Chapter 11 Regulation of the Immune Response
• Many factors govern the outcome of any immune response. These include the antigen itself, its dose and route of administration, and the genetic background of the individual responding to antigenic challenge. A variety of control mechanisms serve to restore the immune system to a resting state when the response to a given antigen is no longer required.
• The APC has an important effect on the immune response through its ability to provide co-stimulation to T cells and by the production of cytokines and chemokines that influence both the nature and make-up of the ensuing reponse. In addition, APC heterogeneity aids in the promotion of different modes of immune response.
• T cells regulate the immune response. Cytokine production by T cells influences the type of immune response elicited by antigen. CD4+ T cells can differentiate into several effector phenotypes such as TH1, TH2 or TH17. These subsets play important roles in the protection of the host against a diversity of pathogen challenges. Regulatory T cells may belong to the CD4 or CD8 subpopulations. They may inhibit responses via a variety of mechanisms such as via cell-to-cell contact or by the production of the anti-inflammatory cytokines IL-10 and TGFβ.
• Immunoglobulins can influence the immune response. They may act positively, through the formation of immune complexes, or negatively, by reducing antigenic challenge or by feedback inhibition of B cells.
• Selective migration of lymphocyte subsets to different sites can modulate the local type of immune response because different TH subsets respond to different sets of chemokines.
• The neuroendocrine system influences immune responses. Cells from both systems share similar ligands and receptors, which permit cross-interactions between them. Corticosteroids in particular downregulate TH1 responses and macrophage activation.
• Genetic factors influence the immune system and include both MHC-linked and non-MHC-linked genes. They affect the level of immune response and susceptibility to infection.
Ideally, an immune response is mounted quickly to clear away a pathogenic challenge with the minimum of collateral damage and then the system is returned to a resting state once the antigen is eliminated. The immune response, like many other biological systems, is therefore subject to a variety of control mechanisms. Additional mechanisms help regulate the levels of immunopathology that are often a necessary sacrifice for pathogen elimination. An insufficient immune response can result in an individual being overwhelmed by infection. An inappropriate, or over vigorous immune response can lead to high levels of immunopathology or even autoimmunity (see Chapter 20). The balance between these two is therefore critical.
• the form, dose, and route of administration of the antigen;
• the antigen-presenting cell (APC);
• the genetic background of the individual;
• any history of previous exposure to the cognate antigen; and
Specific antibodies may also modulate the immune response to an antigen.
Regulation by antigen
Different antigens elicit different kinds of immune response
In some situations, antigens (e.g. those of intracellular microorganisms) may not be cleared effectively leading to a sustained immune response. Chronic immune responses have several possible pathological consequences and can lead to autoimmunity and hypersensitivity (see Chapters 20 and 23–26).
Large doses of antigen can induce tolerance
Very large doses of antigen often result in specific T – and sometimes B – cell tolerance.
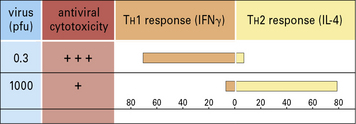
Administration of antigen to neonatal mice often results in tolerance to the antigen. It was once speculated that this might be the result of immaturity of the immune system. However, neonatal mice can develop efficient immune responses (Fig. 11.1) and their non-responsiveness may in some cases be attributable, not to the immaturity of T cells, but to immune deviation. In this case a non-protective TH2-type cytokine response would dominate a protective TH1-type cytokine response.
T-independent polysaccharide antigens have been shown to generate tolerance in B cells after administration in high doses. Tolerance and its underlying mechanisms are discussed in Chapter 19.
Antigen route of administration can determine whether an immune response occurs
The route of administration of antigen has been shown to influence the immune response:
• antigens administered subcutaneously or intradermally evoke an active immune response; whereas
• antigens given intravenously, orally, or as an aerosol may cause tolerance or an immune deviation from one type of CD4+ T cell response to another.
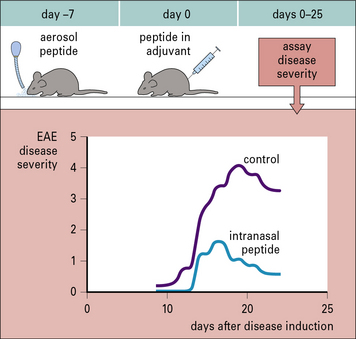
Similar observations have been made when antigen is given as an aerosol. Studies in mice have shown that aerosol administration of an encephalitogenic peptide of myelin basic protein (MBP) inhibits the development of experimental allergic encephalomyelitis (EAE) that would normally be induced by a conventional (subcutaneous) administration of the peptide (Fig. 11.2).
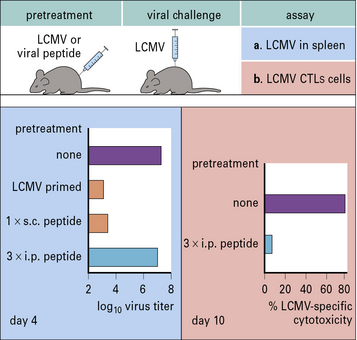
A clear example of how different routes of administration affect the outcome of the immune response is provided by studies of infection with lymphocytic choriomeningitis virus (LCMV). Mice primed subcutaneously with peptide in incomplete Freund’s adjuvant develop immunity to LCMV. However, if the same peptide is repeatedly injected intraperitoneally the animal becomes tolerized and cannot clear the virus (Fig. 11.3).
Regulation by the antigen presenting cell
Neonatal animals are more susceptible to tolerance induction. Therefore mice administered MBP in incomplete Freund’s adjuvant during the neonatal period are resistant to the induction of EAE. This is due to the development of a dominant TH2 response (see Fig. 11.1). The prior TH2 response to MBP prevents the development of the TH1/TH17 pathological response, which mediates EAE. This effect is not restricted to neonatal animals. Indeed, adult Lewis rats can be tolerized to the induction of EAE by similar administration of MBP in incomplete Freund’s adjuvant.
Cytokine production by APCs influences T cell responses
• numerous effects on cell phenotypes; and
• the ability to regulate the type of immune response generated and its extent.
Q. Aside from driving effector T cell responses, how else may APCs affect T cell responses?
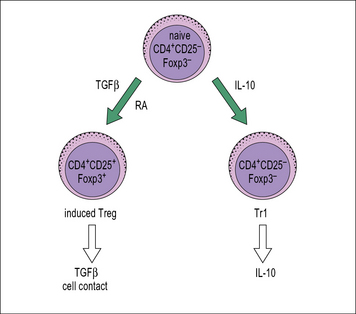
A. Dendritic cells or macrophages may produce cytokines such as TGFβ and IL-10 in response to some antigens. These cytokines can drive the conversion of naive CD4+ T cells into regulatory T cells (see Fig. 11.8), which can act as suppressors.
T cell regulation of the immune response
Differentiation into CD4+ TH subsets is an important step in selecting effector functions
• the sites of antigen presentation;
• co-stimulatory molecules involved in cognate cellular interactions;
• peptide density and binding affinity – high MHC class II peptide density favors TH1 or TH17, low densities favor TH2;
• APCs and the cytokines they produce;
• the cytokine profile and balance of cytokines evoked by antigen;
• receptors expressed on the T cell;
• activity of co-stimulatory molecules and hormones present in the local environment;
The cytokine balance controls T cell differentiation
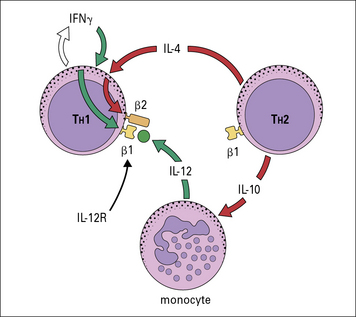
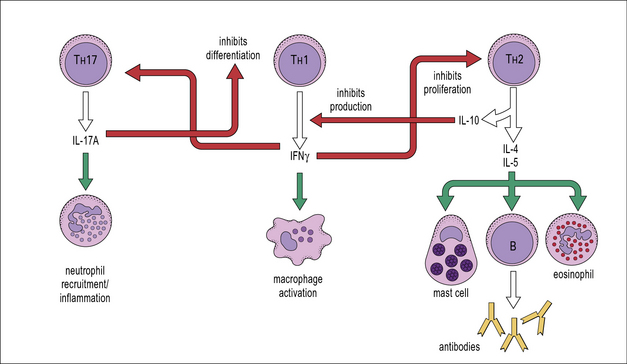
Cytokines from the various TH subsets can cross-regulate each other’s development. Thus, cross-regulation of TH subsets has been demonstrated whereby IFNγ secreted by TH1 cells can inhibit the responsiveness of TH2 cells; also IL-17A can inhibit the development of TH1 responses (Fig. 11.4) whereas IL-10 produced by TH2 cells downregulates B7 and IL-12 expression by APCs, which in turn inhibits TH1 activation.
The TH subset balance is modulated not only by the level of expression of cytokines such as IL-12 or IL-4, but also by expression of cytokine receptors. For instance, the high-affinity IL-12R is composed of two chains, β1 and β2, with both chains being constitutively expressed on TH1 cells. TH1, TH2, and TH17 cells express the β1 chain, but expression of the β2 chain is induced by IFNγ and inhibited by IL-4 (Fig. 11.5). Therefore cytokines reinforce the lineage decisions of the various TH subsets at least in part by controlling the expression of lineage specific receptors.
Q. Apart from the cross-regulation by cytokines described above, in what other ways can a TH1- or TH2-type immune response reinforce itself?
A. The chemokines induced by TH1 and TH2 cells tend to induce the accumulation of the same sets of lymphocytes and their associated effector cells (see Chapter 6).![]()
T cell plasticity
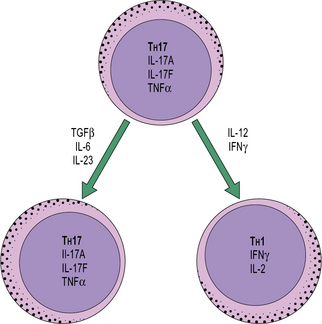
Recently, studies of TH cells have revealed considerable developmental plasticity. In particular, TH17 cells can be induced to produce IFNγ (a TH1 cytokine) and shut down production of IL-17A if they are stimulated in the presence of IL-12 (Fig. 11.w1). Similarly, at least in vitro, TH2 cells can be driven to a mixed TH2/1 phenotype by culture with type one IFNs and IL-12, where cells co-express IL-4 and IFNγ. The reasons for this flexibility in cytokine production remains to be further explored, but it is thought that such plasticity may enable rapid site-specific immune responses to infections to occur.
TH cell subsets determine the type of immune response
• local patterns of cytokine and hormone expression help to select lymphocyte effector mechanism; and
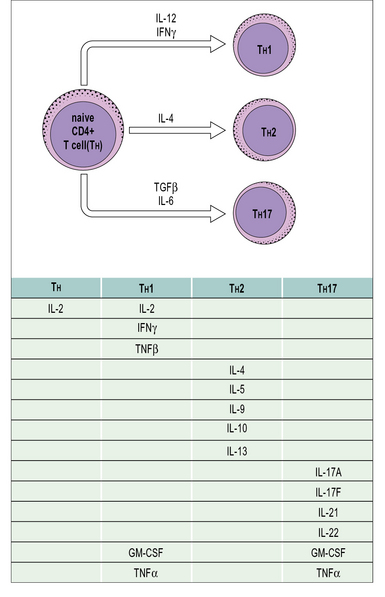
• the polarized responses of CD4+ TH cells are based on their profile of cytokine secretion (Fig. 11.6).
TH1 cytokines including IFNγ, TNFβ, and IL-2 also promote:
TH2 clones are typified by production of IL-4, IL-5, IL-9, IL-10 and IL-13 (see Fig. 11.4). These cells provide optimal help for humoral immune responses biased towards:
• IgG1 and IgE isotype switching;
• stimulation of mast cells, eosinophil growth and differentiation; and
CD8+ T cells can be divided into subsets on the basis of cytokine expression
However, both CTL1 and CTL2 cells can be cytotoxic and kill mainly by a Ca2 +/perforin-dependent mechanism (see Fig. 10.10).
Treg differentiation is induced by Foxp3
Comparison of CD4+CD25+ Tregs with naive and activated CD4+ T cells shows that regulatory cells selectively express Foxp3, a member of the forkhead/winged helix transcription factors (Fig. 11.7) essential for the development and function of CD4+CD25+ Tregs. Mutations in the Foxp3 gene cause immune dysregulation, polyendocrinopathy enteropathy, X-linked syndrome (IPEX). Individuals with this disease have increased autoimmune and inflammatory diseases.
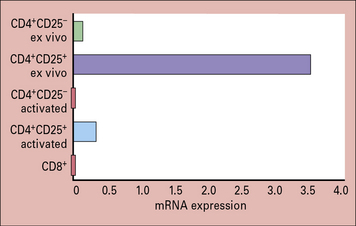
Fig. 11.7 Foxp3 is selectively expressed in Tregs
(Adapted from Fontenot JD, Gavin MA, Rudensky AY. Nat Immunol 2003;4:330–336.)
Reciprocal developmental relationship between induced Tregs and TH17 cells
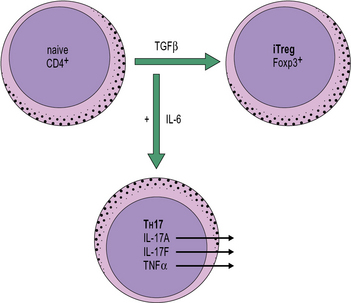
At least in mice, both induced Treg and TH17 cells are dependent on TGFβ for their development. In the presence of TGFβ, naive CD4+ T cells upregulate Foxp3 and develop into induced Tregs. In the additional presence, however, of inflammatory cytokines such as IL-6 or IL-1β, naive T cells develop into inflammatory TH17 cells that secrete cytokines such as IL-17A, IL-17F, and TNFα. This dichotomous relationship implies an important role for cytokines derived from the innate immune system in determining the outcome of an adaptive immune response (Fig. 11.w2).
Tr1 regulatory cells
CD4+ regulatory T cells can also be generated from naive TH cells in the presence of certain cytokines (Fig. 11.8) such as IL-10. These cells do not express CD25 or Foxp3 and they mediate suppression through the secretion of IL-10.
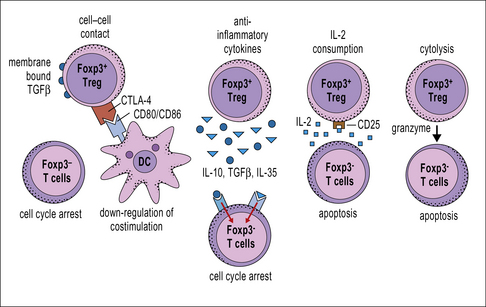
Mechanisms of Treg suppression
In spite of this at least four mechanisms of Treg suppression have been proposed (Fig. 11.9).
Role of Tregs in infection
Although CD4+ Tregs play a vital role in the prevention of autoimmune diseases (see Chapter 20), their role in infections is less clear. CD4+ Tregs have a protective role against immune-mediated pathology and their ability to suppress is important in reducing inflammation. For example, lesions in the eye in stromal keratitis are less severe in the presence of CD4+CD25+ Tregs.
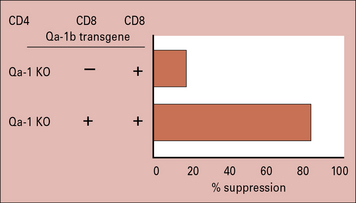
CD8+ T cells suppress secondary immune responses
In mice the non-classical MHC class Ib molecule, Qa-1, is crucial for suppression. Mice lacking Qa-1 are unable to suppress responses. However when Qa-1 is replaced into these cells their ability to suppress immune responses is restored (Fig. 11.10).
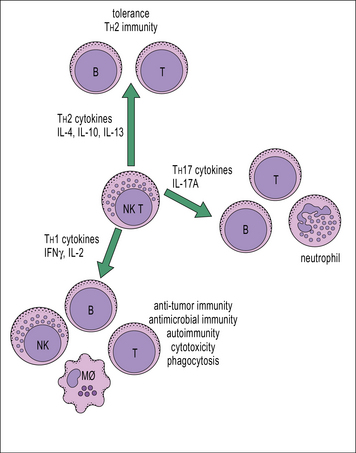
NK and NKT cells produce immunoregulatory cytokines and chemokines
NK cell activity itself is induced by a variety of cytokines including:
NK cells in turn are negatively regulated by cytokines such as IL-10 and TGFβ.
T cells secreting IFNγ are able to induce NK cell activation, increasing both NK proliferation and cytotoxicity. They are capable of making both TH1-type (IFNγ), TH2-type (IL-4), and TH17 (IL-17A) cytokines depending on the cytokines present in the microenvironment when they are activated (Fig. 11.11). These early sources of cytokine are important in influencing the nature of the T cell response.
Regulation of the immune response by immunoglobulins
Antibody has been shown to exert feedback control on the immune response.
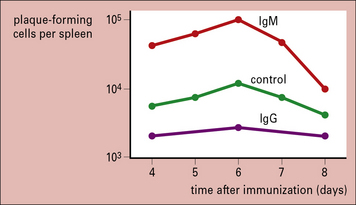
Passive administration of IgM antibody together with an antigen specifically enhances the immune response to that antigen, whereas IgG antibody suppresses the response. This was originally shown with polyclonal antibodies, but has since been confirmed using monoclonal antibodies (Fig. 11.12).
• certain vaccines (e.g. mumps and measles) are not generally given to infants before 1 year of age because levels of maternally derived IgG remain high for at least 6 months after birth and the presence of such passively acquired IgG at the time of vaccination would result in the development of an inadequate immune response in the baby;
• in cases of Rhesus (Rh) incompatibility, the administration of anti-RhD antibody to Rh– mothers prevents primary sensitization by fetally derived Rh+ blood cells, presumably by removing the foreign antigen (fetal erythrocytes) from the maternal circulation (see Chapters 24 and 25).
• IgM-containing immune complexes are taken up by Fc or C3 receptors on APCs and are processed more efficiently than antigen alone;
• IgM-containing immune complexes stimulate an anti-idiotypic response to the IgM, which amplifies the immune response.
IgG antibody can regulate specific IgG synthesis
IgG can suppress antibody responses in a number of ways.
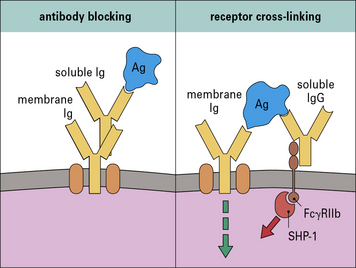
• Passively administered antibody binds antigen in competition with B cells (antibody blocking) (Fig. 11.13). In this case suppression is highly dependent on the concentration of the antibody, and on its affinity for the antigen compared with the affinity of the B cell receptors. Only high-affinity B cells compete successfully for the antigen. This mechanism is independent of the Fc portion of the antibody.
• Immunoglobulin can inhibit B cell differentiation by cross-linking (receptor cross-linking) the antigen receptor with the Fc receptor (FcγRIIb) on the same cell (see Fig. 11.13). In this case, the suppressive antibody and the B cell’s receptor antibody may recognize different epitopes.
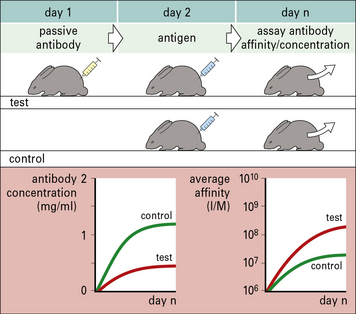
• Doses of IgG that are insufficient to inhibit the production of antibodies completely have the effect of increasing the average antibody affinity because only those B cells with high-affinity receptors can successfully compete with the passively acquired antibody for antigen. For this reason, antibody feedback is believed to be an important factor driving the process of affinity maturation (Fig. 11.14).
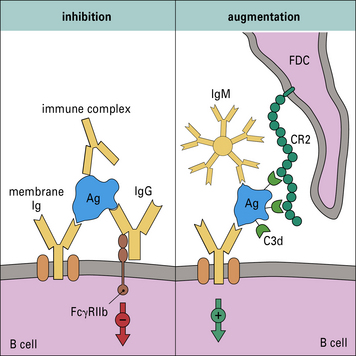
Immune complexes may enhance or suppress immune responses
Immune complexes can inhibit or augment the immune response (Fig. 11.15). By activating complement, immune complexes may become localized via interactions with CR2 on follicular dendritic cells (FDCs). This could facilitate the immune response by maintaining a source of antigen.
Apoptosis in the immune system
Apoptosis is a cellular clearance mechanism through which homeostasis is maintained.
• involved in clearing cells with a high avidity for antigen in the thymus and is an important mechanism of immunological tolerance (see Chapter 19);
• an important mechanism in maintaining homeostasis in the immune system.
A small number of cells are prevented from undergoing apoptosis and enter the memory T cell pool.
Immune regulation by selective cell migration
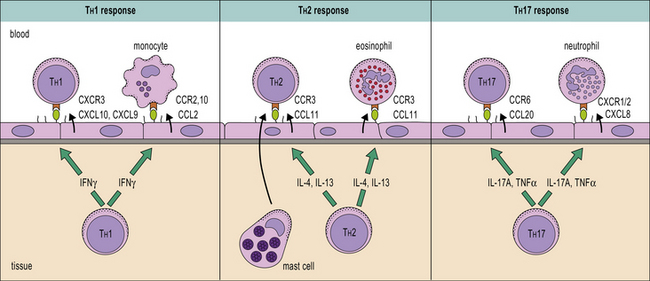
The expression of different chemokine receptors on TH1 cells (CXCR3 and CCR5), TH2 cells (CCR3, CCR4, CCR8), and TH17 (CCR6) allows chemotactic signals to produce the differential localization of T cell subsets to sites of inflammation (see Fig. 6.10).
Chemokines can be induced by cytokines released at sites of inflammation, so providing a mechanism for local reinforcement of particular types of response (Fig. 11.16). Once a response is established the T cells can induce further migration of appropriate effector cells. This is clearly illustrated in TH1 responses where the secondary production of CCL2, CCL3, CXCL10, and CCL5 serves to attract mononuclear phagocytes to the area of inflammation. Production of IL-17A can drive the expression of CCL20, the ligand for CCR6, recruiting more TH17 cells to the site of inflammation. The ability of cytokines such as TGFβ, IL-12, and IL-4 to influence chemokine or chemokine receptor expression provides a further level of control on cell migration or recruitment.
T cell expression of different molecules can mediate tissue localization
It has recently been suggested that there are two types of memory cell:
• central memory cells express CCR7, home to lymphoid tissues, and do not have immediate effector function;
• effector memory cells do not express CCR7, migrate to non-lymphoid tissues, and produce effector cytokines – effector cells in non-lymphoid tissues, such as the skin, can proliferate and senesce and this has a direct effect on the level of local immune responses.
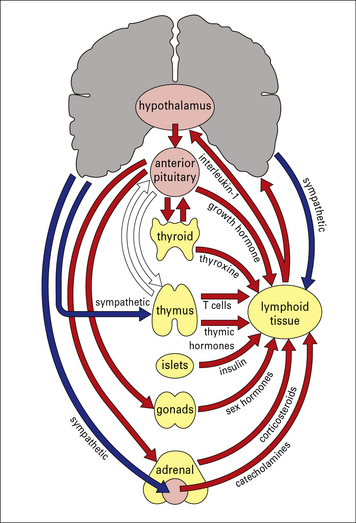
Neuroendocrine regulation of immune responses
It is now widely accepted that there is extensive cross-talk between the neuroendocrine and immune systems. Both systems share similar ligands and receptors that permit intra and inter-system communication. These networks of communication are deemed essential for normal physiological function and good health. For instance they play important roles in modulating the body’s response to stress, injury, disease and infection. The interconnections of the nervous, endocrine, and immune systems are depicted in (Fig. 11.17).
There are several routes by which the central nervous system and immune system can interact:
• most lymphoid tissues (e.g. spleen and lymph nodes) receive direct sympathetic innervation to the blood vessels passing through the tissues and directly to lymphocytes;
• the nervous system directly and indirectly controls the output of various hormones, in particular corticosteroids, growth hormone, prolactin, α-melanocyte-stimulating hormone, thyroxine, and epinephrine (adrenaline);
• immune derived growth factors and cytokines can in turn feed back on the neural and endocrine systems, which probably has an important role in regulating the use of the body’s resources.
• inhibit TH1 cytokine production while sparing TH2 responses; and
• induce the production of TGFβ, which in turn may inhibit the immune response.
Genetic influences on the immune response
• the development of techniques such as microsatellite mapping;
MHC haplotypes influence the ability to respond to an antigen
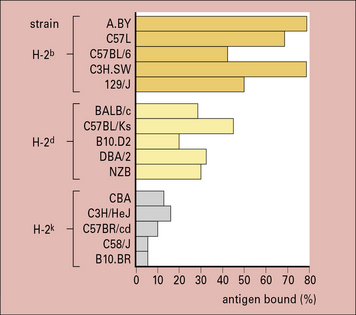
The development of inbred mouse strains conclusively demonstrated that genetic factors have a role in determining immune responsiveness. For example, strains of mice with different MHC haplotypes vary in their ability to mount an antibody response to specific antigens (Fig. 11.18). This function depends on MHC class II molecules (see Chapter 5) and is specific for each antigen – a high responder strain for some antigens may be a low responder strain for others.
MHC-linked genes control the response to infections
MHC-linked genes (see Chapter 5) are involved in the immune response to infectious agents. In some cases the gene involved is the MHC gene itself, but in others it can be a gene linked to the MHC.
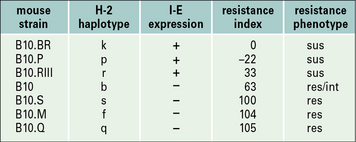
Susceptibility to infection by Trichinella spiralis is affected by the I-E locus in mice
The first observation that genes within the MHC could influence the response to parasites involved the susceptibility to Trichinella spiralis infection. The response of different recombinant mouse strains to infection with T. spiralis is affected by the I-E locus. Mouse strains that express I-E (B10.BR, B10.P) appear to be susceptible whereas those that do not (B10.S, B10.M) are resistant to infection (Fig. 11.19). The response to T. spiralis is also influenced by the MHC-linked gene Ts-2, which maps close to the TNF genes.
Many non-MHC genes also modulate immune responses
• individuals with defects in the complement components C1q, C1r, and C1s (see Chapter 4) are predisposed to develop systemic lupus erythematosus (SLE) and lupus nephritis – the development of SLE-like symptoms in C1q knockout mice parallels the human situation;
• deficiency in C3 leads to an increased susceptibility to bacterial infections and a predisposition to immune complex disease – this is also seen with a deficiency in C2 and C4, both of which are located within the MHC region;
• high IgE production in some allergy-prone families associates with the presence of an ‘atopy gene’ on human chromosome 11q;
• Biozzi generated two lines of mice based on their responsiveness to erythrocyte antigens. The high responder and low responder Biozzi mice make quantitatively different amounts of antibody in response to antigenic challenge and the basis for these differences has in part been attributed to genetic differences in macrophage activity; the high and low responder strains also differ in their ability to respond to parasitic infections, but this does not correlate with the level of antibody produced.
Non-MHC-linked genes affect susceptibility to infection
• upregulation of the oxidative burst;
• enhanced tumoricidal activity;
TLR4 polymorphisms, malaria and septic shock
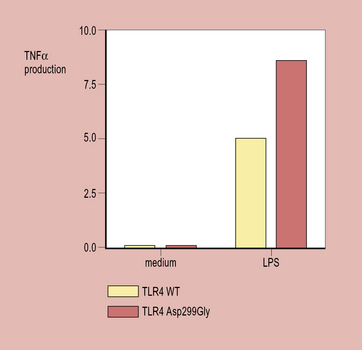
Analysis of the effect on TNF production by whole blood samples taken from individuals with wildtype TLR4 or the Asp299Gly TLR4 polymorphism (the ligand for TLR4) reveals a significant increase in TNF alpha production when samples are stimulated with LPS if the Asp299Gly allele is present (Fig. 11.w3).
Polymorphisms in cytokine and chemokine genes affect susceptibility to infections
The outcome of the mutation is dependent on which cytokine gene is affected.
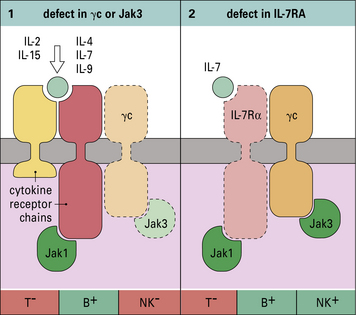
• mutations in the IL-7R α chain have a reduced number of T cells;
• whereas those with deficiency in the common cytokine receptor γ chain (γc), a component of IL-2, IL-4, IL-7, IL-9, and IL-15 receptors, have reduced numbers of T and NK cells and impaired B cell function, in part attributable to the lack of T cell help (Fig. 11.20).
Further examples are the mutations in the IFNγ receptor (IFNγR) or IL-12 receptor (IL-12R), which increase susceptibility to mycobacterial infection. A list of genetic defects that contribute to impaired immune responses is given in Figure 11.21.
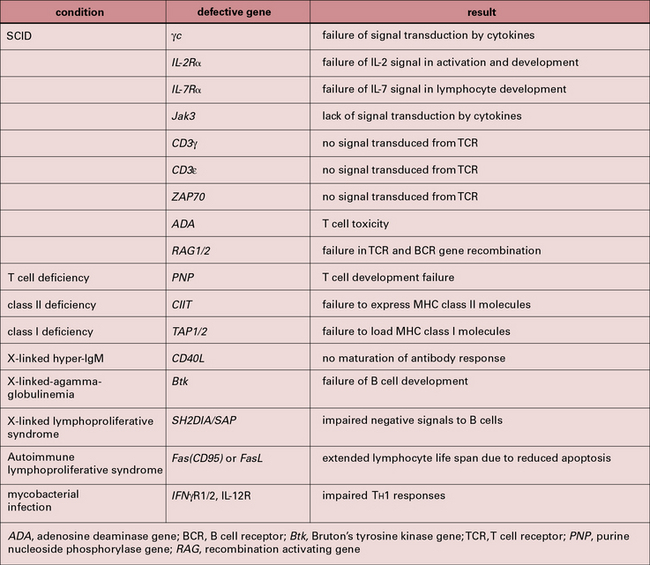
Fig. 11.21 Genetic defects associated with immune deficiency or abnormalities
Genetic defects associated with immune deficiency or abnormalities. See also Chapter 16.
(Based on a review by Leonard, Curr Opin Immunol 2000;12:465–467.)
This polymorphism in the TNFα promoter has also been associated with:
Agnello D., Lankford C.S., Bream J., et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161.
Bettelli E., Carrier Y., Gao W., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238.
Blalock J.E. Shared ligands and receptors as a molecular mechanism for communication between immune and neuroendocrine systems. Ann N Y Acad Sci. 1994;741:292–298.
Chess L., Jiang H. Resurrecting CD8+ suppressor cells. Nat Immunol. 2004;5:569–571.
Cua D.J., Tato D.M. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489.
Ferwerda B., McCall M.B., Alonso S., et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Nat Acad Sci U S A. 2007;104:16645–16650.
Heymann B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–738.
Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517.
Leonard W.J. Genetic effects on immunity. Curr Opin Immunol. 2000;12:465–467.
Metzler B., Wraith D.C. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993;5:1159–1165.
Mills K.H.G., McGuirk P. Antigen-specific regulatory T cells – their induction and role in infection. Semin Immunol. 2004;16:107–117.
Murphy K.M., Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–690.
Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5:285–294.
Shevach E.M. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645.
Taub D.D. Neuroendocrine Interactions in the Immune System. Cell Immunol. 2008;252:1–10.
Van der Vliet H.J.J., Molling J.W., von Blomberg B.M., et al. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin Immunol. 2004;112:8–23.
Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127.