CHAPTER 32 Refractive presbyopia management
Introduction
Fundamental principles and anatomical considerations
The change in optical power of the eye in order to produce a sharp retinal image of objects at different distances is called accommodation. The accommodative apparatus of the eye consists of the ciliary muscle, the zonular fibers which form the suspension apparatus of the crystalline lens, and the crystalline lens itself. During accommodation (far–near focusing) the tension of the zonular fibers is reduced by the contraction of the ciliary muscle ring and under the influence of the elastic lens capsule the shape of the lens changes to its mechanical resting state12. This increases the surface curvature and the anteroposterior thickness of the lens and reduces its equatorial diameter. These changes contribute to an increase in optical power of the eye.
Clinical features and pathophysiology of presbyopia
The decrease of accommodative amplitude begins at an age of 10–12 years and ends with a nearly complete loss of accommodative ability by the age of 50–55. Presbyopia has been attributed to a loss of elasticity of the ciliary muscle and the choroid as well as geometric changes and hardening of the lens, especially the lens nucleus, with advancing age. Although the underlying causes are still a matter of scientific discussion, it is basically accepted that the hardening of the lens substance represents a substantial limiting factor for the accommodation in the presbyopic eye4. Experimental results suggest that other important structures such as ciliary muscle and lens capsule still remain at least partially functional even in the presbyopic eye.
Operation techniques
Pseudoaccommodative procedures
Monovision
The term monovision refers to correcting one eye (usually the dominant) to emmetropia and the other to a myopia between −1.0 and −2.0 D. which can be achieved with a variety of refractive surgical procedures (e.g. excimer laser surgery)2. In hyperopes, a steepening of the cornea with over-correction of one eye can be performed by laser thermokeratoplasty (LTK) or conductive keratoplasty (CK). All surgical techniques which can generate a unilateral myopia can be used in emmetropes.
Multifocal excimer laser treatment of the cornea
Principle
Early attempts at the creation of a multifocal cornea with the excimer laser faced problems such as regression of the treatment effect and reduced visual quality with frequently occurring monocular diplopia. Flying-spot excimer lasers and improved laser profiles have renewed interest in presbyopic LASIK treatment. Profiles for the multifocal corneal ablation with LASIK are currently used almost exclusively in hyperopic and emmetropic eyes. Patterns with a central near zone (‘central multifocal LASIK’) and a central far zone with mid-peripheral near addition (‘peripheral multifocal LASIK’) have been used1.
Corneal implants
Current application
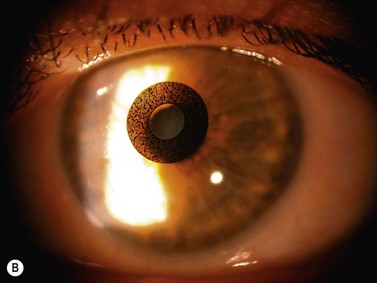
The company AcuFocus (Irvine, CA) produces an implant for presbyopic correction of the emmetropic eye. (AcuFocus ACI-7000, Fig. 32.1), which is supposed to enhance depth of focus using the pinhole principle. It consists of a ring made from polyvinylidene fluoride (PVDF) with a thickness of 10 µm, an outer diameter of 3.8 mm, and a round central opening with a diameter of 1.6 mm and is implanted unilaterally under a corneal lenticle which is prepared as in LASIK13.
Results
In a recently published study, 39 presbyopic patients were treated with the AcuFocus implant13. Twelve patients were emmetropes without previous surgery; 27 had been emmetropized with LASIK for hyperopia. After 12 months mean binocular uncorrected near visual acuity was J1+ with unchanged distance visual acuity. Increased spectacle independence was also reported.
Multifocal intraocular lenses
Refractive multifocal IOLs
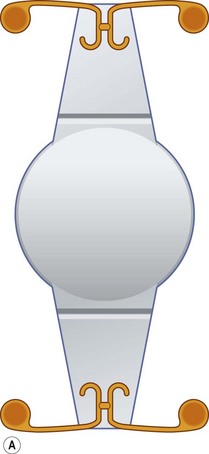
Refractive bifocal or multifocal IOLs have on their optic two or more ring-shaped spherical zones of different refraction (Fig. 32.2A). The near part is usually located in the center of the lens optic. With accommodative miosis, the near part is effective when looking at near targets, and the distance part when looking at far targets. The efficacy depends on the centration of the IOL and on pupil diameter.
Diffractive multifocal IOLs
Diffractive multifocal IOLs part the light to two focus points: one for near and one for distance. They have a diffractive anterior or posterior surface with concentric rings for the diffraction of incoming light rays, which are focused onto a far and a near point (Fig. 32.2B). The bifocality is largely independent of pupil diameter and centration. To avoid unwanted optical effects, with newer diffractive multifocal IOLs the step heights of the diffractive rings are reduced towards the optic margin (apodized diffractive optic)6. Some modern multifocal IOLs use a combination of diffractive and refractive principle.
Results
Several studies for evaluation of multifocal IOLs showed high rates of spectacle independence and patient satisfaction5,7. To achieve a good optical effect with good distance and near visual acuity the eye must be made emmetropic and corneal astigmatism must be treated. This requires exact biometry and IOL calculation. Given these precautions, modern multifocal IOLs provide high predictability and efficacy.
Accommodative procedures
Anterior ciliary sclerotomy and scleral expansion
Principle
An alternative theory of accommodation suggests another pathophysiology of presbyopia11. The basic assumptions are: (i) during near accommodation the lens equator moves outward causing an increase in lens diameter. (ii) The equatorial lens diameter increases with age due to the natural growth of the lens. (iii) Presbyopia is caused by decrease of the distance between lens equator and ciliary muscle leaving not enough space for the lens equator to move outward with accommodation. Assuming this, it seems logical to increase this space to restore accommodation.
Potentially accommodating IOLs
Principle and historical development
Accommodative IOLs are designed to transform the movement of the ciliary muscle into a dynamic change of ocular dioptric power. All currently available accommodative IOLs are based on the principle of optic shift. By use of a hinge or a similar mechanism in the IOL, haptic contraction of the ciliary muscle is supposed to shift the IOL optic anteriorly. This concept allows for only a small accommodative amplitude between 0.5 and 2.5 D for an anterior shift of 1 mm9.
Current models
Eyeonics AT-45 CrystaLens
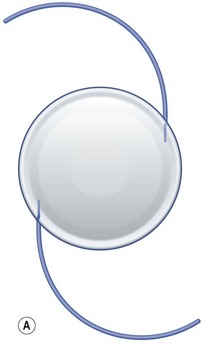
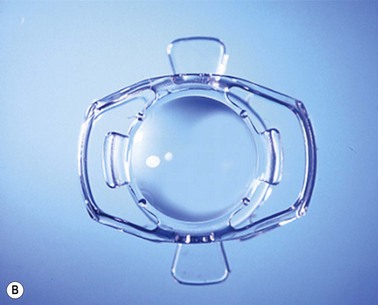
This accommodative IOL (Fig. 32.3A) is the only one approved as such by the FDA (since 2003). It is made of a high refractive silicone material and has a modified plate-haptic design with a sharp-edged optic of 5.0 mm diameter and a groove (hinge) near the optic–haptic junction. At the end of each plate haptic there is a pair of arcuate stabilizing polyimide haptics. A version with an aspherically modified optic aimed at achieving a greater depth of focus named CrystaLens HD has recently obtained FDA approval.
Visiogen synchrony
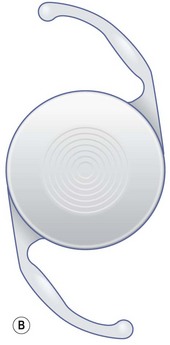
The synchrony IOL (Fig 32.3B) which is manufactured by Visiogen (Aliso Viejo, CA) has two optics, a convex anterior one with a dioptric power of 32 D and a concave posterior one, which are connected by a spring mechanism. The anterior optic is designed to move inside the capsular bag while the posterior one remains stationary and brings the image into focus adapted to the axial length of the eye. This is supposed to result in accommodative amplitude largely independent of IOL power. The material is high–refractive silicone.
Results
Measurements of forward optic movement of single-optic accommodative IOLs under stimulation with pilocarpine yielded variable results ranging from 0.13 mm to 1.04 mm3.
The 1CU IOL showed subjective distance-corrected near visual acuity comparable to a multifocal IOL and better than non-accommodative controls. For the AT-45 CrystaLens, a study from 2004 showed mean distance-corrected near visual acuity of 20/32 and mean uncorrected near visual acuity of 20/25. Another study showed a mean uncorrected near visual acuity of 20/50 and no significant increase in accommodative amplitude3.
The synchrony IOL achieved in a pilot study an average subjective accommodative amplitude of 3.22 ± 0.88 D10.
Complications
Because of the smaller optic diameter compared with monofocal conventional IOL, the mobility inside the capsular bag, and the larger haptics, the barrier effect of sharp optic edges may be less effective in preventing posterior capsule opacification. Another problem is dislocation of the optic and haptics caused by capsular bag shrinkage. Increasing capsule contraction and fibrosis over time may decrease the mobility of accommodating IOLs and the resulting accommodative amplitude9.
1 Alio JL, Amparo F, Ortiz D, et al. Corneal multifocality with excimer laser for presbyopia correction. Curr Opin Ophthalmol. 2009;20:264-271.
2 Evans BJ. Monovision: a review. Ophthalmic Physiol Opt. 2007;27:417-439.
3 Findl O, Leydolt C. Meta-analysis of accommodating intraocular lenses. J Cataract Refract Surg. 2007;33:522-527.
4 Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863-872.
5 Kohnen T, Allen D, Boureau C, et al. European Multicenter Study of the AcrySof ReSTOR Apodized Diffractive Intraocular Lens. Ophthalmology. 2006;113:578-584.
6 Kohnen T, Derhartunian V. Apodisierte Diffraktionsoptik. Neues Konzept in der Multifokallinsentechnologie. [Apodized diffractive optic. New concept in multifocal lens technology]. Ophthalmologe. 2007;104:899-904.
7 Kohnen T, Kook D, Auffarth GU, et al. [Use of multifocal intraocular lenses and criteria for patient selection]. Ophthalmologe. 2008;105:527-532.
8 Mathews S. Scleral expansion surgery does not restore accommodation in human presbyopia. Ophthalmology. 1999;106:873-877.
9 Menapace R, Findl O, Kriechbaum K, et al. Accommodating intraocular lenses: a critical review of present and future concepts. Graefes Arch Clin Exp Ophthalmol. 2007;245:473-489.
10 Ossma IL, Galvis A, Vargas LG, et al. Synchrony dual-optic accommodating intraocular lens. Part 2: pilot clinical evaluation. J Cataract Refract Surg. 2007;33:47-52.
11 Schachar RA. Cause and treatment of presbyopia with a method for increasing the amplitude of accommodation. Ann Ophthalmol. 1992;24:445-452.
12 von Helmholtz H. Über die Accommodation des Auges. Graefes Arch Ophthalmol. 1855;1:1-74. Abt II
13 Yilmaz OF, Bayraktar S, Agca A, et al. Intracorneal inlay for the surgical correction of presbyopia. J Cataract Refract Surg. 2008;34:1921-1927.