Chapter 58 Revision Anterior Cruciate Ligament Reconstruction*
Introduction
Reconstruction of the anterior cruciate ligament (ACL) has become an increasingly common orthopaedic procedure. In the United Kingdom, approximately 5000 ACL reconstructions are performed per year1; in the United States, more than 100,000 procedures are currently performed annually.2 This trend is likely to continue with the general population’s increasing pursuit of an active lifestyle. Although long-term functional stability and symptom relief after primary ACL reconstruction exceed 90% in some studies,3,4 overall clinical failure rates of 10% to 25% have been documented.1 It is currently estimated that between 3000 and 10,000 U.S. patients and approximately 1000 U.K. patients are candidates for revision ACL surgery annually.1
Revision ACL surgery is recommended for patients who have instability or reduced activity with pathological laxity after a failed primary ACL reconstruction. The important stages in assessing a patient with a failed ACL reconstruction include a detailed history, patient selection, physical examination and appropriate investigations, choice of graft, surgical technique, and rehabilitation.5 Eliciting important relevant history from a patient who is usually apprehensive and has some knowledge of the problem (from general practitioners, physiotherapists, and the Internet) can be difficult. One should spend enough time trying to find out the details of the original injury (e.g., high-velocity trauma may suggest multiligament laxity) and also the patient’s experience of previous treatment as well as his or her knowledge of the problem. A full history of occupational and future recreational activities is mandatory. Often the patient’s expectations are not realistic, and therefore despite achieving knee stability, the revision surgery will not result in a contented patient. Instability and/or pain top the list of patient symptoms. It should be clarified with the patient preoperatively that a reduced activity level and/or excellent natural proprioception may result in a reduction or even an abolition in symptoms of instability without the need for surgery. Revision reconstruction should be offered to patients with symptoms of instability or those who wish to increase their activity level to include manoeuvres involving twisting or a sudden change in direction. In such cases symptoms of instability, if left untreated, will contribute to repeated meniscal and chondral damage, leading to an earlier progression of osteoarthritis (OA). At the same time, the patient should be made aware of the risk of a gradual progression of OA, irrespective of the method of treatment but especially if symptoms of instability are ignored. It should be carefully explained that symptoms of pain are likely to be caused by degenerative disease or a torn meniscus and that a revision ACL reconstruction alone is unlikely to be the answer to this problem. One should also alert the patient to the potential need for bone grafting and thus a staged reconstruction.
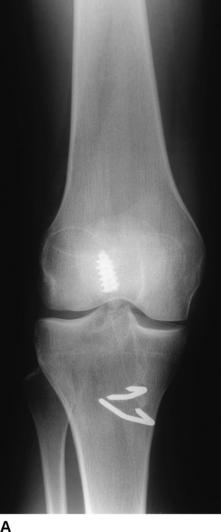
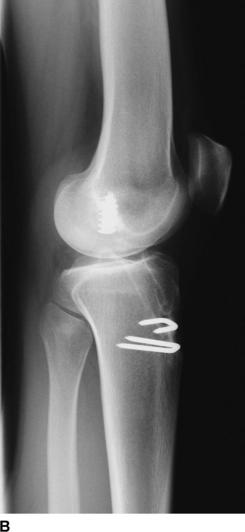
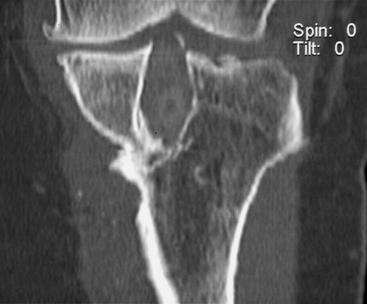
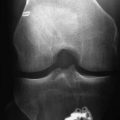
The most useful investigations are the plain x-ray series of an anteroposterior (AP) standing radiograph and a lateral x-ray in full extension, together with skyline and Rosenberg views (Fig. 58-1).1 These will show the original tunnel placement and usually the fixation methods used, tunnel widening,6,7 osteolysis, and the presence and extent of joint space narrowing. If possible, comparison with previous radiographs will help quantify bone loss (tunnel widening). It is our practice to obtain a magnetic resonance imaging (MRI) scan preoperatively, but only rarely has this provided additional information that has changed the course of management. If no reason for the primary failure can be elicited, vigilance for a missed or complex laxity should be exercised. Careful clinical examination and an examination under anesthesia, which may include fluoroscopic stress views, are useful in finding an additional laxity.
Causes Of Failure of Primary Procedure
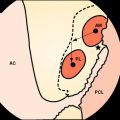
The causes of recurrent patholaxity after primary ACL reconstruction6–26 can be broadly divided into four groups: technical errors, failure due to biological factors, failure due to significant trauma, and failure owing to laxity in the secondary restraints.17 By far the most common cause is error in the surgical technique, with 77% to 95% of all cases of ACL failure attributed to technical error.27 This category includes poor graft selection or harvest, improper tensioning or fixation, and especially incorrect tunnel placement.9,17,18 More than 70% of technical failures, and thus more than 50% of all ACL failures, can be attributed to malpositioned tunnels.8,17 Inappropriate positioning of either the tibial or the femoral tunnel results in excessive length changes in the graft as the knee moves through a range of motion, resulting in either a limited range of motion or excessive graft laxity.8,18 Anterior placement of the femoral tunnel, a common mistake, will result in limited flexion and potential graft failure if full flexion is achieved. Tibial tunnel placement may be somewhat more forgiving, but anterior placement leads to impingement in extension and excessive tension in flexion, whereas posterior placement may cause laxity in flexion.17 Perhaps the most common error in surgical technique involves the anterior placement of femoral and/or tibial tunnels.16 Carson et al recently published their review of 90 failed ACL reconstructions and quoted 52% of the failures as being due to surgical technical errors.28 Several studies have shown that a posterior and proximal placement of the intraarticular exit of the femoral tunnel is advisable and involves minimal lengthening of the ACL substitute toward extension.
Treatment Options
Revision ACL surgery is often considered a salvage procedure with very limited goals, distinctly different from those of primary ACL reconstruction.5,29 Many reports in the literature quote inferior results for revision cases compared with primary reconstruction. However, with many categories of failure, the population of failed ACLs is a diverse group and a difficult subset to study.30
We ask the following questions before deciding on a definitive treatment plan:
Management of previous tunnel malposition is technically demanding, and different surgeons have used various approaches for dealing with bony defects resulting from incorrectly positioned prior tunnels. In some cases of gross tunnel malposition, a new tunnel may simply be drilled without violating the original tunnel or removing any tunnel hardware. Alternatively, tunnels can be oriented in a divergent pathway that maintains the appropriate articular surface attachment. In many cases, however, new tunnels cannot be drilled without overlapping or breaking into a previous tunnel. Two or more screws can be used to supplement fixation and fill the cavity of an enlarged tunnel. Although this might be useful in limited cases, the fixation achieved tends to be inferior and postoperative rehabilitation may be compromised. Graduated tunnel dilators may allow controlled expansion of a previous tunnel, compacting rather than removing additional bone.17 In such instances, options include the use of an allograft tendon with an enlarged bony portion, an oversized interference screw, or stacked interference screws.31 If the original tunnel is correctly positioned and only slightly larger (3–5 mm) than the new graft, stacking two interference screws may be sufficient to fill the tunnel and secure the graft.32,33 Battaglia and Miller34 have described use of freeze-dried allograft bone dowels to address bony defects during revision ACL reconstruction. These allografts are readily available and can be easily used to fill deficiencies resulting from previous tunnels or osteolysis. The grafts provide sufficient structural support to allow redrilling of new tunnels through or next to the bone plug. Unfortunately this option implies slower graft incorporation35 and has implications for the rehabilitation regimen.
Definition of knee instability
The IKDC classifies knees that are within 2 mm of the normal contralateral knee by means of KT-1000 or similar testing as “normal.”36 Knees that have greater than 5 mm of difference are classified as “abnormal.” The KT-1000 applies a force of 134N to assess knee laxity.
For the past 15 years, we have used the Westminster cruciometer (University College, London) for laxity measurements. It is a validated tool that applies an 89N force during the laxity measurement. Similar to the KT-1000, this cruciometer has been shown to give a reproducible quantitative evaluation of the Lachman test, and a previous study has shown average displacement of normal knee to be 3.2 mm as compared with 8.4 mm in the ACL deficient knee.37 A further validation of the Westminster cruciometer was done recently by comparing the laxity measurements in normal, ACL deficient, and ACL reconstructed knees. The correlation between the Westminster cruciometer and KT-2000 was found to be excellent (Pearson’s coefficient: 97%). The KT-2000 reading can be obtained using the following equation:
Surgical procedure
Stage II
The second stage includes a further EUA, arthroscopy, relevant meniscal and chondral surgery, graft harvest, and revision ACL reconstruction. Our choice of graft is described in Table 58-1.
| Primary Graft | Revision Graft |
|---|---|
| Bone–patellar tendon–bone (BPTB) | Four-strand hamstring |
| Four-strand hamstring | BPTB |
| Prosthetic | Ipsilateral BPTB graft or four-strand hamstring |
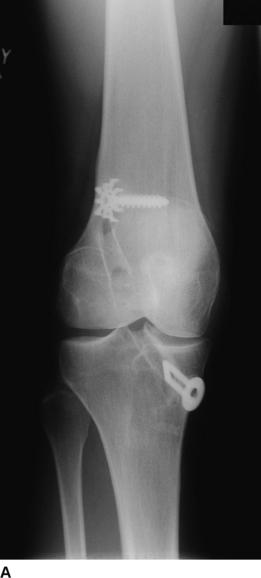
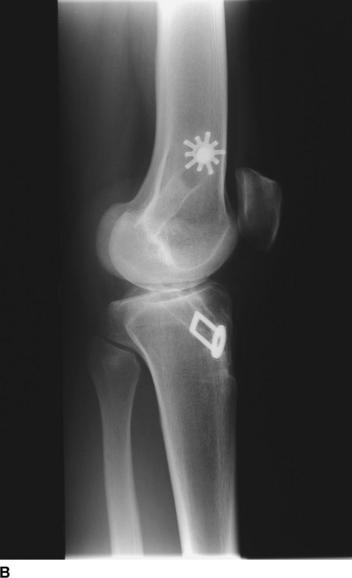
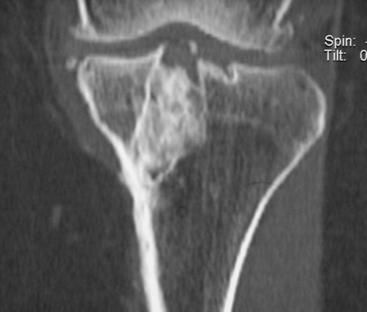
The revision procedure itself is similar to any primary procedure, and attention is given to achieve the correct anatomical placement of the tunnels. Because the landmarks are often less distinct than in a primary case, the tibial tunnel should be referenced off the PCL on the medial side of the mid-intercondylar point and the femoral tunnel referenced from the over-the-top position. We have favored the use of proprietary jigs (femoral Puddu guide, Acufex). Perioperative imaging using an image intensifier may occasionally be necessary to ensure optimal tunnel placement (Fig. 58-2).
Graft Choice: Autograft Versus Allograft
For a long time it has been debated whether an autograft or an allograft should be used for revision ACL reconstruction.38–41 Allografts have certain advantages. The donor site morbidity is eliminated, which may help during the rehabilitation. When weighed against the total costs of a two-staged ACL reconstruction, their use could be financially justified. However, they do have specific risks. Viral transmission of hepatitis, human immunodeficiency (HIV) virus, or other infection is a concern.42 Allografts tend to integrate more slowly than autografts and can cause immunological reactions, which may interfere with the healing process; hence the recommendation of a slower rehabilitation protocol.41 Furthermore, the sterilization process used may decrease the mechanical properties of the allograft. In addition, this increases the cost. As of March 11, 2002, the Centers for Disease Control and Prevention (CDC) had received 26 reports of bacterial infections from musculoskeletal allografts.43 Because the notification of infection secondary to use of allografts is voluntary, it is likely that its true incidence is underestimated. We agree with the concept that every effort should be made to ensure killing of bacteria and bacterial spores with the help of available technologies. These added risks of using an allograft have led us to refrain from their routine use. An allograft should only be considered when host material is scarce. This is sometimes the case in patients with multi-ligament laxity. One can use ipsilateral and contralateral bone–patellar tendon–bone (BPTB) as well as hamstring tendons but may still have to use allografts for a multidirectional laxity involving the ACL, PCL, posterolateral corner (PLC), and/or medial collateral ligament (MCL).44–46
We have always favored the use of autograft rather than allograft. It is not our practice routinely to use the contralateral limb for harvesting the graft, although some surgeons prefer the contralateral limb in the primary or revision setting.46 We do not have any experience of using reharvested BPTB or four-strand hamstring (4-SH) graft; reports in the literature of their use and satisfactory clinical outcome are few.47
Graft Fixation: Cortical or Apertural
The techniques for graft fixation during the revision procedure are similar to those used in the primary procedure. When dealing with bone–bone fixation, the interference screw,48 is our traditional method of fixation, although if the femoral tunnel is a tight fit, Rigidfix (Mitek Products, Ethicon, Edinburgh) is satisfactory. For hamstring fixation, we use IntraFix (Mitek) on the tibial side; for a cortical fixation on the femoral side, a Corin anchor (Corin Group, The Corinium Centre, Cirencester, United Kingdom) is used.
The types of fixation method used on both the femoral and tibial sides play a crucial role in the stability achieved after ACL reconstruction. The fixation devices must be able to withstand early postoperative forces until graft–tunnel healing has occurred. The fixation should facilitate graft tunnel healing, producing a normal histological transition zone between the host bone and the new ligament.45,49
The fixation methods can be broadly classified as cortical (suspensory) or apertural (intratunnel).50 There is a belief that cortical fixation may not perform as well as aperture fixation and that there may be a “bungee effect” causing reduced stability because fixation is farther from the joint, resulting in a longer graft with reduced stiffness. Cyclical elastic stretching under loading can be expected to increase with lengthening of the graft between the points of fixation. Anchoring the graft distant to the joint line may also allow AP movement, described as a “windshield wiper” effect after widening of the tunnel. However, this is debatable. Graft fixation relies on the friction between the graft and the fixation device until the graft integrates with the surrounding host tissues.50 Older methods such as in-line staples (tibial side) or simple buttons (femoral side) have a high chance of graft slippage due to their dependence on “simple friction” between the fixation device and a smooth, compressible soft tissue graft. This prevents the grafts being satisfactorily tightened and held. On the other hand, techniques such as Endobutton (Smith & Nephew, Andover, MA), Corin Anchor, and newer tibial devices rely on “complex friction” and thereby ensure better stability.51–56 Prodromos et al,50 in a recent meta-analysis of ACL reconstruction, considered stability after ACL reconstruction as a function of hamstring versus patellar tendon graft and fixation type. The authors concluded that four-strand hamstrings had overall higher stability than BPTB and the graft stability was fixation dependent. Four-strand hamstring grafts with Endobutton femoral fixation and second-generation tibial cortical fixation (belt-buckle staple configuration or interference screws augmented with staples) resulted in higher stability than all other graft/fixation combinations. Therefore either the bungee effect does not exist or, if it does exist, it seems inconsequential. Also, it is likely that the bungee effect is only likely during the early postoperative period before the bone tunnels have healed around the graft, converting cortical to apertural fixation.50
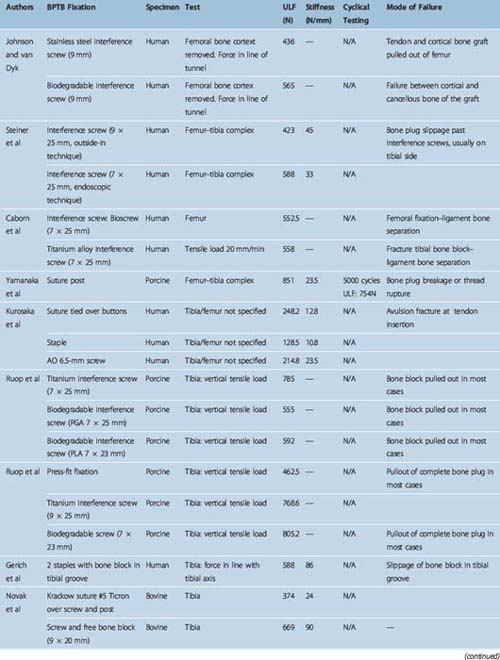
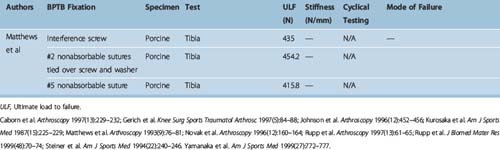
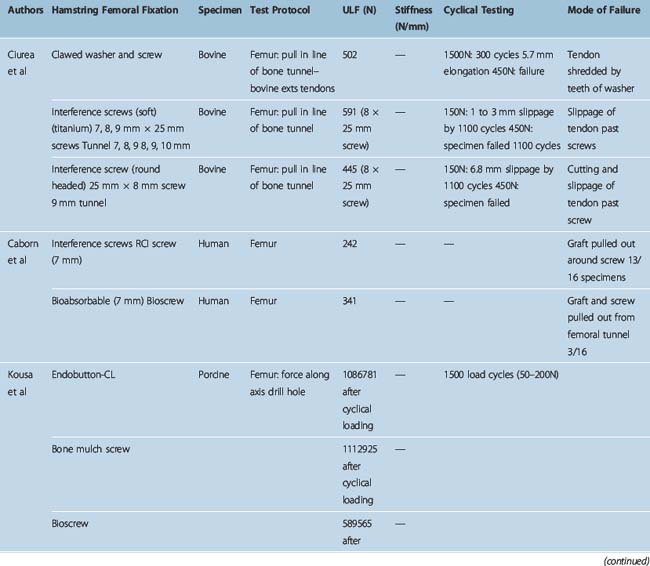
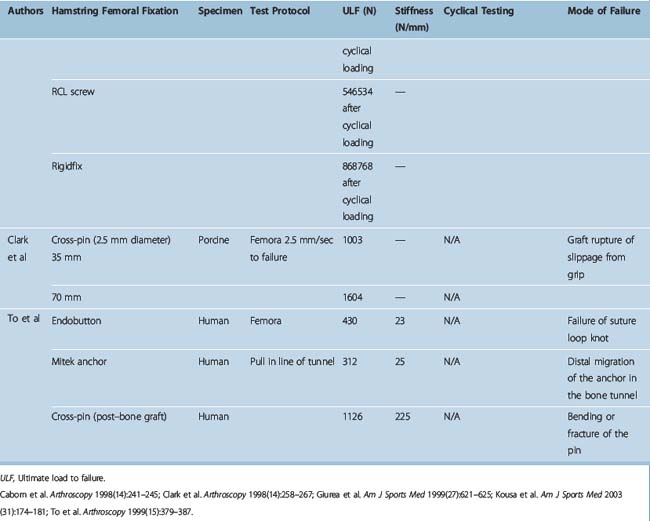
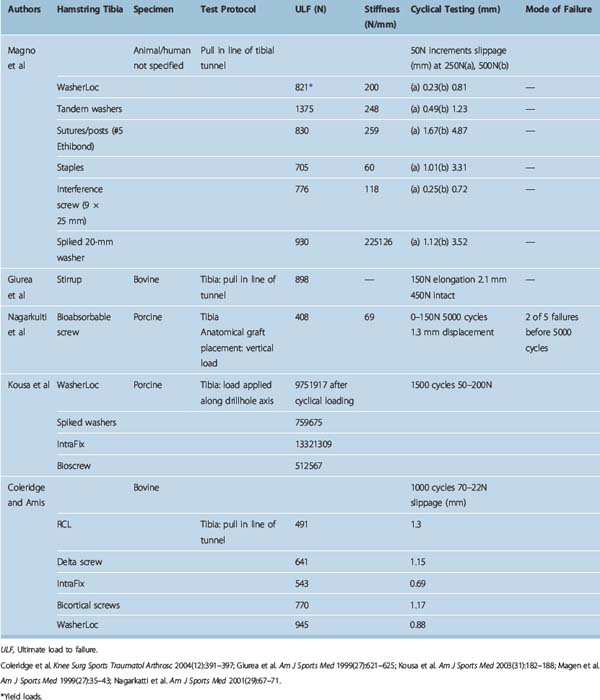
Different authors have assessed34,49,57–60 the fixation strengths of various femoral and tibial fixation devices used for ACL reconstruction. Harvey et al in their succinct review article considered the different types of fixations used along with results of laboratory testing.49 These are summarized in Tables 58-2, 58-3, and 58-4.49
Tibial fixation is commonly considered more problematic than femoral fixation because forces on the ACL substitute are parallel to the tibial drill hole,60,61 the bone quality of the tibial metaphysis is inferior to that of the femur,61,62 and the four-tailed end of the hamstring tendon graft that is fixed to the tibia is more difficult to secure. The WasherLoc secures the graft at the external tibial aperture, and the tandem spiked washers have an even longer working length because they are placed completely outside the tibial tunnel. The IntraFix may be considered a semi-aperture fixation because the 30-mm plastic sheath extrudes distally from the entrance of the tibial drill hole and thus, in a normal tibial tunnel of 35 to 45 mm in length, leaves 5 to 15 mm of free graft within the proximal opening (aperture) of the drill hole. Finally, interference screws can be considered truly anatomical (apertural) fixations because they can be advanced to the internal tibial tunnel orifice.
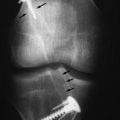
When interference screws are used in the tibia, they are inserted from the outside-in, producing forces that are counter to the direction of the tension on the graft, as opposed to the femoral side, where the screw is placed from the inside-out, thus wedging the graft during screw insertion. The strength of fixation of interference screws is influenced by several variables, such as the density of the bone,61 the insertion torque,63 the geometry55,64,65 and material of the screw,66 and the length and diameter of the screw.67 Different considerations may be important in the fixation of hamstring and BPTB grafts. Increasing the diameter of the screw increases the fixation of the hamstring by a press-fit mechanism that crushes the surrounding cancellous bone. However, poor engagement of the thread into the tendon may make this less important and length of the screw more so. Engagement of the thread into a corticocancellous BPTB block gives good fixation, which is influenced more by the changes in the diameter of the screw and less so by changes in the length (Fig. 58–3).49
Postoperative rehabilitation
Brown and Carson2 suggested that an accelerated rehabilitation program68 for revision ACL reconstruction is not appropriate due to weaker initial graft fixation. We have found that this is not necessary, as using a two-stage technique ensures that there is good quality bone around the tunnels and initial graft fixation is as secure as in the primary reconstruction. We have followed the same rehabilitation program for both primary and revision ACL patients and have not found any significant difference between the objective and subjective laxity assessment at follow-up between the primary and revision ACL reconstruction.
Our experience with a two-stage revision Anterior Cruciate Ligament reconstruction
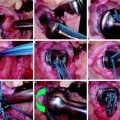
From 1991 to 2003 the senior author performed 75 revision ACL reconstructions. Of these, nine were performed as a single-stage revision, and 11 were performed in patients with multi-ligamentous laxity needing attention. The remaining 55 patients underwent revision ACL reconstruction using a two-stage technique with bone grafting of the tibial tunnel (Fig. 58-4). Of these 55 patients, 49 had a minimum follow-up of 3 years or more, and we compared this group of patients with a matched cohort of 49 patients with primary ACL reconstruction. These results were recently published in the American Journal of Sports Medicine.1 The salient findings of this study are summarized here.
Review of literature
Noyes and Barber-Westin reported on 55 patients who had a revision ACL reconstruction with a BPTB autograft.33 The failure rate, which was determined in a fashion similar to that in their revision allograft report, was 24%. Of these 13 patients, six had a reharvested patellar tendon autograft. This was a heterogenous group, and the authors stratified the group of 55 knees into those who had an ACL reconstruction only, those who required a staged high tibial osteotomy, and those who required a concurrent ligament reconstructive procedure. The group requiring only ACL reconstruction had a failure rate of 16% (5/32 knees). Earlier, the same research group had also published their results of revision ACL surgery with the use of a BPTB allograft.39 They noted an incidence of 33% failure at a mean of 42 months. In this series, the allografts were obtained from tissue banks certified by the American Association of Tissue Banks (AATB) and were fresh-frozen at the time of procurement. The grafts had been sterilized with 25,000 gray of gamma irradiation. This amount of low-dose irradiation probably does not alter the mechanical properties of the graft. In fact, AATB has advocated use of low-dose irradiation for many years and several authors have used it for ACL allografts to improve the protection against bacterial contamination.42,43 Noyes et al advocate that allograft should not be considered as the first choice of graft for revision surgery. If no autograft is available for revision surgery, they advise augmentation of the allograft with the lateral extraarticular iliotibial band procedure to reduce the high failure rate associated with the use of allograft.33
Fox et al recently published their results of revision ACL reconstruction using nonirradiated patellar tendon allograft.69 Thirty-two of 38 patients (84%) were available for follow-up. The mean patient age was 28 years with a mean follow-up of 4.8 years (range 2.1–12.1). This is a good homogenous group of patients with critical evaluation of results. None of the patients in the series had meniscal allograft surgery, posterolateral reconstructions, high tibial osteotomies, or contralateral ACL deficiency or reconstruction. Of this patient group, 87% were subjectively satisfied, 87% had 0/1+ pivot shift, and 84% had a KT-1000 SSD of less than 3 mm. The authors quote a failure rate of 28% using stringent criteria, namely the presence of a positive pivot-shift test (grade 1/2/3) and/or a KT-1000 result of more than 5 mm SSD. If the criteria used for definition are changed to SSD greater than 5 mm and pivot-shift grade 2 or more, then the failure rate is just 6%. The authors very correctly point out that the criteria used for defining failure after revision surgery are variable, which further complicates the comparison.
The Pittsburgh series of Johnson et al20,21,48 used irradiated, fresh-frozen allografts. Nine of the 25 patients had a KT-1000 maximum manual difference of greater than 5.5 mm. Eighty percent had grade 0 or 1 Lachman result, and 20% had grade 2 Lachman result; 76% of patients were satisfied with the results.21
Uribe et al reported on 54 patients with revision ACL reconstruction using a variety of grafts including ipsilateral patellar tendon autograft, contralateral patellar tendon autograft, allograft patellar tendon, and hamstring autograft. All the patients had an improvement in their objective stability; however, only 54% of the patients returned to their pre–ACL injury activity level.70
Battaglia et al34 recently published their experience of revision ACL reconstruction using freeze-dried allograft bone dowels. The advantages of these grafts are their ready availability, the elimination of donor site (iliac crest) morbidity, and their ability to provide sufficient structural support for the new tunnels. Although all these proposed benefits are true, with passage of time the allograft may become resorbed, compromising the stability of the ACL reconstruction.
Our results compare favorably to those published in the literature28,33,34,39,69–71 with regard to laxity measurements, and our failure rate is significantly lower. In only one case the graft had failed at 52 months and the patient complained of instability requiring revision, giving a failure rate of 2.04%. In another patient, the cruciometer reading was 5 mm, suggestive of increased laxity. However, this patient is coping well at present and has not had any further surgical intervention. These results have been achieved despite an uncompromised rehabilitation regimen, and we believe that this can be attributed at least partly to the two-stage technique we used, which allows for the consolidation of the bone graft in the tibial tunnel. These figures also represent the “worst-case” scenario, as no patient was lost to follow-up.
1 Thomas NP, Kankate R, Wandless F, et al. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;33:1701-1709.
2 Brown CHJ, Carson EW. Revision anterior cruciate ligament surgery. Clin Sports Med. 1999;18:109-171.
3 Aglietti P, Buzzi R, Simeone AJV, et al. Arthroscopic-assisted anterior cruciate ligament reconstruction with the central third patellar tendon. A 5–8 year follow-up. Knee Surg Sports Traumatol Arthrosc. 1997;5:138-144.
4 Steiner ME, Hecker AT, Brown CHJ, et al. Anterior cruciate ligament graft fixation. Comparison of hamstring and patellar tendon grafts. Am J Sports Med. 1994;22:240-246. discussion 6–7
5 Thomas NP. The patient with the failed ACL reconstruction. In: Clinical challenges in orthopaedics: the knee. City: Martin Dunitz; 2000:51-57.
6 L’Insalata JC, Klatt B, Fu FH, Harner CD. Tunnel expansion following ACL reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc. 1997;5:234-238.
7 Wilson TC, Kantaras A, Atay A, et al. Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med. 2004;32:543-549.
8 Allen CR, Giffin JR, Harner CD. Revision anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34:79-98.
9 Azer FM. Revision anterior cruciate ligament reconstruction. Instr Course Lect. 2002;51:335-342.
10 Berg EE. Tibial bone plug non-union: a cause of anterior cruciate ligament reconstructive failure. Arthroscopy. 1992;8:380-384.
11 Corsetti JR, Jackson DW. Failure of anterior cruciate ligament reconstruction: the biologic basis. Clin Orthop. 1996;325:42-49.
12 Dunn WR, Lincoln AE, Hinton RY, et al. Occupational disability after hospitalization for the treatment of the anterior cruciate ligament. J Bone Joint Surg. 2003;85A:1656-1666.
13 Dye SF. The future of anterior cruciate ligament restoration. Clin Orthop. 1996;325:130-139.
14 Frank C. Future directions of ACL research. In: The anterior cruciate ligament: current and future concepts. New York: Raven Press; 1993:4449-4450.
15 Friedlaender GE. Immune responses to osteochondral allografts. Current knowledge and future directions. Clin Orthop. 1983;174:58-68.
16 Friedlander GE. Current concepts review. Bone grafts. J Bone Joint Surg. 1987;69A:786-790.
17 Getelman MH, Friedman MJ. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg. 1999;7:189-198.
18 Greis PE, Johnson DL, Fu FH. Revision anterior cruciate ligament surgery: causes of graft failure and technical considerations of revision surgery. Clin Sports Med. 1993;12:839-852.
19 Harner CD. Editorial comment. Failed ACL surgery—a symposium. Clin Orthop. 1996;325:2-3.
20 Johnson DL. Revision ACL surgery. In: Ryder B, editor. Knee surgery. Philadelphia: Williams & Wilkins; 1994:877-895.
21 Johnson DL, Coen MJ. Revision ACL surgery. Etiology, indications, techniques and results. Am J Knee Surg. 1995;8:155-167.
22 O’Brien WR, Friederich NF. Isometric placement of cruciate ligament substitutes. In: The cruciate ligaments. Edinburgh: Churchill Livingstone; 1994:595-604.
23 O’Neill DB. Arthroscopically assisted reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 2001;83:1329-1332.
24 Stapleton TR. Complications in anterior crucitae ligament reconstructions with patellar tendon grafts. Sports Med Arthrosc Rev. 1997;5:156-162.
25 Vergis A, Gillquist J. Graft failure in intra-articular anterior cruciate ligament reconstructions: a review of the literature. Arthroscopy. 1995;11:312-321.
26 Wetzler MJ, Bartolozzi AR, Gillespie MJ, et al. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6:181-189.
27 Wolf RS, Lemak LJ. Revision anterior cruciate ligament surgery. J South Orthop Assoc. 2002;11:25-32.
28 Carson EW, Anisko EM, Restrepo C, et al. Revision anterior cruciate ligament reconstruction: etiology of failures and clinical results. J Knee Surg. 2004;17:127-132.
29 Safran MR, Harner CD. Technical considerations of revision anterior cruciate ligament surgery. Clin Orthop. 1996;325:50-64.
30 Grossman MG, El Attrache NS, Shields CL, et al. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21:418-423.
31 Noyes FR, Barber-Westin SD. Revision anterior cruciate ligament surgery: experience from Cincinnati. Clin Orthop Rel Res. 1996;325:116-129.
32 Noyes FR, Barber-Westin S. Revision anterior cruciate ligament surgery: report of 11-year experience and results in 114 consecutive patients. Instr Course Lect. 2001;50:451-461.
33 Noyes FR, Barber-Westin SD. Revision anterior cruciate surgery with use of bone-patellar tendon-bone autogenous grafts. J Bone Joint Surg. 2001;83A:1131-1143.
34 Battaglia TC, Miller MD. Management of bony deficiency in revision anterior cruciate ligament reconstruction using allograft bone dowels: surgical technique. Arthroscopy. 2005;21:767.
35 Jaureguito JW, Paulos LE. Why grafts fail. Clin Orthop. 1996;325:25-41.
36 Hefti F, Muller W, Jakob RP, et al. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226-234.
37 Crawford E, Dewer M, Aichworth PM. The Westminster cruciometer for measurement of anterior cruciate instability: proceedings of BASK meeting December 1985. J Bone Joint Surg. 1987;69B:159.
38 Getelman MH, Schepsis AA, Zimmer J. Revision ACL reconstruction: autograft versus allograft. Arthroscopy. 1995;11:378.
39 Noyes FR, Barber-Westin SD, Roberts CS. Use of allografts after failed treatment of rupture of the anterior cruciate ligament. J Bone Joint Surg. 1994;76A:1019-1031.
40 Pelker RR, Friedlaender GE, Markham TE, et al. Effects of freezing and freeze-drying on the biomechanical properties of rat bone. J Orthop Res. 1984;1:405-411.
41 Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg. 1987;69:573-582.
42 Asselmeier MA, Caspari RB. A review of allograft processing and sterilization techniques and their role in transmission. Am J Sports Med. 1993;21:170-175.
43 Noyes FR, Barber-Westin SD. Prospective evaluation of allograft meniscus transplantation: a minimum 2-year follow-up. Am J Sports Med. 2006;34:2038-2039.
44 Ritchie JR, Parker RD. Graft selection in anterior cruciate ligament revision surgery. Clin Orthop. 1996;325:65-77.
45 Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg. 1993;75A:1795-1803.
46 Shelbourne KD, O’Shea JJ. Revision anterior cruciate ligament reconstruction using the contralateral bone-patellar tendon-bone graft. Instr Course Lect. 2002;51:343-346.
47 Colosimo AJ, Heidt RSJ, Traub JA, et al. Revision anterior cruciate ligament reconstruction with a reharvested ipsilateral patellar tendon. Am J Sports Med. 2001;29:746-750.
48 Johnson DL, Swenson TM, Irrgang JJ, et al. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop. 1996;325:100-109.
49 Harvey AR, Thomas NP, Amis AA. Fixation of the graft in reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 2005;87A:593-603.
50 Prodromos CC, Joyce BT, Shi K, et al. A meta-analysis of stability after anterior cruciate ligament reconstruction as a function of hamstring versus patellar tendon graft and fixation type. Arthroscopy. 2005;21:1202.
51 Freedman K, D’Amato M, Nedeff D, et al. Arthroscopic anterior cruciate ligament reconstruction. A metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31:2-11.
52 Grover DM, Howell SM, Hull ML. Early tension loss in an anterior cruciate ligament graft. A cadaver study of four tibial fixation devices. J Bone Joint Surg. 2005;87A:381-390.
53 Kawakami H, Shino K, Hamada M. Graft healing in a bone tunnel: bone-attached graft with screw fixation versus bone-free graft with extra-articular suture fixation. Knee Surg Sports Traumatol Arthrosc. 2004;12:384-390.
54 Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27:35-43.
55 Weiler A, Hoffmann RFG, Siepe CJ, et al. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28:356-359.
56 Yunes M, Richmond JC, Engels EA, et al. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction. A meta-analysis. Arthroscopy. 2001;17:248-257.
57 Kousa P, Teppo L, Jarvinen NJ, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31:182-188.
58 Kousa P, Teppo L, Jarvinen NJ, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31:174-181.
59 Kurosaka M, Yoshiya S, Andrish JT. A biomechanical comparison of different surgical techniques of graft fixation in anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:225-229.
60 Malek MM, DeLuca JV, Verch DL, et al. Arthroscopically assisted ACL reconstruction using central third patellar tendon autograft with press fit femoral fixation. Instr Course Lect. 1996;45:287-295.
61 Brand JJr, Weiler A, Caborn DNM, et al. Current concepts. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28:761-774.
62 Vuori I, Heinonen A, Sievanen H, et al. Effects of unilateral strength training and detraining on bone mineral density and content in young women. A study of mechanical loading and deloading on human bones. Calcif Tissue Int. 1994;55:59-67.
63 Brand JCJr, Pienkowski D, Steenlage E, et al. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28:705-710.
64 Giurea M, Zorilla P, Amis A, et al. Comparative pull-out and cyclic-loading strength tests of anchorage of hamstring tendon grafts in anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:621-625.
65 Weiler A, Hoffmann RF, Stahelin AC, et al. Hamstring tendon fixation using interference screws. A biomechanical study in calf tibial bone. Arthroscopy. 1998;14:29-37.
66 Beynnon BD, Meriam CM, Ryder SH, et al. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26:536-539.
67 Harvey AR, Thomas NP, Amis AA. The effect of screw length and position on fixation of four strand hamstring grafts for anterior cruciate ligament reconstruction. Knee. 2003;10:97-102.
68 Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18:292-299.
69 Fox JA, Pierce M, Bojchuk J, et al. Revision anterior cruciate ligament reconstruction with nonirradiated fresh-frozen patellar tendon allograft. Arthroscopy. 2004;20:787-794.
70 Uribe JW, Hechtman KS, Zvijac JE, et al. Revision anterior cruciate ligament surgery: experience from Miami. Clin Orthop Relat Res. 1996:91-99. Apr
71 Woods GW, Fincher AL, O’Connor DP, et al. Revision anterior cruciate ligament reconstruction using the lateral third of the ipsilateral patellar tendon after failure of a central-third graft: a preliminary report on 10 patients. Am J Knee Surg. 2001;14:23-31.