Resuscitative Thoracotomy
In the United States, trauma is the leading cause of death in people aged 1 through 44.1 Blunt trauma accounts for the majority of trauma mortality overall, but in urban settings, penetrating trauma, including firearm-related injuries, accounts for an increased proportion of trauma deaths. In 2007, more than 31,000 firearm-related deaths occurred in the United States,2 with many victims arriving at the emergency department (ED) in extremis.
Penetrating cardiac injuries are associated with a very high mortality rate. On rare occasions, however, an aggressive approach involving the use of emergency department thoracotomy (EDT) leads to survival in patients with impending or recent traumatic arrest. EDT is a dramatic, heroic intervention performed outside the operating room and often in the absence of trained cardiothoracic or trauma surgeons. Though supported as a potential lifesaving procedure, EDT is not a mandated standard of care nor a procedure that is expected to be performed in most EDs. The first successful thoracotomy was reported more than 100 years ago, and the first EDT was reported in 1966.3 Since then, multiple studies have reported outcomes, indications, techniques, and risks associated with the procedure. In 2003, the National Association of EMS Physicians Standards and Clinical Practice Committee and the American College of Surgeons Committee on Trauma (ACSCOT) proposed specific guidelines for EDT.4 However, despite the guidelines, EDT remains a procedure done on a case-by-case basis with controversial evidence regarding the ideal indications.
Indications and Contraindications
In the ED, the vast majority of thoracotomies are performed on penetrating trauma patients in cardiac arrest. Beall and coworkers initially proposed EDT for the treatment of penetrating cardiac injuries in 1966.3 Since then, it has been expanded to include extrathoracic injuries, blunt trauma, and nontraumatic pathology. Studies show wide variation in survival rates and outcomes. Taking 40 years of collective EDT data into account, the survival rate of patients undergoing EDT for blunt trauma is nearly 2%, whereas that for penetrating trauma is nearly 16%,4 but these survival rates depend on many variables and are not applicable to every situation. There are a paucity of data concerning survival rates in patients with EDT performed for nontraumatic causes, and it is not recommended that this procedure be regularly used in these settings.
Make the decision to perform EDT quickly based on whether the patient is likely to benefit from the procedure, has a reasonable chance of survival, and cannot tolerate a delay in operative intervention. Also consider the risks associated with performing the procedure. Trauma researchers have identified several factors that are considered crucial when determining who will benefit from EDT (Box 18-1).
The first assessment is made in the prehospital setting, where determination of the mechanism of injury and the presence or absence of a pulse is critical. Recommendations from the ACSCOT guidelines state that EDT has no role in blunt trauma victims who are apneic and pulseless and lack an organized rhythm.4 Such patients do not survive, regardless of the intervention. In one of the largest EDT series to date, Branney and coworkers5 reviewed 868 consecutive patients over a 23-year period. They found that no blunt trauma patients survived EDT when they had no vital signs in the field but that 2.5% of blunt trauma patients survived EDT when vital signs were present in the field. Rhee and colleagues6 examined 4620 cases of EDT from 24 studies over a 25-year period. The overall survival rate after blunt trauma was just 1.4%, which led to EDT falling out of favor for this indication. Recent articles, however, have challenged the idea of limiting EDT to those in cardiac arrest from penetrating injury only.7–9 Moore and associates recommended considering EDT in blunt trauma victims who have received less than 5 minutes of cardiopulmonary resuscitation (CPR) and possess signs of life.7 The survival rate of pulseless trauma patients sustaining penetrating injury is significantly higher than that of blunt trauma patients. EDT should only rarely be used in patients with blunt trauma mechanisms. Several penetrating injury subtypes have been studied: firearm injuries, stab wounds, and penetrating explosive injuries. Thoracic stab wounds consistently show the highest rates of survival after EDT.5,10–12 This is theoretically due to the decreased amount of tissue damage related to the weapon and the ability to quickly identify anatomic structures and injuries. Penetrating firearm injuries are more likely to result in death because of increased tissue damage from the missile and concussive surrounding forces. Patients with firearm injuries are more likely to have multiple wounds, and the depth of penetration is increased in comparison to stab wounds. One published cohort of combat casualties from explosive penetrating injuries reported similar survival rates as those after firearm-related penetrating injuries.12
The location of the penetrating injury helps determine the futility of EDT. A trend toward increased survival rates in patients with thoracic injuries was found in historical data.4,12–21 Isolated cardiac wounds have the highest survival rate after EDT, with approximately 19% of patients surviving the procedure.8 Penetrating abdominal injuries have beneficial outcomes when EDT is performed to cross-clamp the aorta, with survival rates in the mid-teens.5,12,22,23 Extremity injuries rarely require EDT because the use of a tourniquet can control the hemorrhaging until the patient can be transported to the operating room. When EDT is used for traumatic extremity exsanguination, though, survival rates range from 10% to 25%.12 Patients in cardiac arrest associated with head injuries, especially those with open cranial wounds, have dismal survival rates and are considered poor candidates for further resuscitative efforts, including EDT.7
The type of cardiac electrical activity is helpful in determining who may benefit from EDT. Battistella and colleagues24 reviewed 604 patients undergoing CPR for traumatic cardiopulmonary arrest and found that of the 212 patients who were in asystole, none survived. Fulton and associates25 found that of patients in traumatic arrest, survival was improved when the patients exhibited ventricular fibrillation, ventricular tachycardia, or pulseless electrical activity rather than asystole or an idioventricular rhythm. In another study of EDT for traumatic arrest, asystole, idioventricular rhythm, or severe bradycardia was indicative of poor outcomes or an unsalvageable patient.4 In fact, most emergency medical service providers will not transport trauma patients who are in asystole regardless of the mechanism.26
The duration of resuscitation before EDT can also be used as a decision point. With traumatic injury, survival rates diminish as the duration of CPR increases. The consensus recommendation based on multiple studies is that any trauma patient who has undergone CPR for longer than 15 minutes has an exceedingly dismal survival rate and further resuscitation should be considered futile.4,10,11,25–28 Penetrating trauma patients with signs of life and CPR times of less than 15 minutes are candidates for EDT. In fact, penetrating trauma patients who suffer arrest in the ED have acceptable rates of survival and good neurologic outcomes if EDT is performed promptly.14–17,20,27,29 EDT can be considered in victims of blunt trauma cardiac arrest if CPR has been ongoing for less than 5 minutes.7 This practice is controversial, however, and each institution should address this situation both in their trauma protocol and on an individual basis before arrival of the patient in the ED.
Perhaps the most critical determinant of the appropriateness of EDT is whether the patient demonstrates “signs of life.” Signs of life are objective physiologic parameters that are present in patients who survive EDT. They include pupillary response, extremity movement, cardiac electrical activity, measurable or palpable blood pressure, spontaneous ventilation, or the presence of a carotid pulse. The presence of one or more of these indicators has been associated with good neurologic outcomes and increased rates of survival.3–9,30
Although survival remains the ultimate gauge of the effectiveness of EDT, it is essential to consider quality of life also, especially neurologic function of the patient. It is somewhat surprising that survivors of EDT generally have good neurologic outcomes. Rhee and colleagues6 reported that 280 of 303 (92.4%) patients discharged after EDT were neurologically intact. It is not possible to accurately predict which patients are likely to survive intact, but the study by Branney and coworkers5 demonstrated that all survivors with full neurologic recovery had respiratory effort at the scene and 75% still had respiratory effort on arrival at the ED. The presence or absence of a palpable pulse was not an absolute prognostic indicator in this study. Sixty-six percent of long-term survivors (11 patients with penetrating trauma and 1 with blunt trauma) had no detectable pulse on arrival at the ED.9 The first 24 hours after EDT rapidly demonstrates which patients are likely to become long-term survivors. Baker and associates14 showed that with 168 emergency thoracotomies for mixed trauma, most patients with fatal injuries died within 24 hours. Of patients surviving the first 24 hours, 80% (33 of 41) lived and were discharged from the hospital. Full neurologic recovery occurred in 90% of these survivors. Overall, only 2.4% (4 of 168) remained severely disabled or in a persistent vegetative state. Of these 4 patients, only 1 (0.6%) lived beyond 2 months.
Cardiac Injuries—Penetrating
Sixty percent to 80% of cardiac stab wounds result in pericardial effusion regardless of the presence of shock.31 Depending on which chamber is involved, tamponade can occur if the wound is smaller than 1 cm in size. Wounds larger than 1 cm usually continue to bleed regardless of which chamber is involved. Low-pressure atrial wounds generally form a thrombus before tamponade develops. The thicker-walled left ventricle may spontaneously seal stab wounds up to 1 cm in length. As little as 60 to 100 mL of blood acutely filling the pericardium will impede diastolic filling, reduce stroke volume, decrease cardiac output, and increase release of catecholamine. Catecholamine release may mask the severity of illness because it maintains blood pressure through an increase in peripheral vascular resistance. In penetrating cardiac injury, the right ventricle is the chamber most likely to be involved because of its anterior location, followed by the left ventricle and the atria.32,33
The progression from compensated cardiac function to uncompensated tamponade can be sudden and profound. Although one may suspect tamponade based on well-described signs, clinical diagnosis of pericardial tamponade in an unstable trauma patient is difficult because of the combined effect of hemorrhagic and cardiogenic shock. The classic signs of Beck’s triad (distended neck veins, hypotension, and muffled heart sounds) described in 192634 have limited diagnostic value for acute penetrating cardiac trauma.35 The most reliable signs of tamponade are elevated central venous pressure, hypotension, and tachycardia.
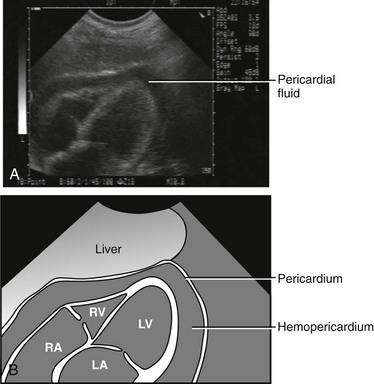
The advent of ultrasound and the focused assessment with sonography in trauma (FAST) examination has improved the diagnosis of pericardial effusion and tamponade. Findings indicative of tamponade include the presence of pericardial fluid with right atrial or ventricular collapse during diastole (Fig. 18-1). FAST is a rapid bedside screening examination used to detect hemopericardium and hemoperitoneum and is now important in the evaluation of unstable trauma patients.36,37 From data collected in 1540 patients, Rozycki and associates38 reported 100% sensitivity and specificity in detecting pericardial and peritoneal fluid in a hypotensive, unstable trauma patient. Ultrasound can have rare false-negative results when pericardial fluid from a cardiac injury decompresses into the thoracic cavity through a wound in the pericardium.39
After EDT for penetrating cardiac wounds, survival is also related to the mechanism of injury. Patients with stab wounds fare better than do patients with gunshot wounds. Rhee and colleagues6 noted that 16.8% of patients with stab wounds survived to hospital discharge after EDT. Branney and coworkers5 reported a 29% survival rate in stab wound patients with tamponade and a 15% survival rate in those without tamponade.
In contrast, gunshot wounds are often large injuries unable to seal themselves; tamponade occurs in only 20%. Patients with penetrating cardiac injuries from gunshot wounds are more likely to initially be seen with profoundly compromised hemodynamics. In addition, the increasing popularity of larger-caliber weapons has made it more difficult to resuscitate patients with gunshot wounds to the chest. Of 112 patients with gunshot wounds to the heart,5 only 2% survived neurologically intact.
Cardiac Injuries—Blunt
Blunt trauma to the heart can range from minor contusion to cardiac rupture. The most common cause of death after nonpenetrating cardiac injuries is myocardial rupture, and in approximately 25% of such patients the ascending aorta is ruptured simultaneously.31 Branney and coworkers5 observed a 2% survival rate in blunt trauma patients resuscitated with EDT. Those who survived had vital signs present in the field. The poor outcomes associated with this type of injury are a result of the poor cardiac function caused by myocardial contusion, even if the hemorrhage has been treated.
Pulmonary Injuries
Pulmonary injuries can be divided into three types: parenchymal, tracheobronchial, and large vessel. Parenchymal and tracheobronchial injuries rarely require EDT because they are either rapidly fatal or treated initially by tube thoracostomy. Tracheobronchial injury is more common in blunt than in penetrating trauma. Bertelson and Howitz40 reviewed 1128 patients at autopsy and found only 3 to have this injury. The airway is usually maintained, even with complete transection. The stiff tracheobronchial cartilage tends to hold the lumen open, and the paratracheal and parabronchial fasciae preserve the relationship of the proximal to distal bronchi. Ninety percent of tracheobronchial tears occur within 2.5 cm of the carina, and most commonly involve the main stem bronchi. Complete division of the trachea is extremely rare. Depending on the size and location of the injury, patients may have massive hemoptysis, airway obstruction, pneumomediastinum, pneumothorax, or tension pneumothorax. Massive subcutaneous emphysema and pneumomediastinum are usually seen, although up to 10% of patients with this injury have no abnormal findings on the initial radiograph.41 If hemorrhage is profuse or if the site of the injury can be determined, use of a bifid endotracheal tube or unilateral intubation of a main stem bronchus will help secure the airway.
Lacerations of the lung parenchyma that are not accompanied by injury to major vessels generally respond to tube thoracostomy. If the initial chest tube drainage is more than 1500 mL or if there is persistent hypotension or cardiac arrest, consider immediate thoracotomy. For pulmonary injuries, survival after EDT is also related to the mechanism of injury. Branney and coworkers5 reported a 17% survival rate after pulmonary stab wounds, 3% after gunshot wounds, and 5% after blunt trauma.
Air Embolism
Air embolism is a complication of pulmonary parenchymal injuries that may require immediate thoracotomy if the patient is hemodynamically unstable. The development of air embolism after penetrating injuries of the lung is often insidious, and the diagnosis is usually made at the time of thoracotomy.42 Preoperative and postmortem diagnosis of air embolism is difficult, and it is likely that most air emboli are not detected. Air embolism is confirmed at thoracotomy by needle aspiration of a foamy air-blood mixture from the left or right ventricle or by visualization of air within the coronary arteries.
Air embolism involving the left side of the circulatory system is referred to as arterial or systemic air embolism. The lethal volume depends on the organs to which it is distributed. As little as 0.5 mL of air in the left anterior descending coronary artery can lead to ventricular fibrillation. Two milliliters of air injected into the cerebral circulation can be fatal. The formation of traumatic bronchovenous fistulas creates potential entry points for air to move into the left side of the circulatory system. The only requirement is the formation of an air-blood gradient conducive to the inward movement of air. Although lowered intravascular pressure from hemorrhage is a risk factor, the most important element in all reports of air embolism has been the use of positive pressure ventilation.43
In a review of 447 cases of major thoracic trauma, Yee and coworkers44 found adequate chart data to suggest the diagnosis of air embolism in 61 patients. About 25% of patients with air embolism have blunt trauma with associated lung injury secondary to multiple rib fractures or hilar disruption. The overall mortality is higher than 50%.
The diagnosis of air embolism is easily overlooked because the signs and symptoms are similar to those of hypovolemic shock. Two valuable signs that are present in 36% of patients are hemoptysis and the occurrence of cardiac arrest after intubation and ventilation. The development of focal neurologic changes, seizures, or central nervous system dysfunction in the absence of head injury is also suggestive of the diagnosis.45 Overall, the diagnosis is subtle and must be considered when there is no evidence of the more common causes of extremis in a trauma patient.
Blunt and Penetrating Abdominal Injury
In the setting of penetrating abdominal injury, thoracotomy with cross-clamping of the thoracic aorta has been advocated as a means of controlling hemorrhage, redistributing blood flow to the brain and heart, and reducing blood loss below the diaphragm. Unfortunately, aortic cross-clamping can also have detrimental effects. Kralovich and colleagues46 studied the hemodynamic consequences of aortic occlusion in a swine model of hemorrhagic arrest. There was no difference between groups in return of spontaneous circulation; however, the occluded aorta group experienced statistically greater impairments in left ventricular function and systemic oxygen utilization in the postresuscitation period. Branney and coworkers5 found that 8 of 76 patients undergoing EDT for penetrating abdominal injury survived neurologically intact. More recently, Seamon and colleagues23 achieved a 16% survival rate with good neurologic outcomes (8 of 50 patients) when EDT was used before laparotomy for abdominal exsanguination from trauma. Of note, none of the survivors in this study were in cardiac arrest at the time of EDT, but they did have severe hemorrhagic shock, and six of the eight had unmeasurable blood pressure. Current recommendations suggest that EDT be performed judiciously in patients with abdominal trauma as an adjunct to definitive repair of the abdominal injury.
Open-Chest Resuscitation for Nontraumatic Arrest
At present, less than 6% of CPR attempts conducted outside hospital special care units result in survival.47 The first case of a human survivor of open-chest cardiac massage (OCCM) was reported in 1901. In 1960, Kouwenhoven and associates48 published favorable survival rates with closed-chest CPR as opposed to OCCM. After further refinement by Pearson and Redding, closed-chest CPR gradually became the preferred method of cardiac compression.49
The goal of CPR is to restore coronary perfusion pressure (CPP), which is the prime determinant for return of spontaneous circulation as established in animal models. Paradis and associates50 found that humans need a minimal CPP of 15 mm Hg to achieve return of spontaneous circulation. Although a CPP of 15 mm Hg does not guarantee return of spontaneous circulation, there is 100% failure of resuscitation if CPP is below this level. Despite the limited number of human studies on OCCM, its hemodynamic superiority over closed-chest CPR is compelling. Del Guercio and coworkers51 measured cardiac output during both closed-chest CPR and OCCM in in-hospital cardiac arrest patients. OCCM produced a mean cardiac index of 1.31 L/min/m2 as opposed to 0.6 L/min/m2 during closed-chest CPR. Boczar and colleagues52 further examined 10 patients who were unresponsive to closed-chest CPR and measured CPP during closed-chest CPR followed by OCCM. Mean CPP in the closed-chest group was 7.3 mm Hg versus 32.6 mm Hg in the open-chest group. All patients achieved a CPP of at least 20 mm Hg at some time during their OCCM phase. This easily surpassed the minimal CPP required for return of spontaneous circulation. Outcomes after OCCM have not been well established. Animal models suggest not only improved hemodynamic parameters but also a possible increase in 24-hour survival rates.53 Neurologic outcomes, however, are unknown, and the American Heart Association guidelines for CPR do not promote the regular use of OCCM in patients with out-of-hospital cardiac arrest.47
Nontraumatic Hypothermic Cardiac Arrest
In the setting of cardiac arrest from hypothermia, consider the use of EDT and OCCM. Cardiopulmonary or venovenous bypass is the most rapid method of core rewarming, but it is rarely available immediately. Open thoracotomy with mediastinal irrigation has been used successfully in cases of severe hypothermia with cardiac arrest. Brunette and McVaney54 reported 11 patients with hypothermic cardiac arrest, 7 of whom underwent EDT with OCCM and mediastinal rewarming. Five patients survived, and all had positive neurologic outcomes despite cardiac arrest times of between 10 and 90 minutes (although one patient died of gastrointestinal hemorrhage and sepsis following resuscitation, the other four patients survived with full neurologic recovery). The other four patients who did not undergo EDT did not survive despite being taken promptly to the operating room for cardiopulmonary bypass rewarming. Although the number of cases is limited, this study is evidence that OCCM can provide prolonged hemodynamic support and good neurologic outcomes. It should be noted that similar case reports also exist in which closed-chest CPR was maintained for prolonged periods and resulted in successful hypothermic resuscitation.55 EDT with mediastinal irrigation can produce core rewarming rates as fast as 8°C/hr, with the heart and lungs preferentially being rewarmed first.54 Mediastinal irrigation involves heating sterile saline in a microwave oven to 40°C and then pouring it slowly over the heart and into the thorax. Performing a thoracotomy for hypothermic arrest does not preclude the use of cardiac bypass inasmuch as it was subsequently used after EDT in three of the survivors from the Brunette and McVaney study.54
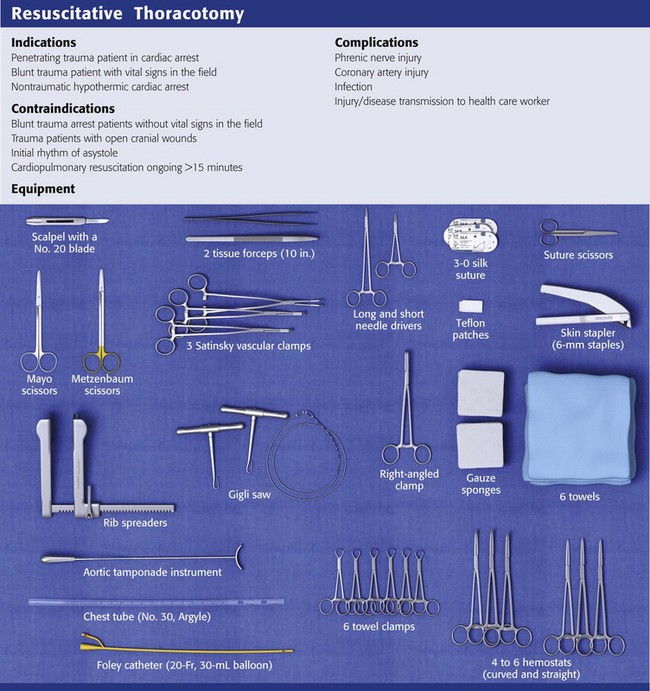
Equipment
Carefully select the instruments to be included in the EDT equipment tray. See Review Box 18-1 for a complete list.
Procedure
All trauma patients arriving at the ED with hypotension must be assumed to be hypovolemic and be treated accordingly. Rapidly exclude other causes as well, including tension pneumothorax, cardiac tamponade, air embolism, and neurogenic or cardiogenic shock. A useful algorithmic overview of the approach to chest trauma is presented in Figure 18-2. The use of autotransfusion, if available, has several benefits, but its use is not widespread or considered standard.56
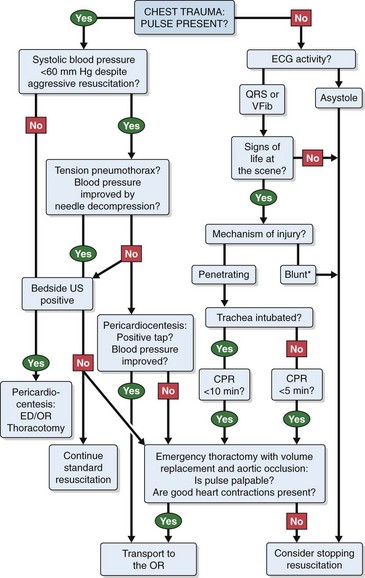
Figure 18-2 An algorithmic approach to chest trauma. CPR, cardiopulmonary resuscitation; ECG, electrocardiographic; ED, emergency department; OR, operating room; QRS, organized electrical activity; tap, pericardial tap yielding blood; US, ultrasonography; VFib, ventricular fibrillation. *Pulseless blunt trauma management is controversial, with some data supporting stopping resuscitation immediately4 and recent data recommending emergency department thoracotomy if CPR has been ongoing for less than 5 minutes.7
Airway Control
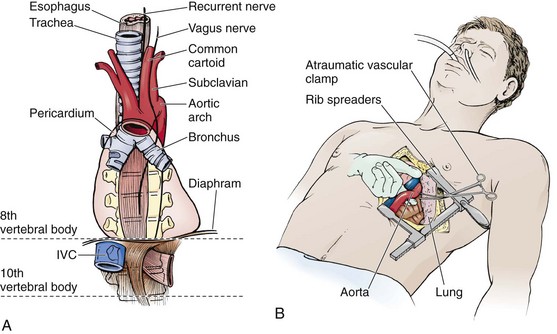
Intubate the patient orotracheally, if possible, but be aware that access to the thoracic organs, surgical repairs, or surgical procedures may be hampered by frequent inflations of the left lung. If necessary, selectively intubate the right lung by blindly advancing a standard single-lumen endotracheal tube to a depth of 30 cm (measured from the corner of the mouth) in adult patients.57 Although the left lung and the right upper lobe are not ventilated with the tracheal tube in this position, animal studies and data from humans suggest that selective right lung ventilation provides adequate oxygenation and ventilation for at least 60 minutes.57 With the left lung deflated one can expedite thoracotomy by maximizing space in the left thoracic cavity. Keep in mind that extending the thoracotomy into the right thoracic cavity may necessitate switching to bilateral lung ventilation or left lung ventilation to allow maximum right thoracic exposure.
Anterolateral Thoracotomy Incision
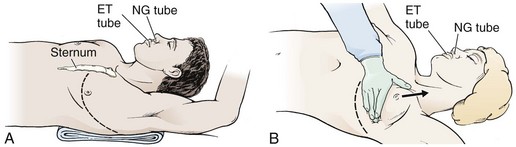
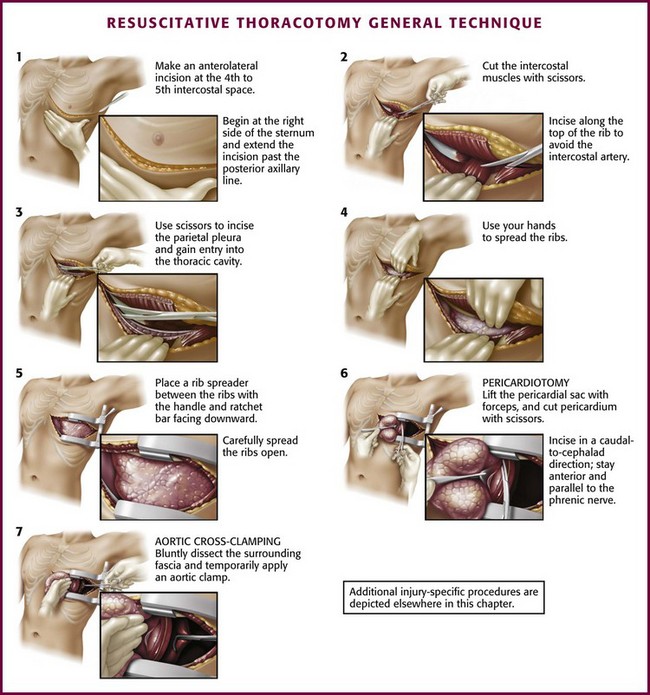
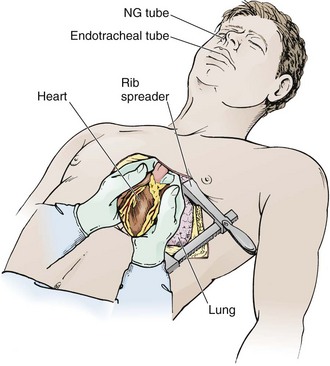
Prepare the skin of the left anterior aspect of the chest with antiseptic if readily available. Wedge towels or sheets under the left posterior part of the chest and place the patient’s left arm above the head (Fig. 18-3A). On the left side of the chest, make an anterolateral incision at the fourth to fifth intercostal space with a scalpel with a No. 20 blade (Fig. 18-4, step 1). Do not take the time to count ribs; simply estimate the location to be just beneath the nipple in males and at the inframammary fold in females (see Fig. 18-3). It is important to establish wide exposure by beginning the incision on the right side of the sternum and extending the skin incision past the posterior axillary line. Beginning here will save time if the right side of the chest needs to be opened as well. Inadequate exposure, rib fractures, and additional delays occur when the skin incision is too limited. With the first sweep of the scalpel, separate skin, subcutaneous fat, and the superficial portions of the pectoralis and serratus muscles.
Cut the intercostal muscles with scissors to expose the thoracic cavity (Fig. 18-4, step 2). Use scissors to divide the intercostal muscles because the risk for lung laceration is greater when a scalpel is used. Make the incision just over the top of the rib to avoid the intercostal neurovascular bundle. Just before opening the pleura, stop ventilations momentarily. This will allow the lung to collapse away from the chest wall. Should the internal mammary artery be transected during the procedure, hemorrhage is generally minimal until after perfusion is reestablished, at which time bleeding may be profuse. If actively bleeding, the internal mammary artery should be ligated or clamped. Do not forget to address the internal mammary artery if perfusion is reestablished because this can be a source of significant bleeding.30
After entering the pleural cavity, gain good exposure. Karmy-Jones and associates58 found that 20% of their patients undergoing EDT via the anterolateral approach needed to have the initial incision extended. To gain better exposure, first use your hands to open the chest cavity. Then place a Finochietto retractor (rib spreader) between the ribs with the handle and the ratchet bar directed downward toward the axilla (Fig. 18-4, step 5). If the retractor were to be placed with the handle up, the ratchet bar would prevent extension of the incision into the right side of the chest. Ribs may be broken during spreading, so be careful to not get cut on the sharp bone edges. If massive hemothorax is encountered, remove the clots manually, suction out the blood, and use towels to absorb any blood spilling from the chest. If the site of injury is to the right of the heart and cannot be reached, extend the incision into the right side of the chest with a Gigli saw, Liebsche knife, sternal osteotome, or standard trauma shears. Before performing EDT it would be prudent to familiarize oneself with the instruments included in the EDT tray, but do not take the time for this during an actual case.
Pericardiotomy
Perform pericardiotomy if tamponade is present or suspected. This procedure is performed anterior and parallel to the left phrenic nerve (Fig. 18-4, step 6). Begin the incision near the diaphragm to avoid injury to the coronary arteries. Lift the pericardial sac with toothed forceps, and use scissors to make a small hole. Extend the incision with scissors in a cephalad direction along the anterior aspect of the pericardium. Extend the incision so that it reaches from the apex of the heart to the root of the aorta. When the pericardium is under tension, it may be difficult to grasp the pericardium with forceps. In this case, use sharp, straight Mayo scissors to divide the pericardium by layers. If the heart is in arrest, speed is important, so use sharp scissors to “catch” the pericardium and start the pericardiotomy. To do this, hold the point of the scissors almost parallel to the surface of the heart and use enough pressure to create a wrinkle in the pericardium to puncture it as the scissors move forward. Apply moderate pressure to puncture the fibrous pericardium. Be cautious because if the point of the scissors is unnecessarily angled toward the heart, the sudden “give” that occurs when you open the pericardium may result in laceration of the myocardium. Remove clots of blood from the pericardial sac with a sweeping motion with your gloved hand, sterile lap sponges, or gauze pads. If cardiac repair or cardiac compressions are needed, deliver the heart from the pericardial sac. To do this, place your right hand through the pericardial incision and encircle the heart, pull it into the left side of the chest, and place the pericardial sac behind the heart.
Direct Cardiac Compressions
Three techniques for cardiac compression have been described: one-handed compression, one-handed sternal compression, and two-handed (bimanual) compression (Fig. 18-5). To perform the one-handed compression method, place your thumb over the left ventricle, the opposing fingers over the right ventricle, and your palm over the apex of the heart. To perform one-handed sternal compression, hold your fingers flat. Keep the fingers tightly together to form a flat surface over the left ventricle and compress the heart up against the sternum with your fingers. To perform two-handed compression, cup the left hand and place it over the right ventricle. Hold the fingers of the right hand tightly together to form a flat surface supporting the left ventricle. Push the flat surface of your right-hand fingers to compress the heart against the cupped surface of the left hand. The bimanual technique has been shown to be consistently superior.59
A difference of opinion exists regarding the optimal rate at which the heart should be compressed. Some recommend a rate of 50 to 60 compressions/min; however, no physiologic data support such a recommendation. Recent American Heart Association guidelines for closed-chest CPR recommend a rate of at least 100 compressions/min.47 It is conceivable that this would also apply to OCCM.
It is important to remember the following points while performing cardiac compression:
1. Use the entire palmar surface of the fingers. Avoid fingertip pressure.
2. For each method, adjust the force of compression so that it is perpendicular to the plane of the septum. The anterior descending coronary artery is located over the interventricular septum, so this is a helpful landmark to orient your hand properly.
3. Position your fingers so that the coronary arteries will not be occluded.
4. Venous filling of the heart is especially sensitive to changes in position. Maintain a relatively normal anatomic position of the heart to prevent kinking of the vena cava and pulmonary veins. Do not angle the heart more than 30 degrees into the left side of the chest.
5. It is essential to completely relax the heart between compressions to allow it to refill completely.
Control of Hemorrhagic Cardiac Wounds
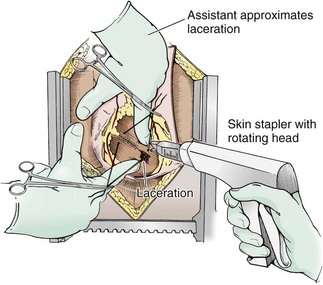
To partially control active bleeding from a ventricular wound, place one finger over the wound and use the other hand to stabilize the beating heart. This maneuver buys time while you begin to repair the injury and continue with resuscitation. If the heart is not beating, you may perform closure of the injury before resuscitation and defibrillation, although this is controversial. If you elect to repair the injury before attempting to restart the heart, perform intermittent cardiac massage. Surgical staples can be used to close a ventricular wound and are an extremely rapid method for controlling hemorrhage (Fig. 18-6).60 This technique is particularly useful with large or multiple lacerations. Another advantage is that stapling does not expose the operator to the risk associated with needlestick. Macho and coworkers61 reported a 93% success rate in temporarily controlling hemorrhage in 28 patients (33 lacerations) with penetrating injuries to both the atria and ventricles by using a standard skin stapler with wide (6-mm) staples placed at 5-mm intervals (Auto-Suture 35W, U.S. Surgical Corp., Norwalk, CT). The rotating long neck of the Ethicon Proximate Quantum Skin Stapler (Model PQW-35, Ethicon, Inc., Somerville, NJ) is helpful in obtaining proper orientation of the staples during placement. The staples can be left in place and reinforced or replaced on further exploration in the operating room.
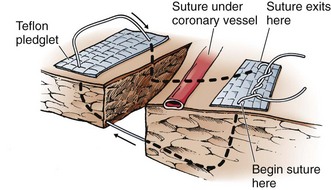
Alternatively, repair the wound by placing several horizontal mattress sutures under the tamponading finger (Fig. 18-7). Polypropylene 2-0 or 3-0 monofilament (Prolene) suture is recommended for cardiac repair, but nonabsorbable silk can also be used. Avoid smaller sutures and nylon sutures. If multiple sutures are needed, lay them all in place before they are tied. This allows you to attain equal distribution of wound tension, which prevents tearing of the myocardium.62 Alternatively, pass the suture underneath, over, and through Teflon pledgets to prevent the suture from cutting through the myocardium. Pledgets are especially important for reinforcement when the myocardium has been weakened by the blast effect of a bullet63 or when suturing the thinner-walled atria or right ventricle. An alternative to Teflon pledgets is to use small rectangles of pericardial tissue cut from the opened pericardium.
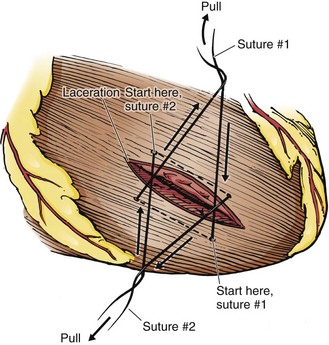
For large wounds that cannot be controlled with pressure, place an incomplete horizontal mattress suture on either side of the wound (Fig. 18-8). Cross the free ends to stop the bleeding. Then the actual reparative sutures can be placed accurately. It must be stressed that suturing the myocardium requires good technique. Excessive tension may tear the myocardium and aggravate the situation. Keys to success include using appropriately sized suture, obtaining a generous “bite” with the needle, and applying only enough tension to control the bleeding.
If exsanguinating hemorrhage is not controlled by the aforementioned methods, temporarily occlude inflow to the heart. Apply inflow occlusion intermittently for 60 to 90 seconds. During occlusion, the heart shrinks, hemorrhage is controlled, and you can place sutures in a decompressed injury. Two techniques that are useful are vascular clamping of the superior and inferior vena cava for partial inflow occlusion64 and the Sauerbruch grip (Fig. 18-9).65 The Sauerbruch grip can be performed quickly with the added advantage of cradling and stabilizing the heart while you repair the wounds over either the ventricle or the left atrium. It involves occlusion of the vena cava between the ring and middle fingers of the left hand to create partial occlusion of inflow. The Sauerbruch grip will interfere only with the repair of wounds involving the right atrium.
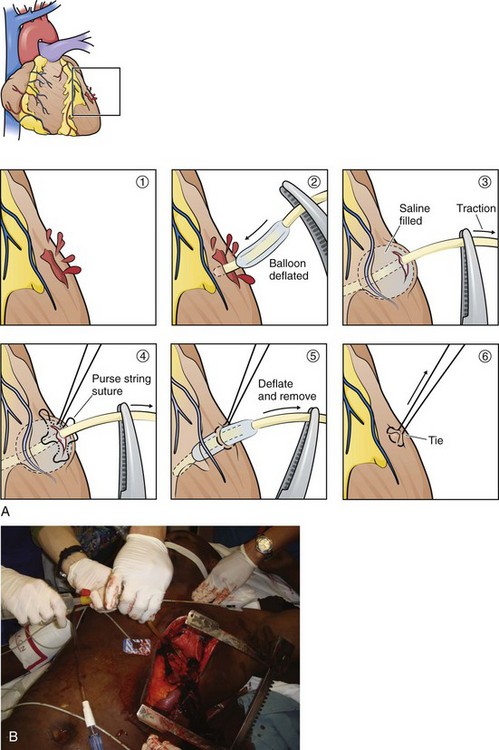
Another technique for temporarily controlling hemorrhage is to insert a Foley catheter (20 Fr with a 30-mL balloon) through a wound.66 After inserting the catheter, inflate the balloon, clamp the catheter to prevent air embolism, and apply gentle traction (Fig. 18-10). Apply enough traction to slow the bleeding and provide an acceptable level to visualize and repair the wound. Excessive traction can pull the catheter out and enlarge the wound. The balloon will effectively occlude the wound internally. A purse-string suture is then used to close the wound. When repairing the wound, be careful with the suture needle because it can easily rupture the balloon. Temporarily pushing the balloon into the ventricular lumen during passage of the needle can be done to avoid this complication. Use normal saline when inflating the balloon because using air could result in air embolism if the suture needle ruptures the balloon.
Foley catheters have several advantages over other methods for controlling cardiac wounds. With the digital method, your fingertip will often slip if there is a strong heartbeat, you cannot visualize the wound during repair, and digital pressure significantly interferes with cardiac massage. Intermittent total venous inflow occlusion is an effective method of controlling bleeding and decompressing the heart, but such control will be at the expense of poor cardiac output. Attempting to elevate the heart for control and repair of posterior cardiac wounds often results in cardiac arrest by reducing both venous and arterial flow. With posterior injuries, use of a Foley catheter does not require continued viewing after initial placement. If bleeding can be controlled, repairs in this location should await full-volume expansion or cardiopulmonary bypass.67 Regardless of location, the most valuable feature of the Foley catheter is that you can control hemorrhage without interfering with cardiac compression. Also, the catheter can be used for infusion of fluids (Fig. 18-10B).68
To initially manage wounds of the atria, use partial-occlusion clamps (Fig. 18-11). Because of the thin structure and instability of the atrial wall, you will not be able to effectively stop bleeding with digital pressure. Injuries near the caval-atrial junction are not amenable to clamping; in this location, use a Foley catheter to tamponade the wound.66 Be careful to not obstruct atrial filling with the inflated balloon. Skin staples may also be used for closure of atrial wounds.61
Control of Hemorrhagic Great-Vessel Wounds
Wounds of the great vessels can be controlled with digital pressure or partial-occlusion clamps. If desired, close small aortic wounds with 3-0 Prolene suture.69 To prevent exsanguinating hemorrhage from the left subclavian artery, try to cross-clamp the intrathoracic portion of the artery. Cross-clamping of the right subclavian artery is very difficult. For injuries to this vessel, use laparotomy pads for compression in the apex of the pleura from below and the supraclavicular fossa from above (Fig. 18-12) to prevent further bleeding as the patient is stabilized and moved to the operating suite.60
Aortic Cross-Clamping
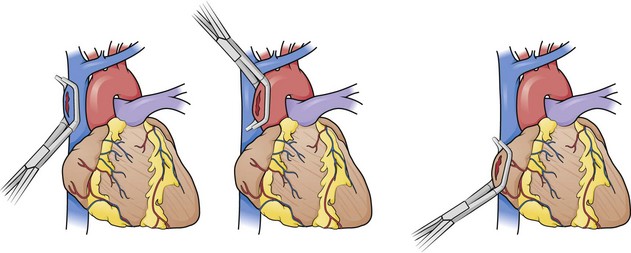
For persistent hypotension (systolic blood pressure <70 mm Hg) after thoracotomy and pericardiotomy, perform temporary occlusion of the descending thoracic aorta. This maneuver can maintain myocardial and cerebral perfusion (Fig. 18-13; also see Fig. 18-4, step 7), although Kravolich and colleagues46 suggested that the benefit of significant improvement in CPP may be overstated. When the aorta has been injured by blunt trauma, selective clamping is necessary (Fig. 18-14). Aortic occlusion has a limited role in controlling hemorrhage below the diaphragm. In patients with a tense abdomen and massive hemoperitoneum, aortic cross-clamping is beneficial when applied just before laparotomy.23
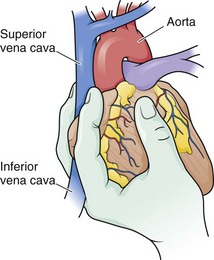
The aorta can be very difficult to identify in an ED, especially when collapsed as a result of exsanguination. It is situated immediately anterior to the vertebrae and actually lies on the vertebral bodies themselves. The esophagus lies anterior and slightly medial to the aorta. Passing a nasogastric tube from above may help in identifying the esophagus. To expose the descending aorta, ask an assistant to retract the left lung in a superomedial direction. To achieve adequate exposure it is sometimes necessary to divide the inferior pulmonary ligament (be careful to not injure the inferior pulmonary vein). Identify the aorta by advancing the fingers of the left hand along the thoracic cage toward the vertebral column. Open the mediastinal pleura and bluntly dissect the aorta away from the esophagus anteriorly and the prevertebral fascia posteriorly before clamping. To locate the aorta, use a DeBakey aortic clamp or a curved Kelly clamp for blunt dissection and spread the pleura open above and below the aorta (Fig. 18-15). Alternatively, if excessive hemorrhage limits direct visualization, bluntly dissect with the thumb and fingertips. Separate the aorta from the esophagus, which lies medially and slightly anteriorly. It may be difficult to separate the esophagus from the aorta by feel in a hypotensive or cardiac arrest situation. When the aorta is completely isolated, use the index finger of the left hand to flex around the vessel and apply a large Satinsky or DeBakey vascular clamp with the right hand. Check brachial blood pressure immediately after the occlusion. If systolic pressure is higher than 120 mm Hg, slowly release the clamp and adjust it to maintain a systolic pressure of less than 120 mm Hg.22
Given the need for timely intervention, the simplest and most desirable approach to aortic occlusion is to have an assistant digitally compress it or use the aortic tamponade instrument (Fig. 18-16). The aortic tamponade instrument, however, may be applied blindly to the vertebral column and permits safe, quick, and complete aortic occlusion.70 This technique may be most prudent when isolation of the aorta is difficult. The instrument’s unique shape allows it to remain in place and to provide atraumatic occlusion with little interference in the operative field when compared with digital compression. The degree of occlusion can be varied by the amount of pressure exerted by the operator.
Potential complications of aortic cross-clamping include ischemia of the spinal cord, liver, bowel, and kidneys. In addition, iatrogenic injury to the aorta and the esophagus may occur. Fortunately, these complications are infrequent. The metabolic penalty of aortic cross-clamping becomes exponential when occlusion time exceeds 30 minutes.71 Whenever possible, unclamp the aorta for 30 to 60 seconds every 10 minutes to increase distal perfusion. Perform final release of the aorta gradually.
Management of Air Embolism
Air emboli traverse capillary beds if the blood pressure is high enough. After controlling the bronchovenous fistula, produce a brief period of proximal aortic hypertension by cross-clamping the descending aorta. Maintain systemic arterial pressure with adequate fluid resuscitation. Vasopressors such as dopamine, epinephrine, or norepinephrine may be required to increase systemic pressure and facilitate the passage of air bubbles from left to right.72
Maintain left atrial pressure at a high level. Keep the ventilator inspiratory pressure as low as possible, and use 100% oxygen to facilitate diffusion of nitrogen from emboli. Consider high-frequency ventilation, which allows the use of small volumes and has been used successfully in individual patients. The most important adjunctive therapy is hyperbaric oxygen. Although it is best to begin treatment within 6 hours of the traumatic insult, there are cases of success and improvement when hyperbaric oxygen has been started even 36 hours after injury.73
Interpretation and Hemodynamic Monitoring
Use systolic blood pressure after the first 30 minutes of resuscitation as a decision point for further treatment. A report of EDT for blunt and penetrating trauma demonstrated a relationship between blood pressure at 30 minutes and the eventual outcome.74 Of the 146 cases reviewed, 45 patients (31%) were transferred to the operating room after initial resuscitation and aortic cross-clamping when necessary. For patients who survived with full neurologic recovery, the average systolic blood pressure after the first 30 minutes of resuscitation was 110 mm Hg. In those who were long-term survivors but had significant brain damage, the average systolic blood pressure was 85 mm Hg. No survivals were recorded when mean systolic blood pressure was lower than 70 mm Hg. Transfer of these patients to the operating room for definitive repair of these mortal wounds would be futile.
EDT in Children
Trauma is the leading cause of death and morbidity in children older than 1 year.1 Just as in adults, improved transportation of injured children to the hospital has resulted in the survival of more patients who would have been pronounced dead at the scene. Although the role of EDT has been reviewed extensively in the adult population, experience and data in the pediatric population are limited. Overall survival rates in children undergoing EDT for penetrating thoracic trauma are approximately 11% to 12% and, for blunt trauma, 1% to 2%.4 General consensus from the practice management guidelines for EDT is to use the same parameters as used for adults.4
Complications
A variety of significant complications occur in patients surviving EDT, but most of them are related to the primary injury rather than the thoracotomy. Techniques to avoid iatrogenic complications include noting the position of the left phrenic nerve and coronary arteries during EDT. Surprisingly, serious infections are uncommon. Patients receiving antibiotics during or immediately after the procedure have a low rate of infectious complications. Standard antibiotic regimens targeting skin flora are recommended but should not delay the procedure.75 As a general rule, excessive attention to antiseptic skin preparation should be avoided because it may delay performance of the procedure.76
Another potentially serious complication of EDT is injury or transmission of disease to health care workers. In an emotionally charged environment in which many clinicians are attempting to perform a lifesaving procedure under difficult conditions, it is easy for a needle, scalpel, or scissors to cause injury. Sharp ribs can cut clinicians quite easily (Fig. 18-17). The seroprevalence of human immunodeficiency virus (HIV) in U.S. EDs is estimated to range from 2% to 6% or 9% in urban areas.77 Tardiff and colleagues77 noted an HIV-positive rate of 7.2% in a study of trauma patients taken to their urban ED. Occupational exposure to both hepatitis B and hepatitis C virus is of concern to health care workers as well. Sloan and associates78 found a 3.1% incidence of hepatitis B in trauma patients brought to their inner-city ED. Hepatitis C is now the most common viral hepatitis seen in health care workers since advent of the hepatitis B vaccine. Approximately 2200 health care workers per year seroconvert after occupational exposure. The risk for seroconversion after occupational exposure to HIV is estimated to be 0.3% for needlestick and as high as 4.5% for deep injury.79 Hepatitis C seroconversion rates after occupational exposure are estimated to be between 1.8% and 10%.79 Keep the risk for exposure to staff in mind, and rigorously follow universal precautions. Although EDT must be performed rapidly to be of value to the patient, excessive haste is not warranted if it threatens the health of members of the medical team.
References
1. Web-based Injury Statistics Query and Reporting Systems (WISQARS). Leading Causes of Death Reports. www.cdc.gov, 2007. [Available at].
2. WISQARS 10 Leading Causes of Injury Deaths by Age Group Highlighting Violence-Related Injury Deaths. www.cdc.gov, 2007. [Available at].
3. Beall, AC, Diethrich, EB, Crawford, HW, et al. Surgical management of penetrating cardiac injuries. Am J Surg. 1966;112:686.
4. Hopson, LR, Hirsh, E, Delgado, J, et al. Guidelines for withholding or termination of resuscitation in prehospital traumatic cardiopulmonary arrest: Joint Position Statement of the National Association of EMS Physicians and the American College of Surgeons Committee on Trauma. J Am Coll Surg. 2003;196:106.
5. Branney, SW, Moore, EE, Feldhaus, KM, et al. Critical analysis of two decades of experience with postinjury emergency department thoracotomy in a regional trauma center. J Trauma. 1998;45:87.
6. Rhee, PM, Acosta, J, Bridgeman, A, et al. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg. 2000;190:288.
7. Moore, EE, Knudson, MM, Burley, CC, et al. Defining the limits of resuscitative emergency department thoracotomy: a contemporary Western Trauma Association perspective. J Trauma. 2011;70:334.
8. Cothren, CC, Moore, EE. Emergency department thoracotomy. In: Feliciano DV, Mattox KL, Moore EE, eds. Trauma. 6th ed. New York: McGraw-Hill; 2008:245–259.
9. Powell, DW, Moore, EE, Cothren, CC, et al. Is emergency department resuscitative thoracotomy futile care for the critically injured patient requiring prehospital cardiopulmonary resuscitation? J Am Coll Surg. 2004;2:211.
10. Durham, LA, Richardson, RJ, Wall, MJ, et al. Emergency center thoracotomy: impact of prehospital resuscitation. J Trauma. 1992;32:775.
11. Milham, FH, Grindlinger, GA. Survival determinants in patients undergoing emergency room thoracotomy for penetrating chest injury. J Trauma. 1993;34:332.
12. Edens, JW, Beekley, AC, Chung, KK, et al. Long-term outcomes after combat casualty emergency department thoracotomy. J Am Coll Surg. 2009;209:188.
13. Mattox, KL, Espada, R, Beall, AC, et al. Performing thoracotomy in the emergency center. JACEP. 1978;7:423.
14. Baker, CC, Thomas, AN, Trunkey, DD. The role of emergency room thoracotomy in trauma. J Trauma. 1980;20:848.
15. Harner, TJ, Oreskovich, MR, Copass, MK, et al. Role of emergency thoracotomy in the resuscitation of moribund trauma victims. Am J Surg. 1981;142:96.
16. Danne, PD, Finelli, F, Champion, HR. Emergency bay thoracotomy. J Trauma. 1984;24:796.
17. Hoyt, DB, Shackford, SR, Davis, JW. Thoracotomy during trauma resuscitations—an appraisal by board-certified general surgeons. J Trauma. 1989;29:1318.
18. DiGiacomo, JC, Odom, JW. Resuscitative thoracotomy and combat casualty care. Mil Med. 1991;156:406.
19. Ivatury, RR, Kazigo, J, Rohman, M, et al. “Directed” emergency room thoracotomy: a prognostic prerequisite for survival. J Trauma. 1991;31:1076.
20. Lorenz, HP, Steinmetz, B, Lieberman, J, et al. Emergency thoracotomy: survival correlates with physiologic status. J Trauma. 1992;32:780.
21. Velmahos, GC, Degiannis, E, Souter, I, et al. Outcome of a strict policy on emergency department thoracotomies. Arch Surg. 1995;130:774.
22. Ledgerwood, AM, Kazmers, M, Lucas, CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma. 1976;16:610.
23. Seamon, MJ, Pathak, AS, Bradley, KM, et al. Emergency department thoracotomy: still useful after abdominal exsanguination? J Trauma. 2008;64:1.
24. Battistella, FD, Nugent, W, Owings, JT, et al. Field triage of the pulseless trauma patient. Arch Surg. 1999;134:742.
25. Fulton, RL, Voigt, WJ, Hilakos, AS. Confusion surrounding the treatment of traumatic cardiac arrest. J Am Coll Surg. 1995;181:209.
26. Brywczynski, J, McKinney, J, Pepe, PE, et al. Emergency medical systems transport decisions in posttraumatic circulatory arrest: are national practices congruent? J Trauma. 2010;69:1154.
27. Mattox, KL, Feliciano, DV. Role of external cardiac compression in truncal trauma. J Trauma. 1982;22:934.
28. Pasquale, MD, Rhodes, M, Cipolle, MD, et al. Defining “dead on arrival:” impact on a level I trauma center. J Trauma. 1996;41:726.
29. Flynn, TC, Ward, RE, Miller, PW. Emergency department thoracotomy. Ann Emerg Med. 1982;11:413.
30. Asensio, JA, Patrizio, P, García-Nuñez, LM, et al. Emergency department thoracotomy. In: Asensio JA, Trunkey DD, eds. Current Therapy of Trauma and Surgical Critical Care. Philadelphia: Mosby, 2008.
31. Eckstein, M, Henderson, SO. Thoracic trauma. In Marks JA, ed.: Rosen’s Emergency Medicine Concepts & Clinical Practice, 7th ed, St. Louis: Mosby, 2009.
32. Asensio, JA, Berne, JD, Demetriades, D, et al. One hundred five penetrating cardiac injuries: a 2-year prospective evaluation. J Trauma. 1998;44:1073.
33. Asensio, JA, Arroyo, H, Jr., Veloz, W, et al. Penetrating thoracoabdominal injuries: ongoing dilemma—which cavity and when? World J Surg. 2002;26:539.
34. Asensio, JA, Patrizio, P, Bruno, P, et al. Penetrating cardiac injuries: a historical perspective and fascinating trip through time. J Am Coll Surg. 2009;208:462.
35. Carrasguilla, C, Wilson, RF, Wait, AF, et al. Gunshot wounds of the heart. Ann Thorac Surg. 1972;13:208.
36. Kirkpatrick, AW, Ball, CG, D’Amours, SK. Acute resuscitation of the unstable adult trauma patient: bedside diagnosis and therapy. Can J Surg. 2008;51:57.
37. Ma, OJ, Mateer, JR, Ogata, M, et al. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38:879.
38. Rozycki, GS, Ballard, RB, Feliciano, DV, et al. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228:557.
39. Ball, CG, Williams, BH, Wyrzykowski, AD, et al. A caveat to the performance of pericardial ultrasound in patients with penetrating cardiac wounds. J Trauma. 2009;67:1123.
40. Bertelsen, S, Howitz, P. Injuries of the trachea and bronchi. Thorax. 1972;27:188.
41. Guest, JL, Anderson, JN. Major airway injury in closed chest trauma. Chest. 1977;72:63.
42. Estrera, AS, Pass, LJ, Platt, MR. Systemic arterial air embolism in penetrating lung injury. Ann Thorac Surg. 1990;50:257.
43. Graham, JJ, Beall, CA, Mattox, KL, et al. Systemic air embolism following penetrating trauma to the lung. Chest. 1977;72:449.
44. Yee, ES, Verrie, ED, Thomas, AN. Management of air embolism in blunt and penetrating thoracic trauma. J Thorac Cardiovasc Surg. 1983;85:661.
45. King, MW, Aitchison, JM, Nel, JP. Fatal air embolism following penetrating lung trauma: an autopsy study. J Trauma. 1984;24:753.
46. Kralovich, KA, Morris, DC, Dereczyk, BE, et al. Hemodynamic effects of aortic occlusion during hemorrhagic shock and cardiac arrest. J Trauma. 1997;42:1023.
47. Hazinski, FM, Nolan, JP, Billi, JE, et al. Part 1: executive summary: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122:S250.
48. Kouwenhoven, WB, Jude, JR, Kickerbocker, GG. Closed-chest cardiac massage. JAMA. 1960;173:1064.
49. Redding, JS, Pearson, JW. Resuscitation from asphyxia. JAMA. 1962;182:283–286.
50. Paradis, NA, Martin, GB, Rivers, EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1105.
51. Del Guercio, LM, Feins, NR, Coghn, JD, et al. Comparison of blood flow during external and internal cardiac massage in man. Circulation. 1965;31:171.
52. Boczar, ME, Howard, MA, Rivers, EP, et al. A technique revisited: hemodynamic comparison of closed- and open-chest cardiac massage during human cardiopulmonary resuscitation. Crit Care Med. 1995;23:498.
53. Alzaga-Fernandez, AG, Varon, J. Open-chest cardiopulmonary resuscitation: past, present and future. Resuscitation. 2005;64:149.
54. Brunette, DD, McVaney, K. Hypothermic cardiac arrest: an 11 year review of ED management and outcome. Am J Emerg Med. 2000;18:418.
55. Roggero, E, Stricker, H, Biegger, P. Severe accidental hypothermia with cardiopulmonary arrest: prolonged resuscitation without extracorporeal circulation. Schweiz Med Wochenschr. 1992;122:161.
56. Brown, CV, Foulkrod, KH, Sadler, HT, et al. Autologous blood transfusion during emergency trauma operations. Arch Surg. 2010;145:690.
57. Lui, RC, Johnson, FE. Selective right-lung ventilation during emergency department thoracotomy. Crit Care Med. 1989;17:1057.
58. Karmy-Jones, R, Nathens, A, Jurkovich, GJ, et al. Urgent and emergent thoracotomy for penetrating chest trauma. J Trauma. 2004;56:664.
59. Barnett, WM. Comparison of open-chest cardiac massage techniques in dogs. Ann Emerg Med. 1983;12:180.
60. Shamoun, JR, Barraza, KR, Jurkovich, GJ, et al. In extremis use of staples for cardiorrhaphy in penetrating cardiac trauma: case report. J Trauma. 1989;29:1589.
61. Macho, JR, Markinson, RE, Schecter, WP. Cardiac stapling in the management of penetrating injuries of the heart: rapid control of hemorrhage and decreased risk of personal contamination. J Trauma. 1993;34:711.
62. Mattox, KL, Von Kock, L, Beall, AC, et al. Logistic and technical considerations in the treatment of the wounded heart. Circulation. 1975;52:210.
63. Evans, J, Gray, LA, Rayner, A, et al. Principles for the management of penetrating cardiac wounds. Ann Surg. 1979;189:777.
64. Trinkle, JK, Toon, RS, Franz, JL, et al. Affairs of the wounded heart: penetrating cardiac wounds. J Trauma. 1979;19:467.
65. Maynard, AD, Cordice, JW, Naclerio, EA. Penetrating wounds of the heart: a report of 81 cases. Surg Gynecol Obstet. 1952;94:605.
66. Wilson, SM. In extremis use a Foley catheter in cardiac stab wound. J Trauma. 1986;26:400.
67. Symbas, PN, Harlaftis, N, Waldo, WJ. Penetrating cardiac wounds: a comparison of different therapeutic methods. Ann Surg. 1976;183:337.
68. Moulton, C, Pennycook, A, Crawford, R. Intracardiac therapy following emergency thoracotomy in the accident and emergency department: an experimental model. Arch Emerg Med. 1992;9:190.
69. Stothert, JC, McBride, L, Tidik, S, et al. Multiple aortic tears treated by primary suture repair. J Trauma. 1987;27:955.
70. Schwab, CW. Emergency department thoracotomy (EDT): a 26-month experience using an agonal protocol. Am Surg. 1986;52:20.
71. Fabian, TC, Richardson, JD, Croce, MA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374.
72. Goldstone, J, Towan, HJ, Ellis, RJ. Rationale for use of vasopressors in treatment of coronary air embolism. Surg Forum. 1978;29:237.
73. Kinwald, EP. Gas embolism. In: Kinwald EP, ed. Hyperbaric Medicine Practice. Flagstaff, AZ: Best Publishing; 1994:327.
74. Moore, EE, Moore, JB, Gallaway, AC, et al. Post injury thoracotomy in the emergency department: a critical evaluation. Surgery. 1979;86:590.
75. Martin, GJ, Dunne, JR, Cho, JM, et al. Prevention of infections associated with combat-related thoracic and abdominal cavity injuries. J Trauma. 2011;71:S270.
76. Mandal, AK. Prophylactic antibiotics and no antibiotics compared in penetrating chest trauma. J Trauma. 1985;25:639.
77. Tardiff, K, Marzuk, PM, Leon, AC, et al. Human immunodeficiency virus among trauma patients in New York City. Ann Emerg Med. 1998;32:151.
78. Sloan, EP, McGill, AB, Zalenski, R, et al. Human immunodeficiency virus and hepatitis B virus seroprevalence in an urban trauma population. J Trauma. 1995;38:736.
79. Sikka, R, Millham, FH, Feldman, JA. Analysis of occupational exposures associated with emergency department thoracotomy. J Trauma. 2004;56:867.