169 Rhabdomyolysis
• The most common causes of rhabdomyolysis include substance abuse, direct muscle injury, infection, strenuous physical activity, medications, and toxic ingestions.
• Only 50% of patients with rhabdomyolysis complain of specific muscle symptoms, and less than 10% have muscle tenderness on examination.
• Acute kidney injury is a potential major complication of rhabdomyolysis.
• Aggressive fluid resuscitation decreases the likelihood of kidney injury.
• Rhabdomyolysis and crush syndrome often emerge as the leading causes of delayed mortality in mass casualty incidents involving building collapse.
• Early hemodialysis is indicated when severe hyperkalemia, refractory metabolic acidosis, or crush injury is present.
• Use of supplemental calcium or loop diuretics should be avoided if possible in the setting of rhabdomyolysis.
DefinItion and Epidemiology
Rhabdomyolysis is a condition characterized by injury to skeletal muscle that results in release of the intracellular contents into the extracellular fluid and circulation. Authoritative thresholds for creatine kinase (CK) range between 1000 and 10,000 U/L, but some definitions additionally mandate the presence of myoglobinuria (Box 169.1).
Rhabdomyolysis can occur secondary to trauma, exertion, muscle hypoxia, genetic defects, infections, changes in body temperature, metabolic and electrolyte disorders, drugs and toxins, and idiopathic causes. Various categorizations of rhabdomyolysis have been proposed: traumatic versus atraumatic, reversible versus irreversible, endogenous versus exogenous, and hereditary versus acquired. More than half of all cases of rhabdomyolysis are multifactorial (Box 169.2).
Box 169.2 Causes of Rhabdomyolysis
Rhabdomyolysis afflicts more than 25,000 individuals in the United States each year. Morbidity and mortality vary tremendously depending on etiology, available treatment, time course, and comorbid factors. Acute kidney injury is a potential major complication of rhabdomyolysis and worldwide occurs in 15% to 45% of cases. In contrast, 7% to 10% of cases of acute kidney injury in the United States are caused by rhabdomyolysis.1 Mortality generally ranges from 3% to 10% but can be as high as 25% in mass casualty incidents that involve crush injuries.
Pathophysiology
Rhabdomyolysis is a condition characterized by injury to skeletal muscle that alters the integrity of the cell membrane. Despite the large number of causes of rhabdomyolysis, the underlying pathology involves direct damage to the sarcolemma or depletion of adenosine triphosphate (ATP) within the myocyte resulting in an unregulated increase in intracellular calcium. This leads to constant contraction, energy depletion, and eventual necrosis and death of the muscle cell with release of its intracellular contents into the circulation.2 The most important products released include potassium, phosphorus, myoglobin, CK, aspartate transaminase, alanine transaminase, lactate dehydrogenase, urate, cytokines, and purines.
Causes of Rhabdomyolysis
Medical Decision Making and Diagnostic Testing
Urinalysis
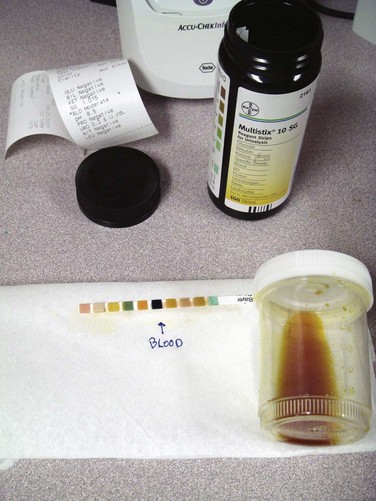
The urine dipstick is a commonly used screening test for rhabdomyolysis. The orthotoluidine test on the urine dipstick will react in the presence of either myoglobin or hemoglobin. A report of “large blood” on the urine dipstick and absence of red blood cells on microscopy classically suggests the presence of free myoglobin in urine. Figure 169.1 shows the typical urine appearance in the setting of myoglobinuria. Unfortunately, clinical data do not fully support this screening practice, with microscopic hematuria occurring in about 30% of patients with rhabdomyolysis. In addition, myoglobinuria may be transient and not identified at the time of urinalysis despite the presence of significant clinical rhabdomyolysis. Dipstick testing will detect a urine myoglobin level higher than 1.0 mg/dL, which correlates with a serum value of approximately 100 mg/dL.
Renal Function Tests
Though not consistently observed in all analyses, creatinine has at times been shown to rise faster with rhabdomyolysis than with other causes of acute renal failure. Markedly high creatinine levels or a relatively low ratio of blood urea nitrogen to creatinine should raise suspicion for rhabdomyolysis (Box 169.3).
Complications
Acute Kidney Injury
Acute kidney injury caused by rhabdomyolysis may be oliguric (most common), nonoliguric, or occasionally anuric. It typically results in a higher anion gap acidosis and higher uric acid levels and often leads to a more rapid increase in serum creatinine than do other forms of acute kidney injury. Fractional excretion of sodium is often less than 1%, in contrast to other forms of acute tubular necrosis. It is difficult to predict the patients in whom acute kidney injury will develop based on laboratory values at initial evaluation.3
Compartment Syndrome
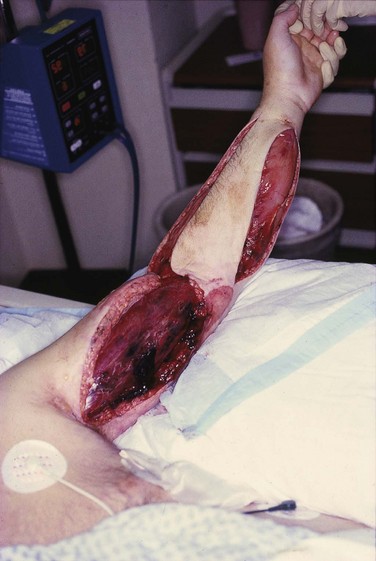
Most striated muscles are contained within rigid compartments formed by fascia and bones. When the muscle is traumatized, marked swelling and edema occur within a closed osteofascial compartment, and muscle perfusion is reduced to a level below that required for cellular viability. As intracompartmental pressure rises above 30 to 35 mm Hg, compartment syndrome develops and significant muscle ischemia ensues and requires decompressive fasciotomy. Figure 169.2 shows an example of compartment syndrome.
Treatment
General Measures
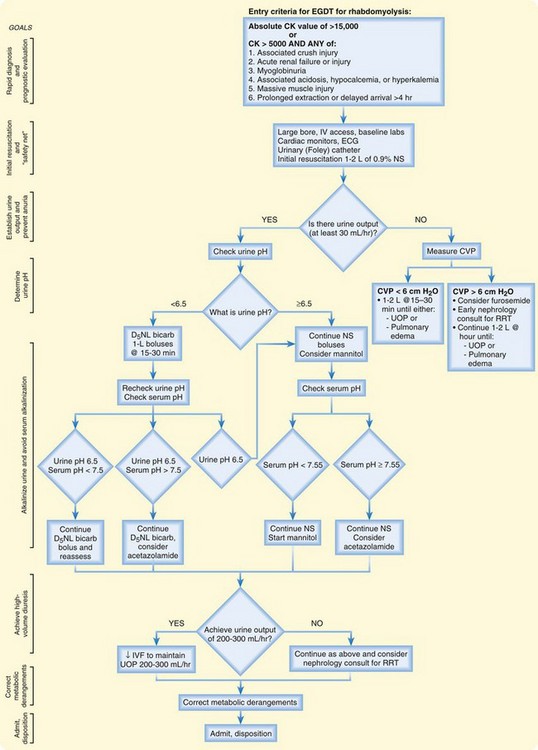
Rhabdomyolysis is physiologically and clinically similar to cell degradation states such as tumor lysis syndrome and sepsis. An organized, aggressive treatment strategy should focus on clinical end points similar to those for other cell lysis conditions. The emergency treatment of rhabdomyolysis, in an early goal-directed fashion, is summarized in Figure 169.3.
The main goal of therapy is prevention of acute renal failure through high-volume resuscitation.4 The two most common reasons for the development of acute kidney injury are slow fluid resuscitation and inadequate fluid resuscitation. Normal saline is superior to lactated Ringer solution for the treatment of rhabdomyolysis because normal saline is not associated with risk for phosphate toxicity. More than 10 L of normal saline is typically administered in the first 24 hours of therapy to maintain high-volume dilute urine output.
Avoid Calcium Supplementation and Loop Diuretics
![]() Red Flags
Red Flags
Cautions for a Physician
Hypercalcemia can develop after recovery of acute renal failure secondary to rhabdomyolysis. Calcium administration should be avoided during renal failure unless the patient has symptomatic hypocalcemia or severe hyperkalemia.
With the large volume of fluid administration typically needed in patients with rhabdomyolysis, it is important to monitor the patient closely for any evidence of fluid overload.
If mannitol is used, serum sodium and osmolarity should be checked frequently to avoid a hyperosmolar state. Acute kidney injury may occur with doses higher than 200 g/day and cumulative doses higher than 800 g.
If alkalinization of urine is initiated, there is a potential for worsening of hypocalcemia. It can also lead to hypokalemia. Urine pH, serum bicarbonate, serum calcium, and serum potassium should be monitored, and if urine pH does not rise after 4 to 6 hours or symptomatic hypocalcemia develops, alkalinization should be stopped.
Crush Syndrome: Disaster and Mass Casualty Considerations
The International Society of Nephrology can be contacted to respond to a mass casualty incident with emergency renal therapy equipment through its Disaster Relief Task Force (http://www.isn-online.org/isn/society/about/isn_20011.html).
Tips and Tricks
Management of Crush Syndrome
Prehospital Phase
Begin high-volume intravenous fluid resuscitation before extrication from collapsed buildings.
Remove constrictive jewelry and clothing.
Flaccid paralysis may mimic spinal cord injury (but rectal tone will be normal).
Prehospital amputation may be necessary to allow extrication of the victim.
“Physiologic amputation” (application of a tourniquet above the point of injury) may minimize the release of toxic substances into the circulation, thereby reducing rates of rhabdomyolysis; ice or dry ice should be applied distal to the tourniquet.
Hospital Phase
Anticipate the need for renal replacement therapy and hemodialysis resources.
The decision for amputation can be aided by use of the mangled extremity severity score (MESS). A MESS score of 7 or higher is a 100% positive predictor of amputation.
Avoid early aggressive surgical débridement unless vascular compromise is obvious.
Bosch X, Poch E, Grau J. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72.
Bywaters E, Beall D. Crush injuries with impairment of renal function. Br Med J. 1941;1:427–432.
Fernandez WG, Hung O, Bruno GR, et al. Factors predictive of acute renal failure and need for hemodialysis among ED patients with rhabdomyolysis. Am J Emerg Med. 2005;23:1–7.
Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19:568–574.
Lippi G, Cervellin G, Comelli I. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749–756.
1 Antons KA, Williams CD, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400–409.
2 Bosch X, Poch E, Grau J. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72.
3 Capacchione JF, Muldoon SM, et al. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065–1069.
4 Chatzizisis YS, Misirli G, Giannoglou GD. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19:568–574.
5 Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678–685.
6 Karajala VW, Mansour W, Kellum JA. Diuretics in acute kidney injury. Minerva Anestesiol. 2009;75:251–257.
7 Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67:272–283.
8 Koyner JL, Sher Ali R, Murray PT. Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury? Nephron Exp Nephrol. 2008;109(4):e109–e117.
9 Litman RS, Rosenberg H. Malignant hyperthermia: update on susceptibility testing. JAMA. 2005;293:2918–2924.
10 Mannix RM, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119–2125.
11 McClure DL, Valuck RJ, Glanz M, et al. Systematic review and meta-analysis of clinically relevant adverse events from HMG CoA reductase inhibitor trials worldwide from 1982 to present. Pharmacoepidemiol Drug Saf. 2007;16:132–143.
12 Ronco C. Extracorporeal therapies in acute rhabdomyolysis and myoglobin clearance. Crit Care. 2005;9:141–142.
13 Patel DR, Gysmfi R, Torres A. Exertional rhabdomyolysis and acute kidney injury. Physician Sportsmed. 2009;37:71–79.
14 Rodríguez-Capote K, Balion CM, Hill SA, et al. Utility of urine myoglobin for the prediction of acute renal failure in patients with suspected rhabdomyolysis: a systematic review. Clin Chem. 2009;55:2190–2197.
15 Sever MS, Lameire N, Vanholder R. Renal disaster relief: from theory to practice. Nephrol Dial Transplant. 2009;24:1730–1735.
16 Vanholder R, Van Biesen W, Lameire N, et al. The role of the International Society of Nephrology/Renal Disaster Relief Task Force in the rescue of renal disaster victims. Contrib Nephrol. 2007;156:325–332.
17 Walsh S, Fan SL. Visualising rhabdomyolysis. Lancet. 2009;373:154.
18 Alpers JP, Jones LK, Jr. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487–491.
19 Thondebhavi Subbaramaiah M, Sapsford D, Banham-Hall E. Acetazolamide as an adjunct to sodium bicarbonate in the treatment of rhabdomyolysis. Anaesth Intensive Care. 2010;38:398.
20 Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749–756.
21 Lai CC, Wang CY, Lin HI. Rhabdomyolysis and acute kidney injury associated with 2009 pandemic influenza A (H1N1). Am J Kidney Dis. 2010;55:615.
22 Hedenmalm K, Alvan G, Ohagen P, et al. Muscle toxicity with statins. Pharmacoepidemiol Drug Saf. 2010;19:223–231.
23 Young SE, Miller MA, Docherty M. Urine dipstick testing to rule out rhabdomyolysis in patients with suspected heat injury. Am J Emerg Med. 2009;27:875–877.
24 Lee PJ, Lachmann RH. Acute presentations of inherited metabolic disease in adulthood. Clin Med. 2008;8:621–624.
25 Clarkson PM. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sports Med. 2007;37:361–363.
26 Hoffman JD, Steiner RD, Paradise L, et al. Rhabdomyolysis in the military: recognizing late-onset very long-chain acyl Co-A dehydrogenase deficiency. Mil Med. 2006;171:657–658.
27 Clarkson PM, Kearns AK, Rouzier P, et al. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med Sci Sports Exerc. 2006;38:623–627.
28 Ghatak A, Faheem O, Thompson PD. The genetics of statin-induced myopathy. Atherosclerosis. 2010;210:337–343.
29 Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf. 2009;4:181–187.
30 Campbell GA, Rosner MH. The agony of ecstasy: MDMA (3,4 methylenedioxymethamphetamine) and the kidney. Clin J Am Soc Nephrol. 2008;3:1852–1860.
31 Silva M, Matthews ML, Jarvis C, et al. Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther. 2007;29:253–260.
32 Peltonen S, Ahlström A, Kylävainio V, et al. The effect of combining intermittent hemodiafiltration with forced alkaline diuresis on plasma myoglobin in rhabdomyolysis. Acta Anaesthesiol Scand. 2007;51:553–558.
33 Hatamizadeh P, Najafi I, Vanholder R, et al. Epidemiologic aspects of the Bam earthquake in Iran: the nephrologic perspective. Am J Kidney Dis. 2006;47:428–438.
34 Brown CV, Rhee P, Chan L, et al. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56:1191.
35 Gonzalez D. Crush syndrome. Crit Care Med. 2005;33(1 Suppl):S34–S41.
36 Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore). 1982;61:141–152.
37 Allison RC, Bedsole DL. The other medical causes of rhabdomyolysis. Am J Med Sci. 2003;326:79–88.
38 Better OS, Stein JH. Early management of shock and prophylaxis of acute renal failure in traumatic rhabdomyolysis. N Engl J Med. 1990;322:825–829.
39 Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med. 1984;144:277–280.
40 Bywaters E, Beall D. Crush injuries with impairment of renal function. Br Med J. 1941;1:427–432.
41 Lippi G, Cervellin G, Comelli I. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749–756.
42 Fernandez WG, Hung O, Bruno GR, et al. Factors predictive of acute renal failure and need for hemodialysis among ED patients with rhabdomyolysis. Am J Emerg Med. 2005;23:1–7.
43 Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19:568–574.
44 Gunal AI, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15:1862–7186.
45 Mullins RJ, Slater MS, Malinosk DJ. Crush injury and rhabdomyolysis. Crit Care Clin. 2004;20:171–192.