Chapter 37 Rehabilitation after Articular Cartilage Procedures
PRINCIPLES OF ARTICULAR CARTILAGE REHABILITATION
Several principles exist that must be considered when designing a rehabilitation program after articular cartilage repair procedures (Table 37–1). These key principles have been designed based upon the authors’ understanding of the basic science and mechanics of articular cartilage. These principles include individualization, creating a healing environment, understanding the biomechanics of the knee, reducing pain and effusion, restoring soft tissue balance, restoring muscle function, restoring proprioception and neuromuscular control, controlling the application of loads, and team communication. Each of these principles is briefly described as they relate to the rehabilitation program after articular cartilage repair procedures.
TABLE 37-1 Key Principles to Consider when Designing Rehabilitation Programs after Articular Cartilage Repair Procedures
Critical Points PRINCIPLES OF ARTICULAR CARTILAGE REHABILITATION
Individualization
Create a Healing Environment
Biomechanics of the Knee
Reduce Pain and Effusion
Restore Soft Tissue Balance
Restore Muscle Function
Control the Application of Loads
Individualization
One of the most important principles involving rehabilitation after articular cartilage repair procedures is the need for an individualized approach for each patient. Several variables must be considered when developing a unique rehabilitation progression for each patient. These include specifics regarding the patient, lesion, and surgery (Table 37–2).
TABLE 37-2 Specific Variables that Must be Considered when Designing Postoperative Rehabilitation Protocols after Articular Cartilage Procedures
| Lesion specifics |
The quality of each individual’s articular cartilage is the result of several factors including age, BMI, general health, nutrition, history of previous injuries, and genetics. The composition of articular cartilage undergoes a gradual degeneration over time that results in a breakdown of tissue matrix and a reduction in the load-bearing capacity of the cartilage.8 The specific factors that contribute to this deterioration remain controversial, but it appears that age, obesity, poor nutrition, and a history of repetitive impact loading (through work or sport activities) may result in osteoarthritic changes.8 Thus, younger patients with isolated defects and relatively healthy surrounding articular cartilage will progress more rapidly than older individuals with more degenerative changes and less dense cartilage structure. Furthermore, the patient’s motivation and previous activity levels must be considered when determining the rehabilitation approach to ensure that the goals of each patient are addressed. The rehabilitation program should be individualized to the specific demands of each patient’s activities of daily living, work, and/or sport activities.
BMI is another factor to consider. Mithoefer and coworkers30 reported a correlation between BMI and outcomes after microfracture, because the greater the BMI, the more likely the clinical outcome would be less favorable.
Create a Healing Environment
The next principle of articular cartilage rehabilitation involves creating an environment that facilitates the healing process while avoiding potentially deleterious forces to the repair site. This involves a thorough knowledge of the physiologic repair process after surgery. Through animal studies, as well as closely monitoring the maturation of repair tissue in human patients via arthroscopic examination, the biologic phases of maturation have been identified after several articular cartilage repair procedures.4,5,16,36,38 Knowledge of the healing and maturation process after these procedures will ensure that the repair tissue is gradually loaded and that excessive forces are not introduced too early in the healing process. These are discussed in detail in regard to each specific surgical procedure.
Two of the most important modes of rehabilitation of articular cartilage procedures are weight-bearing restrictions and range of motion (ROM) limitations. Unloading and immobilization have been shown to be deleterious to healing articular cartilage, resulting in proteoglycan loss and gradual weakening.2,17,50 Therefore, controlled weight-bearing and ROM are essential to facilitate healing and prevent degeneration. This gradual progression has been shown to stimulate matrix production and improve the tissue’s mechanical properties.6,7,51
Controlled compression and decompression forces observed during weight-bearing may nourish the articular cartilage and provide the necessary signals to the repair tissue to produce a matrix that will match the environmental forces.2,17,50 A progression of partial weight-bearing with crutches is used to gradually increase the amount of load applied to the weight-bearing surfaces of the joint. The use of a pool or aquatic therapy may also be beneficial to initiate gait training and lower extremity weight-bearing exercises. The buoyancy of the water has been shown to decrease the amount of weight-bearing forces to approximately 25% of the individual’s body weight when submerged to the level of the axilla and 50% of the individual’s body weight when submerged to the level of the waist.20 Commercially available devices to unload the patient’s body weight during treadmill ambulation may also be useful.
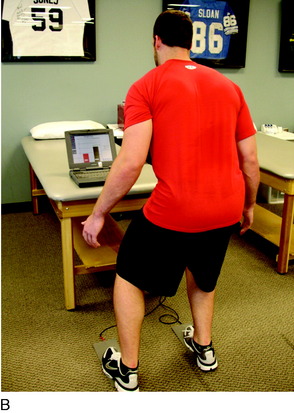
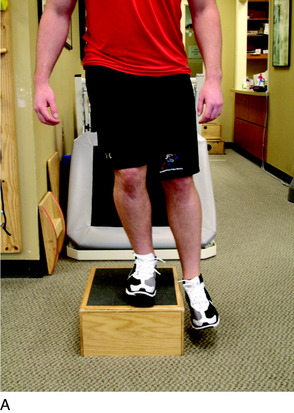
A force platform is another useful tool during the early phases of rehabilitation when weight-bearing is limited. This can be used to monitor the percentage of weight-bearing on each extremity during closed kinetic chain (CKC) exercises such as weight-shifts, mini-squats, and leg press (Fig. 37–1).
Passive range of motion (PROM) activities, such as continuous passive motion (CPM) machines or manual PROM, performed by a rehabilitation specialist are also begun immediately after surgery in a limited ROM to nourish the healing articular cartilage and prevent the formation of adhesions. Motion exercises may assist in creating a smooth low-frictional surface by sliding against the joint’s articular surface and may be an essential component in cartilage repair.44,45 The authors’ opinion is that PROM is a safe and effective exercise to perform immediately postoperatively, with minimal disadvantageous shear or compressive forces, if done with patient relaxation. This ensures that muscular contraction does not create deleterious compressive or shearing forces. Furthermore, the use of CPM has been shown to enhance cartilage healing and long-term outcomes after articular cartilage procedures.41,42 In a study comparing the outcomes of patients after microfracture procedures, Rodrigo and associates41 reported an 85% satisfactory outcome in patients who used a CPM machine for 6 to 8 hours per day for 8 weeks as compared with a 55% satisfactory outcome in patients who did not use a CPM machine. PROM may also be performed on an isokinetic device (Biodex Corporation, Shirley, NY) in the passive mode or with a bike with adjustable pedals that can alter the available ROM (Unicam Corporation, Ramsey, NJ) (Fig. 37–2). The authors advocate low-intensity (light-resistance) bicycling for long duration to stimulate articular cartilage regeneration.
Biomechanics of the Knee
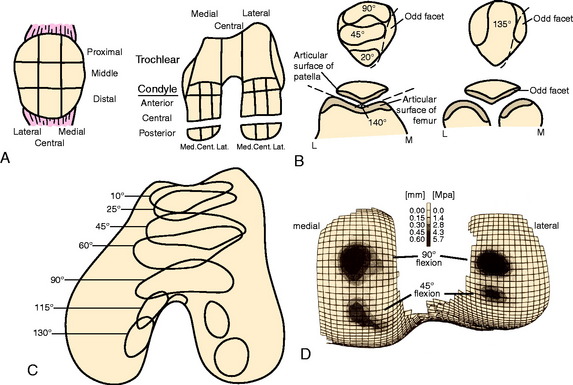
The next rehabilitation principle involves the biomechanics of the tibiofemoral and patellofemoral joints during normal joint articulation. Articulation between the femoral condyle and the tibial plateau is constant throughout knee ROM. The anterior surface of each femoral condyle is in articulation with the middle aspect of the tibial plateau near full knee extension. With weight-bearing, as the knee moves into greater degrees of knee flexion, the femoral condyles progressively roll posteriorly and slide anteriorly causing the articulation to shift posteriorly on the femoral condyle and tibial plateaus.24,28
The articulation between the inferior margin of the patella and the trochlea begins at approximately 10° to 20° of knee flexion depending on the size of the patella and the length of the patella tendon.23 As the knee proceeds into greater degrees of flexion, the contact area of the patellofemoral joint moves proximally along the patella. At 30°, the area of patellofemoral contact (inferior facets) is approximately 2 cm2.23 The area of contact gradually increases as the knee is flexed. At 60° of knee flexion, the middle facets of the patella articulate with the trochlea. At 90° of knee flexion, the contact area increases up to 6 cm2 and the superior facets articulate.23
Using this knowledge of joint arthrokinematics, the rate of weight-bearing, PROM, and exercise progression may be based on the exact location of the lesion (Fig. 37–3).3,12,14,15 For example, a patient with a lesion on the anterior aspect of the femoral condyle may perform exercises into deeper degrees of knee flexion without causing articulation at the repair site. Conversely, lesions on the posterior condyle may require the avoidance of exercise in deep knee flexion owing to the rolling and sliding component of the articulation during deeper knee flexion. Furthermore, the rehabilitation program for lesions on a non–weight-bearing surface, such as the trochlea, may include immediate partial weight-bearing with a brace locked in full knee extension without causing excessive compression on the repair site.
Rehabilitation exercises are altered based on the biomechanics of the knee to avoid excessive compressive or shearing forces. Whereas the exact ROM in which articulation of the lesion occurs is the most important factor to consider when designing the rehabilitation program, the amount of compressive and shear forces observed at the joint also vary throughout the ROM. Open kinetic chain (OKC) exercises, such as knee extension, are commonly performed from 90° to 40° of knee flexion. This ROM provides the lowest amount of patellofemoral joint reaction forces while exhibiting the greatest amount of patellofemoral contact area,22,23,49 thus distributing the force along a greater surface area. CKC exercises such as the leg press, vertical squats, lateral step-ups, and wall-squats are performed initially from 0° to 30° and then progressed to 0° to 60° where tibiofemoral and patellofemoral joint reaction forces are lowered.22,23,49 Clinically, these exercises are begun using a leg press machine rather than the vertical mini-squat owing to the ability to control the amount of weight applied to the lower extremities in the horizontal position in comparison with the vertical squat. As the repair site heals and symptoms subside, the ROM in which exercises are performed is progressed to allow greater muscle strengthening in a larger arc of motion. Exercises are progressed based on the patient’s symptoms and the clinical assessment of swelling and crepitation.
Reduce Pain and Effusion
Numerous authors have studied the effect of pain and joint effusion on muscle inhibition. A progressive decrease in volitional quadriceps activity has been noted as the knee exhibits increased pain and distention.48,52 Therefore, the reduction in knee joint pain and swelling is crucial to minimize this reflexive inhibition and restore normal quadriceps activity. Furthermore, any increase in intra-articular joint temperature has been shown to stimulate proteoglytic enzyme activity, which has a detrimental effect on articular cartilage.21,34
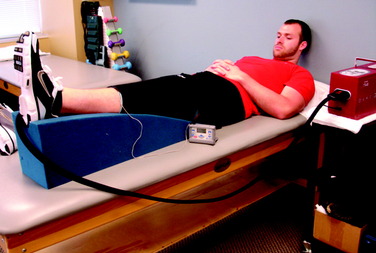
Treatment options for swelling reduction include cryotherapy, elevation, high-voltage stimulation, and joint compression through the use of a knee sleeve or compression wrap (Fig. 37–4). Patients presenting with chronic joint effusion may also benefit from a knee sleeve or compression wrap to apply constant pressure while performing everyday activities in an attempt to minimize the development of further effusion (Fig. 37–5).
Pain can be reduced passively through the use of cryotherapy, transcutaneous electrical nerve stimulation, and analgesic medication. Immediately after injury or surgery, the use of a commercial cold wrap can be extremely beneficial. PROM may also provide neuromodulation of pain during acute or exacerbated conditions.43
Restore Soft Tissue Balance
One of the most important aspects of articular cartilage rehabilitation involves the avoidance of arthrofibrosis, particularly with the OATS and ACI procedures, owing to the large open incision and extensive soft tissue trauma. This is achieved through the restoration of full passive knee extension, patellar mobility, and soft tissue flexibility of the knee and entire lower extremity. The inability to fully extend the knee results in abnormal joint arthrokinematics and subsequent increases in patellofemoral and tibiofemoral joint contact pressure, increased strain on the quadriceps muscle, and muscular fatigue.35 Therefore, a drop-lock postoperative knee brace locked into 0° of extension is used during ambulation, and PROM out of the brace is performed immediately after surgery.
The goal is to achieve at least 0° of knee extension within the first few days after surgery. Specific exercises include manual PROM exercises performed by the rehabilitation specialist, supine hamstring stretches with a wedge under the heel, and gastrocnemius stretching with a towel. Overpressure of 6 to 12 pounds may be used for a low-load long-duration stretch as needed to achieve full extension (Fig. 37–6). Patients are instructed to perform low, long-duration stretches for 10 to 12 minutes several times each day (usually five to six times per day). Modalities such as moist heat and ultrasound may also be applied to facilitate greater ROM improvements before and/or during these stretching techniques.25,40
Restore Muscle Function
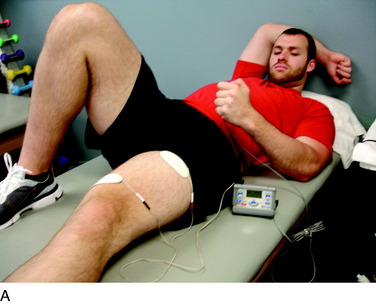
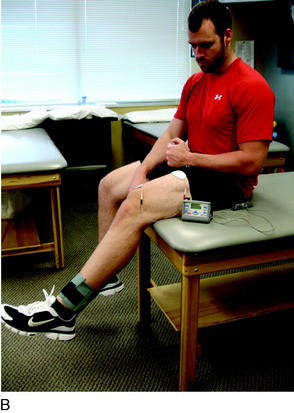
The next principle involves restoring muscle function of the lower extremity. As previously stated, inhibition of the quadriceps muscle is a common clinical enigma in the presence of pain and effusion during the acute phases of rehabilitation. Electrical muscle stimulation (EMS) and biofeedback are often incorporated with therapeutic exercises to facilitate the active contraction of the quadriceps musculature (Fig. 37–7A).
EMS and biofeedback on the quadriceps musculature appear to facilitate the return of muscle activation and may be valuable additions to therapeutic exercises.9,46 Clinically, EMS is begun immediately after surgery while the patient performs isometric and isotonic exercises such as quadriceps sets, straight leg raises, hip adduction and abduction, and knee extensions (see Fig. 37–7B). EMS is used before biofeedback when the patient presents acutely with the inability to activate the quadriceps musculature. EMS is useful to attempt to recruit a maximum amount of muscle fibers during active contraction and may be used throughout the rehabilitation process. Once independent muscle activation is present, biofeedback may also be incorporated to facilitate further neuromuscular activation of the quadriceps. The patient must concentrate on neuromuscular control to independently activate the quadriceps during rehabilitation. The quadriceps and the hip/core muscles are emphasized to assist in dissipating ground reaction forces.
Enhance Proprioception and Neuromuscular Control
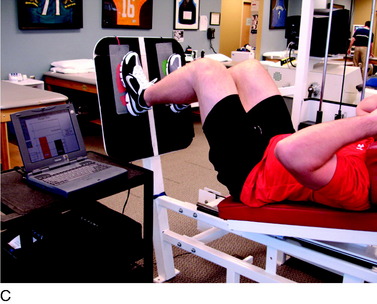
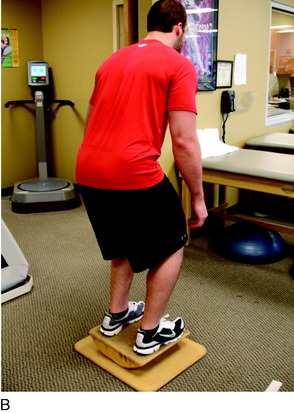
Proprioceptive and neuromuscular control drills of the lower extremities should be included to restore dynamic stabilization of the knee joint postoperatively. Proprioceptive deficits have been noted in the injured and postoperative knee.10,39 Specific drills initially include weight-shifting side-to-side, weight-shifting diagonally, mini-squats, and mini-squats on an unstable surface such as a tilt board (Fig. 37–8). Perturbations can be added to challenge the neuromuscular system as well as additional exercises including lunges, step-ups, and balance onto unstable surfaces (Figs. 37–9 and 37–10).
Control the Application of Loads
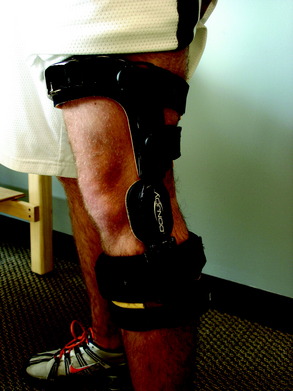
In addition, patients may benefit from the use of orthotics, insoles, and bracing to alter the applied loads on the articular cartilage during functional activities. These devices are used to avoid excessive forces by unloading the area of the knee where the lesion is located. Unloading braces are often used for patients with subtle uncorrected abnormal alignments (genu varum) or large or uncontained lesions or who had concomitant osteotomies and meniscal allografts (Fig. 37–11).
REHABILITATION AFTER ARTICULAR CARTILAGE REPAIR PROCEDURES
The rehabilitation progression is designed based on the four biologic phases of cartilage maturation: proliferation, transitional, remodeling, and maturation.4,5,11,16,19,33,36,38 The length of each phase will vary depending on the lesion, patient’s age and general health, and surgical specifics discussed previously; however, the concepts of each phase are consistent. The following sections overview the generalized rehabilitation process during each of the four phases.
Phase IV: Maturation Phase
The final phase is known as the maturation phase, which typically begins in a range of 4 to 6 months and can last up to 15 to 18 months. This depends on the type of surgery, size, and location of the lesion. During this phase, the repair tissue reaches its full maturation. The duration of this phase varies based on several factors such as lesion size and location and the specific surgical procedure performed. The patient will gradually return to full premorbid activities as tolerated. Impact-loading activities are gradually introduced. Although such procedures as OATS and ACI are designed to restore function rather than to facilitate return to high-impact athletic activities, a return to athletic activities is determined based on the unique presentation of each patient. A return to competitive athletics has been documented after microfracture,44 OATS,19 and ACI27,28 procedures.
SPECIFIC POSTOPERATIVE GUIDELINES
Microfracture
Rehabilitation after a microfracture procedure progresses more cautiously than that of a débridement or chondroplasty (Table 37–3). The program is based on size, location, number of areas treated, and concomitant procedures. The early protection phase begins immediately after surgery and lasts until the 4th week postoperatively. During this time, defects begin to fill with a fibrin clot, although no fibrocartilage is present.11 A period of non–weight-bearing is applied for the first 2 to 6 weeks postoperatively for most lesions. A recent study by Marder and colleagues27 compared the results of patients with small focal lesions of less than 2.0 cm2 using two postoperative rehabilitation programs. Group I used touch-down weight-bearing and a CPM machine for 6 to 8 hours a day for 6 weeks. Group II was allowed weight-bearing as tolerated immediately after surgery and used active-assisted heel-slides for ROM (without the use of a CPM). The authors reported significant improvements in both groups and no significant differences in the subjective or objective outcomes between groups a minimum of 2 years postoperatively. Thus, it appears that it may be possible to begin early controlled weight-bearing for small, focal lesions without applying deleterious forces to the repair site. Initial controlled touch-down weight-bearing is initiated for lesions that are localized and smaller than 2.0 cm2 in patients with good tissue quality. For patients with patellofemoral lesions, immediate weight-bearing is performed owing to the lack of lesion articulation during weight-bearing; however, a drop-locked knee brace is worn to avoid deleterious sheer forces to the healing repair site.
| Phase I: Early Protection Phase (Weeks 0–4) | |
|---|---|
| Goals | |
| Phase II: Transition Phase (Weeks 4–8) | |
|---|---|
| Goals | |
| WB | |
| ROM | |
| Strengthening exercises |
Initiate front lunges, wall-squats, front and lateral step-ups wk 5 for small femoral condyle and patellofemoral lesions, wk 8 for large femoral condyle lesions.
|
| Functional activities | |
| Criteria to progress to phase III | |
| Phase III: Remodeling Phase (Weeks 8–16) | |
|---|---|
| Goals | |
| ROM | Patient should exhibit 125°–135°+ flexion. |
| Exercise program | |
| Functional activities | As patient improves, increase walking (e.g., distance, cadence, incline) |
| Maintenance program | |
| Criteria to progress to phase IV | |
| PHASE IV: MATURATION PHASE (WEEKS 16–26) | |
|---|---|
| Goals | Gradual return to full unrestricted functional activities. |
| Exercises | |
| Functional activities | Patient may return to various sport activities as progression in rehabilitation and cartilage healing allows. Generally, low-impact sports such as swimming, skating, rollerblading, and cycling are permitted at about 2 mo for small femoral condyle and patellofemoral lesions and at 3 mo for large femoral condyle lesions. Higher-impact sports such as jogging, running, and aerobics may be performed at 4 mo for small lesions or 5 mo for larger lesions. High-impact sports such as tennis, basketball, football and baseball are allowed at 6–8 mo. |
CKC, closed kinetic chain; CPM, continuous passive motion; EMS, electrical muscle stimulation; OKC, open kinetic chain; Q/H, quadriceps/hamstring; ROM, range of motion; WB, weight-bearing.
The transition phase begins at week 4 and progresses to week 8. During this phase, the patient may progress to full weight-bearing and more functional CKC exercises. At 6 weeks postoperatively, a thin layer of tissue covers the base of the lesion.13 Although the repair is still incomplete, fibrocartilagenous tissue is present, and by 8 weeks, some tissue with hyaline-like characteristics has been detected.11 By 12 weeks, the defect is completely filled and the quality of cartilaginous tissue improves significantly.13
Clinical outcome studies indicate that a significant number of patients will be able to return to sports after a microfracture procedure. Mithoefer and coworkers31 reported on 32 patients who routinely participated in high-impact and pivoting sports. At a minimum 2-year follow-up, 66% had good to excellent results and 44% had returned to high-impact sports. However, after initial improvement, scores decreased in 47% of athletes. Return to sports was significantly higher in athletes younger than 40 years of age, patients with a lesion size less than 200 mm2, those with preoperative symptoms less than 12 months’ duration, and patients who had no prior surgical intervention. Mithoefer and associates29 reported results of microfracture on isolated femoral chondral lesions. They reported that 67% of patients had a good to excellent result; 25%, a fair result; and 8%, a poor result. The investigators were able to obtain follow-up magnetic resonance imaging (MRI) on 50% of the patients. MRI revealed good tissue healing and filling in 54%, moderate tissue filling in 29%, and poor healing result in 17%. Successful outcomes correlated to a low BMI, good healing or lesion filling, and short duration of symptoms. The worst results were seen in patients with a BMI of 30 kg/m. Kreuz and colleagues26 compared the results of microfracture in patients older than 40 years of age and younger than 40 years of age. The authors reported superior outcomes in patients 40 years or younger. Between 18 and 36 months postoperatively, the investigators found a deterioration in International Cartilage Repair Society (ICRS) scores, which was significantly pronounced in older patients. Furthermore, MRI performed at 36 months after surgery indicated better fill in patients younger than 40 years of age.
OATS
Rehabilitation after OATS procedures requires the avoidance of early deleterious forces to avoid disrupting the healing transplanted bone plugs (Table 37–4). Currently, the pace of the rehabilitation program after OATS procedures is based not only on the size of the lesion but also on the amount of transplanted bone plugs. The program is progressed more cautiously when numerous bone plugs are used owing to the potential for a less congruent surface. The early protection phase lasts until the 8th week postoperatively. During this phase, an initial 44% reduction in the push-in and pull-out strength of the transplanted bone plugs has been observed (at 1 wk postoperatively),52 emphasizing the need for strict non–weight-bearing postoperatively. Partial weight-bearing is usually initiated 2 to 4 weeks after surgery based on the size of the lesion and the number of transplanted bone plugs. Although the original hyaline cartilage remains intact and viable,33 the strength of the bone plugs is the limiting factor when designing the postoperative rehabilitation program.
TABLE 37-4 Rehabilitation after Osteochondral Autograft Transplantation
| Phase I: Early Protection Phase (Weeks 0–6) | |
|---|---|
| Goals | |
| Phase II: Transition Phase (Weeks 6–12) | |
|---|---|
| Goals | |
| Brace | Discontinue brace at 6 wk, consider unloading brace for femoral condyle lesions. |
| WB | |
| ROM | |
| Strengthening exercises |
CKC exercises (leg press) wk 8–10 for femoral condyle lesions: mini-squats 0°–45°, front lunges, step-ups, wall-squats; may need to delay CKC up to 14 wk if large lesions.
Leg press wk 8–10 (0°–90° for femoral condyle, 0°–60° for patellofemoral, progressing to 0°–90° as tolerated).
|
| Functional activities | |
| Criteria to progress to phase III | |
| Phase III: Remodeling Phase (Weeks 12–26) | |
|---|---|
| Goals | |
| ROM | Patient should exhibit 125°–135° flexion—no restrictions. |
| Exercise program | |
| Functional activities | As patient improves, increase walking (e.g., distance, cadence, incline) |
| Maintenance program | |
| Criteria to progress to phase IV | |
| Phase IV: Maturation Phase (Weeks 26–52) | |
|---|---|
| Goals | Gradual return to full unrestricted functional activities. |
| Exercises | |
| Functional activities | Patient may return to various sport activities as progression in rehabilitation and cartilage healing allows. Generally, low-impact sports such as skating, rollerblading, and cycling are permitted at about 6–8 mo. Higher-impact sports such as jogging, running, and aerobics may be performed at 8–10 mo. High-impact sports such as tennis, basketball, and baseball are allowed at 12–18 mo. |
CKC, closed kinetic chain; CPM, continuous passive motion; EMS, electrical muscle stimulation; OKC, open kinetic chain; Q/H, quadriceps/hamstring; ROM, range of motion; WB, weight-bearing.
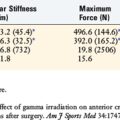
By 4 weeks, the cancellous bone plugs have united,19 and by 6 weeks, there is full subchondral integration and 29% of grafts show bonding between the articular cartilage of the bone plugs and the surrounding articular cartilage.33 Although integration has occurred, a 63% decrease in graft stiffness is still observed at 6 weeks postoperative.33 During this time, weight-bearing is gradually progressed as the strength of the repair increases. At 8 weeks postoperative, fibrocartilage has been observed to grow to the surface and seal the recipient and donor site hyaline cartilage, and weight-bearing is progressed to full. Immediate weight-bearing is initiated for patellofemoral lesions with a drop-lock knee brace and progressed to full without the brace at approximately 6 to 8 weeks postoperative.
Clinical outcome results after mosaicplasty indicate good results. Hangody and Fules18 followed 831 patients and reported a good to excellent result in 92% of those who exhibited a lesion of the femoral condyle, and the same in 87% with a tibial plateau lesion. Lesions of the patella rendered the lowest outcomes, with only 79% exhibiting a good to excellent result. Furthermore, the authors reported a 3% rate of donor site morbidity. Lastly, 36 patients exhibited a painful postoperative hemarthroses. Bartha and coworkers1 reported on 89 patients who underwent second-look arthroscopy, 77% of whom exhibited congruent surfaces and healing of the lesion.
ACI
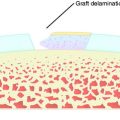
The rehabilitation program after ACI is vital to the success and long-term outcomes of patients (Table 37–5). Early controlled ROM and weight-bearing are necessary to stimulate chondrocyte development, although caution is placed on overaggressive activities that may result in cell damage or graft delamination.
TABLE 37-5 Rehabilitation after Autologous Chondrocyte Implantation
| Phase I: Early Protection Phase (Weeks 0–6) | |
|---|---|
| Goals | |
| Phase II: Transition Phase (Weeks 6–12) | |
|---|---|
| Goals | |
| Brace | |
| WB | |
| ROM | |
| Strengthening exercises | |
| Functional activities | |
| Criteria to progress to phase III | |
| Phase III: Remodeling Phase (Weeks 12–26) | |
|---|---|
| Goals | |
| ROM | Patient should exhibit 125°–135° flexion. |
| Exercise program | |
| Functional activities | As patient improves, increase walking (e.g., distance, cadence, incline) |
| Maintenance program | |
| Criteria to progress to phase IV | |
| Phase IV: Maturation Phase (Weeks 12–26) | |
|---|---|
| Goals | Gradual return to full unrestricted functional activities. |
| Exercises | |
| Functional activities | Patient may return to various sport activities as progression in rehabilitation and cartilage healing allows. Generally, low-impact sports such as swimming, skating, rollerblading, and cycling are permitted at about 6 mo. Higher-impact sports such as jogging, running, and aerobics may be performed at 8–9 mo for small lesions or 9–12 mo for larger lesions. High-impact sports such as tennis, basketball, football and baseball are allowed at 12–18 mo. |
CKC, closed kinetic chain; CPM, continuous passive motion; EMS, electrical muscle stimulation; OKC, open kinetic chain; Q/H, quadriceps/hamstring; ROM, range of motion; WB, weight-bearing.
Knowledge of the biologic healing response is vital for the development of appropriate rehabilitation guidelines. Rehabilitation may begin as early as the recovery room in the form of CPM. At this time, the chondrocytes are aligned and attached to the underlying surface.47 It is imperative that the patient be appropriately positioned to allow for the effect of gravity to evenly distribute the chondrocytes on the base of the defect during these first 4 hours as the cells adhere to the surface. A study by Sohn and associates47 showed that the defect orientation during these first 4 hours can be an important factor in the uniformity of cell distribution in the ACI procedure. These authors advanced the hypothesis, which has not yet been proved clinically, that the effects of gravity within the first few hours of cell implantation could lead to localization of induced cells in one area of the graft but not in other areas, which may affect healing of the defects. This suggests two possibilities. First, that the position of the patient with a patellar defect in the prone position would contraindicate CPM, because it would produce an abnormal gravity effect. Second, that the use of CPM immediately after surgery is warranted to produce a “more even distribution of implanted cells within the defect.” At present, no in vivo or clinical studies have been published to support this hypothesis.
Proliferation of the chondrocytes occurs in the first 6 weeks after cell implantation. During the first 24 hours after cell implantation, the cells line the base of the lesion and multiply several times to produce a matrix that will fill the defect with a soft repair tissue up to the level of the periosteal cover.16,38 At this time, PROM and controlled partial weight-bearing will help to promote cellular nutrition through synovial fluid diffusion as well as provide the proper stimulus for the cells to produce specific matrix markers. During this initial phase, controlled PROM and a gradual weight-bearing progression are two of the most important components of the rehabilitation process.
During the transition phase, which includes weeks 7 through 12, the repair tissue is spongy and compressible with little resistance. Upon arthroscopic examination, the tissue may in fact have a wavelike motion when a probe is slid over the tissue.16,38 During this phase, the patient achieves full ROM and progresses from partial to full weight-bearing. Continued maturation of the repair tissue is fostered through higher level functional and motion exercises. CKC exercises, such as front lunges, step-ups, and wall-squats are performed as well as machine exercises for the entire lower extremity. Again, caution should be placed on exercises that produce sheer forces in patients with patellofemoral lesions.
The remodeling phase occurs from 12 weeks through 32 weeks postoperatively. During this phase, there is a continuous production of matrix with further remodeling into a more organized structural tissue. The tissue at this point has the consistency of soft plastic upon probing.16,38 As the tissue becomes more firm and integrated, it allows for more functional training activities to be performed as well as elliptical, bicycle, and a gradual walking program.
The final maturation phase can last up to 15 to 18 months depending upon the size and location of the lesion. During this phase, the repair tissue reaches its full maturation. The stiffness of the cartilage resembles that of the surrounding tissue.16,38 The duration of this phase varies based on several factors such as lesion size and location.
Basic science studies have shown that it may take up to 6 months for the graft site to become firm and at least 9 months to become as durable as the surrounding healthy articular cartilage.16,38 Thus, low-impact activities (such as golf, swimming, cycling, and walking) are initiated by months 5 to 6 and progressed to moderate-impact activities (such as tennis, hiking, skating) from months 7 to 9 as tolerated. Usually, high-impact activities such as running and skiing are permitted with a gradual return beginning at 11 to 12 months postoperative.
The results of clinical outcomes after ACI are encouraging. Mithofer and colleagues32 reported a 72% good to excellent result and return to play in competitive soccer players, with 80% returning back to the same level of play. Peterson and coworkers37 reported a 90% good to excellent result using ACI on osteochondritis dissecans lesions of the knee in patients with a mean age of 26.4 years (range, 14–52 yr).
1 Bartha L., Vajda A., Duska Z.L., et al. Autologous osteochondral mosaicplasty grafting. J Orthop Sports Phys Ther. 2006;36:739-750.
2 Behrens F., Kraft E.L., Oegema T.R.Jr. Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J Orthop Res. 1989;7:335-343.
3 Blankevoort L., Kuiper J.H., Huiskes R., Grootenboer H.J. Articular contact in a three-dimensional model of the knee. J Biomech. 1991;24:1019-1031.
4 Brittberg M., Lindahl A., Nilsson A., et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
5 Brittberg M., Nilsson A., Lindahl A., et al. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;326:270-283.
6 Buckwalter J.A. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192-202.
7 Buckwalter J.A., Mankin H.J. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477-486.
8 Cohen N.P., Foster R.J., Mow V.C. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28:203-215.
9 Delitto A., Rose S.J., McKowen J.M., et al. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys Ther. 1988;68:660-663.
10 Friden T., Roberts D., Ageberg E., et al. Review of knee proprioception and the relation to extremity function after an anterior cruciate ligament rupture. J Orthop Sports Phys Ther. 2001;31:567-576.
11 Frisbie D.D., Oxford J.T., Southwood L., et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215-227.
12 Fujikawa K., Seedhom B.B., Wright V. Biomechanics of the patello-femoral joint. Part I: a study of the contact and the congruity of the patello-femoral compartment and movement of the patella. Eng Med. 1983;12:3-11.
13 Gill T.J., McCulloch P.C., Glasson S.S., et al. Chondral defect repair after the microfracture procedure: a nonhuman primate model. Am J Sports Med. 2005;33:680-685.
14 Goodfellow J., Hungerford D.S., Woods C. Patello-femoral joint mechanics and pathology. 2. Chondromalacia patellae. J Bone Joint Surg Br. 1976;58:291-299.
15 Goodfellow J., Hungerford D.S., Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58:287-290.
16 Grande D.A., Pitman M.I., Peterson L., et al. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208-218.
17 Haapala J., Arokoski J., Pirttimaki J., et al. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000;21:76-81.
18 Hangody L., Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
19 Hangody L., Kish G., Karpati Z. Autogenous osteochondral graft technique for replacing knee cartilage defects in dogs. Orthop Int. 1997;5:175-181.
20 Harrison R.A., Hillman M., Bulstrode S. Loading of the limb when walking partially immersed. Physiotherapy. 78, 1992.
21 Horvath S.M., Hollander J.L. Intra-articular temperature as a measure of joint reaction. J Clin Invest. 1949;28:469-473.
22 Huberti H.H., Hayes W.C. Patellofemoral contact pressures. The influence of Q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66:715-724.
23 Hungerford D.S., Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;144:9-15.
24 Iwaki H., Pinskerova V., Freeman M.A. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br. 2000;82:1189-1195.
25 Knight C.A., Rutledge C.R., Cox M.E., et al. Effect of superficial heat, deep heat, and active exercise warm-up on the extensibility of the plantar flexors. Phys Ther. 2001;81:1206-1214.
26 Kreuz P.C., Erggelet C., Steinwachs M.R., et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180-1186.
27 Marder R.A., Hopkins G.Jr., Timmerman L.A. Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy. 2005;21:152-158.
28 Martelli S., Pinskerova V. The shapes of the tibial and femoral articular surfaces in relation to tibiofemoral movement. J Bone Joint Surg Br. 2002;84:607-613.
29 Mithoefer K., Williams R.J.3rd, Warren R.F., et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 pt 2):294-304.
30 Mithoefer K., Williams R.J.3rd, Warren R.F., et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-1920.
31 Mithoefer K., Williams R.J.3rd, Warren R.F., et al. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34:1413-1418.
32 Mithofer K., Peterson L., Mandelbaum B.R., Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33:1639-1646.
33 Nam E.K., Makhsous M., Koh J., et al. Biomechanical and histological evaluation of osteochondral transplantation in a rabbit model. Am J Sports Med. 2004;32:308-316.
34 Osbahr D.C., Cawley P.W., Speer K.P. The effect of continuous cryotherapy on glenohumeral joint and subacromial space temperatures in the postoperative shoulder. Arthroscopy. 2002;18:748-754.
35 Perry J., Antonelli D., Ford W. Analysis of knee joint forces during flexed-knee stance. J Bone Joint Surg Am. 1975;57A:961-967.
36 Peterson L., Brittberg M., Kiviranta I., et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12.
37 Peterson L., Minas T., Brittberg M., Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation. Results at two to ten years. J Bone Joint Surg. 2003;85(suppl 2):17-24.
38 Peterson L., Minas T., Brittberg M., et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-234.
39 Roberts D., Friden T., Stomberg A., et al. Bilateral proprioceptive defects in patients with a unilateral anterior cruciate ligament reconstruction: a comparison between patients and healthy individuals. J Orthop Res. 2000;18:565-571.
40 Robertson V.J., Ward A.R., Jung P. The effect of heat on tissue extensibility: a comparison of deep and superficial heating. Arch Phys Med Rehabil. 2005;86:819-825.
41 Rodrigo J.J., Steadman J.R., Silliman J.F. Improvement of full-thickness chondral defect healing in the human knee after débridement and microfracture using continuous passive motion. Am J Knee Surg. 1994;7:109-116.
42 Salter R. The biological concept of continuous passive motion of synovial joints: the first 18 years of basic research and its clinical application. In: Ewing J., editor. Articular Cartilage and Knee Joint Function. New York: Raven, 1990.
43 Salter R.B., Hamilton H.W., Wedge J.H., et al. Clinical application of basic research on continuous passive motion for disorders and injuries of synovial joints: a preliminary report of a feasibility study. J Orthop Res. 1984;1:325-342.
44 Salter R.B., Simmonds D.F., Malcolm B.W., et al. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62:1232-1251.
45 Shimizu T., Videman T., Shimazaki K., Mooney V. Experimental study on the repair of full thickness articular cartilage defects: effects of varying periods of continuous passive motion, cage activity, and immobilization. J Orthop Res. 1987;5:187-197.
46 Snyder-Mackler L., Delitto A., Bailey S.L., Stralka S.W. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166-1173.
47 Sohn D.H., Lottman L.M., Lum L.Y., et al. Effect of gravity on localization of chondrocytes implanted in cartilage defects. Clin Orthop Relat Res. 2002;394:254-262.
48 Spencer J.D., Hayes K.C., Alexander I.J. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171-177.
49 Steinkamp L.A., Dillingham M.F., Markel M.D., et al. Biomechanical considerations in patellofemoral joint rehabilitation. Am J Sports Med. 1993;21:438-444.
50 Vanwanseele B., Lucchinetti E., Stussi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage. 2002;10:408-419.
51 Waldman S.D., Spiteri C.G., Grynpas M.D., et al. Effect of biomechanical conditioning on cartilaginous tissue formation in vitro. J Bone Joint Surg Am. 2003;85(suppl 2):101-105.
52 Whiteside R.A., Bryant J.T., Jakob R.P., et al. Short-term load bearing capacity of osteochondral autografts implanted by the mosaicplasty technique: an in vitro porcine model. J Biomech. 2003;36:1203-1208.