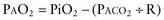Chapter 365 Respiratory Pathophysiology and Regulation
365.1 Lung Volumes and Capacities in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
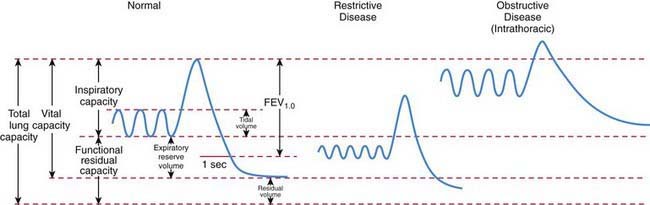
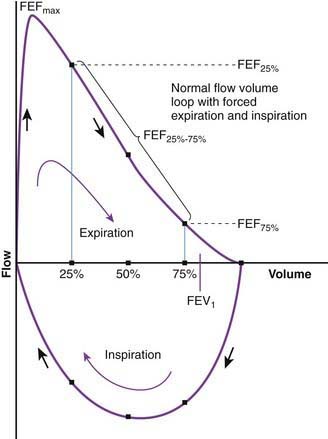
Traditionally, lung volumes are measured with a spirogram (Fig. 365-1). Tidal volume (VT) is the amount of air moved in and out of the lungs during each breath; at rest, tidal volume is normally 6-7 mL/kg body weight. Inspiratory capacity (IC) is the amount of air inspired by maximum inspiratory effort after tidal expiration. Expiratory reserve volume (ERV) is the amount of air exhaled by maximum expiratory effort after tidal expiration. The volume of gas remaining in the lungs after maximum expiration is residual volume (RV). Vital capacity (VC) is defined as the amount of air moved in and out of the lungs with maximum inspiration and expiration. VC, IC, and ERV are decreased in lung pathology but are also effort dependent. Total lung capacity (TLC) is the volume of gas occupying the lungs after maximum inhalation.
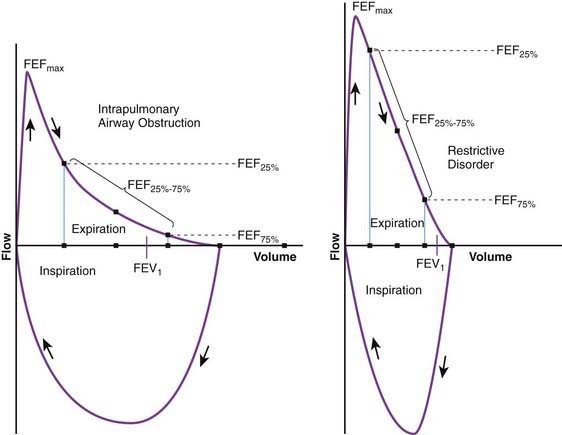
Flow volume relationship offers a valuable means at the bedside or in an office setting to detect abnormal pulmonary mechanics and response to therapy with relatively inexpensive and easy-to-use devices. After maximum inhalation, the patient forcefully exhales through a mouthpiece into the device until residual volume is reached followed by maximum inhalation (Fig. 365-2). Flow is plotted against volume. Maximum forced expiratory flow (FEF max) is generated in the early part of exhalation, and it is a commonly used indicator of airway obstruction in asthma and other obstructive lesions. Provided maximum pressure is generated consistently during exhalation, a decrease in flow is a reflection of increased airway resistance. The total volume exhaled during this maneuver is forced vital capacity (FVC). Volume exhaled in one second is referred to as FEV1. FEV1/FVC is expressed as a percentage of FVC. FEF25%-75% is the mean flow between 25% and 75% of FVC and is considered relatively effort independent. Individual values and shapes of flow-volume curves show characteristic changes in obstructive and restrictive respiratory disorders (Fig. 365-3). In intrapulmonary airway obstruction such as asthma or cystic fibrosis, there is a reduction of FEFmax, FEF25%-75%, FVC, and FEV1/FVC. Also, there is a characteristic concavity in the middle part of the expiratory curve. In restrictive lung disease such as interstitial pneumonia, FVC is decreased with relative preservation of airflow and FEV1/FVC. The flow volume curve assumes a vertically oblong shape compared to normal. Changes in shape of the flow volume loop and individual values depend on the type of disease and the extent of severity.
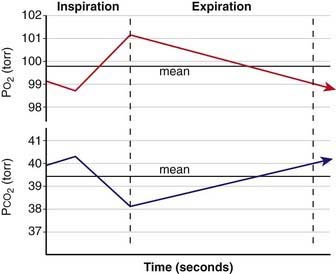
Functional residual capacity (FRC) is the amount of air left in the lungs after tidal expiration. FRC has important pathophysiologic implications. Alveolar gas composition changes during inspiration and expiration. Alveolar PO2 (PAO2) increases and alveolar PCO2 (PACO2) decreases during inspiration as fresh atmospheric gas enters the lungs. During exhalation, PAO2 decreases and PACO2 increases as pulmonary capillary blood continues to remove oxygen from and add CO2 into the alveoli (Fig. 365-4). FRC acts as a buffer, minimizing the changes in PAO2 and PACO2 during inspiration and expiration. FRC represents the environment available for pulmonary capillary blood for gas exchange at all times.
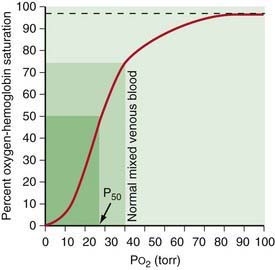
A decrease in FRC is often encountered in alveolar interstitial diseases and thoracic deformities. The major pathophysiologic consequence of decreased FRC is hypoxemia. Reduced FRC results in a sharp decline in PAO2 during exhalation because a limited volume is available for gas exchange. PO2 of pulmonary capillary blood therefore falls excessively during exhalation, leading to a decline in arterial PO2 (PaO2). Any increase in PAO2 (and therefore PaO2) during inspiration cannot compensate for the decreased PaO2 during expiration. The explanation for this lies in the shape of O2-hemoglobin (Hb) dissociation curve, which is sigmoid shaped (Fig. 365-5). Because most of the oxygen in blood is combined with Hb, it is the percentage of oxyhemoglobin (SO2) that gets averaged rather than the PO2. Although an increase in arterial PO2 cannot increase O2-Hb saturation >100%, there is a steep desaturation of hemoglobin below a PO2 of 50 torr; thus, decreased SO2 during exhalation as a result of low FRC leads to overall arterial desaturation and hypoxemia. The adverse pathophysiologic consequences of decreased FRC are ameliorated by application of positive end expiratory pressure (PEEP) and increasing the inspiratory time during mechanical ventilation.
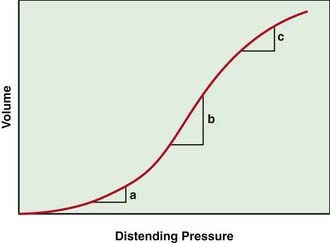
The lung pressure–volume relationship is markedly influenced by FRC (Fig. 365-6). Pulmonary compliance is decreased at abnormally low or high FRC.
365.2 Chest Wall
Ashok P. Sarnaik and Sabrina M. Heidemann
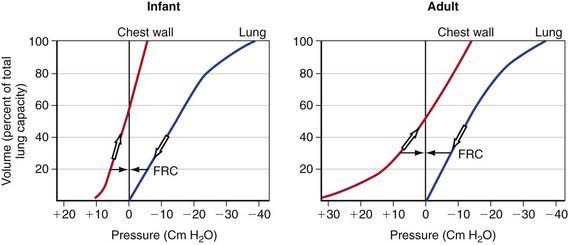
The chest wall and diaphragm of an infant are mechanically disadvantaged compared to that of an adult when required to increase thoracic (and therefore the lung) volume. The infant’s ribs are oriented much more horizontally and the diaphragm is flatter and less domed. The infant is therefore unable to duplicate the efficiency of upward and outward movement of obliquely oriented ribs and downward displacement of the domed diaphragm in an adult to expand the thoracic capacity. Additionally, the infant’s rib cage is softer and thus more compliant compared to an adult’s. Although a soft, highly compliant chest wall is beneficial to a baby in its passage through the birth canal and allows future lung growth, it places the young infant in a vulnerable situation under certain pathologic conditions. Chest wall compliance is a major determinant of FRC. Because the chest wall and the lungs recoil in opposite directions at rest, FRC is reached at the point where the outward elastic recoil of the thoracic cage counterbalances the inward lung recoil. This balance is attained at a lower lung volume in a young infant because of the extremely high thoracic compliance compared to older children (see Fig. 365-7 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). The measured FRC in infants is higher than expected because respiratory muscles of infants maintain the thoracic cage in an inspiratory position at all times. Additionally, some amount of air trapping during expiration occurs in young infants.
Abnormalities of the chest wall are encountered in certain pathologic conditions. Chest wall instability can result from trauma (fractured ribs, thoracotomy) and neuromuscular diseases that lead to intercostal and diaphragmatic muscle weakness. The increased chest wall compliance makes such children more vulnerable to respiratory decompensation when faced with similar pulmonary pathology compared to older children and adults with stiffer chest walls. Children with rigid, noncompliant chest wall (asphyxiating thoracic dystrophy of Jeune [Chapters 411.3 and 691], achondroplasia [Chapter 411.4]) have markedly diminished lung volumes and capacities.
365.3 Pulmonary Mechanics and Work of Breathing in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
where R is resistance, l is length, η is viscosity, and r is the radius. The practical implication of pressure-flow relationship is that airway resistance is inversely proportional to its radius raised to the 4th power. If the airway lumen is decreased in half, the resistance increases 16-fold. Newborns and young infants with their inherently smaller airways are especially prone to marked increase in airway resistance from inflamed tissues and secretions. In diseases in which airway resistance is increased, flow often becomes turbulent. Turbulence depends to a great extent on the Reynolds number (Re), a dimensionless entity, which is calculated as
where r is radius, v is velocity, d is density, and η is viscosity. Turbulence in gas flow is most likely to occur when Re exceeds 2000. Resistance to turbulent flow is greatly influenced by density. A low-density gas such as helium-oxygen mixture decreases turbulence in obstructive airway diseases such as viral laryngotracheobronchitis and asthma. Neonates and young infants are predominantly nose breathers and therefore even a minimal amount of nasal obstruction is poorly tolerated.
365.4 Airway Dynamics in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
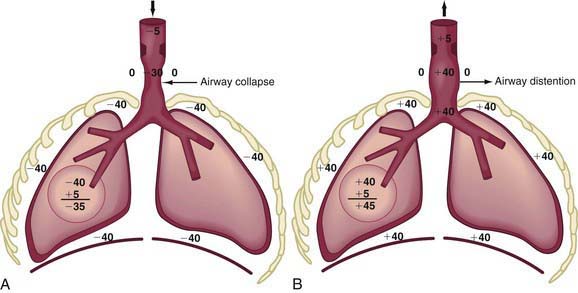
In extrathoracic airway obstruction (choanal atresia [Chapter 368], retropharyngeal abscess, laryngotracheobronchitis [Chapter 377]), the high negative intrapleural pressure during inspiration is transmitted up to the site of obstruction, after which there is a rapid dissipation of pressure. Therefore, the extrathoracic airway below the site of obstruction has markedly increased negative pressure inside, resulting in its collapse, which makes the obstruction worse (Fig. 365-8A). This produces inspiratory difficulty, prolongation of inspiration, and inspiratory stridor. Also, the increased negative intrapleural pressure results in chest wall retractions. During expiration, the increased positive intrapleural pressure is again transmitted up the airways to the site of obstruction, leading to a distention of the extrathoracic airway and amelioration of obstruction (Fig. 365-8B).
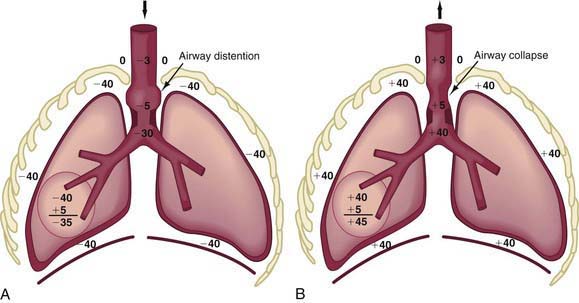
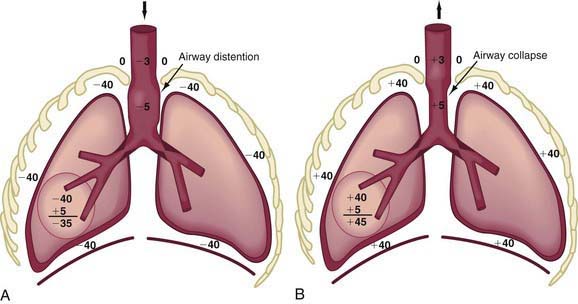
In obstruction of intrathoracic-extrapulmonary airway (vascular ring [Chapter 378], mediastinal tumors) and intrapulmonary airway (asthma, bronchiolitis), the increased negative intrapleural pressure results in a distention of intrathoracic airways during inspiration, thus providing some relief from obstruction (Fig. 365-9A).
During expiration, the increased positive intrathoracic pressure is transmitted up to the site of obstruction, after which it dissipates rapidly. The intrathoracic airway above the site of obstruction is therefore subjected to much greater intrapleural pressure from outside, which cannot be adequately balanced by enough positive pressure inside, resulting in collapse above the site of obstruction (Fig. 365-9B).
The site at which pressures inside and outside the airway during exhalation are equal is referred to as the equal pressure point (EPP). With intrathoracic airway obstruction, the EPP is shifted distally toward the alveolus, causing airway collapse above. Marked inspiratory and expiratory changes in a young infant’s airway lumen above the EPP is often termed collapsible trachea. Tracheal collapse is often a sign of airway obstruction, and it even contributes to its severity, but it is rarely the primary abnormality. With intrapulmonary airway obstruction, an even wider portion of intrathoracic airway is subjected to pressure swings during inspiration and expiration (Fig. 365-10).
365.5 Interpretation of Clinical Signs to Localize the Site of Pathology
Ashok P. Sarnaik and Sabrina M. Heidemann
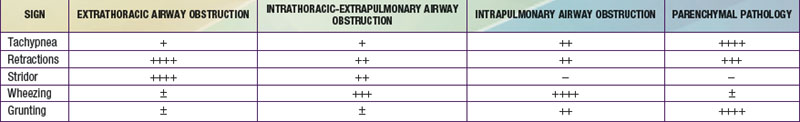
The rate and depth of respiration and the presence of retractions, stridor, wheezing, and grunting are valuable signs in localizing the site of respiratory pathology (Table 365-1 and Fig. 365-11). Rapid and shallow respirations (tachypnea) are characteristic of parenchymal pathology, in which the elastic work of breathing is increased disproportionately to the resistive work of breathing. Chest wall, intercostal, and suprasternal retractions are most striking, with increased negative intrathoracic pressure during inspiration. This occurs in extrathoracic airway obstruction as well as diseases of decreased compliance. Inspiratory stridor is a hallmark of extrathoracic airway obstruction. Expiratory wheezing is characteristic of intrathoracic airway obstruction, either extrapulmonary or intrapulmonary. Grunting is produced by expiration against a partially closed glottis and is an attempt to maintain positive airway pressure during expiration for as long as possible. Such prolongation of positive pressure is most beneficial in alveolar diseases that produce widespread loss of FRC, such as in pulmonary edema, hyaline membrane disease, and pneumonia. Grunting is also effective in small airway obstruction (bronchiolitis) to maintain a higher positive pressure in the airway during expiration, decreasing the airway collapse.
365.6 Ventilation-Perfusion Relationship in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
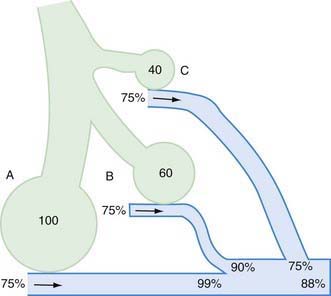
The 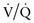 relationship is adversely affected in a variety of pathophysiologic states (Fig. 365-12). Air movement in areas that are poorly perfused is referred to as dead space ventilation. Examples of dead space ventilation include pulmonary thromboembolism and hypovolemia. Perfusion of poorly ventilated alveoli is referred to as intrapulmonary right-to-left shunting or venous admixture. Examples include pneumonia, asthma, and hyaline membrane disease. In intrapulmonary airway obstruction, the closing capacity is abnormally increased and can exceed the FRC. In such situations, perfusion of poorly ventilated alveoli during tidal respiration results in venous admixture.
relationship is adversely affected in a variety of pathophysiologic states (Fig. 365-12). Air movement in areas that are poorly perfused is referred to as dead space ventilation. Examples of dead space ventilation include pulmonary thromboembolism and hypovolemia. Perfusion of poorly ventilated alveoli is referred to as intrapulmonary right-to-left shunting or venous admixture. Examples include pneumonia, asthma, and hyaline membrane disease. In intrapulmonary airway obstruction, the closing capacity is abnormally increased and can exceed the FRC. In such situations, perfusion of poorly ventilated alveoli during tidal respiration results in venous admixture.
365.7 Gas Exchange in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
Venous blood brought to the lungs is “arterialized” after diffusion is complete. After complete arterialization, the pulmonary capillary blood should have the same PO2 and PCO2 as in the alveoli. The arterial blood gas composition is different from that in the alveoli, even in normal conditions because there is a certain amount of dead space ventilation as well as venous admixture in a normal lung. Dead space ventilation results in a higher PaCO2 than PACO2, whereas venous admixture or right-to-left shunting results in a lower PaO2 compared to the alveolar gas composition (see Fig. 365-11). PaO2 is a reflection of the amount of oxygen dissolved in blood, which is a relatively minor component of total blood oxygen content. For every 100 torr PO2, there is 0.3 mL of dissolved O2 in 100 mL of blood. The total blood oxygen content is composed of the dissolved oxygen and the oxygen bound to hemoglobin. Each gram of hemoglobin carries 1.34 mL of O2 when 100% saturated with oxygen. Thus, 15 g of hemoglobin carries 20.1 mL of oxygen. Arterial oxygen content (CaO2), expressed as mL O2/dL blood, can be calculated as (PaO2 × 0.003) + (Hb × 1.34 × SO2), where Hb is grams of hemoglobin per dL blood and SO2 is percentage of oxyhemoglobin saturation. The relationship of PO2 and the amount of oxygen carried by the hemoglobin is the basis of the O2-Hb dissociation curve (see Fig. 365-5). The PO2 at which hemoglobin is 50% saturated is referred to as P50. At a normal pH, hemoglobin is 94% saturated at PO2 of 70, and little further gain in saturation is accomplished at a higher PO2. At PO2 <50, there is a steep decline in saturation and therefore the oxygen content.
365.8 Interpretation of Blood Gases
Ashok P. Sarnaik and Sabrina M. Heidemann
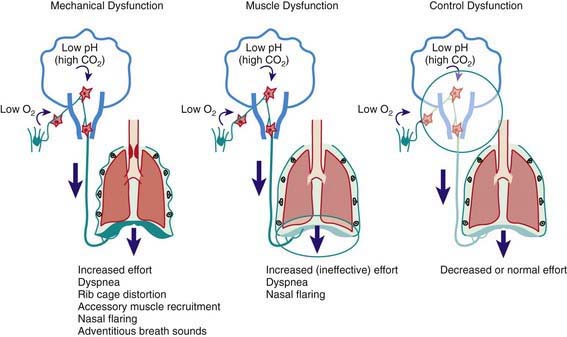
Clinical observations and interpretation of blood gas values are critical in localizing the site of the lesion and estimating its severity (see Table 365-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). In airway obstruction above the carina (subglottic stenosis, vascular ring), blood gases reflect overall alveolar hypoventilation. This is manifested by an elevated PACO2 and a proportionate decrease in PAO2 as determined by the alveolar air equation. A rise in PACO2 of 20 torr decreases PAO2 by 20 × 1.25 or 25 torr. In the absence of significant parenchymal disease and intrapulmonary shunting, such lesions respond very well to supplemental oxygen in reversing hypoxemia. Similar blood gas values, demonstrating alveolar hypoventilation and response to supplemental oxygen, are observed in patients with a depressed respiratory center and ineffective neuromuscular function, resulting in respiratory insufficiency. Such patients can be easily distinguished from those with airway obstruction by their poor respiratory effort.
Table 365-2 INTERPRETATION OF ARTERIAL BLOOD GAS VALUES
In intrapulmonary airway obstruction (asthma, bronchiolitis), blood gases reflect  imbalance and venous admixture. In these diseases, the obstruction is not uniform throughout the lungs, resulting in areas that are hyperventilated and others that are hypoventilated. Pulmonary capillary blood coming from hyperventilated areas has a higher PO2 and lower PCO2, whereas that coming from hypoventilated regions has a lower PO2 and higher PCO2. A lower blood PCO2 can compensate for the higher PCO2 because the Hb-CO2 dissociation curve is relatively linear. In mild disease, the hyperventilated areas predominate, resulting in hypocarbia. An elevated PaO2 in hyperventilated areas cannot compensate for the decreased PaO2 in hypoventilated areas because of the shape of the O2-Hb dissociation curve. This results in venous admixture, arterial desaturation, and decreased PaO2 (see Fig. 365-12). With increasing disease severity, more areas become hypoventilated, resulting in normalization of PaCO2 with a further decrease in PaO2. A normal or slightly elevated PaCO2 in asthma should be viewed with concern as a potential indicator of impending respiratory failure. In severe intrapulmonary airway obstruction, hypoventilated areas predominate, leading to hypercarbia, respiratory acidosis, and hypoxemia. The degree to which supplemental oxygenation raises PaO2 depends on the severity of the illness and the degree of venous admixture.
imbalance and venous admixture. In these diseases, the obstruction is not uniform throughout the lungs, resulting in areas that are hyperventilated and others that are hypoventilated. Pulmonary capillary blood coming from hyperventilated areas has a higher PO2 and lower PCO2, whereas that coming from hypoventilated regions has a lower PO2 and higher PCO2. A lower blood PCO2 can compensate for the higher PCO2 because the Hb-CO2 dissociation curve is relatively linear. In mild disease, the hyperventilated areas predominate, resulting in hypocarbia. An elevated PaO2 in hyperventilated areas cannot compensate for the decreased PaO2 in hypoventilated areas because of the shape of the O2-Hb dissociation curve. This results in venous admixture, arterial desaturation, and decreased PaO2 (see Fig. 365-12). With increasing disease severity, more areas become hypoventilated, resulting in normalization of PaCO2 with a further decrease in PaO2. A normal or slightly elevated PaCO2 in asthma should be viewed with concern as a potential indicator of impending respiratory failure. In severe intrapulmonary airway obstruction, hypoventilated areas predominate, leading to hypercarbia, respiratory acidosis, and hypoxemia. The degree to which supplemental oxygenation raises PaO2 depends on the severity of the illness and the degree of venous admixture.
365.9 Pulmonary Vasculature in Health and Disease
Ashok P. Sarnaik and Sabrina M. Heidemann
The tunica media of the pulmonary arteries of the fetus become more muscular in the last trimester of pregnancy (Chapter 95.1). Up to 90% of the systemic venous return is shunted away from the pulmonary arterial circulation to the systemic arterial circulation through the foramen ovale and the ductus arteriosus. After birth, with functional closure of the foramen ovale and the ductus arteriosus, and dilatation of the pulmonary arterial circulation with consequent decrease in pulmonary vascular resistance (PVR), all of the right ventricular output passes through the lung. The PVR is ∼50% of the systemic arterial resistance 3 days after birth. In the next several wk after birth as pulmonary arterial musculature in the tunica media involutes, there is a further decline in PVR and therefore in pulmonary artery pressure. Two to 3 mo after birth, the PVR and the pulmonary artery pressure are ∼15% of the systemic values, a relationship that exists through childhood and adolescence. Pulmonary vasculature constricts in response to hypoxemia, acidosis, and hypercarbia and dilates with increased alveolar and arterial PO2, alkalosis, and hypocarbia. Younger infants, with their relatively muscular pulmonary arteries, are especially susceptible to pulmonary vasoconstrictive stimuli.
Failure of the pulmonary arterial circulation to dilate after birth results in persistent pulmonary hypertension of the newborn (PPHN; Chapter 95.7). Because of the persistently high PVR, the systemic venous blood returning to the right side of the heart continues to be shunted across the foramen ovale and the ductus arteriosus to the systemic arterial circulation, leading to a vicious cycle of hypoxemia, acidosis, and further pulmonary vasoconstriction.
The relatively high PVR opposes excessive left-to-right shunting in full-term neonates with ventricular septal defect and patent ductus arteriosus (Chapter 420). Such infants do not usually manifest heart failure until 2-3 mo after birth, when PVR has sufficiently declined. Premature infants who have lesions capable of left-to-right shunting are susceptible to developing heart failure earlier in life because of less musculature in pulmonary artery tunica media and, therefore, a lower PVR. Persistent and long-term left-to-right shunting carries the risk of developing secondary pulmonary vascular disease characterized by the postnatal development of medial muscular hypertrophy followed by intimal proliferation and increased PVR. Early changes in pulmonary vasculature are reversible with correction of the congenital heart defect responsible for left-to-right shunting. Advanced pulmonary vascular disease is characterized by irreversible intimal and medial changes. When PVR is increased to suprasystemic levels, right-to-left shunting occurs and is characterized by a cyanotic state (Eisenmenger syndrome), making the heart defect inoperable in the absence of an accompanying lung transplantation (Chapter 427.2).
Pulmonary hypertension can develop without a well-defined etiology (primary pulmonary hypertension) or as a consequence of an underlying disease (secondary pulmonary hypertension) (Chapter 427). Adverse effects of pulmonary hypertension are related to an increased right ventricular afterload, decreased cardiac output, and heart failure characterized by increased systemic venous pressure, hepatomegaly, and edema. In an acute situation, right ventricular failure and decreased cardiac output can worsen oxygen delivery and hypoxemia. Right ventricular failure secondary to pulmonary pathology is referred to as cor pulmonale. Secondary pulmonary hypertension is a common occurrence in end-stage chronic obstructive pulmonary disease (COPD) such as cystic fibrosis and bronchopulmonary dysplasia. Pulmonary arterial involvement is sometimes encountered in collagen vascular diseases such as scleroderma (Chapter 154) and dermatomyositis (Chapter 153). Functional or structural upper airway obstruction can also produce right ventricular failure. Children with marked obesity are also susceptible to chronic alveolar hypoventilation and right heart failure, termed Pickwickian syndrome. Treatment of the underlying cause is the 1st priority in patients with secondary pulmonary hypertension (Chapter 427).
365.10 Immune Response of the Lung to Injury
Ashok P. Sarnaik and Sabrina M. Heidemann
Local and systemic diseases can potentially induce an inflammatory response in the lung. Local diseases of the lung capable of inducing the inflammatory response include infectious processes, aspiration, asphyxia, pulmonary contusion, and inhalation of chemical irritants; systemic diseases include sepsis, shock, trauma, and cardiopulmonary bypass. This inflammatory response is mediated through the release of cytokines and other mediators. In the lung, alveolar macrophages are the chief architects of the early cytokine response, producing tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). These cytokines are involved in initiating the inflammatory cascade, resulting in the production of other cytokines, prostaglandins, reactive oxygen species, and upregulating cell adhesion molecules, which, in turn, leads to white cell migration into the lung tissue. The pathophysiologic consequences of the inflammatory response include injury to pulmonary capillary endothelium and the alveolar epithelial cells. Various cytokines and eicosanoids produce pulmonary vasoconstriction, resulting in pulmonary hypertension and increased right ventricular afterload. Injury to the capillary endothelium results in increased permeability and exudation of protein-rich fluid into the pulmonary interstitium and alveoli. Cellular debris and fibrin form the characteristic eosinophilic hyaline membranes along the walls of the alveolar duct. There is sloughing of type 1 pneumocytes. Interstitial and alveolar edema results in decreased FRC, diffusion barrier, intrapulmonary right-to-left shunting across poorly ventilating alveoli, and increase in the alveolar-arterial (A-aO2) gradient. Clinically, A-aO2 gradient >200 is characterized as acute lung injury and a gradient >300 is termed acute respiratory distress syndrome (ARDS) (Chapter 65).
365.11 Regulation of Respiration
Ashok P. Sarnaik and Sabrina M. Heidemann
Sensors
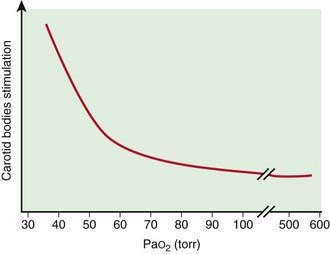
Peripheral chemoreceptors are located in carotid bodies just above the bifurcation of the common carotid arteries, and in the aortic bodies above and below the aortic arch; the carotid bodies are the most important in humans. The most important variable in determining the activity of the carotid bodies is changes in PaO2. Although the carotid bodies have a relatively high metabolic rate, they receive a very high flow for their rather small size. As long as a normal blood flow is maintained, the dissolved oxygen reflected by PaO2 is sufficient for their metabolism. Stimulation of carotid bodies resulting in increased ventilation occurs when their oxygen supply is decreased below their metabolic requirements. This occurs when there is decreased PaO2, decreased blood flow (low cardiac output), and impaired oxygen use (cyanide poisoning). In anemia, carbon monoxide poisoning, and methemoglobinemia, carotid bodies are not stimulated as long as the PaO2 and the cardiac output are not compromised. The relationship of PaO2 and the stimulation of carotid bodies is nonlinear (Fig. 365-13).
Sleep States
Respiratory regulation is considerably affected by sleep. Sleep, in general, decreases central chemosensitivity to CO2. PaCO2 is increased by a few torr compared to that in the wakeful state. Two broad categories of sleep states exist: non–rapid eye movement (NREM) and rapid eye movement (REM) sleep (Chapter 17). NREM sleep is characterized by high-voltage, slow waves on electroencephalogram (EEG) and is associated with fragmented mental activity. Muscle tone and movements are relatively unaffected. NREM sleep is likened to a “relatively inactive brain in a movable body.” REM sleep is so termed because of the presence of episodic bursts of rapid eye movements.
Regulation of Respiration in Special Situations
Fetus, Newborns, and Young Infants
Many neurotransmitters involved in regulation of respiration also undergo developmental maturational changes. Serotoninergic neurons located in the raphe nuclei possess chemosensitive properties and respond to a decrease in pH. An increase in population of these neurons is associated with increasing chemosensitivity in the developing animal. Abnormalities of the arcuate nucleus, the human equivalent of the rat and cat medullary raphe, have been demonstrated at autopsy on infants dying of sudden infant death syndrome (SIDS; Chapter 367). Cohort studies of Japanese, African-American, and white victims of SIDS have implicated a homozygous gene that encodes for the long allele of the serotonin transporter promoter. SIDS victims are more likely to express the long allele of the serotonin transporter promoter and miss the short allele compared to controls. The delay in development of serotoninergic neurons or overexpression of the long allele for serotonin transporter promoter might explain the abnormal respiratory response to adverse conditions, which results in SIDS. Central chemoreception is also severely impaired in congenital central hypoventilation syndrome (CCHS), also known as Ondine’s curse, which results in sleep-associated respiratory arrests. Mutations of PHOX2B gene located on chromosome 4 have been shown to cause CCHS.
Abu-Shaweesh JM. Maturation of respiratory responses in the fetus and neonate. Semin Neonatol. 2004;9:169-180.
Bush A. Update in pediatric lung disease 2006. Am J Respir Crit Care Med. 2007;175(6):532-540.
Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. ed 3. Philadelphia: WB Saunders; 2000:15-25.
Chokroverty S. Physiology of sleep. In: Chokroverty S, Daroff RB, editors. Sleep disorders medicine: basic science, technical considerations, and clinical aspects. ed 2. Boston: Butterworth-Heinemann; 1999:95-126.
Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239-266.
Gozal D. New concepts in abnormalities of respiratory control in children. Curr Opin Pediatr. 2004;19:305-308.
Polgar G, Weng T. The functional development of the respiratory system from the period of gestation to adulthood. Am Rev Respir Dis. 1979;120:625-695.
Polla B, D’Antona G, Bottinelli R, et al. Respiratory muscle fibres: specialisation and plasticity. Thorax. 2004;59:808-817.
Smith JC, Ellenberger HH, Ballanyi K, et al. Pre-Botzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726-729.
Weese-Mayer DE, Berry-Kravis EM, Ceccherine I, et al. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164(1–2):38-48.
West JB. Respiratory physiology: the essentials, ed 7. Baltimore: Lippincott Williams and Wilkins; 2005.

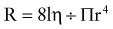
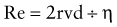
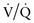 ratios favor ventilation in the nondependent portions and perfusion in the dependent portions. Because the airways in the dependent portion of the lung are narrower, they close earlier during expiration. The lung volume at which the dependent airways start to close is referred to as the closing capacity. In normal children, the FRC is greater than the closing capacity. During tidal respiration, airways remain patent both in the dependent and the nondependent portions of the lung. In newborns, the closing capacity is greater than the FRC, resulting in perfusion of poorly ventilated alveoli during tidal respiration; therefore, normal neonates have a lower Pa
ratios favor ventilation in the nondependent portions and perfusion in the dependent portions. Because the airways in the dependent portion of the lung are narrower, they close earlier during expiration. The lung volume at which the dependent airways start to close is referred to as the closing capacity. In normal children, the FRC is greater than the closing capacity. During tidal respiration, airways remain patent both in the dependent and the nondependent portions of the lung. In newborns, the closing capacity is greater than the FRC, resulting in perfusion of poorly ventilated alveoli during tidal respiration; therefore, normal neonates have a lower Pa