CHAPTER 48 REHABILITATION AFTER STROKE
Stroke, or vascular brain injury, has an incidence of between about 1.1 and 2.6 per 1000 adults1 and a prevalence of 5 to 8 per 1000.2 It is a leading cause of chronic disability worldwide; in the United Kingdom, it is the commonest single cause of severe disability in people living at home.3,4 About 75% of affected patients are older than 65 years, and 10% are younger than 55. Each year in England and Wales, stroke occurs in about 10,000 people younger than 55 and 1000 people younger than 30. Six to 12 months after stroke, only 60% of patients with hemiplegic stroke have achieved independence in personal care, and those with sensory or visual field loss are more disabled; 30% to 40% of survivors are depressed, 10% to 15% severely so; 50% need help with either housework, meal preparation, or shopping; and a similar percentage lack a meaningful social, recreational, or occupational activity during the day.5–8 In addition to the personal consequences, the economic consequences of stroke are also enormous; direct health care costs account for approximately 4% of total health care costs, or about £3 billion in the United Kingdom (about $5.3 billion in U.S. dollars) per annum at 2005 prices9; these costs will increase as the population ages.
Neuroprotective interventions to reverse the immediate consequences of stroke are not widely applicable.10 In these circumstances, when reversal of pathology is incomplete, the majority of stroke survivors need the multidimensional process termed rehabilitation to enhance their functional activity and societal participation and to reduce the effect of limitations in these areas, so that life quality is improved and life is “worth living.”11 Rehabilitation goals are achieved through the prevention of secondary complications; through facilitation of neural protection, restoration, and substitution; and through functional compensation, which involves both behavioral adaptation and substitution, as well as modification of personal, environmental, and social contextual factors (Table 48-1). This process must also be accompanied by often difficult and less frequently admitted adjustment to loss and change, a need that is easily forgotten in the gym or during a functional imaging study. This chapter explores the delivery and effectiveness of these interventions during rehabilitation after stroke.
TABLE 48-1 Mechanisms of Recovery after Stroke
The high incidence and prevalence of stroke have provided an opportunity to test the effectiveness of many components of the rehabilitation process. Trials have shown convincingly that organized care produces better outcomes than disorganized care; this result has far-reaching implications for health care systems in general, let alone those focusing on stroke. Many of the areas addressed in this chapter are included in evidence- and expert opinion–based guidelines produced in the United Kingdom by the Royal College of Physicians12 and the Scottish Intercollegiate Guidelines Network,13 in Australia by the National Stroke Foundation,14 and in the United States by the Department of Veterans Affairs and the Department of Defense.15
COMPLEX ORGANIZATIONAL INTERVENTIONS
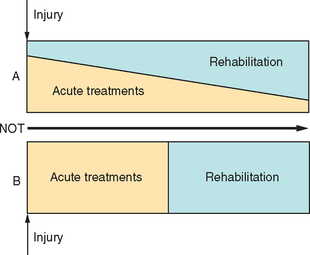
Rehabilitation after single-incident brain injury, including stroke, should be delivered through polymodal inpatient and community-based service delivery systems that differ by structure and process. A classification of these different service options, as a “rehabilitation typology,” remains to be agreed upon, at least cross-culturally, and would facilitate comparisons of different studies.16 The use of each component is determined by a variety of clinical and social factors, including length of time since injury, level of dependency, characteristics of the residual impairment, age of the patient, and resources available.17 Recommendations that different types of care after stroke are incorporated into systems of clinical care (e.g., Schwamm et al18) also emphasize the use of acute and rehabilitation interventions in parallel rather than in series (Fig. 48-1), so that strategies optimizing neural protection acutely are combined with key factors identified in postacute care. The benefits of organized complex polymodal interventions so far reported are likely to reflect mainly the effects of preventing the complications described later in this chapter and shown in Table 48-2, functional interventions that focus on teaching new skills, and the use of aids and appliances and environmental modifications to help patients adapt to their impairments, rather than the effects of neural reorganization.
TABLE 48-2 Complications after Stroke during Inpatient Rehabilitation and Their Management
| Cause and Effect | Stroke Unit Frequency % (95% Confidence Interval) | Management |
|---|---|---|
| Comorbid Conditions | ||
| Hypertension/hypotension | 15/8199 | Antihypertensive/treat cause |
| Cardiac events | 13199 | Specific medical treatments |
| Fever | 20199 | Antipyretics |
| GI bleed | 3196/4199 | ? Prophylaxis; avoid NSAIDs |
| Drug side effects | 9198 | Drug withdrawal |
| Musculoskeletal pain | 14198/38197 | Physical therapies |
| Physical Dependency | ||
| Pressure sores | 4198 | Pressure care, 24-hr handling and positioning |
| Sores and skin breaks | 21 (16-25)195/18196 | |
| Contractures | ||
| PEs and DVTs | 3 (0-3)195/5198 | Thromboprophylaxis |
| Falls | 25 (21-30)195/20256/11198 | Risk assessment |
| Shoulder pain | 9 (6-12)195/24256 | Handling and positioning |
| Neurological Damage | ||
| Malnutrition and dehydration | 5198 and 10198/10199 | NG/PEG feeding and dietetics |
| Chest infections | 22 (18-27)195/4199 | Dysphagia management |
| Urinary tract infections | 23 (18-28)195/15256/31198 | Toileting program |
| Confusion and agitation | 36 (30-41)195/4199 | Environmental management ± drugs |
| Epilepsy | 3 (1-5)195/2256/2198 | AEDs if no other trigger |
| Sleep-disordered breathing | 62257 | CPAP |
| Fatigue | 40-60223 | ? Retraining |
| Vascular Brain Injury | ||
| Mass effects | Neurosurgical decompression | |
| Hydrocephalus | Shunting | |
| Catastrophic illness | ||
| Depression | 16 (12-21)195/26256/13198 | CBT ± drugs |
| Anxiety | 14 (10-18)195/8198 | CBT ± drugs |
| Family breakdown | Information + training | |
AED, antiepileptic drug; CBT, cognitive-behavior therapy; CCF, congestive cardiac failure; CPAP, continuous positive airway pressure; DVT, deep vein thrombosis; GI, gastrointestinal; NG/PEG, nasogastric/percutaneous endoscopic gastrostomy; NSAID, nonsteroidal anti-inflammatory drug; PE, pulmonary embolism.
Until about 1995, 20% to 50% of patients after stroke were managed acutely at home in some European countries,19 despite recommendations to the contrary.20 The benefits of stroke unit care and the gradual introduction of thrombolysis and neuroprotective agents are, alone, likely to reduce this percentage dramatically. Furthermore, patients randomly assigned to receive acute care at home after moderately severe stroke (sufficient to cause persistent neurological deficit affecting continence, mobility, and self-care, and necessitating multidisciplinary treatment), rather than to receive care in an acute and rehabilitation stroke unit or a general ward with stroke team support, were significantly more likely at 1 year to be dead or institutionalized (24% versus 14%; P = 0.03) and less likely to be alive without severe disability (85% versus 71%; P = 0.002) than were patients admitted to the stroke unit.21
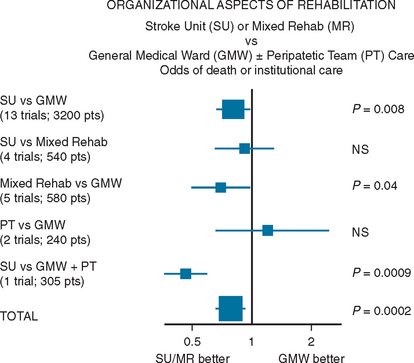
In their meta-analysis of 23 trials, the Stroke Unit Trialists’ Collaboration22 compared care in stroke units, nine of which were “comprehensive” and included both acute and rehabilitation components, with care given by alternative services, usually in a general medical or geriatric ward with or without a visiting stroke team. The meta-analysis showed that patients who receive unit-based inpatient care do not stay longer in hospital and are more likely to be alive, independent, and living at home 1 year after the stroke, regardless of gender, age, and stroke severity (Fig. 48-2). Thus, in comparison with alternative services, stroke unit care produced reductions in the odds of death at 1 year (odds ratio, 0.86; 95% confidence interval, 0.71 to 0.94; P = 0.005), of death or institutionalized care (odds ratio, 0.80; 95% confidence interval, 0.71 to 0.90; P = 0.0002), and of death or dependency (odds ratio, 0.78; 95% confidence interval, 0.68 to 0.89; P = 0.0003).
A subsequent systematic review of results from nine postacute units, which admitted patients more than 1 week after stroke, in comparison with an alternative service, revealed benefits similar to those resulting from combined acute and postacute units,23 and in the United States, compliance with postacute but not acute stroke unit guidelines (Table 48-3) has been shown to be correlated with outcome.16 Secondary analysis has indicated that the reduction in stroke unit deaths probably results from a reduction in the complications of immobility rather than in neurological or cardiovascular complications. The increased number of patients discharged home from stroke units and the reduced requirement for institutional care were attributable largely to an increase in the number of patients returning home physically independent (Rankin score, 0 to 2), rather than dependent (Rankin score, 3 to 5).24
TABLE 48-3 Dimensions of Acute Rehabilitation after Stroke
Compliance with these dimensions of process during postacute rehabilitation after stroke is correlated with outcome.
After Duncan PW, Horner RD, Reker DM, et al: Adherence to postacute guidelines is associated with functional recovery in stroke. Stroke 2002; 33:167-178.
Langhorne and Pollock25 identified aspects of care common to 11 stroke units, 8 of which included acute care, reporting beneficial effects between 1985 and 2000 in the Stroke Unit Trialists’ Collaboration’s systematic review.22 These included comprehensive medical, nursing, and therapy assessments; integration of nursing care within the multidisciplinary team; early mobilization and treatment of hypoxia, hyperglycemia, and suspected infection; avoidance of urinary catheterization; and formalized goal-oriented multidisciplinary team care, with early discharge planning and education and involvement of caretakers.
Evans and associates26 found that many of these aspects of care were not delivered by a peripatetic specialist stroke team (PSST). They compared care delivered to 304 patients randomly assigned to an acute and rehabilitation stroke unit (n = 152) or to general wards supported by a PSST (n = 152). Patients in the stroke unit were monitored more frequently, and more of those patients received oxygen, antipyretics, measures to reduce aspiration, and early nutrition than those in general wards. Many aspects of multidisciplinary care occurred more frequently in the stroke unit, in which complications including stroke progression, chest infections, and dehydration were less frequent. Early feeding, stroke unit management, frequency of complications, and measures to prevent aspiration independently affected outcome. In 1984, a PSST’s input to general ward care was shown to nonsignificantly reduce mortality rates and significantly improve functional recovery in men.27 More recently, in a head-to-head comparison at 1 year of patients randomly assigned to receive care from the PSST with those receiving care in a combined acute and rehabilitation stroke unit, more patients in the PSST group were dead or institutionalized (30% versus 14%; P < 0.001) and fewer were alive without severe disability (66% versus 85%; P < 0.001).21 Retrospective data collected by van der Walt and colleagues28 before and 2 years after the introduction of a PSST to a general ward showed significant improvements in prophylaxis for deep vein thrombosis, incontinence management, premorbid function documentation, frequent neurological observations, and early occupational therapy, with fewer severe complications (9% after versus 24% before; P = 0.004), reduced median length of stay (12.0 after versus 18.5 days before; P = 0.003), and more patients independent at discharge (32% after versus 9% before; P < 0.001). It is thus possible that practice developed in stroke units can now be delivered more effectively by a PSST, and further comparison of these two methods of service delivery remains legitimate.
More recently, high-dependency care has been introduced immediately after stroke, providing continuous rather than manual monitoring for hypoxia, hyperglycemia, hypotension, cardiac arrhythmias, and elevated body temperature during the first 48 to 72 hours after admission.29,30 Currently, its effectiveness is relatively unexplored, but there is a suggestion that it may reduce mortality rates at 3 months and 1 year among patients with severe stroke, without increasing dependency.31,32
Remarkably, the differences between stroke unit and alternative care persist for many years. Indredavik and colleagues33 found that even 10 years after random assignment to an acute and rehabilitation stroke unit or a general ward, fewer patients in the stroke unit had died (75.5% versus 87.3%; P = 0.008), more were at home (19.1% versus 8.2%; P = 0.018), and more were at least partly independent with a Barthel Index score of 60 or higher (20.0% versus 8.2%; P = 0.012) or independent with a Barthel Index score of 95 or higher (12.7% versus 5.4%; P = 0.061). Increased survival times 5 years after random assignment to a stroke rehabilitation unit, versus a general medical or geriatric ward, were also reported by Lincoln and colleagues.34
Early discharge, supported by a multidisciplinary outreach team, of medically stable patients after mild and moderate stroke, with an admission Barthel Index score of more than 9, supplements initial stroke unit gains. A meta-analysis of individual patient data from 11 trials of early supported discharge versus conventional care showed that the patients with early supported discharge had a reduced risk of death or dependency (odds ratio, 0.79; 95% confidence interval, 0.64 to 0.97; P = 0.02), a hospital stay shortened by 8 days (P < 0.0001), and significant improvement in extended activities of daily living (P = 0.05), although not in subjective health status or mood in either patients or caretakers. Patients were moderately disabled at discharge with a median discharge Barthel Index score of 15.35 One study revealed that these gains can include better life quality, assessed by the Nottingham Health Profile, at 1 year,36 and another study revealed that gains in domestic and extended activities of daily living are still evident after 5 years.37
Once in the community, patients are known to be at risk of deteriorating as a result of multiple health problems, including falls, depression, and physical and social inactivity and isolation, in addition to age-related symptoms and comorbidity, and health-related quality of life has been shown to significantly decline in the 6 months after discharge.38 Nursing home care does not substitute for stroke unit care,39 but the place for and the optimal process in other service systems for patients later after stroke (e.g., nurse-led wards, nursing homes, or residential placements) remain to be examined.
A meta-analysis of trials of therapy-based outpatient or domiciliary rehabilitation, delivered by either a multidisciplinary team or by a physiotherapist or occupational therapist, with the goal of improving task-oriented behaviors, has shown that deterioration is prevented (odds ratio, 0.72; 95% confidence interval, 0.57 to 0.92; P = 0.009) and dependency in personal care reduced (95% confidence interval, 0.02 to 0.25; P = 0.02).40 The effective components and best location for this type of service need further exploration, but benefit has been consistent in trials of community-based occupational therapy, which have provided sufficient data for a meta-analysis of eight trials that showed that intervention was associated with improved personal, extended, and leisure-based activities of daily living, depending on the intervention target.41 These findings were confirmed in a more wide-ranging systematic review of occupational therapy for stroke patients by Steultjens and associates,42 who also noted the need for further studies of the effectiveness of splinting.
Most studies of physiotherapy in patients in the community after stroke investigate the effect of a particular physiotherapy treatment on upper or lower limb function at the level of impairment and mobility, which may improve, rather than on limitations in activity and independence, which, if examined, may not improve. Domiciliary physiotherapy within 6 months after stroke has been shown to reduce probability of readmission after an average of only 2.9 (range, 1 to 8) visits43 and to reduce dependency at less cost than for day hospital attendance.44,45 In patients more than 1 year after stroke, only four to five physiotherapy sessions produced a clinically small but significant improvement in mobility.46
Informal caretakers should be recognized as an important resource: They enable patients to remain in the community,47 their support is likely to facilitate patient outcomes,48 and depression is more severe in caretakers who feel poorly supported.49 Formal support for caretakers is difficult to obtain in the United Kingdom. Trials of psychosocial interventions to support caretakers of patients with stroke, involving information packages, specialist nurses, a mental health worker, or family support workers, have failed to show functional or psychological benefit in patients and only modest psychosocial benefit for caretakers.50–57 In contrast, there is evidence that caretaker adjustment is increased by education and counseling or by training in social problem-solving skills.58 Kalra and colleagues59,60 demonstrated that training informal caretakers in basic nursing skills and facilitation of personal care techniques reduced costs and caregiver burden and improved psychosocial outcomes for the caretaker and the patient, although there was still no change in patients’ rates of mortality, institutionalization, and disability.
MANAGEMENT OF NEUROLOGICAL IMPAIRMENTS
Evidence of dysphagia, with consequent risk of dehydration, further malnutrition, and chest infections resulting from aspiration, has been reported to occur clinically in about 50% of all patients with stroke admitted to a hospital, with videofluoroscopic evidence of a swallowing abnormality in up to 65% of patients and of aspiration in about 20%.61,62 Videofluoroscopic and flexible endoscopic evaluation of swallowing increase the reliability with which aspiration can be identified63,64 when clinicians select which patients need tube feeding. However, whether other aspects of dysphagia management, including dietary modification and compensatory swallowing techniques, reduce the need for tube feeding or the risk of aspiration pneumonia remains unclear despite assertion to the contrary.65 The Feed or Ordinary Diet (FOOD) trials66 revealed that in 859 patients randomly assigned to receive nasogastric feeding within a week, in contrast to more than a week, after stroke, absolute mortality rates were reduced by 5.8% (95% confidence interval, −0.8 to 12.5; P = 0.09) in the group fed early; in 321 patients randomly assigned to undergo percutaneous enteral gastrostomy (PEG) feeding or nasogastric tube feeding a median of 1 week after stroke, PEG feeding was associated with an absolute increase in risk of death of 1.0% (95% confidence interval, −10.0 to 11.9; P = 0.9) and an increased risk of death or other poor outcome of 7.8% (95% confidence interval, 0.0 to 15.5; P = 0.05). Thus, as is recommended current practice in hospitalized adult patients in general,67 nasogastric feeding should be used for dysphagic patients soon after stroke, whereas PEG feeding is reserved for patients who do not tolerate nasogastric feeding and as required in the longer-term care of dysphagic patients.
After stroke, early incontinence is predictive of poor outcomes, including death, lengthy hospital stay, institutionalization, and severe disability.68 New urinary incontinence occurs in 40% to 50% of patients during the first week after admission and in 10% to 20% by 6-month follow-up68,69; it appears to remain at this level in the community over the long term.70 The proportions of stroke patients with new fecal incontinence on admission and at 6-month follow-up are lower, at 30% to 40% and 5% to 10%, respectively.68,69 Causes and predictors68,71,72 of fecal and urinary incontinence include age; stroke type and severity; “functional incontinence” resulting from stroke-related cognitive and communication difficulties and mobility problems; autonomic neuropathies, usually diabetic in origin; medications causing bladder and bowel hyporeflexia; preexisting bladder outflow obstruction in men or stress incontinence in women; and perhaps depression and lowered self-esteem that result from the incontinence itself.73,74
Current guidelines (e.g., Royal College of Physicians12) for the management of incontinence after stroke recommend initial definition of its cause and associations through a full clinical assessment. The neurogenic cause after stroke is usually disruption of suprapontine inhibition of bladder contractility resulting in detrusor hyperreflexia, but bladder hyporeflexia may occur after cerebellar stroke.75 Evidence for the effectiveness of methods promoting continence after stroke is currently derived largely from trials in other (nonstroke) patient groups. Management of urinary incontinence76 includes a combination of anticholinergic drug therapy, bladder training by prompted and/or timed voiding77,78 or biofeedback,79 and appropriate management of outflow obstruction and stress incontinence, for which one small study of 26 stroke patients randomly assigned to receive treatment or to control conditions showed no benefit from a 12-week pelvic floor muscle training program.80 Similar general principles apply to the management of fecal incontinence,81 which in inpatients or in discharged patients is associated with needing help to use the toilet82 and thus might be addressed by improving functional independence, whereas constipation but not fecal incontinence after stroke is improved by a single clinical/educational nurse intervention up to 6 months later.83
Impairment of trunk control and sitting balance after acute stroke is common, and recovery is a reliable predictor of functional outcome after stroke. Trunk control, measured by the trunk control test at rehabilitation admission a median of 5 weeks after stroke, accounted for 71% of the variance of the motor component of the Functional Independence Measure at discharge 10 weeks after stroke84; as measured by the Postural Assessment Scale for Stroke Patients within 2 weeks after stroke, trunk control was strongly correlated with functional outcome, rated by a combined score of both personal (Barthel Index) and instrumental (Frenchay Activities Index) activities of daily living at 6 months after stroke.85 A positive correlation has also been found between sitting balance and the Barthel Index score86 and between sitting balance and gait at 6 months.87 Mudie and associates88 found that three specific treatment approaches implemented early after stroke improved sitting balance in the short term in comparison with a nonspecific control approach. Whether early targeting of sitting balance for treatment could improve mobility over the long term is an interesting question that remains to be explored.
Disordered motor control of the limbs after stroke is usually caused by the positive and negative effects of the upper motor neuron syndrome, in which the central abnormality is usually muscle weakness and co-contraction resulting from a failure of coordinated high-frequency motor neuron firing during movement, rather than high tone resulting from spasticity and contracture found at rest,89 which is seen clinically in only 20% to 30% of hemiparetic patients 3 to 12 months after stroke.90,91 Spasticity at rest, when it is a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks, is of diagnostic use but does not contribute significantly to impairment during active voluntary movement and function in either the leg or the arm92,93 and is only one factor contributing to increased muscle tone in the upper motor neuron syndrome, in which co-contraction and biomechanical changes contribute significantly to the resistance to passive movement.94 It has thus been easier to show that pharmacological treatments that reduce the hypertonus of spasticity and co-contraction benefit passive rather than active functional activities, including hand and perineal hygiene, dressing, pain, and limb position,95–100 and even correction of equinovarus at the ankle to improve weight bearing by the affected leg is unlikely to translate into functional improvements in gait101,102 unless it is followed by physical measures to reduce soft tissue shortening and a program of task-related training.103,104
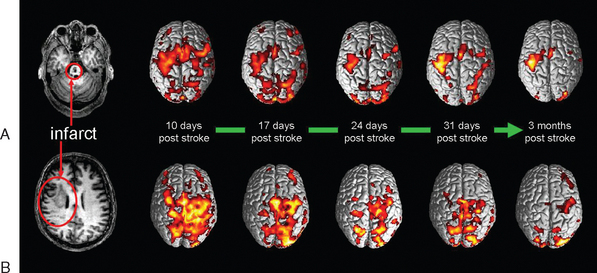
Thus, although treatments for spasticity after stroke achieve passive functional goals by preventing contracture or reduce pain and are of particular value in dependent patients unable to participate in skill learning, the results of treatments for spasticity in general and after stroke have emphasized the fundamental truth of Landau’s statement in 1974105 that “If the major disability of the upper motor neuron syndrome is due to diminished neural input to segmental control of the final common path (negative symptoms), it follows that only some method of provoking [central nervous system] neuronal regeneration or of improving the potency of and control by surviving upper level neurons could provide a direct remedy.” Landau’s next sentence was “No such approach is on the horizon,” and at that time the focus in rehabilitation was on training in compensatory strategies and social and environmental modification. Since the mid-1990s, however, there have been investigations of neurobiological rehabilitation strategies designed to modify the neural processes and behaviors that occur during recovery (Fig. 48-3) (Dobkin, 2004).106
Meta-analyses of physiotherapy after stroke have revealed significant improvements in independence in activities of daily living and reduction in impairments with higher intensities of physiotherapy.107–109 Traditional physiotherapeutic strategies emphasize cutaneous and proprioceptive stimulation, reduction of tone, and therapist-induced central facilitation of normal movement, rather than voluntary activation of proximal or distal musculature to relearn functional tasks. This type of task-related motor relearning was first shown to be effective in improving the ability to balance during seated reaching activities late after stroke.110 Although this approach has not yet been consistently shown to improve functional outcomes and resource use early after stroke,111,112 its principles of repetition, task orientation, attention, and reward are similar to those that result in motor learning and cortical neural reorganization in nonhuman primates with and without ischemic cortical damage.113 It is thus a “neurobiological” approach to skill relearning in which repetition of graded and motivating task-related learning programs is envisaged to drive the acquisition of motor, linguistic, and cognitive skills and more complex behaviors through activity-dependent neural reorganization, regrowth, and replacement.114
Although these techniques can be shown to produce neuroplastic effects—in humans, usually by functional imaging techniques—these changes should not be confused with the potential clinical usefulness of these techniques, which requires a robust behavioral effect if measurable functional benefit is to occur. The effects of task-related training are seen most obviously in the function targeted for treatment; change has been demonstrated after stroke in upper and lower limb function and linguistic skills. Factors that optimize skill learning, including implicit rather than explicit feedback115 and a random rather than blocked learning schedule,116 are likely to affect the rate at which a task is learned and the extent of carryover into novel environments. In the arm, training is focused on practicing graded motivating functional tasks, including grasping, reaching, leaning, and manipulating clothing by the affected limb; in the trunk and leg, training is focused on the recovery of bed mobility, sitting and standing balance, transfers, gait stability and velocity, and stair climbing. Kwakkel and colleagues117 showed that leg training of this sort for an average of 30 minutes of therapy 5 days a week for 20 weeks after stroke onset, in contrast to immobilization of the limb in an inflatable cuff for a similar time, increased performance in activities of daily living (measured by the Barthel Index), walking ability, and also hand dexterity, whereas arm training increased arm dexterity alone.
What determines when, in whom, and how task-oriented training should be carried out after stroke, in an individual as well as in a population, remains open to investigation. The duration in training can be increased: for example, to an impractical 6 hours a day for 10 weekdays late after stroke. Training can be performed solely with a therapist or also automated with a robotic,118,119 most commonly as a treadmill with or without body weight support for gait training120 or in a virtual environment,121 or it can be encouraged, probably at both cortical122 and functional levels, and also in animals,123 by constraint of the unaffected upper limb.124–126 It can also be combined with a variety of impairment-based adjunctive therapies, including muscle-strengthening exercises,127 an orthotic,128 electrical stimulation of the arm or leg,129–131 sensory stimulation,132 mental imagery,133 and musical feedback.134
The available evidence for the effectiveness of these and other physical therapies was systematically reviewed in 2004 by van Peppen and colleagues.135 Which therapy modalities should be used, to what purpose, how they should be combined, at what stage after stroke, at what intensity and for how long, and in which patients are questions that are beginning to be answered clinically with an evidence base, rather than at best theory and at worst belief. The need to be able to do this is emphasized particularly by the evidence beginning to show long-term persistence of task-related training effects136,137 and the useful effects of interventions late after stroke (e.g., Werner and Kessler138 and van der Lee et al139). It is accompanied by a need to explore how the new technologies, which may not always yield benefit (e.g., Richards et al140), can best be put best to work.
Similar principles are beginning to drive trials of rehabilitation therapies of the focal, rather than global,141 cognitive disorders observed after stroke. According to results of clinical measures, language impairments affect about 40% of the stroke population at stroke onset142 and about 10% at 6 months after stroke.143 Minimal spontaneous improvement in language functions occur more than 1 year after stroke. An early meta-analysis144 and review145 of trials of speech and language therapies concluded that there was evidence of effectiveness, but restriction of the evidence to randomized controlled trials, in comparison with no therapy, informal support, or another speech and language therapy, failed to yield clear evidence of effectiveness.146 However, Bhogal and colleagues147 found that increased intensity of therapy was associated with improvement, which occurred in four studies that provided an average of 9 hours of therapy per week for 11 weeks, in contrast to four studies with negative results that provided approximately 2 hours per week for 23 weeks. Although the type of therapy required remains unclear (e.g., Doesborgh et al148), Pulvermüller and colleagues149 suggested that the principles of (a) massed practice—for example, 30 to 35 hours of therapy over 10 days rather than the same therapy time over a longer period—and of (b) constraint (in this case of maladaptive methods of communication), and of (c) everyday behavioral relevance are important factors in generating neuroplastic and thus functional changes in language skills.
The presence of other cognitive problems in the acute phase are important independent predictors of long-term outcome, in addition to neurological deficits rated by standard measures, particularly unilateral neglect150,151 and executive dysfunction.152 Neglect, as currently measured, is seen in more than 60% of patients assessed within 2 to 3 days of admission for stroke, more commonly after right than left hemisphere stroke.153 Of patients with neglect 2 to 4 weeks after stroke, 80% still exhibit aspects of neglect on testing at 6 months,154 and the poor functional outcome predicted by the presence of early neglect is still seen if neglect has resolved on testing,151 possibly as a result of residual problems with nonlateralized attention.155 Neglect, like dysphasia, is heterogeneous; different combinations of lateralized and nonlateralized attentional and motor deficits are identifiable in each patient. A number of techniques have been shown to improve lateralized spatial representation, including caloric stimulation156,157; contralesional neck muscle vibration, in one trial with scanning training158; prism adaptation159; and contralesional limb activation,160 as well as alerting techniques to increase nonlateralized loss of attentional capacity.161 Some of these techniques have also been reported to improve activities of daily living, notably neck muscle vibration with scanning training158 and prism adaptation,162 and contralesional limb activation in one study reduced length of stay by 24 days.150 Whether a treatment battery tailored to the neglect subtypes present in each patient163,164 will prove sufficiently practical and robust to produce significant functional benefits, not yet evident in the literature on systematic review,165 remains to be explored.
In the context of stroke, use-dependent neural reorganization after damage is facilitated by other extrinsic and intrinsic inputs that modulate neural excitability and augment long-term synaptic potentiation. Use-dependent network remodeling at cellular and systems levels is also likely to be needed for the successful application of techniques to promote neural regrowth and/or replacement.166,167 Adjunctive facilitators of this process include manipulation of attention168; increase or decrease of somatosensory input, shown to improve swallowing169 and leg170 and arm122 function; neuromodulatory drugs171; electrical172 and magnetic173 stimulation of the brain; and possibly exercise.174
Pharmacological facilitation and inhibition of neurological recovery after stroke are also likely to be of clinical relevance. The use-dependent plasticity that underpins motor training and perceptual learning is dependent on N-methyl-D-aspartate receptor activation and γ-amino butyric acid–mediated (GABAergic) inhibition,175,176 and these are in turn modulated by cholinergic, noradrenergic, serotonergic, and dopaminergic transmitter systems.171 Early experiments by Feeney and coworkers177 in rats showed that motor recovery after suction ablation of the sensorimotor cortex was improved by amphetamine before practice on a beam-walking task; neither practice nor amphetamine alone had any benefit. Subsequently, α2 antagonists, increasing noradrenergic effects, were found to facilitate motor recovery, and α1 antagonists and α2 agonists, which decrease norepinephrine release or block its postsynaptic effects, reinstate deficit in clinically recovered animals or slow their recovery.178,179 There is also evidence that modulation of other neurotransmitter systems has similar effects.180 The beneficial effect of these pharmacological interventions is dose dependent, and the timing of administration is likely to be important, the effect being dependent on close temporal linkage to behavioral experience. In animals, there is a strong correlation between the functional effects of altering levels of various neurotransmitters and their ability to facilitate increases in synaptic efficacy through long term potentiation.180
Attempts have been made to translate these findings into treatments for stroke patients. In the first clinical trial of noradrenergic enhancement coupled with physical therapy, patients who had suffered from hemiplegic stroke were randomly assigned to receive either 10 mg of dextroamphetamine or placebo 45 minutes before physiotherapy.181 Follow-up assessment 24 hours after treatment revealed a 40% improvement from baseline scores with dextroamphetamine in comparison with placebo. Small numbers of patients in this study made interpretation difficult. A subsequent failure to replicate these findings by Borucki and associates182 was attributed to a different experimental design, particularly a failure to schedule physiotherapy immediately after amphetamine treatment. More recent studies with dextroamphetamine have had conflicting results,183,184 but positive results have been published for daily doses of levodopa (100 mg) used in conjunction with physiotherapy.185 Studies of the effects of drugs used in conjunction with speech therapy have yielded some success.186 Although larger scale studies are under way, the use of pharmacological agents to facilitate the effects of physical and speech therapy is currently only experimental. Conversely, there is some evidence to suggest that patients receiving drugs that reduce the effects of norepinephrine or dopamine, or increase the effects of GABA, at the time of stroke or shortly afterward have poorer outcomes than do patients who do not receive any of these drugs187; thus, care must be taken in their prescription.
Maladaptive or ineffective neural reorganization after stroke may contribute to emotionalism, central poststroke pain (CPSP), and involuntary movement disorders, which include hemiballismus, hemichoreoathetosis, distal resting and/or action tremor, and proximal postural tremor. CPSP, originally described in 1906 as a phenomenon after thalamic stroke by Dejerine and Roussy,188 was found in 16 (8%) of 207 hospitalized stroke patients 6 months after stroke by Andersen and colleagues.48,49 Pain may result from stroke involving spinothalamocortical afferent pathways in the medulla, pons, midbrain, thalamus, subcortical white matter, and cortex. Leijon and colleagues189 found in 27 patients with CPSP that the thalamus was involved in only 9, lesions were in the lower brainstem in 8 and lateral and superior to the thalamus in 6, and onset of the pain was delayed by more than a month in 13 patients. The pain was described as “burning, aching, pricking, and lacerating”; the intensity was increased by external stimuli, either noxious (hyperalgesia) or not noxious (allodynia), the most common being joint movements, cold and light touch, and, in five patients, emotional stimuli. All patients had decreased temperature sensation. Hypersensitivity to cutaneous stimuli was found in 88% of the patients, and sensations of touch and vibration were abnormal in only 52% and 41%, respectively. There were also relatively low incidences of weakness and ataxia, which were present in 48% and 62%, respectively. Treatments in individual patients include all the drugs and physical methods used for neuropathic pain in general; descending modulation of pain pathways by electrical motor cortex stimulation,190 rather than deep brain stimulation,191 and more recently repetitive TMS over the hand area of the motor cortex192 have shown some promise in the treatment of CPSP. Both deep brain stimulation and electrical motor cortex stimulation have been used with some success to treat poststroke movement disorders,191 whereas antidepressants are effective in treating emotionalism.192–194
MANAGEMENT OF SYSTEMIC COMPLICATIONS
In a prospective study of 311 consecutive patients recruited within 7 days of stroke onset, Langhorne and colleagues195 recorded complications in 85% of the patients during hospital admission (see Table 48-3); 6, 18, and 30 months after discharge, there was a high frequency of infections, falls, unexplained blackouts, pain, and symptoms of depression and anxiety. Seizures and chest infections tend to occur soon after admission; other common complications, including venous thromboembolism, skin breakdown, urinary tract infections, falls, and depression, are still occurring at 30 days.196 More than 60% of patients have more than one complication during inpatient rehabilitation, and complications are more common with increasing age, stroke severity, premorbid disability, and low serum albumin level.195–197 The occurrence of a complication is more likely to be associated with death,196 and the causes of death most likely to be prevented by stroke unit care are those resulting from immobility, particularly as a result of infection and venous thromboembolism. More recently, emphasis on acute care after stroke has begun to define potentially avoidable complications that cause disordered physiological homeostasis acutely and may affect mortality rates, morbidity, length of stay, and longer-term outcome31,32,198; these include airway patency and disordered breathing (lowering blood oxygenation), cardiac problems, blood pressure control, temperature regulation, and glycemic control.30 The prevention and early treatment of the acute, early, and later systemic complications of stroke are thus an important aspect of organized multidisciplinary care and rehabilitation after stroke. They require at least easy access to acute care and diagnostic facilities,196,197 if not high-dependency care in the acute phase for some patients, with continuous monitoring of cardiac, respiratory, metabolic, and neurological functions during the first 72 hours after stroke.
Venous thromboembolism was once common after stroke; symptomatic and asymptomatic deep vein thrombosis and pulmonary embolism were present in up to 70% of, respectively, all patients and those studied post mortem.200 In contrast, nearly 20 years later, Kelly and associates200,201 found that in 102 unselected patients receiving stroke unit care, aspirin, and compressive therapy with stockings, symptomatic deep vein thrombosis and pulmonary embolism occurred in only 3% and 5% of patients, respectively; however, noninvasive magnetic resonance direct thrombus imaging revealed the prevalence of venous thromboembolism, deep vein thrombosis, and pulmonary embolism to be high (40%, 18%, and 12%, respectively, and increasing to 63%, 30%, and 20%) in nonambulatory patients with a Barthel Index score of 9 two days after stroke. In the context of early mobilization and stroke unit care, either aspirin or low-dose unfractionated heparin has been shown to reduce the frequency of symptomatic pulmonary embolism to below 1%.203,204 Because there is no evidence that routine low-dose subcutaneous low-molecular-weight heparin is superior to aspirin in preventing pulmonary embolism or deep vein thrombosis,205 current guidelines for routine venous thromboprophylaxis (e.g., Royal College of Physicians12) recommend aspirin, early mobilization, and, for patients with leg weakness, graduated compression stockings, which prevented deep vein thrombosis in all hospitalized patients in one study205 but for which there is insufficient evidence at present to show that this is the case after stroke.206 Factors thought to confer higher risk, such as previous deep vein thrombosis or pulmonary embolism, necessitate individualized treatment decisions, and management in other high-risk groups, such as those initially nonambulatory after stroke, probably requires further definition (e.g., Kelly et al201,202).
The detraining effects of immobility and reduced activity208 compound the increased difficulty in completing activities of daily living that results from comorbid musculoskeletal and cardiorespiratory dysfunction and from the increased aerobic requirements of walking that are secondary to neurological impairments. Even in normal people, bed rest for 4 to 6 weeks results in a loss in exercise capacity of 0.9% per day209 and a decrease in muscle strength of up to 40%.210 After stroke, exercise capacity is reduced to about 60% of normal at 1 month,211 making the performance of activities of daily living effortful and fatiguing. Although activities of daily living exert a training effect, so that exercise capacity improves, it remains reduced at 6 months212 and probably in the long term. Activity intolerance is thus common among stroke survivors, who may work close to their individual maximal exercise capacity while engaged only in domestic chores, in comparison with age- and weight-matched controls.213 Either aerobic training214 or muscle strengthening215,216 or both217 usually improve targeted physiological outcomes and sometimes balance, walking speed, and distance, but not consistently activities of daily living136,218; this finding is sufficient to contribute to recommendations for exercise after stroke from the American Heart Association.219
Detraining is likely to be one of the factors contributing to poststroke fatigue, which occurs in 40% to 70% of patients late after stroke in community studies,220–222 and patient-derived stroke-specific, health-related quality-of-life measures identify lack of energy as one of the four most consequential problems, together with those related to physical, psychosocial, and communication difficulties.223,224 Despite its relevance to quality of life, the natural history, causes, and management of poststroke fatigue remain underinvestigated. It often occurs independently of prestroke fatigue and depression and poststroke depression220,221,225 as “primary” poststroke fatigue, possibly resulting from stroke-disordered attention,226 or in association with physical impairment,221 psychological factors, sleep-disordered breathing and obstructive sleep apnea,227 or incidental systemic comorbid conditions. Management clearly starts with accurate differential diagnosis, but there are no guidelines to direct management of primary poststroke fatigue or evidence that modafinil, which may228 or may not229 be of benefit in multiple sclerosis, is of benefit after stroke.
During hospital admission after stroke, patients suffering from undernutrition have a greater risk of pneumonia, other infections, and gastrointestinal bleeding than that in other patients.230 A higher risk of chest infection and poor nutritional status within the first month after stroke is predicted by abnormal swallowing, which is also associated with increased disability, length of hospital stay, and institutional care and is an independent predictor of mortality during the first 6 months after stroke61,62; rates of death from pneumonia over the longer term are also higher if dysphagia persists.231 Although the prevention of chest infections remains problematic, the prevention of undernutrition in hospital has been clarified. Routine oral supplementation versus no supplementation in admitted stroke patients could not be shown to reduce death or poor outcome when the rate of malnutrition on admission was only 8%232; whether it would with the higher rates of malnutrition on admission of about 40% reported before 1995233 has not been investigated. Nasogastric tube feeding within a week of stroke reduces the mortality rate,66 probably by preventing malnutrition, as it does in other groups of vulnerable hospitalized patients,234 rather than by reducing the incidence of chest infections, which nonetheless occurred, for example, in 44% of nasogastrically fed patients reported by Dziewas and colleagues.235
Pain after stroke is most often musculoskeletal in origin, rather than CPSP, and the result of an exacerbation of preexisting osteoarthritis and/or reduced movement and mobility caused by the stroke. Shoulder pain occurs in more than 60% of patients during the 6 months after stroke and its incidence may increase after discharge.236 Its occurrence is correlated with prestroke shoulder pain, severe upper limb weakness, neglect, sensory loss, visual field defects, and glenohumeral subluxation, but their role in its pathogenesis remains uncertain, and shoulder subluxation is not clearly a cause. Vasomotor changes in the limb, shoulder-hand syndrome, and reflex sympathetic dystrophy may also be present but should not be confused with the painful shoulder, and other disorders of the shoulder including fractures should be ruled out. Shoulder pain causes considerable morbidity and, once established, is difficult to treat. Prevention, particularly in patients at high risk, should include support of the flaccid arm237 and appropriate handling techniques to avoid traction injury. Treatment is likely to be determined partly by the tone in shoulder muscles238: A flaccid shoulder requires support at all times and possibly benefits from functional electrical stimulation (e.g., Renzenbrink and IJzerman239), whereas a spastic shoulder requires maintenance of range of motion by physical techniques and possibly the addition of botulinum toxin. Local steroid injection should be avoided unless there is clear evidence of an inflammatory component to the pain.
There is a high cumulative incidence of psychiatric disorder after stroke. It can be difficult to diagnose in the presence of speech disturbance, anosognosia, and abulia, and it includes major and minor depression, agoraphobia, social withdrawal, apathy and self-neglect, irritability, and pathological emotionalism. However, in 128 community-dwelling patients during the first year after stroke, House and colleagues240 found that little of it persisted, and only two cases of major depression manifested during the whole 12 months. This frequency is lower than that found in other studies: for example, about 25% in a rehabilitation unit within 3 months after stroke241 and 15% in community-dwelling patients 4 months after stroke in the study by Burvill and colleagues242 in Perth, Australia. There remains a suggestion that left-sided anterior strokes affecting frontal-subcortical circuits predispose to depression,243,244 but the majority of studies emphasize that the major risk factors for depression are nonspecific, of the sort seen in older people with other physical illness,245 and they include a history of previous depression, stroke severity, physical dependency, and social inactivity.48,49,246 Over the longer term, any association between stroke and depressive symptoms is attributable largely to physical limitations.247 Although depression soon after stroke probably reduces later functional outcome248 and its presence 1 year later is clearly related to a reduced quality of life,6 two systematic reviews revealed that the evidence of benefit from early prevention or diagnosis and treatment was minimal.249,250
FUTURE DEVELOPMENTS
Because of the evidence for the effectiveness of comprehensive acute and rehabilitation stroke unit care and subsequent supported discharge and intervention in the community, and the national recommendations and guidelines that this evidence makes possible, these service delivery systems of care and rehabilitation can be available to a greater proportion of patients admitted after stroke, particularly when linked to an explicit multisite audit of the organization and process of stroke care of the sort undertaken at intervals by the Royal College of Physicians of London.251 Linkage of these stroke care systems into networks will be gradually developed to facilitate multicenter clinical research, hasten enlargement of the interventional evidence base,252 and promote the delivery of coordinated rather than fragmented systems of care.18 Parallel networks aiming to facilitate the transfer of proof-of-principle studies in the translational restorative neurosciences, while the pharmaceutical industry continues to focus on new methods of neural protection, are likely to follow.
It is now established that the characteristic features of comprehensive stroke unit care include a standardized protocol for acute assessment, intermittent monitoring, medical treatments, and a strategy for the prevention of complications through the work of a multidisciplinary team integrating medical care, nursing, and rehabilitation. Clinical research networks will enable exploration of the effectiveness of further aspects of plant and process that contribute to outcome, including the usefulness of acute continuous physiological monitoring32; other strategies to detect and prevent complications, such as very early mobilization253; and further definition of the aspects of acute and early goal-focused interdisciplinary rehabilitation that optimize functional outcome.17
Since the mid-1990s, the neural reorganization that takes place longitudinally in response to damage and its relation to functional recovery have become better understood.254 This understanding has, for example, provided a biological rationale for the efficacy of task-related training and the need for intensive therapy, and it has led to investigations of how and whether other drivers of neural reorganization can increase function. Although these techniques may reduce impairments in the laboratory and at the proof-of-principle level, the generation of robust functional gains remains elusive, and their introduction and relevance to the clinical domain remain unclear.255 Exploration of how restorative techniques can be applied clinically to generate functionally relevant neural reorganization, regrowth, and repair through novel technologies and drugs, alone or in combination, and in the context of task-related training is a journey of universal interest.
Dobkin BH. Neurobiology of rehabilitation. Ann N Y Acad Sci. 2004;1038:148-170.
Greenwood R, Barnes MP, McMillan TM, et al, editors. Handbook of Neurological Rehabilitation. London: Psychology Press, 2003.
McCrum R. My Year Off; Rediscovering Life after a Stroke. London: Picador, 1998.
Warlow C, Dennis MS, van Gijn J, et al, editors. Stroke. A Practical Guide to Management. London: Blackwell, 2003.
World Health Organization. The International Classification of Functioning, Disability and Health—ICF. Geneva: World Health Organization; 2002. http://www.who.int/classification/icf.
1 Stewart JA, Dundas R, Howard RS, et al. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ. 1999;318:967-971.
2 Feigin VL, Lawes CMM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th Century. Lancet Neurol. 2003;2:43-53.
3 Martin J, Meltzer H, Elliot D. OPCS surveys of disability in Great Britain. Report 1. In: The Prevalence of Disability among Adults. London: HMSO, Office of Population Censuses and Surveys; 1988.
4 Wolfe CDA. The impact of stroke. Br Med Bull. 2000;56:275-286.
5 Thorngren M, Westling B, Norrving B. Outcome after stroke in patients discharged to independent living. Stroke. 1990;21:236-240.
6 Kauhanen ML, Korpelainen JT, Hiltunen P, et al. Domains and determinants of quality of life after stroke caused by brain infarction. Arch Phys Med Rehabil. 2000;81:1541-1546.
7 Patel A, Duncan P, Lai S, et al. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. 2000;81:1357-1363.
8 Mayo NE, Wood-Dauphinee S, Côté R, et al. Activity, participation and quality of life 6 months post stroke. Arch Phys Med Rehabil. 2002;83:1035-1042.
9 Dewey HM, Thrift AG, Mihalopoulos C, et al. Lifetime cost of stroke subtypes in Australia: findings from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2003;34:2502-2507.
10 Wardlaw JM, del Zoppo G, Yamaguchi T, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. (3):2003. CD000213
11 Fuhrer MJ. Subjective well-being: implications for medical rehabilitation outcomes and models of disablement. Am J Phys Med Rehabil. 1994;73:358-364.
12 Royal College of Physicians. National Clinical Guidelines for Stroke, 2nd ed. Available at: http://www.rcplondon.ac.uk/pubs/books/stroke/stroke_guidelines_2ed.pdf. (accessed January 30, 2006).
13 Scottish Intercollegiate Guidelines Network (SIGN). Management of Patients with Stroke. Available at: www.sign.ac.uk/guidelines/published/index.html. (accessed January 30, 2006).
14 National Stroke Foundation (Australia). National Clinical Guidelines for acute stroke management. http://www.strokefoundation.com.au, 2003. (accessed January 30, 2006).
15 Department of Veterans Affairs and Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Stroke Rehabilitation. Washington, DC: Department of Veterans Affairs and Department of Defense, 2003.
16 Duncan PW, Horner RD, Reker DM, et al. Adherence to postacute guidelines is associated with functional recovery in stroke. Stroke. 2002;33:167-178.
17 Meijer R, Ihnenfeldt D, Vermeulen M, et al. The use of a modified Delphi procedure for the determination of 26 prognostic factors in the subacute stage of stroke. Int J Rehabil Res. 2003;26:265-270.
18 Schwamm LH, Pancioli A, Acker JEIII, et al. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association’s Task Force on the Development of Stroke Systems. Stroke. 2005;36:690-703.
19 Shah E, Harwood R. Acute Management: Admission to Hospital in Stroke: Epidemiology, Evidence and Clinical Practice, 2nd ed. Oxford, UK: Oxford University Press, 1999.
20 Aboderin I, Venables G. Stroke management in Europe. Pan European consensus meeting on stroke management. J Intern Med. 1996;240:173-180.
21 Kalra L, Evans A, Perez I, et al. Alternative strategies for stroke care: a prospective randomised controlled trial. Lancet. 2000;356:894-899.
22 Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. (3):2001. CD000197
23 Langhorne P, Duncan P. Does the organization of postacute stroke care really matter? Stroke. 2001;32:268-274.
24 Stroke Unit Trialists’ Collaboration. How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke. 1997;28:2139-2144.
25 Langhorne P, Pollock A. What are the components of effective stroke unit care? Age Ageing. 2002;31:365-371.
26 Evans A, Perez I, Harraf F, et al. Can differences in management processes explain different outcomes between stroke unit and stroke-team care? Lancet. 2001;358:1586-1592.
27 Wood-Dauphinee S, Shapiro S, Bass E, et al. A randomized trial of team care following stroke. Stroke. 1984;15:864-872.
28 van der Walt A, Gilligan AK, Cadilhac DA, et al. Quality of stroke care within a hospital: effects of a mobile stroke service. Med J Aust. 2005;182:160-163.
29 Brown DL, Haley LH. Post-emergency department management of stroke. Emerg Med Clin North Am. 2002;20:687-702.
30 Adams HP, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke. Stroke. 2003;34:1056.
31 Sulter G, Elting JW, Langedijk M, et al. Admitting acute ischemic stroke patients to a stroke care monitoring unit versus a conventional stroke unit: a randomized pilot study. Stroke. 2003;34:101-104.
32 Silva Y, Puigdemont M, Castellanos M, et al. Semi-intensive monitoring in acute stroke and long-term outcome. Cerebrovasc Dis. 2005;19:23-30.
33 Indredavik B, Bakke F, Slordahl SA, et al. Stroke unit treatment. 10-year follow-up. Stroke. 1999;30:1524-1527.
34 Lincoln NB, Husbands S, Trescoli C, et al. Five year follow up of a randomised controlled trial of a stroke rehabilitation unit. BMJ. 2000;320:549.
35 Langhorne P, Taylor G, Murray G, et al. Early supported discharge services for stroke patients: a meta-analysis of individual patients’ data. Lancet. 2005;365:501-506.
36 Fjaertoft H, Indredavik B, Johnsen R, et al. Acute stroke unit care combined with early supported discharge. Long-term effects on quality of life. A randomized controlled trial. Clin Rehabil. 2004;18:580-586.
37 Thorsén AM, Holmqvist LW, de-Pedro CJ, et al. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke: five-year follow-up of patient outcome. Stroke. 2005;36:297-303.
38 Hopman WM, Verner J. Quality of life during and after inpatient stroke rehabilitation. Stroke. 2003;34:801-805.
39 Kramer AM, Steiner JF, Schlenker RE, et al. Outcomes and costs after hip fracture and stroke: a comparison of rehabilitation settings. JAMA. 1997;277:396-404.
40 Outpatient Therapy Trialists. Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. Lancet. 2004;363:352-356.
41 Walker MF, Leonardi BJ, Bath P, et al. Individual patient data meta-analysis of randomized controlled trials of community occupational therapy for stroke patients. Stroke. 2004;35:2226-2232.
42 Steultjens EMJ, Dekker J, Bouter LM, et al. Occupational therapy for stroke patients: a systematic review. Stroke. 2003;34:676-687.
43 Anderson C, Mhurcha CN, Rubenach S, et al. Home or hospital for stroke rehabilitation? Results of a randomised controlled trial. 1: health outcomes at 6 months. Stroke. 2000;31:1024-1031.
44 Young JB, Forster A. The Bradford community stroke trial: results at six months. BMJ. 1992;304:1085-1089.
45 Young J, Forster A. Day hospital and home physiotherapy for stroke patients: a comparative cost-effectiveness study. J R Coll Physicians Lond. 1993;27:252-258.
46 Green J, Young J, Forster A, et al. Combined analysis of two randomized trials of community physiotherapy for patients more than one year post stroke. Clin Rehabil. 2004;18:249-252.
47 Hancock R, Jarvis C. The Long Term Effects of Being a Carer. London: HMSO, 1994.
48 Andersen G, Vestergaard K, Ingemann-Nielsen M, et al. Risk factors for poststroke depression. Acta Psychiatr Scand. 1995;92:193-198.
49 Morris PL, Robinson RG, Raphael B, et al. The relationship between the perception of social support in poststroke depression in hospitalised patients. Psychiatry. 1991;54:306-316.
50 Christie D, Weigall D. Social work effectiveness in two-year stroke survivors: a randomised controlled trial. Community Health Stud. 1984;8:26-32.
51 Friedland JF, McColl M. Social support interventions after stroke: results of a randomised trial. Arch Phys Med Rehabil. 1992;73:573-581.
52 Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ. 1996;312:1642-1646.
53 Dennis M, O’Rourke S, Slattery J, et al. Evaluation of a stroke family careworker: results of a randomised controlled trial. BMJ. 1997;314:1071-1076.
54 Mant J, Carter J, Wade DT, et al. Family support for stroke: a randomised controlled trial. Lancet. 2000;356:808-813.
55 Lincoln NB, Francis VM, Lilley SA, et al. Evaluation of a stroke family support organiser. A randomised controlled trial. Stroke. 2003;34:116-121.
56 Boter H, for the HESTIA Study Group. Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke. 2004;35:2867-2872.
57 Glass TA, Berkman LF, Hiltunen EF, et al. The Families In Recovery From Stroke Trial (FIRST): primary study results. Psychosom Med. 2004;66:889-897.
58 Grant JS, Elliott TR, Weaver M, et al. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke. 2002;33:2000-2065.
59 Kalra L, Evans A, Perez I, et al. Training carers of stroke patients: randomised controlled trial. BMJ. 2004;328:1099-1101.
60 Patel A, Knapp M, Evans A, et al. Training care givers of stroke patients: economic evaluation. BMJ. 2004;328:1102-1104.
61 Smithard D, O’Neill P, Park C, et al. Complications and outcome after acute stroke: does dysphagia matter? Stroke. 1996;27:1200-1204.
62 Mann G, Hankey GJ, Cameron D. Swallowing function after stroke. Prognosis and prognostic factors at 6 months. Stroke. 1999;30:744-748.
63 Splaingard ML, Hutchins B, Sulton LD, et al. Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69:637-640.
64 Leder SB, Espinosa JF. Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17:214-218.
65 Odderson IR, Keaton JC, McKenna BS. Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76:1130-1133.
66 Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:764-772.
67 Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(Suppl 7):vii1-vii12.
68 Brittain KR, Peet SM, Castleden CM. Stroke and Incontinence. Stroke. 1998;29:524-528.
69 Nakayama H, Jørgensen HS, Pedersen PM, et al. Prevalence and risk factors of incontinence after stroke. The Copenhagen stroke study. Stroke. 1997;28:58-62.
70 Jørgensen L, Engstad T, Jacobsen BK. Self-reported urinary incontinence in noninstitutionalized long-term stroke survivors: A population-based study. Arch Phys Med Rehabil. 2005;86:416-420.
71 Gelber DA, Good DC, Laven LJ, et al. Causes of urinary incontinence after acute hemisphere stroke. Stroke. 1993;24:378-382.
72 Barrett JA. Bladder and bowel problems after a stroke. Rev Clin Gerontol. 2001;12:253-267.
73 Barer DH. Continence after stroke: useful predictor or goal of therapy? Age Ageing. 1989;18:183-191.
74 Patel M, Coshal C, Lawrence E, et al. Recovery from poststroke urinary incontinence: associated factors and impact on outcome. J Am Geriatr Soc. 2001;49:1229-1233.
75 Burney TL, Senepati M, Desai S, et al. Acute cerebrovascular accident and lower urinary tract dysfunction: a prospective correlation of the site of brain injury with urodynamic findings. J Urol. 1996;156:1748-1750.
76 Marinkovic S, Badlani G. Voiding and sexual dysfunction after cerebrovascular accidents. J Urol. 2001;165:359-370.
77 Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. (2):2000. CD002113
78 Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. (1):2004. CD002802
79 Middaugh SJ, Whitehead WE, Burgio KL, et al. Biofeedback in treatment of urinary incontinence in stroke patients. Biofeedback Self Regul. 1989;14:3-19.
80 Tibaek S, Jensen R, Lindskov G, et al. Can quality of life be improved by pelvic floor muscle training in women with urinary incontinence after ischemic stroke? A randomised, controlled and blinded study. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:117-123.
81 Cooper ZR, Rose S. Fecal incontinence: a clinical approach. Mount Sinai J Med. 2000;67:96-105.
82 Harari D, Coshall C, Rudd AG, et al. New-onset fecal incontinence after stroke: prevalence, natural history, risk factors, and impact. Stroke. 2003;34:144-150.
83 Harari D, Norton C, Lockwood L, et al. Treatment of constipation and fecal incontinence in stroke patients: randomized controlled trial. Stroke. 2004;35:2549-2555.
84 Franchignoni FP, Tesio L, Ricupero C, et al. Trunk control test (TCT) as an early predictor of stroke rehabilitation outcome. Stroke. 1997;28:1382-1385.
85 Hsieh CL, Sheu CF, Hsueh IP, et al. Trunk control as an early predictor of comprehensive activities of daily living function in stroke patients. Stroke. 2002;33:2626-2630.
86 Sandin KJ, Smith BS. The measure of balance in sitting in stroke rehabilitation prognosis. Stroke. 1990;21:82-86.
87 Feigin L, Sharon B, Czaczkes B, et al. Sitting equilibrium 2 weeks after a stroke can predict the walking ability after 6 months. Gerontology. 1996;42:348-353.
88 Mudie MH, Winzeler-Mercay U, Radwan S, et al. Training symmetry of weight distribution after stroke: a randomized controlled pilot study comparing task-related reach, Bobath and feedback training approaches. Clin Rehabil. 2002;16:582-592.
89 Dietz V. Spastic movement disorder: what is the impact of research on clinical practice? J Neurol Neurosurg Psychiatry. 2003;74:820-821.
90 O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119:1737-1749.
91 Sommerfeld DK, Eek EUB, Svensson AK, et al. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134-139.
92 Ada L, Vattanasilp W, O’Dwyer NJ, et al. Does spasticity contribute to walking dysfunction after stroke? J Neurol Neurosurg Psychiatry. 1998;64:628-663.
93 Burne JA, Carleton VL, O’Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psychiatry. 2005;76:47-54.
94 Sheean GL. Pathophysiology of spasticity. In: Sheean GL, editor. Spasticity Rehabilitation. London: Churchill Communications Europe; 1998:17-38.
95 Bhakta BB, Cozens JA, Chamberlain MA, et al. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial. J Neurol Neurosurg Psychiatry. 2000;69:217-221.
96 Meythaler JM, Guin-Renfroe S, Brunner RC, et al. Intrathecal baclofen for spastic hypertonia from stroke. Stroke. 2001;32:2099-2109.
97 Brashear A, Gordon MF, Elovic E, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347:395-400.
98 Fukuhara T, Kamata I. Selective posterior rhizotomy for painful spasticity in the lower limbs of hemiplegic patients after stroke: report of two cases. Neurosurgery. 2004;54:1268-1273.
99 Francis HP, Wade DT, Turner-Stokes L, et al. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J Neurol Neurosurg Psychiatry. 2004;75:1547-1551.
100 Gordon MF, Brashear A, Elovic E, et al. Repeated dosing of botulinum toxin type A for upper limb spasticity following stroke. Neurology. 2004;63:1971-1973.
101 Hesse S, Lucke D, Malezic M, et al. Botulinum toxin treatment for lower limb extensor spasticity in chronic hemiparetic patients. J Neurol Neurosurg Psychiatry. 1994;57:1321-1324.
102 Hesse S, Krajnik J, Luecke D, et al. Ankle muscle activity before and after botulinum toxin therapy for lower limb extensor spasticity in chronic hemiparetic patients. Stroke. 1996;27:455-460.
103 Richardson D, Sheean G, Werring D, et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychiatry. 2000;69:499-506.
104 Thompson AJ, Jarrett L, Lockley L, et al. Clinical management of spasticity. J Neurol Neurosurg Psychiatry. 2005;76:459-463.
105 Landau WM. Spasticity: the fable of a neurological demon and the emperor’s new therapy [Editorial]. Arch Neurol. 1974;31:217-219.
106 Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528-536.
107 Langhorne P, Wagenaar RC, Partridge C. Physiotherapy after stroke: more is better? Physiother Res Int. 1996;1:75-88.
108 Kwakkel G, Wagenaar RC, Koelman TW, et al. Effects of intensity of rehabilitation after stroke. Stroke. 1997;28:1550-1556.
109 Kwakkel G, van Peppen R, Wagenaar RC, et al. Effects of augmented exercise therapy time after stroke. A meta-analysis. Stroke. 2004;35:2529-2539.
110 Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke. A randomized controlled trial. Stroke. 1997;28:722-728.
111 Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled study. Clin Rehabil. 2000;14:361-369.
112 van Vliet PM, Lincoln NB, Foxall A. Comparison of Bobath based and movement science based treatment for stroke: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2005;76:503-508.
113 Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791-1794.
114 Kempermann G, van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res. 2000;127:35-48.
115 Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learning Memory. 2004;11:388-396.
116 Hanlon RE. Motor learning following unilateral stroke. Arch Phys Med Rehabil. 1996;77:811-815.
117 Kwakkel G, Wagenaar RC, Twisk JWR, et al. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354:191-196.
118 Hesse S, Schmidt H, Werner C, et al. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol. 2003;16:705-710.
119 Lum PS, Taub E, Schwandt D, et al. Automated constraint-induced therapy extension (AutoCITE) for movement deficits after stroke. J Rehabil Res Dev. 2004;41:249-258.
120 Moseley AM, Stark A, Cameron ID, et al. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. (3):2003. CD002840
121 Deutsch JE, Merians AS, Adamovich S, et al. Development and application of virtual reality technology to improve hand use and gait of individuals poststroke. Restor Neurol Neurosci. 2004;22:371-386.
122 Floel A, Nagorsen U, Werhahn KJ, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206-212.
123 DeBow SB, Davies MLA, Clarke HL, et al. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021-1026.
124 Miltner WH, Bauder H, Sommer M, et al. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586-592.
125 Uswatte G, Taub E. Constraint-induced movement therapy: new approaches to outcome measurement in rehabilitation. In: Stuss DT, Winocur G, Robertson IH, editors. Cognitive Neurorehabilitation: A Comprehensive Approach. Cambridge, UK: Cambridge University Press; 1999:215-229.
126 Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19:27-32.
127 Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehab Res Dev. 2004;41:293-312.
128 de Wit DCM, Buurke JH, Nijlant JMM, et al. The effect of an ankle-foot orthosis on walking ability in chronic stroke patients: a randomized controlled trial. Clin Rehabil. 2004;18:550-557.
129 de Kroon JR, van der Lee JH, IJzerman MJ, et al. Therapeutic electrical stimulation to improve motor control and functional abilities of the upper extremity after stroke: a systematic review. Clin Rehabil. 2002;16:350-360.
130 Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in subacute poststroke rehabilitation. J Rehabil Med. 2005;37:32-36.
131 Kottink AIR, Oostendorp LJM, Buurke JH, et al. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004;28:577-586.
132 Peurala SH, Pitkänen K, Sivenius J, et al. Cutaneous electrical stimulation may enhance sensorimotor recovery in chronic stroke. Clin Rehabil. 2002;16:709-716.
133 Liu KP, Chan CC, Lee TM, et al. Mental imagery for promoting relearning for people after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:1403-1408.
134 Schauer M, Mauritz KH. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clin Rehabil. 2003;7:713-722.
135 Van Peppen RPS, Kwakkel G, Wood-Dauphinee S, et al. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18:833-862.
136 Kwakkel G, Kollen BJ, Wagenaar RC. Long term effects of intensity of upper and lower limb training after stroke: a randomised trial. J Neurol Neurosurg Psychiatry. 2002;72:473-479.
137 Feys H, De Weerdt W, Verbeke G, et al. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow-up study of a randomized trial. Stroke. 2004;35:924.
138 Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil. 1996;75:114-120.
139 van der Lee JH, Wagenaar RC, Lankhorst GJ, et al. Forced use of the upper extremity in chronic stroke patients. Results from a single-blind randomized clinical trial. Stroke. 1999;30:2369-2375.
140 Richards CL, Malouin F, Bravo G, et al. The role of technology in task-oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2004;18:199-211.
141 Bowler JV. Dementia after stroke [Editorial]. Stroke. 2004;35:1268-1269.
142 Pedersen PM, Jorgensen HS, Nakayama H, et al. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38:659-666.
143 Wade DT, Hewer RL, David RM, et al. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49:11-16.
144 Robey R. The efficacy of treatment for aphasic persons: a meta-analysis. Brain Lang. 1994;47:582-608.
145 Holland A, Fromm D, DeRuyter F, et al. Treatment efficacy: aphasia. J Speech Hear Res. 1996;39:S27-S36.
146 Greener J, Enderby P, Whurr R. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. (4):1999. CD000425
147 Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke. 2003;34:987-993.
148 Doesborgh SJC, van de Sandt-Koenderman MWE, Dippel DWJ, et al. Effects of semantic treatment on verbal communication and linguistic processing in aphasia after stroke: a randomized controlled trial. Stroke. 2004;35:141-146.
149 Pulvermüller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621-1626.
150 Kalra L, Perez I, Gupta S, et al. The influence of visual neglect on stroke rehabilitation. Stroke. 1997;28:1386-1391.
151 Nys GMS, van Zandvoort MJE, de Kort PLM, et al. The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology. 2005;64:821-827.
152 Pohjasvaara T, Leskelä M, Vataja R, et al. Poststroke depression, executive dysfunction and functional outcome. Eur J Neurol. 2002;9:269-275.
153 Stone SP, Wilson B, Wroot A, et al. The assessment of visuospatial neglect after acute stroke. J Neurol Neurosurg Psychiatry. 1991;54:345-350.
154 Appelros P, Nydevik I, Karlsson GM, et al. Recovery from unilateral neglect after right-hemisphere stroke. Disabil Rehabil. 2004;26:471-477.
155 Mark VW. Acute versus chronic functional aspects of unilateral spatial neglect. Front Biosci. 2003;8:172-189.
156 Rubens AB. Caloric stimulation and unilateral visual neglect. Neurology. 1985;35:1019-1024.
157 Rode G, Perenin MT. Temporary remission of representational hemineglect through vestibular stimulation. Neuroreport. 1994;5:869-872.
158 Schindler I, Kerhoff G, Karnath H-O, et al. Neck muscle vibration induces lasting recovery in spatial neglect. J Neurol Neurosurg Psychiatry. 2002;73:412-419.
159 Frassinetti F, Angeli V, Meneghello F, et al. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain. 2002;125(Pt 3):608-623.
160 Robertson IH, Hogg K, McMillan TM. Rehabilitation of unilateral neglect: improving function by contralesional limb activation. Neuropsychol Rehabil. 1998;8:19-29.
161 Robertson IH, Mattingley JB, Rorden C, et al. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169-172.
162 Rode G, Pisella L, Rossetti Y, et al. Bottom-up transfer of sensory-motor plasticity to recovery of spatial cognition: visuomotor adaptation and spatial neglect. Prog Brain Res. 2003;142:273-287.
163 Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect. Subtypes, neuroanatomy, and disability. Neurology. 2004;62:749-756.
164 Parton A, Malhotra P, Husain M. Hemispatial neglect. J Neurol Neurosurg Psychiatry. 2004;75:13-21.
165 Bowen A, Lincoln NB, Dewey M. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Syst Rev. (2):2002. CD003586
166 Mattsson B, Sorensen JC, Zimmer J, et al. Neural grafting to experimental neocortical infarcts improves behavioral outcome and reduces thalamic atrophy in rats housed in enriched but not in standard environments. Stroke. 1997;28:1225-1231.
167 Cairns K, Finklestein SP. Growth factors and stem cells as treatments for stroke recovery. Phys Med Rehabil Clin North Am. 2003;14(1 Suppl):S135-S142.
168 Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66-72.
169 Fraser C, Power M, Hamdy S, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831-840.
170 Uy J, Ridding MC, Hillier S, et al. Does induction of plastic change in motor cortex improve leg function after stroke? Neurology. 2003;61:982-984.
171 Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815-835.
172 Hummel F, Celnik P, Giraux P, et al. Effects of noninvasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490-499.
173 Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201-206.
174 Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295-301.
175 Dinse HR, Ragert P, Pleger B, et al. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91-94.
176 Dinse HR, Ragert P, Pleger B, et al. GABAergic mechanisms gate tactile discrimination learning. Neuroreport. 2003;14:1747-1751.
177 Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855-857.
178 Sutton RL, Feeney DM. α-Noradrenergic agonists and antagonists affect recovery and maintenance of beam walking ability after sensorimotor cortex ablation in the rat. Restor Neurol Neurosci. 1992;4:1-11.
179 Feeney DM, De Smet AM, Rai S. Noradrenergic modulation of hemiplegia: facilitation and maintenance of recovery. Restor Neurol Neurosci. 2004;22:175-190.
180 Goldstein LB. Pharmacology of recovery after stroke. Stroke. 1990;21(11 Suppl):139-142.
181 Crisostomo EA, Duncan PW, Propst M, et al. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol. 1988;23:94-97.
182 Borucki SJ, Landberg J, Redding M. The effect of dextroamphetamine on motor recovery after stroke. Neurology. 1992;42(Suppl 3):329.
183 Walker-Batson D, Smith P, Curtis S, et al. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Further evidence. Stroke. 1995;26:2254-2259.
184 Sonde L, Nordstrom M, Nilsson CG, et al. A double-blind placebo-controlled study of the effects of amphetamine and physiotherapy after stroke. Cerebrovasc Dis. 2001;12:253-257.
185 Scheidtmann K, Fries W, Muller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet. 2001;358:787-790.
186 Walker-Batson D, Curtis S, Natarajan R, et al. A double-blind, placebo-controlled study of the use of amphetamine in the treatment of aphasia. Stroke. 2001;32:2093-2098.
187 Goldstein LB. Common drugs may influence motor recovery after stroke. The Sygen In Acute Stroke Study Investigators. Neurology. 1995;45:865-871.
188 Dejerine J, Roussy G. Le syndrome douloureux thalamique. Rev Neurol. 1906;14:521-532.
189 Leijon G, Boivie J, Johansson I. Central poststroke pain—neurological symptoms and pain characteristics. Pain. 1989;36:13-25.
190 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78:393-401.
191 Katayama Y, Yamamoto T, Kobayashi K, et al. Deep brain and motor cortex stimulation for poststroke movement disorders and poststroke pain. Acta Neurochir Suppl. 2003;87:121-123.
192 Lefaucheur JP, Drouot X, Menard-Lefaucheur I, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75:612-616.
193 Andersen G, Vestergaard K, Ingeman-Nielsen M, et al. Incidence of central poststroke pain. Pain. 1995;61:187-193.
194 House AO, Hackett ML, Anderson CS, et al. Pharmaceutical interventions for emotionalism after stroke. Cochrane Database Syst Rev. (1):2004. CD003690
195 Langhorne P, Stott DJ, Robertson L, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223-1229.
196 Davenport RJ, Dennis MS, Wellwood I, et al. Complications after acute stroke. Stroke. 1996;27:415-420.
197 Kalra L, Yu G, Wilson K, et al. Medical complications during stroke rehabilitation. Stroke. 1995;26:990-994.
198 Roth EJ, Lovell L, Harvey RL, et al. Incidence of and risk factors for medical complications during stroke rehabilitation. Stroke. 2001;32:523-529.
199 Cavallini A, Micieli G, Marcheselli S, et al. Role of monitoring in management of acute ischemic stroke patients. Stroke. 2003;34:2599-2603.
200 McCarthy ST, Turner J. Low-dose subcutaneous heparin in the prevention of deep-vein thrombosis and pulmonary emboli following acute stroke. Age Ageing. 1986;15:84-88.
201 Kelly J, Rudd A, Lewis RR, et al. Screening for proximal deep vein thrombosis after acute ischemic stroke: a prospective study using clinical factors and plasma D-dimers. J Thromb Haemost. 2004;2:1321-1326.
202 Kelly J, Rudd A, Lewis RR, et al. Venous thromboembolism after acute ischemic stroke: a prospective study using magnetic resonance direct thrombus imaging. Stroke. 2004;35:2320-2325.
203 The International Stroke Trial (IST). a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569-1581.
204 Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86.
205 Gubitz G, Sandercock P, Counsell C. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. (2):2004. CD000024
206 Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. (1):2000. CD001484
207 Mazzone C, Chiodo-Grandi F, Sandercock P, et al. Physical methods for preventing deep vein thrombosis in stroke. Cochrane Database Syst Rev. (4):2004. CD001922
208 Yamey G, Greenwood R. Physical consequences of neurological disablement. In: Greenwood RJ, Barnes MP, McMillan TM, et al, editors. Handbook of Neurological Rehabilitation. Hove, UK: Psychology Press; 2003:191-222.
209 Convertino VA. Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc. 1997;29:191-196.
210 Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29:197-206.
211 Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83:1697-1702.
212 Mackay-Lyons MJ, Makrides L. Longitudinal changes in exercise capacity after stroke. Arch Phys Med Rehabil. 2004;85:1608-1612.
213 Bjurö T, Fugl-Meyer AR, Grimby G, et al. Domestic work in patients with neuromuscular handicap. Scand J Rehabil Med Suppl. 1978;6:193-196.
214 Potempa K, Lopez M, Braun L, et al. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101-105.
215 Weiss A, Suzuki T, Bean J, et al. High intensity strength training improves strength and functional performance after stroke. Am J Phys Med Rehabil. 2000;79:369-376.
216 Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35:1404-1409.
217 Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173-2180.
218 Saunders DH, Greig CA, Young A, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. (1):2004. CD003316
219 Gordon NF, Gulanick M, Fernando CF, et al. Physical activity and exercise recommendations for stroke survivors. Stroke. 2004;35:1230.
220 Ingles JL, Eskes GA, Phillips SJ. Fatigue after stroke. Arch Phys Med Rehabil. 1999;80:173-178.
221 van der Werf SP, van den Broek HL, Anten HW, et al. Experience of severe fatigue long after stroke and its relation to depressive symptoms and disease characteristics. Eur Neurol. 2001;45:28-33.
222 Glader EL, Stegmayr B, Asplund KL. Poststroke fatigue: a 2-year follow-up study of stroke patients in Sweden. Stroke. 2002;33:1327-1333.
223 Williams LS, Weinberger M, Harris LE, et al. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362-1369.
224 Hilari K, Byng S, Lamping DL, et al. Stroke and aphasia quality of life scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke. 2003;34:1944-1950.
225 Choi KS, Han SW, Kwon SU, et al. Poststroke fatigue: characteristics and related factors. Cerebrovasc Dis. 2005;19:84-90.
226 Staub F, Bogousslavsky J. Fatigue after stroke: a major but neglected issue. Cerebrovasc Dis. 2001;12:75-81.
227 Bassetti CL. Sleep and stroke. Semin Neurol. 2005;25:19-32.
228 Rosenberg JH, Shafor R. Fatigue in multiple sclerosis: a rational approach to evaluation and treatment. Curr Neurol Neurosci Rep. 2005;5:140-146.
229 Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005;64:1139-1143.
230 The FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. 2003;34:1450-1456.
231 Iwamoto T, Fukuda S, Kikawada M, et al. Prognostic implications of swallowing ability in elderly patients after initial recovery from stroke. J Gerontol A Biol Sci Med Sci. 2005;60:120-124.
232 Dennis MS, Lewis SC, Warlow C. Routine oral nutritional supplementation for stroke patients in hospital (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:755-763.
233 McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308:945-948.
234 Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review. BMJ. 1999;317:495-501.
235 Dziewas R, Ritter M, Schilling M, et al. Pneumonia in acute stroke patients fed by nasogastric tube. J Neurol Neurosurg Psychiatry. 2004;75:852-856.
236 Wanklyn P, Forster A, Young J. Hemiplegic shoulder pain (HSP): natural history and investigation of associated features. Disabil Rehabil. 1996;18:497-501.
237 Ada L, Foongchomcheay A, Canning C. Supportive devices for preventing and treating subluxation of the shoulder after stroke. Cochrane Database Syst Rev. (1):2005. CD003863
238 Turner-Stokes L, Jackson D. Shoulder pain after stroke: a review of the evidence base to inform the development of an integrated care pathway. Clin Rehabil. 2002;16:276-298.
239 Renzenbrink GJ, IJzerman MJ. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18:359-365.
240 House A, Dennis M, Mogridge L, et al. Mood disorders in the year after first stroke. Br J Psychiatry. 1991;158:83-92.
241 Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31:1829-1832.
242 Burvill PW, Johnson GA, Jamrozik KD, et al. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry. 1995;166:320-327.
243 Robinson RG, Kubos KL, Starr LB, et al. Mood disorders in stroke patients: importance of location of lesion. Brain. 1984;107:81-93.
244 Vataja R, Leppävuori A, Pohjasvaara T, et al. Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci. 2004;16:156-162.
245 Lenze EJ, Rogers JC, Martire LM, et al. The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry. 2001;9:113-135.
246 Singh A, Black HE, Herrmann N, et al. Functional and neuroanatomic correlations in poststroke depression: the Sunnybrook Stroke Study. Stroke. 2000;31:637-644.
247 Bisschop MI, Kriegsman DMW, Deeg DJH, et al. The longitudinal relation between chronic diseases and depression in older persons in the community: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57:187-194.
248 Parikh RM, Robinson RG, Lipsey JR, et al. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol. 1990;47:785-789.
249 Anderson CS, Hackett ML, House AO. Interventions for preventing depression after stroke. Cochrane Database Syst Rev. (1):2004. CD003689
250 Hackett ML, Anderson CS, House AO: Interventions for treating depression after stroke. Cochrane Database Syst Rev 2004; (3): CD003437. DOI: 10.1001/14651858. CD003437. pub 2.
251 Royal College of Physicians. National Sentinel Stroke Audit Report 2004. London: Royal College of Physicians, 2005.
252 Forster A, Young J. Research networks for stroke rehabilitation: opportunities and barriers. Clin Med. 2005;5:42-46.
253 Indredavik B, Bakke F, Slørdahl SA, et al. Treatment in a combined acute and rehabilitation stroke unit: which aspects are most important? Stroke. 1999;30:917-923.
254 Ward NS. Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol. 2004;17:725-730.
255 Academy of Medical Sciences. Restoring neurological function: putting the neurosciences to work in neurorehabilitation. Available at: http://www.acmedsci.ac.uk/index.php?pid=48&prid=15, 2004. (accessed January 30, 2006).