Chapter 86 Rheumatoid Arthritis
Rheumatoid arthritis is a systemic disease of unknown cause that primarily involves small blood vessels and synovium. A chronic disease, it is generally more common in females. This disorder is characterized by polyarticular symmetrical involvement of the smaller joints of the appendicular skeleton. It destroys the articular joint surfaces and the joint capsules as well as the ancillary ligaments that support the joints.1 In addition, rheumatoid arthritis can be associated with osteoporosis and erosion and cyst formation in the bone.2 The extent of myelopathy in patients with rheumatoid arthritis is difficult to evaluate because their disease may be complicated by peripheral joint destruction, peripheral neuropathies, nerve entrapments, and rheumatoid myopathy.3
The most common skeletal manifestation of rheumatoid arthritis occurs with the involvement of the metatarsophalangeal joints of the feet. This is followed in frequency by rheumatoid involvement of the cervical spine and the metacarpophalangeal joints of the hands.4 Cervical spine involvement is very common, and symptoms do not necessarily accompany extensive bony changes of the spine.5 Rheumatoid involvement of the cervical spine may be present with minimal or no clinical or radiologic expression elsewhere in the body.4 The major clinical problems result from erosive changes in the cervical spine that lead to pathologic subluxation or dislocation.6 Involvement of the thoracic and lumbar regions may also occur, although this is much less common.
The three most common lesions that cause neurologic involvement and/or intractable pain are atlantoaxial subluxation, subaxial subluxation, and vertical subluxation of the odontoid process.7 The onset of cervical spine instability is often insidious. It may be masked by weakness and loss of function associated with peripheral joint disease.8 Although any synovial joint in the spine may be involved, the earliest changes are usually observed in the upper cervical region.3 Rheumatoid involvement of the cervical spine appears to begin early and progresses in relationship to peripheral joint involvement. The cervical spine abnormalities are the result of destruction in the joints, ligaments, and bone by synovitis.2
Atlantoaxial subluxation represents the most common and significant manifestation of rheumatoid involvement of the cervical spine.6 The extent of neurologic deficit does not correlate with the degree of subluxation observed on lateral radiographs. This discrepancy may be the result of the formation of a pannus between the dens and the dura mater. This may contribute to spinal cord compression that cannot be visualized on plain radiographs. In 1830, Sir Charles Bell9 reported the first case of an atlantoaxial subluxation resulting from an inflammatory process. Incompetence of the transverse atlantal ligament was demonstrated pathologically.
The rate of development of neurologic signs and symptoms is usually slow. Numerous large surgical and nonsurgical series of patients with rheumatoid arthritis have demonstrated that the average duration of disease before surgery is 15 to 20 years.7,8,10–15 Although life expectancy in patients with moderate or severe rheumatoid arthritis is less than that of the general population, the presence of a cervical subluxation does not necessarily influence life expectancy.14
The most common clinical finding in rheumatoid involvement of the cervical spine is pain. Typically, this pain is severe and persistent and is usually located in the occipital region4,10,16,17 or the neck,18 or it may radiate toward the vertex of the skull.16 Characteristically, this pain is exacerbated by neck motion.4,5,19 Tears in the transverse atlantal and alar ligaments and in the atlanto-occipital membrane may give rise to retro-orbital or temporal region pains.19 Pain in the arms is usually absent. This type of pain helps distinguish these disorders from cervical spondylosis.20 Neurologic signs and symptoms that are useful for detection of the onset of myelopathy in rheumatoid arthritis patients include neck pain, occipital neuralgia, Lhermitte sign (electric-like shocks produced with neck flexion), and the patient’s account of diminished motor ability or a documented worsening in the motor examination from the previous neurologic examination.21
Myelopathy may develop as a result of spinal cord compression. This usually occurs in late middle age, following many years of disability.21 Because of the frequent, severe deforming effects of rheumatoid arthritis in the extremities, the development of long tract signs is useful in detecting myelopathy. These include hyperreflexia and extensor plantar responses.12,17 Additional signs of myelopathy such as spasticity, presence of a Hoffman sign, and ankle clonus may also be detected. The myelopathy that occurs in rheumatoid arthritis is most likely caused by the effects of compression, stretch, and movement, not by ischemia.22
Because of patient selection and referral patterns, there is a marked discrepancy between the incidence of neurologic involvement of rheumatoid disease of the cervical spine in surgical and nonsurgical series. In two large nonsurgical series of more than 2000 patients with rheumatoid arthritis, reported by Smith et al. and Nakano et al., the incidence of cervical myelopathy was less than 3% in each series.12,14 Interosseous erosive disease of the peripheral joints has been correlated to the severity of involvement of the cervical spine.18,24
The executive committee of the American Rheumatism Association established criteria for rheumatoid arthritis that must be applied only to patients with a clear-cut diagnosis. In addition, this committee has adopted a classification of functional capacity that is used in rheumatologic studies.25
Ranawat et al. developed a classification based on signs and symptoms.7 In this surgical series, neurologic deficits were divided into three classes: I, no neurologic deficits; II, subjective weakness, hyperreflexia, and dysesthesias; and III, objective weakness and long tract signs (A, ambulatory, and B, quadriparetic, not ambulatory).
Vertebral artery compression leading to vertebrobasilar insufficiency may occur in rheumatoid arthritis. The most common potential sites for mechanical compression of the vertebral artery include the foramen transversarium, the atlantoaxial joint, and the occipitoatlantal joint. Vertebral artery insufficiency may be the result of kinking of the vertebral artery at one of these locations or the involvement of the brainstem by upward migration of the dens.24 Symptoms that have been attributed to vertebral artery insufficiency secondary to rheumatoid disease of the cervical spine include dizziness, tinnitus, vertigo, diplopia, suboccipital headache, dysphasia, blurring of vision, cortical blindness, nystagmus, transient blackout spells, confusion, and dysarthria.17,24–26 The frequency with which vertebral artery symptoms may occur is poorly documented. Henderson et al. published nine autopsy cases of severe end-stage rheumatoid arthritis patients with vertical subluxation of the dens that showed the vertebral artery to be patent in all specimens.22
The pathologic changes in rheumatoid arthritis of the cervical spine are predominantly secondary to synovitis. Synovitic proliferation destroys the facet joints, erodes and deforms the dens, and weakens the ligamentous attachments.24 A characteristic pannus may form between the odontoid peg and the ventral dura mater that can compress the spinal cord. This pannus is usually firm, gray-pink tissue that shows end-stage chronic inflammation of synovial tissue.21 In addition to this proliferation of the synovial tissue, osteoporosis and destruction of cartilage and subchondral bone can occur.4,26 Localized bone loss around the inflamed joints is the result of prostaglandin and cytokine synthesis during the inflammatory process, which increases bone resorption.27 Inflammatory destruction of the lateral atlantoaxial joints can lead to vertical translocation of the dens.24 When the odontoid process herniates through the foramen magnum, it can cause flattening, softening, and atrophy of the medulla.28
The specific mechanism by which ischemia causes damage to the spinal cord is unclear. Two separate studies have postulated that intermittent compression of the ventral spinal artery was responsible for the spinal cord injury.1,12 However, in a necropsy study of nine patients, Henderson et al. demonstrated that the histopathologic changes were localized principally to the dorsal white matter of the spinal cord. In this study, the territory of the ventral spinal artery was spared.22 O’Brien et al. performed a histologic study of specimens removed during ventral decompressions of the cervicomedullary junction. They determined that repetitive mechanical damage caused by instability at the atlantoaxial joint, rather than an acute compressive effect from an inflammatory pannus, was the cause of spinal cord compression and subsequent axonal injury.29 In addition, Crockard and Grob have stated that there is no evidence that avascular necrosis or vasculitis is involved in the inflammatory process of rheumatoid arthritis involvement of the spine. They believe that it is the repetitive movement of the unstable atlantoaxial joint against the neuraxis that causes a mechanical “wear-and-tear” phenomenon that leads to the development of a myelopathy.21
In rheumatoid arthritis (due to deficient osteogenesis) osteophytes do not form.1,4,16 This may be contrasted to osteoarthritis that is characterized by the development of osteophytic spurs, which often have a stabilizing effect.1,16,19
Imaging Studies
In a study by Younes et al., 72.5% of patients who met American College of Rheumatology criteria for rheumatoid arthritis had pathology of the cervical spine identified in at least one imaging modality (plain radiographs, MRI, or CT).30
On plain radiographs, small or absent osteophytes, osteoporotic vertebrae, and eroded vertebral end plates characterize rheumatoid arthritis.4 In the absence of rheumatoid arthritis, degenerative changes are more marked in the lower cervical spine with advancing age. Radiographic changes at the C1-2 level are not present unless a specific process, such as rheumatoid arthritis, is affecting this region. These changes are independent of age.30 Bland et al. found 86% of patients with classic or definite rheumatoid arthritis to have evidence of cervical spine involvement on plain radiographs. These changes may frequently be asymptomatic and may not be associated with any neurologic deficit.5 In patients with severe polyarticular rheumatoid arthritis for more than 20 years, Santavirta et al. found radiographic subluxation of the cervical spine in more than 80% of patients.13
The most common radiologic abnormality in rheumatoid arthritis is ventral subluxation of the atlas. The atlantodental interval (ADI) measures the distance between the ventral aspect of the dens and the dorsal ring of the atlas. In normal adult patients, this distance is less than 3 mm.31 An ADI of between 3 and 5 mm in an adult is abnormal and indicates a tear or insufficiency of the transverse atlantal ligament. A separation of greater than 5 mm indicates rupture or attenuation of the alar ligaments in addition to the transverse atlantal ligament.16 Many studies have shown a poor correlation between abnormalities of the ADI and neurologic deficits. In an innovative study, Boden et al. defined the dorsal atlanto-odontoid interval (AOI) as the distance between the dorsal surface of the dens and the ventral edge of the dorsal ring of the atlas measured along the transverse axis of the ring of the atlas. They demonstrated an excellent correlation between the dorsal AOI and the severity of the neurologic deficit. A dorsal AOI of less than 14 mm correlated significantly with the presence and severity of a neurologic deficit. This dorsal AOI was also found to be a good predictor of neurologic recovery postoperatively. These authors stated that the weak correlation of the ventral ADI with a neurologic deficit may be due to variations in the diameter of the atlas as well as the presence of a pannus behind the odontoid process.32 Weissman et al. studied 109 patients with rheumatoid arthritis and atlantoaxial subluxations for 5 years. They found that the ADI increased by more than 2 mm in 41%, remained unchanged in 40%, and decreased in 19% by more than 2 mm. Of the patients whose ADI decreased, more than 50% had developed vertical subluxation of the odontoid process.15 Henderson et al. have stated that vertical subluxation may cause a pseudofixation of the ADI. Interpretation of the ADI must therefore be made in conjunction with measurement of the vertical axial subluxation.22
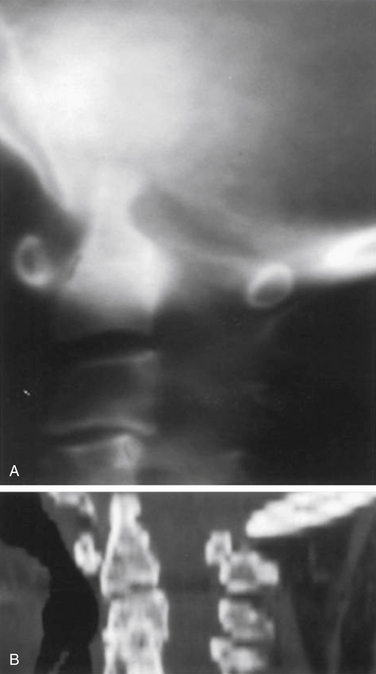
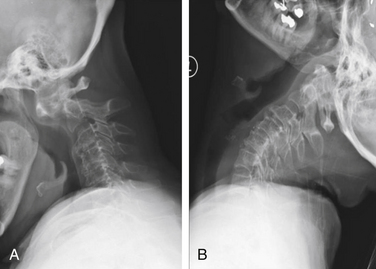
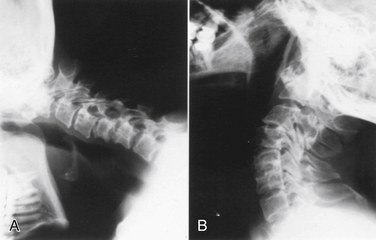
Vertical subluxation of the dens is an upward migration of the dens into the foramen magnum (Fig. 86-1). In this location, the dens competes for space with the spinal cord and the brainstem. Vertical subluxation requires the lateral facet joints to be damaged.12 Vertical subluxation is the second most common upper cervical spine abnormality in rheumatoid arthritis, after atlantoaxial subluxation, and may accompany ventral atlantoaxial subluxation. An increase in the vertical subluxation may actually produce a decrease in the measured ventral atlantoaxial subluxation.15 In vertical subluxation, the presence of a rheumatoid pannus, together with the invaginated dens, may produce ventral compression of the cervicomedullary junction.17
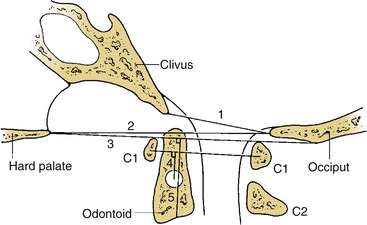
In an attempt to quantify vertical subluxation of the dens, numerous lines and indices have been proposed (Fig. 86-2). These lines are characteristically drawn between bony landmarks that are identifiable on a lateral cervical spine radiograph. The tip of the dens is then measured with respect to the various lines. A McGregor line is drawn from the hard palate to the occiput, and it is probably the most traditional measure.2 A shortcoming of these lines is that the hard palate, the dorsal edge of the foramen magnum, and the tip of the dens are not always visualized on routine lateral cervical spine radiographs in a patient with vertical subluxation of the odontoid. To avoid these difficulties, Ranawat et al. developed an index that measures the distance between the diameter of the ring of the first cervical vertebra to the center of the pedicle of the second cervical vertebra.7 In men and women with normal cervical spines, this distance measured 17 mm in men and 15 mm in women. No normal patients had an interval of less than 15 mm.
A cervical myelogram provides an indirect visualization of the spinal cord and adjacent structures silhouetted by contrast media in the subarachnoid space.33 The information obtained by myelography may be augmented by a postmyelogram CT. Computerized myelotomography, with multiplanar reconstruction, can clearly demonstrate the important contribution of proliferative rheumatoid pannus behind the odontoid peg to ventral cervicomedullary compression. In addition, bone windows on the CT scan can demonstrate bony abnormalities.
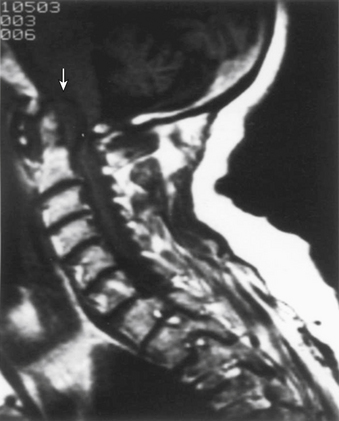
Currently, MRI is considered the ideal study to demonstrate the level and extent of spinal cord compression.33,34 The advantages of MRI include direct imaging of the entire length of the spinal cord and the cervicomedullary junction, direct imaging in multiple planes, superior demonstration of soft tissue structures, and the lack of image degradation by bone artifact at the cervicomedullary junction (Fig. 86-3). In addition, MRI is noninvasive (no intrathecal contrast is needed), can be performed on an outpatient basis, does not expose the patient to the dangers of ionizing radiation, and is generally well tolerated.6,33,35 A limitation of MRI is that it does not image compact bone as accurately as CT.34
Furthermore, Dickman et al. have shown that the transverse atlantal ligament can be consistently and clearly visualized with MRI. This determination of a loss of anatomic continuity of the transverse atlantal ligament is useful for operative planning.36 For patients who are unable to undergo MRI, CT scanning with multiplanar reconstructions provides better information than polytomography. It is important to visualize the neuraxis before surgery; injecting water-soluble contrast into the lumbar subarachnoid space provides additional important anatomic information regarding the flow of cerebrospinal fluid in the upper cervical spine region. Knowledge of the position and course of the spinal vessels, in particular the vertebral arteries, is of extreme importance in many of the surgical approaches to the craniocervical junction. CT angiography provides an excellent means of visualizing the spinal vasculature in relation to the bony anatomy of the upper cervical spine.
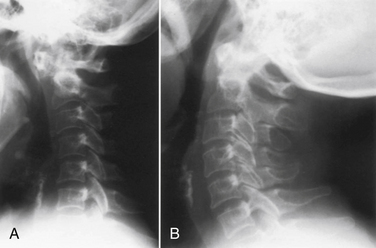
Dynamic views of the cervical spine, in flexion and extension, provide useful information about the degree of instability of the occiput–C1-2 complex (Fig. 86-4). These flexion-extension views can be obtained on plain films, tomograms, computerized myelotomography scans, and MRI studies. Dickman et al. recommend routine lateral cervical radiographs in flexion and extension for all patients undergoing transoral surgery.36 Furthermore, Sharp and Purser recommend lateral flexion and extension radiographs in any patient with severe rheumatoid arthritis undergoing general anesthesia for any surgical procedure.37 Bell and Stearns have found MRI in flexion and extension to be very useful for demonstrating dynamic changes in spinal cord configuration. They have used this information to determine the proximal extent of fusion required and have stated that it provides an accurate and dynamic study of the relationship between the spinal cord and the surrounding bony and soft tissue structures.38 Additionally, upright MRI of the cervical spine can help reveal pathology that may be missed at the craniocervical junction in supine position.39,40
Operative Indications
As a general rule, the presence of myelopathy or a progressive neurologic deficit continues to be a strong indication for surgical intervention in patients with rheumatoid changes of the cervical spine.*
Likewise, intractable pain is an indication for operative intervention.2,3,6,11,18,24,29,32 A much more controversial topic is whether a patient with markedly abnormal radiographs, in the absence of pain or progressive neurologic deficit, should undergo surgery. Numerous authors believe that radiologic evidence of an impending neurologic deficit is an indication for surgery.1,7,41,42 Pellicci et al. believe that radiographic evidence of disease progresses to a greater degree than does neural involvement.18 As such, they believe that no fusion should be recommended on the basis of a radiographic abnormality or radiographic deterioration alone. Others have supported this more conservative viewpoint.2,13,19,28 In an intermediate position, Boden et al. stated that only patients with a neurologic deficit underwent surgery in their series. Nevertheless, they recommended surgery for patients with or without a neurologic deficit, if certain radiographic criteria are met.32
Specific Regions of the Spine
Upper Cervical Spine
To fully understand the complex pathologic changes that occur in the occiput–C1-2 region, a thorough understanding of the biomechanics of this region is necessary. The majority of axial rotation occurs in the upper cervical spine. Approximately 60% of the axial rotation of the entire cervical spine and occiput is found in the upper region (occiput–C1-2) and 40% is found in the subaxial region (below C2). There is no axial rotation between the occiput and C1 unless there has been an occipitoatlantal disruption.44 An extensive amount of axial rotation, 47 degrees, occurs at the C1-2 level.45 White and Panjabi have identified two distinct reasons why this increased motion can occur at the C1-2 level. First, the articular surfaces of the lateral masses of both C1 and C2 have a convex orientation in the sagittal plane that allows for considerable mobility. Second, a taut yellow ligament is not present between the dorsal elements of C1 and C2. Instead, a loose, readily mobile atlantoaxial membrane connects the dorsal elements of C1 and C2, thereby enhancing the motion capacity of this region.45 Craniovertebral junction instability in rheumatoid arthritis patients occurs from a combination of bone softening, ligamentous destruction, and inflammatory pannus formation.46 Unlike the lower cervical spine, the upper cervical spine cannot clearly be divided into ventral and dorsal elements with corresponding mechanical properties.47 White and Panjabi have defined clinical instability as “loss of the capacity of the spine, under physiologic loads, to maintain relationships between vertebrae so that no spinal cord or nerve root damage occurs and no incapacitating deformity or pain develops.”45 This definition has led to certain anatomic measurements from plain film radiographs that can lead to a suspicion of clinical instability. In adults, a ventral ADI of greater than 3 mm on a lateral radiograph is regarded as potentially unstable. Although less widely used, a dorsal AOI of less than 14 mm is also suspicious for clinical instability.28 With this basic understanding of the biomechanical relationships of the upper cervical spine, the two most common abnormalities in this region are ventral atlantoaxial subluxation, caused by incompetent ligaments, and vertical subluxation of the odontoid process that occurs because of lateral mass erosion.
Atlantoaxial Subluxation
Ventral atlantoaxial subluxation is the most common abnormality of the spine in rheumatoid arthritis. Classically, atlantoaxial subluxation is considered present if the ADI is greater than 3 mm in an adult or greater than 4 mm in a child.24 Due to selection biases between surgical and nonsurgical studies of patients with rheumatoid arthritis, there are marked discrepancies in the frequency with which atlantoaxial subluxation occurs. In a large study in the rheumatologic literature on the prevalence of atlantoaxial subluxation in rheumatoid arthritis patients, the authors found that an atlantoaxial subluxation was present in 1 of 30 patients with any evidence of rheumatoid arthritis, in 1 of 15 patients with clinical evidence of the disease, and in 1 of 5 patients with rheumatoid arthritis admitted to the hospital.37 This last group of patients are those more likely to be seen by a spine surgeon. Indeed, in the study by Weissman et al., ventral atlantoaxial subluxation was found to occur in one fourth to one third of rheumatoid arthritis patients.15 Disruption of the transverse ligament of the atlas is the most important pathologic abnormality responsible for atlantoaxial instability.36
The degree of atlantoaxial subluxation is poorly correlated with clinical evidence of a compressive myelopathy.33 Similarly, no correlation between the magnitude of an atlantoaxial subluxation and mortality was found in a 10-year follow-up study.13 In a large radiologic review of 189 rheumatoid arthritis patients with atlantoaxial subluxations, Weissman et al. found the incidence of spinal cord compression to be 11%.15 These findings underscore the necessity of an accurate history and physical examination to determine which patients with atlantoaxial subluxations demonstrate evidence of neurologic abnormalities related to this radiographic abnormality. A characteristic clinical finding of ventral atlantoaxial subluxation is difficulty in raising the head to the neutral position following downward gaze. This sensation of the head “falling forward” has been referred to as the Sharp-Purser sign.37 An extremely rare abnormality is dorsal atlantoaxial subluxation. For a dorsal atlantoaxial subluxation to occur, the dens must be destroyed, fractured, or congenitally absent; alternatively, the ventral arch of the atlas must be destroyed or congenitally absent.15,31,48,49
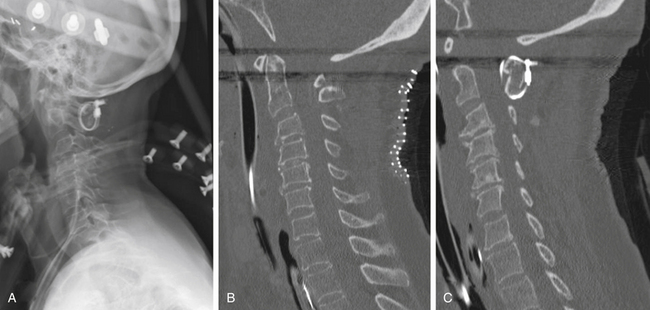
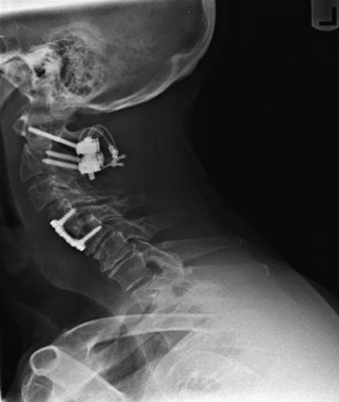
Vertical subluxation of the dens can occur simultaneously with a ventral atlantoaxial subluxation. When this occurs, the ADI can appear to actually decrease in size.50 Isolated ventral subluxation is more common than either vertical subluxation or a combination of ventral subluxation and vertical subluxation. Kraus et al. studied 55 patients with atlantoaxial subluxations who received dorsal fusion and found that none developed subsequent vertical subluxations. They believed that the dorsal fusion operation may have a protective effect in preventing vertical subluxation.11 This protective effect of dorsal fusion has not been demonstrated in a prospective study. Contrary to the findings of Kraus et al.,11 we present the case of a patient with rheumatoid arthritis that initially presented with atlantoaxial subluxation that was treated with a Brooks-type fusion. The patient subsequently developed vertical subluxation 6 months after the operation despite fusion at C1-2, which was treated with occipitocervical fusion (see Figs. 86-8 to 86-11).
Vertical Subluxation of the Odontoid
Vertical subluxation of the dens is referred to by a variety of terms, including atlantoaxial impaction, cranial settling, upward migration of the odontoid, pseudobasilar invagination, translocation of the dens, superior migration of the odontoid, basilar invagination, and vertical settling. These terms have been used interchangeably in the medical literature. Vertical subluxation occurs as a consequence of loss of substance of the lateral masses, usually the atlas. However, the lateral masses of the axis and, less commonly, the occipital condyles may also be involved.22 In vertical subluxation, the ventral arch of C1 gradually articulates with caudal portions of C2, first via the base of the dens and then with the body of C2.15 If there is more than 18 mm of translocation, the ring of the atlas is usually broken and the base of the axis has migrated within the disrupted ring. Significant vertical subluxation of the dens is generally agreed to be an indication for a fusion operation.7,13,15–17,28,42
Neurologic abnormalities are far more common with vertical subluxation of the dens than with atlantoaxial subluxation. The abnormal neurologic symptoms are the result of compression of the brainstem or upper cervical spinal cord. Neurologic abnormalities detected in vertical subluxation of the dens include hyperreflexia, extensor plantar reflexes, limb paresthesias, progressive difficulty with ambulation, a central cordlike syndrome, neurogenic bladder, and lower cranial nerve palsies.17 Rheumatoid arthritis patients with vertical subluxation are more likely to be symptomatic from spinal cord compression.13,15,17,28,51
Lower Cervical Spine
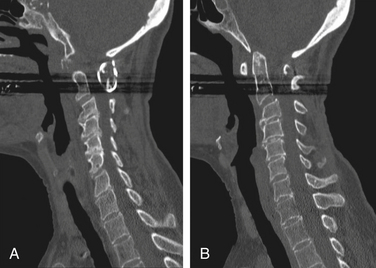
As with abnormalities of the upper cervical spine, the presence of subaxial subluxations of the lower cervical spine varies greatly in reported series. Subaxial subluxation is a gradual process in which there is a forward displacement of one or more vertebral bodies on the vertebral body immediately below (Fig. 86-5). The rheumatoid process attacks the zygapophyseal facet and uncovertebral joints, weakens the supporting ligaments, and erodes the intervertebral discs. These effects loosen the various intervertebral fixations and permit luxation between adjacent vertebral bodies.1 Subaxial subluxations are usually reducible by traction; however, the reduction is difficult to maintain.29 Classically, subaxial subluxation is a translation of one vertebra in relation to an adjacent vertebra of greater than 3.5 mm on a lateral cervical spine radiograph.31 Boden et al. found that the diameter of the subaxial sagittal spinal canal reflected the presence and degree of a neurologic deficit, more often than did the percentage of vertebral body slip.32 In addition to the bony luxations, compression from epidural rheumatoid granulations has been reported.2–4
Subaxial subluxations are usually late developments in the aggressive forms of rheumatoid arthritis.4,13 The severity of these subaxial subluxations is also closely related to the duration of rheumatoid disease.4,14 When compared with patients with atlantoaxial subluxations, the incidence of neurologic deficits is higher in patients with subaxial subluxation, and the final results are poorer.8
Typically, subaxial subluxations show a “staircase” appearance with multiple subluxations observed sequentially in the cervical spine.2,6,12 Subaxial subluxations may result in nerve root impingement from foraminal narrowing.6 The presence of a subaxial subluxation should be suspected when bizarre weakness of the hands is observed in a patient with rheumatoid arthritis. This may be difficult in patients with long-standing disease who already have crippling deformity of the hands.52 This subaxial spinal cord involvement may cause myelopathy and may explain why some patients do not respond to surgical decompression at the craniocervical junction.22 The most common site for subaxial subluxation is at the C3-4 level; however, it often occurs at multiple levels, and these typically lack osteophytes.2,3,53
Subaxial subluxations may be present at the time of surgery for an upper cervical instability. The caudal extent of the instrumentation and fusion must therefore be carefully selected to incorporate any segments with a significant subaxial subluxation.54,55 In an autopsy study of nine patients with severe end-stage rheumatoid arthritis, Henderson et al. found subaxial compression present in eight of the nine patients.22 More commonly, subaxial subluxations may occur after fusion operations in the upper cervical spine.3,6,11,53 As a result, it is essential that patients who undergo upper cervical fusions be followed closely with lateral cervical spine radiographs both for evidence of an acceptable bony fusion and for the development of subaxial subluxations. Kraus et al. found a much higher incidence of subaxial subluxations in patients whose upper cervical fusion incorporated the occiput. They postulated that this was because of a longer lever arm that results in higher forces generated at the lower cervical levels and that leads to a subaxial subluxation.11 Subaxial cervical subluxations in rheumatoid arthritis patients have been associated with quadriplegia, sudden death, and other neurologic complications resulting from damage of the spinal cord or from interference with the flow of the vertebral arteries.56
The most accurate radiographic imaging study currently available to detect the site and type of spinal cord compression in subaxial subluxation of the rheumatoid spine is MRI.13 All patients with subaxial subluxations and atlantoaxial subluxations with neurologic deficits or vertical subluxations of the dens should have an MRI study, if possible.8 In patients who cannot undergo an MRI, a myelogram followed by a postmyelogram CT scan should be obtained.
Thoracic and Lumbar Spines
Involvement of the thoracic and lumbar spines in rheumatoid arthritis patients is rare. Heywood and Meyers have stated that subcervical rheumatoid spondylitis is more common than is generally believed and have found an incidence of 0.9% in their clinic.57 Most other series of rheumatoid arthritis patients do not even address the topic of the thoracic and lumbar spines. In a study by Redlund-Johnell and Larsson, of 100 patients with severe rheumatoid arthritis who had previously undergone occipitocervical fusion, four patients with subluxation of the upper thoracic spine were found. These changes in the upper thoracic spine were radiologically similar to the destructive type of subaxial subluxation typically observed in the cervical spine. The authors believed that this upper thoracic subluxation may have been caused by increased motion as compensation for decreased mobility in the cervical spine.58 The pathogenesis of rheumatoid spondylitis in the thoracic and lumbar spines is believed to be the result of a facet joint synovitis that leads to instability. In addition, involved costovertebral joints can spread their involvement to the disc space, causing a discitis between the disc and the vertebral end plates that can lead to further instability.57 Rheumatoid granulation tissue causing compression of the thoracic spine has been reported.59,60 In addition, an extensive inflammatory synovitis of the costovertebral and costotransverse joints can occur in the thorax.61
Rheumatoid involvement of the lumbar spine is rarely recognized as a cause of nerve root or cauda equina compression. Intraspinal rheumatoid granulomatous nodules can lead to nerve root compression, causing back and leg pain.59,62 In addition, rheumatoid granulation tissue can develop in the lumbar facet joints and spread to the periarticular structures to contribute to cauda equina compression.63,64
The plain radiographic features of subcervical rheumatoid spondylitis include an ill-defined, blurred, and eroded margin to the vertebral end plates and an ill-defined, eroded facet joint complex.57 If not specifically sought, these radiographic abnormalities may be easily overlooked. Frequently, rheumatoid arthritis patients have stiff, high-positioned shoulders that may conceal the cervicothoracic junction on conventional radiographs. If clinically indicated, an MRI study allows for better visualization of the cervicothoracic junction and the remainder of the thoracic and lumbar spines.58
Surgical Management of the Rheumatoid Spine
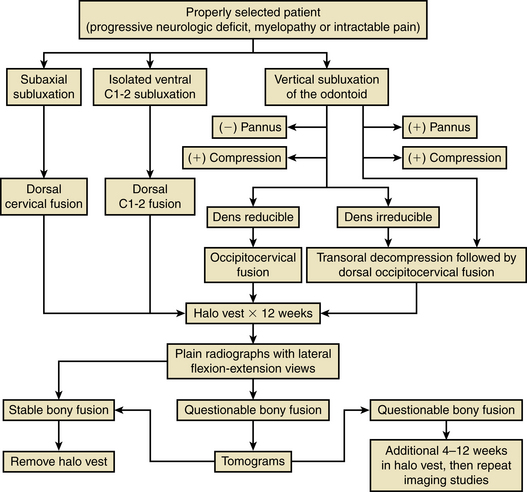
Many different operations have been recommended for patients with rheumatoid arthritis of the spine (Fig. 86-6). The various options include a dorsal cervical fusion of C1-2, an occipitocervical fusion, a transoral approach, ventral and dorsal approaches to the subaxial cervical spine, and various combinations of these approaches. With the advent of spinal instrumentation techniques, surgeons have a wealth of different treatment options available to treat rheumatoid arthritis patients. In addition, nonoperative modalities including traction and halo-vest immobilization, as well as adjuvant medical therapies such as steroids, nonsteroidal anti-inflammatory drugs, penicillamine, and methotrexate, enter into the decision-making process.
Most rheumatoid arthritis patients never develop spinal instability and never require spine surgery. As previously described, large studies of patients from rheumatology clinics have demonstrated that the clinical course of rheumatoid arthritis is usually benign. However, the natural history of the disease is that a certain proportion of affected patients eventually require surgery. Pellicci et al. followed 106 patients for more than 5 years and found that diligent use of a supportive collar does not alter the natural history of the disease.18 Similarly, More and Sen have stated that the realistic treatment goals achieved by conservative measures are pain abatement and reduction of inflammation. The natural history of the disease is not altered.19 Pellicci’s study also documented that rheumatoid involvement of the cervical spine does not appear to shorten life expectancy.18 These findings are most likely a result of the small number of rheumatoid arthritis patients who develop cervical myelopathy. When cervical myelopathy is established, the natural history without surgical intervention is grave.2 In addition, the development of a subaxial subluxation or a vertical subluxation in a patient with a preexisting atlantoaxial subluxation has been found to be a poor prognostic sign.18 The factors that influence the treatment of upper cervical lesions in rheumatoid arthritis are reducibility of the abnormality with traction, the type of compressive lesion (whether of a bony or a soft tissue component), and the direction and mechanics of the spinal cord or brainstem compression.51
With appropriate operative indications, the treatment of an isolated atlantoaxial subluxation is a dorsal C1-2 fusion. In these cases, the occiput should not be incorporated into the fusion because the higher complication rate, lower fusion rate, and morbidity associated with the decreased range of neck motion all mitigate against this procedure.
If vertical subluxation of the dens is present, or a combination of a vertical subluxation with an atlantoaxial subluxation is present, an occipitocervical fusion may be required. To determine whether an occipitocervical fusion alone is adequate, or a ventral decompression is necessary, cervical traction, in addition to the dorsal stabilization, may be used to determine whether the subluxation is reducible.15,17,46 Transoral decompression may also be deemed necessary if significant soft tissue rheumatoid granulation—that is, a rheumatoid pannus—is responsible for compression of the spinal cord.65
Viewpoints in the management of vertical subluxation of the dens are divergent, as has been shown in numerous studies published by Menezes et al.16,17,51 and Crockard et al.21,54,65 Menezes et al. maintain that it is the reducibility of a vertical subluxation that is the primary determinant of whether a transoral decompression is necessary. They determined that in 80% of their patients, an adequate reduction of the vertical subluxation could be achieved with traction and, therefore, only a dorsal occipital cervical fusion was necessary. Despite the reducibility, all 45 patients in this series were symptomatic because of compression of the cervicomedullary junction. In addition, these authors have stated that decompression of the cervicomedullary junction, via a transoral approach, is necessary for irreducible lesions. They found that all patients with greater than 20 mm of penetration of the dens through the foramen magnum were unable to have their deformity reduced with cervical traction and required a transoral decompression.17 Dickman et al. have also recommended a transoral decompressive procedure for irreducible craniovertebral junction compression.46 Both Menezes et al. and Dickman et al. recommend that the ventral decompressive surgery should be performed first and subsequent dorsal internal fixation later.17,46 Transoral surgery involves the resection of the dens, the ventral arch of the atlas, the ventral longitudinal ligament, the apical ligament, the alar ligament, the transverse atlantal ligament, and the tectorial membrane.46 As such, the spine is destabilized after this procedure, necessitating a dorsal fusion. The advantages of the transoral approach to irreducible craniovertebral junction pathology have been described by Menezes and van Gilder. These include performing surgery in an avascular midline plane, accessibility to both bony and soft tissue pathology, and performing surgery with the patient’s head in an extended position (which decreases brainstem angulation and compression during the surgery). This approach allows for exposure from the lower half of the clivus to the C2-3 interspace. These authors emphasized the need for a tracheotomy in all transoral surgeries.51
Crockard et al. have stressed the contribution of a ventral compressive agent, the rheumatoid pannus, to be as important as any bony deformity in the development of spinal cord compression.54 Crockard and Grob stated that the overall results of dorsal cervical surgery alone for patients with cervical myelopathy have been unsatisfactory, with excessively high morbidity and mortality rates.21 For patients with an established myelopathy in whom the pannus has been demonstrated to contribute to the cervicomedullary junction compression, Crockard performs a transoral decompression with a dorsal occipitocervical fusion in a single one-stage operation with the patient in the lateral position. Traditional skull traction is not used at any stage, but the head is held in the Mayfield retractor with a tilt table, which, in effect, functions like skull traction. In Crockard’s experience, this one-stage procedure has led to fewer complications and better long-term results than either a two-stage operation or a dorsal fixation procedure alone.54 Performing both operations at a single sitting avoids the discomfort of prolonged bed rest and the complications associated with prolonged immobilization. In most patients, Crockard has not found it necessary to perform a tracheotomy.21,54,65
As with the operative approaches to vertical subluxations, treatment of subaxial subluxations of the cervical spine is varied. Simpson et al. have stated that surgery for subaxial subluxations involves a dorsal fusion and that the role of ventral surgery is unclear in this condition.6 Santavirta et al. have indicated that reduction of the bony subluxation with a fusion is not adequate in patients with spinal cord compression from subaxial subluxation because rheumatoid granulation tissue in the sublaminar space may continue to compress the neural elements. Therefore, they recommend laminectomy in addition to this fusion procedure.3 King has stated that a dorsal decompression may relieve the spinal cord compression. It may also, however, worsen spinal instability.41 At present, the optimal surgical treatment for subaxial subluxation of the rheumatoid cervical spine is unclear. Further experience with the various ventral and dorsal techniques is necessary to clearly delineate which procedure is most advantageous.
Surgical Techniques
The need for bone grafting in all patients has been challenged. Although it is uniformly agreed that young patients and patients with a reasonable prospect for long-term survival require grafting, Crockard et al.21,54 and Moskovich et al.66 contend that the results of arthrodesis in end-stage rheumatoid arthritis patients are very poor. Therefore, they evaluate each case on an individual basis, and in selected situations, perform a fixation procedure without a supplemental bony fusion. This nonfusion approach has not been used by the authors of this chapter.
C1-2 Stabilization
Dorsal Techniques
Wire and Cable Fixation
In 1910, Mixter and Osgood described a patient with atlantoaxial instability secondary to a nonunion of an odontoid fracture. They successfully treated this fracture by fixing the dorsal arch of the atlas to the spinous process of the axis using a stout, braided silk thread that had been soaked in tincture of benzoin. This case report represented the first documented C1-2 stabilization procedure.67 Although not mentioned in the report by Mixter and Osgood, use of bone graft materials to promote a long-term stable bony fusion of the axis and the atlas was soon recognized to be a necessary portion of any upper cervical spine stabilization procedure.
Gallie-Type Fusion
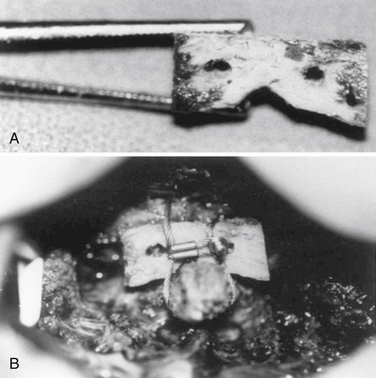
Axial rotation is the major motion that occurs at the C1-2 level. Although this motion is a movement that is clinically important to control, translation is the main pathologic movement at the C1-2 level.45 Gallie, in 1939, described a method to prevent the recurrence of cervical subluxation at the C1-2 level. He fastened the two vertebrae together with a fine steel wire passed beneath the dorsal arch of the atlas and around the spinous process of the axis. He stated that the risk of a late recurrence could be eliminated if bone grafts were applied to the construct. Gallie used a tricortical bone graft in an onlay fashion.44 Dickman et al. have described a modification of a Gallie-type C1-2 fusion. In their modification, they interpose a bicortical iliac graft between the dorsal arch of C1 and the lamina of C2.68 This construct attempts to compress the bone graft between the two bony surfaces of the dorsal elements in an attempt to increase the fusion rate (Fig. 86-7); this type of construct has higher rotational stability as compared with Gallie’s original technique.69
Brooks-Jenkins Fusion
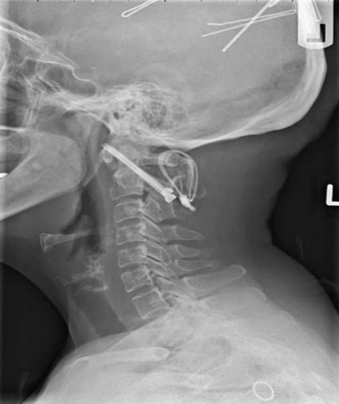
The next major modification to the Gallie-type C1-2 fusion procedure was proposed by Brooks and Jenkins in 1978. They described a wedge compression arthrodesis of the atlantoaxial joint that was performed by placing sublaminar wires beneath the lamina of C2 and the dorsal arch of C1 bilaterally. Separate bone grafts were placed on each side of the midline and were secured separately. The authors stated that the procedure was rarely indicated in patients with long-standing rheumatoid arthritis or in patients with severe osteopenia. Despite this warning, Brooks-type fusions have been performed frequently for rheumatoid arthritis (Figs. 86-8 to 86-11).70
Halifax Interlaminar Clamp
The dangers inherent in both the Brooks-type and the Gallie-type fusions are the necessity to pass the wire beneath the dorsal elements of the upper cervical spine. In doing so, compression from a ventrally located pannus can actually be increased and can lead to neurologic worsening. In an attempt to avoid this complication, techniques were developed that attempted to avoid the need to pass a sublaminar wire. In 1984, Holness et al.71 reported the use of an interlaminar clamp. This device was originally developed in Halifax, Nova Scotia, Canada, and is commonly referred to as the Halifax clamp. The Halifax clamp involves the bilateral placement of hooks over the dorsal arch of C1 and under the lamina of C2, which are held together by a screw. In their original description, Holness et al. did not advocate routine bone grafting. They did, however, endorse bone grafting in rheumatoid arthritis patients. The Halifax clamp provided numerous advantages, including a decreased risk of dural penetration, a decreased risk of neurologic injury, ease of use, elimination of wire cutout in osteoporotic bone, and more recently, the development of MRI-compatible clamps to allow for better postoperative imaging.71 The motion for which the Halifax clamp is particularly suited is the resistance of flexion.20 In rheumatoid C1-2 ventral subluxation, rotation is the motion that must be controlled, and Halifax clamps do not control rotational movement well. As such, rotational instability can occur, and this can lead to failure.20,72
Sonntag described a modification to the Gallie technique providing improved rotational stability without a second sublaminar cable at C2.68 In the Sonntag technique, a sublaminar cable is passed under the dorsal arch of C1 from caudal to rostral. Next, an iliac crest graft is fashioned in an H configuration and placed between the dorsal arch of C1 and the C2 spinous process. The loop of the cable is then brought over the bone graft and fixed to the C2 spinous process. Sonntag has reported 97% fusion rates using this technique when using a halo vest for 3 months postoperatively and a hard collar for 2 to 3 months thereafter. The use of multistranded cables has reduced the risk of sublaminar passage; however, stand-alone cable fixation is rarely used in modern C1-2 fixation.
Screw Fixation
Transarticular Screw
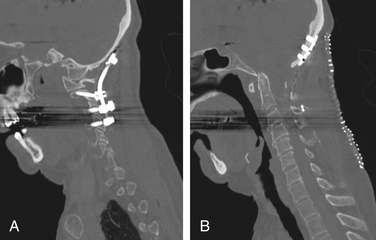
The optimal mechanical construct to provide internal fixation of the C1-2 segment should consist of a three-point fixation.21,47 In 1979, Jeanneret and Magerl described the technique of transarticular screw fixation for odontoid fractures with instability.73 This technique is applicable in reducible subluxations of C1 on C2 and in patients with dorsal element fractures or where a decompressive laminectomy is indicated. This technique limits rotational movement at the atlantoaxial joint (Fig. 86-12).
To avoid vertebral artery injury, it is imperative to have detailed knowledge of its course within the particular patient. Thin-cut CT scans with multiplanar reconstructions are often adequate to identify anomalous vertebral artery location. There is a 19% reported incidence of at least one vertebral artery being in an anomalous location, which would preclude placement of a transarticular screw.74
The operation is performed with exposure of the dorsal elements of C1, C2, and the inferior facet of C2 through a midline incision. The dissection is then further carried out to identify the superior border of the C2 lamina and the dorsal and medial surfaces of the pedicle/pars complex. The entry point is selected in the medial half of the inferior facet of C2. A Penfield instrument is placed against the medial surface of the pedicle to provide tactile feedback. Using fluoroscopic guidance, a tract is drilled through the C1-2 facet joint, into the lateral mass of C1 at the dorsal margin of the anterior tubercle. After tapping with a 3.5-mm tap, a fully threaded cortical screw is placed through this tract.
Pars and Pedicle Screw
C2 pars screw placement is similar to that of the dorsal transarticular screw placement. The difference in the technique is that the pars screw does not enter into the C1-2 facet joint, making it a significantly shorter screw length (Fig. 86-13). This is typically combined with individual lateral mass screws at C1 connected with dorsal rods. The biomechanical strength of a C1 lateral mass–C2 pars screw has been shown to be comparable to that of the C1-2 transarticular screw.75–77
Goel and Laheri described the techniques of C1-2 fixation using C1 lateral mass screws and C2 pedicle screws in 1994.78 Harms and Melcher modified the techniques using polyaxial screws and rods.79 The biomechanical strength of this type of construct has been shown to be superior to both C1-2 transarticular screws and C2 pars screws in terms of both insertional torque and pull-out strength.76 Other advantages of this technique are that (1) anatomic alignment of the C1-2 complex is not necessary prior to instrumentation and that (2) dorsal decompression with laminectomy may be performed, unlike with wiring/clamping techniques. The technique of placing C1 lateral mass screws involves exposing the lateral mass of C1 and the C2 nerve root. The C2 nerve root is protected by retracting it inferiorly. The entry point is the center of the lateral mass and the junction of the lateral mass with the C1 arch. Using fluoroscopic guidance, drilling is started 10 to 15 degrees medially and continues to the posterior border of the anterior tubercle of C1. The trajectory for the C2 pedicle screw is different from that of the C2 pars screw. The entry point for the screw is at a point lateral to the superior margin of the C2 lamina into the superior portion of the C2 pars. The trajectory for drilling the C2 pedicle is 20 degrees superior and 15 to 25 degrees medial from this entry point. The potential risks of the C2 pedicle screw are similar to those of the C1-2 transarticular screw, although the risk of damage to the vertebral artery is somewhat less.20,80
C2 Translaminar Screws
Wright described the placement of translaminar screws to stabilize the C1-2 complex in 2003.81,82 The major advantage of this technique is the decreased risk of damage to the vertebral arteries that exists with the other types of screw fixation described previously. In cases of high-riding vertebral arteries or failed C2 pedicle screw fixation, C2 translaminar screws are a viable option. The screws are placed by first identifying the spinous process, laminae, and lateral masses of C2, as well as the lateral masses of C1. A cortical window is created at the junction of the spinous process and lamina. A hand drill is then used to drill out the contralateral lamina, through which the screw will then be placed. It is advisable to first drill out the smaller lamina because placement of the first screw limits the trajectory of the second.
Biomechanical testing has shown translaminar screws to be stronger in both pull-out strength and insertional torque than C2 pars screws.77 Complications of this technique include cortical breaching, possibly resulting in cerebrospinal fluid leakage or spinal cord injury. Overall, C2 translaminar screw placement is somewhat safer than placement of other types of screws because the length of the screw is visible within the surgical field.
Ventral C1-2 Transarticular Screw
Barbour first described ventral transarticular screw fixation in 1971, but the technique never gained popularity.83 Koller et al. revisited the feasibility of the procedure in 2006.84 This is generally used as a salvage procedure in cases of failed dorsal fusion operations. As with dorsal transarticular screws, motion at C1-2 is essentially eliminated.
Occipitocervical Fusion
When vertical subluxation of the dens is present, with or without a concurrent atlantoaxial subluxation, an atlantoaxial fusion is not adequate treatment. In these cases, it is necessary to extend the fusion to include the occiput. Inclusion of the occiput in the fusion mass significantly decreases head and neck motion, and as a result, it should not be performed in cases with an isolated ventral atlantoaxial subluxation.
In 1987, Wertheim and Bohlman described a technique for occipitocervical fusion using rigid wiring of the occiput through the external occipital protuberance to the cervical spine by use of large iliac crest bone grafts. This technique avoided the need to penetrate both tables of the skull, allowed for immediate rigid internal fixation, permitted early patient mobilization, and resulted in a successful fusion in all 13 patients in their study. Of note, eight of these patients had rheumatoid arthritis.85 Modifications of the technique of Wertheim and Bohlman include placement of occipital bur holes to secure the occipital portion of the fusion.86,87 In addition, Stambough et al. described a technique for occipitocervical fusion in osteopenic rheumatoid arthritis patients using bone in combination with polymethylmethacrylate (PMMA). The authors believed that this technique provided immediate stability and optimized the chances for long-term bony stability.88 Fusion constructs using PMMA for benign disease are, however, prone to failure because the PMMA inhibits fusion.
In 1986, Ransford et al. described a method of occipitocervical stabilization with an anatomically contoured steel loop secured by occipital and sublaminar wires. This rigid dorsal internal fixation system provides immediate stabilization at the craniocervical junction. The authors stated that the loop system provided the necessary stability to allow the patient to mobilize without major external support. The use of this system was originally described in patients after transoral decompressive surgeries. It has, however, been subsequently widely used for primary occipitocervical surgical stabilizations.89 A similar modification of this technique incorporates a malleable rod and segmental wiring. This technique, described by Fehlings et al., also provides immediate rigid stabilization, and the authors have stated that it avoids the need for an external orthosis.55
Rogers et al. have described a useful clinical tool to help determine which surgical techniques are safest. They have stated that although motor examination may be difficult in patients with severe rheumatoid arthritis, testing of sensation remains relatively straightforward. A loss of proprioception suggests a severe dorsal compressive component, usually at the occipitocervical junction, and passage of sublaminar wires in these cases may be hazardous and may result in neurologic morbidity.90 Grob et al. described a technique for occipitocervical plating that uses screws into the occiput and the lateral masses in the cervical spine, obviating the need for sublaminar wires. This technique also provides immediate rigid internal fixation and good rotational stability.91
Several modifications to the technique of occipital screw placement have been proposed in recent years. Screws can be placed either lateral to the midline or in the midline bony keel.91,92 Mingsheng et al. reported a method of placing the screws into the diploe paralleling the occipital table.93 Pait et al. described a technique of placing an “inside-outside” screw by drilling a bur hole and trough in the occipital bone in order to allow for bicortical screw placement while minimizing the risk of dural violation.87 This technique was reviewed in 21 patients with rheumatoid arthritis; no complications or instrumentation failures were noted in the study.94
In a recent review by Winegar et al., several techniques of occipitocervical fusion were compared. Although all methods of fusion were found to have great efficacy, constructs that incorporated rods and screws performed best or near best in all categories studied.95 It should be noted that there is not adequate inherent stability in any bone-wire or bone-cable constructs, and as a result, external immobilization is advisable whenever these techniques are used. Constructs that incorporate plates and screws or rectangles and cable or wire have greater inherent stability and may diminish the requirements for postoperative external immobilization.
Transoral Surgery
In the transoral approach to the odontoid, a midline dorsal pharyngeal incision is made. This allows for exposure of the lower clivus to the level of the C2-3 junction. Depending on the preference of the operating surgeon, splitting the soft palate, performing a tracheotomy, using cervical traction, and using a halo vest postoperatively are all considerations with this surgery. In addition, the transoral decompression can be combined simultaneously with a dorsal cervical stabilization procedure to allow for immediate mobilization of the patient postoperatively.65 On the other hand, the dorsal stabilization can be performed separately later.17,46
Menezes has stated that if the vertical subluxation has caused the dens to penetrate the foramen magnum by greater than 20 mm, an acceptable reduction with prolonged traction cannot be achieved. These patients may require a transoral resection of the dens.16 In 14 rheumatoid arthritis patients with irreducible vertical subluxation, Menezes and van Gilder found a sequestered odontoid process protruding into the pons intradurally in four cases.51
The specific aspects of the surgical procedure are open to debate. However, there appears to be uniform agreement among investigators experienced in this technique that the ventral transoral decompression should precede the dorsal cervical stabilization.17,46 Postoperative swallowing difficulty is not uncommon after transoral surgery, although the rate is lessened when the procedure is performed without splitting the soft palate.96
Subaxial Cervical, Thoracic, and Lumbar Surgery
In cases of subaxial subluxations of the cervical spine, there is not adequate proof that a ventral, dorsal, or combined ventral and dorsal approach is the optimal treatment. Likewise, lesions affecting the thoracic or lumbar spine are so rare that specific treatments must be individualized on a case-by-case basis. Thus, no attempt will be made in this section to analyze the limited information available on these lesions.
Postoperative External Orthoses
There is no consensus in the orthopaedic and neurosurgical literature regarding the optimal type of external orthosis to be used after an upper cervical stabilization procedure. Opinion ranges from the need for postoperative halo vest immobilization for a minimum of 12 weeks, followed by 4 to 8 weeks additional time if flexion-extension views do not show a stable fusion,41 to no need for any external orthosis postoperatively.89 The majority of opinions fall into three categories: routine use of the halo vest postoperatively2,8,41,53,68,88; routine use of an external orthosis other than the halo vest such as a Minerva jacket, Philadelphia collar, or sternal occipital mandibular immobilizer (SOMI)brace*; and use of a halo vest when fixation is deemed tenuous with a lesser device used when the fixation is considered more secure.18,28,46,55,85,86
This lack of agreement regarding the use of external orthoses postoperatively may be the result of varied new surgical procedures currently being developed. In the Magerl transarticular screw fixation of C1-2, if a bone graft is incorporated dorsally, a true three-point fixation can be achieved.99 Similarly, some occipitocervical fusion techniques also allow for a three-point fixation and thus may eliminate the need for a halo vest.66,89,100 Long-term follow-up results are needed to determine whether the benefit of improved patient comfort postoperatively in a lesser orthosis outweighs the risk of an inferior fusion rate if the external support is inadequate. The need for the rigid support of a halo vest orthosis may be unnecessary in patients who are able to have a true three-point fixation internally. It should be noted that halo vests have a higher complication rate in elderly patients.101
Postoperative Results
Neurologic Outcome
Boden et al. found that the severity of the neurologic deficit present preoperatively was an accurate predictor of the postoperative neurologic status. They also stated that the duration of the neurologic deficit preoperatively did not affect the prognosis for neurologic recovery after the operation.32 Hultquist et al. found that most of their patients had relief of their pain and amelioration of their neurologic symptoms; however, there was little evidence of improved overall functional capacity. In spite of this, most of their patients expressed great satisfaction in being pain-free postoperatively.10
Once again, there is considerable discrepancy in the results of neurologic outcome among reported series. In the review by Menezes et al., all 45 patients with vertical subluxation of the odontoid had an improvement in their functional neurologic grade and amelioration of their neurologic dysfunction.17 On the other hand, Chan et al. found that no patient with a preoperative myelopathy improved neurologically in the postoperative period.53
The lack of improvement in some published series once a myelopathy has been established has led some investigators to recommend that early operative stabilization be considered before the onset of neurologic deficit, when radiographic instability is present. Although all patients in their series had evidence of a neurologic deficit before surgery, Boden et al. have recommended that surgery be performed in neurologically intact patients who have a vertical subluxation of the dens compounding a marked atlantoaxial subluxation.32 In pain-free neurologically intact patients with severe radiographic abnormalities, the decision to intervene depends on both accurate serial neurologic examinations and accurate serial radiographic imaging studies.
Fusion Outcome
Moskovich and Crockard have defined the requirements for considering a solid dorsal atlantoaxial fusion a success: lateral flexion and extension radiographs that show no motion and evidence of trabecular bone in continuity between the dorsal elements and the graft.20 In cases of doubt, plain tomograms or multiplanar CT scan reconstructed images are excellent for determining continuity of the fusion. Chan et al. agreed with this definition, and in addition, they defined a fibrous union as the presence of less than 2 mm of motion on flexion-extension radiographs.53 Dickman et al. have stressed that patients with fibrous unions must be followed closely for the signs of delayed instability or wire breakage.68
In rheumatoid arthritis patients, radiographic determination of an occipitocervical fusion may be difficult. Moskovich et al. have reviewed a series of 152 rheumatoid arthritis patients who underwent occipitocervical stabilization procedures. In 80% of patients, no bone grafting was used to augment the stabilization procedure with a contoured steel loop. To evaluate the occipitocervical complex for postoperative stability, these authors measured the angle between the occiput and the upper cervical vertebrae. The difference in the angle between flexion and extension radiographs provides an indication of stability. This method allows one to determine postoperative stability regardless of whether a bone graft is used to supplement the stabilization procedure.66
Variability in the reporting of fusion rates postoperatively in rheumatoid arthritis patients may be a result of discrepancies about whether a given author classifies patients with fibrous union along with the successful bony fusion patients or whether patients with fibrous union are classified along with the nonunion, or pseudarthrosis, patients. When pseudarthrosis occurs after atlantoaxial fusions, it is most common between the dorsal bone graft and the ring of C1.26,102 The reasons for nonunion at this location include the high cortex-to-medulla ratio of the dorsal ring of C1, which is relatively sclerotic,20 and frequent erosion of the dorsal arch of C1 in patients with rheumatoid arthritis.6 In subaxial subluxations, failure of a ventral fusion may be a result of angulation, pseudarthrosis, or collapse of the graft in the bone graft bed.7 Santavirta et al. believed that ventral procedures for subaxial subluxation often fail because of vertebral body osteoporosis, and they stated that acrylic cement and metal rods are of little use as stabilizers in rheumatoid cervical spines.13
Fusion rates vary between 50% to 100% in reported series of atlantoaxial fusions and occipitocervical fusions. Menezes et al. have stated that a stable bony fusion occurred in 100% of their 45 patients with fusion for vertical subluxation of the odontoid.17 Similarly, all seven rheumatoid arthritis patients of Dickman et al. developed stable osseous unions with a bicortical iliac bone graft in a modified Gallie-type fusion.68 Tricortical iliac crest bone grafts in dorsal atlantoaxial fusions are associated with a higher rate of fibrous union.68,103 Fusion rates from 75% to 90% have been reported with Halifax interlaminar clamps,20,72 a modified Gallie approach,41,68 and the transarticular screw fixation technique of Magerl.12,90 Fusion rates between 50% and 75% have been reported with traditional Gallie fusions13 and after combined transoral and dorsal fusion operations for vertical subluxation of the odontoid.46
Mortality
Mortality in rheumatoid arthritis patients varies greatly in reported series. Surgical series must be compared to the natural history of rheumatoid arthritis in patients never subjected to surgical procedures. In a 15-year prospective follow-up study of patients with new-onset rheumatoid arthritis, Corbett et al.104 found only a small increase in mortality in rheumatoid arthritis patients compared with the general population over the same 15-year period.105 They found an increased mortality at 15 years in rheumatoid arthritis patients with concurrent heart disease. Males were overrepresented in this study.104
Davis and Markley reported the first case of an autopsy-proven death in a rheumatoid arthritis patient secondary to compression of the medulla by herniation of the odontoid process through the foramen magnum.106 This was followed by two separate case reports by Martel and Abell102 of autopsy-proven sudden death secondary to atlantoaxial subluxation. As the danger of sudden death was more widely reported, it became apparent that spinal cord damage may not always have been recognized as a cause of death in rheumatoid arthritis patient fatalities in the past. Smith et al. stated that spinal cord involvement may have been responsible for more deaths than had previously been apparent.14
Reported mortality rates in the first month postoperatively have varied between 4% and 10%.11,51,53,66 Many deaths have been attributed to postoperative myocardial infarction; however, it is possible that spinal cord compression may contribute to these deaths. Santavirta et al., while operating on patients with severe rheumatoid arthritis, found that 50% of these patients died over a 10-year follow-up period postoperatively. These authors estimated that the average age of death of these patients was approximately the same as that of other patients with equally severe rheumatoid arthritis.13 Like the nonsurgical long-term follow-up study of Corbett et al., 104 Santavirta et al. reported a significantly increased rate of death postoperatively when cardiac disease was also present.13
Medical Adjuncts
Patients with long-standing rheumatoid arthritis requiring surgery on the cervical spine have often been treated with glucocorticoid medications for many years. It is well known that these medications exert deleterious effects on bone. Skeletal damage resulting from long-term treatment with supraphysiologic doses of glucocorticoids occur in four ways: inhibition of bone growth, delayed union of fractures, osteoporosis, and osteonecrosis.107
Corbett et al. in a 15-year prospective follow-up study of patients with new-onset rheumatoid arthritis, found that long-term use of steroids in rheumatoid arthritis was associated with higher mortality.104 In addition, Hall et al. have stated that the cumulative dose of steroid is more important with respect to this negative effect than is the daily dose.100 Santavirta et al. found that patients who had received steroid treatments for greater than 4 years had more fibrous unions and more pseudarthroses in a 10-year follow-up study.13 Laan et al. found that low-dose prednisone therapy had a deleterious effect on the bone mineral density of postmenopausal women. These postmenopausal patients have an increased risk of developing axial osteopenia and vertebral fractures. A similar bone density loss in men was not demonstrable.103 In patients with active rheumatoid arthritis, low doses of glucocorticoid can cause marked vertebral trabecular bone loss in the initial month of therapy.17,103 After cessation of steroid therapy, this bone loss may be partially reversible.100,108 Papadopoulos et al. found that radiologic progression of atlantoaxial subluxation occurs more rapidly in patients with systemic disease severe enough to require maintenance steroid therapy.42
In an attempt to counteract these deleterious effects of glucocorticoids on bone in rheumatoid arthritis patients, prednisone derivatives have been developed. Deflazacort, an oxazolone derivative of prednisone, has been developed and used to reduce the incidence of the catabolic glucocorticoid actions of prednisone and still maintain its beneficial actions. In a randomized double-blind study, deflazacort was found to decrease the amount of corticosteroid-induced osteoporosis.107
Another medical adjunct that may be of use is estrogen replacement. In postmenopausal women with rheumatoid arthritis, estrogen replacement is protective with regard to the risk of spinal osteoporosis. Sambrook et al. concluded that estrogen therapy should be considered in postmenopausal women with rheumatoid arthritis who are at risk for osteoporosis.109
Additional research is necessary to develop medications that can provide the symptomatic relief of prednisone while eliminating or reducing its harmful side effects on bone in rheumatoid arthritis patients who are predisposed to osteoarthritis by the disease process itself. In a recent review, Klarenbeek et al. concluded that initial treatment for patients diagnosed with rheumatoid arthritis should be with a single disease-modifying antirheumatic drug (DMARD), preferably methotrexate, in combination with a short course of glucocorticoids.110 Patients that have limited response may then be placed on a tumor necrosis factor-α blocker or placed on a combination of DMARDs (such as sulfasalazine, leflunomide, and hydroxychloroquine).111,112
Osteopenia/osteoporosis is a frequently encountered problem in the patient with rheumatoid arthritis. The loss of bone density occurs not only from glucocorticoid use as part of therapy but also from the underlying inflammatory disease.60,113 Bisphosphonates are currently being studied as useful adjuncts to reduce bony erosion in rheumatoid patients with encouraging preliminary results.114,115
Summary
Following strict operative indications, the operative techniques that the authors recommend are as follows. For an isolated ventral C1-2 subluxation, a modified Gallie operation using bicortical iliac crest graft, advocated by Sonntag and others,41,68 is used. The numerous other stabilization techniques for isolated C1-2 instability are generally acceptable. An important consideration is that incorporation of the occiput into the fusion construct may increase the risk of a poor result, and this should only be done if clearly indicated. When a vertical subluxation of the dens is present, an occipitocervical fusion with screws into the occipital keel and fixation of the C2 dorsal elements with pars, pedicle, or laminar screws using contoured rods is ideal. These rods can be extended caudally in cases of subaxial subluxation. If the vertical subluxation is irreducible, or if significant ventral pathology causing compression is present, a transoral decompression may be necessary. This transoral decompression should usually be performed before any dorsal surgery. Depending on the patient’s physical condition, the ventral surgery may be followed by the dorsal operation in the same sitting, or the dorsal surgery can be performed at a later date. The majority of subaxial subluxations of the cervical spine are best treated with lateral mass fixation. Because thoracic and lumbar spine involvement in rheumatoid arthritis is very rare, the surgical management of these cases must be individualized on a case-by-case basis. As a general rule, ventral pathology should be decompressed and stabilized ventrally and dorsal pathology should be decompressed and stabilized dorsally. The authors routinely use cables over wire or twisted wire constructs. In addition, titanium cables, screws, and rods may be used. It should be recognized, however, that titanium implants are more difficult to work with and fail more easily than traditional steel; also, postoperative neuroimaging studies show significant erosion in the immediate regions of the implant. In addition, bending of the titanium rods at the occipitocervical junction may cause them to weaken.
Cohen M.J., Ezekiel J., Persellin R.H. Costovertebral and costotransverse joint involvement in rheumatoid arthritis. Ann Rheum Dis. 1978;37:473-475.
Corbett M., Dalton S., Young A., et al. Factors predicting death, survival and functional outcome in a prospective study of early rheumatoid disease over fifteen years. Br J Rheumatol. 1993;32:717-723.
Friedman H. Intraspinal rheumatoid nodule causing nerve root compression. J Neurosurg. 1970;32:689-691.
Koller H., Kammermeier V., Ulbricht D., et al. Anterior retropharyngeal fixation C1-2 for stabilization of atlantoaxial instabilities: study of feasibility, technical description and preliminary results. Eur Spine J. 2006;15(9):1326-1338.
Messina O.D., Barreira J.C., Zanchetta J.R., et al. Effect of low doses of deflazacort vs. prednisone on bone mineral content in premenopausal rheumatoid arthritis. J Rheumatol. 1992;19:1520-1526.
1. Hughes J.T. Spinal cord involvement by C4-C5 vertebral subluxation in rheumatoid arthritis: a description of 2 cases examined at necropsy. Ann Neurol. 1977;1:575-582.
2. Lipson S.J. Rheumatoid arthritis in the cervical spine. Clin Orthop Relat Res. 1989;239:121-127.
3. Santavirta S., Konttinen Y.T., Sandelin J., Slatis P. Operations for the unstable cervical spine in rheumatoid arthritis. Acta Orthop Scand. 1990;61:106-110.
4. Bland J.H. Rheumatoid arthritis of the cervical spine. J Rheumatol. 1974;1:319-342.
5. Bland J.H., Davis P.H., London M.G., et al. Rheumatoid arthritis of the cervical spine. Arch Intern Med. 1963;112:892-898.
6. Simpson J.M., An H.S., Balderston R.A. Complications of surgery of the spine in rheumatoid arthritis and ankylosing spondylitis. In: Balderston R.A., An H.S., editors. Complications in spinal surgery. Philadelphia: WB Saunders; 1991:169-175.
7. Ranawat C.S., O’Leary P., Pellicci P., et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg [Am]. 1979;61:1003-1010.
8. Stirrat A.N., Fyfe I.S. Surgery of the rheumatoid cervical spine. Clin Orthop Relat Res. 1993;293:135-143.
9. Bell C. The nervous system of the human body. Embracing the papers delivered to the Royal Society on the subject of the nerves. London: Longman, Rees, Orme Brown, & Green; 1830.
10. Hultquist R., Zygmunt S., Saveland H., et al. Characterization and functional assessment of patients subjected to occipito-cervical fusion for rheumatoid atlanto-axial dislocation. Scand J Rheuntatol. 1993;22:20-24.
11. Kraus D.R., Peppelman W.C., Agarwal A.K., et al. Incidence of subaxial subluxation in patients with generalized rheumatoid arthritis who have had previous occipital cervical fusions. Spine. 1991;16(Suppl):S486-S489.
12. Nakano K.X., Schoene W.C., Baker R.A., Dawson D.M. The cervical myelopathy associated with rheumatoid arthritis: analysis of 32 patients, with 2 postmortem cases. Ann Neurol. 1978;3:144-151.
13. Santavirta S., Konttinen Y.T., Laasonen E., et al. Ten-year results of operations for rheumatoid cervical spine disorders. J Bone Joint Surg [Br]. 1991;73:116-120.
14. Smith P.H., Berin R.T., Sharp J. Natural history of rheumatoid cervical luxations. Ann Rheum Dis. 1972;31:431-439.
15. Weissman B.N.W., Aliabadi P., Weinfeld M.S., et al. Prognostic features of atlantoaxial subluxation in rheumatoid arthritis patients. Radiology. 1982;144:745-751.
16. Menezes A.H., van Gilder J.C. Anomalies of the craniovertebral junction. In: Youmans J.R., editor. Neurological surgery. Philadelphia: WB Saunders; 1990:1394-1400.
17. Menezes A.H., van Gilder J.C., Clark C.R., El-Khoury G. Odontoid upward migration in rheumatoid arthritis. J Neurosurg. 1985;63:500-509.
18. Pellicci P.M., Ranawat C.S., Tsairis P., Bryan W.J. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J Bone Joint Surg [Am]. 1981;63:342-350.
19. More J., Sen C. Neurosurgical management of the rheumatoid cervical spine. Mt Sinai J Med. 1994;61:257-264.
20. Moskovich R., Crockard H.A. Atlantoaxial arthrodesis using interlaminar clamps. Spine. 1992;17:261-267.
21. Crockard H.A., Grob D. Rheumatoid arthritis: upper cervical involvement. In: Clark C.R., editor. The cervical spine. Philadelphia: Lippincott-Raven, 1995.
22. Henderson F.C., Geddes J.F., Crockard H.A. Neuropathology of the brainstem and spinal cord in end stage rheumatoid arthritis: implications for treatment. Ann Rheum Dis. 1993;52:629-637.
23. Mueller C.A., Roesseler L., Podlogar M., et al. Accuracy and complications of transpedicular C2 screw placement without the use of spinal navigation. Eur Spine J. 2010;19(5):809-814.
24. Grantham S.A. Rheumatoid arthritis and other noninfectious inflammatory diseases: atlantoaxial instability. In In the Cervical Spine Research Society: The cervical spine. Philadelphia: Lippincott-Raven; 1983.
25. Steinhrocker O., Traeger C.H., Batterman R.C. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
26. Robinson B.P., Seeger J.F., Zak S.M. Rheumatoid arthritis and positional vertebrobasilar insufficiency. J Neurosurg. 1986;65:111-114.
27. Snelling J.P., Pickard J., Wood S.K., Prouse P.J. Reversible cortical blindness as a complication of rheumatoid arthritis of the cervical spine. Br J Rheumatol. 1990;29:228-230.
28. van den Brink H.R., Lems W.F., van Everdingen A.A., Bijlsma J.W. Adjuvant oestrogen treatment increases bone mineral density in postmenopausal women with rheumatoid arthritis. Ann Rheum Dis. 1993;52:302-305.
29. O’Brien MF, Casey ATH, Crockard HA, et al: Histology of the rheumatoid atlantoaxial joints: a clinico-pathologic analysis of 33 operative cases. In Abstracts of the 22nd Annual Meeting of the Cervical Spine Research Society (Baltimore), pp 83–84.
30. Younes M., Belghali S., Kriâa S., et al. Compared imaging of the rheumatoid cervical spine: prevalence study and associated factors. Joint Bone Spine. 2009;76(4):361-368.
31. Redlund-Johnell I., Pettersson H. Radiographic measurements of the cranio-vertebral region. Designed for evaluation of abnormalities in rheumatoid arthritis. Acta Radiot [Diagn]. 1984;25:23-28.
32. Boden S.D., Dodge L.D., Bohhnan H.H., et al. Rheumatoid arthritis of the cervical spine. J Bone Joint Surg [Am]. 1993;75:1282-1297.
33. White A.A.III, Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: Lippincott-Raven, 1990. 92–97, 296
34. Breedveld F.G., Algra P.R., Vielvoye C.J., Gats A. Magnetic resonance imaging in the evaluation of patients with rheumatoid arthritis and subluxations of the cervical spine. Arthritis Rheum. 1987;30:624-629.
35. Smoker W.R.K., Keyes W.D., Dunn V.D., Menezes A.H. MRI versus conventional radiologic examinations in the evaluation of the craniovertebral and cervicomedullary junction. Radiographics. 1986;6:953-994.
36. Dickman C.A., Mamourian A., Sonntag V.K.H., et al. Magnetic resonance imaging of the transverse atlantal ligament for the evaluation of atlantoaxial stability. J Neurosurg. 1991;75:221-227.
37. Sharp J., Purser D.W. Spontaneous atlanto-axial dislocation in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis. 1961;20:47-77.
38. Bell G.R., Stearns K.L. Flexion-extension MRI of the upper rheumatoid cervical spine. Orthopedics. 1991;14:969-974.
39. Ferreiro Perez A., Garcia Isidro M., Ayerbe E., et al. Evaluation of intervertebral disc herniation and hypermobile intersegmental instability in symptomatic adult patients undergoing recumbent and upright MRI of the cervical or lumbosacral spines. Eur J Radiol. 2007;62(3):444-448.
40. Suzuki F., Fukami T., Tsuji A., et al. Discrepancies of MRI findings between recumbent and upright positions in atlantoaxial lesion. Report of two cases. Eur Spine J. 2008;17(Suppl 2):S304-S307.
41. King I.T. Rheumatoid subluxations of the cervical spine (editorial). Ann Rheum Dis. 1985;44:807-808.
42. Papadopoulos S.M., Dickman C.A., Sonntag V.K.H. Atlantoaxial stabilization in rheumatoid arthritis. J Neurosurg. 1991;74:1-7.
43. Peppelman W.C., Krans D.R., Donaldson W.F.III, Agarwal A. Cervical spine surgery in rheumatoid arthritis: improvement of neurologic deficit after cervical spine fusion. Spine. 1993;18:2375-2379.
44. Gallie W.E. Fractures and dislocations of the cervical spine. Am J Surg. 1939;46:495-499.
45. White A.A.III, Panjabi M.M. The clinical biomechanics of the occipitoatlantoaxial complex. Orthop Clin North Am. 1978;9:867-878.
46. Dickman C.A., Locantro J., Fessler R.G. The influence of transoral odontoid resection on stability of the craniovertebral junction. J Neurosurg. 1992;77:525-530.
47. Grob D., Crisco J.J.III, Panjabi M.M., et al. Biomechanical evaluation of four different posterior atlantoaxial fixation techniques. Spine. 1992;17:480-490.
48. Frigaard E. Posterior atlanto-axial subluxation in rheumatoid arthritis. Scand J Rheumatol. 1978;7:65-68.
49. Isdale I.C., Corrigan A.B. Backward luxation of the atlas: two cases of an uncommon condition. Ann Rheum Dis. 1970;29:6-9.
50. Mathews J.A. Atlanto-axial subluxation in rheumatoid arthritis: a 5-year follow-up study. Ann Rheum Dis. 1974;33:526-531.
51. Menezes A.H., van Gilder J.C. Transoral-transpharyngeal approach to the anterior craniocervical junction: ten-year experience with 72 patients. J Neurosurg. 1988;69:895-903.
52. Bailey R.W. Rheumatoid arthritis and other noninfectious inflammatory diseases. In: The Cervical Spine Research Society: The cervical spine. Philadelphia: Lippincott-Raven; 1983.
53. Chan D.P., Ngian K.S., Cohen L. Posterior upper cervical fusion in rheumatoid arthritis. Spine. 1992;17:268-272.
54. Crockard H.A., Calder I., Ransford A.O. One-stage decompression and posterior fixation in rheumatoid atlanto-axial subluxation. J Bone Joint Surg [Br]. 1990;72:682-685.
55. Fehlings M.G., Errico T., Cooper P., et al. Occipitocervical fusion with a five-millimeter malleable rod and segmental fixation. Neurosurgery. 1993;32:198-208.
56. Fam A.G., Cruickshank B. Subaxial cervical subluxation and cord compression in psoriatic spondylitis. Arthritis Rheum. 1982;25:101-106.
57. Heywood A.W., Meyers O.L. Rheumatoid arthritis of the thoracic and lumbar spine. J Bone Joint Surg [Br]. 1986;68:362-368.
58. Redlund-Johnell I., Larsson E.M. Subluxation of the upper thoracic spine in rheumatoid arthritis. Skeletal Radiol. 1993;22:105-108.
59. Friedman H. Intraspinal rheumatoid nodule causing nerve root compression. J Neurosurg. 1970;32:689-691.
60. Walsh N.C., Gravallese E.M. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol. 2004;16:419-427.
61. Cohen M.J., Ezekiel J., Persellin R.H. Costovertebral and costotransverse joint involvement in rheumatoid arthritis. Ann Rheum Dis. 1978;37:473-475.
62. Baggenstoss A.H., Bickel W.H., Ward L.E. Rheumatoid granulomatous nodules as destructive lesions of vertebrae. J Bone Joint Surg [Am]. 1952;34:601-609.
63. Linquist P.R., McDonnell D.E. Rheumatoid cyst causing extradural compression. J Bone Joint Surg [Am]. 1970;52:1235-1240.
64. Magnaes B., Hauge T. Rheumatoid arthritis contributing to lumbar spinal stenosis. Scand J Rheumatol. 1978;7:215-218.
65. Crockard H.A. The transoral approach to the base of the brain and upper cervical cord. Ann R Coll Surg Engl. 1985;67:321-325.
66. Moskovich R., Crockard H.A., Shott S., Ransford A.O. Occipitocervical stabilization for myelopathy in patients with rheumatoid arthritis. Implications of not bone-grafting. J Bone Joint Surg [Am]. 2000;82(3):349-365.
67. Mixter S.J., Osgood R.B. Traumatic lesions of the atlas and axis. Ann Surg. 1910;51:193-207.
68. Dickman C.A., Sonntag V.K.H., Papadopoulos S.M., Hadley M.N. The interspinous method of posterior atlantoaxial arthrodesis. J Neurosurg. 1991;74:190-198.
69. Dickman C.A., Crawford N.R., Paramore C.G. Biomechanical characteristics of C1-2 cable fixations. J Neurosurg. 1996;85(2):316-322.
70. Brooks A.L., Jenkins E.B. Atlanto-axial arthrodesis by the wedge compression method. J Bone Joint Surg [Am]. 1978;60:279-284.
71. Holness R.O., Huestis W.S., Howes W.J. Posterior stabilization with an interlaminar clamp in cervical injuries: technical note and review of the long term experience with the method. Neurosurgery. 1984;14:318-322.
72. Aldrich E.F., Weber P.B., Crow W.N. Halifax interlaminar clamp for posterior cervical fusion: a long-term follow-up review. J Neurosurg. 1983;78:702-708.
73. Jeanneret B., Magerl F. Primary posterior fusion C1/2 in odontoid fractures: indications, technique, and results of transarticular screw fixation. J Spinal Disord Tech. 1992;4:464-475.
74. Paramore C.G., Dickman C.A., Sonntag V.K. The anatomical suitability of the C1-2 complex for transarticular screw fixation. J Neurosurg. 1996;85(2):221-224.
75. Elgafy H., Potluri T., Goel V.K., et al. Biomechanical analysis comparing three C1-C2 transarticular screw salvaging fixation techniques. Spine. 2010;35(4):378-385.
76. Härtl R., Chamberlain R.H., Fifield M.S., et al. Biomechanical comparison of two new atlantoaxial fixation techniques with C1-2 transarticular screw-graft fixation. J Neurosurg Spine. 2006;5(4):336-342.
77. Lehman R.A.Jr., Dmitriev A.E., Helgeson M.D., et al. Salvage of C2 pedicle and pars screws using the intralaminar technique: a biomechanical analysis. Spine. 2008;33(9):960-965.
78. Goel A., Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir (Wien). 1994;129(1–2):47-53.
79. Harms J., Melcher R.P. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26(22):2467-2471.
80. Wright N.M., Lauryssen C. Vertebral artery injury in C1-2 transarticular screw fixation: results of a survey in the AANS/CNS section on disorders of the spine and peripheral nerves. American Association of Neurological Surgeons/Congress of Neurological Surgeons. J Neurosurg. 1998;88:634-640.
81. Wright N.M. Translaminar rigid screw fixation of the axis. Technical note. J Neurosurg Spine. 2005;3(5):409-414.
82. Wright N.M. Posterior C2 fixation using bilateral, crossing C2 laminar screws: case series and technical note. J Spinal Disord Tech. 2004;17(2):158-162.
83. Barbour J.R. Screw fixation in fractures of the odontoid process. S Aust Clinics. 1971;5:20.
84. Koller H., Kammermeier V., Ulbricht D., et al. Anterior retropharyngeal fixation C1-2 for stabilization of atlantoaxial instabilities: study of feasibility, technical description and preliminary results. Eur Spine J. 2006;15(9):1326-1338.
85. Wertheim S.B., Bohlman H.H. Occipitocenical fusion. J Bone Joint Surg [Am]. 1987;69:833-836.
86. Clark C.R. Occipitocervical fusion for the unstable rheumatoid neck. Orthopedics. 1989;12:469-473.
87. Pait T.G., Al-Mefty O., Boop F.A., et al. Inside-outside technique for posterior occipitocervical spine instrumentation and stabilization: preliminary results. J Neurosurg. 1999;90(Suppl 1):1-7.
88. Stambough J.L., Balderston R.A., Grey S. Technique for occipito-cervical fusion in osteopenic patients. J Spinal Dis. 1990;3:404-407.
89. Ransford A.O., Crockard H.A., Pozo J.L., et al. Craniocervical instability treated by contoured loop fixation. J Bone Joint Surg [Br]. 1986;68:173-177.
90. Rogers M.A., Crockard H.A., Moskovich R., et al. Nystagmus and joint position sensation: their importance in posterior occipitocervical fusion in rheumatoid arthritis. Spine. 1994;19:16-20.
91. Grob D., Dvorak J., Panjabi M., et al. Posterior occipitocervical fusion: a preliminary report of a new technique. Spine. 1991;16(Suppl 3):S17-S23.
92. Grob D., Schütz U., Plötz G. Occipitocervical fusion in patients with rheumatoid arthritis. Clin Orthop Relat Res. 1999;366:46-53.
93. Mingsheng T., Huimin W., Xin J., et al. Screw fixation via diploic bone paralleling to occiput table: anatomical analysis of a new technique and report of 11 cases. Eur Spine J. 2007;16(12):2225-2231.
94. Sandhu F.A., Pait T.G., Benzel E., Henderson F.C. Occipitocervical fusion for rheumatoid arthritis using the inside-outside stabilization technique. Spine. 2003;28(4):414-419.
95. Winegar C.D., Lawrence J.P., Friel B.C., et al. A systematic review of occipital cervical fusion: techniques and outcomes. J Neurosurg Spine. 2010;13(1):5-16.
96. Jones D.C., Hayter J.P., Vaughan E.D., et al. Oropharyngeal morbidity following transoral approaches to the upper cervical spine. Int J Oral Maxillofac Surg. 1998;27(4):295-298.
97. Marcotte P., Dickman C.A., Sonntag V.K.H., et al. Posterior atlantoaxial facet screw fixation. J Neurosurg. 1993;79:234-237.
98. Stillerman C.B., Wilson J.A. Atlanto-axial stabilization with posterior trans articular screw fixation: technical description and report of 22 cases. Neurosurgery. 1993;32:948-955.
99. Magerl F., Seeman P.S. Stable posterior fusion of the atlas and axis by transarticular screw fixation. Kehr P., Weidner A., editors. The cervical spine. New York: Springer-Verlag. 1987;vol 1:322-327.
100. Hall G.M., Spector T.D., Griffin A.J., et al. The effect of rheumatoid arthritis and steroid therapy on bone density in post menopausal women. Arthritis Rheum. 1993;36:1510-1516.
101. Daentzer D., Flörkemeier T. Conservative treatment of upper cervical spine injuries with the halo vest: an appropriate option for all patients independent of their age? J Neurosurg Spine. 2009;10(6):543-550.
102. Martel W., Abell M.R. Fatal atlanto-axial luxation in rheumatoid arthritis. Arthritis Rheum. 1963;6:224-231.
103. Laan R.F., van Riel P.L., van Erning L.J., et al. Vertebral osteoporosis in rheumatoid arthritis patients: effect of low dose prednisone therapy. Br J Rheumatol. 1992;31:91-96.
104. Corbett M., Dalton S., Young A., et al. Factors predicting death, survival and functional outcome in a prospective study of early rheumatoid disease over fifteen years. Br J Rheumatol. 1993;32:717-723.
105. Laan R.F., Buijs W.C., Verbeek A.L., et al. Bone mineral density in patients with recent onset rheumatoid arthritis: influence of disease activity and functional capacity. Ann Rheum Dis. 1993;52:21-26.
106. Davis F.W.Jr., Markley H.E. Rheumatoid arthritis with death from medullary compression. Ann Intern Med. 1951;35:451-454.
107. Messina O.D., Barreira J.C., Zanchetta J.R., et al. Effect of low doses of deflazacort vs. prednisone on bone mineral content in premenopausal rheumatoid arthritis. J Rheumatol. 1992;19:1520-1526.
108. Laan R.F., van Riel P.L., van de Putte L.B., et al. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis: a randomized, controlled study. Ann Intern Med. 1993;119:963-968.
109. Sambrook P., Birmingham J., Champion D., et al. Postmenopausal bone loss in rheumatoid arthritis: effect of estrogens and androgens. J Rheumatol. 1992;19:357-361.
110. Klarenbeek N.B., Kerstens P.J., Huizinga T.W., et al. Recent advances in the management of rheumatoid arthritis. BMJ. 2010;341:c6942.
111. Gorter S.L., Bijlsma J.W., Cutolo M., et al. Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69:1010-1014.
112. Smolen J.S., Landewe R., Breedveld F.C., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964-975.
113. Suresh E. Recent advances in rheumatoid arthritis. Postgrad Med J. 2010;86(1014):243-250.
114. Breuil V., Euller-Ziegler L. Bisphosphonate therapy in rheumatoid arthritis. Joint Bone Spine. 2006;73:349-354.
115. Jarret S.J., Conagan P.G., Sloan V.S., et al. Preliminary evidence for a structural benefit of the new bisphosphonate zolendronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54:1410-1414.