CHAPTER 97 Reconstruction of the Mandible
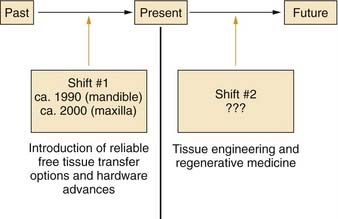
Major advances in patient care generally occur through paradigm shifts (Fig. 97-1). For reconstruction of the mandible and maxilla, the first major paradigm shift occurred with the development of reliable free tissue transfer options along with advances in hardware devices. Before these advances, techniques involving nonvascularized bone grafts, vascularized pedicled bone grafts, and alloplastic materials were not reliably successful for primary reconstruction, especially in the typical defects that were caused by tumor-ablative surgery.1,2 Both advances in surgical techniques and improved hardware options were crucial for this first paradigm shift, which occurred circa 1990 for mandibular reconstruction and circa 2000 for maxillary reconstruction. Although current options have proved to be reliably successful for single-stage primary reconstruction in the mandible and maxilla, these procedures remain highly complex and challenging, and donor sites are still required. In addition, it is difficult to restore the intricate three-dimensional structure precisely in many defects with bone from distant sites because of differences between the size and shape of the bone graft and the defect configuration. Advances in biotechnology, specifically in the field of tissue engineering, will probably have an effect on mandibular and maxillary reconstruction and will cause another paradigm shift within the foreseeable future.3
Anatomy, Physiology, and Pathophysiology
The mandible is the strongest facial bone.4 It is a U-shaped bone with a horizontal portion (body), which carries an alveolar process with teeth, and two vertical portions (rami), which, through the temporomandibular joints, articulate with the skull bilaterally (Fig. 97-2). The mandible is attached to other facial bones by ligaments and muscles, and it articulates with the maxilla through occlusion of the teeth.
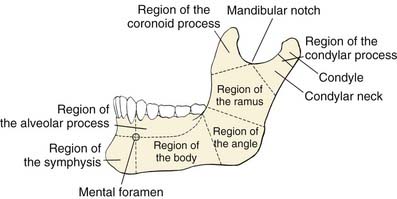
Figure 97-2. Anatomic regions of the mandible.
(From Dingman RO, Natvig P. Surgery of Facial Fractures. Philadelphia: WB Saunders; 1964.)
The body of the mandible has a dense cortical structure on the lateral and medial surfaces with a small core of spongiosa that contains nerves, blood vessels, and lymphatic vessels. The lower portion of the body contains heavy, thick bone that changes very little throughout life. The upper portion of the body, called the alveolar process, contains the dental sockets that support the teeth. Changes in the alveolar process occur throughout adult life, especially after dental extractions, which cause subsequent atrophy of this bony segment (Fig. 97-3). The mental foramen is midway between the inferior border and upper edge of the alveolar process, at the level of the second premolar tooth. The mental nerve branch of the inferior alveolar nerve and the associated vessels pass through this foramen. In an edentulous mandible, the mental foramen drifts to the upper border of the bone as a result of atrophy of the alveolar process (see Fig. 97-3).
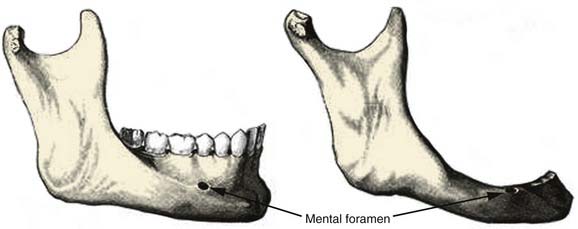
Figure 97-3. Comparison of the anatomy of a dentulous mandible (left) with an edentulous mandible (right).
(From Dingman RO, Natvig P. Surgery of Facial Fractures. Philadelphia: WB Saunders; 1964.)
The movement of the mandible is dependent primarily on two groups of muscles. The depressor-retractor group, which is anterior, is the weaker of the two groups and is composed of the geniohyoid and digastric muscles. These muscles insert into the inferior inner aspect of the symphyseal region and exert downward posterior force. The elevator group, which is posterior, is composed of the masseter, medial pterygoid, and temporalis muscles. The masseter muscle inserts along the outer aspect of the inferior edge of the angle. The medial pterygoid muscle inserts on the inner surface at the mandible angle, thus producing the masseteric-pterygoid sling. The masseter and medial pterygoid muscles always act simultaneously and hold each other in balance, but the angulation of the attachments at the angle is more favorable for the medial pterygoid. Thus, although the medial pterygoid muscle is weaker than the masseter in the intact mandible, it usually overpowers the masseter in segmental defects as a result of this more favorable angulation; this accounts for the typical displacement patterns that occur. Other muscles with more minor influence on the movements of the mandible include the lateral pterygoid and mylohyoid muscles. The lateral pterygoid muscle inserts mostly on the anterior inner aspect of the condylar neck and pulls primarily the neck medially and anteriorly. The mylohyoid muscle has minimal influence on movements of the intact mandible, inasmuch as most of its bundles insert into the muscle’s midline raphe rather than onto the mandible.4
The damage to the oral cavity after tumor extirpation was described by Conley in 1962.5 Options for bone or soft tissue reconstruction were limited at that time, but the serious effects of extirpative surgery on function and cosmesis were clearly recognized. Functions of the oral cavity structures include mastication, swallowing, maintenance of oral competence, articulation, taste, oral hygiene, and airway protection.6 Effective reconstruction of the oral cavity soft tissue components is crucial for functional restoration, especially in preserving mobility, position, and shape of the tongue, which is probably most critical for functional rehabilitation.6
There are two types of mandibulectomies: marginal and segmental (Fig. 97-4). In a marginal mandibulectomy, a rim of bone is resected, but continuity of the mandibular arch is maintained. When this type of mandibulectomy is performed, a curvilinear excision configuration must be used, and a minimal 1 cm height of inferior bone must be preserved, in order to avoid iatrogenic fracture.7,8 The bone defect in a marginal mandibulectomy does not necessitate any specific reconstruction except for soft tissue coverage, usually with a soft tissue flap or skin graft.9 The reconstructive plan in this setting is directed by the characteristics of the soft tissue defect and influenced mostly by the extent of soft tissue loss and whether the tissue has been previously irradiated.
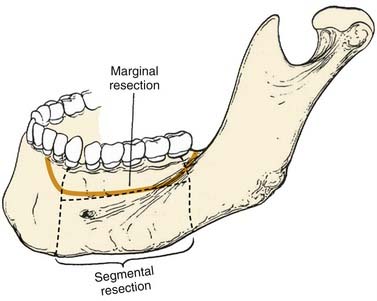
Figure 97-4. Techniques of mandibulectomy: marginal and segmental.
(From Silver CE, Rubin JS. Atlas of Head and Neck Surgery. 2nd ed. London: Churchill Livingstone; 2002.)
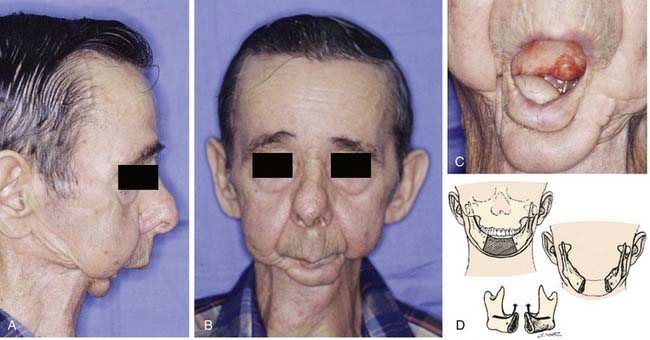
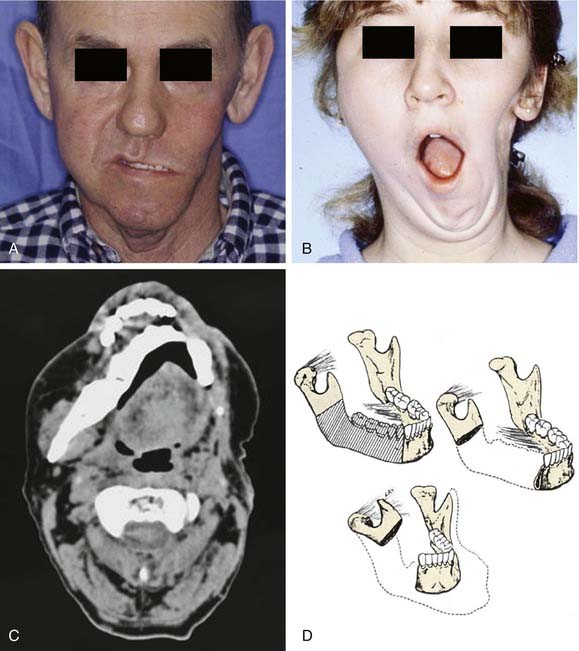
Segmental mandibulectomy involves resection of a full thickness of bone, which creates a discontinuity defect (see Fig. 97-4). Significant cosmetic and functional morbidity are associated with segmental defects. For defects involving the anterior mandible (between both mental foramina), the lateral segments act independently, and the displacement pattern is influenced by the remaining attached muscles (Fig. 97-5).10,11 The medial pterygoid muscle overpowers the masseter and pulls the segments upward, posteriorly, and medially. This movement brings the teeth out of alignment and disrupts mastication. There is complete loss of projection of the lower midline third of the face, which causes an overhanging upper jaw, the so-called Andy Gump appearance. The tongue loses anterior support, thus becoming malpositioned with posterior displacement. The oral sulci become obliterated. The upward movement of the larynx is lost with swallowing as a result of detachment of the suprahyoid musculature. The lower lip is numb as a result of resection of the mental nerves. All these changes lead to a cosmetically and functionally disabling situation for the patient. For defects involving the lateral mandible (posterior to the mental foramina), the remaining bone deviates toward the defect through the action of the remaining muscles of mastication, primarily the medial and lateral pterygoid muscles (Fig. 97-6).10,11 This causes malalignment of the teeth, which affects mastication. The deformity is accentuated by opening the mouth or chewing.
Classification of Defects
A combination of the extent of bone and soft tissue loss determines the reconstructive method. Classification systems that account for this fact are useful. Various classification schemes that have been described for segmental mandibulectomy defects can be used for documenting the defect components, which helps with planning the reconstruction (Fig. 97-7). In Boyd and colleagues’12 classification, H defects are lateral defects of any length up to the midline that include the condyle; L defects are lateral defects that exclude the condyle; and C defects involve the central segment containing the four incisors and two canines. The three lowercase letters in this classification system describe the associated soft tissue components: o (no skin or mucosal component), s (skin), m (mucosa), and sm (skin plus mucosa). Boyd and colleagues recognized the reconstructive and functional problems posed by the absence of the condyle and mentum; thus, their system is similar to Pavlov’s classification system, described before the era of free tissue transfer.12 With loss of the condyle, reconstruction of an articular surface is difficult. Loss of the central segment poses problems with restoration of stomal competence, restoration of an anterior gingivolabial sulcus to fit prosthetics for dental rehabilitation, and restoring the lip height for cosmesis.
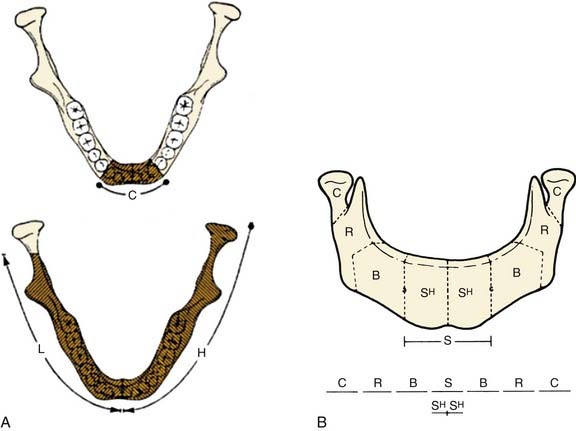
Figure 97-7. Systems of classification of mandible defects described by Boyd and colleagues12 (A) and Urken and associates13 (B).
(A, From Boyd JB, Gullane PJ, Rotstein LE, et al. Classification of mandibular defects. Plast Reconstr Surg. 1993;92:1266-1275. B, From Urken ML, Weinberg H, Vickery C, et al. Oromandibular reconstruction using microvascular composite free flaps. Report of 71 cases and a new classification scheme for bony, soft tissue and neurologic defects. Arch Otolaryngol Head Neck Surg. 1991;117:733-744.)
Another classification scheme, described by Urken and associates,13 is similarly based on functional considerations caused by the detachment of different muscle groups and difficulties with cosmetic restoration. This reconstruction scheme has similar anatomic designations, including separate components of the condyle (C), ramus (R), body (B), total symphysis (S), hemisymphysis (SH), and palate (P). Urken and associates’ classification system also includes a detailed description of soft tissue and neurologic deficits.
Neither of these two classification systems describes the absolute length of the defect. Before the use of free tissue transfer, the chance of healing and bony union depended more significantly on size when nonvascularized bone grafts were used. When osseous free flaps are used, outcomes are less dependent on the length of the graft required to bridge the defect.12
Goals of Mandibular Reconstruction
The primary objective of tumor resections that necessitate mandibulectomy is cure, but the functional and cosmetic rehabilitation are crucial for the physiologic and psychologic well-being of patients. The ideal reconstruction for a segmental mandibulectomy (1) restores oral competency, (2) maintains occlusal relationships with remaining teeth, (3) allows for prosthetic dental restoration, (4) restores bone continuity, and (5) restores facial symmetry and contour to the lower third of the face.14 In addition, wound closure should be immediate and complete in order to maximize early functional recovery, avoid orocutaneous fistula, avoid infection, create a safe wound that can undergo postoperative radiation 6 weeks later, and facilitate early hospital discharge. To achieve these goals, basic principles of reconstructive surgery dictate that the simplest method to achieve the desired results should be chosen for each individual patient.
Background and History
Before the development of advances in surgical techniques and improvements in hardware, reconstructive options were limited and yielded disappointing results. The available options for bone replacement at that time included nonvascularized autogenous bone grafts, allogeneic bone grafts, and vascularized pedicled bone grafts.11 The failure rate of vascularized pedicled bone grafts was as high as 50%.1 Nonvascularized bone grafts, which rely on neovascularization, were found to be prone to infection, extrusion, or resorption when placed into defects in which the oral mucosa was violated or in the setting of perioperative irradiation. Hardware options included alloplastic materials such as trays, prostheses, and Kirschner wires. These devices were designed to prevent displacement of the remaining mandibular segments, support the surrounding soft tissue, and minimize wound contracture.15 Unfortunately, these devices yielded disappointing results because of loosening, migration, exposure, and fracture.
Many studies, including those published by Adamo and Szal,2 Lawson and Biller,1 and Komisar,16 consistently revealed poor results of mandibular reconstruction with the available approaches and technologies. Mandible reconstruction usually proceeded in multiple stages fraught with complications. The management of these complications increased the number of hospitalizations by an average of three per patient.16 These complications sometimes delayed the postoperative treatment with radiation, which affected the ability to cure the cancer. Primary reconstruction yielded the best results but had the highest rate of complications. Secondary reconstruction produced fewer complications but suboptimal results.1,2,16,17 In the secondary setting, soft tissue contracture and fibrosis caused by prior irradiation would fixate the remaining bone segments in a malpositioned orientation, and the fibrosis of the remaining muscles of mastication accentuated this problem.
Even with the successful reconstructions, although most patients demonstrated cosmetic improvement, few experienced significant functional benefits.15–17 The functional limitations resulted from misalignment of the mandible, trismus caused by fibrosis, soft tissue losses, and failure of a bony segment capable of supporting a dental prosthesis. Gullane15 reported that the functional outcomes for patients with primarily lateral defects were more dependent on the soft tissue loss than on the bone reconstruction. At this point, the decision to perform reconstruction needed to be weighed against the overall poor results, increased complications, increased morbidity, and possibility of introducing delays in receiving postoperative radiation treatment. Although the outcomes were suboptimal, many important factors for success that were learned during this era are still basic tenets for mandible reconstruction: (1) stable immobilization of the mandible segments to minimize distortion and maintain occlusal relationships, (2) performance of as much primary reconstruction as possible, (3) importance of soft tissue reconstruction for functional restoration and minimizing hardware complications, and (4) importance of good wound bed vascularity. These early efforts were also important strides toward the development of reliable, one-stage primary reconstructive techniques that are frequently used today.
In 1976, Panje and coworkers18 introduced successful reconstruction for large oral soft tissue defects after tumor-ablative surgery. A free groin flap was used for reconstruction in four patients, and these tissue transfers were successful despite preoperative or postoperative irradiation and oral bacterial contamination. Inclusion of bone with a skin flap was a natural extension of the free flap concept for repairing composite oromandibular defects. Pioneering experimental and anatomic studies proved the feasibility of using composite flaps consisting of bone and soft tissue that were based on a single vascular pedicle.19–22 Subsequently, successful reconstruction for large oromandibular defects with bone from the ilium was described.23–25 The transfer of vascularized fibular bone, which was initially described for complicated extremity reconstruction, was subsequently described for oromandibular reconstruction.26,27 In the majority of medical centers, the fibular osteocutaneous flap is now the most common free tissue transfer used for oromandibular reconstruction.
Hardware for use in the mandible was initially composed of Vitallium (an alloy containing cobalt, chromium, and molybdenum), but because of poor malleability, the metal was subsequently changed to stainless steel and then to titanium for better tissue compatibility.15 In a study of 60 patients treated from 1974 to 1986, Klotch28 described the effectiveness of reconstruction plates for bridging the defect and providing stabilization of the remaining mandibular segments. These plates could be placed rapidly, were well tolerated by tissue, were structurally rigid, and could be readily shaped to the contour of the defect. Hardware problems, however, still occurred frequently with these plates until the development of more reliable methods of soft tissue reconstruction with pedicled and distant flaps.15,28–30 The initial combination of well-vascularized soft tissue reconstruction with reconstruction plates significantly improved the short-term results of primary reconstruction; however, because of continued problems with plate extrusion, plate fracture, and screw loosening, this combination did not provide long-term stability.31 Without bone reconstruction for the defect, the hardware still tended to fail as a result of metal fatigue, leveraged forces with mastication (especially for patients who still had teeth), and volume losses.32 Using this technique anterior mandibulectomy defects continued to have a particularly high rate of extrusion, and affected patients were not considered good candidates for this approach.29,33 The subsequent development and application of osseous free flaps in combination with plates further improved the reliability (short- and long-term) and outcomes of one-stage primary reconstruction for both anterior and lateral mandible defects.34
Current Methods
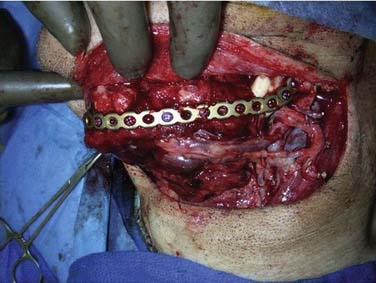
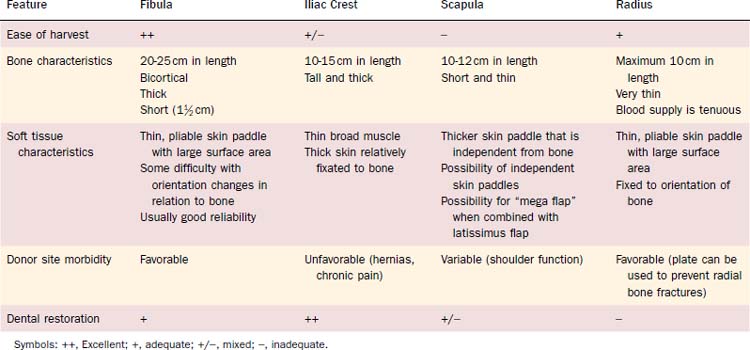
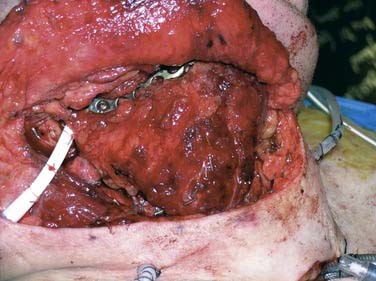
Reconstruction plate combined with an osseous free tissue transfer is the preferred reconstructive method for anterior defects because other methods have a high rate of hardware extrusion and because of the significant morbidity associated with not performing reconstruction for the defect.29,33 For lateral defects, this approach has several advantages: (1) restoration of the bone continuity of the mandible, (2) facilitation of dental restoration, (3) the most consistent long-term outcomes and stability of the reconstruction with minimal hardware complications. The disadvantages of this approach include (1) donor site morbidity, (2) technical difficulty of complicated tissue inset in accurately reconstituting the three-dimensional shape of the resected bone, and (3) the possibility that the associated soft tissue defect is not suitable for reconstruction with the soft tissue available with the bone flap, which would then necessitate two flaps. Figure 97-8 depicts typical harvested fibular and scapular osseous free flaps, and Figure 97-9 depicts the scapular flap inset into an anterior mandibulectomy defect. Table 97-1 summarizes and compares the major characteristics of each of the bone donor sites commonly used for oromandibular reconstruction.35–46 The decision of which flap to use is complex and dependent on the defect characteristics, the patient’s characteristics and preferences, and plans for dental restoration.
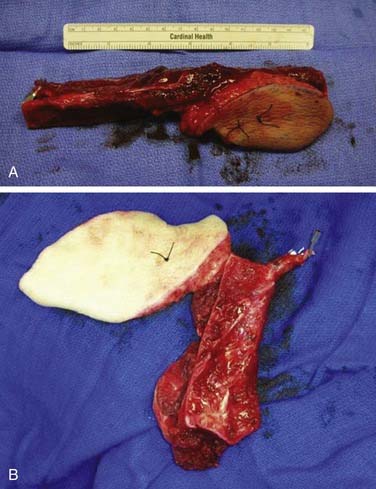
Figure 97-8. Vascularized flaps commonly used for oromandibular reconstruction. A, Fibular. B, Scapular.
Osseous free flap reconstruction for an oromandibular defect can be divided into three steps: planning, harvest, and inset. The considerations for planning include the anticipated bone defect (height, length, and location) and the anticipated soft tissue defect (surface area, volume, and subunits involved). If a single flap cannot provide both the soft tissue and bone needed for the reconstructive needs of the defect, then two flaps must be considered, versus using a flap-plate option. In this alternative approach a reconstruction bar is used alone to maintain mandibular continuity, and a soft tissue flap is used to reconstruct the soft tissue components in the defect. Surgical planning also involves positioning of the patient for flap harvest. The scapular osteocutaneous flap needs to be harvested with the patient in at least a 30-degree lateral decubitus position, whereas the other options can be harvested with the patient in a supine position, which facilitates two-team surgery. Planning the side of the flap harvest is also an important consideration. For fibular flaps, preoperative vascular imaging is recommended because anatomic variations in the vascular blood supply to the foot and atherosclerotic disease might preclude the use of the flap. With conventional angiography, the test results help determine which side to use in up to 25% of patients.47 Other methods described for preoperative evaluation before fibular flap harvest include magnetic resonance angiography, computed tomography–angiography, and color-flow Doppler imaging.48–50 Preoperative evaluation before a radial forearm osteocutaneous flap consists of Allen’s test.46 For flaps from the scapula and iliac crest, preoperative imaging or tests are not necessary to determine which side to use to harvest the flap.
The hardware used during inset is usually a locking reconstruction plate, although the alternative use of miniplates has been described.51 The plate should be bent to the preexisting mandible contour before resection. At the same time, holes should be drilled for fixation screws to ensure that the plate can be reapplied after mandibulectomy, with the bone fragments maintaining anatomic alignment. In general, the reconstruction bar is placed on the lower border of the mandible to avoid tooth roots, maintain optimal aesthetic facial contour, and to lessen chances for intraoral exposure.32 In fibular flaps, however, this can make restoration with osseointegrated implants problematic because of the short bone height in comparison with a dentulous mandible.52 In these cases, several options should be considered: placing the reconstruction plate 1 cm above the inferior mandible border, using a double-barrel fibular flap construct, or choosing an alternative donor site with more bone height, such as an iliac crest flap.
Reconstruction plate combined with a soft tissue flap is generally an option only for lateral defects; when this approach is used for anterior defects, the rate of hardware complications is high. There are several advantages to using this approach as an alternative to osseous free flaps in reconstruction for lateral defects: (1) Application is easy and quick, (2) rigid fixation of the remaining mandible segments is provided, (3) morbidity of bone donor site is avoided, (4) cosmetic restoration is adequate, and (5) the range of flap choices is wider for optimal reconstruction in the soft tissue defect, which can have a more significant effect on functional recovery. Screw loosening is not a problem with locking plates, because the stability of the fixation is not dependent on the screw-bone interface. One consideration with this approach, however, is the overall poor rate of survival that has been consistently reported from multiple institutions; thus, a reconstructive technique that maximizes functional restoration and minimizes operative risk is favored.16,53–56 Because of this and other disadvantages, including subacute and long-term hardware complications (extrusion and fracture) and the difficulty with dental restoration, this approach is not widely favored.
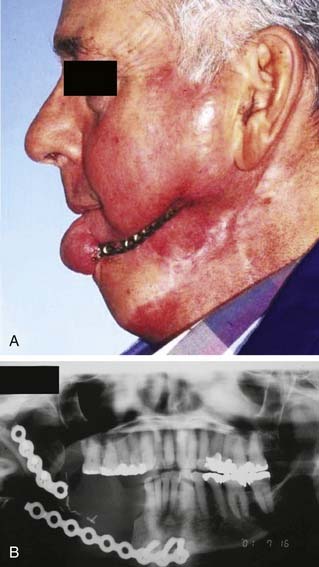
An example of plate extrusion is shown in Figure 97-10A. Reconstruction plate extrusion usually occurs extraorally, and the mean time from surgery to exposure is approximately 10 months.54,57 The pathophysiologic development of plate exposure is postulated to be related to plate characteristics and the size of the defect. The extrusion rates for stainless steel and titanium plates are not significantly different, but the use of low-profile plates has decreased the extrusion rate from 29% to 7%.56–58 The volume of the bone and soft tissue deficit has also been shown to be predictive of exposure.31,32 Without replacing bone within the defect, wound contracture medial to the plate can occur during wound healing as a result of inadequate volume replacement; the contracture causes a slow but steady pressure of the plate on the overlying skin, thus resulting in extrusion. A volume-based approach with “over-reconstructing” the soft tissue defect medial to the plate can be used to avoid a dead space in this area; this approach has been shown to decrease the exposure rate from 38% to 8% (Fig. 97-11).54
An example of plate fracture is depicted in Figure 97-10B. The average time from surgery to plate fracture is 21 months.54 Plate fractures occur primarily because of metal fatigue. This is accentuated by the forces involved in mastication, especially in dentulous patients. Although reconstruction plates can fracture in any patient, those with teeth are at particularly high (up to 26%) risk.30,54 Therefore, dentulous patients who require reconstruction for lateral mandible defects should be considered better candidates for osseous free flaps than for soft tissue flaps, especially when the associated soft tissue defect is not complex. In addition, dentulous cancer survivors who undergo reconstruction with a plate combined with a soft tissue flap are at particularly high risk for subsequent plate fracture; to avoid this complication, planned secondary bone reconstruction should be considered for these patients.59
Outcomes
Cordeiro and colleagues60 reported on short-term results of oromandibular reconstruction in which osseous free flaps were used in 150 consecutive patients during a 10-year period. Of these flaps, 90% were fibular, and testing was performed at least 6 months after surgery. Swallowing, speech, and aesthetic results were all favorable. Of the patients, 45% tolerated an unrestricted diet and 45% were on a soft food diet. Only 9% had unintelligible speech, and only 14% were considered to have poor aesthetic results. Hidalgo and Pusic39 subsequently reported long-term outcomes of oromandibular reconstruction after a follow-up period of at least 10 years. Of the 82 patients who had undergone osseous free flap reconstruction for oromandibular defects from 1987 to 1990, only 32 were still alive in 2002, and 20 agreed to participate in the study. The mean bone defect length was 13 cm, and 75% of defects were associated with radiation exposure either before (n = 2) or after surgery (n = 13). The aesthetic result was judged to be excellent for 55% of patients and good for 20%. Seventy percent of patients tolerated a regular diet, and the rest were on soft-food diets. Speech was easily intelligible in 85%. There were no cases of fracture or osteoradionecrosis of the transferred bone. This study demonstrated that the favorable functional and aesthetic results remain stable over time.
The value of mandible reconstruction has also been measured through objective testing and patient questionnaires. Urken and associates61 studied multiple different functional and aesthetic outcome measures in a group of patients who underwent placement of an iliac crest free flap with dental implant, in comparison with patients with similar defects who underwent no bone reconstruction. Patients were also compared to normal denture wearers and normal dentulous patients. Overall, patients with bone free flap reconstruction had better oral competence, mastication, and cosmesis. In Wilson and coworkers’ study,62 patients who had undergone hemimandibulectomy were surveyed with regard to whether mandible reconstruction was performed. Mandible reconstruction was associated with a better appearance, better eating ability, and a better overall quality of life.
Long-term follow-up has revealed that the bone transferred with fibular flaps maintains bone mass and height, even in the presence of postoperative radiation.39,63 On average, bone height decreased between 4% and 7%. The retention of bone height is not affected by site of reconstruction, patient’s age, length of follow-up, adjuvant irradiation, or osseointegrated implants.
Developments
For osseous free flap reconstructions, establishing proper contour of the reconstruction plate is crucial for obtaining correct alignment of the temporomandibular joints, reestablishing normal occlusion, and providing an aesthetically appropriate shape to the mandible.52 Ideally, the plate is contoured to the native mandible shape before the bone cuts for the mandibulectomy are made. This technique is not possible when the tumor extends through the buccal-labial cortex of the mandible or if the tumor margin might be violated in attempts to expose the outer cortex of the mandible. In addition, secondary mandibular reconstruction in the presence of preexisting occlusal problems is a challenge to contouring a reconstruction plate accurately. Previous techniques to overcome these challenges have included placing the patient in temporary maxillomandibular fixation to align the mandibular fragments before or after tumor resection and then contouring the plate “free hand” with a best estimation to ensure tandem movement of the temporomandibular joints and correct positioning of the occlusion.
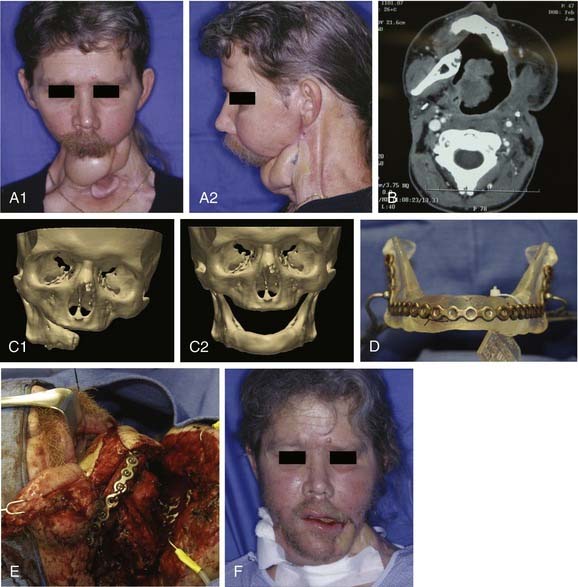
For these challenging cases, techniques are now commercially available for using stereolithographic modeling of patients’ anatomy for surgical planning and accurately contouring the reconstruction plate. These models can be made from acrylic, cellulose, or plaster. They are constructed in a computer-assisted process based on computed tomography imaging. The models are accurate to within 1 mm. The computer-assisted design process can be used to manipulate the three-dimensional image and mirror normal anatomy in order to create a “perfected” mandible for which a model can be made, and then the reconstruction plate is contoured to the model during surgery. An example of the effective use of this technique is shown in Figure 97-12. Advantages of this approach for selective cases include providing an accurate template for contouring the reconstruction plate, saving time during the operation by bending the reconstruction plate preoperatively, allowing for surgical simulation, improving treatment planning, and assisting with preoperative counseling. The disadvantages are the necessity for additional time to make the model (usually can be done in less than 1 week) and the high cost (up to $3000). D’Urso and colleagues64 prospectively studied the use of medical models in 45 patients with various sites of disease in the craniofacial region. Use of models decreased operating room time by 18%, improved planning, and improved measurement accuracy. In addition, patients found the education provided for informed consent helpful.
Future Approaches
Although current methods of reconstruction for mandibular defects are effective, limitations are still significant. These limitations include measurable donor site morbidity, increased surgical time for harvesting the flap, possible complications from flap harvest, difficulty of tissue inset, and unavailability of autologous bone grafts that truly reflect the three-dimensional shape of the resected bone. Therefore, advancements in tissue engineering are starting to be applied for mandible reconstruction. Tissue engineering can be defined as the regeneration or repair of new tissues through the use of biologic mediators and matrices. This regeneration occurs through the recapitulation of key events that occur during tissue formation and growth, which includes cellular migration, proliferation and apoptosis, differentiation, inductive and inhibitory interactions, and tissue patterning and morphogenesis. Signaling molecules, cells, and a scaffold are required for most tissue engineering approaches in order to reproduce this developmental sequence of events.3
Most tissue engineering strategies for mandible reconstruction have been based on bone morphogenetic proteins (BMPs). BMPs were discovered by Urist65 in 1965 as the osteoinductive component of demineralized bone matrix, and these proteins were subsequently isolated, cloned, and purified in the 1980s.66 BMPs are a large subgroup within the transforming growth factor β (TGF-β) family of growth and differentiation proteins.67 The human genome encodes 20 known BMPs; BMP-2, BMP-4, BMP-6, BMP-7, and BMP-9 have demonstrated osteoinductive activity. Human recombinant BMP-2 and BMP-7 are now commercially available, and human recombinant BMP-2 was approved by the U.S. Food and Drug Administration in March 2007 for specific orodental applications (sinus floor augmentation, localized alveolar ridge augmentation for extraction socket defects).
Preclinical data regarding the use of osteoinductive BMP therapies for healing mandibular and calvarial defects in animal models (mice, rats, rabbits, dogs, pigs, and nonhuman primates) are extensive. More recently, case reports and small case series have documented the use of BMPs for reconstruction for segmental mandibulectomy defects in humans.68–70 An example is shown in Figure 97-13. In the cases so far described, BMPs have been used in defects caused by resection of benign neoplasms or caused by trauma. Use of BMPs is currently contraindicated in wounds caused by tumor-ablative surgery because the effects of these growth factors on the cancer cells are unknown. Also, their use in previously irradiated wounds or wounds that will receive postoperative radiation is not currently advisable.71,72 At the time this chapter was written, use of BMPs for mandible reconstruction was recommended only in clinical trials in carefully selected patients because the ultimate success of this technique was unknown, although very promising.
Baker SR, Sullivan MJ. Osteocutaneous free scapular flap for one-stage mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1988;114:267-277.
Blackwell KE, Lacombe V. The bridge lateral mandibular reconstruction plate revisited. Arch Otolaryngol Head Neck Surg. 1999;125:988-993.
Chepeha DB, Teknos TN, Fung K, et al. Lateral oromandibular defect: when is it appropriate to use a bridging reconstruction plate combined with a soft tissue revascularized flap? Head Neck. 2008;30:709-717.
Conley JJ. The crippled oral cavity. Plast Reconstr Surg. 1962;30:469-478.
Deleyiannis FWB, Dunkelbarger J, Lee E, et al. Reconstruction of the marginal mandibulectomy defect: an update. Am J Otolaryngol. 2007;28:363-366.
Gullane P. Primary mandibular reconstruction: analysis of 64 cases and evaluation of interface radiation dosimetry on bridging plates. Laryngoscope. 1991;101:1-24.
Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71-79.
Hidalgo DA, Pusic AL. Free flap mandibular reconstruction: a 10-year follow up study. Plast Reconstr Surg. 2002;110:438-449.
Melugin MB, Oyen OJ, Indresano AT. The effect of rim mandibulectomy configuration and residual segment size on postoperative fracture risk: an in vitro study. J Oral Maxillofac Surg. 2001;59:409-413.
Moscoso JF, Keller J, Genden E, et al. Vascularized bone flaps in oromandibular reconstruction. A comparative anatomic study of bone stock from various donor sites to assess suitability for endosseous dental implants. Arch Otolaryngol Head Neck Surg. 1994;120:36-43.
Moscoso JF, Urken ML. The iliac crest composite flap for oromandibular reconstruction. Otolaryngol Clin North Am. 1994;27:1097-1117.
Ostrup LT, Fredrickson JM. Distant transfer of a free, living bone graft by microvascular anastomosis. Plast Reconstr Surg. 1974;54:274-285.
Panje WR. Free compound groin flap reconstruction of anterior mandibular defect. Arch Otolaryngol. 1981;107:17-22.
Taylor GI. Reconstruction of the mandible with free composite iliac bone grafts. Ann Plast Surg. 1982;9:361-376.
Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124:46-55.
Urken ML, Buchbinder D, Weinberg H, et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: a comparative study of reconstructed and nonreconstructed patients. Laryngoscope. 1991;101:935-950.
Wei FC, Celik N, Yang WG, et al. Complications after reconstruction by plate and soft-tissue free flap in composite mandibular defects and secondary salvage reconstruction with osteocutaneous flap. Plast Reconstr Surg. 2003;112:37-42.
Werle AH, Tsue TT, Toby B, et al. Osteocutaneous radial forearm free flap: its use without significant donor site morbidity. Otolaryngol Head Neck Surg. 2000;123:711-717.
1. Lawson W, Biller HF. Mandibular reconstruction: bone graft techniques. Otolaryngol Head Neck Surg. 1982;90:589-594.
2. Adamo AK, Szal RL. Timing, results, and complications of mandibular reconstructive surgery: reports of 32 cases. J Oral Surg. 1979;37:755-763.
3. Nussenbaum B, Krebsbach PH. The role of gene therapy for craniofacial and dental tissue engineering. Adv Drug Deliv Rev. 2006;58:577-591.
4. Dingman RO, Natvig P. Surgery of Facial Fractures. Philadelphia: WB Saunders; 1964.
5. Conley JJ. The crippled oral cavity. Plast Reconstr Surg. 1962;30:469-478.
6. Urken ML. Reconstruction of the oral cavity following resection for cancer. Johnson JJ, Didolkar MS, editors. Head and Neck Cancer, Volume III. Amsterdam: Elsevier Science, 1993.
7. Melugin MB, Oyen OJ, Indresano AT. The effect of rim mandibulectomy configuration and residual segment size on postoperative fracture risk: an in vitro study. J Oral Maxillofac Surg. 2001;59:409-413.
8. Barttelbort SW, Bahn SL, Ariyan SA. Rim mandibulectomy for cancer of the oral cavity. Am J Surg. 1987;154:423-428.
9. Deleyiannis FWB, Dunkelbarger J, Lee E, et al. Reconstruction of the marginal mandibulectomy defect: an update. Am J Otolaryngol Head Neck Surg. 2007;28:363-366.
10. Marchetta FC. Function and appearance following surgery for intraoral cancer. Clin Plast Surg. 1976;3:471-479.
11. Cohen M, Schultz RC. Mandibular reconstruction. Clin Plast Surg. 1985;12:411-422.
12. Boyd JB, Gullane PJ, Rotstein LE, et al. Classification of mandibular defects. Plast Reconstr Surg. 1993;92:1266-1275.
13. Urken ML, Weinberg H, Vickery C, et al. Oromandibular reconstruction using microvascular composite free flaps. Report of 71 cases and a new classification scheme for bony, soft tissue and neurologic defects. Arch Otolaryngol Head Neck Surg. 1991;117:733-744.
14. Tucker HM. Nonrigid reconstruction of the mandible. Arch Otolaryngol Head Neck Surg. 1989;115:1190-1192.
15. Gullane P. Primary mandibular reconstruction: analysis of 64 cases and evaluation of interface radiation dosimetry on bridging plates. Laryngoscope. 1991;101:1-24.
16. Komisar A. The functional result of mandibular reconstruction. Laryngoscope. 1990;100:364-374.
17. Lawson W, Loscalzo LJ, Baek SM, et al. Experience with immediate and delayed mandibular reconstruction. Laryngoscope. 1982;92:5-10.
18. Panje WR, Bardach J, Krause CJ. Reconstruction of the oral cavity with a free flap. Plast Reconstr Surg. 1976;58:415-418.
19. Ostrup LT, Fredrickson JM. Distant transfer of a free, living bone graft by microvascular anastomosis. Plast Reconstr Surg. 1974;54:274-285.
20. Ostrup LT, Fredrickson JM. Reconstruction of mandibular defects after radiation, using a free, living bone graft transferred by microvascular anastomosis. Plast Reconstr Surg. 1975;55:563-572.
21. Taylor GI, Townsend P, Corlett R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps. Plast Reconstr Surg. 1979;64:745-759.
22. Taylor GI, Wilson KR, Rees MD, et al. The anterior tibial vessels and their role in epiphyseal and diaphyseal transfer of the fibula: experimental study and clinical applications. Br J Plast Surg. 1988;41:451-469.
23. Panje WR. Free compound groin flap reconstruction of anterior mandibular defect. Arch Otolaryngol. 1981;107:17-22.
24. Taylor GI. Reconstruction of the mandible with free composite iliac bone grafts. Ann Plast Surg. 1982;9:361-376.
25. Fredrickson JM, Man SC, Hayden RE. Revascularized iliac bone graft for mandibular reconstruction. Acta Otolaryngol. 1985;99:214-223.
26. Taylor GI, Miller GDH, Ham FJ. The free vascularized bone graft: a clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533-544.
27. Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71-79.
28. Klotch DW. Mandibular reconstruction using AO plates. Am J Surg. 1987;154:384-387.
29. Schusterman MA, Reece GP, Kroll SS, et al. Use of the AO plate for immediate mandibular reconstruction in cancer patients. Plast Reconstr Surg. 1991;88:588-593.
30. Irish JC, Gullane PJ, Gilbert RW, et al. Primary mandibular reconstruction with the titanium hollow screw reconstruction plate: evaluation of 51 cases. Plast Reconstr Surg. 1995;96:93-99.
31. Arden RL, Rachel JD, Marks SC, et al. Volume-length impact of lateral jaw resections on complication rates. Arch Otolaryngol Head Neck Surg. 1999;125:68-72.
32. Boyd JB. Use of reconstruction plates in conjunction with soft-tissue free flaps for oromandibular reconstruction. Clin Plast Surg. 1994;21:69-77.
33. Nicholson RE, Schuller DE, Forrest LR, et al. Factors involved in long- and short-term mandibular plate exposure. Arch Otolaryngol Head Neck Surg. 1997;123:217-222.
34. Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps. Arch Otolaryngol Head Neck Surg. 1998;124:46-55.
35. Thoma A, Levis C, Young JEM. Oromandibular reconstruction after cancer resection. Clin Plast Surg. 2005;32:361-375.
36. Moscoso JF, Keller J, Genden E, et al. Vascularized bone flaps in oromandibular reconstruction. A comparative anatomic study of bone stock from various donor sites to assess suitability for endosseous dental implants. Arch Otolaryngol Head Neck Surg. 1994;120:36-43.
37. Frodel JL, Funk GF, Capper DT, et al. Osseointegrated implants: a comparative study of bone thickness in four vascularized bone flaps. Plast Reconstr Surg. 1993;92:449-457.
38. Urken ML. Composite free flaps in oromandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1991;117:724-732.
39. Hidalgo DA, Pusic AL. Free flap mandibular reconstruction: a 10-year follow up study. Plast Reconstr Surg. 2002;110:438-449.
40. Anthony JP, Rawnsley JD, Benhaim P, et al. Donor leg morbidity and function after fibula free flap mandible reconstruction. Plast Reconstr Surg. 1995;96:146-152.
41. Shindo M, Fong BP, Funk GF, et al. The fibula osteocutaneous flap in head and neck reconstruction: a critical evaluation of donor site morbidity. Arch Otolaryngol Head Neck Surg. 2000;126:1467-1472.
42. Moscoso JF, Urken ML. The iliac crest composite flap for oromandibular reconstruction. Otolaryngol Clin North Am. 1994;27:1097-1117.
43. Baker SR, Sullivan MJ. Osteocutaneous free scapular flap for one-stage mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1988;114:267-277.
44. Coleman SC, Burkey BB, Day TA, et al. Increasing use of the scapula osteocutaneous free flap. Laryngoscope. 2000;110:1419-1424.
45. Villaret DB, Futran NA. The indications and outcomes in the use of osteocutaneous radial forearm free flap. Head Neck. 2003;25:475-481.
46. Werle AH, Tsue TT, Toby B, et al. Osteocutaneous radial forearm free flap: its use without significant donor site morbidity. Otolaryngol Head Neck Surg. 2000;123:711-717.
47. Carroll WR, Esclamado R. Preoperative vascular imaging for the fibular osteocutaneous flap. Arch Otolaryngol Head Neck Surg. 1996;122:708-712.
48. Lorenz RR, Esclamado R. Preoperative magnetic resonance angiography in fibular free flap reconstruction of head and neck defects. Head Neck. 2001;23:844-850.
49. Rozen WM, Ashton MW, Stella DL, et al. Magnetic resonance angiography and computed tomography angiography for free fibula flap transfer. J Reconstr Microsurg. 2008;24:457-458.
50. Futran ND, Stack BC, Zaccardi MJ. Preoperative color flow Doppler imaging for fibula free tissue transfers. Ann Vasc Surg. 1998;12:445-450.
51. Shaw RJ, Kanatas AN, Lowe D, et al. Comparison of miniplates and reconstruction plates in mandibular reconstruction. Head Neck. 2004;26:456-463.
52. Schrag C, Chang YM, Tsai CY, et al. Complete rehabilitation of the mandible following segmental resection. J Surg Oncol. 2006;94:538-545.
53. Disher MJ, Esclamado RM, Sullivan MJ. Indications for the AO plate with a myocutaneous flap instead of revascularized tissue transfer for mandibular reconstruction. Laryngoscope. 1993;103:1264-1268.
54. Chepeha DB, Teknos TN, Fung K, et al. Lateral oromandibular defect: when is it appropriate to use a bridging reconstruction plate combined with a soft tissue revascularized flap? Head Neck. 2008;30:709-717.
55. Deleyiannis FWB, Lee E, Gastman B, et al. Prognosis as a determinant of free flap utilization for reconstruction of the lateral mandibular defect. Head Neck. 2006;28:1061-1068.
56. Klotch DW, Gal TJ, Gal RL. Assessment of plate use for mandibular reconstruction: has changing technology made a difference? Otolaryngol Head Neck Surg. 1999;121:388-392.
57. Blackwell KE, Buchbinder D, Urken ML. Lateral mandibular reconstruction using soft-tissue free flaps and plates. Arch Otolaryngol Head and Neck Surg. 1996;122:672-678.
58. Blackwell KE, Lacombe V. The bridge lateral mandibular reconstruction plate revisited. Arch Otolaryngol Head Neck Surg. 1999;125:988-993.
59. Wei FC, Celik N, Yang WG, et al. Complications after reconstruction by plate and soft-tissue free flap in composite mandibular defects and secondary salvage reconstruction with osteocutaneous flap. Plast Reconstr Surg. 2003;112:37-42.
60. Cordeiro PG, Disa JJ, Hidalgo DA, et al. Reconstruction of the mandible with osseous free flaps: a 10-year experience with 150 consecutive patients. Plast Reconstr Surg. 1999;104:1314-1320.
61. Urken ML, Buchbinder D, Weinberg H, et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: a comparative study of reconstructed and nonreconstructed patients. Laryngoscope. 1991;101:935-950.
62. Wilson KM, Rizk NM, Armstrong SL, et al. Effects of hemimandibulectomy on quality of life. Laryngoscope. 1998;108:1574-1577.
63. Disa JJ, Winters RM, Hidalgo DA. Long term evaluation of bone mass in free flap mandible reconstruction. Am J Surg. 1997;174:503-506.
64. D’Urso PS, Barker TM, Earwaker WJ, et al. Stereolithographic biomodeling in cranio-maxillofacial surgery: a prospective trial. J Craniomaxillofac Surg. 1999;27:30-37.
65. Urist MR. Bone formation by autoinduction. Science. 1965;150:893-899.
66. Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A. 1988;85:9484-9488.
67. Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001;83(Suppl 1, Pt 1):S1-S6.
68. Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein–2. J Oral Maxillofac Surg. 2008;66:616-624.
69. Clokie CML, Sandor GKB. Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc. 2008;74:67-72.
70. Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766-770.
71. Nussenbaum B, Rutherford RB, Krebsbach PH. Bone regeneration in cranial defects previously treated with radiation. Laryngoscope. 2005;115:1170-1177.
72. Nussenbaum B, Rutherford RB, Teknos TN, et al. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther. 2003;14:1107-1115.