.
217 Wright R.W., Fetzer G.B. Bracing after ACL reconstruction: a systematic review. Clin Orthop Relat Res. 2007;455:162-168.
218 Wu G.K., Ng G.Y., Mak A.F. Effects of knee bracing on the functional performance of patients with anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2001;82:282-285.
219 Wu G.K., Ng G.Y., Mak A.F. Effects of knee bracing on the sensorimotor function of subjects with anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29:641-645.
220 Yack H.F., Collins C.E., Whieldon T.J. Comparison of closed and open kinetic chain exercise in the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21:49-54.
221 Yack H.J., Riley L.M., Whieldon T.R. Anterior tibial translation during progressive loading of the ACL-deficient knee during weight-bearing and nonweight-bearing isometric exercise. J Orthop Sports Phys Ther. 1994;20:247-253.
222 Yasuda K., Sasaki T. Exercise after anterior cruciate ligament reconstruction. The forces exerted on the tibia by the separate isometric contractions of the quadriceps or the hamstrings. Clin Orthop Relat Res. 1987;220:275-283.
223 Yasuda K., Tsujino J., Ohkoshi Y., et al. Graft site morbidity with autogenous semitendinosus and gracilis tendons. Am J Sports Med. 1995;23:706-714.
224 Young A., Hughes I., Round J.M., Edwards R.H. The effect of knee injury on the number of muscle fibres in the human quadriceps femoris. Clin Sci (Lond). 1982;62:227-234.
225 Yu J.K., Paessler H.H. Relationship between tunnel widening and different rehabilitation procedures after anterior cruciate ligament reconstruction with quadrupled hamstring tendons. Chin Med J (Engl). 2005;118:320-326.