Opening Round
Case 1
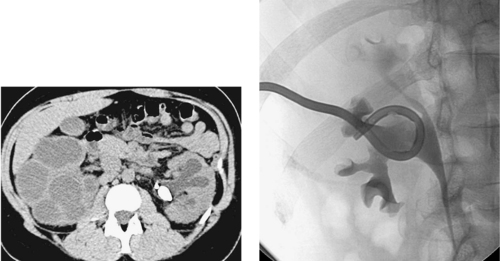 |
1. What treatment would you recommend for this 32-year-old woman with fever, flank pain, leukocytosis, and urinalysis consistent with urinary tract infection?
2. Unilateral or bilateral?
3. When should this procedure be performed?
4. What is the most common cause of this diagnosis?
ANSWERS: CASE 1
Pyonephrosis
1. Percutaneous nephrostomy tube insertion.
2. Bilateral.
3. Urgently.
4. Urinary tract stones.
Reference
Watson, R.A.; Esposito, M.; Richter, F., Percutaneous nephrostomy as adjunct management in advanced upper urinary tract infection, Urology 5 4 (1999) 234–239.
Cross-Reference
Comment
The CT examination demonstrates bilateral hydronephrosis. Although percutaneous nephrostomy tubes can be inserted electively for relief of urinary obstruction, clinical signs of infection are concerning for pyonephrosis: infected urine within the obstructed urinary collecting system. Such patients are at risk for sepsis, and percutaneous nephrostomy should be performed urgently. In the patient who already exhibits signs of sepsis, percutaneous nephrostomy can be life-saving and must be performed emergently.
Percutaneous nephrostomy tubes can be placed using a variety of imaging guidance techniques. If contrast material is already present from an existing study or if an existing ureteral stent or radiopaque renal calculus is present, then fluoroscopic guidance alone may be sufficient. Otherwise, ultrasound- or CT-guided puncture of a posterior calyx can be performed (one-pass method). Alternatively, if a dilated posterior calyx is not clearly visible, then the renal pelvis can be punctured with a 22-gauge needle. A small amount of contrast and air are injected to opacify a posterior calyx, which is then definitively punctured using an 18-gauge needle under fluoroscopic guidance (two-pass method).
Although any cause of urinary tract obstruction can lead to hydronephrosis or pyonephrosis, more than 50% of cases result from urinary tract calculi. Another common cause is ureteral compression by a pelvic mass lesion. Definitive removal of the obstructing agent should be deferred to a time when the patient’s infection has resolved, because excessive manipulation can precipitate sepsis.
Notes
Case 2
 |
1. What abnormality is seen in the first image?
2. What is the most likely etiology?
3. Would you describe this as an acute event or a chronic event?
4. How is blood reaching the lower extremities in this patient?
ANSWERS: CASE 2
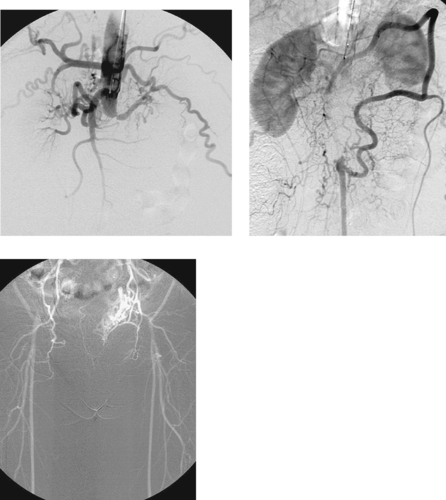
Abdominal Aortic Occlusion (Leriche’s Syndrome)
1. Occlusion of the infrarenal abdominal aorta with enlarged lumbar collaterals.
2. Atherosclerosis.
3. This most likely represents thrombosis at the site of a chronic stenosis.
4. Superior mesenteric artery to inferior mesenteric artery via an enlarged marginal artery of Drummond (second image), to the internal iliac arterial system and finally to the common femoral arteries.
Reference
Bosch, J.L.; Huninck, M.C., Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aorto-iliac occlusive disease, Radiology 204 (1997) 87–96.
Cross-Reference
Comment
Occlusion of the abdominal aorta can result from a variety of causes including trauma, thromboembolism, or iatrogenic dissection. In this case, thrombosis is superimposed on chronic atherosclerotic stenosis, a condition known as Leriche’s syndrome. In these patients, symptoms of ischemia might not appear until occlusion is imminent, and some patients present after the vessel has occluded. The classic symptoms of Leriche’s syndrome are seen in men and include hip and buttock claudication, absent femoral pulses, impotence (due to limitation of blood flow into the internal iliac artery territories), and cool lower extremities.
The slow progression of the atherosclerotic occlusion allows the development of large intercostal, lumbar and epigastric collaterals that provide flow to the iliac arteries and lower-extremity vessels. The treatment of choice for Leriche’s syndrome is surgical bypass graft placement; in this case, an aorto-bifemoral graft would be appropriate. In patients who are not surgical candidates, endoluminal recanalization and stenting of the occluded aorta and iliac arteries can be performed.
Notes
Case 3
 |
1. What is the most likely diagnosis?
2. Does this represent a vascular ring?
3. Does this finding usually indicate the presence of congenital heart disease?
4. Does this finding typically cause airway compression?
ANSWERS: CASE 3
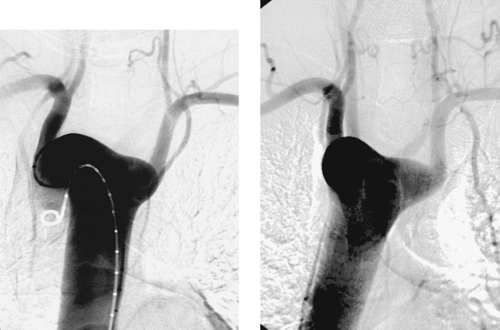
Variant Anatomy: Right-Sided Aortic Arch
1. Right aortic arch with aberrant left subclavian artery.
2. Yes.
3. No.
4. No.
References
Franquet, T.; Erasmus, J.J.; Gimenez, A., The retrotracheal space: Normal anatomic and pathologic appearances, Radiographics 22 (2002) S231–S246.
Donnelly, L.F.; Fleck, R.J.; Pacharn, P.; et al., Aberrant subclavian arteries: Cross-sectional imaging findings in infants and children referred for evaluation of extrinsic airway compression, Am J Roentgenol. 178 (2002) 1269–1274.
Cross-Reference
Comment
A right-sided aortic arch with aberrant left subclavian artery occurs in 0.05% to 0.10% of the population. The aortic arch passes over the right mainstem bronchus and descends to the right of the esophagus and trachea. The left subclavian artery arises as the last branch, often from a diverticulum of Kommerell, as in this case, and passes behind the trachea and esophagus to supply the left arm. Symptoms of respiratory and esophageal compression are seen in only 5%. Only 10% of patients have associated congenital heart disease, most commonly tetralogy of Fallot.
Right aortic arch can also be seen with mirror-image branching. In this situation, the aortic branch vessels in order are the left brachiocephalic artery, right common carotid artery, and right subclavian artery. More than 98% of these patients have cyanotic congenital heart disease, most commonly tetralogy of Fallot.
Notes
Case 4
 |
1. What vessel does this dialysis catheter traverse just before reaching the heart?
2. How does blood typically reach the right atrium in this anatomic variant?
3. Is there an association with congenital heart disease?
4. What anatomic variant is in the differential diagnosis?
ANSWERS: CASE 4
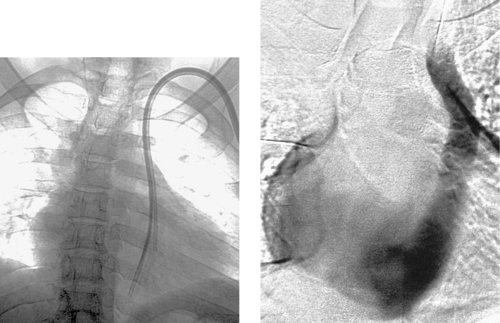
Variant Anatomy: Left Superior Vena Cava
1. Left superior vena cava.
2. Via the coronary sinus.
3. Yes.
4. Duplicated superior vena cava.
Reference
Minniti, S.; Visentini, S.; Procacci, C., Congenital anomalies of the venae cavae: Embryological origin, imaging features and report of three new variants, Eur Radiol. 12 (2002) 2040–2055.
Cross-Reference
Comment
A left superior vena cava results from persistence of the left anterior cardinal vein. This usually occurs in association with congenital heart disease, although it occurs rarely as an isolated abnormality associated with situs inversus. Typically, the left superior vena cava drains to the right atrium via the coronary sinus, but occasionally it drains directly into the left atrium.
Duplication of the superior vena cava is more common and is even more often associated with congenital heart disease. It can be associated with anomalous pulmonary venous return. Similar to the isolated left superior vena cava, the left moiety typically drains into the coronary sinus.
Notes
Case 5
 |
1. What type of examination is depicted above and how was it performed?
2. What is the primary abnormality in the right leg?
3. What surgical treatment can be used for this problem?
4. Would endovascular stent placement be a good alternative to surgery?
ANSWERS: CASE 5
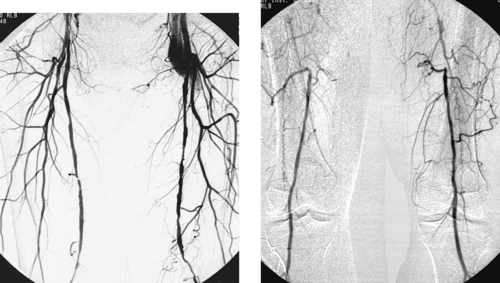
Occluded Superficial Femoral Artery
1. Bilateral lower-extremity digital subtraction arteriogram. A catheter has been placed via the right common femoral artery (CFA) into the aorta or into the right iliac artery.
2. Right superficial femoral artery (SFA) occlusion with popliteal reconstitution.
3. Femoropopliteal bypass graft placement.
4. Self-expanding nitinol stents may be used for treating this condition, but they should be reserved for patients who are not candidates for surgery.
Reference
Hunink, M.; Wong, J.; Donaldson; et al., Revascularization for femoropopliteal disease: A decision and cost-effectiveness analysis, JAMA. 274 (1995) 165–171.
Cross-Reference
Comment
The SFA represents an extremely common site of atherosclerotic disease. Stenotic lesions in this vessel are most commonly observed at the level of the adductor (Hunter’s) canal. These lesions are a common cause of calf claudication and can contribute (in the presence of other lesions) to rest pain and limb-threatening ischemia. Progressive SFA stenosis often leads to complete SFA occlusion.
When describing a lesion in the SFA, several observations are important to make: (1) The status of the ipsilateral CFA is important because this vessel nearly always represents the source vessel for a therapeutic bypass graft; (2) The point at which the distal circulation reconstitutes, as well as its continuity with pedal flow, determines the distal anastomotic site of the bypass graft; (3) The status of the ipsilateral profunda femoral artery often determines the clinical status of the limb, because this vessel provides the source for the collaterals that reconstitute the distal circulation.
The standard treatment of isolated SFA occlusion with popliteal reconstitution is femoropopliteal bypass graft placement. This procedure has a 5-year patency of 50% to 80%, depending on whether the distal anastomosis is placed above or below the knee and depending on the number and quality of patent runoff vessels. Catheter-directed thrombolysis with subsequent angioplasty and stenting can be used to recanalize native SFA occlusions, but patency rates following this procedure are less than those of surgical therapy.
Case 6
 |
1. What vessel is selectively injected?
2. What is the abnormality?
3. When is treatment recommended?
4. What treatment methods can be used?
ANSWERS: CASE 6
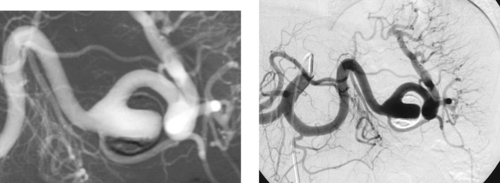
Splenic Artery Aneurysm
1. Celiac artery.
2. Splenic artery aneurysm.
3. Treatment is recommended when the aneurysm is large (>2.5cm), symptomatic, or rapidly expanding and in women who are or might become pregnant.
4. Transcatheter embolization (preferred in most cases) or surgical resection.
Reference
Stanley, J.C., Mesenteric arterial occlusive and aneurysmal disease, Cardiol Clin. 20 (2002) 611–622.
Cross-Reference
Comment
The splenic artery is the most common site of visceral artery aneurysms, followed by the hepatic artery. Splenic artery aneurysms are more common in women than men. The most common etiology is medial degeneration with superimposed atherosclerosis. There appears to be some relationship to pregnancy because the majority of women who have splenic artery aneurysms have had at least two pregnancies, and pregnancy is associated with an increased risk of rupture. Other causes include trauma, pancreatitis, infection, congenital portal hypertension, collagen vascular disease, hypersplenism, fibromuscular dysplasia, and vasculitis.
Many aneurysms are discovered incidentally on cross-sectional imaging. The main risk is aneurysm rupture, which carries a high mortality. These patients typically present with abdominal pain and/or hypotension. Nevertheless, less than 10% of aneurysms rupture, and the majority of ruptures are associated with pregnancy. Therefore, treatment is not recommended for all unruptured aneurysms.
Treatment is directed at eliminating flow into the aneurysm sac. Transcatheter embolization is the preferred treatment, and coils are the most commonly used agent. It is important to embolize from distal to proximal across the aneurysm neck to prevent retrograde flow into the aneurysm from collateral vessels filling the distal splenic artery. Splenic infarction or abscess formation is rare owing to collateral flow to the spleen. Splenic artery aneurysms can be treated surgically by removing the spleen and aneurysm or by ligating the artery proximal and distal to the aneurysm.
Case 7
 |
1. What is the main angiographic finding?
2. Is this pathologic?
3. What vascular beds most commonly demonstrate this finding?
4. What is the likely explanation?
ANSWERS: CASE 7
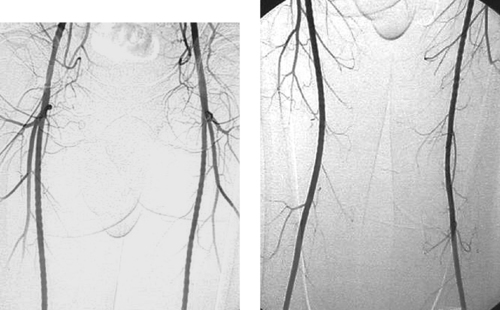
Normal Finding: Standing Waves
1. A regular corrugated appearance of the arteries, known as standing waves.
2. No.
3. The extremity arteries and mesenteric vessels.
4. Flow and pressure changes during contrast injection into a high-resistance vascular bed.
Reference
Reuter, S.R.; Redman, H.C.; Cho, K.J., Vascular diseases, In: Gastrointestinal Angiography3rd ed. (1986) Saunders, Philadelphia, pp. 120–121.
Cross-Reference
Comment
Standing waves are occasionally observed during arteriography of the extremities or mesenteric vessels. Appearing as regular alternating areas of constriction, they likely result from focal, circular vasoconstriction. Various hypotheses exist to explain this benign finding. All center on changes in flow and pressure resulting from injection of contrast media.
Case 8
 |
1. What is the likely diagnosis?
2. Is this disorder more common in men or women?
3. Name three conditions that this disorder can be associated with.
4. Name three long-term complications of this disorder.
ANSWERS: CASE 8
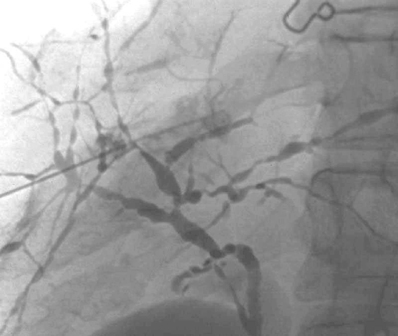
Sclerosing Cholangitis
1. Sclerosing cholangitis.
2. Men (70% of cases).
3. Inflammatory bowel disease, pancreatitis, mediastinal or retroperitoneal fibrosis.
4. Acute cholangitis episodes, biliary cirrhosis, cholangiocarcinoma.
Reference
Bader, T.R.; Beavers, K.L.; Semelka, R.C., MR imaging features of primary sclerosing cholangitis: Patterns of cirrhosis in relationship to clinical severity of disease, Radiology 226 (2003) 675–685.
Cross-Reference
Comment
Sclerosing cholangitis is an insidious progressive disease in which chronic inflammation and obliterative fibrosis affect the intrahepatic and extrahepatic biliary ductal system. Patients often present with chronic or intermittent obstructive jaundice, abdominal pain, fatigue, and/or fever. The hallmark cholangiographic findings of sclerosing cholangitis include multifocal strictures, saccular outpouchings and areas of dilation, a beaded appearance of the biliary ductal system, and/or a pruned-tree appearance of the biliary system. The common bile duct is almost always involved. The differential diagnosis of these findings includes multifocal cholangiocarcinoma and primary biliary cirrhosis.
Sclerosing cholangitis can occur as a primary idiopathic disorder or in association with other inflammatory conditions, including ulcerative colitis, Crohn’s disease, pancreatitis, retroperitoneal fibrosis, or mediastinal fibrosis. The dreaded long-term sequelae of this disorder are biliary cirrhosis with portal hypertension, and cholangiocarcinoma.
Medical management of primary sclerosing cholangitis is not particularly effective. Percutaneous biliary drainage or hepaticojejunostomy can provide palliation to carefully selected patients with appropriate anatomy, but the only truly effective treatment for this disorder is liver transplantation.
Case 9
 |
1. What type of study has been performed?
2. What is the diagnosis?
3. How long have the patient’s symptoms likely been present?
4. What treatment options might be considered?
ANSWERS: CASE 9
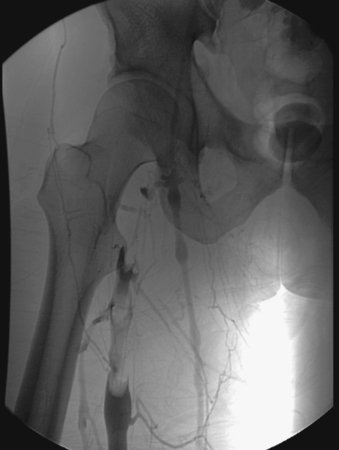
Acute Deep Vein Thrombosis
1. Lower-extremity venogram.
2. Acute femoral deep vein thrombosis (DVT).
3. Less than 2 weeks.
4. Anticoagulation is the traditional standard of care. Selected patients with acute iliofemoral DVT may be treated with catheter-directed thrombolysis or surgical thrombectomy. Elastic compression stockings should be worn for at least 2 years to prevent post-thrombotic syndrome. Patients who have contraindications to anticoagulation should undergo filter placement in the inferior vena cava for prophylaxis of pulmonary embolism.
Reference
Society of Interventional Radiology: Society of Interventional Radiology position statement Vedantham, S.; Millward, S.F.; Cardella, J.F.; et al., Treatment of acute iliofemoral deep vein thrombosis with use of adjunctive catheter-directed intrathrombus thrombolysis, J Vasc Interv Radiol. 17 (4) (2006) 613–616.
Cross-Reference
Comment
Lower-extremity DVT is a significant cause of morbidity and long-term disability. The immediate complications of DVT can include pulmonary embolism and limb-threatening phlegmasia. In the long term, a large fraction of DVT patients experience post-thrombotic symptoms, which include limb heaviness and aching, ambulatory edema, venous claudication, and lower extremity ulcerations.
The diagnosis of DVT in the femoropopliteal veins can be made with high accuracy using duplex ultrasound. In contrast, lower-extremity venography, the gold standard for the diagnosis of DVT, is only infrequently required for diagnosis. Venography is more often used to evaluate for DVT in the iliac venous system, which is often not well visualized by ultrasound. Venographic findings of acute (< 2 weeks) DVT typically include globular filling defects within a vein, abrupt occlusion of the vein, and/or dilation of the distal venous system. In this case, globular elongated filling defects are present within the femoral vein.
The standard treatment for patients with DVT is anticoagulation using low-molecular-weight or unfractionated heparin followed by coumadin for at least 3 months in most instances.
Patients with iliofemoral DVT have a particularly high risk of developing a severe form of post-thrombotic syndrome. For this reason, selected patients with acute iliofemoral DVT are treated with catheter-directed thrombolysis or surgical venous thrombectomy.
Notes
Case 10
 |
1. What abnormality is present?
2. What is the likely etiology of this abnormality?
3. How can patients with this problem be treated?
4. What imaging modality is best used for follow-up after treatment?
ANSWERS: CASE 10
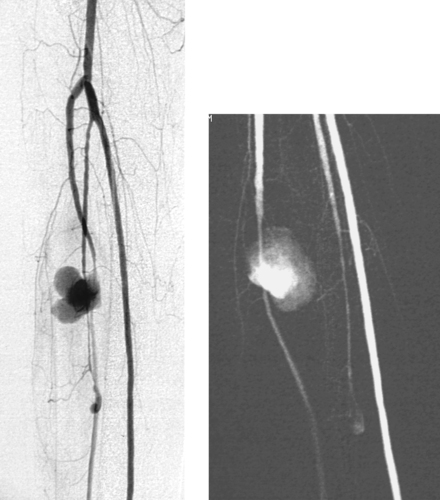
Tibial Artery Pseudoaneurysm
1. Large saccular pseudoaneurysm of the anterior tibial artery.
2. Trauma, iatrogenic vascular injury, or infection.
3. Surgical repair of the vessel is usually employed. In select cases where the vessel does not provide significant arterial supply to the foot, angiographic embolization may be performed.
4. Duplex ultrasound.
Reference
Wolford, H.; Peterson, S.L.; Ray, C.; et al., Delayed arteriovenous fistula and pseudoaneurysm after an open tibial fracture successfully managed with selective angiographic embolization, J Trauma. 51 (2001) 781–783.
Cross-Reference
Comment
Peripheral pseudoaneurysms of medium-sized vessels most commonly occur in the setting of trauma. In this case, the trauma was probably caused by Fogarty catheter embolectomy of the tibial arteries following revision of a thrombosed femoropopliteal bypass graft (not shown). Although mycotic aneurysm is rare, the possibility should be considered when saccular or irregular aneurysms are seen in the periphery.
Although duplex ultrasound can diagnose and localize the pseudoaneurysm, arteriography is important in planning appropriate therapy. It is important to determine the exact location of the entry into the pseudoaneurysm and to completely evaluate the arterial runoff to the extremity. When the injured vessel still makes a significant contribution to perfusion of the lower extremity, surgical repair of the vessel or ligation with distal bypass is clearly indicated. In select instances when this is not the case, arterial embolization may be undertaken. In this instance, it is important to place coils within the artery both proximal and distal to the origin of the pseudoaneurysm.
Notes
Case 11
 |
1. What type of examination is depicted?
2. What symptoms are likely to be present?
3. What abnormality is seen on the image?
4. How might this problem be treated?
ANSWERS: CASE 11
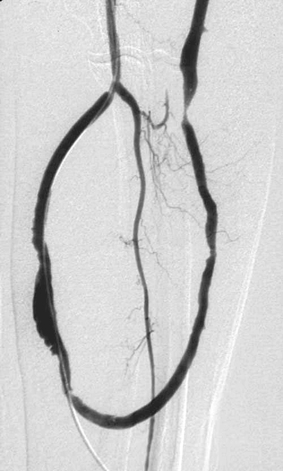
Dialysis Graft Stenoses
1. Dialysis fistulogram.
2. Low flows or high recirculation during dialysis.
3. Three stenoses are present: a tight stenosis at the venous anastomosis of the graft and two mild stenoses within the graft.
4. Angioplasty of the stenoses, or surgical graft revision.
Reference
National Kidney Foundation, Guidelines for vascular access, In: Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines (2000) National Kidney Foundation, New York.
Cross-Reference
Comment
Hemodialysis can be performed from a variety of access routes: (1) A native fistula can be created between an extremity artery—usually a radial artery or brachial artery—and an adjacent vein; (2) A prosthetic graft—usually polytetrafluoroethylene—can be placed to attach a parent artery to an outflow vein; (3) A dialysis access catheter may be placed from a suitable central vein, preferably an internal jugular vein.
When a native fistula or prosthetic graft is used, suboptimal dialysis can occur due to formation of stenotic lesions somewhere in the vascular access circuit. The venous anastomosis of the graft is by far the most common site of stenosis in patients with prosthetic grafts, as in this case. However, suboptimal dialysis can also result from flow-limiting stenoses within the graft, within the outflow veins draining the graft, within the central venous system, at the arterial anastomosis, or even in the parent artery. Such lesions, in addition to causing suboptimal dialysis, ultimately lead to graft thrombosis in the majority of cases.
Improved duration of graft patency and improved dialysis quality can be achieved via successful treatment of stenotic lesions. This can be achieved surgically or via percutaneous transluminal angioplasty. The mean graft patency following angioplasty of a venous stenosis of a patent graft is approximately 6 months, but repeat angioplasty can often delay graft replacement for several years.
Notes
Case 12
 |
1. On an anteroposterior view of an angiogram, name the three tibial runoff arteries from lateral to medial.
2. Do most patients have a true trifurcation of the popliteal artery?
3. Which vessel continues into the foot as the dorsalis pedis artery?
4. Which vessel normally terminates above the ankle level?
ANSWERS: CASE 12
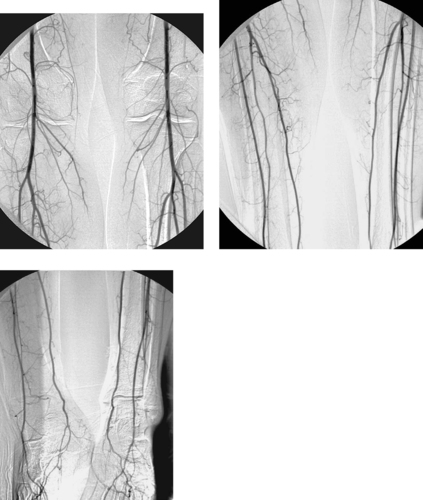
Normal Tibial Artery Anatomy
1. Anterior tibial artery, peroneal artery, and posterior tibial artery.
2. No.
3. Anterior tibial artery.
4. Peroneal artery.
Reference
Toussarkissian, B.; Mejia, A.; Smilanich, R.P., Noninvasive localization of infrainguinal arterial occlusive disease in diabetics, Ann Vasc Surg. 13 (2001) 714–721.
Cross-Reference
Comment
The popliteal artery begins distal to the adductor canal and passes through the popliteal fossa between the heads of the gastrocnemius muscle. At its terminal aspect it typically bifurcates into the anterior tibial artery and tibioperoneal trunk. After 2 to 3cm, the tibioperoneal trunk in turn bifurcates into the peroneal and posterior tibial arteries. A true trifurcation of the popliteal artery is only present in a minority of patients.
In the normal patient, the anterior tibial artery continues across the ankle as the dorsalis pedis artery; the posterior tibial artery passes behind the medial malleolus into the foot, where it divides into the lateral and medial plantar arteries. These vessels then anastomose through dorsal and plantar arches within the foot. The peroneal artery runs in the interosseous membrane and typically gives off terminal branches proximal to the ankle that anastomose with branches from the posterior tibial artery and anterior tibial artery.
Notes
Case 13
 |
1. What angiographic abnormality is depicted?
2. What symptoms are likely being experienced by the patient?
3. Does this abnormality occur more commonly on the left or the right?
4. What is the most common etiology of this abnormality?
ANSWERS: CASE 13
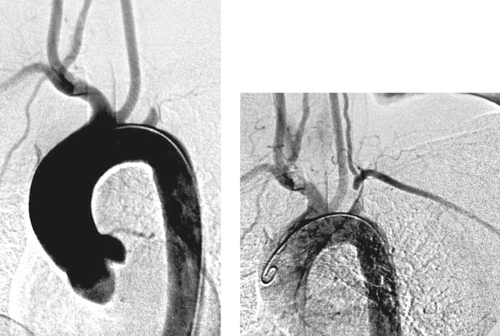
Subclavian Steal Syndrome
1. Subclavian steal syndrome.
2. Symptoms of vertebrobasilar insufficiency or, less likely, symptoms of brachial insufficiency.
3. Left (3:1 ratio).
4. Atherosclerosis.
Reference
Taylor, C.L.; Selman, W.R.; Ratcheson, R.A., Steal affecting the central nervous system, Neurosurgery 50 (2002) 679–688.
Cross-Reference
Comment
Subclavian steal syndrome can occur due to stenosis or occlusion of the proximal segment of the subclavian artery. Most commonly, this lesion causes signs of vertebrobasilar insufficiency, including syncopal or near-syncopal episodes initiated by exercising the ipsilateral arm, headaches, nausea, vertigo, and other neurological symptoms. In a minority of patients, signs of brachial insufficiency are present, including upper-extremity pain, paresthesias, coolness, weakness, or fingertip necrosis.
The diagnosis is often suspected based on the clinical history and the physical finding of diminished pulse and/or diminished systolic blood pressure in the affected limb. Duplex ultrasound often demonstrates reversal of vertebral artery flow. The classic angiographic features of this diagnosis are the presence of stenosis or occlusion of the subclavian artery proximal to the vertebral artery origin, with retrograde flow down the vertebral artery seen later in the angiographic run. The lesion can be treated with either surgical bypass or angioplasty.
Notes
Case 14
 |
 |
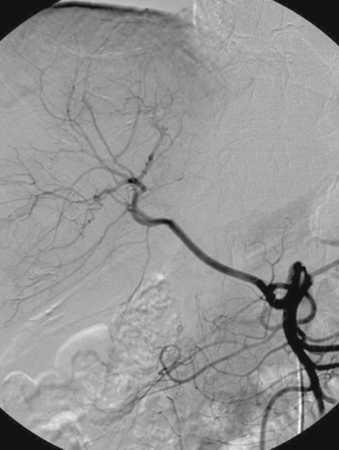
1. What anatomic variant is depicted in the first image?
2. Where does this vessel arise from?
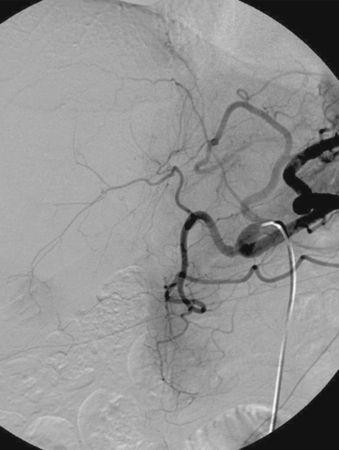
3. What anatomic variants are depicted in the second image?
4. Where do these vessels arise from?
ANSWERS: CASE 14
Variant Anatomy: Replaced Hepatic Arteries
1. Replaced right hepatic artery.
2. Superior mesenteric artery.
3. Replaced left hepatic artery and middle hepatic artery.
4. Left gastric artery and common hepatic artery.
Reference
Covey, A.M.; Brody, L.A.; Maluccio, M.A.; et al., Variant hepatic arterial anatomy revisited: Digital subtraction angiography performed in 600 patients, Radiology 224 (2002) 542–547.
Cross-Reference
Comment
It is important to recognize the common variants in the arterial supply to the liver. Vessels can be replaced or accessory, but vessels supplying the liver are typically not redundant. A replaced artery exists when the vessel supplying an entire hepatic lobe arises aberrantly. An accessory artery exists when a portion of a hepatic lobe is supplied by a vessel of normal origin but an additional vessel of aberrant origin also supplies a portion of the lobe.
Estimates vary, but approximately 15% to 25% of people have an accessory or replaced left hepatic artery arising from the left gastric artery. When it is difficult to determine whether left hepatic branches are arising from the left gastric artery, steep left anterior oblique and lateral views can help to separate hepatic branches (which run anteriorly to the left liver lobe) and gastric fundal branches.
Approximately 15% to 20% of people have an accessory or replaced right hepatic artery arising from the superior mesenteric artery. The replaced hepatic artery is almost invariably the first branch from the superior mesenteric artery in such cases. Additional rare variants have been described in which the entire hepatic arterial supply is derived from the superior mesenteric artery or aorta.
Notes
Case 15
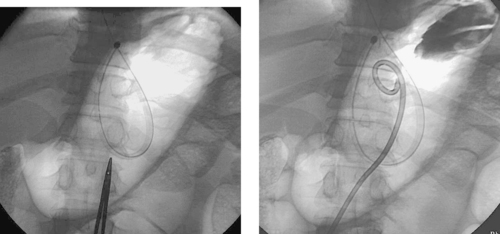 |
1. What procedure has been performed?
2. Is general anesthesia required for this procedure?
3. Name two major complications of this procedure.
4. After the catheter is placed, what is the earliest time it can be removed?
ANSWERS: CASE 15
Percutaneous Gastrostomy Placement
1. Percutaneous gastrostomy tube placement.
2. No.
3. Peritonitis and hemorrhage.
4. One month; a mature transperitoneal tract must be present.
Reference
Laasch, H.U.; Wilbraham, L.; Bullen, K., Gastrostomy insertion: Comparing the options—PEG, RIG or PIG?Clin Radiol. 58 (2003) 398–405.
Cross-Reference
Comment
Percutaneous gastrostomy tube (G-tube) placement is performed for nutritional support in patients with inadequate oral intake or for gastric decompression in patients with chronic obstruction of the small bowel. Advantages of percutaneous G-tube placement over surgical placement include elimination of general anesthesia and its associated morbidity. Endoscopic placement has a higher incidence of aspiration and wound infection.
The basic method of G-tube placement involves the following steps: (1) The stomach is insufflated with air through a nasogastric tube; (2) Fluoroscopic confirmation of a safe access window into the stomach is confirmed, with careful attention to avoiding transcolonic or transhepatic puncture; (3) Percutaneous gastropexy may be performed via insertion of two to four metallic T-fasteners, which bring the anterior gastric wall up to the anterior abdominal wall. This is done routinely in some centers and selectively in others. Selected patients include those with ascites and patients who have diminished ability to form a secure transperitoneal tract around the catheter (e.g., patients receiving steroids); (4) A needle is placed in the gastric body, and contrast is injected to confirm intragastric positioning; (5) Over a guidewire, the tract is enlarged using sequential dilators; (6) The gastrostomy catheter is positioned in the stomach and contrast is injected to confirm adequate positioning.
Complications of percutaneous G-tube placement can include peritonitis, aspiration, hemorrhage, and tube migration. Contraindications include uncorrectable bleeding diatheses, lack of a safe access window into the stomach, massive ascites, anterior gastric wall neoplasm, and the presence of a ventriculoperitoneal shunt.
Notes
Case 16
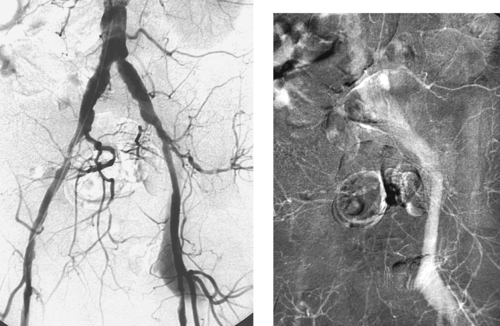 |
1. What abnormality is depicted in the images above?
2. What are the likely etiologies of such a lesion in this location?
3. What clinical problems can be associated with such a lesion?
4. What is the standard treatment for this lesion?
ANSWERS: CASE 16
Femoral Arteriovenous Fistula
1. Early filling of the left iliofemoral venous system during the early arterial phase of the angiogram, indicating a left femoral arteriovenous fistula (AVF).
2. Iatrogenic catheterization injury, trauma, postsurgical complication.
3. Vascular steal of blood from the ipsilateral limb, high-output cardiac failure.
4. Surgical repair of the femoral artery.
References
Perings, S.M.; Kelm, M.; Jax, T., A prospective study on incidence and risk factors of arteriovenous fistulae following transfemoral cardiac catheterization, Int J Cardiol. 88 (2003) 223–228.
Cross-Reference
Comment
The proper evaluation of an angiogram starts with several important observations: (1) The type of examination should be stated; (2) The catheter position and vascular approach should be noted, and the reader should specify whether nonselective or selective catheter placement has been performed; (3) The reader should be sure to observe whether the image viewed was obtained in the early arterial, late arterial, parenchymal, or venous phases of the dynamic angiographic run.
Complications of arteriography include groin infection, groin hematomas, contrast-related renal dysfunction or allergy, and arterial injuries. Injury to the femoral artery at the puncture site can result in formation of a pseudoaneurysm (which occurs in 1% of angiograms) with or without an arteriovenous fistula. The optimal site of femoral artery puncture is at the femoral head level; at this level, the common femoral artery and vein lie side by side. However, abnormally low punctures of the femoral artery can result in traversal of the femoral vein, with formation of an AVF upon removal of the catheter.
AVFs are usually asymptomatic, but they occasionally enlarge and cause arterial steal or high-output cardiac failure. Surgical ligation of femoral AVFs may be performed in patients who have symptomatic AVFs that fail to spontaneously close.
Notes
Case 17
 |
1. Is this more likely an acute or chronic process?
2. What is the most likely cause?
3. Would you expect the pulmonary artery pressures to be normal?
4. How does primary pulmonary hypertension differ on arteriography?
ANSWERS: CASE 17
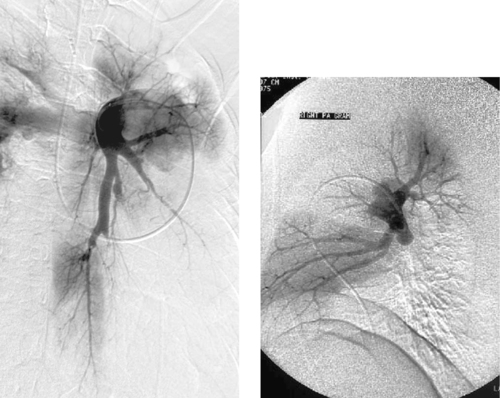
Chronic Pulmonary Embolism
1. Chronic.
2. Thromboembolic disease.
3. No.
4. In primary pulmonary hypertension, acute or organized thrombus is not seen, and there is widespread tortuosity and severe tapering of the distal arterial branches.
Reference
Hansell, D.M., Small-vessel diseases of the lung: CT–pathologic correlates, Radiology 225 (2002) 639–653.
Cross-Reference
Comment
In most patients with acute pulmonary embolism, the emboli resolve partially or completely within several weeks of the event. However, in some patients the emboli do not resolve, and 0.1% to 0.2% of patients develop pulmonary hypertension as a result of multiple episodes of pulmonary embolism. These patients typically present with dyspnea and fatigue that is often progressive. Many patients have no history of deep venous thrombosis or prior known pulmonary embolism.
Angiography remains the gold standard for diagnosis. The findings are characteristic and include enlarged central pulmonary arteries, intra-luminal webs, pulmonary arterial branch stenoses, luminal irregularities, outpouchings often with a rounded-off appearance (as in this case), regional perfusion defects, and frank obstruction of vessels. The mean pulmonary artery pressure is typically elevated.
Notes
Case 18
 |
1. What are two commonly used classification systems for this disorder?
2. What imaging modalities are most accurate in the diagnosis of this disorder?
3. Based upon the second image, what complications of this disorder might be expected in this patient?
4. What role does angiography have in the management of this disorder?
ANSWERS: CASE 18
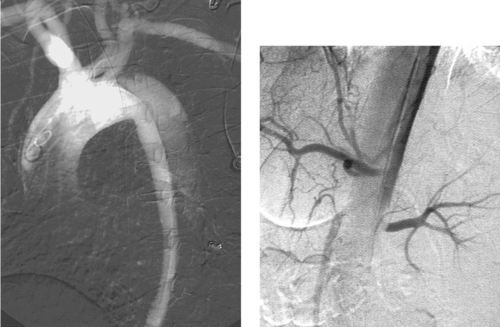
Aortic Dissection
1. DeBakey and Stanford.
2. Helical CT, magnetic resonance imaging, and transesophageal echocardiography have superseded angiography in the primary diagnosis of aortic dissection.
3. Renal and/or mesenteric ischemia.
4. Angiography is used primarily to define branch vessel anatomy and lumen of origin in patients undergoing surgery or endovascular therapy.
Reference
Vedantham, S.; Picus, D.; Sanchez, L.A.; et al., Percutaneous management of ischemic complications in patients with type-B aortic dissection, J Vasc Intervent Radiol. 14 (2003) 181–193.
Cross-Reference
Comment
Acute aortic dissection is a life-threatening disease with a mortality of about 1% per hour during the first 48 hours. The definitive diagnosis of aortic dissection is made using helical computed tomography, magnetic resonance imaging, or multiplanar transesophageal echocardiography. Angiography is used when branch vessels must be assessed before surgical aortic repair or endovascular therapy.
Stanford type A dissections involve the ascending aorta with (DeBakey type I) or without (DeBakey type II) concomitant involvement of the descending aorta. Stanford type B dissections (DeBakey type III) involve only the aorta distal to the left subclavian artery. Patients with Stanford type A dissections undergo emergent surgical ascending aortic replacement to prevent the complications of coronary artery occlusion, aortic valvular insufficiency, and rupture into the pericardial sac. Patients with uncomplicated Stanford type B dissections are treated with pharmacologic therapy to reduce the systemic blood pressure and cardiac impulse force.
Treatment options for Stanford type B dissections complicated by branch vessel ischemia include surgical aortic replacement and endovascular therapy. Endovascular interventions include balloon fenestration of the dissection flap, stenting of compromised branch vessels, and placement of aortic stents or stent grafts.
Notes
Case 19
 |
1. How would you describe the findings?
2. What endovascular treatment options might be considered for this lesion?
3. What is the immediate success rate for stenting of this lesion? What is the rate of primary patency at one year? Primary patency at 4 years?
4. What parameters would you use to select an angioplasty balloon and stent?
ANSWERS: CASE 19
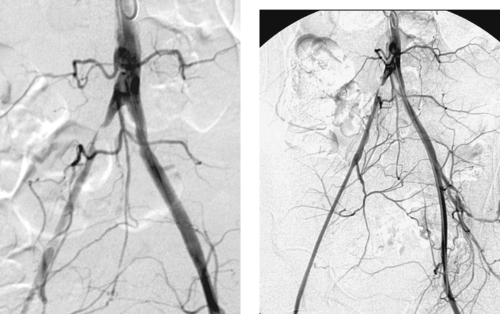
Iliac Artery Stenosis
1. High-grade stenosis of the proximal right common iliac artery.
2. Percutaneous transluminal angioplasty (PTA) or primary stenting.
3. Technical success rate is 91% to 100%. Primary patency rate is 80% to 90% at 1 year and 75% to 85% at 4 years.
4. An angioplasty balloon should be oversized by approximately 10% of vessel diameter in the iliac arteries. An expanded stent should be 1 to 2cm longer than the stenotic lesion and 1mm wider in diameter than normal adjacent vessel lumen.
Reference
Bosch, J.L.; Tetteroo, E.; Mall, W.P.; et al., Iliac arterial occlusive disease: Cost-effectiveness analysis of stent placement versus percutaneous transluminal angioplasty, Radiology 208 (1998) 641–648.
Cross-Reference
Comment
PTA and endoluminal stenting are well-accepted procedures for treating aortoiliac occlusive disease. Treatment is appropriate in patients with claudication that limits lifestyle and in patients with limb-threatening ischemia. Concentric, noncalcified stenoses less than 3cm long have the best long-term patency after treatment and often respond well to angioplasty alone.
Stent insertion following angioplasty is appropriate for greater than 30% residual stenosis, a residual systolic pressure gradient of greater than 10mm Hg at rest or greater than 20mm Hg after administration of a vasodilator, development of a hemodynamically significant dissection, or late restenosis at the angioplasty site. Primary stenting is useful when there is a higher risk of dissection or distal embolization with angioplasty alone—generally for eccentric, calcified plaques or those with small amounts of associated fresh thrombus.
Generally, the secondary patencies of iliac artery angioplasty and stenting are comparable to those of surgical reconstruction but with lower morbidity and mortality rates.
Notes
Case 20
 |
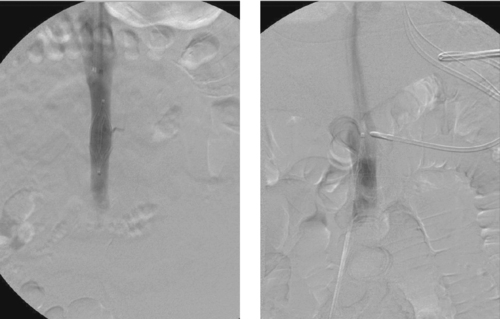 |
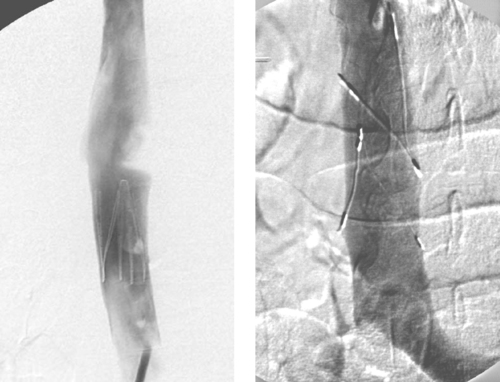
1. Name the devices in the images.
2. Are these devices retrievable?
3. In what situation is it necessary to use the device used in the second image?
4. What are the indications for placement of these devices?
ANSWERS: CASE 20
Inferior Vena Cava Filters
1. Vena Tech, bird’s nest, TrapEase, and Greenfield inferior vena cava filters.
2. No.
3. Megacava–caval diameter greater than 28mm.
4. Deep vein thrombosis (DVT) or pulmonary embolism (PE) in patients with contraindications to, complications of, or failure of anticoagulation; patients with poor cardiopulmonary status, for whom a single pulmonary embolus is likely to be fatal; patients with free-floating inferior vena cava (IVC) thrombus.
Reference
Grassi, C.J.; Swan, T.L.; Cardella, J.F.; et al., Quality improvement guidelines for percutaneous permanent inferior vena cava filter placement for the prevention of pulmonary embolism, J Vasc Intervent Radiol. 12 (2001) 137–141.
Cross-Reference
Comment
Different IVC filters vary in terms of their physical characteristics, cost, and deployment method, but all serve to prevent PE in patients with documented lower-extremity DVT and as prophylaxis in selected patients who are at high risk for developing lower-extremity DVT.
An inferior vena cavogram is performed to obtain four specific pieces of information that guide filter selection and placement: (1) Presence of IVC thrombus: This can interfere with proper seating of the filter and might mandate placement at a higher level in the IVC; (2) Location of the lowermost renal vein on each side, because the preferred location of IVC filters is immediately below the renal vein entry; (3) Presence of caval or renal vein anomalies; (4) IVC diameter: Any filter (Greenfield, Boston Scientific; Venatech, Braun; or Simon-Nitinol, Bard) can be inserted into an IVC that measures 18 to 28mm. However, between 1% and 3% of patients have a megacava, in which the IVC diameter is greater than 28mm. The TrapEase filter (Cordis) can be inserted into an IVC measuring 18 to 30mm, and the bird’s nest filter (Cook) can be inserted into an IVC measuring up to 40mm. In the rare situation where the IVC diameter is greater than 40mm, two filters can be inserted, one into each common iliac vein.
Notes
Case 21
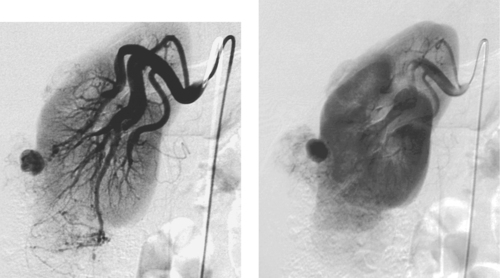 |
1. How would you describe the findings?
2. What is the likely diagnosis?
3. What complication commonly results from these lesions?
4. What congenital syndrome is found with high frequency in patients with these lesions?
ANSWERS: CASE 21
Renal Angiomyolipoma
1. Rounded enhancing parenchymal mass lesion.
2. Renal angiomyolipoma (AML).
3. Spontaneous hemorrhage.
4. Tuberous sclerosis is present in 30% to 40% of patients with angiomyolipoma.
Reference
Siegel, C., Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture, J Urol. 169 (2003) 1598–1599.
Cross-Reference
Comment
AMLs are hamartomatous lesions containing fat, smooth muscle, and blood vessels. Approximately 80% of patients with tuberous sclerosis have AMLs, which are often multiple and bilateral in these patients.
AMLs are often diagnosed on cross-sectional imaging owing to the presence of fat within a renal lesion, which nearly always indicates AML. AMLs are usually hypervascular, and this property gives them a characteristic appearance on angiography as well as strong contrast enhancement on MRI and CT. Angiographic features include hypervascularity with large, tortuous feeding arteries arranged circumferentially, occasional small arterial aneurysms, and a sunburst appearance in the parenchymal phase. Arteriovenous shunting does not commonly occur in these lesions. Angiographic differentiation from renal cell carcinoma is usually not definitive.
AMLs are generally treated if they are large or symptomatic or if they bleed spontaneously. Treatment may include surgical resection or percutaneous embolization.
Notes
Case 22
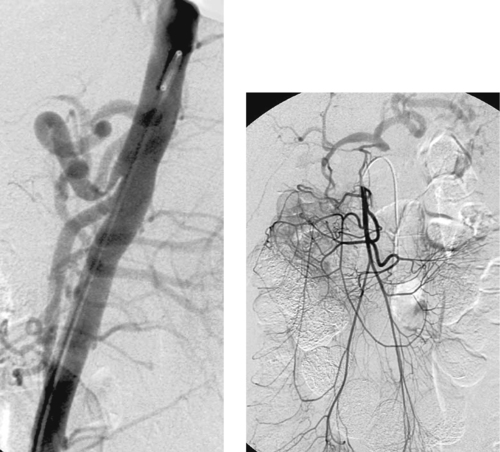 |
1. What is the diagnosis?
2. What vessel is being selectively injected in the second image? What additional vascular distribution is being filled?
3. Does the obstruction typically worsen during inspiration or expiration?
4. What is the treatment of choice?
ANSWERS: CASE 22
Median Arcuate Ligament Syndrome
1. Median arcuate ligament syndrome or celiac artery compression syndrome.
2. Superior mesenteric artery. Celiac artery territory.
3. Expiration.
4. Surgery.
Reference
Douard, R.; Ettore, G.M.; Chevalier, J.M.; et al., Celiac trunk compression by arcuate ligament and living-related liver transplantation: A two-step strategy for flow-induced enlargement of donor hepatic artery, Surg Radiol Anat. 24 (2002) 327–331.
Cross-Reference
Comment
Median arcuate ligament syndrome or celiac artery compression syndrome is the most common visceral arterial compression syndrome. Patients with this syndrome have extrinsic compression of the celiac artery by the median crus of the diaphragm and/or the celiac neural plexuses and connective tissues. The majority of patients with this diagnosis are young, thin women who are asymptomatic, despite stenoses of more than 50% diameter. However, some patients do develop crampy abdominal pain and malabsorption that has been attributed to celiac artery compression.
The arterial compression usually varies with respiration and worsens with expiration. As in this case, the compression can be severe enough that injection of the superior mesenteric artery produces celiac artery opacification in retrograde fashion via the gastroduodenal and pancreaticoduodenal arteries.
Surgery to enlarge the diaphragmatic hiatus or resect the celiac ganglion is the preferred therapy because this compression does not respond to angioplasty. Stents are contraindicated due to possible device fatigue.
Notes
Case 23
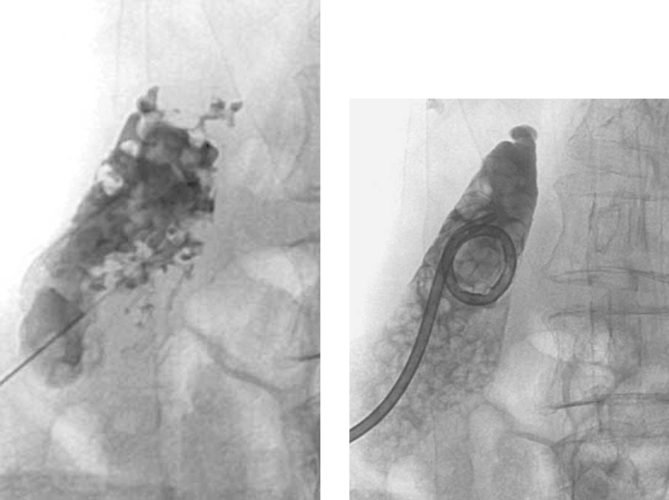 |
1. What are the imaging findings?
2. What treatment was provided?
3. What are the two possible routes of percutaneous drainage?
4. What definitive treatment options exist?
ANSWERS: CASE 23
Percutaneous Cholecystostomy
1. Multiple filling defects within the gallbladder and nonfilling of the cystic duct.
2. Percutaneous insertion of a cholecystostomy tube.
3. Transhepatic and through the free peritoneal surface of the gallbladder.
4. Cholecystectomy and percutaneous gallstone removal.
Reference
Menu, Y.; Vuillerme, M.P., Non-traumatic abdominal emergencies: Imaging and intervention in acute biliary conditions, Eur Radiol. 12 (2002) 2397–2406.
Cross-Reference
Comment
The current indications for percutaneous gallbladder drainage include acute calculous or acalculous cholecystitis, access for percutaneous stone dissolution or removal, diagnostic cholangiography, and drainage of the biliary system when the common bile duct is obstructed. For many of these patients the diagnosis of cholecystitis is difficult because the patients are unable to provide history owing to mechanical ventilation or depressed mental status.
There are two different potential routes to percutaneously drain the gallbladder, each with advantages and disadvantages. Transhepatic access is favored by many because there is a reduced incidence of bile leakage into the peritoneum due to the fixation of the gallbladder to the liver surface. However, disadvantages include the potential for liver laceration and bleeding. Therefore, some favor the transperitoneal route. The aspirated bile should be cultured, although negative cultures are found in as many as 40% of patients despite obvious cholecystitis.
To reduce the risk of sepsis, the tube should not be manipulated until the patient improves clinically. Subsequently, cholangiography via the tube is performed to establish cystic and common bile duct patency and to establish the presence or absence of stones that will require further treatment. No drainage catheter should be removed until the underlying problem has resolved and a complete fibrous tract has developed around the catheter from the gallbladder to the skin surface to prevent bile peritonitis.
Notes
Case 24
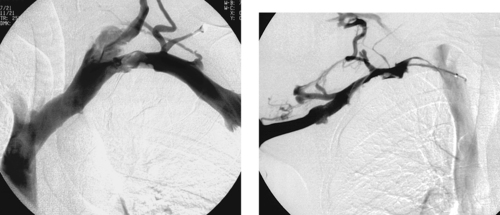 |
1. Describe the common abnormality seen in these two patients.
2. Name two common etiologies of this lesion.
3. What are the standard treatment options?
4. Is stent placement an effective treatment method?
ANSWERS: CASE 24
Subclavian Vein Occlusion
1. Subclavian vein stenosis with associated thrombus formation and collateral vein development.
2. Primary subclavian vein occlusion (this case) is caused by thoracic outlet syndrome (Paget-Schroetter syndrome). Secondary subclavian vein occlusion is currently most commonly the result of central venous catheters and pacemakers.
3. Primary subclavian vein occlusion: catheter-directed thrombolysis followed by surgical thoracic outlet decompression and anticoagulation therapy. Secondary subclavian vein occlusion: anticoagulation alone for most patients.
4. No.
Reference
Meissner, M.H., Axillary–subclavian venous thrombosis, Rev Cardiovasc Med. 3 (Suppl 2) (2002) S76–S83.
Cross-Reference
Comment
Primary axillosubclavian vein occlusion is caused by mechanical compression of the vein at its point of entry into the thorax. This disorder most commonly is seen in young patients, particularly those with well-developed musculature. The compression induces an intimal reaction in the vein, causing stenosis, which produces symptoms of upper extremity swelling and/or pain. If the occlusion is left undiagnosed, thrombosis often occurs.
The diagnosis of subclavian vein stenosis or thrombosis is usually made by venography. When evaluating a patient for primary axillosubclavian vein occlusion, it is important to perform venographic runs with the arm abducted and during arm abduction with pectoralis flexion. These maneuvers often demonstrate pronounced compression or occlusion of the vein. The disorder is commonly bilateral, so it is important to also evaluate the contralateral upper extremity.
The treatment of primary axillosubclavian occlusion centers on early removal of thrombus via catheter-directed thrombolysis, followed by surgical thoracic outlet decompression. Angioplasty of the underlying subclavian vein stenosis is not performed for fear of further injuring the subclavian vein. Stent placement is contraindicated as initial therapy because of the risk of stent fracture resulting from mechanical compression in this area.
Case 25
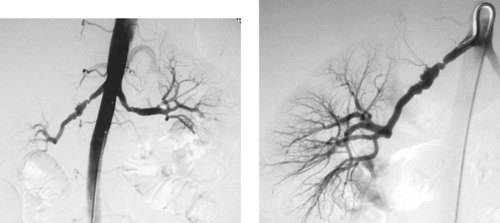 |
1. How often is this disease bilateral?
2. Is this disease more common in men or in women?
3. What arterial abnormalities can be seen?
4. What is the accepted treatment?
ANSWERS: CASE 25
Fibromuscular Dysplasia with Renal Artery Stenosis
1. Two thirds of the time.
2. More common in women than men (ratio 3:1).
3. Alternating areas of stenosis and dilation, focal or long segmental stenoses, aneurysms, dissection.
4. Percutaneous transluminal angioplasty.
Reference
Gowda, M.S.; Loeb, A.L.; Crouse, L.J., Complementary roles of color-flow duplex imaging and intravascular ultrasound in the diagnosis of renal artery fibromuscular dysplasia (FMD): Should renal arteriography serve as the “gold standard”?J Am Coll Cardiol. 41 (2003) 1305–1311.
Cross-Reference
Comment
FMD is a disorder of unknown cause that most commonly affects the renal arteries. It is the second most common cause of renovascular hypertension (behind atherosclerotic renal artery stenosis). The mid-and distal portions of the main renal artery are most commonly affected, but the entire artery can be involved. Rarely, only the proximal portion is affected. When FMD is unilateral, the right renal artery is more commonly affected. The disease involves renal artery branch vessels in nearly 20%. In less than 5% of cases, only the branch vessels are diseased.
The most common angiographic finding is the string-of-beads appearance seen in the medial fibroplasia variant. Five additional variants have been described. In decreasing order of frequency, these are perimedial fibroplasia, medial hyperplasia, medial dissection, intimal fibroplasia, and adventitial fibroplasia.
In this case, the preferred treatment modality is percutaneous transluminal angioplasty. The expected technical success is similar to angioplasty of atherosclerotic stenoses, but the expected clinical success is better. About 40% of patients are cured of their hypertension, and an additional 40% demonstrate significantly improved blood pressure control following angioplasty. The 5-year patency rates following angioplasty for renal artery FMD are about 90%. Stents are reserved for cases in which iatrogenic dissection occurs following angioplasty.
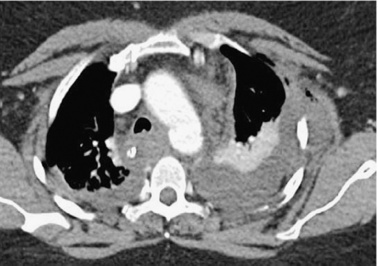
Case 26
 |
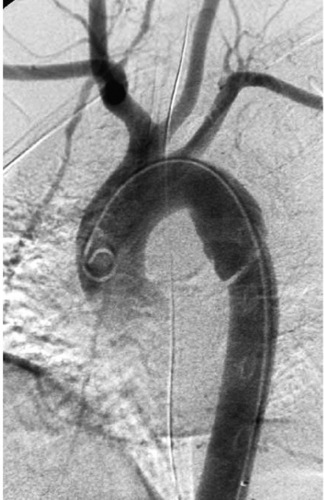 |
1. What is the most common angiographic finding in acute traumatic aortic injury (ATAI)?
2. How often is free extravasation seen at angiography?
3. What CT findings may be present in ATAI?
4. How common is great vessel injury with ATAI?
ANSWERS: CASE 26
Acute Traumatic Aortic Injury
1. Pseudoaneurysm.
2. Rarely.
3. Mediastinal hematoma, changes in aortic caliber, irregularity of aortic contour, and/or presence of an intimal flap.
4. From 15% to 30% of cases.
Reference
Fattori, R.; Napoli, G.; Lovato, L.; et al., Indications for, timing of, and results of catheter-based treatment of traumatic injury to the aorta, AJR Am J Roentgenol. 179 (2002) 603–609.
Cross-Reference
Comment
ATAI is associated with significant mortality. Aortic rupture is the cause of death in approximately 16% of motor vehicle crashes involving sudden deceleration. Only 15% of patients with ATAI live long enough to survive transfer to the hospital, and the mortality when ATAI is undiagnosed is 30% at 6 hours, 50% at 24 hours, and 90% within 4 months.
Sudden deceleration causes stress at points of maximal fixation in the aorta. The most common traumatic aortic injury seen on angiography (80%) is a laceration just distal to the left subclavian artery at the aortic isthmus, resulting in development of a pseudoaneurysm. The pseudoaneurysm typically appears as a bulge of the aortic contour that can involve the entire circumference of the aorta or only a portion of it. As in the case shown, a linear lucency representing a flap of involved intima and media may be identified. Less-common sites of injury seen on angiography include the ascending aorta (most patients with this injury expire before angiography can be obtained) and the descending thoracic aorta at the diaphragm level.
The treatment of choice for ATAI is emergency surgical aortic repair. In carefully selected patients with associated injuries and comorbidities, stent grafts have also been employed in the management of ATAI.
Notes
Case 27
 |
1. What surgical procedure has been performed?
2. What was the probable indication for selecting this procedure?
3. Does this conduit appear normal?
4. What factors are most important in determining long-term patency?
ANSWERS: CASE 27
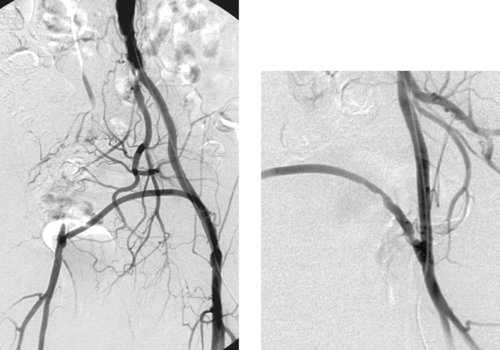
Patent Femorofemoral Bypass Graft
1. Femorofemoral bypass graft.
2. Right common iliac artery occlusion.
3. No. A stenosis is present just beyond the left femoral anastomosis.
4. The severity of disease in the inflow and outflow vessels.
Reference
Nazzal, M.M.; Hoballah, J.J.; Jacobovicz, C.; et al., A comparative evaluation of femorofemoral crossover bypass and iliofemoral bypass for unilateral iliac artery occlusive disease, Angiology 49 (1998) 259–265.
Cross-Reference
Comment
A femorofemoral bypass graft is an extraanatomic vascular bypass usually used to treat unilateral iliac artery occlusion. Extraanatomic bypass grafts are preferred in patients with unilateral iliac disease, patients who are poor candidates for surgery, patients with severe scarring from prior vascular procedures, patients with current abdominal or groin infections, or patients in whom one limb of an aortobifemoral bypass graft is occluded.
The degree of disease in the native donor and recipient arteries typically determines the long-term patency of the graft. Disease in the donor iliac artery can result in significant flow reduction to both limbs, and in severe cases it can lead to flow reversal in the graft. The presence of superficial femoral artery occlusion reduces the duration of graft patency as well as the likelihood of achieving symptomatic relief. Complications of femorofemoral bypass placement can include graft thrombosis, femoral steal phenomenon, anastomotic pseudoaneurysms, and anastomotic stenoses.
Arteriography is typically performed by catheterizing the donor femoral artery, but other approaches include the axillary artery, the translumbar aorta, and direct graft puncture. Due to the tendency for complications to occur at the anastomoses, it is important to obtain images in different projections to optimally profile these regions.
Notes
Case 28
 |
1. What is the most likely diagnosis?
2. What chromosomal abnormality has been identified?
3. How do these patients usually present?
4. What is the embolization material of choice?
ANSWERS: CASE 28
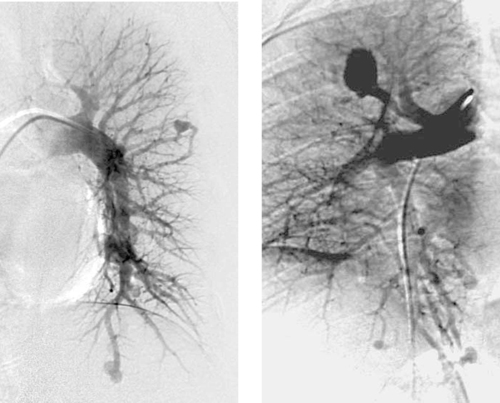
Pulmonary Arteriovenous Malformation
1. Hereditary hemorrhagic telangiectasia (HHT), which is also known as Osler–Weber–Rendu syndrome.
2. An abnormality in a gene called endoglin on chromosome 9, which codes for an endothelial cell receptor for transforming growth factor β.
3. Stroke, transient ischemic attack, or brain abscess.
4. Coils.
References
Zylak, C.J.; Eyler, W.R.; Spizamy, D.L.; et al., Developmental lung anomalies in the adult: Radiologicpathologic correlation, Radiographics 22 (2002) S25–S43.
Haitjema, T.; Disch, F.; Overtoom, T.T.; et al., Screening family members of patients with hereditary hemorrhagic telangiectasia, Am J Med. 99 (1995) 519–524.
Cross-Reference
Comment
HHT has a reported prevalence of 2 to 3 per 100,000, but it is probably more common because in many patients with mild symptoms it can go undiagnosed. The disorder has a classic triad of mucocutaneous telangiectasias, epistaxis, and autosomal dominant inheritance. About 15% of patients with HHT have pulmonary arteriovenous malformations (AVM), but the risk is higher when there is a family member with a pulmonary AVM.
Although most patients present with the central nervous system manifestations (stroke and brain abscess), the most common clinical manifestations are dyspnea, fatigue, cyanosis, clubbing, and polycythemia.
Treatment options include surgical resection of the involved lung and embolotherapy with coils or detachable balloons. Because the overwhelming majority of pulmonary AVMs have a single feeding artery and a single draining vein, with an intervening thin-walled aneurysm, the goal of therapy is to eliminate arterial inflow. This is in contrast to peripheral (nonpulmonary) AVMs, in which the goal of therapy is to eliminate the nidus when possible. Family members of patients with HHT should undergo screening.
Notes
Case 29
 |
1. What abnormality is seen?
2. Is this abnormality likely to be associated with significant symptoms?
3. What specific symptoms are likely to be present?
4. What is the preferred therapy?
ANSWERS: CASE 29
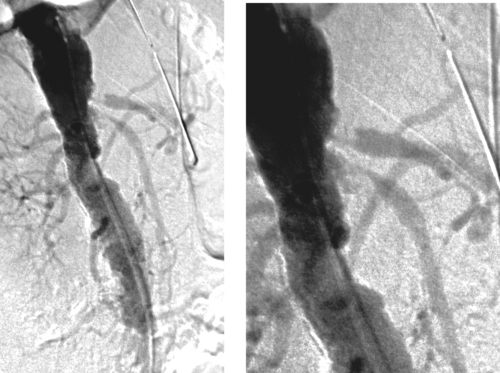
Chronic Mesenteric Ischemia
1. Tight origin stenoses of the celiac artery and superior mesenteric artery (SMA).
2. Yes.
3. Intestinal angina (postprandial abdominal pain), food fear, anorexia, weight loss.
4. Surgical aortomesenteric bypass.
References
Park, W.M.; Cherry, K.J.; Chua, H.K., Current results of open revascularization for chronic mesenteric ischemia: A standard for comparison, J Vasc Surg. 35 (5) (2002) 853–859.
Matsumoto, A.H.; Angle, J.F.; Spinosa, D.J., Percutaneous transluminal angioplasty and stenting in the treatment of chronic mesenteric ischemia: Results and longterm followup, J Am Coll Surg. 194 (1 Suppl) (2002) S22–S31.
Cross-Reference
Comment
Chronic mesenteric ischemia is less common than acute mesenteric ischemia. The primary cause is atherosclerosis, usually due to aortic plaques that involve the mesenteric artery ostia. These stenoses are typically circumferential and can exhibit post-stenotic dilation. The stenosis can progress to thrombotic occlusion. Most patients are elderly women with risk factors for atherosclerosis. In addition to the classic symptoms listed above, some patients develop nausea, vomiting, and/or diarrhea due to malabsorption. Physical examination is typically unremarkable, although an epigastric bruit may be heard.
It is generally agreed that the diagnosis requires that at least two of the three mesenteric arteries to have significant stenoses or occlusions, as seen in this case, because the splanchnic system has efficient collateral channels. However, milder degrees of stenosis can become symptomatic when cardiac output is reduced. Typically, enlarged collaterals are identified at arteriography because the disease is chronic. The main collaterals between the celiac and SMA circulations are the gastroduodenal and pancreaticoduodenal arteries. The primary collaterals between the SMA and IMA circulations are the marginal artery of Drummond and the arc of Riolan. The IMA can receive flow from the internal iliac arteries via the hemorrhoidal arterial system as well.
Surgical revascularization remains the mainstay of therapy. However, balloon angioplasty with or without stent insertion is an alternative in patients who are poor surgical candidates.
Notes
Case 30
 |
1. What is the diagnosis?
2. What is the best method to diagnose and initially characterize this lesion?
3. For what purpose is angiography being performed?
4. What treatment methods could be used in this patient?
ANSWERS: CASE 30
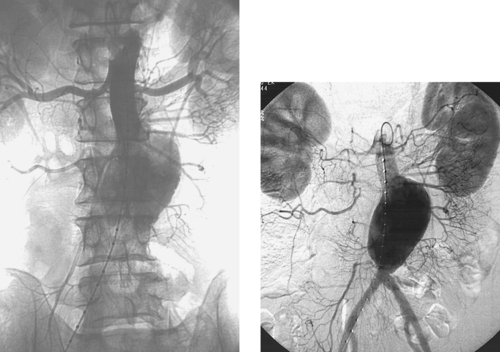
Abdominal Aortic Aneurysm
1. Abdominal aortic aneurysm (AAA).
2. Cross-sectional imaging modalities including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI).
3. To evaluate the relationship of the aneurysm to branch vessels, to determine if the patient is a candidate for endovascular aneurysm repair, and to make the relevant measurements in the event endovascular repair is used.
4. Surgical repair would likely consist of aortobifemoral bypass graft placement. Endovascular stent-graft placement is another option.
Reference
Golzarian, J., Imaging after endovascular repair of abdominal aortic aneurysm, Abdom Imaging. 28 (2003) 236–243.
Cross-Reference
Comment
AAAs usually result from atherosclerosis. The most important complications of AAAs are aneurysm rupture and distal embolization. Rupture carries a high mortality rate, and rupture risk increases with larger aneurysm size. AAA treatment is indicated for aneurysms greater than 5cm in diameter or those that cause distal embolization; a lower size threshold is used in women and in patients with connective tissue disorders.
Imaging diagnosis of AAA is usually made by ultrasonography or CT, both of which are highly sensitive for AAA. These modalities enable precise diameter measurements of the aneurysm and adjacent normal aortic segments to be obtained. In contrast, when thrombus is present within an aneurysm sac, arteriography can be insensitive for the diagnosis of AAA and often underestimates aneurysm diameter. CT angiography is invaluable in obtaining measurements needed for planning stent-graft repair of AAAs. Arteriography is primarily used intraprocedurally to evaluate visceral branch vessel relationships to the aneurysm, to evaluate the suitability of the iliac arteries as access vessels for stent-graft placement, and to enable proper stent-graft device selection and sizing.
The traditional standard treatment of AAA is operative repair with bypass graft placement. In recent years, endovascular stent-graft placement has been employed in patients with suitable anatomy and is clearly indicated in high-risk patients with medical contraindications to aortic surgery. Compared with operative repair, stent grafts have demonstrated diminished periprocedural morbidity and hospital stay.
Notes
Case 31
 |
1. What is the arteriographic diagnosis?
2. What additional radiographic findings are present?
3. What is the preferred treatment of the vascular injury?
4. Why is this preferred over surgery?
ANSWERS: CASE 31
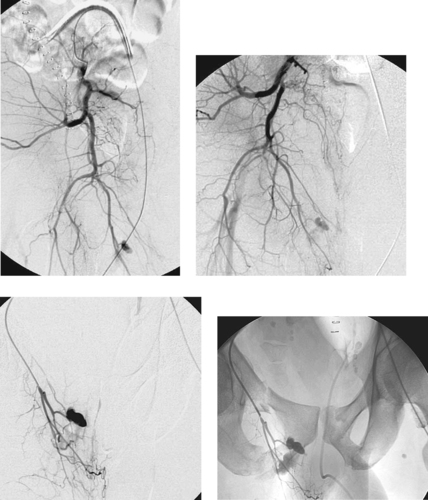
Pelvic Trauma with Pseudoaneurysm
1. Pseudoaneurysm and active extravasation from a right internal iliac artery branch.
2. Fractures of the right superior and inferior pubic rami and the left pubis.
3. Transcatheter embolization.
4. Operative exploration can lead to uncontrolled bleeding due to loss of the tamponade effect of hematoma below the pelvic peritoneum. Surgery has a high failure rate and an increased risk of infection in this clinical setting.
Reference
Velmahos, G.C.; Toutozas, K.J.; Vassiliu, P.; et al., A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries, J Trauma. 53 (2002) 303–308.
Cross-Reference
Comment
Blunt pelvic trauma usually results from motor vehicle crashes, falls from a height, and crush injuries. Approximately 10% of patients with pelvic fractures have pelvic bleeding that requires therapy. These patients are often hypotensive and have multiple organ injuries. Prompt treatment of active hemorrhage is necessary because mortality is primarily related to hemorrhage and sepsis. Commonly injured vessels are the superior gluteal and internal pudendal arteries, and injury results from adjacent pelvic fractures.
The focus of extravasation may be evident on nonselective angiography of the abdominal aorta. However, it is necessary to select both internal iliac arteries to exclude a vascular injury, and the entire pelvis including the femoral regions should be studied because there may be multiple sites of bleeding. Arteriographic findings can include contrast extravasation, pseudoaneurysm, vasospasm, vascular occlusion, and hematoma (displacement, compression, and/or stretching of arterial branches), and/or arteriovenous fistula.
Transcatheter embolization with coils or Gelfoam is the preferred treatment. Both internal iliac artery branches should be treated if the bleeding site is midline so as to prevent continued hemorrhage from collateral flow. Selective embolization is preferred if possible, but in the unstable patient, embolization of the proximal internal iliac artery may be necessary and is usually well tolerated.
Notes
Case 32
 |
1. What is the most likely diagnosis based on this CT examination performed in a young woman with fevers and leukocytosis 8 days following colorectal surgery?
2. What are the main treatment options?
3. What preprocedure management is appropriate?
4. What criteria determine the timing of catheter removal?
ANSWERS: CASE 32
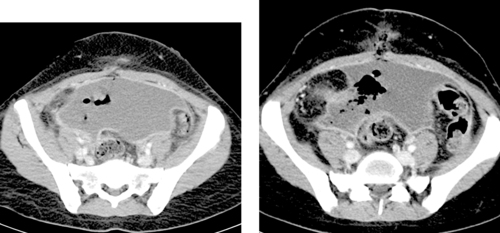
Percutaneous Abscess Drainage
1. Intraabdominal abscess.
2. Percutaneous catheter drainage or surgical exploration and washout.
3. History and physical, evaluation of coagulation profile, and administration of antibiotics appropriate to cover the likely infectious organisms.
4. Clinical improvement of the patient, reduction of drain catheter output, resolution of the fluid collection, collapse of the cavity, and absence of fistula.
Reference
Lee, M.J., Non-traumatic abdominal emergencies: Imaging and intervention in sepsis, Eur Radiol. 12 (2002) 2172–2179.
Cross-Reference
Comment
Percutaneous catheter drainage of an infected fluid collection is a common procedure performed by the interventional radiologist. Percutaneous drainage has nearly replaced surgical drainage as the treatment of choice for abscesses or other fluid collections because it is less invasive and has lower morbidity and expense. A safe route to the fluid collection is required for percutaneous drainage, and typically fluoroscopy, ultrasound, or CT can be used to guide safe passage of the needle so that vital structures are not transgressed. The best candidate fluid collections are those that are unilocular, well-defined, and free-flowing. More-complex collections (e.g., multilocular or debris laden) can be drained percutaneously, but complete drainage may be slow or impossible.
Because drainage of infected collections can cause transient bacteremia, all patients should receive antibiotics before and after the procedure. A sample of the fluid should be acquired for culture, so that the antibiotics can be tailored to treat the organisms involved. The clinical condition of most patients improves significantly within 24 to 48 hours of effective drainage. Follow-up imaging is necessary to establish satisfactory drainage and exclude the presence of undrained components or fistulas, particularly when the clinical condition does not improve or there is continued daily output that does not taper in volume
Notes
Case 33
 |
1. What anatomic variant is depicted in the first image?
2. What caconym is often used to describe this variant?
3. What anatomic variant is depicted in the second image?
4. How common are these two variants?
ANSWERS: CASE 33
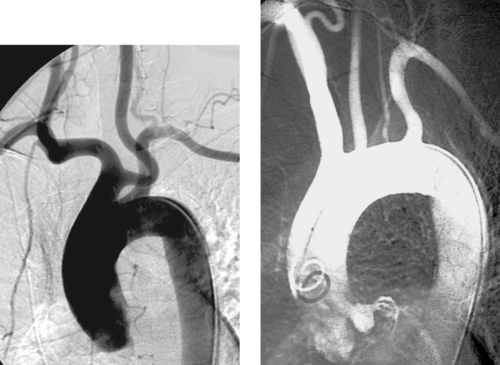
Variant Anatomy: Aortic Arch
1. Common origin of the brachiocephalic artery and left common carotid artery.
2. Bovine arch.
3. Origin of the left vertebral artery directly from the aortic arch.
4. Bovine arch in 15% to 25% of the population, left vertebral artery origin from the aortic arch in 5% of the population.
Reference
Morgan-Hughes, G.; Roobottom, C.; Ring, N., Anomalous aortic arch anatomy: Three dimensional visualisation with multislice computed tomography, Postgrad Med J. 79 (929) (2003) 167.
Cross-Reference
Comment
Normally there are three branch vessels arising from the aortic arch. In order, these are the brachiocephalic artery, the left common carotid artery, and the left subclavian artery. Although this is the typical configuration, it is seen in only about 70% of patients. Multiple anatomic variants comprise the remainder. The most common of these is a common origin of the right brachiocephalic and left common carotid arteries. Following this in decreasing order of frequency include direct origin of the left vertebral artery from the aortic arch, common origin of the common carotid arteries, presence of two brachiocephalic arteries, and separate origin of each of the four great vessels from the aortic arch.
Notes
Case 34
 |
1. What procedure was performed between the times at which the two images above were obtained?
2. Based upon the first image alone, if no other vascular lesions were present, what symptom did the patient likely have?
3. Based upon the first image alone, if no other vascular lesions were present, what was the patient’s ankle-brachial index (ABI) likely to be?
4. Is the procedure that was performed associated with a higher patency rate than the surgical procedure that would be used to treat this problem?
ANSWERS: CASE 34
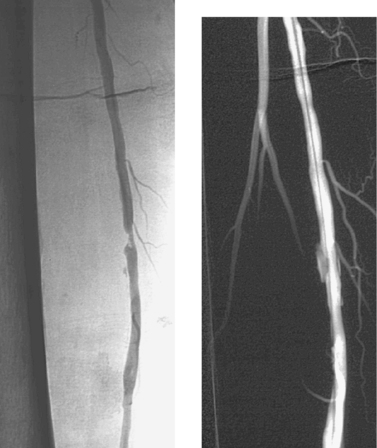
Superficial Femoral Artery Stenosis
1. Percutaneous balloon angioplasty of a right superficial femoral artery stenosis.
2. Right calf claudication.
3. Likely 0.5 to 0.9.
4. No. In the superficial femoral artery, the expected patency following angioplasty is lower than that associated with femoropopliteal bypass. However, the morbidity of angioplasty compares favorably with that of surgery.
Reference
Hunink, M.; Wong, J.; Donaldson, M.; et al., Revascularization for femoropopliteal disease: A decision and cost-effectiveness analysis, JAMA. 274 (1995) 167–171.
Cross-Reference
Comment
In general, patients with a single vascular level of disease are likely to experience intermittent claudication without rest pain and are likely to have an ABI of 0.5 to 0.9. Patients with rest pain nearly always have more than one level of vascular disease (for example, iliac artery disease and femoral artery disease), and they typically have an ABI under 0.4.
The major indications for invasive treatment of lower-extremity atherosclerotic disease are limb-threatening rest pain, lifestyle-limiting intermittent claudication, and the presence of a lesion that is suspected of being a source of distal embolization.
The expected 5-year patency of a femoropopliteal bypass graft is 50% to 80% depending upon the level of the distal anastomosis and the quality of the runoff vessels. In contrast, the expected 2-year patency of a superficial femoral artery angioplasty procedure is 50% to 70%, with 5-year patency well under 50%. However, the morbidity of angioplasty is significantly lower than that of surgical bypass grafting, and bypass can always be performed following failure of angioplasty or recurrence after it. For these reasons, patients with isolated superficial femoral artery stenoses thought to be amenable to angioplasty (short, concentric stenoses) are often treated with angioplasty first. Nitinol stents can be used to improve patency in patients who have poor technical results with angioplasty or in more-complex lesions.
Notes
Case 35
 |
1. What pathologic abnormality is present?
2. What normal variant is present?
3. If an inferior vena cava (IVC) filter is required, where should it be placed?
4. Is this patient at significant risk for pulmonary embolism?
ANSWERS: CASE 35
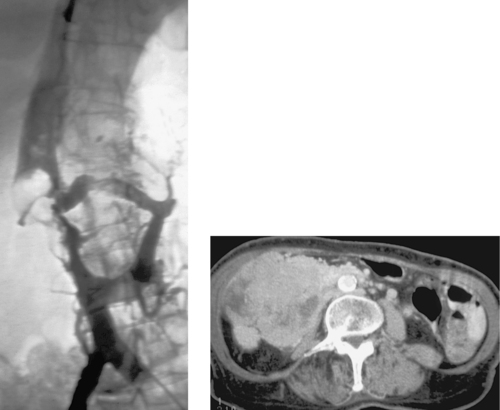
Inferior Vena Cava Thrombosis
1. Acute thrombosis of the inferior vena cava.
2. Circumaortic left renal vein.
3. Suprarenal IVC.
4. Yes.
Reference
Razavi, M.K.; Hansch, E.C.; Kee, S.T.; et al., Chronically occluded inferior venae cavae: Endovascular treatment, Radiology 214 (2000) 133–138.
Cross-Reference
Comment
The etiologies of IVC thrombosis include IVC filter placement, abdominal malignancy with mass effect upon the IVC, sepsis, dehydration, and hypercoagulability. Depending upon collateral formation and the extent of venous involvement, the symptoms can include bilateral lower-extremity and lower-body wall edema and lower-extremity pain. Anticoagulation therapy is typically given to minimize the acute symptomatology and to decrease the substantial risk of pulmonary embolism. In carefully selected patients, catheter-directed thrombolysis can be used to achieve venous recanalization as well.
Abdominal tumors can cause IVC thrombosis via compression or outright invasion. The most common histology of such lesions is renal cell carcinoma, although other lesions such as hepatocellular carcinoma, adrenal carcinoma, nodal metastases, and IVC leiomyomas (as in this case) can cause IVC thrombosis as well. In patients with renal cell carcinoma, the presence of IVC involvement does not usually preclude surgical resection.
Notes
Case 36
 |
1. Why might the study above have been performed?
2. What main findings are present?
3. How often does this anatomic variant occur?
4. With conventional anatomy, which kidney is harvested for transplant and why?
ANSWERS: CASE 36
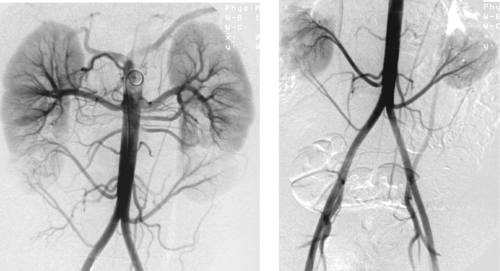
Variant Anatomy: Multiple Renal Arteries
1. Transplant donor, evaluation for possible renal artery stenosis (due to hypertension or chronic renal insufficiency).
2. Three renal arteries on the right and two renal arteries on the left.
3. About 30% of patients have more than one renal artery.
4. All other factors being equal, the left kidney is generally harvested for transplantation because the longer left renal vein facilitates easier anastomosis.
Reference
Liem, Y.S.; Kock, M.C.; Ijzermans, J.N.; et al., Living renal donors: Optimizing the imaging strategy–decision and cost-effectiveness analysis, Radiology 226 (2003) 53–62.
Cross-Reference
Comment
Patients being considered as donors for renal transplantation generally undergo preoperative angiographic evaluation directed at determining several specific pieces of information. (1) The size and number of renal arteries on each side and the presence of any anatomic variants: Generally, kidneys with a single renal artery of significant size are preferred; (2) The presence of early bifurcation of the renal artery: This is important because there must be sufficient room to place a clamp (which measures approximately 1cm in width) across the donor main renal artery before its division; (3) The presence of stenosis or any evidence of fibromuscular dysplasia: Patients with these disorders are not considered candidates for transplantation; (4) The presence of parenchymal or ureteral anomalies that are not detected on other imaging examinations.
Multiple renal arteries occur in 30% of patients, and they represent the most common anomaly of the renal arteries. Accessory renal arteries most commonly arise from the aorta inferior to the main renal artery, but they can arise anywhere in the abdominal aorta or from the iliac arteries, as in the case depicted above. If the flush aortogram does not clearly demonstrate what parenchymal distribution is supplied by a particular artery suspected of providing renal supply, then selective arteriography can be performed to clarify this.
In recent years, many institutions rely instead upon noninvasive modalities such as MR angiography or CT arteriography to evaluate renal donors.
Case 37
 |
1. What is the diagnosis?
2. Where is this abnormality typically located?
3. How common is this abnormality?
4. What percentage of lower gastrointestinal (GI) bleeds are attributable to this diagnosis?
ANSWERS: CASE 37
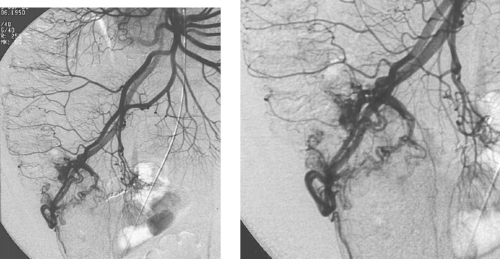
Angiodysplasia
1. Angiodysplasia.
2. Right colon, especially the cecum.
3. About 2% of autopsies.
4. From 3% to 5%.
Reference
Junquera, F.; Quiroga, S.; Saperas, E.; et al., Accuracy of helical computed tomographic angiography for the diagnosis of colonic angiodysplasia, Gastroenterology 119 (2000) 293–299.
Cross-Reference
Comment
Angiodysplasia is a vascular abnormality that may be the cause of chronic intermittent GI bleeding or, rarely, acute massive bleeding in up to 50% of persons older than 55 years. However, because it has been identified incidentally in 15% of nonbleeding patients at mesenteric angiography, the presence of angiodysplasia does not confirm that it is the source of bleeding. Active contrast extravasation is only identified in 10% of cases. Therefore, in the evaluation of GI bleeding, one should diligently search for an alternate cause of bleeding if angiodysplasia without active extravasation is identified.
Although these lesions can be located anywhere along the GI tract, they are typically located within the right colon and particularly within the cecum. They range in size from very tiny to very large, as in this case. The imaging characteristics include a vascular tuft or tangle of vessels with early, intense filling of the draining vein that then slowly empties. To make the diagnosis one needs to see simultaneous opacification of the artery and vein that, because they run in parallel, typically creates a tram-track appearance. This lesion can be missed at colonoscopy, so angiography is very useful in diagnosis.
Because of the abnormal vessels, bleeding from angiodysplasia is typically not responsive to vasopressin infusion. Surgical resection is curative. Embolization has been attempted with variable success.
Case 38
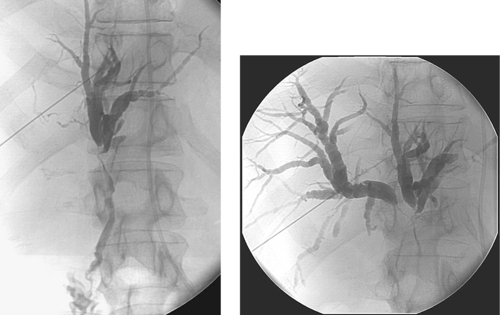 |
1. What examination is depicted?
2. Where should the needle be inserted to evaluate the right hepatic ducts?
3. What is the differential diagnosis?
4. What are the major complications of this procedure?
ANSWERS: CASE 38
Cholangiocarcinoma (Klatskin Tumor)
1. Percutaneous transhepatic cholangiogram (PTC).
2. Low intercostal approach (preferably below the 10th rib) in the right midaxillary line.
3. Cholangiocarcinoma, gallbladder cancer, hepatocellular carcinoma, metastases, extrinsic adenopathy.
4. Biliary sepsis and hemorrhage.
Reference
Szklaruk, J.; Tamm, E.; Charnsangevej, C., Preoperative imaging of biliary tract cancers, Surg Oncol Clin N Am. 11 (2002) 865–876.
Cross-Reference
Comment
PTC has largely been replaced by endoscopic retrograde cholangiopancreatography (ERCP). The primary indications for PTC are evaluation and treatment of biliary obstructive disease in symptomatic patients who are not amenable to or who fail ERCP.
Bacterial overgrowth is common in patients with biliary obstruction. Therefore, all patients should receive preprocedure antibiotics to cover gram-negative species, even in the absence of overt signs and symptoms of cholangitis. A bile duct sample should be sent for culture. Aspirated bile can be sent for cytologic analysis when neoplasm is suspected. Material can also be acquired using a brush biopsy device or fine needle aspiration. Larger specimens can be obtained using biopsy forceps or atherectomy devices.
If it is necessary to drain the liver percutaneously, a peripheral biliary radical should be selected from the cholangiogram images for needle and subsequent catheter insertion, if the initial needle entry site is unfavorable. Manipulation should be minimized in patients with signs and symptoms of suppurative cholangitis, and in these patients temporary external drainage is preferred. Definitive internal drainage via internal–external biliary drains or internal stents may be delayed several days to allow resolution of cholangitis.
Contraindications to PTC include uncorrectable coagulopathy and massive ascites.
Notes
Case 39
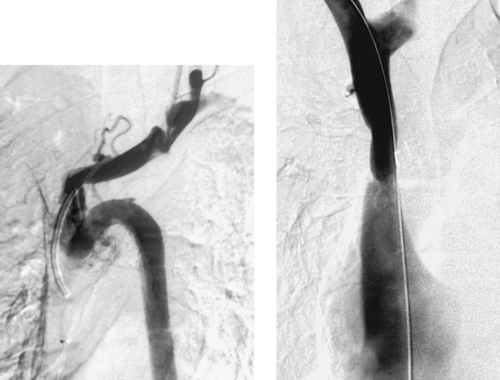 |
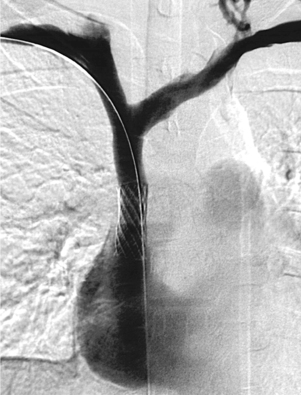 |
1. What symptoms are experienced by the patient with the venograms in the first two images?
2. What is the most common underlying etiology of this syndrome?
3. What other risk factors can contribute to the development of this abnormality?
4. What endovascular treatment modality is effective in treating this abnormality?
ANSWERS: CASE 39
Superior Vena Cava Obstruction
1. Swelling of the face, head, neck, and arms.
2. Intrathoracic malignancy.
3. Central venous catheters, pacemakers, prior radiation therapy.
4. Placement of an endovascular stent (seen in the third image).
References
Nicholson, A.A.; Ettles, D.F.; Arnold, A.; et al., Treatment of malignant superior vena cava obstruction: Metal stents or radiation therapy, J Vasc Intervent Radiol. 8 (1997) 781–788.
Vedantham, S., Endovascular strategies for superior vena cava obstruction, Tech Vasc Intervent Radiol. 3 (2000) 29–39.
Cross-Reference
Comment
Patients with superior vena cava obstruction (SVCO) commonly experience pronounced facial and arm swelling, headaches, hoarseness, dysphagia, and dyspnea. In severe cases, SVCO can cause syncope, visual and cognitive disturbances, seizures, and even coma. The most common cause of SVCO is intrathoracic malignancy, commonly bronchogenic carcinoma or lymphoma. Other risk factors for the development of SVCO include the use of central venous catheters, pacemakers, and prior radiation therapy.
Patients with malignant SVCO are typically treated with external-beam irradiation with or without adjunctive chemotherapy depending upon tumor histology and stage. This approach produces symptomatic improvement in 60% to 75% of patients within 2 to 4 weeks. Patients with benign causes of SVCO are usually treated with anticoagulation, and selected patients are offered surgical venous bypass.
Endovascular methods have been used to treat SVCO with good short-term success. When SVCO is caused by SVC stenosis without thrombus formation (as in the top right-hand image), endovascular stents can be placed (bottom image). When SVC thrombosis is present, catheter-directed thrombolysis can first be used to remove the thrombus before the stent is placed. Response to endovascular therapy occurs in 95% of patients within a few days.
Notes
Case 40
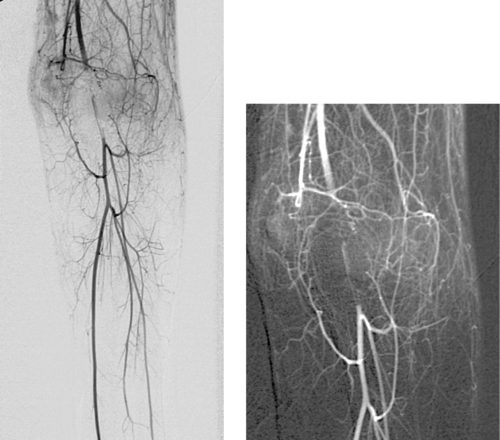 |
1. What are the hard clinical signs of a major arterial injury?
2. Is the presence of a peripheral pulse a reliable indicator that arterial injury is not present?
3. Should the abnormality in the images be treated percutaneously?
4. What angiographic findings of traumatic arterial injury are commonly seen?
ANSWERS: CASE 40
Traumatic Brachial Artery Occlusion
1. Pulsatile bleeding, expanding hematoma, pulse deficits, distal ischemia, bruit or thrill at the injury site.
2. No.
3. No. Surgical thrombectomy with brachial artery repair is indicated.
4. Intimal defects indicating dissection, intraluminal filling defects indicating thrombosis, vasospasm, extrinsic mass effect or defects suggesting extrinsic or intramural hematoma, pseudoaneurysm, distal embolization of thrombus, frank contrast extravasation indicating major active bleeding or arterial transection, and early venous filling indicative of arteriovenous fistula formation.
Reference
Soto, J.A.; Munera, F.; Morales, C., Focal arterial injuries of the proximal extremities: Helical CT arteriography as the initial method of diagnosis, Radiology 218 (2001) 188–194.
Cross-Reference
Comment
When hard clinical signs of upper extremity arterial injury are present, angiography should be performed promptly to guide surgical repair. When hard clinical signs are absent, indications for angiography include a shotgun blast etiology, a bullet following the course of a major artery over a long segment, history of peripheral vascular disease in the involved limb, thoracic outlet injury location, or the presence of extensive injury to bone or soft tissue. Angiography can be performed electively in proximity injuries.
Catheter angiography is the gold standard for evaluating injuries to upper-extremity arteries. Guidewire and catheter manipulation within the area suspected of being injured should be avoided whenever possible in order to avoid inducing vasospasm that could be mistaken for an injury. Angiography provides information about the presence and location of arterial injury as well as the extent of injury. The presence of unsuspected injuries and the etiology of any pulse deficits might also be indicated by angiography.
Common pitfalls in diagnosis include mistaking lacerated intima for thrombus or embolus, mistaking vasospasm for occlusion, and missing an intimal injury owing to dense contrast or overlying bone. Two projections should be used for a complete study.
Notes
Case 41
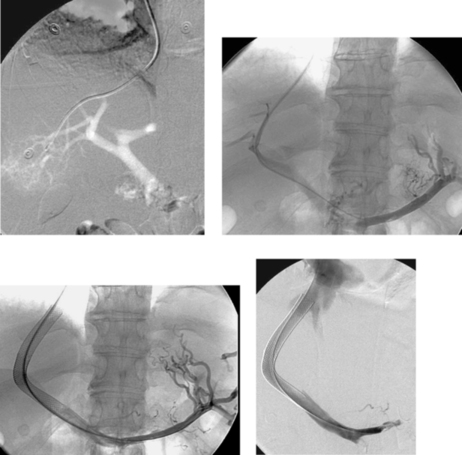 |
1. What are the primary indications for the procedure depicted above?
2. How was the first image above obtained?
3. What portosystemic gradient is associated with an increased risk for gastroesophageal variceal hemorrhage?
4. What adjunctive procedures are commonly performed during this procedure?
ANSWERS: CASE 41
Transjugular Intrahepatic Portosystemic Shunt
1. Intractable ascites, bleeding gastroesophageal varices that have failed endoscopic management, and refractory hepatic hydrothorax.
2. Carbon dioxide portography performed by injection through a catheter in the right hepatic vein.
3. Greater than 12mm Hg.
4. Percutaneous drainage of ascites and/or coil embolization of persistent varices.
References
Boyer, T.D., Transjugular intrahepatic portosystemic shunt: Current status, Gastroenterology 124 (2003) 1700–1710.
Biecker, E.; Roth, F.; Heller, J.; et al., Prognostic role of the initial portal pressure gradient reduction after TIPS in patients with cirrhosis, Eur J Gastroenterol Hepatol. 19 (2007) 846–852.
Cross-Reference
Comment
A transjugular intrahepatic portosystemic shunt (TIPS) is intended to decrease portal vein pressures in patients with portal hypertension by creating a conduit for blood to bypass the hepatic parenchyma and enter the systemic circulation. Therefore, the best candidates for TIPS are those who either have recurrent ascites requiring repeated large-volume paracentesis or bleeding gastroesophageal varices that have failed initial endoscopic treatment or have recurred despite treatment.
A long curved needle is passed through the hepatic parenchyma from a hepatic vein (usually the right hepatic vein) to a nearby portal vein (usually the right portal vein). When portal vein access is successfully attained, pressures are measured in the portal vein and right atrium, the hepatic tract is dilated, and a stent graft is inserted. Post-TIPS portal vein and right atrial pressures are then acquired. The goal of TIPS placement is to reduce this portosystemic gradient to less than 12mm Hg to decrease the risk of gastroesophageal variceal hemorrhage. The portosystemic gradient should be reduced to between 6 and 8mm Hg if the indication is intractable ascites.
The decision to place a TIPS is best made in conjunction with the hepatologist, gastroenterologist, and transplant surgeon, because not all patients benefit from a TIPS, and some patients worsen after TIPS placement. The Childs-Pugh classification system and the MELD scoring system are used to classify patients and gain an estimate of the relative benefits and risks of the procedure.
Notes
Case 42
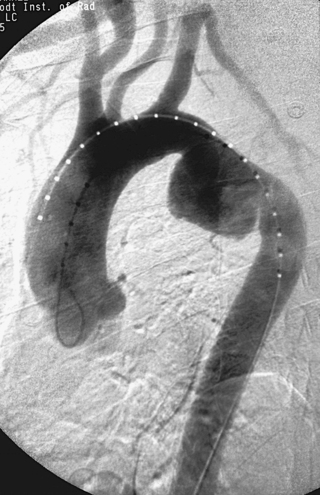 |
1. What is the diagnosis?
2. What is the likely etiology of this particular lesion?
3. What is the purpose of the radiopaque markers on the catheter?
4. Can this lesion be treated in an endovascular fashion?
ANSWERS: CASE 42
Thoracic Aortic Aneurysm
1. Thoracic aortic aneurysm.
2. Atherosclerosis or prior trauma.
3. The marker catheter is used to provide precise longitudinal measurements of the length of the aneurysm and its proximal and distal necks.
4. Yes, using a stent graft.
Reference
Mitchell, R.S., Stent grafts for the thoracic aorta: A new paradigm?Ann Thorac Surg. 74 (5) (2002) S1818–S1820.
Cross-Reference
Comment
Thoracic aortic aneurysms (TAAs) are commonly the result of atherosclerotic disease, although they can also result from trauma, connective tissue disorders, syphilis, infection, and other conditions. The main complication of TAAs is that of rupture, which occurs in 30% of aneurysms greater than 6cm in diameter. For these reasons, TAAs 5.5 to 6.0cm in diameter are generally repaired surgically. In female patients and those with connective tissue disorders, TAAs tend to rupture at smaller diameters, and these patients are therefore usually referred earlier for surgical repair.
The diagnosis of TAA is generally made using CT or MRI. Angiography is rarely used for the diagnosis of TAA, and it does not provide accurate aneurysm diameter measurements in comparison with CT and MRI. However, angiography provides fairly accurate measurements of the length of the aorta and the degree of angulation in the proximal neck of the aneurysm, and it accurately depicts the aneurysm’s relationship to the origins of the great vessels. These findings can be extremely important in surgical planning and in selecting a device and access route for endovascular stent-graft repair.
The most important drawbacks of surgical TAA repair are significant rates of perioperative mortality (6%–12%), paraplegia (3%–16%), and cardiopulmonary complications (5%–30%). Early results suggest that significant improvements in perioperative mortality and morbidity are likely to be achieved with endovascular repair, although the durability of these procedures is not known.
Notes
Case 43
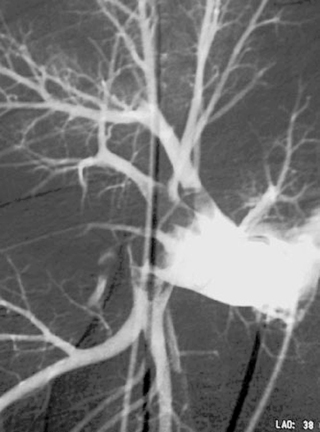 |
1. What is the diagnosis?
2. What is the most common and reliable angiographic finding in this disorder?
3. What electrocardiographic finding is of particular concern in a patient who is about to undergo pulmonary arteriography, and how is this problem circumvented?
4. What are normal right atrium, right ventricle, and pulmonary artery pressures?
ANSWERS: CASE 43
Acute Pulmonary Embolism
1. Acute pulmonary embolism.
2. Intraluminal filling defect at least partially surrounded by contrast material.
3. Left bundle branch block. A transvenous pacer can be placed.
4. Right atrium: 0–8mm Hg mean. Right ventricle: 15–30mm Hg systolic, 0–8mm Hg diastolic. Pulmonary artery: 15–30mm Hg systolic, 3–12mm Hg diastolic.
Reference
Harvey, R.B.; Gester, W.B.; Hrung, J.M.; et al., Accuracy of CT angiography versus pulmonary angiography in the diagnosis of acute pulmonary embolism: Evaluation of the literature with summary ROC curve analysis, Acad Radiol. 7 (2000) 786–797.
Cross-Reference
Comment
With the advent of spiral CT angiography, pulmonary arteriography is being performed much less often in the diagnosis of pulmonary embolism. Pulmonary arteriography has a negative predictive value of nearly 100% for vascular thromboembolism within 3 months.
The range of angiographic findings in acute pulmonary embolism includes abrupt vessel cutoff, intraluminal filling defects manifested by the tram-track sign, wedge-shaped parenchymal oligemia, absence of a draining vein from the affected segment, arterial collaterals, and hypervascularity of an infarcted segment. Pulmonary emboli are typically multiple and bilateral, and they tend to lodge in the lower lobe vessels.
The accuracy of pulmonary arteriography can be optimized with attention to several facts. Emboli lyse rapidly, so arteriography should be performed within 24 to 48 hours of symptoms. Multiple projections of both lungs should be obtained. Ventilation–perfusion scans and CT can be used to focus the examination to suspicious areas. Careful attention to small vessels, particularly in the lower lobes, is critical because many patients only have subsegmental emboli. Emboli can be missed because of overlapping vessels, small size of the emboli, or respiratory or patient motion, so additional imaging, including oblique or magnification views or selective vessel injection, may be necessary. False positives can occur from overlapping of vessels, mock lines, cystic air spaces, or poor opacification.
Notes
Case 44
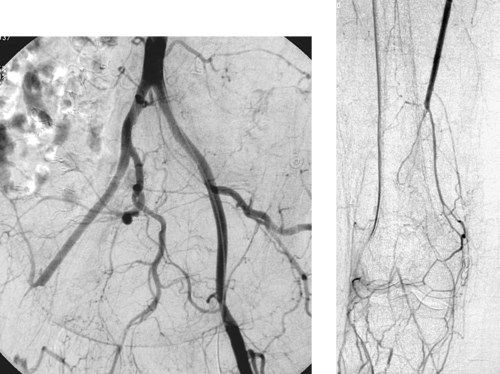 |
1. What are the most common causes of acute lower-extremity ischemia?
2. What is the diagnosis in this case?
3. What angiographic signs suggest this diagnosis?
4. What is the most common source of arterial emboli?
ANSWERS: CASE 44
Embolic Femoral Artery Occlusion
1. Embolization, thrombosis of atherosclerotic native arteries, and occlusion of bypass grafts.
2. Acute emboli to the right common femoral artery and superficial femoral artery.
3. Abrupt vessel cutoff, meniscus configuration of cutoff, lack of significant atherosclerotic disease elsewhere, poor development of collaterals.
4. Embolization of left-heart thrombus.
Reference
Ouriel, K., Current status of thrombolysis for peripheral arterial occlusive disease, Ann Vasc Surg. 16 (2002) 797–804.
Cross-Reference
Comment
Patients with acute limb ischemia typically present with a cold painful leg with pallor, cyanosis, and/or paresthesias. A careful pulse examination often suggests the level of obstruction. Profound sensory loss, muscle weakness, or paralysis are concerning for irreversible ischemia. Most macroemboli lodge near branch points in the femoral or popliteal arteries.
The role of angiography in the evaluation of acute limb ischemia is fourfold: (1) Determine the level of arterial obstruction and reconstitution; (2) Determine whether the occlusion is embolic or thrombotic; (3) Assist in finding the source of embolus. The most common source of emboli by far is the left heart, due to left atrial or ventricular dilation, dysrhythmia, valvular heart disease, left ventricular aneurysm, or rarely left heart tumor; for this reason, echocardiography is often indicated. Angiography can sometimes localize the source of noncardiac emboli, which can originate from aneurysms or ulcerated plaque in the aortoiliac vessels; (4) In selected cases, reopen the artery.
Treatment is chosen based upon the presumed cause, the severity of the symptoms, and the patient’s clinical condition. Acute embolization is treated with surgical embolectomy or bypass grafting if the limb appears severely threatened. Thrombolytic therapy can reduce the thrombus burden, but it can be ineffective in lysing the embolic nidus itself, which is composed of organized thrombus or plaque material. Ultimately, the embolic source must be treated to prevent recurrence.
Notes
Case 45
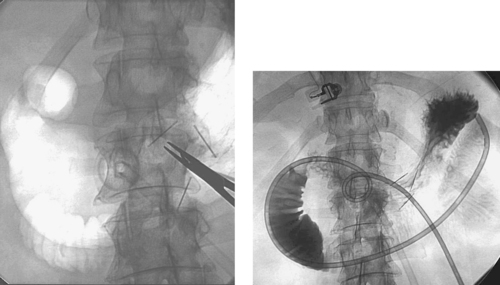 |
1. What procedure has been performed?
2. What do the three short linear opacities overlying the gastric body represent?
3. On average, is this procedure technically more challenging than percutaneous gastrostomy tube placement?
4. Is the frequency of catheter occlusion equal to that associated with percutaneous gastrostomy tubes?
ANSWERS: CASE 45
Percutaneous Gastrojejunostomy Placement
1. Percutaneous gastrojejunostomy (GJ) tube placement.
2. Gastropexy T-fasteners.
3. Yes.
4. No. GJ tubes tend to occlude more often owing to their longer length and the smaller diameter of the jejunal lumen.
Reference
Bell, S.D.; Carmody, E.A.; Yeung, E.Y.; et al., Percutaneous gastrostomy and gastrojejunostomy: Additional experience in 519 procedures, Radiology 194 (1995) 817–820.
Cross-Reference
Comment
The indications for placement of a percutaneous GJ tube (either de novo or via conversion of an existing gastrostomy tube to a GJ tube) include aspiration with gastric feeding, known severe gastroesophageal reflux, gastric outlet obstruction, and decreased gastric motility (e.g., patients with diabetic gastroparesis).
From a technical standpoint, three differences from percutaneous gastrostomy tube placement are present. First, because of the increased catheter manipulation required for GJ-tube placement, gastropexy with placement of two to four T-fasteners is strongly recommended. Second, catheterization of the duodenum can be challenging in patients with pyloric stenosis or unfavorable angulation of the initial gastrostomy tract. Third, extreme care must be used to avoid buckling a loop of catheter into the stomach during placement; this can result in loss of hard-earned access into the duodenum and can lengthen procedure time. The optimal positioning of the distal part of the tube is in the proximal jejunum beyond the ligament of Treitz.
Notes
Case 46
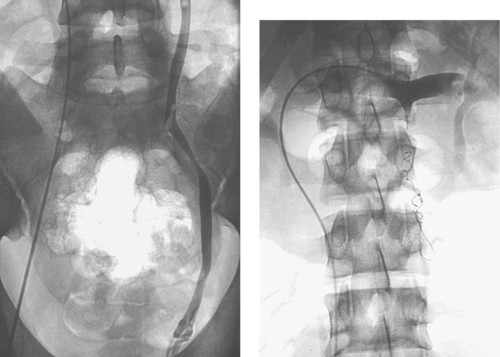 |
1. What vein is selectively catheterized?
2. Is this disorder more common on the left or right?
3. What are the indications for treatment?
4. What treatment options exist?
ANSWERS: CASE 46
Male Varicocele
1. Left gonadal vein.
2. Left.
3. Spermatic dysfunction (male infertility), scrotal pain, scrotal enlargement or disfigurement, and testicular hypoplasia in adolescent boys.
4. Surgical ligation and percutaneous transluminal embolization.
Reference
Fretz, P.C.; Sandlow, J.I., Varicocele: Current concepts in pathophysiology, diagnosis, and treatment, Urol Clin North Am. 29 (2002) 921–937.
Cross-Reference
Comment
Varicocele is a dilation of the pampiniform plexus that affects 10% to 15% of males. Many theories have been proposed to explain primary varicocele, including abnormalities of the internal spermatic vein valves and left renal vein compression causing venous hypertension. A unilateral right varicocele or sudden onset of a varicocele in an older man should raise concern for an abdominal or pelvic mass causing venous compression and impaired venous drainage.
Most men are asymptomatic, but infertility resulting from impaired sperm motility and number, scrotal pain, and scrotal swelling and disfigurement can result. Nearly 50% of men evaluated for infertility have unilateral or bilateral varicocele. Leading theories suggest a relation to elevated scrotal temperature or reflux of metabolites from the renal and adrenal veins. Most varicoceles are detected on physical examination, but sonography, scintigraphy, and MRI are useful when the examination is normal or equivocal.
Surgical ligation and embolotherapy are the two primary treatment modalities. Embolotherapy involves performing a diagnostic venogram to confirm valvular incompetence followed by embolization of the internal spermatic vein from the level of the superior pubic ramus to the vein orifice with occlusion of collateral channels as necessary. Surgical ligation and embolotherapy demonstrate similar rates of technical success, improvement in sperm density and motility, subsequent pregnancy, and complications.
Notes
Case 47
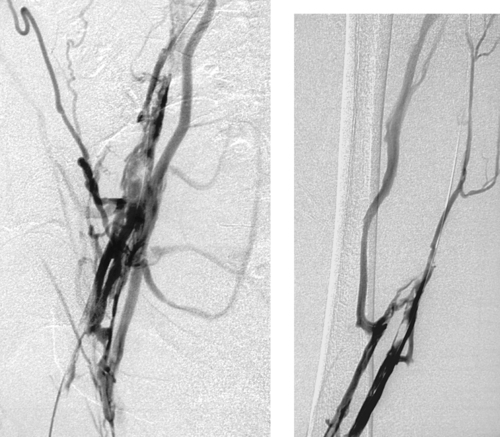 |
1. What type of examination has been performed?
2. What are the main clinical findings?
3. Is catheter-directed thrombolysis a good treatment option for this patient?
ANSWERS: CASE 47
Chronic Deep Vein Thrombosis
1. Lower-extremity digital subtraction venogram.
2. Irregular mural filling defects, septated venous channels with surrounding collaterals, consistent with chronic deep vein thrombosis (DVT).
3. No. Thrombolysis is less likely to succeed when DVT is chronic in duration.
Reference
Prandoni, P.; Lensing, A.W.A.; Cogo, A.; et al., The long-term clinical course of acute deep-vein thrombosis, Ann Intern Med. 125 (1996) 1–7.
Cross-Reference
Comment
From 25% to 50% of patients with deep vein thrombosis develop post-thrombotic syndrome (PTS). Patients with PTS often manifest symptoms of limb edema, aching, venous claudication, hyperpigmentation, and/or ulcerations. The symptoms are typically worse near the end of the day after the patient has been ambulatory. PTS is known to impair quality of life.
The etiology of post-thrombotic symptoms is ambulatory venous hypertension, which has two distinct components: Completed valvular damage with subsequent reflux and persistent venous obstruction due to intraluminal thrombus and debris.
Venographic findings of chronic (>2 weeks) DVT often include a septated and contracted appearance of the involved vein(s) with multiple channels, relative lack of venous dilation, and the presence of large collaterals. Sonographic findings parallel the venographic findings, and include increased echogenicity of the intraluminal material, a laminar distribution of the filling defects, and a septated appearance.
Notes
Case 48
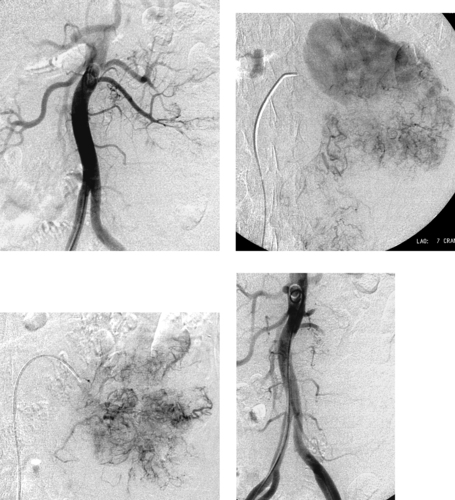 |
1. What is the primary angiographic finding?
2. What is the likely diagnosis?
3. What is the likely treatment for this disorder?
4. What is the role of angiography in this disorder?
ANSWERS: CASE 48
Renal Cell Carcinoma
1. Large hypervascular mass replacing the lower part of the left kidney.
2. Renal cell carcinoma.
3. Radical nephrectomy (common) or partial nephrectomy (carefully selected cases).
4. Presurgical planning to determine if a partial nephrectomy will be appropriate. Also, angiography may be performed to enable preoperative embolization (as seen in the last figure), performed to diminish perioperative blood loss.
Reference
Zielinski, H.; Szmigielski, S.; Petrovich, Z., Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma, Am J Clin Oncol. 23 (2000) 6–12.
Cross-Reference
Comment
Risk factors for renal cell carcinoma include tobacco use, long-term phenacetin use, von Hippel–Lindau disease (which can cause bilateral tumors), and chronic hemodialysis. Clinically, the disorder commonly manifests with gross hematuria, flank pain, and/or weight loss; less commonly, the disorder is suspected based upon the presence of a palpable mass or a paraneoplastic syndrome (hypertension, erythrocytosis, or hypercalcemia).
Imaging findings of renal cell carcinoma include a solid mass lesion on cross-sectional imaging, which can be calcified, necrotic, and/or hemorrhagic. The tumor might extend into the renal vein and/or inferior vena cava. Angiographically, 95% of tumors are hypervascular, and arteriovenous shunting and venous lakes are common. Preoperative angiographic embolization is often performed to diminish tumor vascularity and thereby reduce perioperative blood loss.
Notes
Case 49
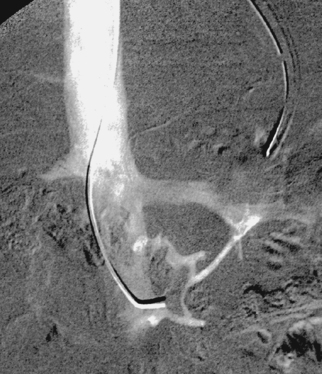 |
1. What anatomic variant is depicted in the first image?
2. Why is this important to diagnose when placing an inferior vena cava (IVC) filter?
3. What other venous anomalies are important to exclude before placing a filter?
4. Where should an IVC filter be?
ANSWERS: CASE 49
Variant Anatomy: Circumaortic Left Renal Vein
1. Circumaortic left renal vein.
2. This segment can serve as a collateral pathway for emboli to circumvent an IVC filter and reach the lungs.
3. IVC duplication, left-sided IVC, and accessory renal veins.
4. Below the lowest renal vein if this is possible.
Reference
Trigaux, J.P.; Vandroogenbroek, S.; Wilpelaere, J.F., Congenital anomalies of the inferior vena cava and left renal vein: Evaluation with spiral CT, J Vasc Interv Radiol. 9 (1998) 339–345.
Cross-Reference
Comment
A circumaortic left renal vein is found in 1.5% to 8.7% of the population. It represents a congenital variant and results from persistence of the infrarenal segment of the left supracardinal vein. The preaortic portion of the venous ring is located in the usual position of the left renal vein. The retroaortic portion of the ring courses downward, joining the IVC in the lower lumbar region. There may be associated anomalies of drainage of the lumbar veins and left gonadal vein.
An IVC filter placed below the upper insertion but above the lower insertion of the venous ring allows the ring to serve as a potential pathway for emboli from the lower extremity or pelvis to pass up the IVC, into the lower limb of the left renal vein, and out the upper limb into the IVC above the filter. To exclude this pathway, the filter should preferably be deployed below all of the renal veins.
Accessory renal veins have a similar theoretical risk and can occur on either the right or left. As above, if possible, the IVC filter should be placed below all renal veins.
Case 50
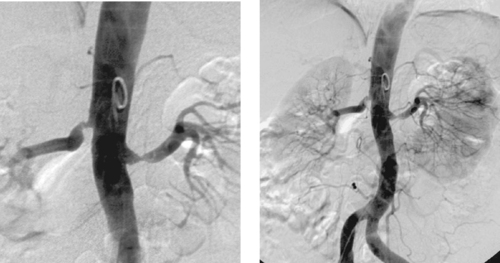 |
1. What are the common presenting symptoms experienced by patients with this disorder?
2. Name three potential etiologies for the lesions visualized in the images.
3. What is the most likely cause of the lesions seen in the images?
4. What treatment options are available for patients with this disorder?
ANSWERS: CASE 50
Atherosclerotic Renal Artery Stenosis
1. Hypertension and chronic renal failure.
2. Atherosclerosis, fibromuscular dysplasia, neurofibromatosis.
3. Atherosclerosis.
4. Aortorenal arterial bypass, percutaneous transluminal angioplasty, endovascular stent placement.
References
Gill, K.S.; Fowler, R.C., Atherosclerotic renal arterial stenosis: Clinical outcomes of stent placement for hypertension and renal failure, Radiology 226 (3) (2003) 821–826.
Olin, J.W., Atherosclerotic renal artery disease, Cardiol Clin. 20 (2002) 547–562.
Cross-Reference
Comment
Patients with renal artery stenosis (RAS) typically present with hypertension refractory to multiple medications or chronic renal failure resulting from ischemic nephropathy. The most common cause of RAS by far is atherosclerosis. Other causes include fibromuscular dysplasia, aortic dissection, and neurofibromatosis. Atherosclerotic lesions are most commonly located in the ostial and periostial portions of the main renal artery, as in this case, but they can also be seen distally.
Surgical bypass is most commonly performed in patients with lesions not amenable to percutaneous treatment. RAS caused by atherosclerosis has a 60% to 70% patency at 5 years when treated by percutaneous balloon angioplasty; however, angioplasty of ostial lesions is associated with substantially lower patencies (25%-50%). For this reason, endovascular stents are usually used to treat ostial renal artery stenosis.