24 Reticular formation
Organization
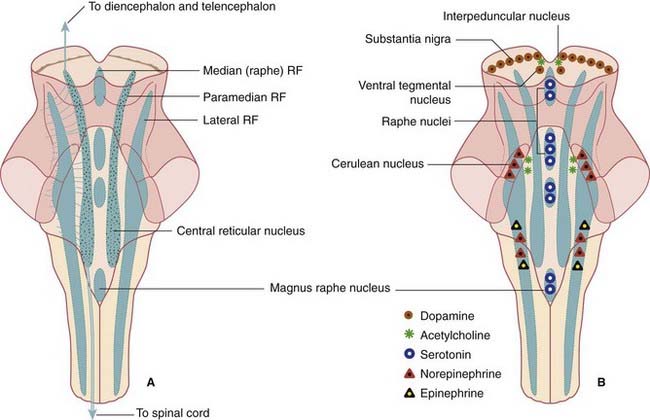
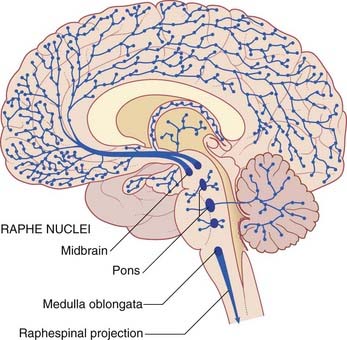
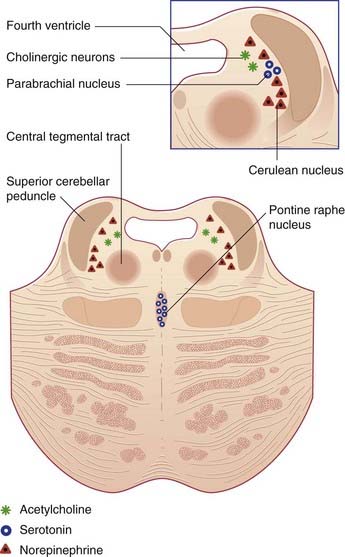
The ground plan is shown in Figure 24.1A. In the midline, the median reticular formation comprises a series of raphe nuclei (pron. ‘raffay’ and derived from the Greek word for seam). The raphe nuclei are the major source of serotonergic projections throughout the neuraxis (see next section).
Aminergic neurons of the brainstem
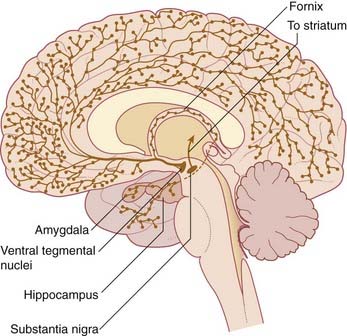
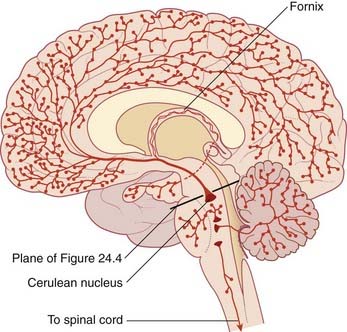
Embedded in the reticular formation are sets of aminergic neurons (Figure 24.1B). They include one set producing serotonin (5-hydroxytryptamine) and three sets producing catecholamines, as listed in Table 24.1.
| Transmitter | Location |
|---|---|
| Serotonin | Raphe nuclei of midbrain, pons, medulla |
| Dopamine | Tegmentum of midbrain |
| Norepinephrine | Midbrain, pons, medulla |
| Epinephrine | Medulla |
Functional Anatomy
The range of functions served by different parts of the reticular formation is indicated in Table 24.2.
Table 24.2 Elements of the reticular formation and their perceived functions
| Element | Function |
|---|---|
| Premotor cranial nerve nuclei | Patterned cranial nerve activities |
| Pontine locomotor center | Pattern generation |
| Magnocellular nuclei | Posture, locomotion |
| Salivatory nuclei | Salivary secretion, lacrimation |
| Pontine micturition center | Bladder control |
| Medial parabrachial nucleus | Respiratory rhythm |
| Central reticular nucleus of medulla oblongata | Vital centers (circulation, respiration) |
| Lateral medullary nucleus | Conveys somatic and visceral information to the cerebellum |
| Ascending reticular activating system (ARAS) | Arousal |
| Aminergic neurons | Sleeping and waking, attention and mood, sensory modulation, blood pressure control |
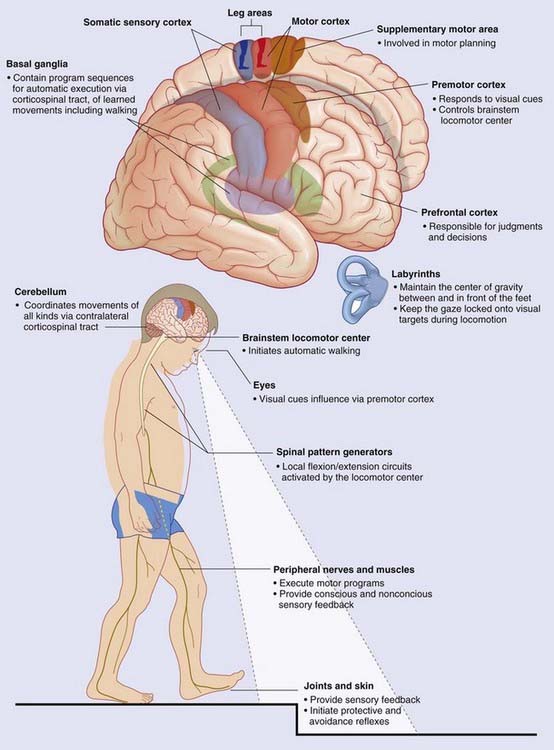
Pattern generators
Patterned activities involving cranial nerves include:
Locomotor pattern generators are described in Box 24.1. An overview of gait controls is provided in Box 24.2. Higher-level bladder controls are described in Box 24.3.
Box 24.1 Locomotor pattern generators
The MLR contains the pedunculopontine nucleus, close to the superior cerebellar peduncle where this passes along the upper corner of the fourth ventricle to enter the midbrain (Figure 17.16). These nuclei send fibers down the central tegmental tract to the oral and caudal pontine nuclei serving extensor motor neurons and to medullary magnocellular neurons serving flexor motor neurons.
Blessing WW. Lower brainstem regulation of visceral, cardiovascular and respiratory function. In: Paxinos G, Mai JK, editors. The human nervous system. ed 2. Amsterdam: Elsevier; 2004:465-479.
Fouad K, Pearson K. Restoring walking after spinal cord injury. Progr Neurobiol. 2004;73:107-126.
Grasso R, Ivanenko YP, Zago M, et al. Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain. 2004;127:1029-1034.
Jahn K, Deutschlander A, Stephan T, et al. Supraspinal locomotor control in quadriceps and humans. Prog Brain Res. 2008;171:353-372.
McCrea DA, Ryback IA. Organization of mammalian locomotor system and pattern generation. Brain Res Rev. 2008;57:134-146.
Box 24.3
The assistance of Professor Mary Pat FitzGerald, Department of Urogynecology, Stritch School of Medicine, Loyola University, Chicago, is gratefully acknowledged.
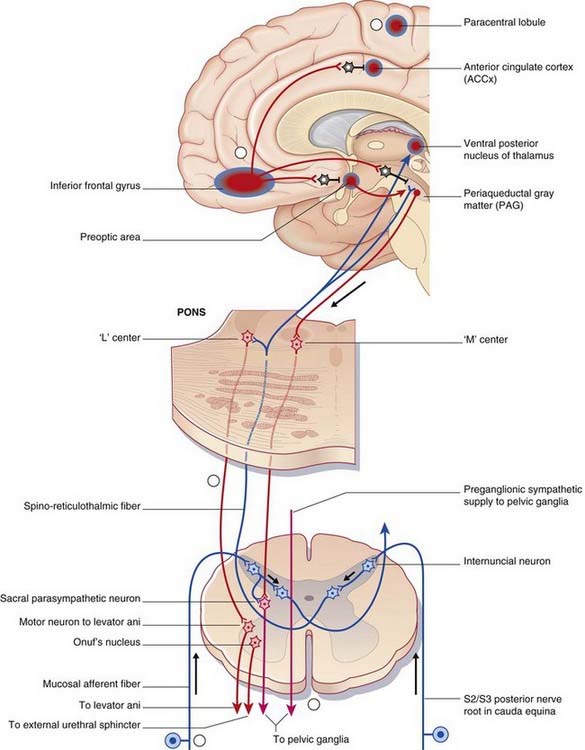
Higher-level bladder controls
The micturition control center is in the paramedian pontine reticular formation on each side, with interconnections across the midline. Magnocellular neurons project from here all the way to micturition-related parasympathetic neurons in segments S2–4 of the spinal cord (Figure Box 24.3.1).
Activation of the micturition control center produces not only a rise in intravesical pressure but also relaxation of the external urethral striated sphincter, brought about by simultaneous excitation of GABAergic internuncials synapsing in the nucleus of Onuf in sacral segments of the spinal cord (Ch. 13).
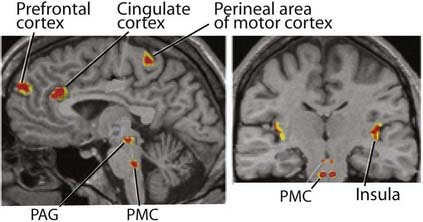
This right-sided bias is thought to be related to emotional aspects of micturition.
The micturition cycle
Role of monoamines
The motor and sensory spinal cord nuclei serving the bladder are abundantly supplied with serotonergic neurons descending from the magnus raphe nucleus (MRN) in the medulla oblongata. Animal experiments have shown that local applications of 5 HT are excitatory to pelvic sympathetic and sacral anterior horn neurons, inhibitory to pelvic parasympathetic and sacral posterior horn neurons. Distension of the bladder is known to stimulate the MRN (via spinoreticular activation of the periaqueductal gray matter). A quick review of the lower-level bladder controls (Box 13.3) suggests that the MRN sets the general tone in favor of bladder filling.
Respiratory control
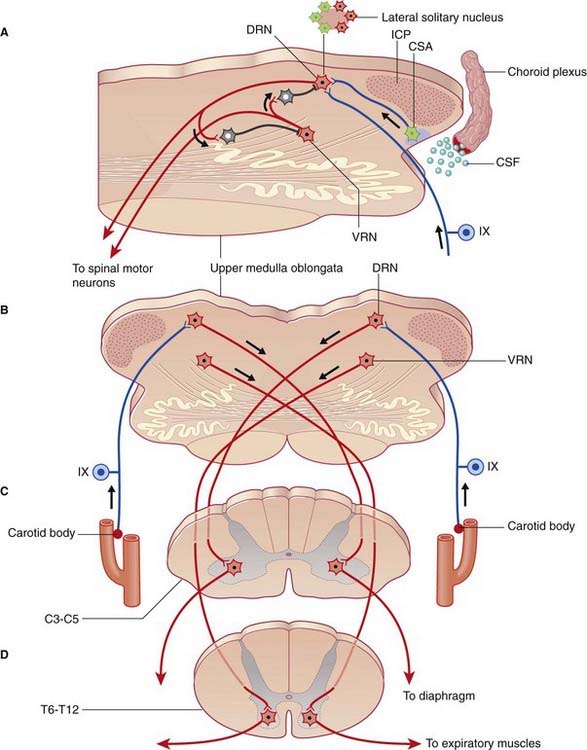
The respiratory cycle is largely regulated by dorsal and ventral respiratory nuclei located at the upper end of the medulla oblongata on each side. The dorsal respiratory nucleus occupies the midlateral part of the solitary nucleus. The ventral nucleus is dorsal to the nucleus ambiguus (hence the term retroambiguus nucleus in Figure 17.11). A third, medial parabrachial nucleus, adjacent to the cerulean nucleus, seems to have a pacemaker function governing respiratory rate (cycles per minute). As will be seen in Chapter 34, stimulation of this nucleus by the amygdala, in anxiety states, results in characteristic hyperventilation.
Medullary chemosensitive area
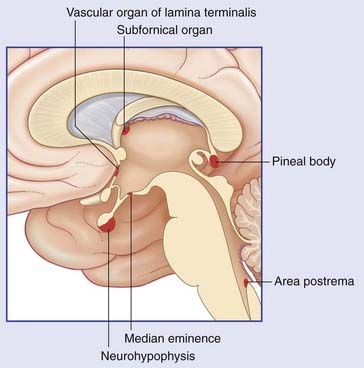
Close to the site of attachment of the glossopharyngeal nerve to the medulla oblongata, the choroid plexus of the fourth ventricle pouts through the lateral aperture of the fourth ventricle (Figure 24.6). At this location, cells of the lateral reticular formation at the medullary surface are exquisitely sensitive to the H+ ion concentration in the neighboring cerebrospinal fluid. In effect, this chemosensitive area samples the Pco2 level in the blood supplying the brain. Any increase in H+ ions stimulates the dorsal respiratory nucleus through a direct synaptic linkage. (Several other nuclei within the medulla are also chemosensitive.)
Carotid chemoreceptors
The pinhead carotid body, close to the stem of the internal carotid artery (Figure 24.6), receives from this artery a twig which ramifies within it. Blood flow through the carotid body is so intense that the arteriovenous Po2 changes by less than 1% during passage. The chemoreceptors are glomus cells to which branches of the sinus nerve (branch of IX) are applied. The carotid chemoreceptors respond to either a fall in Po2 or a rise in Pco2 and cause reflex adjustment of blood gas levels.
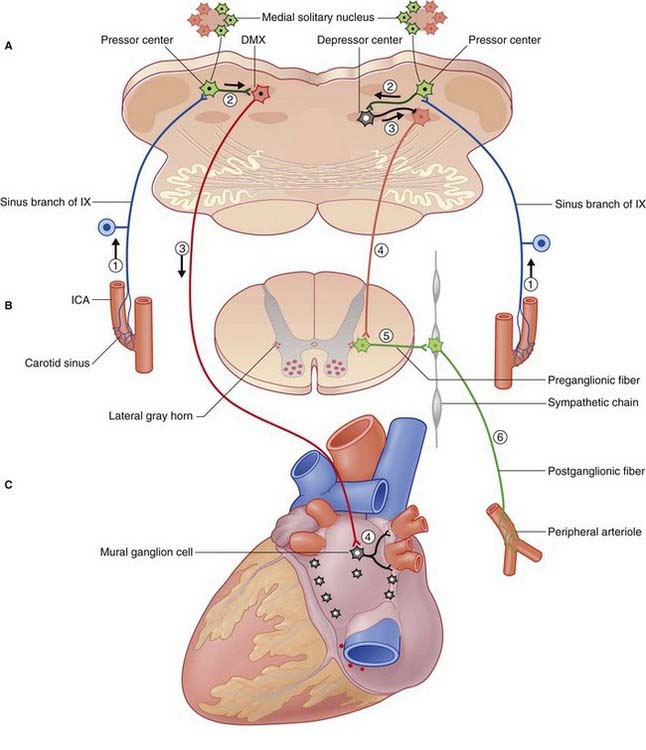
Cardiovascular control
Afferents signaling increased arterial pressure arise in stretch receptors (a multitude of free nerve endings) in the wall of the carotid sinus and aortic arch (Figure 24.7). Known as baroreceptors, these afferents project to medially placed cells of the solitary nucleus constituting the baroreceptor center. Afferents from the carotid sinus travel in the glossopharyngeal nerve; those from the aortic arch travel in the vagus nerve. The baroreceptor nerves are known as ‘buffer nerves’ because they act to correct any deviation of the arterial blood pressure from the norm.
Cardiac output and peripheral arterial resistance depend on a balance in the activity of sympathetic and parasympathetic efferents. Two major reflexes, barovagal and barosympathetic, help to lower a raised blood pressure as detailed in the caption to Figure 24.7.
Sleeping and wakefulness
Electroencephalography (EEG) reveals characteristic patterns in the electrical activity of cerebral cortical neurons that accompany various states of consciousness. The normal waking state is characterized by rapid, low-amplitude waves. The onset of sleep is accompanied by slow, high-amplitude waves, the higher amplitude being due to the synchronized activity of larger numbers of neurons. This type of sleep is called S (synchronized) sleep. It lasts for about 90 minutes before being replaced by D (desynchronized) sleep in which the EEG pattern resembles the waking state. Dreams occur during D sleep and there are rapid eye movements (hence the more usual term, REM sleep). Several S and D phases occur during a normal night’s sleep, as described in Chapter 30.
Ascending reticular activating system
This term refers to the participation of reticular formation neurons in activation of the cerebral cortex, as shown by a change in EEG records from high-amplitude, slow waves to low-amplitude, fast waves during spontaneous arousal from sleep. The strongest candidates for such a role are sets of cholinergic neurons close to the cerulean nucleus (Figure 24.4). In addition to supplying the above-mentioned fibers to the ocular motor nuclei, these neurons project to almost all thalamic nuclei, and they have an excitatory effect upon thalamic neurons projecting to the cerebral cortex.
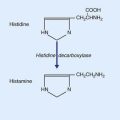
The hypothalamus is an important control center for various body rhythms, including sleep and wakefulness. A second candidate for cortical activation has been found in the hypothalamus, namely the tuberomammillary nucleus. This nucleus contains histaminergic neurons having widespread projections to the cerebral cortex (Ch. 26).
Following arousal, the waking-state EEG pattern seems to be sustained by the continuing discharge of the brainstem and hypothalamic neurons mentioned; also by a third set of neurons, embedded in the basal forebrain immediately above the optic chiasm. The third set occupies the basal nucleus of Meynert (Ch. 34) projecting cholinergic axons to most parts of the cerebral cortex.
Sensory modulation: gate control
Tactile sensory transmission is gated at the level of the posterior column nuclei. Corticospinal neurons projecting from the postcentral gyrus may facilitate or inhibit sensory transmission at this level, as mentioned in Chapter 16.
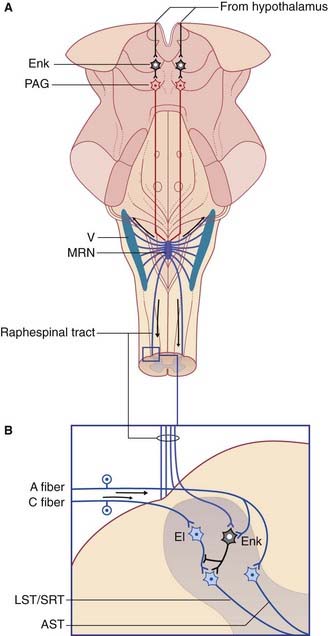
Segmental antinociception
Large (A category) mechanoreceptive afferents from hair follicles synapse upon anterior spinothalamic relay cells (and their trigeminal equivalents). They also give off collaterals to inhibitory (mainly GABA) gelatinosa cells which synapse in turn upon lateral spinothalamic relay cells (Figure 24.8). Some of the internuncials also exert presynaptic inhibition upon C-fiber terminals, either by axo-axonic contacts (which are very difficult to find in experimental material), or by dendro-axonic contacts. Gating of the spinothalamic response to C-fiber activity can be induced by stimulating the mechanoreceptive afferents, thereby recruiting inhibitory gelatinosa cells. This simple circuit accounts for the relief afforded by ‘rubbing the sore spot’. It also provides a rationale for the use of transcutaneous electrical nerve stimulation (TENS) by physical therapists for pain relief in arthritis and other chronically painful conditions. The standard procedure in TENS is to apply a stimulating electrode to the skin at the same segmental level as the source of noxious C-fiber activity, and to deliver a current sufficient to produce a pronounced buzzing sensation.
Supraspinal antinociception
Magnus raphe nucleus (Figure 24.8)
In addition to the segmental and supraspinal controls of nociceptive transmission from primary to secondary afferents, gating occurs within the thalamus (see Ch. 27).
Furthermore, perception of the aversive (unpleasant) quality of pain seems to require participation of the anterior cingulate cortex (Ch. 34), which is rich in opiate receptors.
Clinical Panel 24.1 Urge incontinence
As mentioned in Chapter 8, G-protein-gated muscarinic receptors, activated by postganglionic fibers from pelvic ganglia, are abundant on the detrusor muscle of the bladder. Accordingly, the drugs of choice are muscarinic receptor antagonists. However, antimuscarinic side-effects such as dry mouth and constipation may require this therapy to be withdrawn.
FitzGerald MP, Thom DH, Wassel-Fyr C, et al. Childhood urinary symptoms predict adult overactive bladder symptoms. J Urol. 2006;175:989-993.
Griffiths D, Tadic SD. Bladder control, urgency and urge incontinence: evidence from functional brain imaging. Neurourol Urodynam. 2008;27:466-474.
Nitti VW. Botulinum toxin for the treatment of idiopathic and neurogenic overactive bladder: state of the art. Rev Urol. 2006;8:198-208.
Benarroch E. Descending monoaminergic pain modulation. Neurology. 2008;71:218-221.
Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology. 2008;71:1733-1738.
Bianchi AL, Denavit-Saube M, Champagnet J. Central control of breathing. Physiol Rev. 1995;75:1-45.
Edgerton VR, Roy RR. Paralysis recovery in humans and model systems. Curr Opin Neurobiol. 2002;12:658-667.
Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829-898.
Rosenfeld JP. Interacting brain components of opiate-activated, descending, pain-inhibitory systems. Neurosci Behav Rev. 1994;18:403-409.
Siddall PJ. Pain mechanisms and management. Clin Exp Pharm Physiol. 1995;22:679-688.