33 Sciatic Neuropathy
Anatomy
The sciatic nerve is derived from the L4–S3 roots, carrying fibers that eventually will become the tibial and common peroneal nerves. It leaves the pelvis through the sciatic notch (greater sciatic foramen) under the piriformis muscle accompanied by the other branches of the lumbosacral plexus (inferior and superior gluteal nerves and posterior cutaneous nerve of the thigh). In some individuals, fibers destined to become the common peroneal nerve run through the piriformis muscle before joining the sciatic nerve. Covered by the gluteus maximus, the sciatic nerve next runs medial and posterior to the hip joint between the ischial tuberosity and the greater trochanter of the femur (Figure 33–1). The knee flexors, including the medial hamstrings (semimembranosus and semitendinosus) and lateral hamstrings (long and short heads of the biceps femoris), and the lateral division of the adductor magnus are all supplied by the sciatic nerve.
Clinical
Sciatic neuropathies caused by trauma, injection, infarction, or compression present acutely. Otherwise, most sciatic neuropathies present in a progressive, subacute fashion. Patients with a complete sciatic neuropathy have paralysis of knee flexion and all movements about the ankle and toes. Sensation is lost in several areas (Figure 33–2), including the lateral knee (lateral cutaneous nerve of the knee), lateral calf (superficial peroneal nerve), dorsum of the foot (superficial peroneal nerve), web space of the great toe (deep peroneal nerve), posterior calf and lateral foot (sural nerve), and sole of the foot (distal tibial nerve). Pain may be perceived in the proximal thigh, radiating posteriorly and laterally into the leg, but it usually does not affect the back. The ankle reflex is depressed or absent on the involved side.

FIGURE 33–2 Sensory loss in sciatic neuropathy (in green).
(Adapted from Haymaker, W., Woodhall, B., 1953. Peripheral nerve injuries. WB Saunders, Philadelphia, with permission.)
This complete deficit is seen only in severe lesions or late in the course of sciatic neuropathy. Initially, the clinical presentation most often mimics peroneal neuropathy. It has long been recognized that the peroneal fibers are preferentially affected in most sciatic nerve lesions. Thus, it is not unusual for a patient with sciatic neuropathy to present with a footdrop and sensory disturbance over the dorsum of the foot and lateral calf. Indeed, early sciatic nerve lesions may be nearly impossible to differentiate clinically from peroneal nerve lesions at the fibular neck (Table 33–1).
Etiology
Sciatic neuropathy is distinctly uncommon and is associated with a limited differential diagnosis (Box 33–1). As the sciatic nerve runs posterior to the hip joint, one of the most common presentations occurs following hip or femur fracture (especially posterior dislocation) or as a complication of the subsequent surgery to repair the fracture. As a complication of surgery, sciatic neuropathy may occur due to retraction or stretch, as well as a result of methylmethacrylate cement forming spurs and then eroding into the nerve months to years later, which has been well documented in several case reports.
Another common cause of sciatic neuropathy is tumor (neurofibroma, schwannoma, neurofibrosarcoma, lipoma, and lymphoma). Tumors affecting the sciatic nerve usually can be imaged quite well as a mass lesion on computed tomography or magnetic resonance imaging (MRI) scanning (Figure 33–3). Other rare mass lesions also may affect the sciatic nerve. An enlarged Baker’s cyst in the popliteal fossa may compress the distal sciatic nerve as it bifurcates into the tibial and common peroneal nerves. Several unusual vascular abnormalities, including aneurysms of the inferior gluteal, iliac, or persistent sciatic arteries and arteriovenous malformations near the piriformis muscle, have been associated with sciatic neuropathy.

FIGURE 33–3 Mass lesion of the sciatic nerve.
(Adapted from Preston, D.C., Shapiro, B.E., 2001. Lymphoma of the sciatic nerve. J Clin Neuromuscul Dis 2, 227–228.)
Disorders that result in a mononeuritis multiplex syndrome (see Chapter 26) may affect the sciatic nerve. For example, vasculitic neuropathy commonly results in infarction of the sciatic nerve in the proximal thigh, which is a watershed area for nerve ischemia. The neuropathy often is acute and begins with prominent pain. Until additional nerve lesions develop, recognition of the underlying mononeuritis multiplex pattern is difficult or impossible.
Piriformis Syndrome
As the sciatic nerve leaves the pelvis, it runs under or through the piriformis muscle (Figure 33–4). The piriformis muscle originates from the sacrum, the sciatic notch and the sacrotuberous ligament, and then runs through the greater sciatic foramen to attach to the greater trochanter of the femur. The main action of the piriformis is to externally rotate the hip. When the hip is in a flexed position, it also acts as a partial hip abductor. Theoretically, a hypertrophied piriformis muscle could compress the sciatic nerve (piriformis syndrome), somewhat comparable to compression of the median nerve by the pronator teres muscle in pronator teres syndrome. In the past, many cases of “sciatica” were attributed to piriformis syndrome. However, most, if not all, cases of sciatica are due to lumbosacral radiculopathy and not sciatic neuropathy from piriformis syndrome. Piriformis syndrome is considered by many to be a controversial entity. There are very few reported cases of patients who meet the criteria for definite piriformis syndrome, which include (1) sciatic neuropathy clinically, (2) electrophysiologic evidence of sciatic neuropathy, (3) surgical exploration showing entrapment of the sciatic nerve within a hypertrophied piriformis muscle, and (4) subsequent improvement following surgical decompression.

FIGURE 33–4 Anatomic relationships of the sciatic nerve to the piriformis muscle.
(Adapted from Beaton, L.E., Anson, B.J., 1938. The sciatic nerve and the piriformis muscle: their interrelation as possible cause of coccygodynia. J Bone J Surg 20, 686–688.)
• The Freiberg maneuver: with the patient lying supine, the examiner forcefully internally rotates the leg, stretching the piriformis muscle.
• The Pace maneuver: in the seated position, the patient abducts the hip against resistance, activating the piriformis muscle.
• The Beatty maneuver: lying on their side, the patient abducts the hip, activating the piriformis muscle.
• The FAIR (flexion, adduction, internal rotation) maneuver: with the patient lying supine, the examiner passively flexes, adducts, and internally rotates the hip, stretching the piriformis muscle. This maneuver is also reported to be useful in the EDX of piriformis syndrome (see below).
Electrophysiologic Evaluation
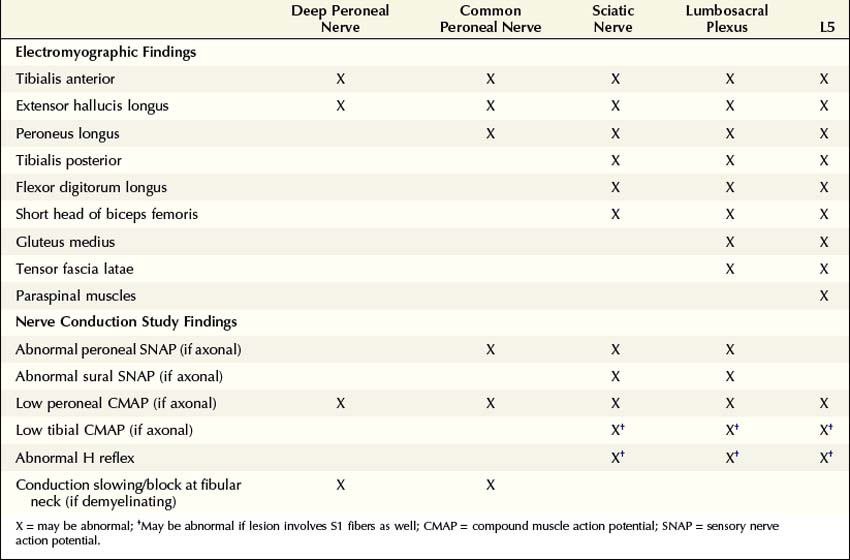
The electrophysiologic evaluation plays a key role in the assessment of a possible sciatic neuropathy. The electrophysiologic approach is similar to the clinical approach: evaluate and exclude disorders that can mimic sciatic neuropathy, including peroneal palsy at the fibular neck, lumbosacral plexopathy, and lumbosacral radiculopathy (Table 33–2).
Table 33–2 Electromyographic and Nerve Conduction Study Abnormalities Localizing the Lesion Site in Sciatic Neuropathy
Nerve Conduction Studies
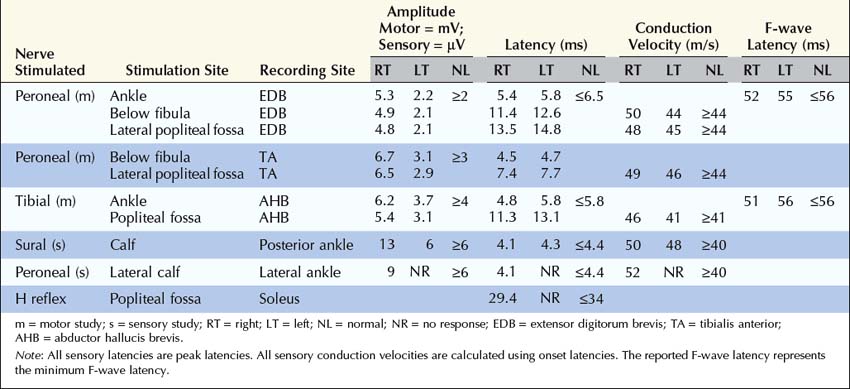
The nerve conduction evaluation of sciatic neuropathy is straightforward (Box 33–2). Routine peroneal and tibial motor studies should be performed bilaterally, recording the extensor digitorum brevis (EDB) and abductor hallucis brevis, respectively. Careful attention must be paid to the peroneal motor study, with the electromyographer looking for evidence of peroneal palsy at the fibular neck (either focal slowing or conduction block). In this regard, it is useful to perform peroneal motor studies recording the tibialis anterior as well as the extensor digitorum brevis. In sciatic nerve lesions with axonal loss, the amplitude of the peroneal or tibial compound muscle action potentials (CMAPs) may be reduced on the symptomatic side compared with normal control values or, more importantly, when compared with the contralateral asymptomatic leg. The peroneal fibers often are affected out of proportion to the tibial fibers. If there has been loss of the fastest conducting axons, there may be mild prolongation of the distal motor latency and some slowing of conduction velocity, but never into the demyelinating range.
Box 33–2
Recommended Nerve Conduction Study Protocol for Sciatic Neuropathy
1. Tibial motor study, recording abductor hallucis brevis and stimulating medial ankle and popliteal fossa; bilateral studies
2. Peroneal motor study, recording extensor digitorum brevis and stimulating ankle, below fibular neck and lateral popliteal fossa; bilateral studies. In patients with an isolated footdrop and clinical findings limited to the distribution of the peroneal nerve, the study should be performed, recording the tibialis anterior and stimulating below fibular neck and lateral popliteal fossa, to increase the yield of demonstrating conduction block or focal slowing across the fibular neck.
3. Sural sensory study, stimulating posterior lateral calf, recording posterior ankle; bilateral studies
4. Superficial peroneal sensory study, stimulating lateral calf, recording lateral ankle; bilateral studies
Special Studies in Suspected Piriformis Syndrome
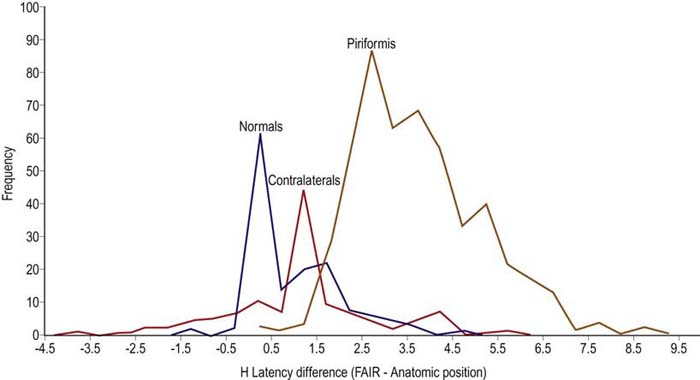
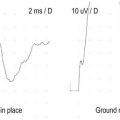
Most often, standard nerve conduction studies and needle EMG are normal in patients who are clinically diagnosed with piriformis syndrome. The one electrophysiologic test proposed to be of value is a modification of the H reflex. In piriformis syndrome, the H reflex is reported to be prolonged when performed with the hip in flexion, adduction, and internal rotation (FAIR test) compared to the normal anatomic position (Figure 33–5). This position stretches the piriformis muscle and theoretically may put pressure on the sciatic nerve.
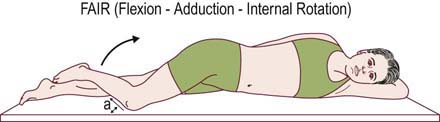
FIGURE 33–5 Flexion, adduction, and internal rotation (FAIR) position.
(From Fishman, L.M., Zybert, P.A., 1992. Electrophysiological evidence of piriformis syndrome. Arch Phys Med Rehabil 73, 359–364.)
In the largest reported study of this test, in patients with clinical criteria suggestive of piriformis syndrome, the mean prolongation of the H reflex in the FAIR position was 3.39 ms, which is equivalent to 5.45 standard deviations above the mean for a normal population. Compare this to the mean delay of the H reflex in 88 normal persons in the FAIR position compared to the anatomic position, which was 0.01 ms, with a standard deviation of 0.62 ms (Figure 33–6). However, the asymptomatic population was not normally distributed. Using a cutoff of 3 standard deviations (1.86) resulted in a specificity of 83% (i.e., 17% of a normal control population would be misidentified as abnormal). In addition, the contralateral, asymptomatic limbs of the patient group often demonstrated abnormalities, although they were less marked than in the symptomatic limbs.
Electromyographic Approach
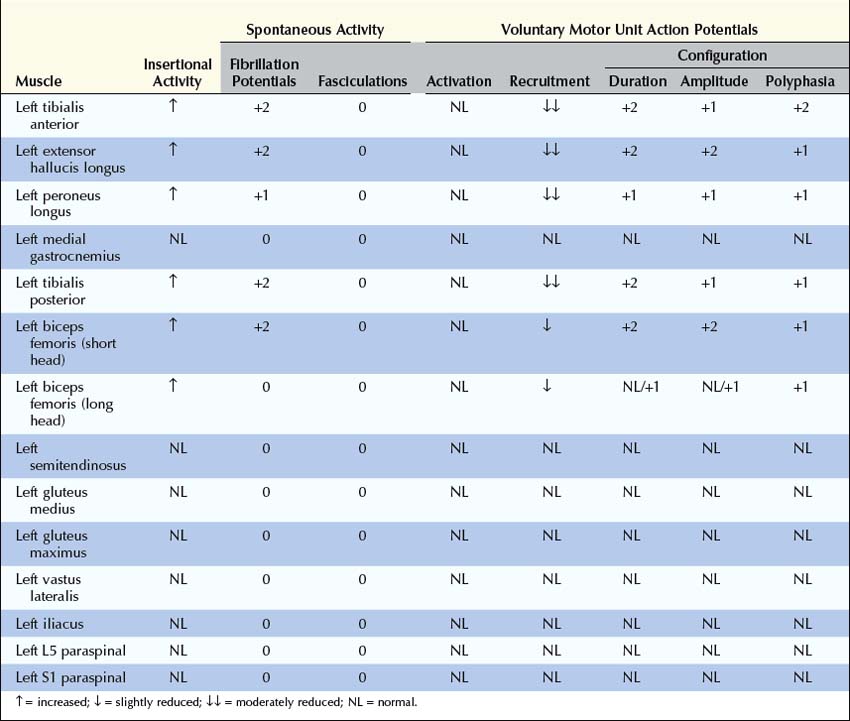
After the nerve conduction studies are completed, EMG is used to further localize the lesion and assess its severity (Box 33–3). First, muscles innervated by the deep and superficial peroneal nerves should be sampled (e.g., tibialis anterior, extensor hallucis longus, peroneus longus). Abnormalities in these muscles are consistent with a lesion of the peroneal nerve, sciatic nerve, lumbosacral plexus, or L5–S1 nerve roots. Next, tibial-innervated muscles in the calf should be sampled, including the medial gastrocnemius and especially the tibialis posterior or flexor digitorum longus. If abnormalities are found in any of these muscles, as well as in the peroneal-innervated muscles, an isolated lesion of the peroneal nerve has been excluded. The differential at this point includes a lesion of both the tibial and peroneal nerves versus a lesion of either the sciatic nerve, lumbosacral plexus, or L5–S1 nerve roots.
Box 33–3
Recommended Electromyographic Protocol for Sciatic Neuropathy
1. At least two peroneal-innervated muscles (tibialis anterior, extensor hallucis longus, peroneus longus)
2. At least two tibial-innervated muscles (medial gastrocnemius, tibialis posterior, flexor digitorum longus)
3. Short and long heads of the biceps femoris
4. At least one superior gluteal-innervated muscle (gluteus medius, tensor fascia latae)
5. At least one inferior gluteal-innervated muscle (gluteus maximus)
6. L5 and S1 paraspinal muscles
7. At least two non-sciatic, non-L5–S1-innervated muscles (vastus lateralis, iliacus, thigh adductors) to exclude a more widespread lesion
![]() Example Case
Example Case
 Case 33–1
Case 33–1
Summary
What is the Most Likely Clinical Diagnosis
Although the prior hip surgery suggests a possible sciatic lesion adjacent to the site of the surgery, the slowly progressive nature of the clinical presentation is worrisome for an expanding or infiltrating mass lesion, such as a tumor. Of course, the possibility of methylmethacrylate cement from the hip replacement forming spurs and then slowly eroding into the nerve must be considered in this context. The combination of the clinical history, neurologic examination, and electrodiagnostic studies provides a basis for imaging studies that now can be done in a more intelligent manner. In this case, subsequent MRI scanning of the left thigh showed a large enhancing lesion of the sciatic nerve in the mid-thigh. A subsequent biopsy revealed large cell lymphoma (see Figure 33–3).
Chiao H.C., Marks K.E., Bauer T.W., et al. Intraneural lipoma of the sciatic nerve. Clin Orthop. 1987;221:267.
Cusimano M.D., Shedden P.M., Hudson A.R., et al. Arteriovenous malformation of the pyriformis muscle manifesting as a sciatic nerve tumor. Neurosurgery. 1992;31:151.
Edwards M.S., Barbaro N.M., Asher S.W., et al. Delayed sciatic palsy after total hip replacement: case report. Neurosurgery. 1981;9:61.
Eusebi V., Bondi A., Cancellieri A., et al. Primary malignant lymphoma of sciatic nerve. Report of a case. Am J Surg Pathol. 1990;14:881.
Fishman L.M., Dombi G.W., Michaelsen C., et al. Piriformis syndrome: diagnosis, treatment, and outcome – a 10-year study. Arch Phys Med Rehabil. 2002;83:295–301.
Fishman L.M., Zybert P.A. Electrophysiological evidence of piriformis syndrome. Arch Phys Med Rehabil. 1992;73:359–364.
Gasecki A.P., Ebers G.C., Vellet A.D., et al. Sciatic neuropathy associated with persistent sciatic artery. Arch Neurol. 1992;49:967.
Kirschner J.S., Foye P.M., Cole J.L. Piriformis syndrome, diagnosis and treatment. Muscle Nerve. 2009;40:10–18.
Mohan S.R., Grimley R.P. Common iliac artery aneurysm presenting as acute sciatic nerve compression. Postgrad Med J. 1987;63:903.
Papadopoulos S.M., McGillicuddy J.E., Messina L.M. Pseudoaneurysm of the inferior gluteal artery presenting as sciatic nerve compression. Neurosurgery. 1989;24:926.
Pillay P.K., Hardy R.W., Jr., Wilbourn A.J., et al. Solitary primary lymphoma of the sciatic nerve: case report. Neurosurgery. 1988;23:370.
Sieb J.P., Schultheiss R. Segmental neurofibromatosis of the sciatic nerve: case report. Neurosurgery. 1992;31:1122.
Stewart J.D., Fishman L.M., Schaefer M.P. Issues & opinions: piriformis syndrome. Muscle Nerve. 2003;11:644–649.
Stillman M.J., Christensen W., Payne R., et al. Leukemic relapse presenting as sciatic nerve involvement by chloroma (granulocytic sarcoma). Cancer. 1988;62:2047.
Yeun E.C., Olney R.K., So Y.T. Sciatic neuropathy: clinical and prognostic features in 73 patients. Neurology. 1994;44:1669.
Yuen E.C., So Y.T. Sciatic neuropathy. Neurol Clin. 1999;17:617–631.
Young J.N., Friedman A.H., Harrelson J.M., et al. Hemangiopericytoma of the sciatic nerve. Case report. J Neurosurg. 1991;74:512.