Retroviruses
I Retroviridae: General Features
• Midsized viruses with an enveloped capsid containing two copies of a single-stranded (+) RNA genome, transfer RNA (tRNA), reverse transcriptase, integrase, and protease.
• Two human pathogens—human immunodeficiency virus (HIV) and human T lymphotropic virus (HTLV)—are retroviruses.
1. All retroviral genomes contain three genes—gag, pol, and env—and are flanked by long-terminal repeats (Table 26-1).
TABLE 26-1
Retrovirus Genes and Their Function
| Gene | Virus | Function |
| gag | All | Group-specific antigen: core and capsid proteins |
| int | All | Integrase |
| pol | All | Polymerase: reverse transcriptase, protease, integrase |
| pro | All | Protease |
| env | All | Envelope: glycoproteins |
| tax | HTLV | Transactivation of viral and cellular genes |
| tat | HIV-1 | Transactivation of viral and cellular genes |
| rex | HTLV | Regulation of RNA splicing and promotion of export to cytoplasm |
| rev | HIV-1 | Regulation of RNA splicing and promotion of export to cytoplasm |
| nef | HIV-1 | Alteration of cell activation signals; progression to AIDS (essential) |
| vif | HIV-1 | Virus infectivity, promotion of assembly, blocks a cellular antiviral protein |
| vpu | HIV-1 | Facilitates virion assembly and release, decrease of cell surface CD4 |
| vpr (vpx*) | HIV-1 | Transport of complementary DNA to nucleus, arresting of cell growth |
| LTR | All | Promoter, enhancer elements |
From Murray PR, Rosenthal KS, Pfaller MA: Medical Microbiology, 6th ed. Philadelphia, Mosby, 2009, Table 64-2.
2. Complex retroviruses, such as HIV, have several other genes encoding auxiliary and regulatory proteins (e.g., nef, tat, and rev)
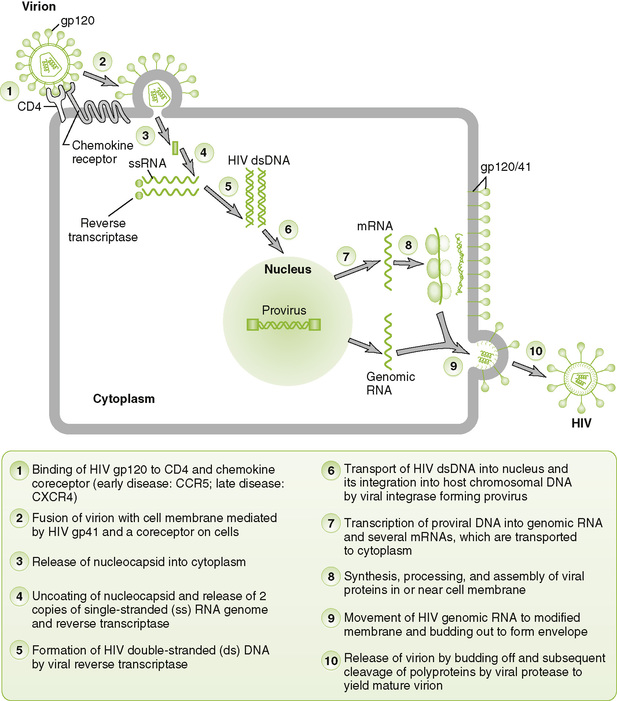
B Key HIV proteins (Fig. 26-1)
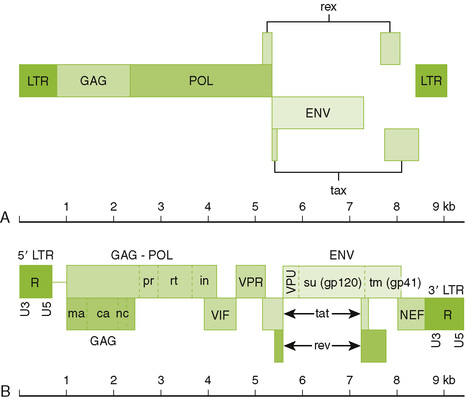
26-1 Genome structure of human retroviruses: (A) HTLV-1 and (B) HIV. The basic retrovirus genome consists of the long terminal repeat (ltr) group–specific antigen (capsid proteins)(gag)-enzymes(polymerase, integrase, protease)(env) and the glycoproteins (env). Complex retroviruses, such as HIV have additional proteins that enhance their virulence. These are described in Table 26-1. (Redrawn from Belshe RB: Textbook of Human Virology, 2nd ed. St Louis, Mosby, 1991. In Murray PR, Rosenthal KS, Pfaller MA: Medical Microbiology, 6th ed. Philadelphia, Mosby, 2009, Fig. 64-4.)
1. Major envelope glycoprotein consists of two associated proteins: an attachment protein (HIV gp120) and transmembrane fusion protein (HIV gp41) both cleaved from gp160 precursor.
2. Three enzymes are carried within the nucleocapsid: reverse transcriptase, integrase, and protease.
3. Matrix protein (HIV p17) forms a layer underlying the envelope.
4. Nucleocapsid protein (HIV p24) forms the outer layer of the virion core, and detection in blood indicates infection.
• Binding of viral gp120/gp41 to CD4 and a chemokine coreceptor is required for HIV infection of cells.
• Reverse transcriptase carried in the virion synthesizes a complementary DNA (cDNA) from viral genome, forming an RNA-DNA hybrid. The same enzyme then degrades the RNA strand and synthesizes a complementary DNA strand, forming double-stranded viral DNA.
• Viral integrase catalyzes integration of viral DNA into host nuclear DNA, forming provirus.
3. Viral messenger RNA (mRNA) and genome replication
• Host DNA-dependent RNA polymerase transcribes a full-length (+) RNA copy of the integrated genome and several shorter mRNA copies for individual proteins and polyproteins.
• After synthesis of viral proteins, nucleocapsids containing two copies of genome associate with glycoprotein modified plasma membrane and bud off.
• After budding, protease cleaves gag-pol polyprotein to produce mature (cone-shaped) nucleocapsid and functional integrase and reverse transcriptase enzymes.
1. High rate of mutation in retroviruses results from numerous alterations introduced by reverse transcriptase.
2. Generation of new HIV strains occurs during the course of infection of an individual, leading to changes in tissue tropism, antigenicity, and other properties of the virus.
3. Generation of drug resistance occurs readily, requiring multiple drug therapy (highly active antiretroviral therapy [HAART]).
• HIV is a lentivirus that causes immunosuppression and neurologic disorders.
• HIV has a characteristic morphology with a cone-shaped capsid surrounded by an envelope (see Fig. 26-1).
1. Serologic and molecular tests
a. Enzyme-linked immunosorbent assay (ELISA) detects anti-HIV antibodies as the standard screening test.
b. Western blot for anti-HIV antibodies is used to confirm ELISA test for diagnosis of HIV infection.
c. Viral RNA and antigens can be detected early in infection (before the appearance of antibody) and late in infection (when antibody titer falls).
1) Viral RNA (genome load) is detected by reverse transcriptase–polymerase chain reaction (RT-PCR)
2) Genome load is an early indicator of infection, course of disease, and efficacy of therapy.
3) Quantitative RT-PCR and other assays are used to evaluate genome load.
d. HIV p24 antigen is an early marker of infection and active virus replication.
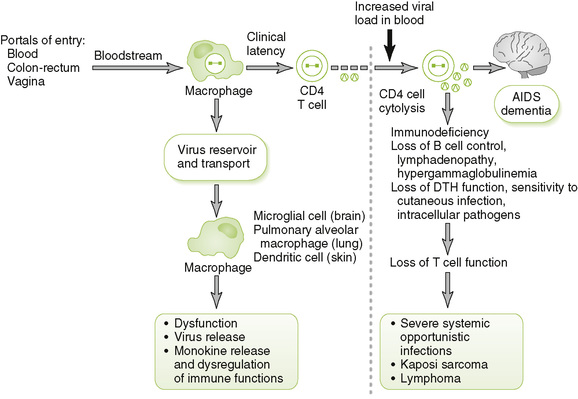
• HIV primarily infects helper T (TH) cells and myeloid-lineage cells, which express CD4 and a chemokine coreceptor.
2. Consequences of target cell infection
• Persistently infected macrophages may act as the major reservoir and distribution vehicle for HIV in the body.
• Killing of infected CD4 T cells leads to decreased CD4 T cell count and eventually to other immune system abnormalities. Examples include:
a. Decreased proliferation of CD8 T cells (due to reduced interleukin [IL]-2 production)
(1) Increased susceptibility to viruses
b. Decreased macrophage function due to reduced interferon-γ production
(1) Reduced macrophage function increases susceptibility to fungi, mycobacterial recurrences, and bacterial infections.
c. Changes in the balance of cytokines
e. Uncontrolled nonspecific antibody production (hypergammaglobulinemia)
• Neurologic abnormalities may result from infection of brain macrophages, microglial cells, and neurons.
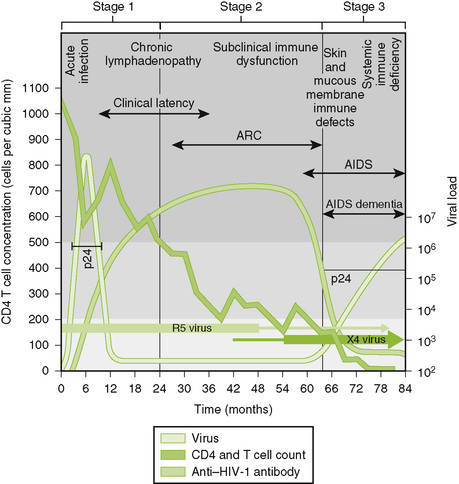
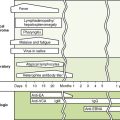
C Clinical course of HIV infection (Fig. 26-4)
• Asymptomatic or resembles mononucleosis and may have aseptic meningitis or a rash; symptoms subside spontaneously.
• Viral load increases and then decreases, but HIV persists in the host for months to years with few symptoms.
• Onset is marked by reduction of CD4 T cell count below 500 cells/μL and reduced delayed-type hypersensitivity responses.
• Symptoms include lymphadenopathy and fever, weight loss, malaise, diarrhea, night sweats, fatigue, and relatively mild opportunistic infections such as oral thrush (oropharyngeal candidiasis), hairy leukoplakia, and listeriosis.
• AIDS is defined by CD4 T cell count of less than 200 cells/μL and usually is accompanied by the appearance of one or more indicator diseases (Table 26-2).
TABLE 26-2
Selected AIDS Indicator Diseases
| Type | Specific disease* |
| Bacterial infection | Mycobacterium avium-intracellulare complex infection, disseminated |
| Extrapulmonary tuberculosis (Mycobacterium tuberculosis) | |
| Salmonella septicemia, recurrent | |
| Viral infection | Cytomegalovirus disease |
| Herpes simplex virus infection, chronic or disseminated | |
| Progressive multifocal leukoencephalopathy (JC virus) | |
| Fungal infection | Candidiasis of the esophagus, trachea, or lungs (Candida albicans) |
| Cryptococcal meningitis (Cryptococcus neoformans) | |
| Histoplasmosis (Histoplasma capsulatum) | |
| Pneumocystis jiroveci pneumonia | |
| Protozoal infection | Cryptosporidiosis, chronic with diarrhea (Cryptosporidium species) |
| Toxoplasmosis of the brain (Toxoplasma gondii) | |
| Neoplasia | Cervical cancer (invasive) |
| Kaposi sarcoma | |
| Primary lymphoma of the brain | |
| Other non-Hodgkin lymphomas | |
| Other | HIV wasting syndrome |
*A diagnosis of AIDS is made for HIV-infected patients who manifest any of these diseases regardless of their T cell count.
• Progression of AIDS is marked by an increase in detectable viral load and further reduction of CD4 T cell count to very low levels.
• AIDS-related dementia, marked by a slow deterioration of intellect and other neurologic disorders, occurs in some AIDS patients.
1. Inoculation with infected blood: transfusion of blood or blood products, sharing of needles by intravenous drug abusers, use of infected tattoo needles, accidental needle sticks, and contact with contaminated blood through open wounds and mucous membranes
• Screening of the blood supply, organ transplants, and clotting factors used by hemophiliacs for the presence of HIV has reduced transmission by these routes.
2. Sexual contact: anal or vaginal intercourse
3. Intrauterine or perinatal transmission or through breast milk to the newborn
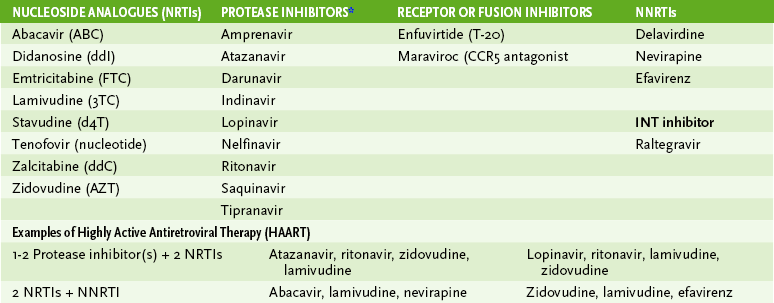
• Antiviral drug therapy delays the onset and severity of symptoms and decreases virus production and shedding.
• Targets for therapy are polymerase, protease, integrase, viral binding, and fusion to cell.
1. Treatment can be initiated when CD4 T cell numbers are less than 500.
2. Treatment with AZT alone or in combination with lamivudine or other drug treatment of pregnant mother can prevent transmission to fetus.
• HTLVs are oncoviruses associated with cancer and neurologic disorders.
• Only HTLV-1 has been directly associated with human disease, although infection is usually asymptomatic.
1. CD4 T cells are the primary targets of HTLV-1 infection, but the virus can also infect neurons.
2. Viral tax protein stimulates proliferation of infected T cells in the absence of antigen by activating production of IL-2 and the IL-2 receptor.