Chapter 15 Sacroiliac Joint Injections and Lateral Branch Blocks, Including Water-Cooled Neurotomy
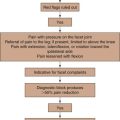
 Pain attributable to SIJ dysfunction is an area approximately 3 × 10 cm just inferior to the posterior superior iliac spine and usually not superior to L5.
Pain attributable to SIJ dysfunction is an area approximately 3 × 10 cm just inferior to the posterior superior iliac spine and usually not superior to L5. Referral patterns in patients with SIJ dysfunction are usually reported as radiating into the buttock.
Referral patterns in patients with SIJ dysfunction are usually reported as radiating into the buttock. Combination of tests increases the likelihood of a diagnosis of SIJ dysfunction and positive response to SIJ injections.
Combination of tests increases the likelihood of a diagnosis of SIJ dysfunction and positive response to SIJ injections. Controlled, comparative, image-guided local anesthetic diagnostic blocks are beneficial in the diagnosis of SIJ dysfunction.
Controlled, comparative, image-guided local anesthetic diagnostic blocks are beneficial in the diagnosis of SIJ dysfunction. Minimally invasive procedures (intraarticular injections, ablative procedures) are minimally destructive to anatomy and have shown benefit in symptom attenuation.
Minimally invasive procedures (intraarticular injections, ablative procedures) are minimally destructive to anatomy and have shown benefit in symptom attenuation.Establishing a Diagnosis
Goldthwaite and Osgood, in 1905, first described “sacroiliac strain” as a possible source of low back pain1 and the sacroiliac joint (SIJ) was considered the primary source of low back pain in the early 20th century.2 In 1936, Pitkin and Pheasant described lower extremity pain as originating in the sacroiliac and lumbosacral joints and their accessory ligaments and coined the term sacroarthrogenic telalgia. SIJ fusion became the treatment of choice for radicular pain originating from the low back.3 It was not until Mixter and Barr that focus was placed on the lumbar spine, the intervertebral disc, and the herniation of nucleus pulposus contents.4 Today, there is a reemergence implicating the SIJ as a potential source of low back pain with an estimated prevalence among patients with low back pain of 18% to 30%.5,6
Pathology of the SIJ has been documented in a variety of conditions; structural abnormalities, joint infections, metabolic and inflammatory disorders, and degeneration have all been implicated.7 SIJ dysfunction or SIJ syndrome is a condition in which pain localized to the SIJ cannot otherwise be explained by an identifiable pathological process (e.g., joint infection). Clinically distinguishing primary SIJ dysfunction from the other etiologies that overlap in pain referral patterns may be difficult. Much of the confusion regarding the diagnosis of SIJ dysfunction revolves around the inability to differentiate SIJ pain from pain that originates from surrounding structures. For example, discogenic and facet joint pathology have a clinical presentation similar to that of SI joint pathology. Not only are the pain distributions considered similar in many references, but the clinical tests used in diagnosis (e.g., Patrick’s, Gaenslen’s) are known to stress surrounding structures.7 The familiar symptom in SIJ dysfunction is the pain or other symptoms ascribed to a rectangular area approximately 3 × 10 cm just inferior to the posterior superior iliac spine (PSIS) identified on a pain drawing; however, no clinical studies have demonstrated this as a consistent finding for diagnosing SIJ dysfunction.8 Through patient history and diagnostic testing, several referral patterns have been proposed, although these referral patterns may be present in other sources of low back pain.9
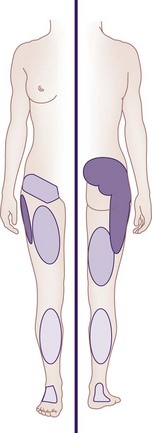
Furthermore, referral of pain into various locations of the lower extremity does not distinguish SIJ pain from other pain states.5,10 However, only 4% of patients with SIJ pain mark any pain above the L5 on self-reported pain drawings.10 The multiple patterns of SIJ pain referral zones may arise for several reasons: SIJ innervation is highly variable and complex; pain may be somatically referred from other primary osseous and ligamentous nociceptors such as the zygapophyseal joint, intervertebral disc, or adjacent structures (e.g., piriformis muscle, sciatic nerve, and L5 nerve root); and the referral pattern may be affected by intrinsic joint pathology and become active nociceptors.1 Slipman et al11 examined pain referral patterns in patients with SIJ dysfunction and reported that 94% of patients had pain radiating into the buttock, lower lumbar region (72%), lower extremity (50%), groin area (14%), upper lumbar region (6%), and abdomen (2%). In 28% of patients, the pain radiated distal to the knee, and 14% described foot pain (Fig. 15-1).
SIJ syndrome may occur acutely from trauma, sudden heavy lifting, prolonged lifting and bending, torsional strain, rising from a stooped position, a fall onto the buttock, or rear-end motor vehicle accident with the ipsilateral foot on the brake.1 Characteristically associated with SIJ dysfunction include buttock pain caudal to the PSIS; pseudoradiculopathy with pain radiating to the posterolateral aspect of the thigh (at times caudal to the knee); and aggravation of pain in sitting position, rising from a sitting position (exiting an automobile), and relieved by changing positions. The most consistent factor for identifying patients with unilateral SIJ pain is unilateral pain localized predominantly below the L5 spinous process.5,7,8,10,12
Physical Examination
In 1994, the International Association for the Study of Pain (IASP) proposed a set of criteria for diagnosing SIJ pain, referring to patients with pain in the area of the SIJ, which should be reproducible by performing specific pain provocation tests or should be completely relieved by infiltration of the symptomatic SIJ with local anesthetics.13 An assortment of SIJ examination maneuvers has been described to diagnose SIJ dysfunction (Box 15-1). Problematic to this is that provocation in this area may incite pain in adjacent structures, confounding the diagnosis of SIJ dysfunction. Additionally, using individual tests (provocative, motion, or palpation) has yielded low sensitivity or specificity for detecting a pathological SIJ.14–16 Dreyfuss et al,10 examining the usefulness of medical history and physical examination, concluded that no historical feature, none of the 12 tested SIJ maneuvers, and no ensemble of these 12 maneuvers demonstrated worthwhile diagnostic value. These results are replicated in a prospective cohort study in which provocative maneuvers were not predictive of diagnosis of SIJ dysfunction.17 Additionally, 20% of asymptomatic patients have been found to possess pain on provocative tests at the SIJ.18 In contrast, other reports indicate the usefulness of a combination of tests that increases the likelihood of response to SIJ injections.19–21 In a study examining the diagnostic validity of tests that could be ascribed to the IASP criteria for diagnosing SIJ pain, tests such as pain mapping or pain referral patterns have an ability to correctly identify patients with SIJ pain. However, they fail in discriminating patients without SIJ pain. The compression and thigh thrust test were regarded as positive examination finding in diagnosing SIJ pain.22 The general consensus appears to be that SIJ pain can be diagnosed with reasonable certainty with controlled comparative local anesthetic diagnostic blocks.23,24
Box 15-1 Sacroiliac Joint Examination Stress Maneuvers
| Patrick (FABERE) test | With the patient supine, the thigh is flexed, and the ankle is placed above the knee of the contralateral leg. The ipsilateral knee is depressed, the contralateral hip is stabilized, and the ankle is maintained in its position above the contralateral knee. A positive test result is indicated by the patient’s complaints of pain over the ipsilateral SIJ as the knee is depressed toward the examination table. |
| Gaenslen’s test | With the patient supine, the ipsilateral leg hangs over the examination table and the contralateral leg is flexed, bringing the knee towards the abdomen. Counterpressure is applied to the knee of the hanging leg, toward the floor, and the contralateral leg toward the abdomen. A positive test result is indicated by the patient’s complaints of pain over the ipsilateral SIJ as counterpressure is applied. |
| Fortin’s finger test (sacral sulcus tenderness) | The patient is asked to point to the region of pain with one finger. A positive test result is indicated by consistent localization of pain in an area immediately inferomedial to the PSIS within 1 cm. |
| Shear test | With the patient prone, pressure is applied in a caudal direction. A positive test result is indicated by the patient’s complaints of pain over the SIJ as pressure is applied. |
| Compression test | With the patient in lateral decubitus position, the hips and knees are flexed. Downward force is applied to the uppermost iliac crest. A positive test result is indicated by the patient’s complaints of pain over the SIJ as pressure is applied. |
| Gillet test | With the patient standing, the PSIS is palpated along with the sacral spinous processes. The patient flexes the ipsilateral hip and knee to a minimum of 90 degrees. A positive test result is indicated by the thumb on the PSIS moving cephalad in relation to the thumb on the sacrum. |
| Yeoman’s test | With the patient prone, the hip is extended, and the ipsilateral ilium is rotated. A positive test result is indicated by the patient’s complaints of pain over the SIJ as pressure is applied. |
PSIS, posterior superior iliac spine; SIJ, sacroiliac joint.
Anatomy
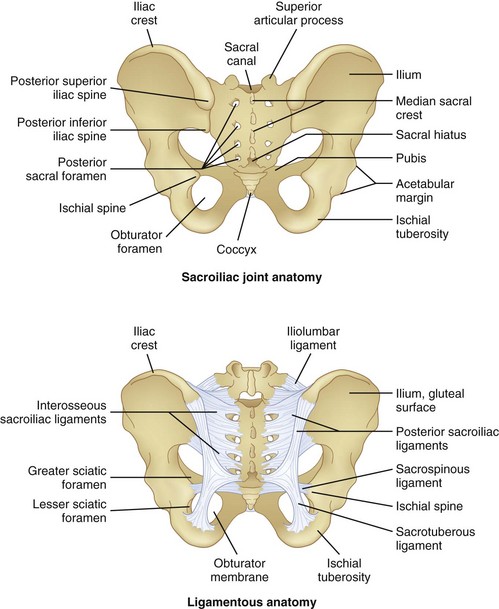
The SIJ is the largest axial joint in the body, with an average surface area of 14 to 17.5 cm2.25,26 It is a diarthrodial joint, with the anterior third being synovial and the remaining posterior aspect a syndesmosis, composed of interosseous ligament connections (Fig. 15-2). An auricular-shaped joint oriented in an oblique medial to lateral direction, morphological variability exists in the adult SIJ with respect to size, shape, and surface contour.27 The posterior aspect of the joint has a fibrous capsule but may lack synovial continuity with the anterior aspect of the joint. Designed for stability, the SIJ is a triplanar shock absorber, transmitting and dissipating upper trunk loads to the pelvis, force from the lower extremities during ambulation, and facilitates parturition. The SIJ rotates about all three axes, although the predominant motion appears to be x-axis rotation with some z-axis translation.1 Motion of the SIJ is usually limited to 1 to 3 degrees of rotation and 1.6 mm of translation, with 90 percent of rotation occurring along the x-axis.1 The SIJ motion progressively decreases, and motion at the joint may become markedly restricted secondary to increased cartilage; reduced density of chondrocytes, causing deep fissures in the cartilage; and fibrous connections among the auricular facets.28 As the capsule becomes increasingly collagenous and fibrous, ankylosis occurs, and by the eighth decade of life, erosions and plaque formation are inevitable and ubiquitous.29
Innervation
Variability and lack of precise innervation of the SIJ remains, but it has been suggested that the ventral side of the SIJ is usually supplied by L4 through S2 spinal nerves, the caudal side by the superior gluteal nerve, and the dorsal side by S1 and S2 spinal nerves.30 Others have implicated the ventral rami of L4 and L5, the superior gluteal nerve, and the dorsal rami of L5, S1, and S2.31 Some authors have even suggested that the anterior SIJ is devoid of nervous tissue32 and that the posterior SIJ is supplied by L3 and S4.33 Additionally implicated are the L5 dorsal ramus and the lateral branches of the S1-S3,34 and this description is the focus of ablative therapy in the attenuation of SIJ pain.
Basic Science
Intraarticular structures possess nerve fibers, which are sensitive for pain and support the theory that nociceptive signals may originate from the intraarticular structures of the SIJ.35 Additionally, as previously described, innervation exists for the SIJ, and the intraarticular nociceptive fibers and the lumbosacral innervation are intended for interventions. There is not a “gold standard” for the diagnosis of SIJ dysfunction, but the rationale for the use of SIJ blocks as standard for diagnosing SIJ pain is based on the fact that SIJs are richly innervated and have been shown to be capable of being a source of low back pain and referred pain in the lower extremity.36
Therapeutic interventions in the treatment of SIJ pain include manual manipulation, prolotherapy, intraarticular injections, ablative procedures, and surgical interventions. Minimally invasive procedures, including intraarticular injections and ablative procedures, are minimally destructive to anatomy. Serial intraarticular injections of local anesthetics and steroids are thought to reduce the inflammatory response and the resultant joint symptomatology. Multiple studies have evaluated the effectiveness of intraarticular injections and ablative procedures (see Outcomes Evidence). Ablative procedures have included conventional radiofrequency (RF), pulsed RF (PRF) denervation, and cooled RF denervation of the SIJ. Conventional RF37 and PRF38 of the lateral branches have been reviewed and discussed by others.
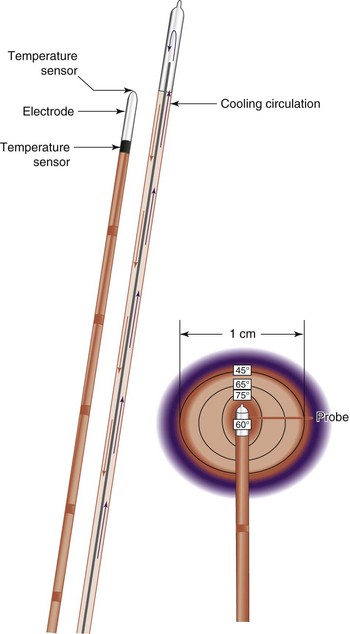
The inherent challenge when treating patients with SIJ pain with RF energy is the inconsistent location of targeted lateral branch nerves.39,40 The lateral branches supplying afferent information from pain-generating SIJs form a complex arcade of small nerve fibers anastomosing with multiple dorsal rami at each foramen. The location of these branches is unpredictable, varying from patient to patient, side to side, and level to level.39 Additionally, conventional RF tissue ablation efficacy is limited by the lesion size secondary to impedance created by tissue desiccation, tissue boiling, and carbonization around the electrode tip. Carbonization is probably the most important cause of the increased impedance and leads to an abrupt decrease in lesion current (and delivered power), such that no more energy is delivered around the electrode and no additional tissue heating occurs.41 Cooled RF energy generates heat in the surrounding tissue, and internal cooling of the electrode moderates temperature near the tip (Fig. 15-3). Internal cooling enhances lesion size by removing the constraint of high-temperature charring in tissue adjacent to the electrode, thus allowing effective ionic heating at a greater distance.42
Imaging
No imaging studies consistently provide findings that are helpful to diagnose primary SIJ pain. Computed tomography (CT), magnetic resonance imaging (MRI), and bone scan are done predominantly to exclude other causes of pain rather than to diagnose idiopathic SIJ pain.9 Because of limitations of the history, physical examination, and imaging modalities, controlled fluoroscopically-guided or CT-guided, contrast-enhanced injections may be the only methods for definitively diagnosing or excluding the SIJ as a source of pain.9
The SIJ has several unique anatomical features that make it one of the more challenging joints to image. The joint is difficult to profile well on radiographic views, and therefore the radiographic findings of sacroiliitis are often equivocal.43 MRI without and with intravenous gadolinium is currently the recommended modality for imaging patients with clinically suspected sacroiliitis and negative or equivocal radiographic findings.43 MRI does not use ionizing radiation, but CT typically uses 1 to 2 rad compared with 0.5 rad for an anteroposterior (AP) radiograph,44 and MRI can detect edema and enhancement before bone changes are visible on CT. MRI is also helpful in identifying active disease and following therapy response in patients with moderate and severe radiographic changes;43 however, CT is superior to MRI for diagnosing chronic bone changes in the superior ligamentous aspect of the SIJ.45 Imaging for diagnostic and therapeutic SIJ injections and lateral branch blocks (LBBs) requires fluoroscopic guidance, but ultrasound guidance has been used with varying success.46,47 In SIJ RF neurotomy, radiographic assistance is necessary for placement of lesioning probes.
Guidelines
SIJ injections with local anesthetic and steroid serves as a diagnostic tool and therapeutic intervention. LBBs may serve as diagnostic or prognostic procedures, for lateral branch denervation. As previously stated, controlled fluoroscopically-guided or CT-guided, contrast-enhanced injections may be the only methods for definitively diagnosing or excluding the SIJ as a source of pain. Analgesic response to a diagnostic block serves as a method to diagnose SI joint pain; however, it is imperative that before performing an SIJ injection that proper history, symptom presentation, physical examination, and imaging studies are reviewed to assess the SIJ as a pain generator and other structures as a mimicker of SIJ pain. Single diagnostic blocks carry a false-positive rate of 20%6 and 22%,48 so it is imperative to assess every patient for a true positive response and not the other factors contributing to analgesia or as a pain generator. These factors may include the placebo effect, convergence and referred pain, neuroplasticity and central sensitization, expectation bias, unintentional sympathetic blockade, systemic absorption of local anesthetic, and psychosocial issues.8
Guidelines for diagnostic and therapeutic treatment of SIJ dysfunction with injections at the SIJ and SIJ RF neurotomy has been proposed (Boxes 15-2 and 15-3).24
Box 15-2 Criteria for Diagnostic and Therapeutic Sacroiliac Joint Injections
 In the diagnostic phase, a patient may receive two procedures at intervals of no sooner than 2 weeks.
In the diagnostic phase, a patient may receive two procedures at intervals of no sooner than 2 weeks. In the therapeutic phase (after the diagnostic phase is completed), the suggested frequency is at least 2 months between injections, provided that >50% relief is obtained for 6 weeks.
In the therapeutic phase (after the diagnostic phase is completed), the suggested frequency is at least 2 months between injections, provided that >50% relief is obtained for 6 weeks. If the procedures are done for different joints, they should be performed at intervals no sooner than 2 weeks. It is suggested that therapeutic frequency remain at least 2 months for each joint. It is further suggested that both joints be treated at the same time, provided the injections can be performed safely.
If the procedures are done for different joints, they should be performed at intervals no sooner than 2 weeks. It is suggested that therapeutic frequency remain at least 2 months for each joint. It is further suggested that both joints be treated at the same time, provided the injections can be performed safely.Box 15-3 Selection and Exclusion Criteria for Sacroiliac Joint Neurotomy
Selection Criteria
 Greater than 80% pain relief from two separate intraarticular blocks with no more than 2 cc of injectate per block. It is recommended to use higher concentration anesthetic such as 0.75% bupivacaine or 4% lidocaine for a more effective block.
Greater than 80% pain relief from two separate intraarticular blocks with no more than 2 cc of injectate per block. It is recommended to use higher concentration anesthetic such as 0.75% bupivacaine or 4% lidocaine for a more effective block.Indications and Contraindications
Indications for sacral joint RF neurotomy include axial low back or buttock pain lasting longer than 3 to 6 months, tenderness overlying the SIJ, positive physical examination findings on more than two provocative maneuvers that stress the SIJ and ligaments, failure to respond to conservative therapy, pain attenuation upon SIJ corticosteroid injections, or pain relief with LBBs at L4 and L5 primary dorsal rami and at S1-S3. Cohen et al49 examined outcome predictors for RF denervation of the LBB and reported that no single variable strongly predicted outcome, suggesting that most patients with SIJ pain, irrespective of cause, can potentially benefit from the procedure.
Equipment
Sacroiliac Joint Injection
Lateral Branch Block
Cooled Radiofrequency Sacroiliac Joint Neurotomy
 Preparation kit, sterile gloves, surgical cap and mask, sterile surgical drape, 27-gauge 3.5-inch spinal needle, extension tubing
Preparation kit, sterile gloves, surgical cap and mask, sterile surgical drape, 27-gauge 3.5-inch spinal needle, extension tubingTechnique
Sacroiliac Joint Injection
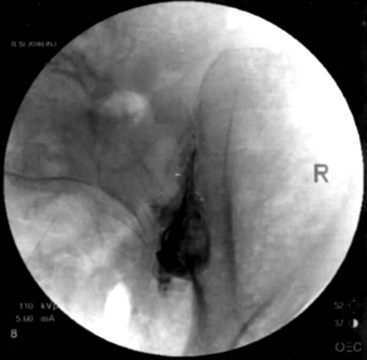
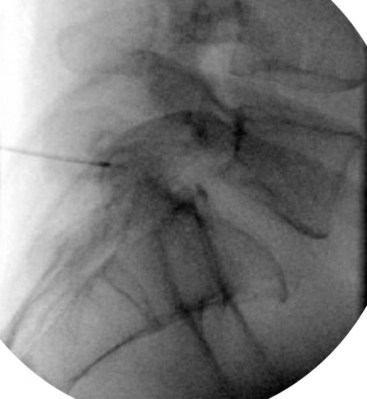
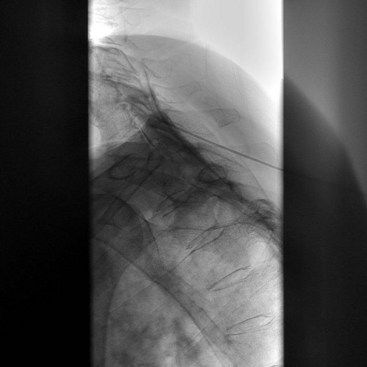
Fluoroscopy guidance is recommended and is described. The patient is placed on the fluoroscopy table in the prone position. After sterile preparation and drape have been accomplished, an AP fluoroscopic image of the SIJ is obtained. The fluoroscope is angled caudad, placing the PSIS and iliac crest overlying the joint image. The fluoroscope is obliqued to the contralateral side (20 to 30 degrees), placing the anterior and posterior aspect of the joint in line. A point along the inferior third of the joint is the target for injection. This area is anesthetized, and a 22-gauge spinal needle is advanced under coaxial technique and intermittent fluoroscopy until the joint has been entered; adjustment should be made accordingly. Entrance into the joint experienced as lack of ease to withdrawal the needle and arcing of the needle along the curvilinear joint and is confirmed by delivery of radiopaque contrast showing an arthrogram indicating intraarticular spread of contrast (Fig. 15-4). A solution containing local anesthetic and steroid is delivered showing spread of the residual intraarticular contrast. The needle is retracted from the joint, subcutaneous tissue, and skin.
Cooled Radiofrequency Sacroiliac Joint Neurotomy
Needle Placement
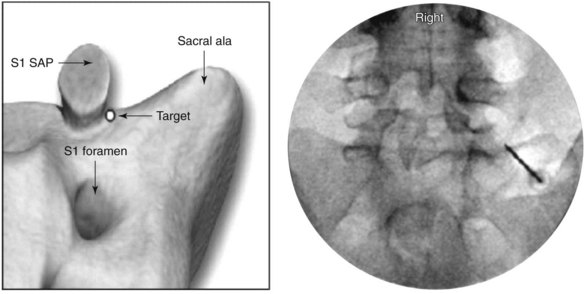
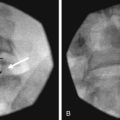
For the L5 dorsal ramus, needle placement is not necessary, and the introducer cannula is advanced without prior needle placement. The puncture point on the skin is just lateral and inferior to the target. The introducer is advanced until contact is made with the periosteum (Fig. 15-5). In the AP fluoroscopic view, the tip of the needle should be immediately adjacent to the base of the SAP and under its lateral margin. A lateral view is obtained to confirm depth of needle placement. The most ventral extent of the cannula in lateral view should be the zygapophyseal joint (Fig. 15-6). The stylet is removed, and bupivacaine 0.5% or lidocaine 2% can be delivered but is optional before lesioning. The SInergy probe is placed through the introducer cannula. The probe is 2 mm shorter than the stylet and will seat in the cannula 2 mm off of the dorsal sacral surface. Displayed on the pain management generator, impedance should not exceed 500 ohm. Impedances greater than 500 ohm may indicate that the probe is seated in tissue not suitable for lesioning or that the probe is not fully seated in the hub of the introducer cannula. The “skin stopper” is advanced along the shaft of the introducer cannula to serve as a depth marker, ensuring that the cannula and probe are not inadvertently advanced.
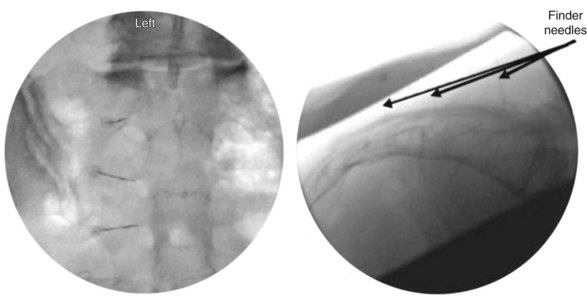
The lateral branches at S1-S3 are approached by raising a skin wheal over the skin entrance sites and subcutaneous tissue with lidocaine 1% to 2% toward the lateral aspect of S1-S3 dorsal sacral foramina. The 25- and 27-gauge spinal needles are advanced to the lateral aspect of the dorsal sacral foramina. These needles function as reference points for the placement of the introducer and probe in the absence of reliable osseous structures. A lateral fluoroscopic view is obtained to confirm entrance of the dorsal sacral foramina canal (Fig. 15-7).
Probe Placement
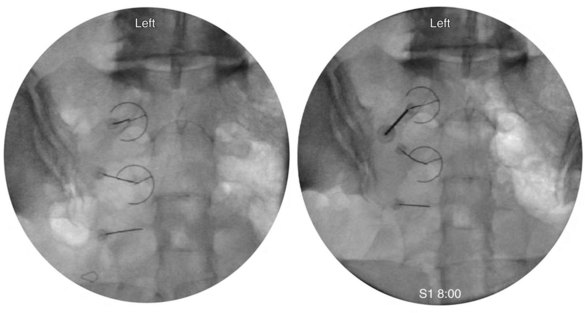
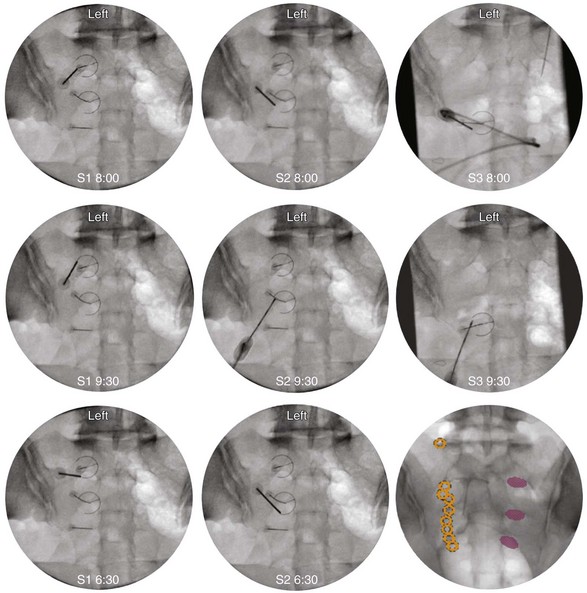
The first target for the introducer cannula placement is the 4 : 00 position for the right side and 8:00 for the left side (Fig. 15-8). Using coaxial technique and intermittent fluoroscopy, the cannula is advanced until the stylet has made contact with the dorsal sacral surface. A lateral fluoroscope view is obtained to confirm depth and contact with the dorsal surface and is not transforaminal. The stylet is removed, and bupivacaine 0.5% or lidocaine 2% can be delivered but is optional before lesioning. The SInergy probe is placed through the introducer cannula. The probe is 2 mm shorter than the stylet and will seat in the cannula 2 mm off of the dorsal sacral surface. Displayed on the pain management generator, the impedance should not exceed 500 ohm. The “skin stopper” is advanced along the shaft of the introducer cannula to serve as a depth marker, ensuring that the cannula and probe are not inadvertently advanced into the foramen. The same sequence is used to place SInergy probes at the 2:30 position and the 5:30 position for the right side (9:30 and 6:30 for left) at the S2 level. The S3 level varies in that two lesions are created by placing probes at 2:30 and 4:00 for the right side and 9:30 and 8:00 for the left side (Fig. 15-9). Because simultaneous lesions cannot be created with the present pain management generator, the sequence is repeated for each probe placement.
Patient Management and Evaluation
Postprocedure instructions are presented in Box 15-4. A benchmark for successful treatment of SIJ pain has been reported in the literature as a 50% reduction on visual analog scale (VAS) scores from baseline.40 Patients should be evaluated within 4 weeks for efficacy and complications. If bilateral neurotomies are indicated, the contralateral side can be lesioned 7 to 10 days after the initial procedure.
Outcomes Evidence
Cooled RF neurotomy for SIJ pain is novel procedure presently without substantial outcome evidence. Although a new technology, Kapural et al40 in a retrospective study investigated cooled RF of the lateral branches. The primary outcome measures were pain relief (VAS scores), changes in function (pain disability index [PDI]), and global patient satisfaction (GPE). Improvements were observed in VAS pain scores 7.1 ± 1.6 to 4.2 ± 2.5 (P <.001) and PDI from 32.7 ± 9.9 to 20.3 ± 12.1 (P <.001) at 3 to 4 months after the procedure. Of 26 patients, 18 rated their improvement in pain scores using GPE as improved or much improved, and eight claimed minimal or no improvement. In a randomized placebo-controlled study evaluating lateral branch RF denervation, Cohen et al50 used the numeric rating scale (NRS) as a primary outcome measure and Oswestry Disability Index (ODI) score, reduction in analgesic medications, and GPE as secondary measures. At 1 month, the treatment group had significantly lower NRS scores than the placebo group (2.4 ± 2.0; range, 0 to 8 vs. 6.3 ± 2.4; range, 2 to 10; P <.001). One, 3, and 6 months after the procedure, 11 (79%), nine (64%), and eight (57%) RF-treated patients experienced pain relief of 50% or greater and significant functional improvement. The authors concluded L4 and L5 primary dorsal rami and S1-S3 lateral branch RF denervation may provide intermediate-term pain relief and functional benefit in selected patients with suspected SIJ pain.
Risk and Complication Avoidance
Fluoroscopic guidance and a firm grasp of anatomy are paramount to performing this procedure safely and effectively. Reported complications from the procedure include temporary worsening of pain attributed to procedure-related pain or temporary neuritis50,51 and temporary, self-limiting (<14 days) paresthesias.49,51 Before lesioning, corticosteroid delivery may attenuate the symptoms secondary to neuritis.52
1 Slipman CW, Whyte WS2nd, Chow DW, et al. Sacroiliac joint syndrome. Pain Physician. 2001;4(2):143-152.
2 Smidt GL, Wei SH, McQuade K, et al. Sacroiliac motion for extreme hip positions. A fresh cadaver study. Spine (Phila Pa 1976). 1997;22(18):2073-2082.
3 Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. AJNR Am J Neuroradiol. 1999;20(8):1429-1434.
4 Mixter W, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
5 Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
6 Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976). 1996;21(16):1889-1892.
7 Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
8 Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
9 Dreyfuss P, Dreyer SJ, Cole A, et al. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
10 Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976). 1996;21(22):2594-2602.
11 Slipman CW, Jackson HB, Lipetz JS, et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000;81(3):334-338.
12 Fortin JD, Aprill CN, Ponthieux B, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: clinical evaluation. Spine (Phila Pa 1976). 1994;19(13):1483-1489.
13 Merskey H Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Seattle: IASP Press; 1994.
14 Forst SL, Wheeler MT, Fortin JD, et al. The sacroiliac joint: anatomy, physiology and clinical significance. Pain Physician. 2006;9(1):61-67.
15 Harrison DE, Harrison DD, Troyanovich SJ. The sacroiliac joint: a review of anatomy and biomechanics with clinical implications. J Manipulative Physiol Ther. 1997;20(9):607-617.
16 Dreyfuss P. The sacroiliac joint: a review. Int Spinal Injection Soc. 1994;2:21-58.
17 Slipman CW, Sterenfeld EB, Chou LH, et al. The predictive value of provocative sacroiliac joint stress maneuvers in the diagnosis of sacroiliac joint syndrome. Arch Phys Med Rehabil. 1998;79(3):288-292.
18 Dreyfuss P, Dryer S, Griffin J, et al. Positive sacroiliac screening tests in asymptomatic adults. Spine (Phila Pa 1976). 1994;19(10):1138-1143.
19 Laslett M, Aprill CN, McDonald B, et al. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
20 Stuber KJ. Specificity, sensitivity, and predictive values of clinical tests of the sacroiliac joint: a systematic review of the literature. J Can Chiropr Assoc. 2007;51(1):30-41.
21 Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16(10):1539-1550.
22 Szadek KM, van der Wurff P, van Tulder MW, et al. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
23 McKenzie-Brown AM, Shah RV, Sehgal N, et al. A systematic review of sacroiliac joint interventions. Pain Physician. 2005;8(1):115-125.
24 Boswell MV, Shah RV, Everett CR, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician. 2005;8(1):1-47.
25 Miller JA, Schultz AB, Andersson GB. Load-displacement behavior of sacroiliac joints. J Orthop Res. 1987;5(1):92-101.
26 Bernard T, Cassidy JD. The sacroiliac syndrome. Pathophysiology, diagnosis and management. In: Frymoyer J, editor. The adult spine: principles and practice. New York: Raven; 1991:2107-2130.
27 Bernard T, Cassidy JD. The sacroiliac joint syndrome. Pathophysiology, diagnosis and management. In: Frymoyer J, editor. The adult spine: principles and practice. New York: Raven; 1991:2343-2363.
28 Kampen WU, Tillmann B. Age-related changes in the articular cartilage of human sacroiliac joint. Anat Embryol (Berl). 1998;198(6):505-513.
29 Bowen V, Cassidy JD. Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life until the eighth decade. Spine (Phila Pa 1976). 1981;6(6):620-628.
30 Solonen KA. The sacroiliac joint in the light of anatomical, roentgenological and clinical studies. Acta Orthop Scand Suppl. 1957;27:1-127.
31 Nakagawa T. [Study on the distribution of nerve filaments over the iliosacral joint and its adjacent region in the Japanese]. Nippon Seikeigeka Gakkai Zasshi. 1966;40(4):419-430.
32 Grob KR, Neuhuber WL, Kissling RO. [Innervation of the sacroiliac joint of the human]. Z Rheumatol. 1995;54(2):117-122.
33 Murata Y, Takahashi K, Yamagata M, et al. Sensory innervation of the sacroiliac joint in rats. Spine (Phila Pa 1976). 2000;25(16):2015-2019.
34 Fortin JD, Kissling RO, O’Connor BL, et al. Sacroiliac joint innervation and pain. Am J Orthop (Belle Mead NJ). 1999;28(12):687-690.
35 Szadek KM, Hoogland PV, Zuurmond WW, et al. Possible nociceptive structures in the sacroiliac joint cartilage: an immunohistochemical study. Clin Anat. 2010;23(2):192-198.
36 Hansen HC, McKenzie-Brown AM, Cohen SP, et al. Sacroiliac joint interventions: a systematic review. Pain Physician. 2007;10(1):165-184.
37 Ferrante FM, King LF, Roche EA, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137-142.
38 Vallejo R, Benyamin RM, Kramer J, et al. Pulsed radiofrequency denervation for the treatment of sacroiliac joint syndrome. Pain Med. 2006;7(5):429-434.
39 Yin W, Willard F, Carreiro J, et al. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: technique based on neuroanatomy of the dorsal sacral plexus. Spine (Phila Pa 1976). 2003;28(20):2419-2425.
40 Kapural L, Nageeb F, Kapural M, et al. Cooled radiofrequency system for the treatment of chronic pain from sacroiliitis: the first case-series. Pain Pract. 2008;8(5):348-354.
41 Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 1996;3(7):556-563.
42 de Baere T, Denys A, Wood BJ, et al. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol. 2001;176(1):187-192.
43 Tuite MJ. Sacroiliac joint imaging. Semin Musculoskelet Radiol. 2008;12(1):72-82.
44 Carrera GF, Foley WD, Kozin F, et al. CT of sacroiliitis. AJR Am J Roentgenol. 1981;136(1):41-46.
45 Puhakka KB, Jurik AG, Egund N, et al. Imaging of sacroiliitis in early seronegative spondyloarthropathy. Assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44(2):218-229.
46 Klauser A, De Zordo T, Feuchtner G, et al. Feasibility of ultrasound-guided sacroiliac joint injection considering sonoanatomic landmarks at two different levels in cadavers and patients. Arthritis Rheum. 2008;59(11):1618-1624.
47 Hartung W, Ross CJ, Straub R, et al. Ultrasound-guided sacroiliac joint injection in patients with established sacroiliitis: precise IA injection verified by MRI scanning does not predict clinical outcome. Rheumatology (Oxford). 2009;49(8):1479-1482.
48 Manchikanti L, Singh V, Pampati V, et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001;4(4):308-316.
49 Cohen SP, Strassels SA, Kurihara C, et al. Outcome predictors for sacroiliac joint (lateral branch) radiofrequency denervation. Reg Anesth Pain Med. 2009;34(3):206-214.
50 Cohen SP, Hurley RW, Buckenmaier CC3rd, et al. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
51 Kapural L, Stojanovic M, Bensitel T, et al. Cooled radiofrequency (RF) of L5 dorsal ramus for RF denervation of the sacroiliac joint: technical report. Pain Med. 2010;11(1):53-57.
52 Dobrogowski J, Wrzosek A, Wordliczek J. Radiofrequency denervation with or without addition of pentoxifylline or methylprednisolone for chronic lumbar zygapophysial joint pain. Pharmacol Rep. 2005;57(4):475-480.