Chapter 105 Role of Gamma Knife Radiosurgery in the Management of Arteriovenous Malformations
Following Roentgen’s discovery of x-rays, there was early interest in the use of irradiation for arteriovenous malformation (AVM) treatment from 1914 to 1950.1 In spite of occasional successes, until 1970, there was consensus among neurosurgeons that radiation had little practical use in the management of AVMs.2
Things began to change with the development of the concept of stereotactically guided treatment of intracranial lesions using a single high dose of ionizing beams by Lars Leksell.3 He defined the term and initiated investigation, first using an orthovoltage x-ray tube combined with his initial rectilinear stereotactic coordinate frame. In 1951, working with Borje Larsson, a physicist, Leksell subsequently reported the first clinical use of stereotactic guidance linked with a cyclotron-generated proton beam.4
In 1968, continuing their work, Leksell built the first dedicated radiosurgical unit, the gamma knife.4,5 The potential value of irradiation in the treatment of vascular malformations was subsequently reassessed. Encouraging factors were evidence that the vessels were radiosensitive and a long-term angiographic follow-up of a small series of AVMs treated with fractionated irradiation by Johnson in the 1950s confirming that the AVM was obliterated in 45% of cases.6
Stereotactic radiosurgery is the closed skull destruction of an intracranial target using ionizing beams of radiation, directed in a focused manner with the help of an extracranial guiding device.7 The application of stereotactic radiosurgery for cerebral arteriovenous malformations began in earnest in the early 1970s.10
Radiosurgical Treatment of Arteriovenous Malformations
It has been estimated that as many as 500,000 people in the United States and Canada have AVMs.8,9 During the last 40 years, the overall role of stereotactic radiosurgery has been established in the management of selected vascular malformations. Together with advances in microsurgical techniques, postoperative neurointensive care management, and advances in intravascular microcatheter techniques for AVM characterization and embolization, radiosurgery alone or in combination represents a moderately low-risk and effective strategy for properly selected patients.11,12
A cure for every patient treated with radiosurgery has not yet been achieved. The obliteration rates at 3 years (“angiographic cure”) range from 70% to 85%.13–17 Several factors have been associated with the likelihood of obliteration, such as AVM volume, prescription dose, location, angioarchitecture, and patient age and lack of history of embolization. Radioresistance has been reported in some lesions that show no response, despite appropriate doses of radiation.17,19
Small treatment volume, a 23- to 25-Gy prescription dose, and a small number of draining veins18–23 are factors associated with a more favorable prognosis. Conversely, factors such as a large AVM’s volume, site, and the radiation dose to the exposed brain tissue, have been associated with the likelihood of complications, including bleeding during the latency period, cerebral edema, and/or radionecrosis.23–34
Some researchers, including Chang et al.,35 have suggested that other factors in addition to volume should be considered when predicting obliteration results, such as the AVM’s flow pattern and the number of draining veins. Furthermore, some lesions that appear to be similar in vessel architecture, location, and volume, respond differently or unexpectedly to treatment plans based on the dose–volume relationship. Some AVMs are obliterated within 2 years, whereas others do so after 3 or 4 years.
In terms of angiographic features associated with lower likelihood of obliteration, Meder et al.36 reported that radiosurgery is less effective in AVMs with direct intranidal fistulas and single draining vessels and suggest that radiosurgery is more likely in patients with plexiform or compact-type AVMs.
Similarly, in a semiquantitative flow study using phase-contrast two-dimensional (2D) magnetic resonance angiography (MRA), Petereit et al.37 reported a negative effect on the obliteration of AVMs by flow speeds greater than 100 ml/s. We reviewed our own experience by defining a critical zone for treatment, the high flow nidus excluding the perilesional draining veins in AVMs using dynamic analysis of circulation times through the AVMs.
Improved Imaging in Radiosurgery
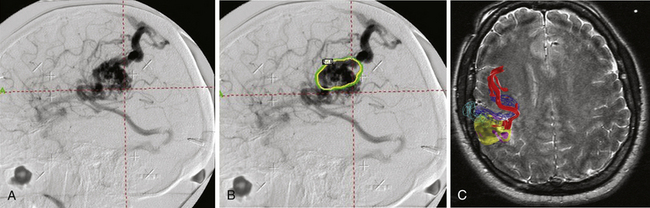
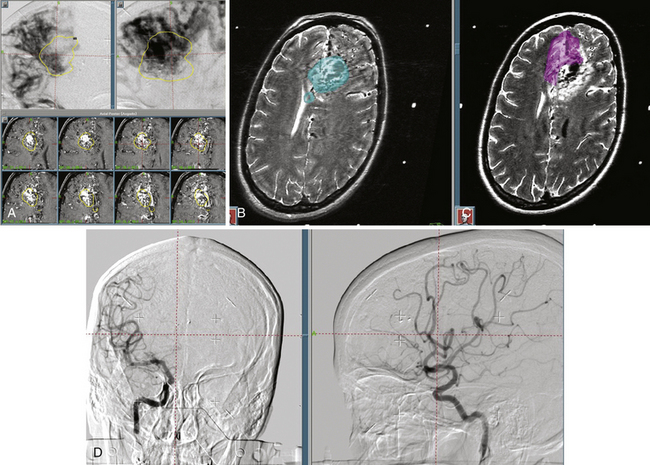
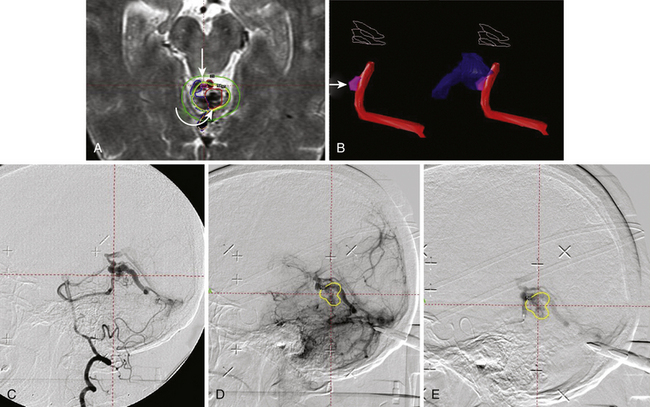
The accuracy of radiosurgical treatment is dependent on the accuracy of target delineation by radiological studies performed with a stereotactic frame in place. In the management of vascular malformations, the primary imaging modality is angiography. Magnetic resonance imaging (MRI) examinations are also recommended for obtaining further information, to fully understand the topographic and tomographic anatomy of the malformation as well as the relationship between the targeted AVM and the non-targeted anatomical structures adjacent to the AVM. The gamma surgery planning program allows target outlines marked on the angiogram to be projected on the MRIs and vice versa (Fig. 105-1). Thus, both modalities can be used in the planning procedure.
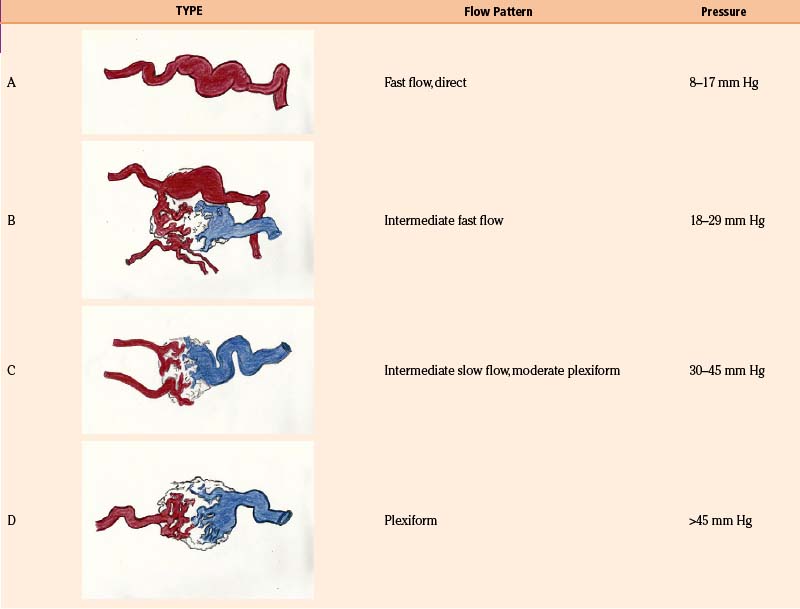
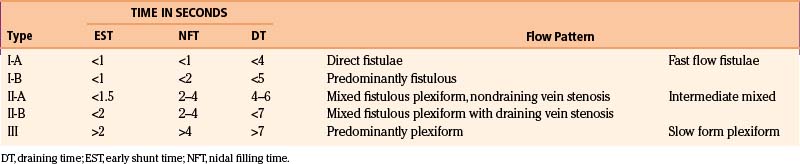
With the goal of improving imaging, and therefore targeting of the AVM, our protocol focuses on the definition of the AVM’s critical zone, which corresponds to the least-resistant portion of the lesion with the predominant flow draining to the main vein. Our experience in measuring the pedicular pressure in feeders, when treating malformations with superselective embolization, was taken into account in a dynamic study of malformations.38 Pedicular pressures lower than 25 mm Hg are observed in direct AV fistulas, whereas plexiform AVM pressures increase gradually to nearly 50 mm Hg (Table 105-1).
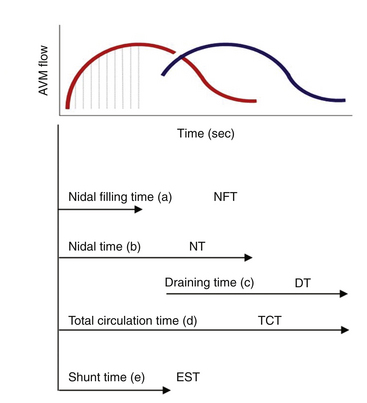
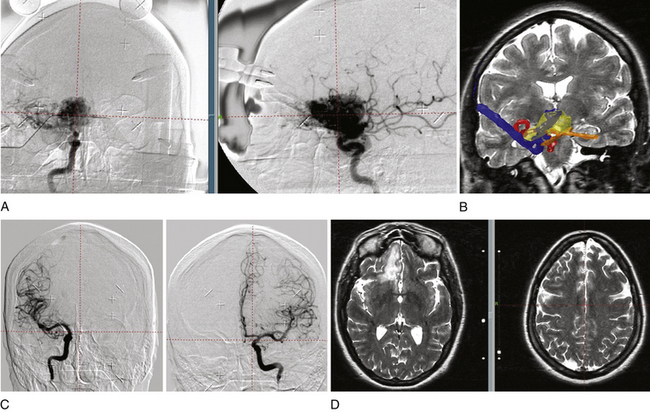
In our protocol, once the Leksell stereotaxic frame (Elekta) is in place on the patient’s head, three-dimensional (3D) volume MRIs are acquired in T1 simple and gadolinium-enhanced sequences, together with axial T2 and coronal sequences for 3D images of the AVM. For digital subtraction angiography (DSA), Axiom Artis equipment is used with software for automated outline correction (Artis&Syngo, Siemens). Currently, images are obtained at 10.5 frames/seconds with a digital contrast injector (Mark V Pro Vis, Medrad), with contrast injected at 7 ml/s in the proximal portion of the carotid and vertebral arteries at the skull base. With superselective contrast injection, the volume is reduced to 3 ml/s. Several circulation times (Fig. 105-2) are then measured frame by frame in the angiography console: nidal filling time (NFT), time from the first frame with nidal staining to the corresponding frame at maximum arterial enhancement; nidal time (NT), includes NFT up to the last frame outlining the arterial nidus; draining time (DT), from the time of the first or predominant draining vein image, when venous draining is multiple, to the time when the abnormal draining vein or veins are no longer seen; total circulation time (TCT), time elapsed from the first frame of the nidal filling time to the last draining frame; and early shunt time (EST), time from the first frame of nidal filling to the first frame of venous draining time.
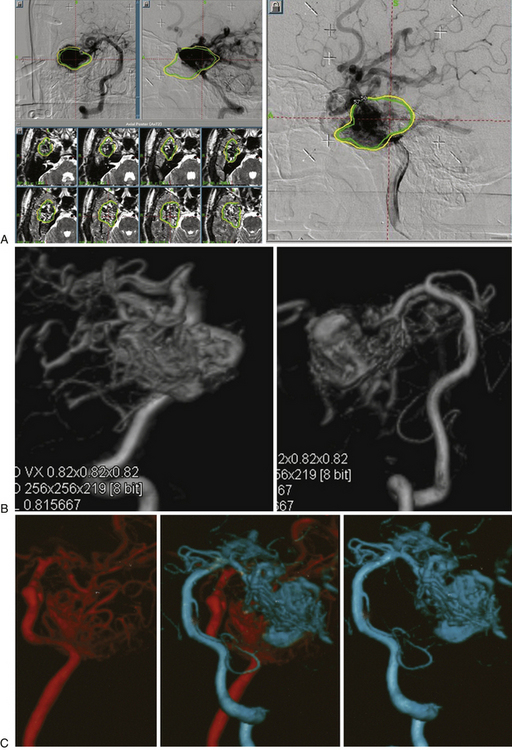
As the circulation times are studied and recorded, the various AVM components are defined, including the single or multiple compartments (Fig. 105-3), the type of venous drainage, and several flow patterns, depending on the circulation time compatible with the three types of circulation: I-A direct fistulae, I-B predominantly fistulous, II-A mixed fistulous-plexiform without stenosis of the draining vein, II-B mixed with draining vein stenosis, and III predominantly plexiform (Table 105-2).
Nidal circulation times (NTs) less than 2 seconds and a total circulation of less than 6 seconds correlate with the I-A and I-B fistulous flow patterns. Shunt (EST) times of less than 2 seconds and nidal filling times (NFTs) of 2 to 4 seconds and total circulation times of up to 8 seconds correlate with the mixed fistulous-plexiform flow pattern, and nidal times greater than 4 seconds and total circulation time greater than 9 seconds reflect predominantly plexiform flow. It is important to note that a patient with a II-B flow pattern with an early shunt time less than 1.5 seconds and a slow draining time greater than 7 seconds, resulting from stenosis of the predominant venous drainage, should be considered a candidate for embolization to reduce the risk of bleeding.
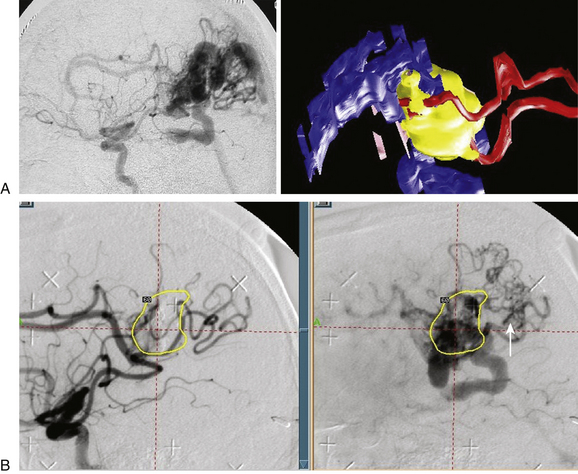
Our embolization protocol for radiosurgery depends on the volume of the AVM, the corresponding flow pattern, and the risk factors for bleeding. If the size of the pre-embolization target is less than 10 ml, the treatment volume should include all the original volume. If the pre-embolization volume is greater than 10 ml, the prescription dose is delivered to the residual volume in the critical zone. In terms of the flow pattern, it is advisable to use embolization to reduce the flow in patterns I and II-B. Endovascular therapy is also used to eliminate high-flow aneurysms or reduce volume in prospective staged protocols (Fig. 105-4).
Patients Characteristics
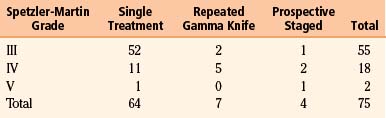
From 1995 to 2008, a total of 250 patients with AVMs underwent gamma knife (Elekta) radiosurgery. Of these, 75 patients were treated after dynamic measurement planning to accurately define the critical zone of the AVM, including the dynamic angiographic criteria that categorize the type of AVM: fistulous versus plexiform or mixed, and Spetzler-Martin (S-M) classification into grade III (55), IV (18), or V (2). According to radiosurgery-based grading system (RBGS) proposed by Pollock and Flickinger,39 the minimum score was 0.6 and the maximum was 2.68, with a mean score of 1.64.
Endovascular therapy was performed in 56% of patients and superselective angiography without embolization in 29%. Radiosurgery was administered as a single treatment in 64 patients, repeat radiosurgery in 7 patients, or prospective staged radiosurgery in four patients (Tables 105-3 and 105-4). The AVM original volume was up to 120 ml, with a critical zone target volume of up to 15 ml in the single treatment group, a median target volume of 8.5 ml, and 28 ml in the prospective staged radiosurgery group. The marginal dose of radiation to the AVM was 18 to 22 Gy, with a median dose of 20 Gy.
Table 105-3 Dynamic Protocol for Arteriovenous Malformations in 75 Patients
| Spetzler-Martin Grade | Embolization plus Gamma Knife | Superselective Angiography for Gamma Knife Planning |
|---|---|---|
| III | 20 (29%) | 15 |
| IV | 20 (86%) | 5 (4 staged) |
| V | 2 (67%) | 2 |
| Total | 42/75 (56)% | 22/75 (29%) |
Results
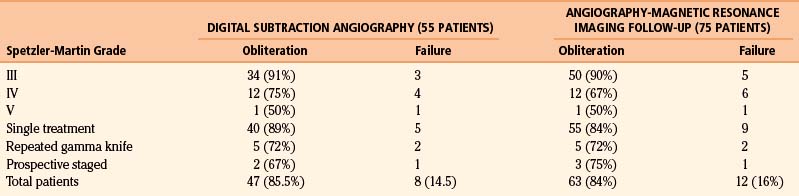
In 55 patients, DSA was used to review the treatment response (Table 105-5). In 75 patients, the evaluation was performed with MRA at 12 to 40 months (mean follow-up 28 months). Obliteration was observed in 90%, 67%, and 50% of patients in groups with S-M grades III, IV, and V, respectively. In the single treatment group, obliteration was 84% and obliteration was complete in 5 out of 7 patients who underwent repeat radiosurgery. In 4 patients who underwent prospectively staged radiosurgery, the lesion was obliterated in 3. In 2 patients, this was confirmed by DSA, and in the other one by MRA. One patient displayed subtotal obliteration after 40 months of follow-up and was completely obliterated at 5 years. The overall MRA obliteration rate was 84%.
Discussion
Some researchers, including Pan et al.,23 Chang et al.,35 Meder et al.,36 and Petereit et al.,37 among others, have suggested that flow pattern–related factors and even the fistulous type of AVM have a negative effect on obliteration. Frame-by-frame dynamic flow analysis and multiplanar MRI allow the identification of failure-related factors more objectively, including prenidal fistulas with early shunt times (<1 second; Fig. 105-5). The presence of a prenidal fistula could represent one of the factors related to the radiobiological resistance published by the Pittsburgh group.17,19
The (Controversial) Role of Embolization
Luessenhop and Spence performed the first cerebral AVM embolization in 1959.40 Flow-directed catheters have been most commonly used since 1990, and the most common complications are aberrant embolization, embolization of the normal proximal arteries, venous system occlusion, and arterial perforation.41,42 Recommendations for embolization are supported by anecdotal case series and nonrandomized cohort studies.
Andrade Souza et al.43 addressed the question of whether preradiosurgical AVM embolization affects obliteration or clinical outcomes. They found that embolization reduced the obliteration rate and that outcomes were better in the nonembolized group, but the differences were not statistically significant. Obliteration was achieved in 70% of patients receiving radiosurgery alone and in 47% of patients treated with embolization before radiosurgery.
Unfortunately, there has been no standardization of the embolization technique. In reports of endovascular therapy, there are distinct outcomes from different groups. Some series have combined the use of particles and cyanoacrylate. There is also no clear information about results and more specifically in terms of the volume of the AVM, flow patterns, risk factors for bleeding, or nidus fragmentation. Furthermore, it is usually not specified whether an endovascular therapist worked together with the neurosurgeon. There is also no systemization of the two-dimensional versus 3D volume measurements,44 or of quantitative flow measurements before and after embolization.45
We are of the opinion that it is important in order to better understand the contribution of embolization, to define whether the radiosurgical treatment volume is based on the global initial volume before embolization or whether the radiation was targeted to the residual volume. We also recommend that neurosurgeons work very closely with endovascular therapists in real time. In some AVMs, stereotactically guided embolization and correlation with 3D topography of the pedicle on MRI using Gamma Plan (Elekta) can optimize the preradiosurgery results by estimating the objective volume and flow reduction, and by providing information about the hemodynamic factors that work against favorable radiosurgery outcomes (Fig. 105-6).
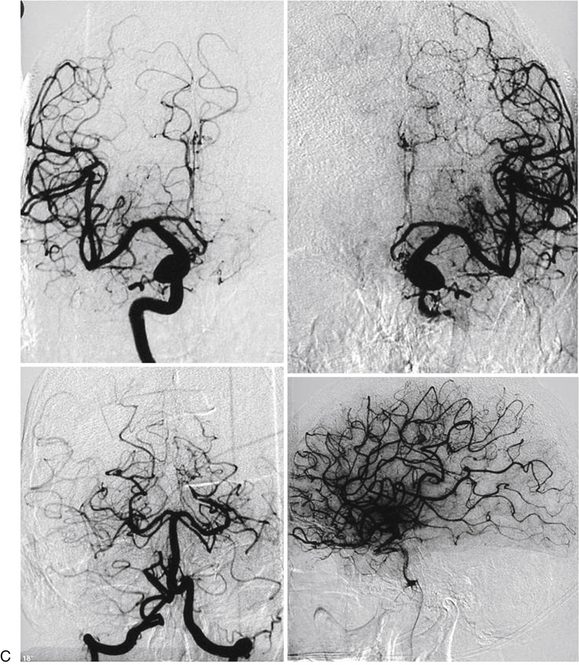
Ideally, the embolization procedure should be concentric to simulate the usual surgical approach, thus avoiding inconvenient postembolization fragmentation of the target. Furthermore, it should be recorded whether the treated area belongs to the early filling and dominant flow zone, which is related to predominantly venous drainage, while excluding pseudonidus (Fig. 105-7). Special attention must be paid to postembolization fragmentation, because the outcomes and complications are operator-dependent.
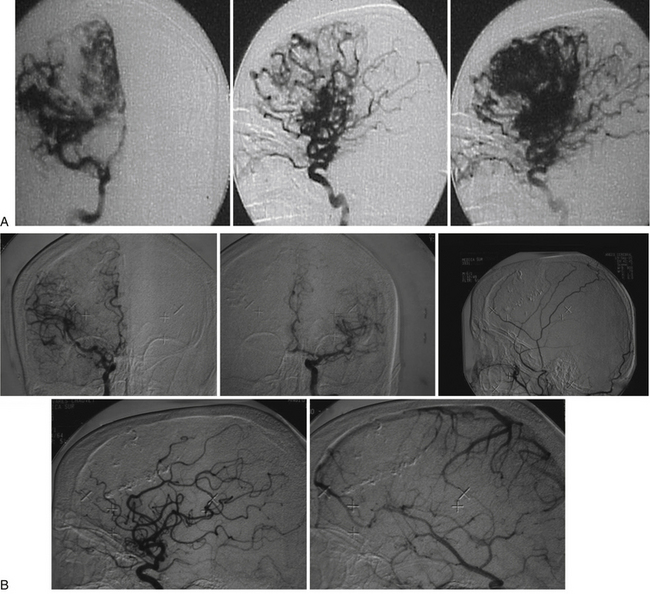
Contrary to our expectations, 2 patients with complex AVMs and steroid treatment who underwent embolization and radiosurgery on the same day remained obliterated 10 years later (Fig. 105-8A and B). We cannot rule out a synergistic effect between radiation damage and the inflammatory response due to embolization. Furthermore, in this series the mean RBGS of 1.64 would represent an expected 60% to 70% mean obliteration rate.
Conclusion
In order to improve the outcomes of AVM radiosurgery, our protocol is designed to be easily followed by any radiosurgical center. Instead of retrospectively, as has usually been suggested, our method can be prospectively and systematically used to evaluate hemodynamic variables of AVMs undergoing radiosurgery. Using digital quantitative methods to measure flow and volume, it is possible to obtain an objective measurement of the flow pattern and volume modifications in an embolized AVM, which will be a helpful tool in endovascular therapy. Building a clear and reliable database that includes data from other radiosurgery centers should help to standardize indications, and to analyze and share review and research results of endovascular therapy for AVMs, which until now have been anarchic and heterogeneous. A dynamic definition of the AVM critical zone might increase the obliteration rate and decrease the treatment volume in big lesions by removing the pseudonidus from the target.
Andrade Souza Y.M., Ramani M., Scora D., et al. Embolization before radiosurgery reduces the obliteration rates of arteriovenous malformations. Neurosurgery. 2007;60:443-445.
Chang J.H., Chang J.W., Park Y.G., Chung S.S. Factors related to complete occlusion of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2000;93:96-101.
Chang S.D., Marcellus M.L., Marks M.P., et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-13.
Del Valle R., Perez M., Ortiz J., et al. Stereotactic noninvasive volume measurement compared with geometric measurement for indications and evaluation of gamma knife treatment. J Neurosurg. 2005;102:140-142.
Del Valle R., Zenteno M., Jaramillo J., et al. Definition of the key target volume in radiosurgical management of arteriovenous malformations:a new dynamic concept based on angiographic circulation time. J Neurosurg. 2008;109:41-50.
Flickinger J.C. An integrated logistic formula for prediction of complications from radiosurgery. Int J Radiat Oncol Biol Phys. 1989;17:879-885.
Flickinger J.C., Pollock B.E., Kondziolka D., Lunsford L.D. A dose–response analysis of arteriovenous malformations obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
Friedman W.A., Bova F.J., Bollampaly S., Bradshaw P. Analysis of factors predictive success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-308.
Haw C.S., terBrugge K., Willinsky R., Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226-232.
Karlsson B., Lax I., Sodermann M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49:1045-1051.
Karlsson B., Lax I., Soderman M. Can the probability for obliteration after radiosurgery for arteriovenous malformations be accurately predicted. Int J Radiat Oncol Biol Phys. 1999;43:313-319.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797-803.
Lunsford L.D. Stereotactic radiosurgery of intracranial arteriovenous malformations. In: Wilkins R.H., Rengachary S.S. Neurosurgery Update II: Vascular, Spinal, Pediatric and Functional Neurosurgery. New York: McGraw Hill; 1991:175-185.
Maruyama K., Shin M., Tago M., et al. Radiosurgery to reduce the risk of first hemorrhage from brain arteriovenous malformations. Neurosurgery. 2007;60:453-459.
Meder J.F., Oppenheim C., Blustajn J., et al. Cerebral arteriovenous malformations: the value of radiologic parameters in predicting response to radiosurgery. AJNR Am J Neuroradiol. 1997;18:1473-1483.
Pan H.C. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000;93:113-119.
Petereit D., Metha M., Turski P., et al. Treatment of arteriovenous malformations with stereotactic radiosurgery employing both magnetic resonance angiography and standard angiography as a database. Int J Radiat Oncol Biol Phys. 1993;25:309-313.
Pollock B.E., Flickinger J.C. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
Pollock B.E., Flickinger J.C., Lunsford L.D., et al. Factors associated with successful arteriovenous malformations radiosurgery. Neurosurgery. 1998;42:1239-1244.
Pollock B.E., Kondziolka D., Lunsford L.D., et al. Repeat stereotactic radiosurgery of arteriovenous malformations: factors associated with incomplete obliteration. Neurosurgery. 1996;38:318-324.
Steiner L., Leksell L., Greitz T., et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Acta Chir Scand. 1972;138:459-464.
Steiner L., Rankin P.B., Prasad D., Steiner M. Gamma surgery for vascular disorders of the brain. In: Germano I., editor. LINAC and Gamma Knife Radiosurgery. Neurosurgical Topics. New York: Thieme/AANS; 2000:91-140.
Zenteno M., Jaramillo J. The predictive value of intrapedicle sodium amobarbital injection in conjunction with pressure recording. AJNR Am J Neuroradiol. 1991;12:1241-1249.
1. Ray B.S. Cerebral arteriovenous aneurysms. Surg Gynecol Obstet. 1941;73:615-648.
2. Steiner L., Rankin P.B., Prasad D., Steiner M. Gamma surgery for vascular disorders of the brain. In: Germano I., editor. LINAC and Gamma Knife Radiosurgery. Neurosurgical Topics. New York: Thieme/AANS; 2000:91-140.
3. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
4. Larsson B., Leksell L., Rexed B., et al. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182:1222-1223.
5. Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797-803.
6. Johnson R. Surgery of cerebral haemorraghe. In: Barain L., Wilkinson M. Recent Advances in Neurology and Neuropsychiatry. 8th ed. London: Churchill; 1969:102-128.
7. Lunsford L.D. Stereotactic radiosurgery of intracranial arteriovenous malformations. In: Wilkins R.H., Rengachary S.S. Neurosurgery Update II: Vascular, Spinal, Pediatric and Functional Neurosurgery. New York: McGraw Hill; 1991:175-185.
8. Drake C.G. Arteriovenous malformations of the brain: the operations for management. N Engl J Med. 1983;309:308-310.
9. Steiner L., Linquist C. Radiosurgery in cerebral arteriovenous malformations. In: Tasker R.R., editor. Neurosurgery: State-of-the-Art Review. Stereotactic Surgery. Philadelphia: Hanley & Belfus; 1987:783-903.
10. Steiner L., Leksell L., Greitz T., et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Acta Chir Scand. 1972;138:459-464.
11. Dawson R.C., Tarr R.W., Hechst S.T., et al. Treatment of arteriovenous malformations of the brain with combined embolization and stereotactic radiosurgery: results after 1 and 2 years. AJNR Am J Neuroradiol. 1990;11:856-864.
12. Chang S.D., Marcellus M.L., Marks M.P., et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-13.
13. Lunsford L.D., Kondziolka D., Flickinger J.C., et al. Stereotactic radiosurgery for arteriovenous malformations. J Neurosurg. 1991;75:512-524.
14. Friedman W.A., Bova F.J. Linear accelerator radiosurgery for arteriovenous malformations. J Neurosurg. 1992;77:832-841.
15. Friedman W.A., Bova F.J., Mendenhall W.M. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995;82:180-189.
16. Flickinger J.C., Pollock B.E., Kondziolka D., Lunsford L.D. A dose–response analysis of arteriovenous malformations obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:873-879.
17. Pollock B.E., Kondziolka D., Lunsford L.D., et al. Repeat stereotactic radiosurgery of arteriovenous malformations: factors associated with incomplete obliteration. Neurosurgery. 1996;38:318-324.
18. Karlsson B., Linquist C., Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40:425-431.
19. Pollock B.E., Flickinger J.C., Lunsford L.D., et al. Factors associated with successful arteriovenous malformations radiosurgery. Neurosurgery. 1998;42:1239-1244.
20. Ellis T.L., Friedman W.A., Bova F.J., et al. Analysis of treatment failure after radiosurgery for arteriovenous malformations. J Neurosurg. 1998;89:104-110.
21. Karlsson B., Lax I., Soderman M. Can the probability for obliteration after radiosurgery for arteriovenous malformations be accurately predicted. Int J Radiat Oncol Biol Phys. 1999;43:313-319.
22. Chang J.H., Chang J.W., Park Y.G., Chung S.S. Factors related to complete occlusion of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 2000;93:96-101.
23. Pan H.C. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000;93:113-119.
24. Flickinger J.C. An integrated logistic formula for prediction of complications from radiosurgery. Int J Radiat Oncol Biol Phys. 1989;17:879-885.
25. Lax I., Karlsson B. Prediction of complication in gamma knife radiosurgery of arteriovenous malformations. Acta Oncol. 1996;35:49-55.
26. Flickinger J.C., Kondziolka D., Pollock B., et al. Complications from arteriovenous malformation radiosurgery: multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys. 1997;38:485-490.
27. Flickinger J.C., Lunsford L.D., Kondziolka D. Dose prescription and dose-volume effects in radiosurgery. Neurosurg Clin North Am. 1992;3:51-59.
28. Karlsson B., Lax I., Sodermann M. Factors influencing the risk for complications following gamma knife radiosurgery for cerebral arteriovenous malformations. Radiother Oncol. 1997;43:275-280.
29. Friedman W., Blatt D., Bova F., et al. The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg. 1996;84:912-919.
30. Pollock B.E., Flickinger J.C., Lunsford L.D., et al. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1996;38:652-659.
31. Karlsson B., Linquist C., Steiner L. Effect of gamma knife surgery on the risk of rupture prior to AVM obliteration. Minim Invasive Neurosurg. 1996;39:21-27.
32. Karlsson B., Lax I., Sodermann M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49:1045-1051.
33. Friedman W.A., Bova F.J., Bollampaly S., Bradshaw P. Analysis of factors predictive success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296-308.
34. Maruyama K., Shin M., Tago M., et al. Radiosurgery to reduce the risk of first hemorrhage from brain arteriovenous malformations. Neurosurgery. 2007;60:453-459.
35. Chang S.D., Marcellus M.L., Marks M.P., et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-13.
36. Meder J.F., Oppenheim C., Blustajn J., et al. Cerebral arteriovenous malformations: the value of radiologic parameters in predicting response to radiosurgery. AJNR Am J Neuroradiol. 1997;18:1473-1483.
37. Petereit D., Metha M., Turski P., et al. Treatment of arteriovenous malformations with stereotactic radiosurgery employing both magnetic resonance angiography and standard angiography as a database. Int J Radiat Oncol Biol Phys. 1993;25:309-313.
38. Zenteno M., Jaramillo J. The predictive value of intrapedicle sodium amobarbital injection in conjunction with pressure recording. AJNR Am J Neuroradiol. 1991;12:1241-1249.
39. Pollock B.E., Flickinger J.C. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002;96:79-85.
40. Luessenhop A.J., Spence W.T. Artificial embolization of cerebral arteries: report of use in a case of arteriovenous malformation. JAMA. 1960;172:1153-1155.
41. Frizzel R.T., Fisher W.S. Cure, morbidity and mortality associated with embolization of brain arteriovenous malformations: a review of 1,246 patients in 32 series of a 35-year period. Neurosurgery. 1995;37:1031-1040.
42. Haw C.S., terBrugge K., Willinsky R., Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006;104:226-232.
43. Andrade Souza Y.M., Ramani M., Scora D., et al. Embolization before radiosurgery reduces the obliteration rates of arteriovenous malformations. Neurosurgery. 2007;60:443-445.
44. Del Valle R., Perez M., Ortiz J., et al. Stereotactic noninvasive volume measurement compared with geometric measurement for indications and evaluation of gamma knife treatment. J Neurosurg. 2005;102:140-142.
45. Del-Valle R., Zenteno M., Jaramillo J., et al. Definition of the key target volume in radiosurgical management of arteriovenous malformations: a new dynamic concept based on angiographic circulation time. J Neurosurg. 2008;109:41-50.