Chapter 6 Retinoids
MOLECULAR BIOLOGY OF RETINOIDS
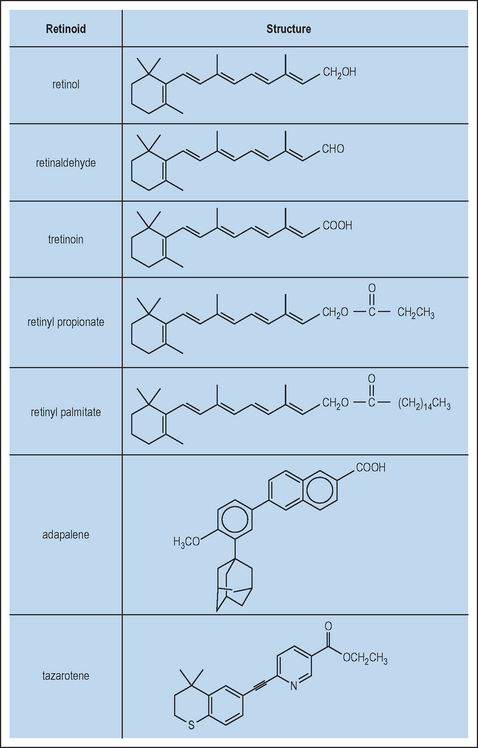
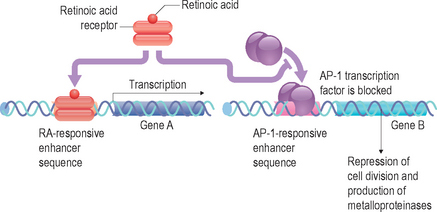
Retinoids are naturally occurring derivatives of beta-carotene and ascribed as vitamin A and its direct metabolites. These include retinol, retinaldehyde, retinyl esters, and retinoic acid (Fig. 6.1). These compounds have an essential role in such processes in higher order mammals as development (including ocular), angiogenesis, and dermatologic homeostasis. One of the key biologically relevant retinoids is retinoic acid, which exists as several isomeric forms (e.g. all-trans, 9-cis and 13-cis) and is essentially an oxidized form of retinol. This molecule has been shown at the molecular level to function as an agonist for the retinoic acid receptors (RAR) and retinoid X receptors (RXR), a specific subgroup from the broader family of nuclear receptors. In this subclass, there exist three isoforms of respective receptors, labeled α, β, and γ. Upon binding of the retinoic acid ligand, RAR and RXR will form a heterodimer that then is capable of interacting with specific DNA sequences located in the promoter regions of retinoid-regulated genes. These nucleic acid sequences are termed retinoic acid response elements (RARE). More recently, it has become apparent that the transcription factor AP-1 also has a significant effect upon regulating activation of genes through its interactions at the RARE site.
In summary, retinoic acid can influence the function of a cell by altering gene expression patterns through its facilitated binding to RAREs of a dimerized RAR/RXR complex (Fig. 6.2). This knowledge of the mechanistic role in retinoid regulation of gene expression patterns allowed for the synthesis of novel pharmacologic classes of compounds that have a broader structural diversity with varying pharmacologic properties than natural retinoids. Additionally, it appears that the majority of biologic effects observed from topical delivery of various retinoids are mediated by interaction through the RAR/RXR complex, including in some cases any obligatory metabolic conversion to retinoic acid.
METABOLISM OF CUTANEOUSLY DELIVERED RETINOIDS
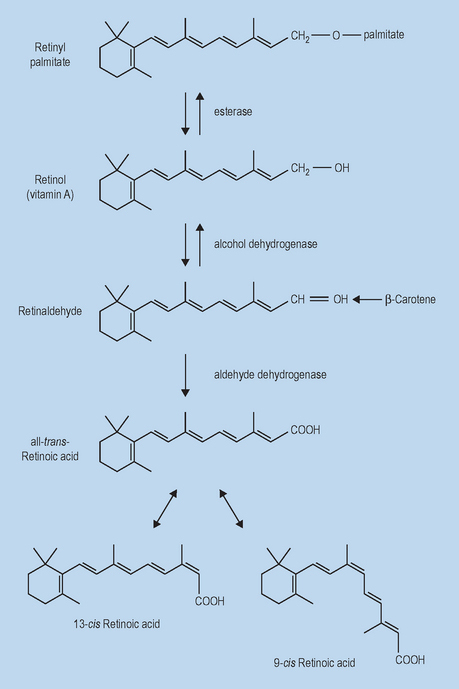
The metabolic pathways that have been identified as involved in retinoid metabolism in the digestive system have been confirmed in large part as existing in human skin (Fig. 6.3). While much of free retinol is esterified via lecithin:retinol acyltransferase (LRAT) or acyl CoA:retinol acyltransferase (ARAT) to retinyl palmitate for storage, a small percentage is further oxidized to the active acid form. The oxidation of free retinol to retinoic acid is the limiting step in the generation of active retinoid metabolites within cells. This process is begun when free retinol associates with a specific cytoplasmic retinol-binding protein (CRBP). The retinol-CRBP complex is a substrate for retinol dehydrogenase, a microsomal enzyme uniquely capable of catalyzing the conversion of retinol to retinaldehyde. Retinaldehyde is then rapidly and quantitatively oxidized to retinoic acid by retinaldehyde oxidase. Once converted, retinoic acid regulates gene expression profiles via RAR/RXR for skin keratinocyte growth and differentiation.
This multistep processing of retinyl esters serves as a point of regulation to control the level of active retinoid in the skin and may thus contribute to the lower irritation potential of these derivatives. Ultimately, retinoic acid can be metabolized irreversibly via hydroxylation to 4-hydroxy-retinoic acid and 4-oxo-retinoic acid via various cytochrome P450. It is important to note that the majority of retinoid metabolism that occurs is mediated via retinoid bound to cytosolic lipid binding proteins. This family of proteins with high retinoid specificity includes CRBP and cytoplasmic retinoic acid-binding protein (CRABP), of which there are two isoforms, I and II.
 irritation that, in some instances, does not mitigate itself completely even after long-term chronic exposure
irritation that, in some instances, does not mitigate itself completely even after long-term chronic exposure
Thus, a significant effort has been expended to identify retinoids that are efficacious and have an overall lower irritation profile and lessened teratogenic safety concerns.
• Retinyl esters
RETINYL PROPIONATE
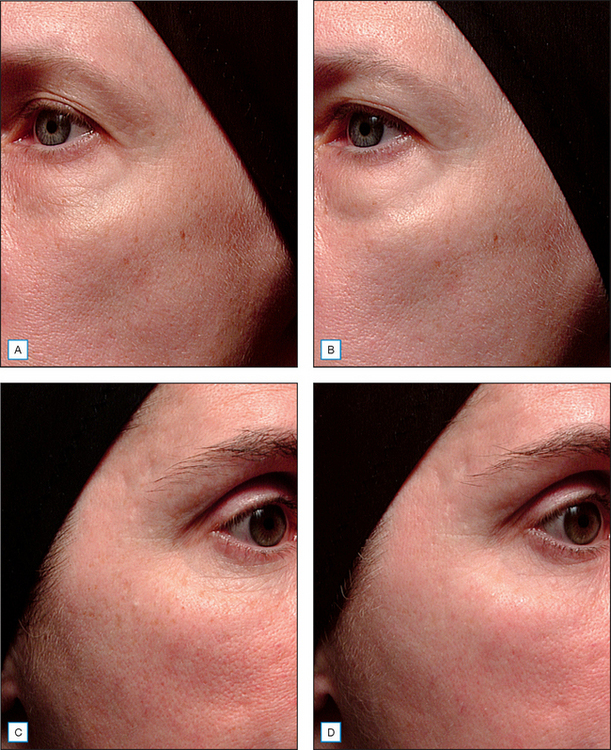
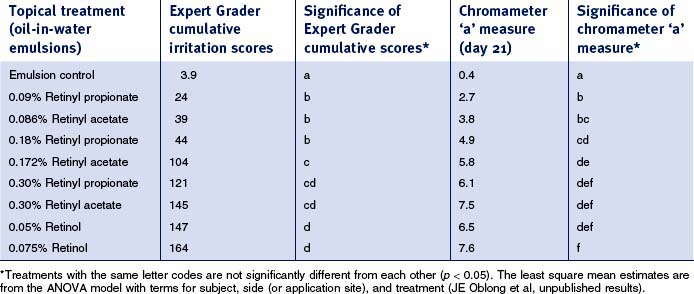
Retinyl propionate has been reported to be active in human skin and to have less irritation than other active retinoid options. More recently, it has been observed that this particular ester is capable of eliciting retinoid-like effects in human skin via both histologic assessments and clinical measures of photodamage changes (Fig. 6.4). Furthermore, retinoid-induced irritation appears to be less evident from retinyl propionate in comparison with retinol or retinyl acetate (Table 6.1). As with the palmitate ester of retinol, retinyl propionate must be hydrolyzed to free retinol, a process that occurs via skin esterases. Additionally, it has been reported that the propionate ester has an improved stability profile relative to other esters, thereby increasing half-life upon skin during topical delivery.
• Tretinoin
The usage of tretinoin, also known as trans-retinoic acid, in the dermatologic field has an extensive history, due in large part to pioneering work to understand the pharmacologic and molecular impact of the active upon photodamaged skin and acne lesions, as well as its role in cellular differentiation and developmental biology. Topical retinoic acid (Retin-A, Renova, Ortho-Neutrogena) is well known for its activity in improving the appearance of the signs of skin photodamage such as fine lines, wrinkles, and pigmentation. However, it has also been found to elicit significant irritation and dryness. Tretinoin (Retin-A) was originally approved up to 0.1% levels for the treatment of acne and was later approved, under the name Renova, at 0.025 and 0.05% to be used for topical treatment of photodamaged skin.
CONCLUSION
Retinoids are a broad family of molecules whose primary function is to serve as agonists for members of the RAR and RXR nuclear receptor family. In turn, this denotes their role in regulating gene expression profiles via RAR/RXR binding to RAREs. In dermatology, retinoids have been shown to have beneficial effects upon acne, psoriasis, actinic keratosis, and photodamaged/aging skin attributes. The ability of synthetic retinoid analogs to mimic the effects of endogenous ligands such as retinoic acid is based in part on similar mechanisms of action but with variant pharmacokinetic properties that renders them as more functional for specific purposes, based on the disease state being targeted. Future research directions in better understanding the connections between efficacy and retinoid-induced irritation should allow for the identification of analogs or optimized therapies that decouple these two phenomena.
Bailey JS, Siu CH. Purification and partial characterization of a novel binding protein for retinoic acid from neonatal rat. Journal of Biological Chemistry. 1988;263:9326–9332.
Bhawan J. Assessment of the long-term safety of topical retinoids. Cutis. 2005;75:25–31.
Boehnlein J, Sakr A, Lichtin JL, Bronaugh RL. Characterization of esterase and alcohol dehydrogenase activity in skin. Metabolism of retinyl palmitate to retinol (vitamin A) during percutaneous absorption. Pharmacy Research. 1994;11:1155–1159.
Creidi P, Humbert P. Clinical use of topical retinaldehyde on photoaged skin. Dermatology. 1999;199(Suppl 1):49–52.
Dosik JS, Homer K, Arsonnaud S. Cumulative irritation potential of adapalene 0.1% cream and gel compared with tazarotene cream 0.05% and 0.1%. Cutis. 2005;75:289–293.
Duell EA, Kang S, Voorhees JJ. Unoccluded retinol penetrates human skin in vivo more effectively than unoccluded retinyl palmitate or retinoic acid. Journal of Investigative Dermatology. 1997;109:301–305.
Effendy I, Kwangsukstith C, Lee JY, Maibach HI. Functional changes in human stratum corneum induced by topical glycolic acid: comparison with all-trans retinoic acid. Acta Dermatologica Venereologica. 1995;75:455–458.
Fluhr JW, Vienne MP, Lauze C, Dupuy P, Gehring W, Gloor M. Tolerance profile of retinol, retinaldehyde and retinoic acid under maximized and long-term clinical conditions. Dermatology. 1999;199(Suppl 1):57–60.
Galvin SA, Gilbert R, Baker M, Guibal F, Tuley MR. Comparative tolerance of adapalene 0.1% gel and six different tretinoin formulations. British Journal of Dermatology. 1998;139(Suppl 52):34–40.
Goodman DS. Retinoid-binding proteins. Journal of the American Academy of Dermatology. 1982;6(4 Pt 2 Suppl):583–590.
Green C, Orchard G, Cerio R, Hawk JLM. A clinicopathological study of the effects of topical retinyl propionate cream in skin photoageing. Clinics in Experimental Dermatology. 1998;23:162–167.
Harrison EH. Enzymes catalyzing the hydrolysis of retinyl esters. Biochimica et Biophysica Acta. 1993;1170:99–108.
Kafi R, Kwak HSR, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Archives of Dermatology. 2007;143:606–612.
Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75:10–13.
Kang S, Duell EA, Fisher GJ, et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels of irritation. Journal of Investigative Dermatology. 1995;105:549–556.
Kang S, Leyden JJ, Lowe NJ, et al. Tazarotene cream for the treatment of facial photodamage: a multicenter, investigator-masked, randomized, vehicle-controlled, parallel comparison of 0.01%, 0.025%, 0.05%, and 0.1% tazarotene creams with 0.05% tretinoin emollient cream applied once daily for 24 weeks. Archives of Dermatology. 2001;137:1597–1604.
Kim MS, Lee S, Rho HS, et al. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clinica Chimica Acta. 2005;362:161–169.
Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. Journal of the American Academy of Dermatology. 1986;15:836–859.
Kurlandsky SB, Xiao J-H, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of all-trans retinol requires metabolic conversion to all-trans-retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. Journal of Biological Chemistry. 1994;269:32821–32827.
Kurlandsky SB, Duell EA, Kang S, Voorhes JJ, Fisher GJ. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. Journal of Biological Chemistry. 1996;271:15346–15352.
Leyden JJ. Retinol for the treatment of photoaged skin. Cosmetic Dermatology. 2005;18(Suppl 1):14–17.
Navarro JM, Casatorres J, Jorcano JL. Elements controlling the expression and induction of the skin: hyperproliferation-associated keratin K6. Journal of Biological Chemistry. 1995;270:21362–21367.
Phillips TJ. An update on the safety and efficacy of topical retinoids. Cutis. 2005;75:14–22.
Phillips TJ, Gottlieb AB, Leyden JJ, et al. Tazarotene Cream Photodamage Clinical Study Group. Efficacy of 0.1% tazarotene cream for the treatment of photodamage: a 12-month multicenter, randomized trial. Archives of Dermatology. 2002;138:1486–1493.
Randolph RK, Simon M. Characterization of retinol metabolism in cultured human epidermal keratinocytes. Journal of Biological Chemistry. 1993;268:9198–9205.
Ridge BD, Batt MD, Palmer HE, Jarrett A. The dansyl chloride technique for stratum corneum renewal as an indicator of changes in epidermal mitotic activity following topical treatment. British Journal of Dermatology. 1988;118:167–174.
Sachsenberg-Studer EM. Tolerance of topical retinaldehyde in humans. Dermatology. 1999;199(Suppl 1):61–63.
Sefton J, Kligman AM, Kopper SC, Lue JC, Gibson JR. Photodamage pilot study: a double-blind, vehicle-controlled study to assess the efficacy and safety of tazarotene 0.1% gel. Journal of the American Academy of Dermatology. 2000;43:656–663.
Semenzato A, Bovenga L, Faiferri L, Austria R, Bettero A. Stability of vitamin A propionate in cosmetic formulations. SÖFW Journal. 1997;123:151–154.
Singh M, Griffiths CEM. The use of retinoids in the treatment of photoaging. Dermatologic Therapy. 2006;19:297–305.
Sorg O, Antille C, Kaya G, Saurat J-H. Retinoids in cosmeceuticals. Dermatologic Therapy. 2006;19:289–296.
Stratigos AJ, Katsambas AD. The role of topical retinoids in the treatment of photoaging. Drugs. 2005;65:1061–1072.
Verschoore M, Poncet M, Czernielewski J, Sorba V, Clucas A. Adapalene 0.1% gel has low skin-irritation potential. Journal of the American Academy of Dermatology. 1997;36(6 Pt 2):S104–109.
Vienne MP, Ochando N, Borrel MT, Gall Y, Lauze C, Dupuy P. Retinaldehyde alleviates rosacea. Dermatology. 1999;199(Suppl 1):53–56.
Weindl G, Roeder A, Schafer-Korting M, Schaller M, Korting HC. Receptor-selective retinoids for psoriasis: focus on tazarotene. American Journal of Clinical Dermatology. 2006;7:85–97.
Weiss JS, Ellis CN, Headington JT, et al. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. Journal of the American Medical Association. 1988;259:527–532.