Renal Failure and Transplantation
Acute Renal Failure
Overview: Acute renal failure is defined as a sudden loss of renal function that may be due to inadequate renal perfusion, renal cell injury, or obstruction to urine flow. Recently, the term “acute kidney injury” (AKI) has been proposed to replace the term acute renal failure and is gaining wide acceptance.1 AKI usually develops in hospitalized children as a result of systemic illness or its treatment and not from primary renal disease. The most common causes of AKI in children are renal ischemia, nephrotoxic drugs, and sepsis.2 Other important causes are listed in Box 118-1. AKI from any cause can lead to chronic kidney disease.3–7 Recovery of renal function depends on the underlying events leading to injury.
Imaging: In general, sonography should be the first diagnostic imaging study performed in the workup of persons with AKI, and in most cases, it is the only imaging required. The role of sonography is to determine renal size and exclude anatomic abnormalities as the cause of AKI. The renal cortex, medulla, and collecting system have different acoustic properties, and pathologic changes are readily detected and correlate well with histologic findings. Doppler techniques provide information about renal perfusion and vascular abnormalities. Sonography also is used to localize the kidneys for percutaneous biopsy. More precise functional information may be obtained with nuclear medicine studies that can aid in differentiating between prerenal, renal, and postrenal causes of AKI.
Prerenal Injury: In persons with prerenal injury, the kidney is intrinsically normal, and renal function is diminished as a result of decreased renal perfusion. Sonographic examination of the kidneys is normal. Restoration of renal perfusion results in a prompt return of renal function.
Intrinsic Renal Disease: Renal ischemia due to a prolonged prerenal injury or a severe hypoxic/ischemic insult can lead to acute tubular necrosis. Imaging findings depend on the severity of parenchymal injury. A generalized increase in renal cortical echogenicity indicates intrinsic renal disease (Fig. 118-1). In mild cases, the kidneys may appear normal or may demonstrate slightly increased renal cortical echogenicity. Decreased corticomedullary differentiation may be noted. Doppler flow may be normal in mild disease, whereas poor peripheral perfusion and diminished arterial diastolic flow due to increased peripheral vascular resistance occur in more severe cases.
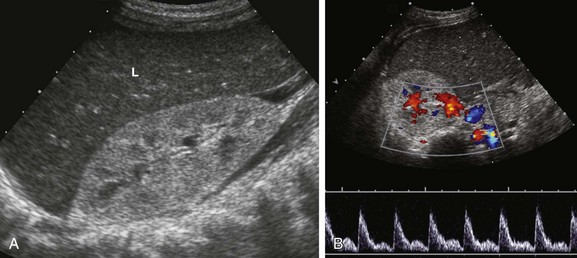
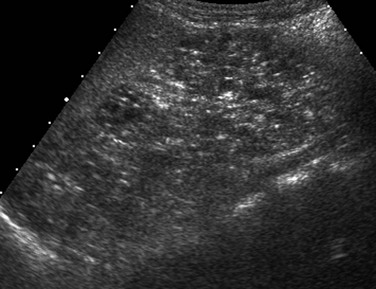
Figure 118-1 Hemolytic-uremic syndrome.
A, A sonogram of an 18-month-old boy with impaired renal function shows an enlarged right kidney with parenchymal echogenicity greater than that of the adjacent liver (L) and decreased corticomedullary differentiation. B, A pulsed Doppler waveform from the main renal artery shows diminished diastolic flow.
Nephrotoxic drugs commonly associated with AKI include antibiotics, chemotherapeutic agents, and nonsteroidal antiinflammatory medications.8 Some agents such as aminoglycoside antibiotics cause tubular injury. Methicillin and other penicillin analogues, cimetidine, sulfonamides, rifampin, and nonsteroidal antiinflammatory drugs may cause acute interstitial nephritis, which is thought to be related to a hypersensitivity reaction with development of antitubular basement membrane antibodies in some cases. Children with acute lymphocytic leukemia and B-cell lymphoma are at risk for the development of uric acid nephropathy and tumor lysis syndrome. One mechanism of injury believed to be of importance in uric acid nephropathy is precipitation of uric acid crystals in the renal microvasculature leading to obstruction of renal blood flow, or precipitation in the renal tubules leading to obstruction of urine flow. Tumor lysis syndrome is a constellation of metabolic abnormalities resulting from spontaneous or treatment-related tumor necrosis. As tumor cells are lysed, rapid increases in serum potassium, uric acid, and phosphorus occur, with a decrease in serum calcium. Patients who are treated with allopurinol, a purine analog used to diminish the excretion of uric acid, instead excrete large quantities of the uric acid precursors xanthine and hypoxanthine. Precipitation of these compounds is thought to play a role in the development of AKI. In patients who are in septic shock, AKI can develop from hypotension, leading to renal ischemia and acute tubular necrosis, or AKI can develop as a result of the use of nephrotoxic medications.
Obstructive Uropathy and Lower Tract Lesions: A urinary tract obstruction can develop in persons with AKI if the obstruction affects a solitary kidney, both ureters, or the urethra. Congenital causes include ureteropelvic junction obstruction, ureterovesical junction obstruction, and posterior urethral valves. Acquired urinary tract obstruction can be caused by urinary tract stones or, rarely, tumors. Bladder rupture is another rare cause of AKI associated with ascites. Bladder rupture in children usually is due to trauma; it also can occur as a complication of infection, chemotherapy, and radiotherapy.9,10
Chronic Renal Failure
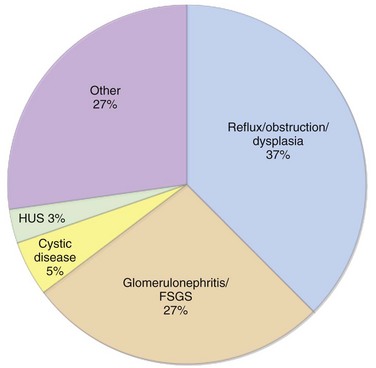
Overview: The terms “chronic renal failure” and “chronic renal insufficiency” have been used historically to describe varying degrees of renal dysfunction. Currently, the term chronic kidney disease (CKD) is used by both adult and pediatric nephrology communities throughout the world.11 The classification of CKD published by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative is based on the estimated glomerular filtration rate and is applicable to children older than 2 years and to adults.12 The classification categorizes CKD into five different stages and is widely used in clinical practice (Table 118-1). Recently it has been recognized that most patients with CKD (estimated to be one in eight adults) never reach end-stage renal disease and the need for renal replacement therapy because they are at risk for accelerated cardiovascular disease and are more likely to die prematurely from cerebrovascular or cardiovascular disease.13 Therefore clinical management requires measures to diminish cardiovascular risk factors in both adult and pediatric patients. According to the North American Pediatric Renal Trials and Collaborative Studies 2007 report,14 the most common causes of pediatric CKD in North America are congenital disorders such as obstructive uropathy, renal dysplasia, and reflux nephropathy (Fig. 118-2; Table 118-2). In contrast, in Japan, 34% of pediatric CKD is due to glomerulonephritis, primary focal segmental glomerulosclerosis, and immunoglobulin A nephropathy.15 In Jordan16 and Iran,17 where consanguinity is more common, heritable disorders such as cystic kidney disease, primary hyperoxaluria, cystinosis, Alport syndrome, and congenital nephrotic syndrome represent a greater proportion of cases of CKD. In the developing world, acquired causes of CKD predominate, particularly infection-related glomerulopathies.18,19 The worldwide incidence and prevalence of CKD is greater for boys than for girls because of the higher incidence of congenital causes of CKD in boys.14–21
Table 118-1
Kidney Disease Outcomes Quality Initiative Stages of Chronic Kidney Disease
| Stage | Description | GFR (mL/min/1.73 m2) |
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mild decrease in GFR | 60-89 |
| 3 | Moderate decrease in GFR | 30-59 |
| 4 | Severe decrease in GFR | 15-29 |
| 5 | Kidney failure | <15 (or dialysis) |
GFR, Glomerular filtration rate.
From VanDeVoorde RG, Warady BA. Management of chronic kidney disease. In: Avner ED, ed. Pediatric nephrology. 6th ed. Berlin: Springer-Verlag; 2009.
Table 118-2
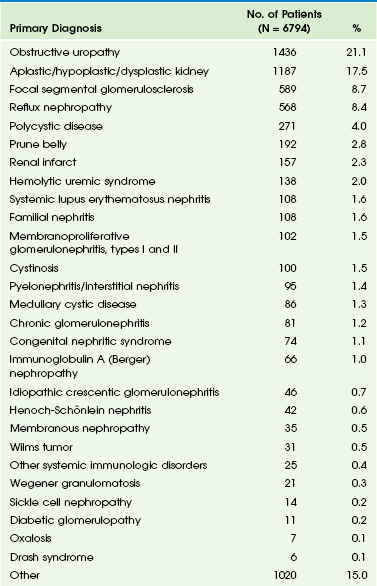
From VanDeVoorde RG, Warady BA. Management of chronic kidney disease. In: Avner ED, ed. Pediatric nephrology. 6th ed. Berlin: Springer-Verlag; 2009.
Imaging: Imaging plays an essential role in diagnosis, assessment of function, and monitoring of the effects of treatment at all stages of CKD, from the antenatal period to pretransplantation evaluation. Many urinary tract abnormalities are discovered by prenatal sonography. Postnatal imaging usually focuses on growth, function, and assessment of complications. A multimodality approach often is required, including radiography, sonography, fluoroscopy, nuclear medicine, and magnetic resonance imaging.
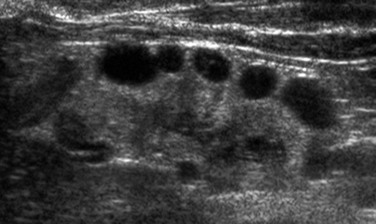
As with AKI, sonography is the primary imaging modality used to investigate CKD and guide performance of a biopsy. Assessment of renal and bladder morphology permits detection of congenital anomalies such as ureteropelvic junction obstruction, posterior urethral valves, duplex collecting systems and ureters, and ectopic ureteral insertion. Determination of kidney size is critical for long-term follow-up. Enlarged kidneys may occur in the setting of obstruction, cystic disease, and glomerulonephritis. Hypoplastic and scarred kidneys usually are small. A gradual reduction in kidney size usually occurs in persons with CKD. With progressive CKD, a concomitant loss of corticomedullary differentiation usually occurs, and most patients will have echogenic renal parenchyma. However, increased renal parenchymal echogenicity is nonspecific and is not linked to the severity of disease.22 Dysplastic kidneys have disordered development that generally is manifested as a combination of echogenic parenchyma and cysts (Fig. 118-3). Dysplasia may be focal or diffuse and generally appears sonographically as echogenic tissue without recognizable corticomedullary differentiation. Total bilateral renal dysplasia is incompatible with life. Multicystic dysplastic kidney is the most common form of unilateral dysplasia and consists of multiple large, noncommunicating cysts of varying size and minimal intervening echogenic parenchyma. Most of these kidneys eventually will involute.23 Monitoring of the contralateral functioning kidney is critical in these patients, because associated abnormalities are common.24 Renal scarring may occur as a sequela of infection or vesicoureteral reflux. Sonography is relatively insensitive in the detection of scarring, and nuclear scintigraphy with dimercaptosuccinic acid may be required for more precise assessment of renal parenchymal integrity.25 Patients with autosomal-recessive polycystic kidney disease have large, echogenic kidneys with poor corticomedullary differentiation due to the presence of microscopic cysts. Visible cysts less than 1 cm in diameter are detected in about 50% of children (Fig. 118-4). Evaluation of the liver is necessary because of the high association of parenchymal cysts and fibrosis. Autosomal-dominant polycystic kidney disease may manifest in childhood, although associated CKD is rare. The kidneys usually appear normal initially, with cystic changes developing later in life. Occasionally, cysts may be present at birth.26–29 Renal vascularity reflects functional status and will decrease in persons with CKD. Several recent publications suggest that the renal arterial resistive index may correlate with creatinine levels and may be an independent risk factor for the progression of CKD.30,31
Transplantation
Overview: Renal transplantation is the treatment of choice in children with end-stage renal disease, with improved patient survival and quality of life compared with dialysis. However, renal transplantation in children is associated with a number of specific problems, including a higher incidence of graft failure and posttransplant malignancy than in adults, as well as growth retardation. Furthermore, it may be challenging technically to transplant a relatively large adult kidney into a small pediatric abdomen. Transplantation of kidneys from donors younger than 5 years usually is avoided because the risk of early graft failure is increased, mainly as a result of graft thrombosis.32 Adult kidneys almost always are used. An allograft may be from a living, related donor, from a living, unrelated donor, or it may be cadaveric in origin. In the United States, more than 50% of transplanted kidneys in children are from living, adult donors.33
Imaging: Assessment of the transplant kidney is similar to that of a native kidney, with an emphasis on graft perfusion and vascular anastomoses; sonography is the primary imaging technique. Familiarity with the surgical anatomy is essential to a complete evaluation of the vasculature. In small children, the kidney is placed intraperitoneally with anastomosis of the donor renal artery and vein to the recipient distal aorta and inferior vena cava, respectively. In older children, the graft is placed in a retroperitoneal location within an iliac fossa. The donor renal artery is anastomosed either to the recipient external (more commonly) or to the internal iliac artery, and the donor vein is anastomosed to the recipient external iliac vein. The donor ureter is anastomosed to the recipient urinary bladder (ureteroneocystostomy).
A normal kidney transplant has a sonographic appearance similar to that of a normal native kidney. The close proximity of the graft to the body surface accentuates the distinction between the renal cortex, medullary pyramids, and central sinus. Doppler evaluation reveals continuous antegrade flow within the main renal artery throughout the cardiac cycle, with low-impedance arterial waveforms in the intrarenal vessels. The intrarenal arterial resistive indices range from 0.4 to 0.8, with a mean value of 0.6.34 Flow within the main renal vein and intrarenal veins is opposite in direction to that in the arteries and usually is mildly pulsatile, reflecting normal cardiac and respiratory motion.
In the immediate postoperative period, the most common complications are acute tubular necrosis, vascular thrombosis, and rejection. These entities may be difficult to distinguish with imaging techniques. In all three entities, the graft will appear swollen, with loss of normal corticomedullary differentiation and elevated arterial resistive indices. Renal vein thrombosis usually occurs in the first postoperative week and manifests as decreased or absent flow within the main renal vein associated with elevated arterial resistive indices (Fig. 118-5). Additional acute complications include urine leaks, other fluid collections (e.g., hematoma or abscess), urinary obstruction, and vesicoureteral reflux, all of which are readily demonstrated by imaging.35–37 Lymphoceles are the most common fluid collections and are caused by lymphatic disruption. They usually develop weeks to months after transplantation (Fig. 118-6).38
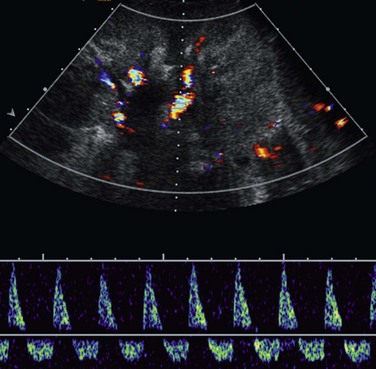
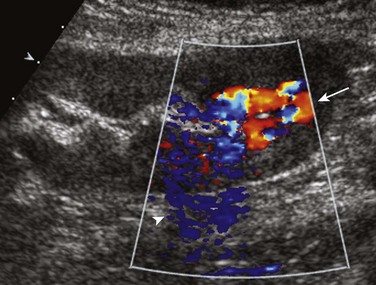
Figure 118-5 Transplant renal vein thrombosis.
A longitudinal sonogram of a 20-month-old boy 1 day after living–related donor transplantation shows a swollen, echogenic kidney with diminished corticomedullary differentiation. Marked pulsatility of the main renal arterial waveform is noted with reversed diastolic flow, reflecting increased peripheral resistance.
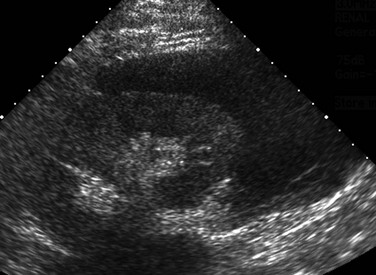
Figure 118-6 Lymphocele.
A sagittal sonogram of a 16-year-old boy obtained 5 weeks after living–related donor transplantation depicts a large fluid collection surrounding the lower pole of the left-sided kidney.
Renal artery stenosis usually occurs within the first 3 years after transplantation.39,40 Doppler sonography may demonstrate an elevated velocity within the main renal artery of greater than 200 cm/sec (Fig. 118-7), as well as a pulsus parvus et tardus waveform in the arteries distal to the stenotic site. Although Doppler sonography is the screening modality of choice, significant stenotic lesions can be missed. Computed tomographic angiography and magnetic resonance angiography are useful problem-solving techniques when sonography is inconclusive. However, catheter angiography remains the “gold” standard imaging technique for renal artery imaging.40–42
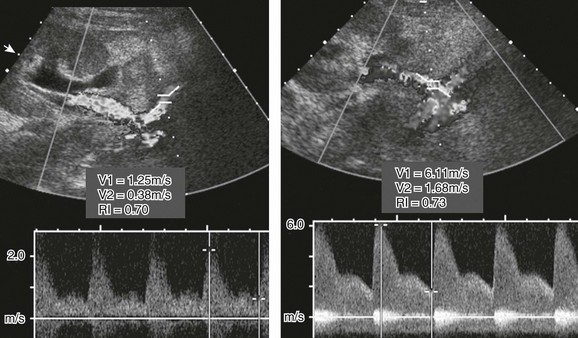
Figure 118-7 Transplant renal arterial stenosis.
A Doppler sonogram of a patient with a rising creatinine level shows a nearly fivefold increase in renal arterial peak systolic velocity (right image) compared with the supplying external iliac artery (left image), indicating hemodynamically significant stenosis.
Important late complications include chronic rejection and posttransplant lymphoproliferative disorder (PTLD). Chronic rejection is manifested sonographically by a gradual reduction in graft size, increased parenchymal echogenicity, and decreased perfusion.37 In the pediatric renal transplant population, two large series have reported an incidence of 1.2%43 and 4.5%.44 PTLD is the most common neoplastic disorder in pediatric transplant recipients, accounting for 52% of all malignancies in this patient population,45 and is associated with Epstein-Barr virus (EBV) infection. In immune-suppressed persons with impaired T-cell immune surveillance, EBV infection can cause abnormal B-cell proliferation, especially in persons who were seronegative at the time of transplantation.45,46 Patients at risk will have elevated EBV titers, and sonographic follow-up must include a search for lymph node enlargement and solid masses of the abdominal and pelvic viscera (Fig. 118-8).
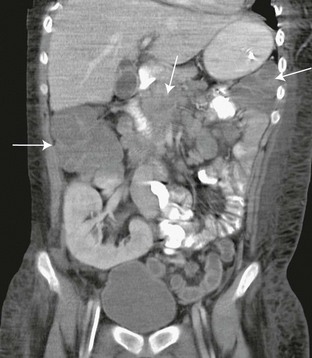
Figure 118-8 Posttransplant lymphoproliferative disorder.
A coronal reformatted image from contrast-enhanced computed tomography in a 17-year-old boy shows a right lower quadrant renal transplant. Multiple low-density conglomerate masses within the abdomen represent lymphoproliferative tissue (arrows).
Renal biopsy may be indicated at any stage after transplantation and often is performed under sonographic guidance. Iatrogenic complications of biopsy include hemorrhage and arteriovenous fistula formation (Fig. 118-9). Sonographic monitoring of the native kidneys always should be performed because of an increased risk of malignancy.47,48
Akbar, SA, Jafri, SZ, Amendola, MA, et al. Complications of renal transplantation. Radiographics. 2005;25:1335–1356.
Borhani, AA, Hosseinzadeh, K, Almusa, O, et al. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics. 2009;29:981–1000.
Irshad, A, Ackerman, SJ, Campbell, AS, et al. An overview of renal transplantation: current practice and use of ultrasound. Semin Ultrasound CT MRI. 2009;30:298–314.
Kalantarinia, K. Novel imaging techniques in acute kidney injury. Curr Drug Targets. 2009;10:1184–1189.
Khati, NJ, Hill, MC, Kimmel, PL. The role of ultrasound in renal insufficiency: the essentials. Ultrasound Q. 2005;21:227–244.
References
1. American Society of Nephrology. Renal research report. J Am Soc Nephrol. 2005;16:1886–1903.
2. Goldstein, SL, Devarajan, P. Acute kidney injury in childhood: should we be worried about progression to CKD? Pediatr Nephrol. 2011;26:509–522.
3. Nash, K, Hafeez, A, Hou, S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936.
4. Uchino, S, Kellum, JA, Bellomo, R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818.
5. Chertow, GM, Soroko, SH, Paganini, EP, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126.
6. Basile, DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156.
7. Hsu, CY, McCulloch, CE, Fan, D, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212.
8. Hui-Stickle, S, Brewer, ED, Goldstein, SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005;45:96–101.
9. Sivit, CJ, Cutting, JP, Eichelberger, MR. CT diagnosis and localization of rupture of the bladder in children with blunt abdominal trauma: significance of contrast material extravasation in the pelvis. AJR Am J Roentgenol. 1995;164:1243–1246.
10. Redman, JF, Seibert, JJ, Arnold, W. Urinary ascites in children owing to extravasation of urine from the bladder. J Urol. 1979;122:409–411.
11. VanDeVoorde, RG, Warady, BA. Management of chronic kidney disease. In Avner ED, ed.: Pediatric nephrology, 6th ed, Berlin: Springer-Verlag, 2009.
12. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis. 2002;39:S46–S75.
13. Coresh, J, Selvin, E, Stevens, LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047.
14. North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) 2007 annual report. NAPRTCS, Boston, MA.
15. Hattori, S, Yosioka, K, Honda, M, et al. The 1998 report of the Japanese National Registry data on pediatric end-stage renal disease patients. Pediatr Nephrol. 2002;17:456–461.
16. Hamed, RM. The spectrum of chronic renal failure among Jordanian children. J Nephrol. 2002;15:130–135.
17. Madani, K, Otoukesh, H, Rastegar, A, et al. Chronic renal failure in Iranian children. Pediatr Nephrol. 2001;16:140–144.
18. Anochie, I, Eke, F. Chronic renal failure in children: a report from Port Harcourt, Nigeria (1985-2000). Pediatr Nephrol. 2003;18:692–695.
19. Sirin, A, Emre, S, Alpay, H, et al. Etiology of chronic renal failure in Turkish children. Pediatr Nephrol. 1995;9:549–552.
20. Ardissino, G, Daccò, V, Testa, S, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382–e387.
21. Gulati, S, Mittal, S, Sharma, RK, et al. Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. 1999;13:594–596.
22. Moccia, WA, Kaude, JV, Wright, PG, et al. Evaluation of chronic renal failure by digital gray-scale ultrasound. Urol Radiol. 1980;2:1–7.
23. Strife, JL, Souza, AS, Kirks, DR, et al. Multicystic dysplastic kidney in children: US follow-up. Radiology. 1993;186:785–788.
24. Schreuder, MF, Westland, R, van Wijk, JA. Unilateral multicystic dysplastic kidney: a meta-analysis of observational studies on the incidence, associated urinary tract malformations and the contralateral kidney. Nephrol Dial Transplant. 2009;24:1810–1818.
25. Moorthy, I, Wheat, D, Gordon, I. Ultrasonography in the evaluation of renal scarring using DMSA scan as the gold standard. Pediatr Nephrol. 2004;19:153–156.
26. Turkbey, B, Ocak, I, Daryanani, K, et al. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKDS/CHF). Pediatr Radiol. 2009;39:100–111.
27. Barua, M, Pei, Y. Diagnosis of autosomal-dominant polycystic kidney disease: an integrated approach. Semin Nephrol. 2010;30:356–365.
28. Rosetti, S, Harris, PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374–1380.
29. Gunay-Aygun, M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:296–306.
30. Sugiura, T, Wada, A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24:2780–2785.
31. Parolini, C, Noce, A, Staffolani, E, et al. Renal resistive index and long-term outcome in chronic nephropathies. Radiology. 2009;252:888–896.
32. Singh, A, Stablein, D, Tejani, A. Risk factors for vascular thrombosis in pediatric renal transplantation: a special report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation. 1997;63:1263–1267.
33. Hwang, AH, Cho, YW, Cicciarelli, J, et al. Risk factors for short- and long-term survival of primary cadaveric renal allografts in pediatric recipients: a UNOS analysis. Transplantation. 2005;80:466–470.
34. Surratt, JT, Siegel, MJ, Middleton, WD. Sonography of complications in pediatric renal allografts. Radiographics. 1990;10:687–699.
35. Salvatierra, O, Jr., Millan, M, Concepcion, W. Pediatric renal transplantation with considerations for successful outcomes. Semin Pediatr Surg. 2006;15:208–217.
36. Kayler, L, Kang, D, Howard, R. Kidney transplant ureteroneocystostomy techniques and complications: review of the literature. Transplant Proc. 2010;42:1413–1420.
37. Carmichael, J, Easty, M. Imaging chronic renal disease and renal transplant in children. Pediatr Radiol. 2010;40:963–974.
38. Kobayashi, K, Censullo, ML, Rossman, LL, et al. Interventional radiologic management of renal transplant dysfunction: indications, limitations, and technical considerations. Radiographics. 2007;27:1109–1130.
39. Dodd, GD, III., Tublin, ME, Shah, A, et al. Imaging of vascular complications associated with renal transplants. AJR Am J Roentgenol. 1991;157:449–459.
40. Singh, AK, Sahani, DV. Imaging of the renal donor and transplant recipient. Radiol Clin North Am. 2008;46:79–93.
41. Browne, RF, Tuite, DJ. Imaging of the renal transplant: comparison of MRI with duplex sonography. Abdom Imaging. 2006;31:461–482.
42. Glockner, JF, Vrtiska, TJ. Renal MR and CT angiography: current concepts. Abdom Imaging. 2007;32:407–420.
43. Dharnidharka, VR, Sullivan, EK, Stablein, DM, et al. Risk factors for post transplant lymphoproliferative disorder (PTLD) in pediatric kidney transplantation: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Transplantation. 2001;71:1065–1068.
44. Funch, DP, Brady, J, Ko, HH, et al. Methods and objectives of a large US multicenter case-control study of post-transplant lymphoproliferative disorder in renal transplant patients. Recent Results Cancer Res. 2002;159:81–88.
45. Penn, I. De novo malignancies in pediatric organ transplant recipients. Pediatr Transplant. 1998;2:56–63.
46. Pinkerton CR, Hann I, Weston CL, et al. Immunodeficiency-related lymphoproliferative disorders: prospective data from the United Kingdom Children’s Cancer Study Group Registry. Br J Haematol. 2002;118:456-461.
47. Tsaur, I, Obermüller, N, Jonas, D, et al. De novo renal cell carcinoma of native and graft kidneys in renal transplant recipients. BJU Int. 2011;108:229–234.
48. Tsaur, I, Karalis, A, Blaheta, R, et al. Transitional cell carcinoma of the native urinary tract after kidney transplantation: recommendations following a long-term retrospective analysis. Am J Med Sci. 2011;341:478–483.