Chapter 80 Recurrent Lumbar Disc Herniation
Lumbar discectomy represents the most commonly performed spinal surgical procedure.1 Approximately 300,000 lumbar discectomy procedures are performed each year in the United States.2 In general, the clinical outcome for this procedure is favorable, with 80% to 90% of patients undergoing surgery reporting good or excellent results.3–6
Despite these favorable results, a relatively small number of patients who have had an initial good outcome following surgery will redevelop symptoms similar to those of their preoperative state owing to a recurrence of herniated disc material at the previous surgical site. The reported incidence of these recurrent lumbar disc herniations ranges from 5% to 15%.7–11
The patient with a symptomatic recurrent disc herniation typically undergoes several weeks or months of conservative management. This treatment may be followed by surgical reexploration in those individuals whose symptoms remain unresponsive. Surgery may involve simply removing the reherniated disc material or a fusion and fixation across the affected disc space. Regardless of the management approach, a recurrent disc herniation creates a substantial economic impact.12 This impact is compounded by time lost from work and the need for many of these patients to be retrained for lighter-duty positions.
Risk Factors for Recurrent Disc Herniations
The risk factors for a primary disc herniation have been noted to include exposure to repetitive lifting, exposure to vibrations, smoking, and a constitutional weakness of the anular tissue.13–15 Isolated trauma or injury has not been found to be a consistent risk factor, occurring in only 0.2% to 10.7% of adults with a herniation.14,16 Conversely, Cinotti et al. found that 42% of patients with a recurrent disc herniation related the onset of radicular pain to an isolated injury or precipitating event.17 Similarly, Suk et al. reported the rate of an isolated injury as a cause of recurrence in 32.1%. This study also noted that 71.4% of the patients with recurrence were males and 57.1% were smokers.18
Despite these findings, other studies have found that gender, age, smoking status, level of herniation, and duration of symptoms were generally not associated with higher rates of recurrence.8,9,17–19 Additionally, the degree of anular incision and the extent of the discectomy (partial or complete) have not been found to affect the potential for recurrence. 9,17–19
One factor that potentially increases the likelihood of a recurrent disc herniation is diabetes. In general, patients with diabetes have been noted to have lower clinical success rates following the initial lumbar discectomy than do nondiabetic patients. Simpson et al. reported an excellent to good outcome following the initial discectomy of 95% in nondiabetic patients but only 39% in diabetic patients.20 Mobbs et al. reported success rates of 86% in nondiabetic patients and 60% in diabetic patients.9 Although these clinical outcome differences were generally felt to be attributable to lower quality-of-life indicators in diabetic patients, Robinson et al. investigated the differences in the proteoglycan profile of the discs in the two groups. This study found that diabetic patients had fewer proteoglycans in the disc material, potentially increasing their susceptibility to recurrent disc prolapse.21
Another proposed risk factor is the configuration of the initial disc herniation. Suk et al. and Grane et al. noted that preoperative disc configuration does not affect the rate of recurrence.18,22 Alternatively, Carragee et al. prospectively evaluated herniated disc configurations along with the rate of reherniation and the rate of reoperation. Disc herniations were divided into four shaped-based groups: (1) fragment-fissure herniations (disc fragment and small anular defect), (2) fragment-defect herniations (large disc fragment with massive dorsal anular tear), (3) fragment-contained discs (incomplete anular tear), and (4) absence of fragment-contained herniations (anular prolapse). Of the four groups, the fragment-fissure type herniations (group 1) were associated with the best outcomes and the lowest rate of reherniation (1%) and required the fewest reoperative procedures (1%). Those with anular prolapse (group 4) were associated with poorer clinical outcomes, with 38% of patients experiencing recurrent or persistent symptoms.8
Evaluation of Recurrent Disc Herniation
The patient who presents with a recurrent disc herniation has generally had a period of clinical improvement following the initial discectomy procedure. A retrospective review of 28 patients with recurrent disc herniation found a pain-free interval ranging from 7 to 168 months (mean of 60.8 months).18 Patients typically report radicular signs and symptoms similar or identical to those of their preoperative clinical state.
Pathologic changes in the ventral epidural space may reflect mass effect due to perineural scarring or recurrent disc herniation.7,23 Scarring is most pronounced before 9 months and primarily involves the anulus fibrosus.24 The scar may surround the nerve roots and cause symptoms by means of neural tension, decreased axoplasmic transport, restriction of blood flow, or restriction of venous return.7
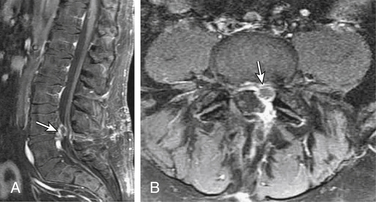
MRI, with and without gadolinium contrast, is the preferred imaging modality for the assessment of a recurrent disc herniation.7,10,25,26 The use of contrast material helps to differentiate normal postoperative anatomic changes from a recurrent herniation. Peridural scarring will typically enhance heterogeneously because of its vascular supply. A recurrent disc herniation usually appears as a polypoid mass with a low signal on T1- and T2-weighted sequences. It is usually contiguous with the parent disc unless sequestered. There can be a hypointense rim of the posterior longitudinal ligament and outer anular fibers that outline the herniation. This rim will enhance with contrast administration (Fig. 80-1). The disc itself will not enhance, because it has no blood supply.7,27
MRI findings will vary according to the time period during which the study is obtained relative to the primary procedure. In the early (1- to 6-month) postoperative period, MRI demonstrates a high-intensity signal band extending from the nucleus pulposus to the site of anular disruption. This is particularly noticeable in the first 2 months following surgery. The anulus is typically hyperintense, and the nucleus is typically hypointense. There is loss of disc space height. The end plates and marrow will frequently have a low signal on T1-weighted images and a high signal on T2-weighted images, suggesting inflammation and edema. The ventral epidural space initially reveals an increase in soft tissue mass, evidence of tissue disruption, edema, and hemorrhage, with the appearance of mass effect.10
Nerve root enhancement with gadolinium in the first few months following surgery is normal. This typically indicates a breakdown of the blood-nerve barrier but usually resolves within 6 months. Postoperative changes at the laminectomy site depend on the extent of surgery, the extent of ligamentum flavum removal, and whether a fat graft was placed in the epidural space. Facet joint enhancement occurs as a local response to dissection and persists long (>6 months) after surgery in more than half of the patients in whom imaging is performed.7,28,29
Late (>6 months) MRI findings include a low-intensity signal band in the disc space representing a healing anular defect. The mass effect that was seen earlier in the ventral epidural space may have resolved29 or may persist as a masslike scar.23 The laminotomy defect contains mature scar with peripheral enhancement identifying granulation tissue. Facet joint enhancement is visible after contrast administration in approximately half of the patients 6 months postoperatively.7
Retraction of the thecal sac toward a soft tissue lesion is suggestive of scar, while displacement away from such a mass is suggestive of a herniated disc.10 Although a pseudomeningocele may also be seen as a mass, its signal characteristics are different, demonstrating cerebrospinal fluid intensity on T1- and T2-weighted images and often an enhancing fibrous capsule.7
Despite the imaging advantages that MRI provides over other techniques, there can be a significant degree of discordance between MRI findings and intraoperative findings. This discordance can occur in 18% to 33% of cases that are proven surgically.30 As with the initial procedure, the successful outcome of any surgery for recurrent disc herniation depends on close correlation between the clinical and radiographic findings.
Management of Recurrent Disc Herniation
The indications for revision surgery are the persistence of radicular symptoms despite a course of conservative treatment and the presence of a clinically correlative finding on radiographic imaging. It is important to determine that the radiographic finding is actually recurrent disc material rather than perineural scar formation because the clinical outcome of surgery for these two findings is different. Jonsson et al. reported on revision surgery for recurrent disc herniation versus perineural scar formation. Surgery for a recurrent disc herniation was found to yield clinical results that were as good as those of the primary discectomy procedure. However, when only perineural scar was present, the results of revision surgery were not as good.31
The most common surgical option used for the management of recurrent disc herniation is a reexploration of the previous surgical site with additional widening of the laminotomy defect and removal of the recurrent disc material. This technique should begin with exposure of normal anatomy immediately above and below the previous laminotomy defect to help orient the surgeon to the pertinent anatomy. Curettes are then used to cautiously dissect the scar from the lateral bony margins. Identification of the medial wall of the pedicles on either side of the disc space allows for further orientation to the neural anatomy. As with the primary procedure, the use of a surgical microscope greatly enhances illumination and visualization of the surgical field.
Suk et al. reviewed 28 patients who had undergone open conventional discectomy for a recurrent disc herniation. Although the length of the revision surgery was significantly longer than that of the primary procedure, there was no significant difference in length of hospital stay or clinical outcome. Age, gender, smoking, occupation, level of herniation, degree of herniation, and pain-free interval did not affect the clinical outcomes of repeat discectomy.18
A limited number of studies have investigated the use of lumbar fusion to manage the patient with a recurrent disc herniation. Chitnavis et al. reported good clinical outcomes in 50 patients with recurrent disc herniation managed by a posterior lumbar interbody fusion.32 Vishteh et al. reported a good outcome in six patients who were managed with an anterior lumbar interbody fusion.33 Proponents of discectomy with fusion have proposed that fusion has several theoretical advantages. Specifically, fusion reduces or eliminates segmental motion, immobilizes the spine, and limits mechanical stresses across the degenerated disc space and may lower the potential for any additional herniation at the affected level.
Fu et al. reported on the long-term outcome in patients who had undergone a dorsolateral fusion compared to a comparable group of patients who were managed with only a conventional discectomy. The follow-up period ranged from 6 to 134 months. The clinical outcome was good or excellent in 78.3% of the patients who had undergone conventional discectomy compared to 83.3% of patients who were fused. This difference was not clinically significant. The difference in the postoperative back pain score was also insignificant. However, the fusion group did have significantly higher intraoperative blood loss, length of surgery, and length of postoperative hospitalization compared to the nonfusion group. The study concluded that disc excision alone is the recommended surgical procedure for managing recurrent disc herniation.34 In the rare case of a recurrent disc herniation that presents with segmental instability (i.e., spondylolisthesis) or in the patient who has had multiple disc herniation recurrences, an interbody or dorsolateral fusion may be a reasonable option to consider.
Babar S., Saifuddin A. MRI of the post-discectomy lumbar spine. Clin Radiol. 2002;57:969-981.
Carragee E.J., Han M.Y., Suen P.W., et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and annular competence. J Bone Joint Surg [Am]. 2003;85:102-108.
Erbayraktar S., Acar F., Tekinsoy B., et al. Outcome analysis of reoperations after lumbar discectomies: a report of 22 patients. Kobe J Med Sci. 2002;48:33-41.
Jonsson B., Stromqvist B. Clinical characteristics of recurrent sciatica after lumbar discectomy. Spine (Phila Pa 1976). 1996;21:500-505.
Suk K.S., Lee H.M., Moon S.H., et al. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976). 2001;26:672-676.
1. Deyo R.A., Weinstein J.N. Low back pain. N Engl J Med. 2001;344:363-370.
2. Bruske-Hohlfeld I., Merritt J.L., Onofrio B.M., et al. Incidence of lumbar disc surgery: a population-based study in Olmsted County, Minnesota, 1950–1979. Spine (Phila Pa 1976). 1990;15:31-35.
3. Henriksen L., Schmidt K., Eskesen V., Jantzen E. A controlled study of microsurgical versus standard lumbar discectomy. Br J Neurosurg. 1996;10:289-293.
4. Moore A.J., Chilton J.D., Uttley D. Long-tern results of microlumbar discectomy. Br J Neurosurg. 1994;8:319-326.
5. Wenger M., Mariani L., Kalbarczyk A., Gröger U. Long-term outcome of 104 patients after lumbar sequestrectomy according to Williams. Neurosurgery. 2001;49:329-335.
6. Williams R.W. Microlumbar discectomy: a 12-year statistical review. Spine (Phila Pa 1976). 1986;11:851-852.
7. Babar S., Saifuddin A. MRI of the post-discectomy lumbar spine. Clin Radiol. 2002;57:969-981.
8. Carragee E.J., Han M.Y., Suen P.W., et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and annular competence. J Bone Joint Surg [Am]. 2003;85:102-108.
9. Mobbs R.J., Newcombe R.L., Chandran K.N. Lumbar discectomy and the diabetic patient: incidence and outcome. J Clin Neurosci. 2001;8:10-13.
10. Ross J.S. MR imaging of the postoperative lumbar spine. Magn Reson Imaging Clin N Am. 1999;7:513-524. viii
11. Suk K.S., Lee H.M., Moon S.H., et al. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976). 2001;26:672-676.
12. Garces Ambrossi G.L., McGirt M.J., Sciubba D.M., et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 2009;65(3):575-578.
13. An H.S., Silveri C.P., Simpson J.M. Comparison of smoking habits between patients with surgically confirmed herniated lumbar and cervical disc disease and controls. J Spinal Disord. 1994;7:369-373.
14. Kelsey J.L., Githens P.B., O’Connor T. Acute prolapsed lumbar intervertebral disc: an epidemiologic study with special reference to driving automobiles and cigarette smoking. Spine (Phila Pa 1976). 1984;9:608-613.
15. Mundt D.J., Kelsey J.L., Golden A.L. An epidemiologic study of non-occupational lifting as a risk factor for herniated lumbar intervertebral disc. Spine (Phila Pa 1976). 1993;18:595-602.
16. Terhaag D., Frowein R.A. Traumatic disc prolapse. Neurosurg Rev. 1989;12(Suppl 1):588-594.
17. Cinotti G., Roysam G.S., Eisenstein S.M., et al. Ipsilateral recurrent lumbar disc herniation. J Bone Joint Surg [Br]. 1998;80:825-832.
18. Suk K.S., Lee H.M., Moon S.H., et al. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976). 2001;26(6):672-676.
19. Graver V., Haaland A.K., Magnaes B., et al. Seven-year clinical follow-up after lumbar disc surgery: results and predictors of outcome. Br J Neurosurg. 1999;13:178-184.
20. Simpson J.M., Silveri C.P., Balderston R.A., et al. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg [Am]. 1993;75:1823-1829.
21. Robinson D., Mirovsky Y., Halperin N., et al. Changes in proteoglycans of intervertebral disc in diabetic patients. A possible cause of increased back pain. Spine (Phila Pa 1976). 1998;23:849-856.
22. Grane P., Tullberg T., Rydberg J., et al. Postoperative lumbar MR imaging with contrast enhancement. Comparison between symptomatic and asymptomatic patients. Acta Radiol. 1996;37:366-372.
23. Deutsch A.L., Howard M., Dawson E.G., et al. Lumbar spine following successful surgical discectomy. Magnetic resonance imaging features and implications. Spine (Phila Pa 1976). 1993;18:1054-1060.
24. Glickstein M.F., Sussman S.K. Time-dependent scar enhancement in magnetic resonance imaging of the postoperative lumbar spine. Skeletal Radiol. 1991;20:333-337.
25. Barrera M.C., Alustiza J.M., Gervas C., et al. Post-operative lumbar spine: comparative study of TSE T2 and turbo-FLAIR sequences vs contrast-enhanced SE T1. Clin Radiol. 2001;56:133-137.
26. Georgy B.A., Hesselink J.R., Middleton M.S. Fat-suppression contrast-enhanced MRI in the failed back surgery syndrome: a prospective study. Neuroradiology. 1995;37:51-57.
27. Haughton V., Schreibman K., De Smet A. Contrast between scar and recurrent herniated disk on contrast-enhanced MR images. AJNR Am J Neuroradiol. 2002;23:1652-1656.
28. Boden S.D., Davis D.O., Dina T.S., et al. Contrast-enhanced MR imaging performed after successful lumbar disk surgery: prospective study. Radiology. 1992;182:59-64.
29. Van de Kelft E.J., van Goethem J.W., de La Porte C., et al. Early postoperative gadolinium-DTPA-enhanced MR imaging after successful lumbar discectomy. Br J Neurosurg. 1996;10:41-49.
30. Erbayraktar S., Acar F., Tekinsoy B., et al. Outcome analysis of reoperations after lumbar discectomies: a report of 22 patients. Kobe J Med Sci. 2002;48:33-41.
31. Jonsson B., Stromqvist B. Clinical characteristics of recurrent sciatica after lumbar discectomy. Spine (Phila Pa 1976). 1996;21:500-505.
32. Chitnavis B., Barbagallo G., Selway R., et al. Posterior lumbar interbody fusion for revision disc surgery: review of 50 cases in which carbon fiber cages were implanted. J Neurosurg. 2001;95(Suppl 2):190-195.
33. Vishteh A.G., Dickman C.A. Anterior lumbar microdiscectomy and interbody fusion for the treatment of recurrent disc herniation. Neurosurgery. 2001;48:334-338.
34. Fu T.S., Lai P.L., Tsai T.T., et al. Long-term results of disc excision for recurrent lumbar disc herniation with or without posterolateral fusion. Spine (Phila Pa 1976). 2005;30:2830-2834.