25 Regional anesthesia
Analgesia: Medications administered to reduce or relieve painful stimuli.3
Anesthesia: Medications administered to render the entire or portion of the body insensible to painful stimuli.3
Alpha-Adrenergic Vasopressors: Medications resulting in peripheral vascular constriction to increase blood pressure. Phenylephrine, a pure alpha agonist with a fast onset and duration of 5 to 20 minutes, is most commonly administered. Phenylephrine can cause reflex bradycardia secondary to baroreceptor stimulation. Norepinephrine is less commonly administered and in low doses has primarily an alpha agonist effect (see Chapter 11).4
Combined Spinal Epidural Technique: Combines the administration of a spinal anesthetic with the placement of an epidural catheter. An epidural needle is placed into the epidural space. A spinal needle is then passed through the epidural needle and medication is injected into the subarachnoid space for spinal anesthesia. The spinal needle is withdrawn and an epidural catheter is placed. The epidural catheter can be used to prolong the duration of anesthesia or to administer postoperative analgesia.5
Dermatome Level: Spinal nerve roots innervate skin in horizontal 1- to 2-inch bands. Each dermatome corresponds to a specific spinal nerve root. Knowledge of dermatome levels allows the perianesthesia nurse to assess the level of neuraxial blockade. Assessment of dermatome levels can be accomplished with a small pin, wet cotton ball, or alcohol wipe.6
Ephedrine: A vasopressor that has direct effects on alpha and beta receptors. It indirectly stimulates the release of catecholamines. Onset is fast, with a duration of action of 1 to 1.5 hours.4
Epidural Block: Placement of local anesthetic in the epidural space that results in blockade of the spinal nerves as they exit the subarachnoid space. An epidural can be used as a sole anesthetic, with general anesthesia, or postoperative analgesia. It can be placed in the thoracic or lumbar epidural space.6
High Spinal Block: Excessive spread of local anesthetic within the subarachnoid space which leads to apnea, loss of consciousness, and hypotension.7
Neuraxial Blockade: Generic term applied to spinal, epidural, or caudal anesthetics.6
Palsy: Traumatic injury to a peripheral nerve that results in a loss of motor and normal sensory function.3
Paralysis: Traumatic injury to a central nervous system nerves resulting in the loss of motor function and normal sensory function.3
Postdural Puncture Headache (Spinal Headache): Headache characterized by an increased intensity of symptoms when in an upright position and a decrease or absence when supine; caused by a leak of cerebral spinal fluid after the dura has been transversed with a needle.7
Tuffier’s Line: Landmark used to identify appropriate levels for administration of neuraxial blockade. A line drawn across the iliac crests will generally cross the interspace of L3-L4.5
Regional anesthesia provides several benefits over general anesthesia. It reduces the number of medications required to induce and maintain general anesthesia, avoids insertion of an airway device, and decreases mortality and morbidity. Specifically it has been found that regional anesthesia decreases the incidence of deep vein thrombosis, pulmonary embolism, transfusion, pneumonia, respiratory depression, myocardial infarction, and renal failure while preserving immune function that may decrease the incidence of infection. In addition, regional anesthesia may have a positive effect on postoperative cognitive function and enhance the return of gastrointestinal mobility.1 Risks include transient or permanent nerve trauma, local anesthetic toxicity, and technique specific complications.2 Considerations include patient selection, surgeon preference, and surgical procedure. Regional anesthetic techniques should increase as technological advances improve block placement, research supports its benefits, and pharmacologic advances improve the safety of local anesthetics.
Neuraxial anesthesia and analgesia
Anatomy of the spine
The vertebral column provides support and attachment to the skull, thorax, and pelvis. It is composed of 33 vertebrae (7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal). Each thoracic vertebra articulates to a corresponding rib. Individual vertebra consists of a pedicle, transverse body, superior articular process, inferior articular process, and spinous process. Individual vertebral bodies have an intervertebral disk connecting them while two superior and inferior articular processes articulate above and below each vertebra. Pedicles are notched superiorly and inferiorly; this allows a space for individual spinal nerves to exit from the spinal cord. The anatomy of the bony spine affects the administration of neuraxial blocks. The spinous process and lamina of a vertebra create the posterior boundary of the spinal canal. Angulation of the spinous process affects needle orientation during insertion. In the lumbar area, spinous processes are almost horizontal with flexion. In the thoracic area they are angled in a caudad fashion with flexion. A second factor is the size of the interlaminar spaces. The larger the interlaminar space the easier the access. Generally the lumbar interlaminar spaces are larger than those in the thoracic region.5,8,9
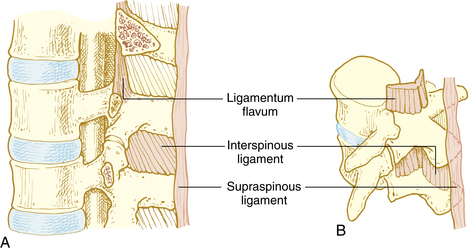
Ligaments of the vertebral column are responsible for maintaining shape of the column, providing support, and protection of the spinal cord. Vertebral bodies and disks are connected by the anterior and posterior longitudinal ligament on the anterior side of the vertebral column and the supraspinous ligament, intraspinous ligament, and ligamentum flavum on the posterior side (Fig. 25-1). When a midline spinal puncture is made, the needle traverses these three ligaments.5,8,9
The spinal cord is a continuation of the medulla oblongata, occupying the upper two thirds of the vertebral canal. It is approximately 18 inches long and ends at the lower border of L1 in most adults. The spinal cord ends in the conus medullaris and cauda equina. Nerve roots are not fixed, as the spinal cord is, making it difficult to cause trauma to nerve roots; this makes it safe to place a spinal needle below L2 in the adult patient. The lower portion of the spinal cord becomes the filum terminale, which connects to the bone of the coccyx and holds the spinal cord in place.8–10 The spinal cord is encased by three membranes: dura, arachnoid, and pia mater. The outermost membrane, dura mater, consists of two layers. Between the dura and the ligamentum flavum is the epidural space, a potential space filled with loose fatty tissue and blood vessels. Local anesthetics are injected in this space for epidural anesthesia. The arachnoid layer consists of a thin membranous sheath. The innermost layer is called the pia mater and is separated from the arachnoid mater by a subarachnoid space filled with cerebrospinal fluid (CSF). The subarachnoid space is where local anesthetics are deposited for spinal anesthesia (Fig. 25-2). The total CSF volume in the adult is approximately 100 to 150 mL, with 25 to 35 mL of the volume residing within the subarachnoid space. CSF, continually produced by the choroid plexuses, is reabsorbed into the blood through the arachnoid villi, granulation, and to a small extent through epidural veins. The specific gravity of CSF, which ranges between 1.004 and 1.009, affects the spread of local anesthetic solution depending on its baracity. A hyperbaric medication will move toward dependent areas because it is heavier than CSF, whereas a hypobaric solution will do the opposite. Isobaric medications stay within the general area of injection.5,8,9
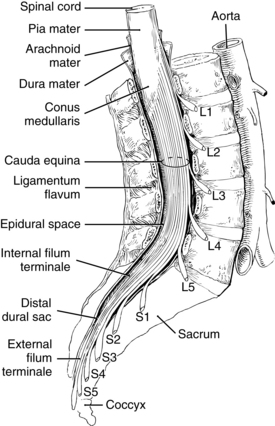
FIG. 25-2 Extension of the spinal cord to the second lumbar vertebra.
(From Nagelhout J, Plaus K, editors: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.)
Thirty-one pairs of spinal nerves travel from the spinal cord and exit at their respective intervertebral foramina (C1-S5). There are 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal pair of spinal nerves. These nerves are blocked by the local anesthetic medication to produce anesthesia or analgesia. Spinal nerve roots vary in size and can be divided into dorsal roots (primarily sensory) and anterior roots (primarily motor).5,8,9
Spinal versus epidural blocks
Spinal and epidural blocks are neuraxial blocks. Both block spinal nerve roots, the site of administration differs as does the mechanism of action (Table 25-1). A spinal anesthetic blocks spinal nerve roots as they pass through the CSF, resulting in blockade of sensory, motor, and autonomic impulse transmission whereas epidural anesthesia and analgesia blocks spinal nerve roots outside the subarachnoid space. Epidurals require a large dose of local anesthetic to allow for diffusion through anatomic barriers, such as the dura, and spreads horizontally and vertically within the epidural space. The epidural space is vascular, and there is a risk of local anesthetic toxicity (see Chapter 24). Spinal anesthesia is accomplished by administering a small dose of local anesthetic within the subarachnoid space. Spinal anesthesia is a single injection through a needle and reduces the time for administration but does not allow for repeated dosing. Epidural anesthesia is administered through a catheter that is placed through a needle, may require more time to place, may be dosed again, and used for postoperative analgesia. Spinal anesthesia is limited to placement in the lower lumbar region below the termination of the spinal cord. Epidural catheter placement can occur in the lumbar or thoracic region. Spinal anesthesia is rapidly achieved and produces an intense sensory and motor block. Rapid onset can lead to profound hypotension. Epidural anesthesia onset is gradual, blockade is less profound, and decreases in blood pressure are less intense.7,9
Table 25-1 Differences Between Spinal and Epidural Anesthesia
| SPINAL | EPIDURAL | |
|---|---|---|
| Site or mechanism of action | Nerve roots blocked as they pass through the CSF | Nerve roots blocked outside the cerebrospinal fluid |
| Site of administration | Lower lumbar below the termination of spinal cord | Lumbar or thoracic area |
| Dose of local anesthetic | Small | Large |
| Instrument for administration | Needle | Catheter |
| Ability to repeat dose | No | Yes |
| Onset | Rapid, intense blockade, may lead to hypotension | Gradual; may have less intense blockade, decrease in blood pressure is more gradual |
Contraindications
Absolute contraindications include patient refusal; cardiovascular diseases such as severe aortic–mitral stenosis and asymmetrical septal hypertrophy; severe uncorrected hypovolemia; documented allergy to local anesthetic; increased intracranial pressure; infection at the site of injection; sepsis or bacteremia; and coagulopathy.1,5,7,9 Coagulation-altering medications should be reviewed and current recommendations should be followed to avoid the potentially catastrophic complication of a neuraxial hematoma.10 Current guidelines related to anticoagulation therapy and regional anesthesia can be accessed and reviewed at http://asra.com/publications-anticoagulation-3rd-edition-2010.php.11 Relative contraindications include preexisting neurologic disease, musculoskeletal abnormalities, prior back surgery, and untreated hypertension.1,5,7,9 Placing a neuraxial block through a tattoo is controversial.12 The perianesthesia nurse should alert the anesthesia provider to potential contraindications.
Spinal anesthesia
Universal protocol, as outlined by The Joint Commission, should be followed and medications should be labeled before the procedure.13 An initial set of vital signs should be documented. Patients may be positioned in a lateral decubitus or sitting position with the back flexed to maximize the intervertebral opening (Figs. 25-3 and 25-4). Tuffier’s line is generally used to identify the 3rd and 4th lumbar spaces, but may not always be reliable.14 After the anesthesia provider has identified the desired interspace, he or she will follow sterile technique (mask, sterile gloves, hat) and proceed with preparing and placing sterile drapes. The perianesthesia nurse should maintain verbal contact with the patient to reassure and inform them of what to expect. Local anesthetic is used to anesthetize the skin and underlying tissue. A 25-gauge or smaller spinal needle is used in patients at risk for a postdural puncture headache (PDPH): patients younger than 50 years, females, pregnancy, and a prior history of PDPH. Needle design is an important consideration in at risk groups, with pencil point needles being less likely to cause a PDPH.1,15 A 22-gauge cutting tip needle can be used in patients older than 50 years. The spinal needle will transverse the supraspinous and interspinous ligaments, ligamentum flavum, and dura; this is noted by a change in the resistance. The stylet is removed and CSF is noted at the hub of the needle. Procaine, bupivacaine, or tetracaine are commonly used for spinal anesthesia. Epinephrine (0.1 to 0.2 mg) or phenylephrine (2 to 5 mg) can be added to increase duration of action. Local anesthetic is administered and patient positioned. Vital signs should be monitored at 1-minute intervals for the first 10 minutes and then, if stable, every 5 minutes. Blood pressure decreases of 20% or more should be treated with fluids and vasopressors (ephedrine or phenylephrine).1,5,7,9
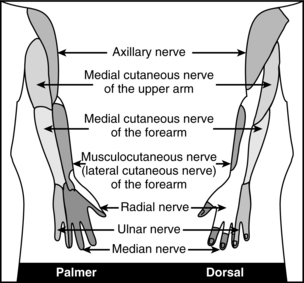
Progression of onset is noted by loss of autonomic or sympathetic function, sense of temperature, pain, touch, movement and proprioception. During recovery, function will return in the opposite order (Box 25-1). Spinal anesthetics are dosed for specific surgical procedures (Table 25-2). Level of anesthesia is monitored by dermatome level (Fig. 25-5). Monitoring regression of spinal anesthetic is aided with the knowledge of the dermatome level and can be assessed by the use of an alcohol wipe, a blunt needle that does not break the skin, or a light pinch.5,6
From Schick L, Windle PE, editors: Perianesthesia nursing core curriculum: preprocedure, Phase I and Phase II PACU nursing, ed 2, St. Louis, 2010, Saunders.
Table 25-2 Dermatome Level and Surgical Procedure
| SENSORY LEVEL | SURGICAL PROCEDURE | CUTANEOUS LEVEL |
|---|---|---|
| Above T4 | Pinky, inner arm, apex of axilla | |
| T4 | Cesarean section, upper abdomen, uterine | Nipple |
| T6-7 | Lower abdomen | Xiphoid |
| T7-8 | Tourniquet pain | |
| T10 | Hip and genitourinary | Umbilicus |
| L1-3 | Lower extremities | |
| S2-5 | Perineal region (genitalia/buttocks) |
Modified from Schick L, Windle PE, editors: Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2, St. Louis, 2010, Saunders.
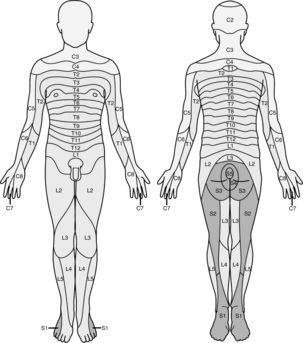
FIG. 25-5 Dermatomes.
(From Nagelhout J, Plaus K, editors: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.)
Epidural anesthesia
Epidural anesthesia or analgesia is similar to spinal anesthesia in regard to positioning, sterile technique, monitoring, progression, and assessment. Epidural anesthesia can be a single shot technique (i.e., caudal anesthesia) or more commonly as an intermittent or continuous technique for anesthesia or analgesia. Anesthesia may be administered for the same type of surgical procedures as spinal anesthetics (see Table 25-2), but may be less effective for procedures of the perineum because of sacral sparing. Epidurals are versatile for analgesic purposes and may be used for labor and delivery or postoperative analgesia for surgical procedures ranging from the thorax to lower extremities. Epidural needles are larger than spinal needles to allow for the insertion of a catheter. The average needle is 9 cm and is marked to indicate the depth of insertion. An epidural needle transverses the supraspinous and intraspinous ligament and seated in the ligamentum flavum. A special loss-of-resistance syringe with preservative-free saline is attached to the hub, and the needle is advanced until a loss of resistance is noted upon entering the epidural space. An epidural catheter is then inserted to a desired depth. Epidural catheters are marked to indicate the depth of insertion.1,5,7,9,16 A 3-mL test dose of 1.5% lidocaine with 1:200,000 epinephrine is administered to help detect intravascular, subarachnoid, or subdural placement.17 If positive, the heart rate and systolic blood pressure will increase by at least 20 beats/min and 15 mm Hg, but may not be reliable in the presence of beta blockers, advanced age, and general anesthesia.18 A subarachnoid injection will result in a spinal anesthetic, whereas a subdural injection can result in an extensive block relative to a small dose of local anesthetic.19 Common local anesthetics used for epidural anesthesia include 2-chloroprocaine, lidocaine, bupivacaine, and ropivacaine. Epidurals are dosed based on the number of dermatomes that are to be blocked (1 to 2 mL per dermatome). Dosing occurs slowly with no more than 5 mL of local anesthetic administered at a time because there is a risk of local anesthetic toxicity. Epinephrine (1:200,000) can be used to decrease absorption and prolong duration. Epidural catheters should be monitored for signs of drainage or dislodgement. The site is covered with a sterile dressing and connections should be tight. The American Society of PeriAnesthesia Nurses position statement on safe medication administration20 and local institutional policy should be followed for the administration of medications. When initiating a continuous infusion check dose and concentration of local anesthetic solution and confirm the infusion rate.
Combined spinal-epidural is a versatile technique that allows for the administration of a spinal anesthetic for immediate anesthesia and an epidural to extend the duration of the anesthetic or provide for postoperative analgesia. It is similar to spinal–epidural anesthesia in regard to positioning, sterile technique, monitoring, progression, and assessment.
Neuraxial complications
High spinal block
Excessive spread of local anesthetic that occurs with spinal or epidural anesthesia is a high spinal block. Signs and symptoms include high sensory block resulting in upper extremity sensory and motor changes, nausea and vomiting, loss of consciousness, anxiety, hypotension, bradycardia or asystole, respiratory distress or apnea. Treatment is dependent on extent of spread. Airway, breathing, and circulation are primary considerations. Supplemental oxygen, assistance with a bag-mask-valve device, or intubation may be required. The patient may be aware of the surrounding environment and verbal reassurance is important to reduce anxiety. Hypotension is caused by an extensive sympathectomy and treated with fluid bolus, elevation of the legs, and vasopressors (see Chapter 11), which may include phenylephrine, norepinephrine, ephedrine, or epinephrine. Bradycardia is treated with ephedrine or atropine. Phenylephrine and norepinephrine administration can cause reflex bradycardia and should be used with caution in the setting of significant bradycardia. Asystole is treated with established ACLS protocol. High spinal blocks are generally self limiting, and the patient will improve as the concentration of local anesthetic declines.1,5,7,9
Hypotension
Postoperative hypotension can be caused by sympathectomy and can occur with spinal or epidural anesthesia. Treatment consists of raising the legs, fluid bolus, and vasopressors (i.e., ephedrine or phenylephrine). Refractory hypotension can be treated with a continuous infusion of phenylephrine, norepinephrine, or low-dose epinephrine. It is important to rule out other causes of hypotension during stabilization, including hemorrhage or cardiovascular complications (see Chapter 29).1,5,7,9
Nausea and vomiting
When associated with neuraxial blockade, the precipitating factor of nausea and vomiting is often hypotension. Supplemental oxygen should be applied and blood pressure should be measured. Treat hypotension, and if nausea does not resolve seek other causes.1,5,7,9
Urinary retention
Urinary retention is often caused by autonomic blockade of the sacral nerves, resulting in a hypotonic bladder. Other causes such as surgical genitourinary trauma, pain, opioids, and renal failure should be ruled out. Patients may exhibit restlessness and incoherence. A neurologic insult, electrolyte disorders, hypoxia, pain, and abnormal glucose levels should also be ruled out. A bladder scan can help to confirm retention, and insertion of a urinary catheter is the treatment.9,21
Hypothermia
Regional anesthesia results in centrally mediated vasodilatation and inhibits peripheral vasoconstriction, transferring body heat from the core to periphery through radiation, convection, conduction, and evaporation. Recognition of hypothermia and initiation of measures to return the patient to a normal temperature are important considerations.22 (see Chapter 53).
Postdural puncture headache
This condition is caused by leaking CSF through the dura, which can occur with spinal anesthesia or inadvertent dural puncture with an epidural. Symptoms usually occur 24 to 48 hours after dural puncture. Symptoms include a headache in the occipital-frontal region that intensifies when upright and declines when supine, nuchal rigidity, visual and auditory disturbances, and nausea and vomiting. Significant neurologic complications such as meningitis, intracranial tumors, and hemorrhage should be ruled out. Treatment is initially symptomatic and includes bed rest, fluids, caffeine, and analgesics. If persistent, an epidural blood patch is the definitive treatment and is highly successful (95% success rate). The anesthesia provider accesses the epidural space at one space below the dural puncture; 15 to 20 mL of the patient’s blood is drawn aseptically and administered through the epidural needle. The patient remains on bed rest for 30 to 60 minutes before ambulation. Complications include back pain, fever, meningitis, and arachnoiditis.5,9,23,24
Neurologic complications
The overall incidence after neuraxial blockade is 0.04%, and permanent injury is rare.25 Palsies, paralysis, or pain should be reported immediately to the anesthesia provider and surgeon. This complication may be related to traumatic needle placement, bleeding or hematoma formation, surgical trauma, or injury related to a portion of the body being insensate. A neurologic consultation may be required to help diagnose and manage the patient’s condition.5,9
Transient neurologic symptoms
These symptoms are most often associated with spinal lidocaine, although it can occur with any local anesthetic. Signs and symptoms usually appear within 24 hours of block resolution and consist of low back pain with radiation to the lower extremities. It is important to rule out other more serious causes. It is generally self limiting with full recovery occurring in 2 days.26 Cauda equina syndrome is associated with lidocaine and continuous spinal microcatheters. It manifests with low back pain with radiation to the lower extremities as well as sensory and motor changes. Prospects of recovery are poor.9 Infectious complications such as meningitis, epidural abscess, and arachnoiditis can occur but are extremely rare.5,9
Spinal or epidural hematoma
Spinal or epidural hematoma is rare but devastating. It is more likely to occur in patients with altered homeostasis. Symptoms include low back pain, motor changes, and bowel or bladder dysfunction. Treatment is emergent decompression, but recovery is poor if surgery is delayed more than 8 hours. Awareness of coagulation-altering medications and notification of anesthesia personnel may prevent this complication from occurring.5,10
Care of patients after neuraxial blockade
In addition to routine PACU Phase I care for patients receiving sedation, the perianesthesia nurse should perform a continual assessment for complications as outlined. Rapid intervention is required for hypotension or bradycardia. Care should be taken not to rapidly change the patient’s position, as this may cause hypotension related to residual sympathectomy. Ensure the patient’s insensate limbs are positioned in correct anatomic alignment and padded to prevent injury.27 Any concerns should be reported to the anesthesia provider. Before discharge, the patient should meet the standards for discharge criteria that are applicable to all patients recovering from anesthesia. Additional concerns include a less than 10% decrease in blood pressure with position change and a sensory level equal or less than T10 with evidence that the block is receding by at least two sensory segments. There is no required minimum stay time, but length of stay should be tailored to the individual patient’s needs.28
Peripheral nerve blocks
Peripheral nerve blocks may be used as a sole anesthetic, in combination with general or neuraxial anesthesia, or for postoperative analgesia. Advantages include a reduction in postoperative pain, decreased nausea and vomiting, reduces complications associated with general anesthesia, and may reduce time to discharge for outpatient procedures.29 Generalized absolute contraindications include patient refusal, documented allergy to local anesthetics, coagulopathy, and infection at the site of injection.30,31 Specific peripheral nerve block contraindications are noted in their descriptions. Relative contraindications include uncooperative patients and neurologic disorders.30,31 If the perianesthesia nurse identifies contraindications, the anesthesia provider should be notified.
Ultrasound
Ultrasound technology furnishes anesthesia providers with anatomic images. Ultrasound guidance for regional anesthesia was first described in 1978.32 Technological advancement, affordability of equipment, and research has increased its use and acceptance. Benefits include anatomic identification of structures in real time; confirmation of local anesthetic spread; increased success rate, quality, and faster onset time for sensory blockade; decrease in number of attempts; and identification of vascular structures. At this time there is no conclusive evidence that it decreases the risk for local anesthetic toxicity. Successful and safe use of ultrasound is user dependent.33–37
Ultrasound waveforms are created by piezoelectric crystals. Electrical impulses cause each crystal to produce sound waves, called an ultrasound beam, which travel through tissue. Echoes are transmitted back to the transducer, converted to an electrical impulse, and then to an image. Biologic tissue varies in its resistance to sound waves, and echoes are reflected back at varying speeds. In general the best position for a transducer, in relation to an anatomic structure, is 90 degrees because the transducer will capture most of the reflected echoes.38,39 See Box 25-2 for common terms associated with the use of ultrasound.
BOX 25-2 Terms Associated with the Use of Ultrasound
Anechoic: Tissue with no reflective index appears gray (fluid filled structures are anechoic).
Curved Array Probe: Ultrasound waves are transmitted in a fanlike fashion.
Echo: The reflection of acoustic impedance is collected by the probe from the tissue.
Hyperechoic: Tissue with a high reflective index appears brightly (bones and tendons).
Hypoechoic: Tissue with a low reflective index appears dark.
In Axis: Ultrasound beam is orientated to view the nerve in its entirety.
In Plane: Ultrasound beam is orientated to view the needle in its entirety.
Linear Array Probe: Ultrasound waves are transmitted in a straight, frontal direction.
Out of Axis or Off Axis: Ultrasound beam is orientated to view the nerve as a cross section.
Out of Plane or Off Plane: Ultrasound beam is orientated to view the needle as a cross section.
(From Chan VWS, et al: Ultrasound imaging for regional anesthesia: a practical guide, ed 2, Toronto, 2007, Toronto Printing Company; Falyar CR: Ultrasound in anesthesia: applying scientific principles to clinical practice, AANA J 78:332-341, 2010.)
Basic ultrasound anatomy and characteristics
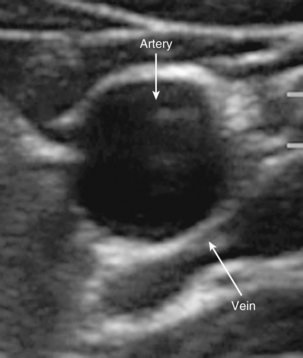
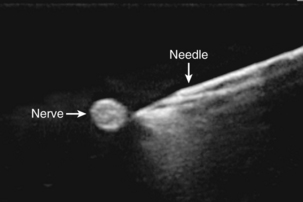
Fat is hypoechoic with hyperechoic lines. Fascial layers appear hyperechoic. Muscle appears hypoechoic with several hyperechoic striations more numerous than those seen in fat. Ultrasound waves do not penetrate bone and appear hyperechoic with a dark shadow below. Arteries are round and pulsating, whereas veins are rounded and compressible. Because arteries and veins contain blood, they appear as anechoic (do not reflect echoes; Fig. 25-6). Color Doppler can help to identify vasculature. A red image indicates flow toward the transducer, and blue indicates the opposite. Orientation of the transducer is important because a 90-degree angle might not detect any flow. If the transducer is oriented to the vessel so that the blood flow is going away from the transducer, it will appear blue despite being an artery.38
Transducer orientation
To obtain an optimal image, the anesthesia provider manipulates the transducer by aligning, tilting, rotating, and sliding. Transducer position relative to nerve position is termed axis. In axis means that the beam is oriented to view the nerve in its length, and out of axis indicates that the beam is oriented to view the nerve as a cross section. Transducer-to-needle orientation is termed plane. An in-plane orientation uses the ultrasound beam to view the needle in its length, and out-of-plane orientation indicates that the needle is viewed as a cross section. Combining terms of axis and plane define the overall approach to a peripheral nerve block. Approaches include in axis/in plane, in axis/off plane, off axis/in plane, and off axis/off plane. Two of the most common approaches are off axis/off plane (Fig. 25-7) and off axis/in plane.38,39
Assisting with ultrasound guided peripheral nerve blocks
Perianesthesia nurses may be involved with placement of peripheral nerve blocks with ultrasound. Sterile preparation and basic ultrasound controls should be understood. Anesthesia providers choose the transducer based on the peripheral nerve block. Low-frequency probes are used to identify anatomic structures that are more than 4 cm below the skin surface (i.e., the sciatic nerve). A frequency of less than 7 MHz allows for deeper penetration at the expense of image quality. High-frequency probes are used for superficial structures (less than 4 cm, brachial plexus or femoral nerve) and have a frequency greater than 7 MHz, limiting tissue penetration but producing a superior image. Transducers are linear or curved. A linear probe will provide better images because waves are transmitted in a linear fashion, allowing transmission of sound waves back to the transducer at the cost of a narrow field of view. Curved probes provide a wide field of vision, but lower resolution because of scattering of returning sound waves.38–40
For a single injection, clear sterile occlusive dressings can be applied to the transducer after gel has been applied. Sterile gel should be available for the skin. Alternatively, a sterile sleeve may be applied for single injection techniques or insertion of a peripheral nerve catheter. Meticulous attention to sterile technique is an important role of the perianesthesia nurse to avoid the introduction of contaminants to the injection site. Notify the anesthesia provider of inadvertent breaks in sterility.40 Knowledge of basic ultrasound controls is important because adjustments to frequency, gain, and depth may be required. Frequency is determined by the transducer; however fine adjustments may improve the image. Gain increases or decreases amplification of ultrasound echoes. Increases brighten the image but produce artifact, whereas decreasing gain darkens the image. Key anatomic structures may be either more superficial or deep than initially anticipated necessitating adjustments in depth. Application of the color Doppler function may be required to determine whether a structure is a nerve, vein, or artery.38,40, 41
Peripheral nerve stimulators
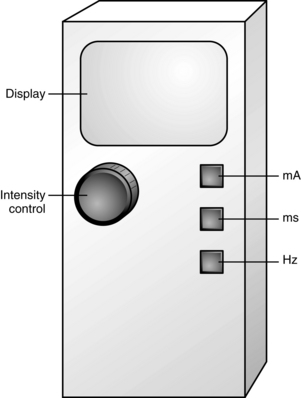
Peripheral nerve stimulators can be used as a standalone technique or as an adjunct to ultrasound for the placement of peripheral nerve blocks. Electrical stimulation of large alpha motor fibers occur at low intensities (measured in milliamps). Pain is a function of small delta fibers and can be elicited with high-intensity stimulation. Use of low-intensity currents elicits a motor response without patient discomfort. The patient should be forewarned that they will experience involuntary movement of muscles. Insulated needles are used to concentrate electrical current at the tip of the needle. The intensity is decreased as the nerve is located. Positive and negative leads are required to complete the circuit. A negative lead is attached to the needle and a positive lead is attached to the patient with an electrocardiogram pad. Good contact with the skin is important for accurate use.30,40,42,43
The perianesthesia nurse may be asked to assist in adjusting the stimulator, subject to state board of nursing regulations and institutional policy. There are three common controls on a nerve stimulator that the perianesthesia nurse should know: current (intensity of stimulation in milliamperes), pulse width (duration of stimulation in milliseconds), and frequency (frequency of stimulation in hertz; Fig. 25-8). A short pulse width of 100 to 200 μs allows the identification of alpha fibers for most peripheral nerves, while the optimal frequency is 2 to 2.5 Hz. The milliampere control is manipulated during block placement; initially it is set at a higher level of intensity. The greater the distance the needle is from the nerve, the greater the intensity required to elicit a response. As the needle is advanced toward the target nerve, the intensity is decreased while still obtaining a motor response. The perianesthesia nurse may be asked to assist in administering the injection under the guidance of the anesthesia provider, subject to state board of nursing regulations and institutional policy. When the nerve is located, aspiration should occur to verify that the needle is not within a vessel. If the needle is not within a vessel, a small test dose of local anesthetic is administered to confirm placement. Injection of 2 to 3 mL of local anesthetic will cause contractions to stop; this is a positive Raj test result. Significant resistance to injection may indicate an intraneural injection and should be immediately stopped. Injection should be slow and in increments of 5 mL, with aspiration occurring before each injection. The patient should be closely monitored for signs and symptoms of complications.30,40,42,43
Peripheral nerve block catheters
Peripheral nerve catheters are used for postoperative analgesia. Markings on the catheter measure the depth of insertion. The thick black line that indicates the needle length is followed by smaller markings measured in centimeters. Catheters may be stimulating or nonstimulating. Stimulating catheters are used for placement with a peripheral nerve stimulator. The target nerve is located with a needle. After the desired stimulation is achieved, the catheter is placed inside the needle. The negative lead is attached and the catheter is advanced while maintaining muscle contractions at the desired level of stimulation. Catheters can be tunneled under the skin and are inserted to the desired depth. A test dose of local anesthetic helps to determine correct placement. When in place, the catheter is secured and a bolus can be given, followed by a continuous infusion. Nonstimulating catheters can be placed under the guidance of ultrasound. Because the catheter is placed under direct visualization, nerve stimulation is not required. Some providers use ultrasound and a stimulating catheter to confirm placement. Peripheral nerve catheters may be placed during the preoperative or postoperative periods. Perianesthesia nurses may assist placement. Reassuring the patient, monitoring vital signs, monitoring for inadvertent vascular injection, and attention to sterile technique are important roles.40
Peripheral nerve catheters should be monitored for signs of drainage or dislodgement. The perianesthesia nurse should ensure the site is covered with a sterile dressing, and that connections are tight. When initiating a continuous infusion, check the dose and concentration of the local anesthetic solution and confirm the infusion rate. Any concerns should be addressed with the anesthesia provider.40
Nursing considerations for peripheral nerve blocks
Perianesthesia nursing actions are outlined in Box 25-3.
BOX 25-3 General Perianesthesia Nursing Actions for Peripheral Nerve Blocks*
Before peripheral nerve blockade
• Notify the anesthesia provider of potential contraindications.
• Document baseline vital signs.
• Medications in syringes should be clearly labeled.
• Resuscitative equipment (i.e., crash cart, bag-mask apparatus, lipid emulsions) should be immediately available.
• Time out. The anesthesia provider and perianesthesia nurse should confirm that consent has been obtained and identify correct patient, peripheral nerve block, limb, and side.
During peripheral nerve blockade
• Patient monitoring should include intermittent blood pressure recordings, continuous electrocardiography, pulse oximetry, mental status, and end tidal carbon dioxide if indicated.
• Fear, anxiety, and pain can cause vasovagal reactions.
• Assist in positioning of the patient.
• Continually reassure and communicate with the patient during and after peripheral nerve blockade.
• Institute meticulous attention to sterile technique and potential breaks.
• Administer sedatives and opioids as directed by the anesthesia provider to decrease anxiety, but not so excessive that the patient can communicate subjective sign and symptoms that may indicate a complication.
• Ensure familiarity with basic controls that may require adjustment during blockade for ultrasound and peripheral nerve stimulator.
• Check for adequate electrogram pad contact with patient skin for the positive lead and a tight connection to the negative lead with peripheral nerve catheters.
• Patients may require verbal reassurance with involuntary muscle movements associated with peripheral nerve stimulator use.
• Administer local anesthetics as directed by the anesthesia provider. The syringe should be clearly labeled with local anesthetic and concentration. Always aspirate before injection. If resistance is encountered, stop immediately and notify the anesthesia provider. Inject slowly in 5-mL increments with aspiration occurring in between.
Postperipheral blockade: recovery
• Institute routine postoperative care. There are no specific time limitations or criteria concerning sensation and motor function with peripheral nerve blocks.
• Monitor for block-specific complications.
• Protect the insensate limb and caution the patient on movement that could lead to injury because of partial motor blockade.
• Avoid direct pressure on injection sites. Assist the patient in repositioning.
• Assess the injection site if possible for hematoma formation.
• For patients with peripheral nerve catheters, ensure that connections are tight and secure.
• Check the insertion site if it can be visualized and ensure that sterile dressings remain intact.
• Report bleeding, dislodgement, or drainage to the anesthesia provider.
• Ensure that the correct local anesthetic solution is infusing at the ordered infusion rate.
• Avoid dislodgement during repositioning or patient transfers.
Patient teaching
• Patient teaching should be tailored to specific blocks.
• Patients should use slings or braces as ordered to prevent injury because of partial motor blockade and insensate limb.
• Duration of the blockade can be variable and largely dependent on whether single injection or continuous infusion is used.
• As sensation returns, the patient may experience numbness, tingling, and possible burning sensation as well as a return of discomfort.
• Be proactive with pain medications to avoid uncontrollable discomfort as the block resolves.
Upper extremity peripheral nerve blocks
Brachial plexus
The brachial plexus is created primarily by the ventral rami of C5 to T1 (Fig. 25-9). C5 and C6 form the superior trunk, C7 from the middle trunk, and C8 and T1 from the inferior trunk as they exit their intervertebral foramina. As the brachial plexus continues its course, trunks split into anterior and posterior divisions. The anterior division of the upper and middle trunk forms the lateral cord; posterior division of the upper, middle, and lower trunk will form the posterior cord; and the anterior division of the lower trunk forms the medial cord. The lateral cord divides into musculocutaneous nerve and contributes to the median nerve. The posterior cord divides into the axillary and radial nerves. The medial cord divides into the ulnar and medial antebrachial cutaneous nerves, and contributes to the median nerve. Individual cutaneous nerve supply to the upper extremity and major motor function are noted in Fig. 25-10 and Table 25-3. The anatomy of the brachial plexus allows the placement of local anesthetic at the level of trunks, cords, or terminal branches. The most common blocks include the interscalene, supraclavicular, infraclavicular, and axillary approach. Determining the approach is dependent on the surgical procedure. Basic knowledge of each approach will help the perianesthesia nurse monitor for potential complications. Techniques include anatomic landmarks, patients’ report of paresthesia, use of a nerve stimulator, and ultrasound guided techniques.30,31,40,44–46
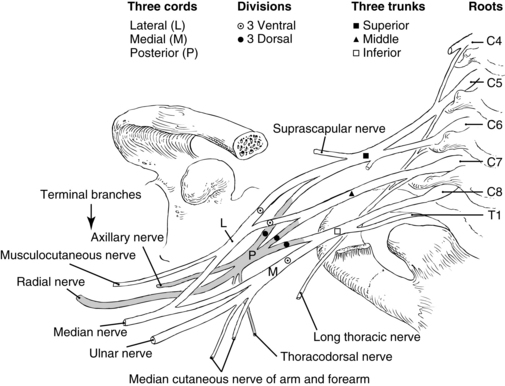
FIG. 25-9 Deviation of the brachial plexus from the cervical spine.
(From Nagelhout J, Plaus K, editors: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.)
Table 25-3 Motor Function of Individual Nerves of the Brachial Plexus
| NERVE | MAJOR MOTOR FUNCTION |
|---|---|
| Axillary nerve | Abduction of the shoulder |
| Musculocutaneous nerve | Flexion of the elbow |
| Radial nerve | Extension of the elbow, wrist, and finger |
| Median nerve | Flexion of the wrist and finger |
| Ulnar nerve | Flexion of the wrist and finger |
Interscalene
Upper, middle, and lower trunks are located in between the anterior and middle scalene muscles. The trunks are named based on their vertical arrangement within this groove. This approach is used for surgical procedures of the shoulder and spares the ulnar distribution. Surgical procedures of the arm and hand use other approaches to the brachial plexus. Positioning of the patient is dependent on the approach. Patients may be in the supine or sitting position with their head turned 30 degrees toward their nonoperative side. Additional positions include lateral decubitus. Key anatomic landmarks include the sternocleidomastoid muscle, interscalene groove, and cricoid cartilage. Paresthesia techniques rely on subjective description of paresthesias in the upper arm or shoulder. Nerve stimulator techniques rely on identification of muscle contractions of the deltoid, biceps, triceps, pectoralis major, forearm, or hand. Ultrasound techniques help to identify specific nerve roots or trunks to guide placement. Complications include blockade of the phrenic nerve resulting in dyspnea, making it an unsuitable block for patients with chronic respiratory conditions. Vascular structures located in close proximity mandate monitoring for signs and symptoms of local anesthetic toxicity (see Chapter 24). As little as 1 to 3 mL of local anesthetic placed within a vertebral artery can result in seizure activity. Pneumothorax is also a potential complication. Signs and symptoms include dyspnea, decreased or absent breath sounds on the affected side, chest pain with deep inspiration, hyper resonance with percussion, and hypoxemia as indicated by arterial blood gases and pulse oximetry. Immediate notification of the provider, anticipation of a chest radiograph, and possible chest tube insertion should be considered. Blockade of the stellate ganglion results in Horner syndrome. The patient may experience hoarseness of the voice, constriction of the pupil (myosis), drooping of the upper eyelid (ptosis), decreased sweating (anhidrosis), and nasal congestion on the affected side. This experience is disconcerting but self limiting, and reassurance is an important consideration. Local anesthetic may be inadvertently placed with the epidural, spinal, or subdural space, leading to a high spinal block (see High Spinal Block). Additional complications include nerve trauma, hematoma, and infection.30,31,40,43–46
Supraclavicular
As the brachial plexus passes between the first rib and clavicle, individual trunks are closely compacted and found slightly posterior to the subclavian artery. This approach is used for surgical procedures of the arm and hand. It is not suitable for shoulder surgery. The patient is placed in a supine position with the head turned at a 30-degree angle toward the nonoperative arm. Anatomic landmarks include the clavicle, sternocleidomastoid, and subclavian artery. A paresthesia of the arm or hand may be sought. With a peripheral nerve stimulator technique, muscle contraction of the fingers is desired. Ultrasound techniques rely on identification of the subclavian artery and trunks of the brachial plexus, which are located posterior to the artery. An in-plane technique is used to identify the needle tip as it is advanced to avoid vasculature and pleura. Complications are similar to interscalene approaches with the addition of hemothorax. Respiratory complications may not be immediate and nursing staff should be sensitive to changes in the patient’s respiratory status.30,31,40,43–46
Infraclavicular
After the first rib and clavicle, the brachial plexus divides into anterior and posterior divisions and then forms the lateral, medial, and posterior cords (named by their relationship to the subclavian artery). This approach is used for surgical procedures of the arm and hand; it is not suitable for shoulder surgery. Patients may be positioned supine with the head turned slightly toward the nonoperative side and the operative limb abducted at 90 degrees or at their side. Anatomic landmarks include the clavicle, coracoid process, and subclavian artery. Infraclavicular techniques are limited to peripheral nerve stimulator and ultrasound techniques. Peripheral nerve stimulators rely on muscle contraction of the median nerve (wrist–finger flexion), radial nerve (elbow–wrist extension) and ulnar nerve (wrist–finger flexion). Complications are similar to interscalene approaches with the addition of hemothorax and chylothorax (accumulation of lymphatic fluid within the chest and associated with left sided blocks). Signs and symptoms of chylothorax are similar to a pleural effusion.30,31,40,43–46
Axillary
Trunks become terminal braches at the pectoralis minor. Blockade here results in anesthesia–analgesia of the median, radial, and ulnar nerves. As the terminal nerves enter the axilla, the musculocutaneous nerve has already left the brachial plexus and is located within the coracobrachialis muscle and must be blocked separately. If a tourniquet is used, a skin wheal of local anesthetic will block the intercostobrachial and medial brachial cutaneous nerves. This approach is acceptable for surgical procedures of the hand, forearm, and elbow. The patient is in the supine or sitting position. The arm is abducted and the elbow is flexed at a 90-degree angle. The primary landmark is the axillary artery. Approaches include transarterial, paresthesia, nerve stimulation, and ultrasound. During a transarterial technique, a needle is inserted through the axillary artery. When blood can no longer be aspirated, local anesthetic is slowly injected with frequent aspirations to ensure that the needle tip is not within the artery. Continuous monitoring of the patient is essential to detect complications such as intravascular injection. Paresthesia techniques rely on subjective reporting by the patient. The anesthesia provider may elect to inject a paresthesia in the surgical distribution or elicit several paresthesias. Nerve stimulator techniques rely on muscle contractions of the hand. Ultrasound techniques identify the axillary artery and vein and the individual nerves that surround them. This approach has a low complication rate but may include intravascular injection, local anesthetic toxicity, nerve trauma leading to neuropathy, hematoma formation, and infection.30,31,40,43–46
Bier block
Bier blocks produce anesthesia by injecting a large volume of 0.5% lidocaine into an exsanguinated limb with an inflated tourniquet. A Bier block is primarily used for the upper extremities, but is also suitable for surgical procedures of the distal forearm and hand. A history of Raynaud or sickle cell disease are contraindications to a Bier block. Before surgery, an intravenous line started in the dorsum of the operative hand is required. A second running intravenous line is started in the nonoperative arm. In the operating room the arm will be exsanguinated, a double tourniquet will be inflated, and 0.5% lidocaine will be injected. Local anesthetic is distributed systemically once the tourniquet is deflated, and anesthesia and analgesia will dissipate. The nurse should anticipate the administration of analgesics for postoperative pain and monitor the patient for signs and symptoms of local anesthetic toxicity.30,31,40,43–46 There is a low incidence of complications; however, cardiac arrest, seizures, compartment syndrome, nerve injury, thrombophlebitis, and bruising have been reported in the literature.47
Lower extremity peripheral nerve blocks
Anesthesia of the lower extremities requires blockade of the lumbar or sacral plexus, or both. The lumbar plexus is formed after the anterior rami of T12-L4 and emerges from their intervertebral foramina. It forms several nerves including the iliohypogastric, ilioinguinal, genital femoral, lateral femoral cutaneous, femoral, and obturator nerves (Fig. 25-11). The sacral plexus is formed by the ventral rami of L4-S4 and forms the gluteal and sciatic nerves.30,31,40,45,46,48
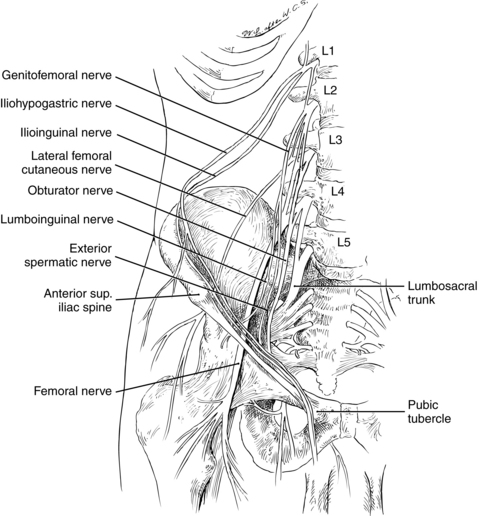
FIG. 25-11 Origin and position of nerves of the lower extremity.
(From Nagelhout J, Plaus K, editors: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.)
Femoral
The femoral nerve divides from the plexus and enters lateral to the femoral artery under the inguinal ligament. It is located below the fascia iliaca and lateral to the femoral artery and vein, which are located between the fascia lata and iliaca. The femoral nerve innervates the quadriceps, pectineus, and sartorius muscles, providing sensation to the anterior and medial thigh as well as medial ankle and foot. Localized groin infections or a history of a femoral vascular graft are contraindications. This block can provide effective anesthesia and analgesia to the anterior portion of the leg including the thigh, knee, and medial ankle and foot along the saphenous nerve distribution. The block can be used in conjunction with a sciatic nerve block to effectively block the remaining portion of the leg innervated by the sacral plexus. Placement of peripheral nerve catheters allow for postoperative analgesia. Common approaches include peripheral nerve stimulator and ultrasound. Peripheral nerve stimulation relies on muscle contraction of the quadriceps and patellar movement. The patient is positioned supine and landmarks are identified, to include the anterior superior iliac spine and superior corner of the pubic tubercle identifying the inguinal ligament and femoral artery. The femoral nerve is located lateral to the artery. Complications include hematoma, local anesthetic toxicity, nerve trauma, and infection. Patients with a femoral nerve block should have a knee immobilizer placed and assisted with ambulation. Local anesthetics block the quadriceps and the patient is at risk for falls.30,31,40,45,46,48
Sciatic
The sciatic nerve is the largest nerve in the human body and originates from the sacral plexus (L4-S4). The sciatic nerve leaves the pelvis through the sacrosciatic foramen down the posterior portion of the buttocks and is located between the greater trochanter and ischial tuberosity. It provides innervation to the hamstrings and all the muscles below the knee. Sensory innervation includes the posterior portion of the knee as well as the lower leg with the exception of the medial portion of the lower leg, which is supplied by the saphenous nerve (a branch of the femoral nerve). The sciatic nerve has poor blood supply and is prone to trauma through pressure exerted through large volumes of local anesthetic, tourniquet, and positioning. Solutions containing epinephrine are avoided secondary to vasoconstriction. Diabetes and neuropathies are relative contraindications. Sciatic nerve blocks are placed with ultrasound or peripheral nerve stimulators. Single injection and peripheral nerve catheters may be placed. Nerve stimulator techniques rely on contraction of the hamstring or gastrocnemius muscle or dorsal–plantar flexion of foot, or both. Positioning of the patient varies and can include lateral decubitus position, lithotomy, or supine position. Major landmarks for a posterior approach include the greater trochanter, posterior superior iliac spine, and sacral hiatus. Landmarks for a lithotomy approach include the ischial tuberosity and greater trochanter, whereas the anterior approach uses the inguinal ligament and greater trochanter. Complications include intraneural injection (resulting in foot drop), local anesthetic toxicity, and hematoma formation. It is important to position the patient so that pressure on the sciatic nerve injection site is reduced. 30,31,40,45,46,48
Popliteal
The sciatic nerve divides into the common peroneal and tibial nerve 7 to 10 cm above the popliteal crease within the popliteal fossa. The lateral border of the fossa includes the biceps femoris while the semitendinosus and semimembranous form the medial border. Popliteal blocks provide surgical anesthesia and analgesia for the ankle and foot. The saphenous nerve provides sensory innervation to the medial ankle and foot and may be supplemented. Peripheral nerve stimulators and ultrasound are common techniques. Peripheral nerve stimulator use seeks motor movement of the ankle, foot, or toes. Patient positioning is either prone or supine. Local anesthetic solutions containing epinephrine are avoided. Contraindications and complications are similar to sciatic nerve block.30,31,40,45,46,48
Ankle
An ankle block is purely sensory and requires blockade of five nerves. Superficial, deep peroneal nerves and posterior tibial and sural nerves originate from the sciatic nerve. The saphenous nerve is a branch of the femoral nerve. Major landmarks include medial and lateral malleolus, posterior tibial artery, Achilles tendon, and flexor hallucis longus tendons. The patient is positioned supine. Ultrasound may be used, but this is a basic block and can be placed easily with landmarks. Solutions containing epinephrine are avoided because they may result in ischemia. Contraindications include infection, severe diabetes, and decreased blood flow to a compromised foot. Serious complications are unlikely. Patients should be cautioned that they can easily injure their foot, and care should be taken to avoid injury.30,31,43,45,46
Summary
Regional anesthesia continues to be a mainstay of anesthetic care and postoperative analgesia. Continued progress in the knowledge of neural blockade, advances in pharmacology, and technology should serve to propel the use of regional anesthesia in the future. Perianesthesia nurses with a foundational knowledge of basic anatomy, common techniques, technology, and complications will be well equipped to manage patients under their watchful eyes.
1. Kleinman W, Mikhail M. Spinal, epidural, & caudal blocks. Morgan GE, et al. Clinical anesthesiology, ed 4, New York: McGraw-Hill Medical, 2006.
2. Greensmith JE, Murray WB. Complications of regional anesthesia. Curr Opin Anaesthesiol. 2006;19:531–537.
3. Venes D, ed. Tabor’s cyclopedic medical dictionary, ed 20, Philadelphia: F.A. Davis, 2005.
4. Nagelhout J. Autonomic and cardiac pharmacology. Nagelhout J, Plaus K. Nurse anesthesia, ed 4, St. Louis: Saunders, 2010.
5. Olson RL, et al. Regional anesthesia: spinal and epidural anesthesia. Nagelhout J, Plaus K. Nurse anesthesia, ed 4, St. Louis: Saunders, 2010.
6. Brown C. Anesthetic agents and adjuncts. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. ed 2. St. Louis: Saunders; 2010.
7. Drasner K, Larson MD. Spinal and epidural anesthesia. Stoelting RK, Miller RD. Basics of anesthesia, ed 5, Philadelphia: Churchhill Livingstone, 2007.
8. Patton KT, Thibodeau GA. Anatomy & physiology, ed 7, St. Louis: Mosby, 2010.
9. Reese CA. Clinical techniques of regional anesthesia, ed 3. Park Ridge, Ill: AANA Publishing; 2007.
10. Horlocker TT, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anesth Pain Med. 2010;35:64–101.
11. American Society of Regional Anesthesia (ASRA): Consensus Statements. available at: http://asra.com/publications-anticoagulation-3rd-edition-2010.php, December 15, 2010. Accessed
12. Welliver D, et al. Lumbar epidural catheter placement in the presence of low back tattoos: a review of the safety concerns. AANA J.2010;78:197–200.
13. The Joint Commission: Accreditation program hospital national safety goals. available at: http://www.jointcommission.org/patientsafety/nationalpatientsafetygoals/, November 29, 2010. Accessed
14. Margarido CB, et al. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anesth. 2011;58(3):262–266.
15. O’Connor G, et al. The effect of spinal needle design, size, and penetration angle on dural puncture cerebral spinal fluid loss. AANA J. 2007;75:111–116.
16. Fischer B. Techniques of epidural block. Anaesth Intensive Care. 2009;10:552–556.
17. Guay J. The epidural test dose: a review. Anesth Analg.2006;102:921–929.
18. Mulroy MF. Systemic toxicity and cardiotoxicity from local anesthetics: incidence and preventative measures. Reg Anesth Pain Med.2002;27:556–561.
19. Kalil A. Unintended subdural injection: a complication of epidural anesthesia-a case report. AANA J.2006;74:207–211.
20. American Society of PeriAnesthesia Nurses: Position statement 9: a position statement on safe medication administration. In American Society of PeriAnesthesia Nurses: Perianesthesia nursing standards and practice recommendations 2010-2012. Cherry Hill, NJ: ASPAN; 2010.
21. Baldini G, et al. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology.2009;110:1139–1157.
22. Burns SM, et al. Unintentional hypothermia: implications for perianesthesia nurses. J Perianesth Nurs.2009;24:167–176.
23. Boyle JAH, Stocks GM. Post-dural puncture headache in the parturient—an update. Anaesth Intensive Care. 2010;11:302–304.
24. Apfel CC, et al. Prevention of postdural puncture headache after accidental dural puncture: a quantitative systematic review. Br J Anaesth.2010;105:255–263.
25. Brull R, et al. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg.2007;104:965–974.
26. Zaridc D, Pace NL. Transient neurologic symptoms (TNS) following spinal anesthesia with lidocaine versus other local anesthetics. Cochrane Database Sys Rev. 2, 2009. Art. No.: CD003006
27. Redmond MC. Immediate postoperative assessment and postanesthesia assessment phase II. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. ed 2. St. Louis: Saunders; 2010.
28. Fetzer SJ. Phase I discharge criteria. Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. ed 2. St. Louis: Saunders; 2010.
29. Yauger YJ, et al. Patient outcomes comparing CRNA-administered peripheral nerve blocks and general anesthetics: a retrospective chart review in a U.S. Army same-day surgery center. AANA J.2010;78:215–220.
30. Burkard JF, Vacchiano CA. Regional anesthesia: upper and lower extremity blocks. Nagelhout JJ, Plaus KL. Nurse anesthesia, ed 4, St. Louis: Saunders, 2010.
31. Gray AT, et al. Peripheral nerve blocks. Stoelting RK, Miller RD. Basics of anesthesia, ed 5, Philadelphia: Churchill Livingstone, 2007.
32. LaGrange P, et al. Application of the doppler ultrasound bloodflow detector in supraclavicular brachial plexus block. BJ Anaesth. 1978;50:965–967.
33. Neal JM, et al. The ASRA evidence based medicine assessment of ultrasound-guided regional anesthesia and pain medicine: executive summary. Reg Anesth Pain Med.2010;35:S1–S9.
34. Liu SS, et al. Evidence basis for ultrasound-guided block characteristics: onset, quality, and duration. Reg Anesth Pain Med.2010;35:S26–S35.
35. Neal JM. Ultrasound-guided regional anesthesia and patient safety: an evidence based analysis. Reg Anesth Pain Med.2010;35:S59–S67.
36. McCartney CJL, et al. Evidence basis for the use of ultrasound for upper extremity blocks. Reg Anesth Pain Med. 2010;35:S10–S15.
37. Salinas FV. Ultrasound and review of evidence for lower extremity peripheral nerve blocks. Reg Anesth Pain Med. 2010;35:S16–S25.
38. Chan VWS, et al. Basic principles & physics of ultrasound. Chan VWS, et al. Ultrasound imaging for regional anesthesia, ed 2, Toronto: Toronto Printing Company, 2008.
39. Falyar CR. Ultrasound in anesthesia: applying scientific principles to clinical practice. AANA J.2010;78:332–341.
40. Moos DD. Understanding peripheral nerve blocks. OR Nurse. 2011;5(5):24–32.
41. Brull R, et al. Practical knobology for ultrasound-guided regional anesthesia. Regional Anesthesia and Pain Medicine. 2010;35(2):S68–S73.
42. Hadzic A, Vloka JD. Peripheral nerve stimulators and nerve stimulation. Hadzic A, Vloka JD. Peripheral nerve blocks: principles and practice. New York: McGraw-Hill; 2004.
43. McCamant KL. Peripheral nerve blocks: understanding the nurse’s role. J Perianesth Nurs.2006;21:16–23.
44. Neal JM, et al. Upper extremity regional anesthesia, essentials of our current understanding,. Reg Anesth Pain Med. 2009;34:134–170.
45. Morgan GE, et al. Peripheral nerve blocks. Morgan GE, et al. Clinical anesthesiology, ed 4, New York: McGraw-Hill, 2005.
46. Wedel DJ, Horlocker TT. Peripheral nerve blocks. In: Longnecker DE, et al. Anesthesiology. New York: McGraw-Hill Medical, 2008.
47. Guay J. Adverse events associated with intravenous regional anesthesia (Bier bock): a systematic review of complications. J Clin Anesth.2009;21:585–594.
48. Enneking FK, et al. Lower-extremity peripheral nerve blockade: essentials of our current understanding. Reg Anesth Pain Med.2005;30:4–35.