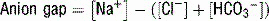Chapter 43 Renal Replacement Therapy and Rhabdomyolysis
Renal Replacement Therapy
1 What are the indications for renal replacement therapy (RRT)?
Indications can be grouped by using the AEIOU mnemonic:
A: (Metabolic) Acidosis refractory to bicarbonate administration.
E: Electrolyte imbalances, of which hyperkalemia is the most life threatening.
I: Ingestions. Some drugs and toxins (and their toxic metabolites) can be cleared with dialysis, including aspirin, lithium, methanol, or ethylene glycol.
O: Overload. Ultrafiltration with dialysis can relieve hypoxemia resulting from volume overload, which may be particularly problematic in the setting of oliguria or anuria.
U: Uremia. Symptoms and signs of uremia can range from mild (anorexia, nausea, pruritus) to severe (encephalopathy, asterixis, pericarditis); patients may also have clinical platelet dysfunction (bleeding) due to uremia.
2 List the different modes of RRT
6 Define hemofiltration, hemodialysis, and hemodiafiltration
 Hemofiltration: Plasma is forced from the blood space into the effluent via the application of pressure across a highly permeable membrane. This results in convective clearance of small and middle-sized molecules through the physical property of solvent drag. This modality does not significantly change the concentration of serum electrolytes and waste products unless a replacement fluid is infused into the blood, effectively diluting out those solutes the physician wishes to remove (e.g., urea nitrogen and potassium) and increasing the concentration of those solutes in which the patient might be deficient (e.g., bicarbonate in a patient with acidemia).
Hemofiltration: Plasma is forced from the blood space into the effluent via the application of pressure across a highly permeable membrane. This results in convective clearance of small and middle-sized molecules through the physical property of solvent drag. This modality does not significantly change the concentration of serum electrolytes and waste products unless a replacement fluid is infused into the blood, effectively diluting out those solutes the physician wishes to remove (e.g., urea nitrogen and potassium) and increasing the concentration of those solutes in which the patient might be deficient (e.g., bicarbonate in a patient with acidemia).
 Hemodialysis: Blood flows on one side of a semipermeable membrane, and the dialysate, which contains various electrolytes and glucose, flows along the other side, usually in the opposite (countercurrent) direction. A concentration gradient drives electrolytes and water-soluble waste products from the plasma compartment into the dialysate. The dialysis machine generates a pressure across the membrane to drive plasma water from the blood side to the dialysate side. Dialysis results in diffusive clearance, preferentially of small molecules.
Hemodialysis: Blood flows on one side of a semipermeable membrane, and the dialysate, which contains various electrolytes and glucose, flows along the other side, usually in the opposite (countercurrent) direction. A concentration gradient drives electrolytes and water-soluble waste products from the plasma compartment into the dialysate. The dialysis machine generates a pressure across the membrane to drive plasma water from the blood side to the dialysate side. Dialysis results in diffusive clearance, preferentially of small molecules.
 Hemodiafiltration: This technique makes simultaneous use of hemofiltration and hemodialysis, resulting in both diffusive and convective clearance.
Hemodiafiltration: This technique makes simultaneous use of hemofiltration and hemodialysis, resulting in both diffusive and convective clearance.
7 List the basic components of a prescription for IHD and for CRRT
 Dialysis access: Arteriovenous fistula, arteriovenous graft, tunneled dialysis catheter, or temporary dialysis catheter
Dialysis access: Arteriovenous fistula, arteriovenous graft, tunneled dialysis catheter, or temporary dialysis catheter
 Treatment duration: For most patients with end-stage renal disease, this ranges between 3 and 4 hours. When a patient with acute renal failure or acute kidney injury (AKI) starts hemodialysis, initial sessions may be as short as 1 to 1.5 hours to decrease the risk of dialysis disequilibrium syndrome.
Treatment duration: For most patients with end-stage renal disease, this ranges between 3 and 4 hours. When a patient with acute renal failure or acute kidney injury (AKI) starts hemodialysis, initial sessions may be as short as 1 to 1.5 hours to decrease the risk of dialysis disequilibrium syndrome.
 Filter size and type: Biocompatible dialysis membranes are now routinely used.
Filter size and type: Biocompatible dialysis membranes are now routinely used.
 Blood flow rate: Blood flow rates of up to 400 to 450 mL/min can be achieved with an arteriovenous fistula or graft and up to 350 mL/min with a tunneled or temporary catheter. Generally, the faster the flow, the more efficient the dialysis.
Blood flow rate: Blood flow rates of up to 400 to 450 mL/min can be achieved with an arteriovenous fistula or graft and up to 350 mL/min with a tunneled or temporary catheter. Generally, the faster the flow, the more efficient the dialysis.
 Dialysate flow rate: Typical flow rates range from 500 mL/min to 800 mL/min.
Dialysate flow rate: Typical flow rates range from 500 mL/min to 800 mL/min.
 Dialysate bath: Concentrations of potassium, sodium, calcium, and bicarbonate can be customized on the basis of the patient’s laboratory studies.
Dialysate bath: Concentrations of potassium, sodium, calcium, and bicarbonate can be customized on the basis of the patient’s laboratory studies.
 Ultrafiltration goal: This is the amount of fluid to be removed from the patient over the course of the session; determined by clinical assessment of the patient’s volume status.
Ultrafiltration goal: This is the amount of fluid to be removed from the patient over the course of the session; determined by clinical assessment of the patient’s volume status.
 Anticoagulation: Clotting within the dialysis circuit can result in significant blood loss; heparin is typically used unless the patient has a contraindication.
Anticoagulation: Clotting within the dialysis circuit can result in significant blood loss; heparin is typically used unless the patient has a contraindication.
 As in IHD, the prescription includes dialysis access, filter size and type, hourly fluid balance, and anticoagulation. An alternative to heparin anticoagulation often used with CRRT is regional citrate anticoagulation, in which citrate is administered to chelate calcium, a critical cofactor in the clotting cascade. Arteriovenous fistular and grafts are not used for CART.
As in IHD, the prescription includes dialysis access, filter size and type, hourly fluid balance, and anticoagulation. An alternative to heparin anticoagulation often used with CRRT is regional citrate anticoagulation, in which citrate is administered to chelate calcium, a critical cofactor in the clotting cascade. Arteriovenous fistular and grafts are not used for CART.
 Blood flow rates are typically slower than in intermittent dialysis (150-200 mL/min).
Blood flow rates are typically slower than in intermittent dialysis (150-200 mL/min).
 Mode of therapy: CVVH, CVVHD, or CVVHDF
Mode of therapy: CVVH, CVVHD, or CVVHDF
 Dialysate or replacement fluid: The specific fluid is based on the metabolic parameters of the patient, including the patient’s acid-base status and serum potassium concentration.
Dialysate or replacement fluid: The specific fluid is based on the metabolic parameters of the patient, including the patient’s acid-base status and serum potassium concentration.
 Dialysate or replacement fluid flow rate: Dosing is weight based and is typically prescribed at a dose ranging from 20 mL/kg/hr to 35 mL/kg/hr, based on the patient’s weight. Studies have shown no mortality difference between patients with renal replacement therapy administered at these two rates.
Dialysate or replacement fluid flow rate: Dosing is weight based and is typically prescribed at a dose ranging from 20 mL/kg/hr to 35 mL/kg/hr, based on the patient’s weight. Studies have shown no mortality difference between patients with renal replacement therapy administered at these two rates.
9 What are nutrition considerations for patients with AKI receiving RRT?
 Amino acids are lost in both IHD and CRRT. Critically ill patients with AKI are often highly catabolic; many patients receiving CRRT will require at least 1.5 to 2 g/kg/day of protein or amino acids.
Amino acids are lost in both IHD and CRRT. Critically ill patients with AKI are often highly catabolic; many patients receiving CRRT will require at least 1.5 to 2 g/kg/day of protein or amino acids.
 Water-soluble vitamins are lost in both IHD and CRRT. Replacement of these vitamins can be achieved with the daily administration of a vitamin complex specifically designed for patients receiving RRT.
Water-soluble vitamins are lost in both IHD and CRRT. Replacement of these vitamins can be achieved with the daily administration of a vitamin complex specifically designed for patients receiving RRT. Fat-soluble vitamins are protein or lipoprotein-bound and are therefore not significantly cleared by CRRT or IHD.
Fat-soluble vitamins are protein or lipoprotein-bound and are therefore not significantly cleared by CRRT or IHD. Trace minerals, such as zinc, may be dialyzed with IHD or CRRT; the benefit of supplementation in this situation remains unproved. Aluminum-containing products, which were used in the past as phosphorus binders, should be avoided because of central nervous system toxicity.
Trace minerals, such as zinc, may be dialyzed with IHD or CRRT; the benefit of supplementation in this situation remains unproved. Aluminum-containing products, which were used in the past as phosphorus binders, should be avoided because of central nervous system toxicity.
Rhabdomyolysis
11 What causes rhabdomyolysis?
Muscle ischemia, damage, and eventual necrosis lead to rhabdomyolysis. The various causes are grouped into physical and nonphysical causes in Box 43-1. Both groups of causes probably share a common pathway in which increased demand on muscle cells and their mitochondria, because of intrinsic deficiencies or extrinsic forces (i.e., decreased oxygen delivery or increased metabolic demands), leads to ischemia and eventual damage.
Box 43-1 Major Causes of Rhabdomyolysis
Nonphysical Causes
 Metabolic myopathies, including McArdle disease, mitochondrial respiratory chain enzyme deficiencies, carnitine palmitoyl transferase deficiency, phosphofructokinase deficiency
Metabolic myopathies, including McArdle disease, mitochondrial respiratory chain enzyme deficiencies, carnitine palmitoyl transferase deficiency, phosphofructokinase deficiency
 Endocrinopathies, including hypothyroidism and diabetic ketoacidosis (due to electrolyte abnormalities)
Endocrinopathies, including hypothyroidism and diabetic ketoacidosis (due to electrolyte abnormalities)
 Drugs and toxins, including medications (antimalarials, colchicine, corticosteroids, fibrates, HMG-CoA reductase inhibitors, isoniazid, zidovudine), drugs of abuse (alcohol, heroin), and toxins (insect and snake venoms)
Drugs and toxins, including medications (antimalarials, colchicine, corticosteroids, fibrates, HMG-CoA reductase inhibitors, isoniazid, zidovudine), drugs of abuse (alcohol, heroin), and toxins (insect and snake venoms)
 Infections (either local or systemic)
Infections (either local or systemic)
 Electrolyte abnormalities: hyperosmotic conditions, hypokalemia, hypophosphatemia, hyponatremia, or hypernatremia
Electrolyte abnormalities: hyperosmotic conditions, hypokalemia, hypophosphatemia, hyponatremia, or hypernatremia
Acid-base interpretation
20 What is the anion gap, how is it calculated, and why is it important in understanding a patient’s acid-base status?
21 Describe an approach to a comprehensive interpretation of a patient’s acid-base status using the arterial blood gas and the serum chemistry values
 Identify whether the patient has acidemia or alkalemia: If the pH is less than 7.37, the patient has acidemia, and, if the pH is greater than 7.43, the patient has alkalemia. Importantly, a pH between 7.37 and 7.43 does not imply that the patient does not have an acid-base disturbance; rather it suggests the presence of a mixed acid-base disorder.
Identify whether the patient has acidemia or alkalemia: If the pH is less than 7.37, the patient has acidemia, and, if the pH is greater than 7.43, the patient has alkalemia. Importantly, a pH between 7.37 and 7.43 does not imply that the patient does not have an acid-base disturbance; rather it suggests the presence of a mixed acid-base disorder.
 Determine whether the primary disturbance is respiratory or metabolic: If the patient has acidemia and the PCO2 is greater than 40 mm Hg, then the primary process is respiratory; if the patient has acidemia and the serum bicarbonate concentration is less than 24 mEq/L, then the primary process is metabolic. If the patient has alkalemia and the PCO2 is less than 40 mm Hg, then the primary process is respiratory; if the patient has alkalemia and the serum bicarbonate concentration is greater than 24 mEq/L, then the primary process is metabolic.
Determine whether the primary disturbance is respiratory or metabolic: If the patient has acidemia and the PCO2 is greater than 40 mm Hg, then the primary process is respiratory; if the patient has acidemia and the serum bicarbonate concentration is less than 24 mEq/L, then the primary process is metabolic. If the patient has alkalemia and the PCO2 is less than 40 mm Hg, then the primary process is respiratory; if the patient has alkalemia and the serum bicarbonate concentration is greater than 24 mEq/L, then the primary process is metabolic.
 Determine whether appropriate compensation for the primary disorder is present: To determine how the kidneys compensate for a primary respiratory process and vice versa, see Table 43-1. If the compensation is less than or greater than predicted, then another primary acid-base disturbance might be present. For example, in presence of a metabolic acidosis, if the PCO2 is lower than expected a concomitant primary respiratory alkalosis is present, whereas if the PCO2 is higher than expected a concomitant primary respiratory acidosis is present.
Determine whether appropriate compensation for the primary disorder is present: To determine how the kidneys compensate for a primary respiratory process and vice versa, see Table 43-1. If the compensation is less than or greater than predicted, then another primary acid-base disturbance might be present. For example, in presence of a metabolic acidosis, if the PCO2 is lower than expected a concomitant primary respiratory alkalosis is present, whereas if the PCO2 is higher than expected a concomitant primary respiratory acidosis is present.
 Calculate the anion gap to look for the presence of an anion gap metabolic acidosis.
Calculate the anion gap to look for the presence of an anion gap metabolic acidosis.
 Calculate the delta-delta: In the presence of an isolated anion gap metabolic acidosis, the serum bicarbonate concentration should fall by an amount that equals the degree to which the anion gap is raised. If this is not the case, another metabolic disorder (either a non–anion gap metabolic acidosis or a metabolic alkalosis) is present. This can be determined by calculating the delta-delta, which is mathematically expressed as follows:
Calculate the delta-delta: In the presence of an isolated anion gap metabolic acidosis, the serum bicarbonate concentration should fall by an amount that equals the degree to which the anion gap is raised. If this is not the case, another metabolic disorder (either a non–anion gap metabolic acidosis or a metabolic alkalosis) is present. This can be determined by calculating the delta-delta, which is mathematically expressed as follows:
Table 43-1 Appropriate Compensation for Primary Acid-Base Disturbances and Their Common Causes
| Primary acid-base disturbance | Subtype | Expected compensation |
|---|---|---|
| Metabolic acidosis | Anion gap | Decrease in PCO2 = 1.2 × ΔHCO3 or PCO2 = (1.5 × HCO3) + 8 ± 2 |
| Non–anion gap | ||
| Metabolic alkalosis | Increase in PCO2 = 0.7 × ΔHCO3 | |
| Respiratory acidosis | Acute | Increase in HCO3 = 0.1 × ΔPCO2 |
| Chronic | Increase in HCO3 = 0.35 × ΔPCO2 | |
| Respiratory alkalosis | Acute | Decrease in HCO3 = 0.2 × ΔPCO2 |
| Chronic | Decrease in HCO3 = 0.4 × ΔPCO2 | |
22 List the differential diagnoses of the major acid-base disturbances
Each of the primary acid-base disturbances has its own differential diagnosis, and many acronyms have been generated to help the student or physician remember them. Of these, the most popular is the MUDPILERS acronym for the differential diagnosis of an anion-gap metabolic acidosis. If an anion gap acidosis is present, the osmolar gap should be measured and calculated; the presence of an osmolar gap in addition to an anion gap suggests a toxic alcohol ingestion, such as ethylene glycol, methanol, or ethanol. A more comprehensive differential diagnosis for each of the primary disturbances is presented in Box 43-2.
Box 43-2 Differential Diagnoses of the Primary Acid-Base Disturbances
Anion Gap Metabolic Acidosis
Common causes can be remembered with the MUDPILERS mnemonic:
Uremia with accumulation of organic anions (phosphates, sulfates, urate)
Diabetic ketoacidosis (and other forms of ketoacidosis: alcoholic, starvation)
Paraldehyde and propylene glycol (carrier for certain medications, including intravenous lorazepam and diazepam)
Non–Anion Gap Metabolic Acidosis
Metabolic Alkalosis
 Gastrointestinal loss of hydrogen ions: removal of gastric secretions (vomiting, nasogastric tube suction)
Gastrointestinal loss of hydrogen ions: removal of gastric secretions (vomiting, nasogastric tube suction)
 Renal loss of hydrogen ions: primary mineralocorticoid excess, administration of thiazide or loop diuretics, posthypercapneic alkalosis, milk-alkali syndrome with associated hypercalcemia, congenital syndromes (Bartter syndrome and Gitelman syndrome)
Renal loss of hydrogen ions: primary mineralocorticoid excess, administration of thiazide or loop diuretics, posthypercapneic alkalosis, milk-alkali syndrome with associated hypercalcemia, congenital syndromes (Bartter syndrome and Gitelman syndrome)
Respiratory Acidosis
 Neuromuscular diseases: Guillain-Barré syndrome, myasthenia gravis, botulism, hypophosphatemia and hypokalemia, poliomyelitis, diaphragmatic dysfunction
Neuromuscular diseases: Guillain-Barré syndrome, myasthenia gravis, botulism, hypophosphatemia and hypokalemia, poliomyelitis, diaphragmatic dysfunction
 Central hypoventilation: congenital central hypoventilation syndrome (Ondine curse), obesity hypoventilation syndrome, Cheyne-Stokes breathing
Central hypoventilation: congenital central hypoventilation syndrome (Ondine curse), obesity hypoventilation syndrome, Cheyne-Stokes breathing
 Medications that depress respiratory drive: narcotics, benzodiazepines, barbiturates, heroin
Medications that depress respiratory drive: narcotics, benzodiazepines, barbiturates, heroin
 Endocrine causes: hypothyroidism
Endocrine causes: hypothyroidism
 Airway obstruction: epiglottis, chronic obstructive pulmonary disease, severe and late phase asthma
Airway obstruction: epiglottis, chronic obstructive pulmonary disease, severe and late phase asthma
 Trauma leading to chest wall abnormalities or restrictive lung disease from severe kyphoscoliosis
Trauma leading to chest wall abnormalities or restrictive lung disease from severe kyphoscoliosis
Key Points Acid-Base Disorders
1. An organized approach to the analysis of acid-base disorders is key.
2. The approach starts by determining whether the patient has acidemia or alkalemia; note that the presence of a normal serum pH does not imply that an acid-base disorder is not present.
3. Determine whether the primary process is metabolic or respiratory.
4. Determine whether there is appropriate compensation for the primary process.
5. Calculate the anion gap and the “delta-delta” to determine whether unrecognized metabolic disturbances exist, including gap and non-gap metabolic acidosis and metabolic alkalosis.
1 Bellomo R., Cass A., Cole L., et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638.
2 Huerta-Alardin A.L., Varon J., Marik P.E. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158–169.
3 Malinoski D.J., Slater M.S., Mullins R.J. Crush injury and rhabdomyolysis. Crit Care Clin. 2004;20:171–192.
4 Marshall MR, Golper TA: Sustained low efficiency or extended daily dialysis: www.uptodate.com. Accessed March 27, 2012
5 McClave S.A., Martindale R.G., Vanek V.W., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. J Parenter Enteral Nutr. 2009;33:277–316.
6 Palevsky PM: Renal replacement therapy (dialysis) in acute kidney injury (acute renal failure) in adults: indications, timing, and dialysis dose: www.uptodate.com. Accessed March 27, 2012
7 Palevsky P.M., Zhang J.H., O’Connor T.Z., et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20.
8 Seifter J. Acid-base disorders. Goldman L., Schafer A. Goldman’s Cecil Medicine, 24th ed, St. Louis: Saunders, 2011.
9 Vanholder R., Sever M.S., Erek E., et al. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561.