Safety Issues
After reading this chapter, the student will be able to:
• Define the various safety issues and safety precautions laboratory workers and administrators need to consider
• Identify the agencies involved in the development, control, and oversight of safety issues in the laboratory environment
• Name and explain the different levels of biosafety
• Give examples of organisms handled in the different biosafety levels
• Describe the different equipment used in a laboratory setting and explain the role of each
• Explain the role of protective gear used in the laboratory and discuss the use of each
• Discuss the safety regulations and concerns in healthcare facilities
• Specify the regulatory and safety issues in physicians’ offices and clinics
• Describe the general safety requirements for hospitals, nursing homes, and personal care facilities
• Name the agencies involved in the surveillance, regulations, and support of the emergency response
Laboratory Safety
Biosafety
In 1992, OSHA published a rule that deals with the occupational health risk caused by exposure to human blood and other potentially infectious materials. A Biosafety Program was developed as an information management system to provide a process and tools to assess the safety, needs, and precautions in the planning, initiation, and termination of activities involving biological materials. The program is intended to protect personnel from exposure to infectious agents and to comply with federal, state, and local requirements. At present the program includes four major components (Box 5.1):
• Lawrence Berkeley National Laboratory (LBNL) Biosafety Manual, which provides guidelines, policies, and procedures for the management of hazardous and potentially hazardous biological materials
• “On-line” Exposure Control Plan development tool to assist principal investigators in the preparation of the Exposure Control Plan required by OSHA for research involving human source material
• Blood-borne training program for the initial OSHA training requirements for individuals who are at risk of occupational exposure to blood and other bodily fluids
• Biosafety training program, which is designed to provide information about safe procedures and practices in biological/biomedical research
Different biosafety levels were developed for microbiological and medical laboratories for personal and environmental protection. Specifically, four levels of containment have been defined and these are termed biosafety levels (BSL-1 to BSL-4), depending on the agent to be handled. The National Institutes of Health (NIH) has introduced the concept of “risk groups,” in which agents are classified into four risk groups (RGs) on the basis of their relative pathogenicity (Table 5.1). The description of biosafety levels and procedures are presented as introductions and are not intended to be an in-depth treatise. In addition, they are being continuously updated by the NIH, and the NIH websites should be consulted for the most recent information (http://www.cdc.gov/OD/ohs/biosfty/bmbl5/bmbl5toc.htm).
TABLE 5.1
Summary of Recommended Biosafety Levels for Infectious Agents
| Risk Group 1 (RG1) | Agents not associated with disease in healthy adult humans |
| Risk Group 2 (RG2) | Agents associated with human disease but generally not serious and for which preventive and therapeutic interventions are available |
| Risk Group 3 (RG3) | Agents associated with serious or lethal human disease. Preventive or therapeutic interventions may be available |
| Risk Group 4 (RG4) | Agents likely to cause serious or lethal human disease. Preventive or therapeutic interventions are not usually available |
Biosafety Level 1 (BSL-1)
BSL-1 applies to working with microorganisms that are generally not disease causing in healthy humans and therefore are of minimal potential hazard to laboratory personnel and the environment (Box 5.2). This safety level is used in municipal water-testing laboratories, high school laboratories, and in some community colleges teaching introductory microbiology classes with organisms that are not considered to be pathogenic and/or hazardous. These laboratories typically include a sink for hand washing, benchtops, sturdy furniture, windows with fly screens if they can be opened, and readily available disinfectants and antiseptics. The laboratory should be easily cleaned, decontaminated, and have procedures posted for the safe disposal of materials being used.
The laboratory at this safety level does not have to be isolated from other parts of the building; however, a door that can be closed while work with agents is in progress is highly desirable. Hazard warning signs (Figure 5.1) should be posted on doors, indicating any hazards that may be present. A sink for hand washing needs to be available because hand washing is one of the simplest yet most important procedures used by laboratory personnel to remove unwanted microbial agents or chemicals used in the laboratory.
Biosafety Level 2 (BSL-2)
BSL-2 is similar to BSL-1 regarding the agents that are being handled. However, for BSL-2 work, the facility, containment devices, administrative controls, practices and procedural standards, and guidelines are designed to maximize safe working conditions for laboratories working with agents of moderate risk to personnel and the environment. Agents manipulated at BSL-2 are considered a moderate risk. They often include pathogens to which personnel have previously been exposed and to which they have had an immune response (e.g., childhood diseases) or against which they have received immunization (Box 5.3). Immunization is recommended before working with certain agents, for example, immunization against the hepatitis B virus which is recommended by OSHA for people at high risk of exposure to blood and blood products. In addition to procedures established in the BSL-1 level laboratory, the BSL-2 laboratory requires that:
• Laboratory personnel receive specific training in handling pathogenic agents and be directed by trained competent scientists
• Access to the laboratory be limited while work is in progress
• Extreme precautions be taken with contaminated sharp items and their disposal
• Specific procedures for dealing with infectious aerosols or splashes be developed for work carried out in biological safety cabinets, hoods, or other physical containment equipment
Biosafety Level 3 (BSL-3)
BSL-3 applies to clinical, diagnostic, teaching, research, or production laboratories using original or exotic agents (Box 5.4). Such agents can potentially cause serious disease or even lethality if exposure occurs. In addition to the laboratory procedures described in BSL-1 and BSL-2, laboratory personnel require specific training in handling pathogenic and potentially lethal agents with on-site supervision by scientists experienced and qualified in working with such agents. Many additional standards are necessary to qualify as a BSL-3 laboratory. Some of the additional procedures include but are not limited to:
• Control of access to the laboratory. This is the responsibility of the laboratory director, who restricts access to personnel required for program conduct or support purposes. Other personnel at risk of acquiring infection, such as those who are immunocompromised or immunosuppressed, should not have access. No minors are allowed in the laboratory.
• All laboratory personnel are required to receive appropriate immunizations and/or tests for sensitivities to the presence of the agents that will be handled or would potentially be present in the laboratory. Periodic testing is recommended and current records of all data kept.
• All procedures involving the manipulation of infectious material are done in biological safety cabinets or other physical containment devices, or by personnel with appropriate protective clothing and equipment.
• The laboratory should have specially engineered design features for BSL-3 laboratory work, for example, special exhaust air ventilation systems. (Please refer to the NIH guidelines for specifics: http://www.cdc.gov/OD/ohs/biosfty/bmbl5/bmbl5toc.htm).
• The laboratory is located separate from areas that are open and accessible to unrestricted traffic flow within the building.
• All windows in the laboratory are closed and sealed.
• Emergency treatment equipment must be readily accessible and kept updated.
• A change-of-clothes room may be included in entrance/exit passageways with appropriate disposal containers for lab-used clothing.
• The Biosafety Level 3 facility design and operational procedures must be documented and tested for appropriate design and parameters before, during, and after use. Data on all operations are kept current and all facilities should be reverified at least annually.
The National Institute of Allergy and Infectious Diseases (NIAID) in partnership with the American Society of Microbiology (ASM) conducted a brief survey of academic, biotechnology, and pharmaceutical facilities in the United States to provide the NIAID with information about the location, capacity, and status of existing and operating facilities capable of BSL-3 containment. This information can be viewed at http://www.asm.org/Policy/index.asp?bid=37789.
Biosafety Level 4 (BSL-4)
BSL-4 is required for working with dangerous and exotic agents that present a high risk of aerosol-transmitted laboratory infections and life-threatening disease (Box 5.5). All laboratory staff is required to have specific training in handling extremely hazardous infectious agents. The facility is located either in a separate building or in a controlled area within a building that is completely isolated from all other areas of that building. In addition to all other laboratory procedures described previously, additional protocols and procedures are necessary for BSL-4. Some of these include but are not limited to the following:
• Personnel enter and leave the laboratory only through clothing-change and personal shower rooms. They are required to take a decontamination shower each time they leave the laboratory.
• All personal clothing is removed and replaced with completely decontaminated laboratory clothing before entering the laboratory. This laboratory clothing is removed and placed in appropriate containers before showering at the time of leaving the laboratory.
• All supplies and materials (laboratory and other) needed in the BSL-4 facility enter via a double-doored autoclave, fumigation chamber, or airlock device.
• A daily inspection of all containment parameters and life support systems will be completed and recorded before laboratory work is initiated. Data on all safety procedures will be kept current.
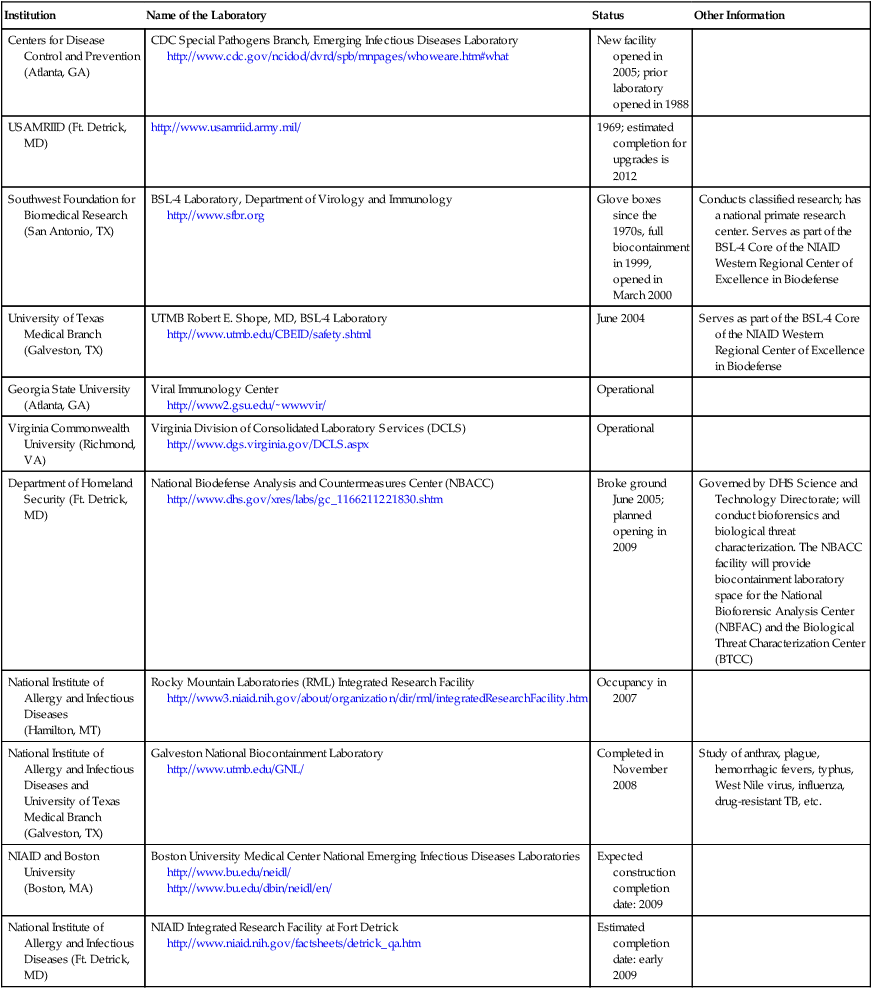
It is important to note that the description of the different biosafety levels (BSL-1 to BSL-4) in this chapter is an introduction only and does not reflect complete, more detailed, and specific governmental suggestions, standards, and regulations. Information about BSL-4 laboratories in the United States is summarized in Table 5.2.
TABLE 5.2
BSL-4 Laboratories in the United States
| Institution | Name of the Laboratory | Status | Other Information |
| Centers for Disease Control and Prevention (Atlanta, GA) | CDC Special Pathogens Branch, Emerging Infectious Diseases Laboratory http://www.cdc.gov/ncidod/dvrd/spb/mnpages/whoweare.htm#what |
New facility opened in 2005; prior laboratory opened in 1988 | |
| USAMRIID (Ft. Detrick, MD) | http://www.usamriid.army.mil/ | 1969; estimated completion for upgrades is 2012 | |
| Southwest Foundation for Biomedical Research (San Antonio, TX) | BSL-4 Laboratory, Department of Virology and Immunology http://www.sfbr.org |
Glove boxes since the 1970s, full biocontainment in 1999, opened in March 2000 | Conducts classified research; has a national primate research center. Serves as part of the BSL-4 Core of the NIAID Western Regional Center of Excellence in Biodefense |
| University of Texas Medical Branch (Galveston, TX) | UTMB Robert E. Shope, MD, BSL-4 Laboratory http://www.utmb.edu/CBEID/safety.shtml |
June 2004 | Serves as part of the BSL-4 Core of the NIAID Western Regional Center of Excellence in Biodefense |
| Georgia State University (Atlanta, GA) | Viral Immunology Center http://www2.gsu.edu/∼wwwvir/ |
Operational | |
| Virginia Commonwealth University (Richmond, VA) | Virginia Division of Consolidated Laboratory Services (DCLS) http://www.dgs.virginia.gov/DCLS.aspx |
Operational | |
| Department of Homeland Security (Ft. Detrick, MD) | National Biodefense Analysis and Countermeasures Center (NBACC) http://www.dhs.gov/xres/labs/gc_1166211221830.shtm |
Broke ground June 2005; planned opening in 2009 | Governed by DHS Science and Technology Directorate; will conduct bioforensics and biological threat characterization. The NBACC facility will provide biocontainment laboratory space for the National Bioforensic Analysis Center (NBFAC) and the Biological Threat Characterization Center (BTCC) |
| National Institute of Allergy and Infectious Diseases (Hamilton, MT) |
Rocky Mountain Laboratories (RML) Integrated Research Facility http://www3.niaid.nih.gov/about/organization/dir/rml/integratedResearchFacility.htm |
Occupancy in 2007 | |
| National Institute of Allergy and Infectious Diseases and University of Texas Medical Branch (Galveston, TX) |
Galveston National Biocontainment Laboratory http://www.utmb.edu/GNL/ |
Completed in November 2008 | Study of anthrax, plague, hemorrhagic fevers, typhus, West Nile virus, influenza, drug-resistant TB, etc. |
| NIAID and Boston University (Boston, MA) |
Boston University Medical Center National Emerging Infectious Diseases Laboratories http://www.bu.edu/neidl/ http://www.bu.edu/dbin/neidl/en/ |
Expected construction completion date: 2009 | |
| National Institute of Allergy and Infectious Diseases (Ft. Detrick, MD) | NIAID Integrated Research Facility at Fort Detrick http://www.niaid.nih.gov/factsheets/detrick_qa.htm |
Estimated completion date: early 2009 |
Available at http://www.liebertonline.com/toc/bsp/5/1
From Gronvall GK, Fitzgerald J, Chamberlain A, et al: High-containment biodefense research laboratories: meeting report and center recommendations, Biosecurity and Bioterrorism 5(1), 75–85, 2007 (accessed November 15, 2008). © Mary Ann Liebert, Inc. Reprinted with permission. DOI: 10.1089/bsp.2007.0902.
Chemicals
• Chemical name: First name of a drug and a precise description of its chemical composition
• Structural formula: A precise description of the atomic groups or arrangements of atoms
• CAS number: The Chemical Abstracts Service (CAS) registry number is a unique number assigned to each chemical to aid in searching on computerized databases
• RTECS number: Likewise, the Registry of Toxic Effects of Chemical Substances (RTECS) is a unique number that aids in finding additional toxicological information about a specific substance
• DOT ID and guide number: This is a listing of the U.S. Department of Transportation (DOT) identification numbers of the chemicals regulated by the DOT and serves a similar purpose as the numbers listed previously
• Synonyms: Names with similar meanings
• Trade names: Also called a proprietary name; it is selected by the pharmaceutical company and may reflect its use. For example, the “-caines” in general are local anesthetics such as procaine in the United States, which is reflected by at least by 24 different “-caines” in worldwide trade names
• Exposure limits: These are the recommended exposure limits (RELs) established by NIOSH
• IDLH: This lists the concentrations of chemicals that are immediately dangerous to life or health
• Physical description: Provides brief information about the appearance and odor of a given substance
• Chemical and physical properties
• Incompatibilities and reactivities
• Personal protection and sanitation: This is a summary of recommended practices for safe handling of each substance
Furthermore, each laboratory should have a chemical hygiene plan, including guidelines on labeling of chemical containers and manufacturer’s material safety data sheets (MSDSs) for each chemical used in that particular laboratory (Figure 5.2). An MSDS form contains data regarding the property of a particular substance intended to provide workers and emergency personnel with procedures for handling that particular substance in a safe manner. Sections on an MSDS data sheet usually include but are not limited to:
This illustration shows the cover page of a typical MSDS data sheet. In all laboratories these sheets will be kept in a dedicated binder for quick reference by laboratory workers and emergency personnel.
• Name, address, and telephone number of the manufacturer
• Physical and chemical properties
• Health effects and first aid
A searchable MSDS database of chemicals and chemical compounds is available at http://hazard.com/msds/.
Equipment
Fire Extinguishers
In addition to fire alarms, fire extinguishers are required in all laboratories. They are classified and assigned a letter or symbol for that classification to a particular fire type they are designed to extinguish (Figure 5.3). However, multipurpose extinguishers are often recommended because they are effective against different types of fires. Extinguishers are located at specified places in a laboratory. They should be inspected at least every 12 months and records kept readily visible and easily accessible by appropriately trained persons in a given institution, usually safety officers. Fire extinguishers are classified as:


This photo shows a typical multipurpose fire extinguisher used in many laboratories. Note the easy access location near the laboratory door, the inspection tag indicating regular inspections/maintenance, and the letters on the extinguisher indicating the class of fires it is designed to extinguish. All the classes and symbols are listed next to the photo.
• Type A: For use on fires from combustibles such as wood, cloth, paper, rubber, and plastics
• Type B: For use on fires from flammable liquids such as grease, gasoline, oil, and paint thinners
• Type C: For use on electrically energized fires (electrical equipment such as electrophoresis units)
• Type D: For use on fires from flammable metals such as magnesium, titanium, sodium, lithium, and potassium
• Type K: For use against fires involving grease fire associated with cooking equipment
• Multipurpose fire extinguishers: These work effectively against type A, B, and C fires. Multipurpose fire extinguishers are recommended for home use
Fume Hoods
• General-purpose hoods: These include standard fume hoods, bypass hoods, constant volume hoods, variable air volume hoods, and auxiliary air supply hoods.
• Radioisotope hoods: These are tested and authorized by radiation safety personnel for use with volatile radioactive materials.
• Biosafety cabinets: These are specialized hoods to prevent or minimize the exposure of humans or the environment to biohazardous agents or materials.
• Perchloric acid hoods: These must be used when performing any laboratory procedure that requires the use of perchloric acid.
Autoclave
An autoclave (see Figure 4.3 in Chapter 4, Microbiological Laboratory Techniques) is a common, routinely used piece of equipment in biological laboratories. It is a device that uses superheated steam under pressure to sterilize equipment and other objects. Sterilization is the elimination of all transmissible agents (see Chapter 4, Microbiological Laboratory Techniques) and autoclaves are widely used for this purpose. However, as an exception, it has been shown that certain archaea (strain 121) and prions have the capacity to survive autoclaving.
Eyewashes and Safety Showers
Eyewashes and safety showers are emergency units present in both public and private industry to protect employees from injury in case of contact with hazardous materials. Employees must be instructed and trained in the proper use of eyewashes and safety showers regardless of their previous experience background. The first seconds after exposure to hazardous materials are critical and decontamination equipment must be in reach for the exposed individual within 10 or fewer seconds after exposure. Therefore, eyewashes and safety showers should be in a clearly marked, accessible location within a laboratory. They provide effective protective treatment in the event of contact from a chemical or biological spill onto the skin or clothing. Many OSHA standards address problems that potentially can occur in the laboratory environment and protocols have been developed for the emergency eyewash and shower equipment (ANSI [American National Standards Institute] Z358.1-2004). The types of equipment addressed by the ANSI standard are listed below and in Figure 5.4.


Although many styles and types are available for laboratory use, all showers and eyewashes have the same function and basic features. These photos show A, a basic plumbed shower activated by pulling on a triangular rod, and B, a basic plumbed eyewash activated by a push paddle.
• Emergency shower: Provides a continuous water flow and is operated by grasping a ring chain or triangular rod
• Plumbed and self-contained eyewash units: Includes devices permanently connected to a source of potable water, and those not permanently installed, but requiring refilling or replacement after use
• Personal eyewash: Used on site for immediate flushing. It has less of a water supply than the plumbed self-contained unit and is used until the victim reaches another unit
• Hand-held drench hose: A flexible hose connected to a water supply for use to irrigate and flush eyes, face, and body areas
• Eye/face wash: Used to irrigate and flush both the face and the eyes
• Combination unit: A shower combined with an eyewash or eye/face wash
Refrigerators/Freezers
Laboratory refrigerators, freezers, and ultralow freezers (down to −85° C) need to be carefully selected for specific chemical or biological needs and records of routine, periodical inspections as well as removal of contents noted. The laboratory refrigerator, freezer, cooling unit, or ultralow freezer should be labeled as such with appropriate hazard signs posted on them (Figure 5.5). Containers placed in a given unit should be completely sealed or capped, securely placed, and have readily visible permanent labels.

This photo shows a chest-type ultralow temperature freezer with appropriate safety labeling such as temperature, a biohazard label, and a warning not to store volatile liquids in the box. If the gases from volatile liquids should accidentally ignite in the freezer, the tight seal of the box could cause a powerful explosion.
Disposal of Hazardous Waste
Materials used in any laboratory environment must be disposed of in designated containers. Broken glass is placed in cartons labeled for broken glass and plastics in plasticware containers (Figure 5.6). Biohazardous wastes are disposed of in autoclave bags (Figure 5.7) and used sharps are placed in biohazard sharps containers (Figure 5.8).

The size and shape of these containers may vary but certain features are common, such as a heavy-duty plastic liner contained in a sturdy cardboard box.

Autoclave bag come in many shapes and sizes but all have some common features: the color of the bags is red or orange, and they have the biohazard symbol printed on the bag. Some bags also incorporate a heat sensitive ink on the bag which will indicate when a bag has been autoclaved.

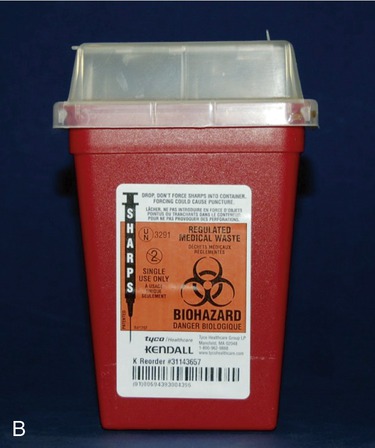
All waste items that have the potential to puncture or cause any break in the skin are considered “sharps” and must be disposed of in a container that is puncture proof. As these photos show, these containers come in many shapes and sizes and are usually made of hard plastic or metal. Sharps containers that may contain sharps contaminated with biological waste must also be labeled with a biohazard symbol.
Protective Gear
Eye Protection
Eye protection is mandatory in many laboratories where a potential for injury exists. The type of eye protection required depends on the hazard present in a given environment. For most situations safety glasses with side shields are adequate, but when there is danger of splashing of any kind goggles are required. The wearing of corrective lenses is no substitute for wearing protective eye equipment such as goggles or transparent face shields as required (Figure 5.9).
Safety in Healthcare Facilities
Some potential chemical hazards include exposure to formaldehyde, a fixative used for the preservation of specimens for pathology; and ethylene oxide, glutaraldehyde, and peracetic acid, which are used for sterilization as already discussed in Chapter 4 (Microbiological Laboratory Techniques).
HEALTHCARE APPLICATION
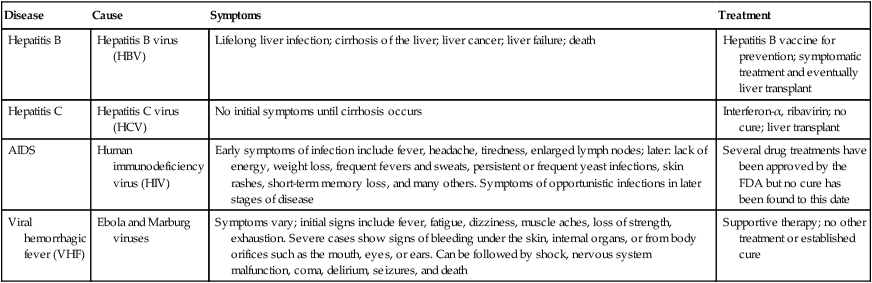
Blood-borne Pathogens in Healthcare Settings
| Disease | Cause | Symptoms | Treatment |
| Hepatitis B | Hepatitis B virus (HBV) | Lifelong liver infection; cirrhosis of the liver; liver cancer; liver failure; death | Hepatitis B vaccine for prevention; symptomatic treatment and eventually liver transplant |
| Hepatitis C | Hepatitis C virus (HCV) | No initial symptoms until cirrhosis occurs | Interferon-α, ribavirin; no cure; liver transplant |
| AIDS | Human immunodeficiency virus (HIV) | Early symptoms of infection include fever, headache, tiredness, enlarged lymph nodes; later: lack of energy, weight loss, frequent fevers and sweats, persistent or frequent yeast infections, skin rashes, short-term memory loss, and many others. Symptoms of opportunistic infections in later stages of disease | Several drug treatments have been approved by the FDA but no cure has been found to this date |
| Viral hemorrhagic fever (VHF) | Ebola and Marburg viruses | Symptoms vary; initial signs include fever, fatigue, dizziness, muscle aches, loss of strength, exhaustion. Severe cases show signs of bleeding under the skin, internal organs, or from body orifices such as the mouth, eyes, or ears. Can be followed by shock, nervous system malfunction, coma, delirium, seizures, and death | Supportive therapy; no other treatment or established cure |
Summary
• All laboratory environments are governed by regulations to ensure the safety of the laboratory personnel and the general environment.
• Microbiological and clinical laboratories are regulated by biosafety level standards, depending on the organisms handled or manipulated in the particular laboratory.
• Standards for the safe use and handling of chemicals apply to all laboratory settings.
• Laboratory safety equipment, an important component in all laboratory settings, is designed to protect the health and safety of all laboratory personnel.
• Safety equipment includes, but is not limited to, fire extinguishers, fume hoods, autoclaves, eyewash and safety showers, and refrigerators/freezers.
• Personal protective gear is used to protect the wearer from specific hazards, but does not protect the immediate environment.
• Safety regulations mandated by federal and state agencies apply to all healthcare facilities, nursing homes, personal care facilities, as well as public institutions.
• All personnel in laboratory facilities and in healthcare and other public facilities should be aware of the specific emergency procedures required for their environment.
Review Questions
2. There are __________ levels of biosafety depending on the organisms handled.
3. The biosafety level necessary in water-testing facilities is level:
4. Which of the following bacteria should be handled in a Biosafety Level 2 facility?
5. Agents associated with human disease, but generally not a serious health risk, are classified in which of the following risk groups?
6. Ebola viruses need to be handled in which of the following biosafety levels?
7. Fires from flammable metals require type __________ fire extinguishers.
8. Bypass fume hoods belong to the group of:
9. Which of the following eyewash/safety showers should be used for immediate flushing only, until the victim reaches another safety unit?
10. All of the following are blood-borne pathogens in the healthcare setting except:
11. Ergonomic guidelines for nursing homes are issued by __________.
12. PASS stands for pull, aim, squeeze, and __________.
13. CDC stands for the Centers for Disease Control and __________.
14. Dangerous and exotic agents need to be handled in a BSL __________ environment.
15. The type of fire extinguisher used on fires from flammable liquids such as gasoline would be a type __________ extinguisher.
16. List five specific areas for which clinical laboratories are responsible.
17. Name the types of personal protective equipment/gear.
18. Describe the different eyewash and safety showers found in laboratories.
19. Name five pieces of information provided by the NIOSH Pocket Guide to Chemical Hazards.
20. Name the different blood-borne pathogens that can be a hazard in healthcare settings.



